NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lubiprostone is an activator of chloride channels (ClC-2) in the intestine and is used for treatment of chronic constipation and irritable bowel syndrome. Lubiprostone has not been linked to serum enzyme elevations during treatment or to episodes of clinically apparent liver injury.
Background
Lubiprostone (loo” bi pros’ tone) is a fatty acid metabolite of prostaglandin E1 that activates the chloride channel protein 2 in the intestine, which increase fluid secretion into the lumen and thus promote intestinal transport. Short term use of lubiprostone increases the number of spontaneous bowel movements and can alleviate symptoms of constipation in patients with chronic idiopathic constipation and irritable bowel syndrome. Lubiprostone was approved for use in the United States in 2006 for short term therapy of chronic idiopathic constipation and indications subsequently expanded to opioid-induced constipation in adults with chronic non-cancer pain and to management of adult women with irritable bowel syndrome and constipation. Lubiprostone is available in capsules of 8 and 24 mcg under the brand name Amitiza. The recommended dose for chronic constipation is 24 mcg twice daily and for irritable bowel syndrome with constipation 8 mcg twice daily. Systemic absorption is minimal, but may be greater in patients with liver disease. Side effects include nausea, diarrhea, abdominal pain and bloating, headache and chest tightness with shortness of breath.
Hepatotoxicity
In clinical trials, lubiprostone therapy was not associated with significant changes in serum enzyme levels or episodes of clinically apparent liver injury. Since its approval and marketing, isolated case reports of serum aminotransferase elevations have been reported to the sponsor, but there have been no published reports of clinically apparent liver injury attributable to lubiprostone. Thus, liver injury from lubiprostone must be extremely rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Lubiprostone is largely active on the epithelial cells in the intestinal tract and has minimal absorption. The lack of systemic absorption of lubiprostone and the low daily doses used (in micrograms rather than milligrams) probably account for its lack of causing liver injury.
Drug Class: Gastrointestinal Agents, Drugs for Constipation, Irritable Bowel Syndrome Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lubiprostone – Amitiza®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lubiprostone | 136790-76-6 | C20-H32-F2-O5 |
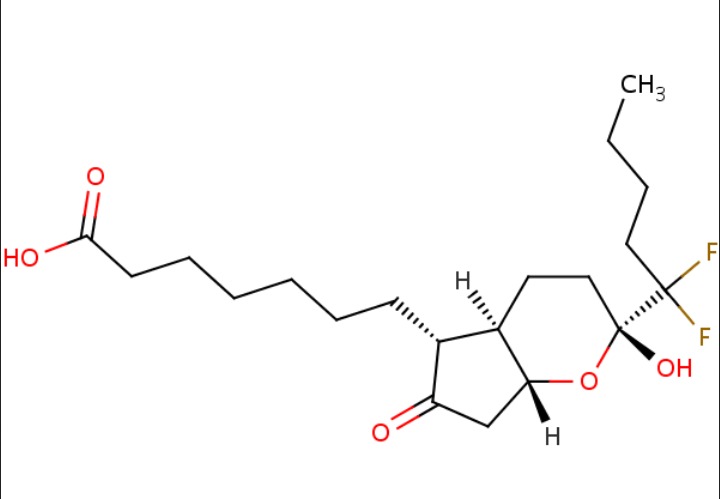 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 April 2019
- Zimmerman HJ. Laxatives. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 721-3.(Expert review of hepatotoxicity of laxatives published in 1999 before the availability of lubiprostone).
- Sharkey KA, MacNaughton WK. Gastrointestinal motility and water flux, emesis; agents used in biliary and pancreatic disease. In, Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 921-44.(Textbook of pharmacology and therapeutics; lubiprostone is discussed as an agent for constipation).
- Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther 2007; 25: 1351-61. [PubMed: 17509103](Among 129 patients with chronic constipation treated with 3 doses of lubiprostone or placebo for 3 weeks, there was no "clear relationship between the dose of lubiprostone and type or frequency of abnormalities" of blood chemistry results).
- Lubiprostone (Amitiza) for irritable bowel syndrome with constipation. Med Lett Drugs Ther 2008; 50 (1290): 53-4. [PubMed: 18617872](Concise review of mechanism of action, safety and efficacy of lubiprostone for irritable bowel syndrome with constipation shortly after its approval for this indication in the United States; side effects include nausea, diarrhea, and chest tightness with difficulty breathing; no mention of ALT elevations or hepatotoxicity).
- Barish CF, Drossman D, Johanson JF, Ueno R. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci 2010; 55: 1090-7. [PubMed: 20012484](Among 237 patients with chronic constipation treated with lubiprostone vs placebo, side effects included nausea [24%] and headache [7%], but there were "no clinically relevant changes in laboratory values").
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to agents used for constipation).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children; there were no gastrointestinal agents in the top 41 causes).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to gastrointestinal agents, laxatives or drugs for constipation).
- Lembo AJ, Johanson JF, Parkman HP, Rao SS, Miner PB Jr, Ueno R. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig Dis Sci 2011; 56: 2639-45. [PMC free article: PMC3169778] [PubMed: 21769655](Among 248 patients with chronic constipation treated with lubiprostone for up to a year, adverse events occurred in 75% of patients, mostly nausea [21%], diarrhea [11%], and headache [10%]; and "there were no clinically meaningful trends" in ALT or AST).
- Chamberlain SM, Rao SS. Safety evaluation of lubiprostone in the treatment of constipation and irritable bowel syndrome. Expert Opin Drug Saf 2012; 11: 841-50. [PubMed: 22834474](Review of the safety of lubiprostone based upon data from 522 patients with irritable bowel syndrome; no mention of ALT elevations or hepatotoxicity).
- Shah E, Kim S, Chong K, Lembo A, Pimentel M. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med 2012; 125: 381-93. [PubMed: 22444104](Systematic review of adverse side effects of drugs used to treat irritable bowel syndrome, including 3 trials of lubiprostone in which there was no difference in dropout rates due to adverse events between drug and placebo, and the principal side effects seen more often with lubiprostone were nausea and diarrhea).
- Chey WD, Drossman DA, Johanson JF, Scott C, Panas RM, Ueno R. Safety and patient outcomes with lubiprostone for up to 52 weeks in patients with irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2012; 35: 587-99. [PubMed: 22251419](Among 520 patients with irritable bowel syndrome treated with lubiprostone for up to 52 weeks, none had a serious adverse event that was attributed to the drug and "Significant changes from baseline...in biochemical laboratory values...were not seen").
- Hyman PE, Di Lorenzo C, Prestridge LL, Youssef NN, Ueno R. Lubiprostone for the treatment of functional constipation in children. J Pediatr Gastroenterol Nutr 2014; 58: 283-91. [PubMed: 24048162](Among 124 children with chronic idiopathic constipation treated with one of 3 doses of lubiprostone, spontaneous bowel movements increased in all groups, adverse events included nausea, diarrhea and abdominal pain and there "were no clinically significant trends in clinical laboratory tests" or serious adverse events judged to be related to lubiprostone therapy).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to lubiprostone or other agents used for chronic idiopathic constipation).
- Spierings ELH, Drossman DA, Cryer B, Mazen Jamal M, Losch-Beridon T, Mareya SM, Wang M. Efficacy and safety of lubiprostone in patients with opioid-induced constipation: phase 3 study results and pooled analysis of the effect of concomitant methadone use on clinical outcomes. Pain Med 2018; 19: 1184-94. [PubMed: 29016868](Among 451 patients with opioid induced constipation enrolled in a 12 week controlled trial, increases in frequency of spontaneous bowel movements were similar with lubiprostone and placebo therapy, while adverse events rates were minimally higher in the drug-treated group; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Lubiprostone: RU 0211, SPI 0211.[Drugs R D. 2005]Review Lubiprostone: RU 0211, SPI 0211.. Drugs R D. 2005; 6(4):245-8.
- Review Clinical pharmacology of lubiprostone, a chloride channel activator in defecation disorders.[Expert Opin Drug Metab Toxicol...]Review Clinical pharmacology of lubiprostone, a chloride channel activator in defecation disorders.Ginzburg R, Ambizas EM. Expert Opin Drug Metab Toxicol. 2008 Aug; 4(8):1091-7.
- Review Lubiprostone: chronic constipation and irritable bowel syndrome with constipation.[Expert Opin Pharmacother. 2009]Review Lubiprostone: chronic constipation and irritable bowel syndrome with constipation.Lacy BE, Chey WD. Expert Opin Pharmacother. 2009 Jan; 10(1):143-52.
- Lubiprostone for chronic idiopathic constipation and irritable bowel syndrome with constipation.[Expert Rev Gastroenterol Hepat...]Lubiprostone for chronic idiopathic constipation and irritable bowel syndrome with constipation.Saad R, Chey WD. Expert Rev Gastroenterol Hepatol. 2008 Aug; 2(4):497-508.
- Review Lubiprostone--a novel treatment for irritable bowel syndrome with constipation.[Drugs Today (Barc). 2008]Review Lubiprostone--a novel treatment for irritable bowel syndrome with constipation.Owen RT. Drugs Today (Barc). 2008 Sep; 44(9):645-52.
- Lubiprostone - LiverToxLubiprostone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...