NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Ampicillin is an oral, third generation aminopenicillin and is used widely to treat mild-to-severe infections due to susceptible organisms. Ampicillin has been linked with idiosyncratic liver injury, but very rarely and in largely in isolated case reports.
Background
Ampicillin (am" pi sil' in) is an oral, third generation penicillin that is one of the most commonly used antibiotics worldwide. Ampicillin has been available in the United States since the mid-1960s and continues to be widely used for bacterial infections in both children and adults. Ampicillin is indicated for mild-to-severe upper respiratory tract infections caused by susceptible agents, such as (but not limited to) Escherichia coli, Hemophilus influenzae, Listeria monocytogenesis, Neisseria gonorrhoeae, Proteus mirabilis, Salmonella, Shigella, Staphylococcus aureus (non-penicillinase producing), Staphylococcus epidermidis, and Streptococcus pneumoniae. Ampicillin is also used for pneumonia, meningitis, endocarditis and uncomplicated gonorrhea. Ampicillin is available orally in multiple generic formulations as capsules of 250 and 500 mg and is usually given in doses of 250 to 500 mg every 6 to 8 hours for 7 to 14 days. Ampicillin is also available in parenteral form (intramuscular and intravenous) and recommended doses are 2 to 4 grams daily in divided doses given every 4 to 6 hours. Side effects are usually mild and self-limited and can include diarrhea, nausea and vomiting, fatigue, headache, and rash. Rare but potentially serious adverse events include hypersensitivity reactions, anaphylaxis, severe skin rash, Stevens Johnson syndrome, C. difficile diarrhea, neutropenia, aplastic anemia and thrombocytopenic purpura.
Hepatotoxicity
Rare instances of idiosyncratic liver injury have been reported in persons receiving the aminopenicillins. The incidence is far lower with ampicillin than occurs with amoxicillin, occurring probably in less than 1 in 100,000 exposed persons. Cases are characterized by a short latency period of a few days to as long as two weeks. The onset of liver injury can occur after the antibiotic is stopped. The serum enzyme pattern associated with aminopenicillin liver injury has included a hepatocellular pattern with marked elevations in ALT and AST, and minimal elevations in alkaline phosphatase and rapid recovery after withdrawal. In addition, cholestatic forms of hepatic injury with marked alkaline phosphatase elevations (as also seen with penicillin-induced liver injury) have also been described, some of which have been associated with prolonged cholestasis and, rarely, with vanishing bile duct syndrome. The onset of hepatic injury may be accompanied by skin rash, toxic epidermal necrolysis or Stevens Johnson syndrome. Autoantibodies are uncommon.
Likelihood score: C (probable but rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver injury associated with ampicillin use is probably hypersensitivity or allergy. Cases of recurrence upon rechallenge or reexposure have been reported.
Outcome and Management
In the few cases of ampicillin related acute liver injury that have been described, most patients have recovered, although recovery has been slow in some cholestatic instances (2 to 6 months). Rare instances of acute liver failure and several cases of vanishing bile duct syndrome have been reported with ampicillin induced liver injury. Corticosteroids have often been used to treat the allergic manifestations and, while they may improve fever and rash promptly, their efficacy in ameliorating the accompanying liver disease has not been shown. Instances of recurrence of liver injury with reexposure to the aminopenicillins and recurrence with exposure to cephalosporins have been reported. Patients with aminopenicillin induced hepatitis should avoid reexposure to other penicillins and should take cephalosporins with caution.
Drug Class: Antiinfective Agents, Aminopenicillins
Other Drugs in the Subclass, Aminopenicillins: Amoxicillin, Amoxicillin-Clavulanate, Ampicillin-Sulbactam, Bacampicillin, Pivampicillin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ampicillin – Generic, Principen®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
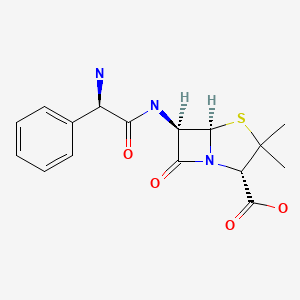
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ampicillin | 69-53-4 | C16-H19-N3-O4-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Penicillins. In, Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams and Wilkins, 1999. p. 595-6.(Expert review of penicillins and liver injury published in 1999; the penicillins commonly lead to hypersensitivity reactions but rarely to liver injury).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics mentions that penicillins rarely cause liver injury and both hepatocellular and cholestatic patterns of injury have been described).
- MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1023-38.(Textbook of pharmacology and therapeutics).
- Knirsch AK, Gralla EJ. Abnormal serum transaminase levels after parenteral ampicillin and carbenicillin administration. N Engl J Med. 1970;282:1081–2. [PubMed: 5438429](Elevations in AST and CPK, but not ALT, occurred after intramuscular injections of ampicillin and carbenicillin, but not after cephalosporins or saline; thus, the elevations probably reflected muscle rather than liver injury).
- McArthur JE, Dyment PG. Stevens-Johnson syndrome with hepatitis following therapy with ampicillin and cephalexin. N Z Med J. 1975;81:390–2. [PubMed: 1057088](Stevens-Johnson syndrome developed in a 9 month old child given ampicillin [rash] and cephalexin, with subsequent hepatocellular injury [bilirubin 13 mg/dL], resolving with prednisone therapy).
- Tamarkina AD, Dement'eva ES, Krylova NI, Minasova GS, Kuleshova EE. Antibiot Med Biotekhnol. 1985;30:62–4. [Enzymological evaluation of the hepatotoxicity of ampicillin and its therapeutic form, roscillin, in the treatment of pyelonephritis in pregnancy] Russian. [PubMed: 3994343](Prospective analysis of serum enzyme levels in 12 patients receiving ampicillin found no changes during 12 days of therapy, although minor changes occurred when it was stopped).
- Lees L, Milson JA, Knirsch AK, Greenhalgh K. Sulbactam plus ampicillin: interim review of efficacy and safety for therapeutic and prophylactic use. Rev Infect Dis. 1986;8 Suppl 5:S644–S650. [PubMed: 3026019](Review of 45 studies of ampicillin-sulbactam in 899 patients found successful outcome in 92%; ALT elevations in 6.9%, AST in 6.2%, but all resolved with stopping and similar rates were reported with use of comparative agents).
- Galante D, Esposito S, Barba D, Ruffilli MP. Clinical efficacy and safety of sulbactam/ampicillin in patients suffering from chronic liver disease. J Antimicrob Chemother. 1987;19:527–32. [PubMed: 3034850](41 patients with advanced liver disease were given sulbactam/ampicillin: there was no worsening of liver disease or enzyme elevations attributed to medication).
- Cavanzo FJ, Garcia CF, Botero RC. Chronic cholestasis, paucity of bile ducts, red cell aplasia, and the Stevens-Johnson syndrome. An ampicillin-associated case. Gastroenterology. 1990;99:854–6. [PubMed: 2116345](A 35 year old woman developed Stevens-Johnson syndrome with cholestatic hepatitis and red cell aplasia 4 days after starting oral ampicillin and subsequently developed vanishing bile duct syndrome and prolonged cholestasis, but with gradual ultimate improvement after several years).
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232:133–8. [PubMed: 1506809](Adverse drug reaction reports in Denmark from 1978 to 1987; no mention of aminopenicillins).
- Köklü S, Yüksel O, Filik L, Usküdar O, Altundag K, Altiparmak E. Recurrent cholestasis due to ampicillin. Ann Pharmacother. 2003;37:395–7. [PubMed: 12639171](23 year old man had recurrent bouts of liver injury 12, 8, and 5 days after starting three 7 day courses of ampicillin, last episode marked by bilirubin 2.1 mg/dL, ALT 265 U/L, and Alk P 455 U/L, resolving within 10 days of stopping).
- Köklü S, Yüksel O, Yolcu OF, Arhan M, Altiparmak E. Cholestatic attack due to ampicillin and cross-reactivity to cefuroxime. Ann Pharmacother. 2004;38:1539–40. [PubMed: 15266040](Follow up on 23 year old man in previous report, who redeveloped liver injury 17 days after starting a 10 day course of cefuroxime [bilirubin 0.7 mg/dL, ALT 427 U/L, Alk P 646 U/L], resolving within 2 months, suggesting cross sensitivity to hepatic injury between cephalosporins and ampicillin).
- Köklü S, Köksal AS, Asil M, Kiyici H, Coban S, Arhan M. Probable sulbactam/ampicillin-associated prolonged cholestasis. Ann Pharmacother. 2004;38:2055–8. [PubMed: 15494387](74 year old developed cholestatic hepatitis arising 1 week after 7 day course of ampicillin-sulbactam with prolonged jaundice [bilirubin 31 mg/dL, ALT 33 U/L, Alk P 519 U/L], resolving after 7 months, except mild GGT elevations).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure; one case was attributed to amoxicillin-clavulanate, but none to penicillin, ampicillin or amoxicillin alone).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 23 cases were attributed to amoxicillin-clavulanate, 2 cases to amoxicillin, but none to ampicillin).
- Björnsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50:511–7. [PubMed: 19155082](Among 685 patients identified an average of 10 years after an episode of drug induced liver injury, 23 [3.4%] had continuing liver disease, 8 with cirrhosis. One patient who died of cirrhosis had ampicillin-attributed liver injury 3 years previously).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; 3 [1%] were attributed to the combination of amoxicillin and clavulanate, but none were attributed to ampicillin or amoxicillin alone].
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 66 due to antimicrobial agents, including two attributed to amoxicillin but none to ampicillin; no details given).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, amoxicillin-clavulanate accounted for 38 cases [0.4%] for an adjusted odds ratio of 1.7, whereas neither amoxicillin or ampicillin alone were listed among the 41 most common causes [linked to at least 30 cases]).
- Köksal AS, Yildiz H, Onder O, Avci S, Kayaåetin E. Sulbactam/ampicillin associated hepatocellular type liver injury. Acta Gastroenterol Belg. 2012;75:66–7. [PubMed: 22567755](47 year old woman developed jaundice 8 days after a 4 day course of ampicillin-sulbactam [bilirubin 18.9 mg/dL, ALT 1344 U/L, Alk P 208 U/L], resolving within 10 weeks of stopping).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, Presentation and Outcomes in Patients with Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, the most commonly implicated agent being amoxicillin with clavulanate [15 cases]; none were attributed to amoxicillin or ampicillin alone).
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [PubMed: 23350857](Among 17 cases of suspected drug induced liver injury seen at a single referral hospital in Tasmania over a 12 month period, 11 were due to antibiotics including flucloxacillin in 4, amoxicillin in 2, amoxicillin-clavulanate in 2, and rifampin, moxifloxacin and ciprofloxacin in 1 each).
- Ferrajolo C, Verhamme KM, Trifirò G, 't Jong GW, Giaquinto C, Picelli G, Oteri A, et al. Idiopathic acute liver injury in paediatric outpatients: incidence and signal detection in two European countries. Drug Saf. 2013;36:1007–16. [PubMed: 23591830](Analysis of 3 electronic healthcare databases from Italy and the Netherlands from 2000-2008 identified 785 cases of unexplained acute liver injury in children, linked to 110 possible medications, with increased adjusted relative risk [RR] of recent exposure to amoxicillin-clavulanate [RR=18.6] and amoxicillin [RR=7.5]).
- Lim R, Choudry H, Conner K, Karnsakul W. A challenge for diagnosing acute liver injury with concomitant/sequential exposure to multiple drugs: can causality assessment scales be utilized to identify the offending drug? Case Rep Pediatr. 2014;2014:156389. [PMC free article: PMC4260426] [PubMed: 25506455](12 year old boy was treated with oral ampicillin for suspected Streptococcal sore throat and developed evidence of neck cellulitis and was treated with intravenous ampicillin-sulbactam, vancomycin, and oral clindamycin at which point he developed jaundice [bilirubin 3.0 mg/dL, ALT 406 U/L, Alk P 404 U/L, INR 1.1] and was continued on ampicillin-sulbactam, developing rash and deep and prolonged jaundice and itching which lasted 5 months and resolved, but with persistence of alkaline phosphatase elevations, the causative agent being unclear but most likely ampicillin-sulbactam).
- Kaye JA, Castellsague J, Bui CL, Calingaert B, McQuay LJ, Riera-Guardia N, Saltus CW, et al. Risk of acute liver injury associated with the use of moxifloxacin and other oral antimicrobials: a retrospective, population-based cohort study. Pharmacotherapy. 2014;34:336–49. [PMC free article: PMC4260122] [PubMed: 24865821](In a US healthcare database with 1.3 million antimicrobial users, there were 607 cases of liver injury and 11 cases of liver failure, the highest relative risk for current single use being 3.2 for levofloxacin, 2.5 for amoxicillin-clavulanate, 2.5 for doxycycline, 2.3 for moxifloxacin and 2.3 for amoxicillin).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, of which 37 [19%] were attributed to antimicrobials, including one to benzathine penicillin and 3 to amoxicillin-clavulanate, but none to 2nd or 4th generation penicillins).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics of which 106 [12%] were due to penicillins including one to 1st, three to 2nd [all due to oxacillin], 97 to 3rd [91 to amoxicillin-clavulanate, and 6 to amoxicillin alone] and five to 4th generation penicillins [all 5 to piperacillin/tazobactam]).
- Guéant JL, Romano A, Cornejo-Garcia JA, Oussalah A, Chery C, Blanca-López N, Guéant-Rodriguez RM, et al. HLA-DRA variants predict penicillin allergy in genome-wide fine-mapping genotyping. J Allergy Clin Immunol. 2015;135:253–9. [PubMed: 25224099](In a genome wide association study of 387 patients with immediate allergic reactions to beta-lactam antibiotics, several class 2 HLA associations [HLA-DRA regions] were found for penicillin responses, but they did not apply to cephalosporin cases).
- Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327–34. [PubMed: 25618544](Review of the most common causes of drug induced liver injury in 3 recent surveys, all of which listed amoxicillin-clavulanate as the most frequent cause; none listed other penicillins).
- Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–89. [PMC free article: PMC5367948] [PubMed: 28043905](A genome wide association study done on 862 Caucasian patients with drug induced liver injury demonstrated a strong link with HLA-A*33:01 in those with cholestatic liver injury, particularly in cases attributed to terbinafine, fenofibrate and ticlopidine).
- Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS, Walensky RP, Nelson SB. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol. 2015;136:1288–94.e1. [PMC free article: PMC4640981] [PubMed: 25981739](Among 824 patients who underwent outpatient parenteral antibiotic therapy for at least 2 weeks, 210 [25%] developed eosinophilia including 58 of 207 [28%] who received “penicillins” of whom 3 developed signs of “possible” DRESS syndrome; specific penicillins accounting for the cases were not provided).
- Munz M, Grummich H, Birkmann J, Wilhelm M, Holzgrabe U, Sörgel F. Severe drug-Induced liver injury as an adverse drug event of antibiotics: a case report and review of the literature. Chemotherapy. 2017;62:367–73. [PubMed: 28934748](20 year old woman received 4 antibiotics [amoxicillin, ciprofloxacin, cefazolin and clindamycin] and acetaminophen within weeks of developing rash, fever and jaundice [bilirubin 3.7 mg/dL, ALT 1219 U/L, Alk P 143 U/L, GGT 201 U/L, INR 1.6], with worsening and signs of hepatic failure but subsequent spontaneous and complete resolution, the specific cause being obscure because of the many drug exposures).
- Takeuchi Y, Shinozaki T, Kumamaru H, Hiramatsu T, Matsuyama Y. Analyzing intent-to-treat and per-protocol effects on safety outcomes using a medical information database: an application to the risk assessment of antibiotic-induced liver injury. Expert Opin Drug Saf. 2018;17:1071–9. [PubMed: 30252549](Cohort matching of cases with vs without antibiotic therapy in a large electronic medical record database from the University of Tokyo Hospital from 2011 to 2015 with adjustments found rates of liver test abnormalities within 30 days of starting penicillins [25.2 per 1000] was higher than that of fluoroquinolones [11.4] and macrolide antibiotics [8.1] as well as controls [6.5 to 7.1]).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–16.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome wide association studies on 2048 patients with drug induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Amoxicillin.[LiverTox: Clinical and Researc...]Review Amoxicillin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ampicillin-Sulbactam.[LiverTox: Clinical and Researc...]Review Ampicillin-Sulbactam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Penicillins (3rd Generation).[LiverTox: Clinical and Researc...]Review Penicillins (3rd Generation).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Penicillins (4th Generation).[LiverTox: Clinical and Researc...]Review Penicillins (4th Generation).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Intravenous sulbactam/ampicillin in the treatment of pediatric infections.[Diagn Microbiol Infect Dis. 1989]Intravenous sulbactam/ampicillin in the treatment of pediatric infections.Lee CY, Lin TY, Chu ML, Lee MJ, Hsu CY, Huang LM, Chen CM. Diagn Microbiol Infect Dis. 1989 Jul-Aug; 12(4 Suppl):179S-183S.
- Ampicillin - LiverToxAmpicillin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...