12.1. INTRODUCTION
The ability to molecularly manipulate protein expression in the rodent brain has proven to be a powerful tool in neuroscience. Specifically, the generation of transgenic mouse lines has allowed scientists to begin to answer important questions about the functional role of specific proteins. However, this technique still suffers from the lack of strict temporal and spatial regulation of protein expression as well as the possibility of genetic compensation. More recently, the expression of heterologous proteins in organotypic brain slices and dissociated neuronal cultures has become another valuable method to study protein function in neurons. In this system, temporal regulation is achieved by acute expression of proteins using various transfection techniques or viruses encoding the protein of choice. A major advantage of this approach is that it allows the direct comparison of cellular properties, such as dendritic spine morphology or synaptic function, between neurons expressing the protein of interest and neighboring control neurons from the same animal. However, a drawback of this technique is that placing dissociated neurons or brain slices in culture invariably results in a degree of cell death and abnormal synaptic rewiring that can perturb the results obtained from various cellular assays. In this chapter, we describe the use of Sindbis viral-mediated gene transfer to acutely express proteins in vivo in the rat brain. This relatively new technique allows acute expression of a protein of choice in a temporally and spatially restricted manner. Importantly, the neurons expressing the recombinant proteins are allowed to do so while remaining in their physiological environment in freely behaving animals. Cell-based assays on the infected neurons can then be performed using well-established standard preparations, such as acute brain slices.
12.2. GENERATION OF SINDBIS VIRAL PARTICLES
Several viral vectors, including the herpes simplex virus, the lentivirus and Sindbis virus can be used to acutely introduce genes in neurons in vivo. We have chosen the Sindbis virus system because it produces the recombinant protein of interest rapidly and is neurotropic [1]. The Sindbis virus is a member of the alphavirus family. These viruses are small-enveloped viruses with single-stranded RNA genomes [2]. The Sindbis expression system is a transient expression system in which the Sindbis life-cycle is exploited to produce recombinant proteins. Green fluorescent protein (GFP) is one obvious marker of choice for monitoring the successful infection of neurons. It can be fused to the recombinant cDNA of interest or encoded as a separate protein as part of a bicistronic cDNA using an internal ribosomal entry site (IRES). We found that either combination allowed enough GFP expression for good visualization of infected neurons within 24 hours of in vivo expression.
The generation of infective Sindbis virus particles has been described previously [3,4]. Briefly, the cDNA of interest is cloned into a carefully designed plasmid vector (pSinRep5) under the control of the Sindbis virus subgenomic promoter. This DNA construct is linearized and used to make recombinant RNA in vitro, which is capped, polyadenylated and then introduced into BHK-21 cells by electroporation. This RNA contains both the inserted gene of interest and the Sindbis viral genome components that are essential for the replication of the viral genome. However, it does not contain the genes for the structural proteins that are needed to generate the virus particles. These genes are provided by another RNA that is also transcribed in vitro from the linearized helper virus plasmid DH(26S). This helper RNA needs to be co-transfected into the BHK21 cells to provide the structural proteins in trans. Because the helper RNA lacks a packaging signal, the particles released by the transfected cells contain only the recombinant RNA and are ready to infect new cells but will only undergo one round of infection. Such infection is thus termed a “dead-end” infection.
After 36 to 48 hours of expression, most BHK21 cells should show signs of cytotoxicity. At this point, the supernatant of these cells containing the viral particles is collected and concentrated. We found that simple sedimentation of virus particles by centrifugation yields titers that are sufficient for in vivo infection. Briefly, the supernatant is first centrifuged at 2000 rpm for 5 min to remove cell debris. This supernatant is then ultracentrifuged in a swinging-bucket rotor (e.g., Beckman SW40) at 30,000 rpm for 90 min (derived from [3]) and carefully aspirated from the top, leaving approximately 200 μl. The remaining, usually invisible, pellet is resuspended, aliquoted in small amounts and stored at 80°C.
The Sindbis virus has been classified as a Biosafety Level-2 (BL-2) agent by the NIH Recombinant DNA Advisory Committee due to its low level of pathogenicity in humans. Experiments using this virus need to be approved by the administrative panel on biosafety of your institution. BL-2 facilities are used to synthesize the viruses and personnel needs to be trained to work with BL-2 agents. The viral particles can be inactivated by organic solvents, bleach or autoclaving.
The high and rapid onset of expression of this virus is accomplished by progressively shutting off protein expression of the infected host cell. This behavior has raised legitimate concerns about the cytotoxicity of Sindbis viruses. However, we have found that neurons expressing these vectors in vivo for periods up to 30 hours showed no electrophysiological or morphological signs of toxicity. Other investigators have described “non-toxic” in vivo expression up to 48 hours [5,6]. Furthermore, less toxic, modified versions of the Sindbis vectors have now been engineered [7,8]. Alternatively, the lentiviral expression system can be used. Lentiviruses have much lower potential for cytotoxicity but this comes at the cost of much lower levels of expression of the protein of interest [9]. This system is also less neurotropic and requires, in our hands, up to seven days of in vivo expression for suitable visualization of the infected neurons.
For in vivo injection of viruses, optimizing the conditions of infection and collection of the viral particles is essential. Electroporation of the BHK21 cells should yield more than 80% transfection, as visualized by GFP expression. This result can be achieved by obtaining high-quality RNA and optimizing the electroporation conditions. We also found that a few rounds of freeze-thaw cycles of the aliquots did not deteriorate the quality of the virus for in vivo infection.
12.3. IN VIVO INJECTION OF VIRAL PARTICLES
We have adapted standard stereotaxic injection techniques to allow micro-injection of viral particles into the CA1 region of hippocampi of young adult rats of post-natal days (PND) 21 through 28. Of course, of the utmost importance is that procedures for humane treatment of animals must be observed at all times. Below, we describe the procedure that we have developed after careful evaluation of the guidelines and options provided by our animal facility veterinary specialists.
A PND21–28 rat is anesthetized with a ketamine/xylazine (50/4.4 mg/kg body weight) cocktail by intraperitoneal (IP) injection. As the animal becomes drowsy 5 to 10 min after the IP injection, we increase analgesia locally by injecting bupivacaine (2 mg/kg) subcutaneously at the site of the incision. Once the animal is in deep anesthesia, as illustrated by the pinch test (i.e., no reflex should be observed when vigorously pinching its paw), we shave its head using an electric clipper. This first part of the procedure needs to be performed away from the surgical area, as shaven hair is a potential hazard for infection.
The animal is then moved to the clean surgical area and is immobilized in a stereotaxic apparatus. The scalp is scrubbed using betadine, rinsed with 70% ethanol and, using sterile instruments, a scalp incision is made. The skin can be immobilized away from the skull using hemostatic forceps. A cannula previously filled with viral solution is placed at the site of injection using the antero-posterior and lateral coordinates assigned to the CA1 region of the hippocampus (see paragraph below). Using a hand-held drill, a hole (1 to 2 mm across) is made in the skull and the dura mater is opened using the bent end of a hypodermic needle. We lower the cannula to the appropriate depth, at which point we inject the viral solution (total volume of 0.5 μl). The injection is made using a Harvard Apparatus injection pump at a flow rate of 0.1 μl/min to minimize tissue damage. When the injection is complete, the cannula is removed slowly at a rate of 0.5 mm/min and the skin is sealed with super glue. To minimize post-operation pain, the animal is given additional analgesia in the form of a subcutaneous injection of buprenorphine (0.03 mg/kg). The animal is then returned to its cage and allowed to recover. A heating lamp is used to avoid post-operative hypothermia and the animal is monitored for heart rate and respiration during recovery. The surgical procedure needs to be performed in a BL-2 approved area and the animals need to be housed in a BL-2 facility overnight.
A major task for the successful use of this technique is to find the stereotaxic coordinates that allow reliable injections into the area of interest. Several adult rat brain atlases are currently available [10] but no atlases are found for the developing rat brain. Therefore, we obtained the appropriate coordinates for our PND21–28 rats by initially using the adult coordinates for the CA1 pyramidal cell layer of the hippocampus and modifying these coordinates by trial and error. Bregma, the point of intersection of the sagittal suture with the coronal suture, was used as the point of reference for the lateral and antero-posterior coordinates (see [10] for details on stereotaxic coordinates nomenclature). We use the following coordinates: antero-posterior, 4 mm; lateral, 2.5 mm, vertical: 2.4 mm, for injection in the CA1 pyramidal neuron layer of the hippocampus.
If you plan to use an injection pump, we have found the following procedures help optimize the chances of obtaining a successful injection. We start the pump flowing upon entering the brain and place the injection cannula 0.5 mm above the final site of injection. At that point, the flow of solution has often been greatly reduced or stopped and therefore we wait until enough pressure has built in the pump to resume the flow. We then lower the cannula to its final destination. This trick ensures that you do not damage the area to be injected by the sudden gush of viral solution that often occurs when the flow of solution resumes.
We have found that keeping the weight of the animal constant yields more accurate injections than solely choosing the animal by age. Also, systematically using the coordinates by carefully locating bregma for each injection is crucial to obtain reliable injections at the desired location. Judging the best place of injection by eye is never reliable.
12.4. USE OF ACUTE IN VIVO EXPRESSION OF RECOMBINANT PROTEINS FOR CELL-BASED ASSAYS
Figure 12.1 shows a successful injection of a GFP-expressing Sindbis virion in the CA1 layer of a PDN24 rat hippocampus after 24 hours of expression. Note that the virus only infects the neurons of the CA1 pyramidal layer. Thus, by expressing GFP along with the protein of interest (either as a fusion protein or via the use of an IRES), identifying the cells that have been infected in living tissue using simple epifluorescent light microscopy and performing any number of cell-restricted assays is straightforward. For example, we were able to compare the detailed electrophysiological properties of infected versus uninfected neighbor neurons in acutely dissected hippocampal slices from infected animals. The infected neurons showed healthy and stable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor– and N-methyl-D-aspartate (NMDA) receptor–mediated excitatory post-synaptic currents (EPSCs) comparable to those recorded from uninfected neurons in the same slice preparation and also comparable to neurons from uninfected animals. We could maintain recordings for times sufficient to examine long-term potentiation (LTP) and long-term depression (LTD) (Figure 12.2a), and also perform many difficult electrophysiological assays such as those that require minimal stimulation techniques [11]. Because standard acute hippocampal slices were used, recordings did not suffer from the drawbacks that often accompany the use of organotypic brain slices, such as epileptiform activity and small, unstable responses. Other investigators have found that normal extracellular field potential recordings could be obtained from areas of the slice containing a high proportion of infected cells, thus confirming the overall good heath of the slices [5]. Furthermore, this technique has been successfully applied to other regions of the brain such as the rat, somatosensory “barrel” cortex [5,6].
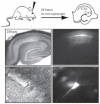
FIGURE 12.1
The diagram shows a schematic of the experimental protocol. Photos show low resolution (4×, top panels) and high resolution (40×, bottom panels) images of a hippocampal slice (left panels show DIC images; right panels show GFP fluorescence) (more...)
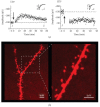
FIGURE 12.2
(a) Example of a whole-cell recording obtained from a GFP-infected CA1 pyramidal neuron (PND24 rat) showing long-term potentiation (LTP) of the AMPA receptor-mediated EPSC. Sample traces were averaged over the baseline period (1) and 45 to 60 min post (more...)
This approach also facilitates the study of the effects of in vivo molecular manipulations on the morphology of neurons. By adding a fluorescent dye (e.g., Alexa 568 fluor hydrazide from Molecular Probes) to the whole-cell pipette recording solution, we were able to fill cells with the dye during electrophysiological recordings. After fixing and mounting the tissue, this enabled visualization of the detailed morphology of the infected neurons using a Zeiss LSM 510 laser-scanning confocal microscope. For example, by collecting Z-stacks of parts of apical secondary dendrites and reconstructing these in 3-D using Volocity software (Improvision), we could compare the density and morphology of dendritic spines between infected and uninfected neurons (Figure 12.2b).
12.5. CONCLUSIONS
In this chapter, we have briefly described the use of a viral-based gene expression system to acutely express heterologous proteins in the rat brain in vivo. We believe that this technique offers several advantages over other approaches that are used to express recombinant proteins in vivo. In particular, it allows evaluation of the effect of acute expression of any protein of interest in a temporally and spatially restricted manner while minimizing the possibility of time-dependent compensations in response to the molecular manipulation. It also permits direct comparison of molecularly-manipulated and neighboring control neurons within the same tissue under close-to-ideal physiological conditions. However, one drawback of the use of Sindbis viruses is that they often result in high over-expression of the recombinant protein. In theory, this could affect the normal functioning of the neuron as well as result in the recombinant protein having effects that the endogenous protein does not. Thus, we encourage the reader to keep up-to-date with the latest versions of the Sindbis viruses that show the least cytotoxicity and to consider the use of other viral-expression systems such as lentiviruses, which permit lower-level and longer-term expression of recombinant proteins. Lentiviruses can be particularly advantageous when using it to express RNAi to knockdown the expression of endogenous proteins.
REFERENCES
- 1.
- Washbourne P, McAllister AK. Techniques for gene transfer into neurons. Curr Opin Neurobiol. 2002;12:566–573. [PubMed: 12367637]
- 2.
- Strauss JH, Strauss EG. The alphaviruses: Gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. [PMC free article: PMC372977] [PubMed: 7968923]
- 3.
- Yuste R, Lanni F, Konnerth A. Imaging Neurons, A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 2000. pp. 58.1–58.8.
- 4.
- Bredenbeek PJ, Frolov I, Rice CM, Schlesinger S. Sindbis virus expression vectors: Packaging of RNA replicons by using defective helper RNAs. J Virol. 1993:6439–6446. [PMC free article: PMC238079] [PubMed: 8411346]
- 5.
- D’Apuzzo M, Mandolesi G, Reis G, Schuman EM. Abundant GFP expression and LTP in hippocampal acute slices by in vivo injection of sindbis virus. J Neurophys. 2001;86:1037–1042. [PubMed: 11495971]
- 6.
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–1588. [PubMed: 12624270]
- 7.
- Jeromin A, Yuan LL, Frick A, Pfaffinger P, Johnston D. A modified Sindbis vector for prolonged gene expression in neurons. J Neurophys. 2003;90:2741–2745. [PubMed: 12853440]
- 8.
- Kim J, Dittgen T, Nimmerjahn A, Waters J, Pawlak V, Helmchen F, Schlesinger S, Seeburg PH, Osten P. Sindbis vector SINrep(nsP2S726): A tool for rapid heterologous expression with attenuated cytotoxicity in neurons. J Neurosci Meth. 2004;133:81–90. [PubMed: 14757348]
- 9.
- Quinonez R, Sutton RE. Lentiviral vectors for gene delivery into cells. DNA Cell Biol. 2002;21:937–951. [PubMed: 12573051]
- 10.
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998.
- 11.
- Isaac JT, Hjelmstad GO, Nicoll RA, Malenka RC. Long-term potentiation at single fiber inputs to hippocampal CA1 pyramidal cells. Proc Natl Acad Sci USA. 1996;93:8710–8715. [PMC free article: PMC38738] [PubMed: 8710936]
- Review In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.[In Vivo Optical Imaging of Bra...]Review In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.Rector DM, Yao X, Harper RM, George JS. In Vivo Optical Imaging of Brain Function. 2009
- Biolistic transfection and expression analysis of acute cortical slices.[J Neurosci Methods. 2020]Biolistic transfection and expression analysis of acute cortical slices.Hamad MIK, Daoud S, Petrova P, Rabaya O, Jbara A, Melliti N, Stichmann S, Reiss G, Herz J, Förster E. J Neurosci Methods. 2020 May 1; 337:108666. Epub 2020 Feb 28.
- A modified Sindbis vector for prolonged gene expression in neurons.[J Neurophysiol. 2003]A modified Sindbis vector for prolonged gene expression in neurons.Jeromin A, Yuan LL, Frick A, Pfaffinger P, Johnston D. J Neurophysiol. 2003 Oct; 90(4):2741-5. Epub 2003 Jul 9.
- The Effects of Sindbis Viral Vectors on Neuronal Function.[Front Cell Neurosci. 2019]The Effects of Sindbis Viral Vectors on Neuronal Function.Uyaniker S, van der Spek SJF, Reinders NR, Xiong H, Li KW, Bossers K, Smit AB, Verhaagen J, Kessels HW. Front Cell Neurosci. 2019; 13:362. Epub 2019 Aug 8.
- Review Techniques and Methods of Animal Brain Surgery: Perfusion, Brain Removal, and Histological Techniques.[Brain Neurotrauma: Molecular, ...]Review Techniques and Methods of Animal Brain Surgery: Perfusion, Brain Removal, and Histological Techniques.Soueid J, Nokkari A, Makoukji J. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. 2015
- Acute In Vivo Expression of Recombinant Proteins in Rat Brain Using Sindbis Viru...Acute In Vivo Expression of Recombinant Proteins in Rat Brain Using Sindbis Virus - The Dynamic Synapse
Your browsing activity is empty.
Activity recording is turned off.
See more...
