NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gottfried JA, editor. Neurobiology of Sensation and Reward. Boca Raton (FL): CRC Press/Taylor & Francis; 2011.
4.1. INTRODUCTION
In this chapter we ask: “What can brains do with reward?” Because different brains can do different things with reward, we focus on three kinds of brains: those of vertebrates, mammals, and primates. We do so not only because we are all of those things, but because about 530 million years ago the early vertebrates evolved a brain that we can recognize as reasonably like our own. That brain helps us, as it helped our ancient ancestors, deal with an intricate calculus of costs and benefits, some of which we call rewards. So at one level the answer to our question is relatively simple: Brains cause vertebrates to move as a coherent whole in order to obtain reward benefits at minimal cost. The complexity of cost-benefit calculations has multiplied manyfold since the early vertebrates first evolved, but they have bequeathed to us their life—our life—of decisions and goal-directed movement.
Although rewards can be understood in terms of costs and benefits, there is more to them than that. Along with vertebrates and other animals, plants endure costs and receive benefits, but these benefits do not count as rewards. Producing certain kinds of flowers and fruits, for example, incurs a metabolic and energetic cost while yielding a reproductive benefit. Most plants require water to produce these benefits. So why is fluid not a “reward” for a plant in need of turgor? The answer can be found in the word animal itself, which refers to a life of movement. Animals first appeared approximately 700–900 million years ago (Vermeij 1996), during a time of increasing atmospheric oxygen pressure, a contribution of our chloroplast-containing cousins. Higher oxygen pressure allowed organisms to use more energy, permitting the evolution of multicellular animals from unicellular ancestors. More energy, however, required more nutrients, and animals evolved the neural mechanisms for coordinated movements to obtain them. Movement, however, is both expensive and dangerous. A life of movement necessitates decisions about where to move, as well as when and if to do so. Animals must decide whether to abandon the relative safety that often comes from staying still in order to forage for nutrients and other rewards. When these decisions lead to actions having a beneficial outcome, we call that outcome reward (see Chapter 3). In short, animals evolved as reward seekers, and reward-seeking behavior (foraging) entails serious costs as well as benefits.
In psychology, a reward is defined operationally as anything that increases the behavior that leads to obtaining it. When reward acts in this way, psychologists also call it a positive reinforcer because it reinforces or strengthens the underlying associations in the brain that are said to “control” the reward-seeking behavior. The concept of reward can be divided into primary and secondary rewards, with primary rewards being those that directly meet biological needs (e.g., food, water, salt, and sex) and secondary rewards (also known as conditioned reinforcers) being stimuli that have acquired rewarding properties through their association with primary rewards.
Two important aspects of reward reflect its evolutionary history. First, evolution did not develop dedicated reward receptors. Instead, animals must learn the sensory properties of reward for both reward-seeking and reward-consuming behaviors (Hall, Arnold, and Myers 2000; Changizi, McGehee, and Hall 2002), and these sensory signals come from every modality. Second, although every sensory modality contributes to reward processing, the brain deals with a variety of rewards in similar ways. So, for example, even a uniquely human kind of reward, such as listening to a favorite piece of music, activates many of the same brain regions as a primary reward such as food (Blood and Zatorre 2001; and see Chapter 19 in this volume).
In what follows, we first sketch some current thinking on the evolution of some of the brain structures that deal with reward, with emphasis on those that likely appeared at three times during the history of animals (Figure 4.1): with early vertebrates, with early mammals, and during primate evolution. Next we use clues from laboratory research, mainly from studies of rats and rhesus monkeys, to paint a picture of structure-function relationships in each of these three clades. We recognize the hazards of this approach. Rats and rhesus monkeys are crown species, each with a long, separate evolutionary history and a complex suite of adaptations and specializations. The preferred way to understand the role of structures in early vertebrates or early mammals would involve the study of a diversity of animals in each group, selected according to the principles of evolutionary biology. Although a limited amount of such research is available, the literature from comparative psychology has not yet reached a point where we can use the preferred approach effectively. Accordingly, we use what hints come from rat and monkey research, in the context of comparative neuroanatomy, to explore what we have inherited from various ancestors. In doing so, we assume that structure-function relationships do not change haphazardly during evolution. Our approach will perhaps seem most peculiar when we use insights gained from the study of macaque monkeys and humans to explore the function of dopamine neurons and the basal ganglia in Section 4.3: “What Can Vertebrate Brains Do With Reward?” We adopt this approach because the original work was based on monkey research, and because we believe that the principles discerned from that research apply to other vertebrates as well. Our assumption is that such principles are highly conserved, although future work on a broad array of vertebrates is needed to confirm that view.
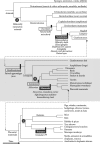
FIGURE 4.1
Three cladograms, arranged from top to bottom. The middle and bottom cladograms each develop one of the lineages from the cladogram above, as shown by the connecting arrows. The circled letters, A, B, and C, reference common ancestors referred to in the (more...)
4.2. WHAT KINDS OF BRAINS DEAL WITH REWARD?
4.2.1. Brain History and Historiography
The field of brain evolution seeks to discern the history of the brain, but that field itself has a history, and not always an attractive one. Readers might wonder why the outline of brain evolution provided here is not widely known and why statements about brain evolution have an aura of controversy. In part, this feeling results from the bad reputation that brain evolution, as a field, has developed in certain circles. Nevertheless, progress has been made in understanding the history of brains, and we attempt to summarize some of that progress as it relates to reward processing.
Unfortunately, neuroscientists have often written about brain evolution in erroneous ways and in what follows we hope to avoid the mistakes of the past. In the mid-1980s, for example, one eminent neuroscientist (Jones 1985, 763) claimed that “no writer in the field today would seriously propose that the brains of existing nonmammalian vertebrates formed a scala naturae,” while another did exactly that by speculating about how a certain behavioral capacity applied “across the entire phyletic scale” (Mishkin, Malamut, and Bachevalier 1984, 73). Evolutionary biologists long ago rejected the concept of a phyletic scale, also called the scale of nature or the scala naturae, in favor of the science of cladistics, which uses the evolutionary innovations of particular lineages to discern “evolutionary trees” through the principle of parsimony. Instead of ranking species as a linear progression from “lower” to “higher” forms—e.g., rats-cats-monkeys-chimpanzees-humans in the mammalian clade—most neuroscientists now understand that phylogenetic relationships have a branching architecture (Figure 4.1), with any two existing lineages sharing one most recent common ancestor. No existing species represents that common ancestor perfectly, and most, like laboratory rats and rhesus monkeys, do so rather poorly. Rats, for example, are highly specialized “gnawers,” with a host of evolutionary innovations that the rodent-primate common ancestor lacked. Although we draw on data from rats and rhesus monkeys to explore what we have inherited from ancestral vertebrates, mammals, and primates, these groups do not make up a “phyletic scale” or anything of the sort. We discuss these groups in that order simply because we evolved from all of them and because they first appeared in that order.
We also discuss structures that evolved before or after others in evolutionary history, a topic that has many pitfalls. For example, one of the neuroscientists quoted above claimed that “the basal ganglia antedates both the cerebral cortex and the limbic system in phylogenesis” (Mishkin, Malamut, and Bachevalier 1984, 74). To the contrary, comparative neuroanatomy shows that homologues of the basal ganglia and many limbic structures, including parts of the cerebral cortex such as the hippocampus, evolved at about the same time, in relatively early vertebrates (Northcutt 1996; Striedter 2005).
Other discredited doctrines have also muddied the waters considerably. Some, such as MacLean’s triune brain theory, have spawned nonsense such as “reptilian brains” inside human heads (MacLean 1985). In this chapter, we assume that structures such as the basal ganglia perform some conserved function, not that humans or other mammals have the same basal ganglia that some ancestral species or group had. (Also, technically, we did not evolve from “reptiles” but instead descended from ancestral amniotes.) Another discredited doctrine invokes the notion of “evolutionary trends” and schemes of connectional and functional hierarchies based on these imaginary trends. Sanides (1970) imagined that a group of medial cortical areas “arose” from the hippocampus, whereas a group of lateral cortical areas arose from the piriform cortex. He claimed that he could spot “trends” in the cytoarchitecture of these two groups of areas from phylogenetically older to newer parts of the cortex. Unfortunately, comparative evidence shows that many of his claims are wrong, while others are plausible but wholly lacking in evidentiary support. For example, whereas Sanides imagined that primary sensory areas appeared in later stages of mammalian evolution, comparative evidence shows that they were among the earliest areas to appear in mammals (Kaas 1995, 2008). Although Sanides’s views were purely speculative, some neuroanatomists and others have adopted his opinions and interpreted their data according to those views (Barbas and Pandya 1989; Mesulam 1990; Cummings 1993). Nothing here should be construed as agreeing with any of that. When we talk about medial and lateral parts of the frontal cortex, we do not do so in the context of imaginary “evolutionary trends” of the sort popularized by Sanides and his followers.
To sketch the history of the brain, three concepts (homology, analogy, and homoplasy) and just five taxonomic terms (protostome, deuterostome, gnathostome, tetrapod, and amniote) clarify matters considerably. We explain the five taxonomic terms below, but take up the three evolutionary concepts here. Homology is a statement about ancestry, not function. A structure or behavior is homologous to another if two or more descendant species have inherited it from their most recent common ancestor. Analogy is a statement about function, not ancestry. A structure or behavior is analogous to another if it subserves the same function. The classic example involves wings. Insects, birds, and bats have wings, and they all perform the same function: producing lift for flight. We can be certain that they are only analogous, and not homologous, because the last common ancestor of each pair (birds and bats, birds and insects, bats and insects) lacked wings of any kind. Furthermore, insect wings evolved about 390 million years ago, hundreds of millions of years before the origin of either birds or bats (Grimaldi and Engel 2005), in a lineage that produced neither bats nor birds as descendants (Figure 4.1). A recent common ancestor of birds and insects is marked with an A in Figure 4.1, which also applies to bats and insects. This extinct species was a worm-like animal with no capacity for flight whatsoever. It had no wings or, indeed, appendages of any kind. Similarly, the last common ancestor of birds and bats is marked with a B. These ancient amniotes, which lived roughly 320 million years ago, also lacked wings. So like bird and insect wings, bird and bat wings are analogous, not homologous. This fact leads to the concept of homoplasy: something similar that has evolved in different lineages through parallel or convergent evolution. For example, the green lizard Lacerta adopts the habit of resting on green leaves. The color of lizard and leaf resemble each other, but this trait is neither analogous nor homologous. The trait (of being green) is not analogous because the color of the lizard performs a camouflage function, but the leaf has no interest in camouflage. The trait is not homologous because the lizard and plant did not inherit these characters from their nearest common ancestor, which was a single-cell eukaryote that lacked color in this sense. Instead, lizard and leaf exhibit homoplasy in being green. Some similarities stem from common developmental constraints and others result from the necessity of dealing with the physical and physiological realities of the world. For example, wings must produce lift from muscle power by interacting with air, so insect, bat, and bird wings (not to mention those of pterodactyls) inevitably shared certain features once they independently evolved.
Because of the prevalence of homoplasy, when we make statements such as “the amygdala and the dopamine system evolved in the early vertebrates,” these conclusions are based on the identification of homologues of those structures in a diverse set of vertebrates, according to the principles of cladistics. Such statements do not imply that vertebrates are the only animals with neurons analogous to those in the mammalian amygdala or that they are the only animals with dopaminergic neurons.
4.2.2. Vertebrate Brains
A key development in the history of animals occurred when vertebrates evolved from invertebrate ancestors (Figure 4.1). Note that the term “invertebrates” refers to an arbitrary grouping of animals rather than a clade (a progenitor species and all of its descendants, living and extinct). The animals that most people think of as “invertebrates” are the protostomes, a group that includes insects, mollusks (such as squid and octopus), and segmented worms. Additional kinds of “invertebrates” are found among the deuterostomes. These two lineages, protostomes and deuterostomes, diverged more than 600 million years ago, and their last common ancestor was a worm-like animal with a diffuse nerve net (marked by an A in Figure 4.1). Both groups formed their nervous systems from ancestral conditions characterized by such nerve nets, which during parallel evolution condensed into concentrated neural structures independently (Valentine 2004; Grimaldi and Engel 2005). Many protostomes evolved to a highly advanced level, producing intelligent animals with extraordinary sensory sensitivity, motor capacity, and social organization. Nevertheless, the brains in these animals evolved independently from those in vertebrates and are not homologous to vertebrate brains. (This conclusion is not without controversy, but the analysis of the options proposed by Schmidt-Rhaesa (2007) in the context of the recent advances in understanding animal phylogeny (Dunn et al. 2008) supports this view.) The brains of protostomes differ in so many crucial respects from vertebrate brains that it is not particularly useful to apply the same name to them, although it is probably unavoidable. Most important for the present purposes, protostome brains lack homologues of the midbrain dopamine system, amygdala, hippocampus, and striatum; they lack anything remotely resembling the cerebral cortex of mammals; and to consider any part of their brains homologous to the prefrontal cortex of primates is preposterous. The brains that protostome invertebrates use to deal with reward have a long evolutionary history, one entirely separate from ours. Their brains are not only unlike those of vertebrates, they have virtually no resemblance to our common ancestral condition either.
Instead, as shown in Figure 4.1, vertebrates and their brains evolved in the deuterostome lineage, long after diverging from the line leading to modern protostomes. With these animals came a complex central nervous system, an ability to swim and eat, and to direct those actions with a brain and sensory organs on and in the head (Northcutt 1996; Holland and Holland 1999; Butler 2000; Nieuwenhuys 2002). (Protostomes developed similar features, too, but did so independently.) For the present purposes, the most important evolutionary developments involved the invention of the telencephalon and the vertebrate dopamine system. The early telencephalon included the olfactory bulb and a homologue of the piriform cortex of mammals (Wicht and Northcutt 1992, 1998; Northcutt 1996, 2001; Striedter 2005). Crucially, the telencephalon of early vertebrates contained homologues of the basal ganglia and, with somewhat less certainty, the amygdala and hippocampus, to use their mammalian names. (The strongest evidence traces all of these structures back to the early tetrapods (see Figure 4.1), but suggestive anatomical evidence indicates that homologues exist in various extant fish groups that make these structures likely to be innovations of relatively early vertebrates, if not the earliest ones (Neary 1990; Ulinski 1990; Northcutt 1996, 2008; Smeets, Marín, and Ganzález 2000; Wullimann and Mueller 2002; Wullimann and Rink 2002; Medina et al. 2005; Striedter 2005; Moreno and Gonzalez 2007).) Vertebrates also innovated paired eyes as part of their central nervous system, which, along with their olfactory system, supplied their brains with information about distant features of their environment, and these organs make crucial contributions to reward-seeking behavior. Thus, roughly 530 million years ago, early vertebrates developed the brain, several remote sensors on the head, and a motor apparatus, and they used these newly evolved systems to guide decisions and goal-directed movements. These evolutionary developments changed forever what brains—our brains by descent—can do with reward.
4.2.3. Mammalian Brains
Mammals deal with reward with a mixture of old and new features, including those inherited from the early vertebrates: the dopamine system, amygdala, hippocampus, basal ganglia, olfactory system (with its olfactory bulb and piriform cortex), paired eyes, and so forth. In addition to these traits, shared with other vertebrates, the early mammals developed a new structure that deals with reward: the neocortex.
The top part of Figure 4.1 shows something about how this came about. Fairly early in vertebrate history, jawed fish (gnathostomes) evolved from jawless ancestors. Their descendants include all of the vertebrates in the bottom two parts of Figure 4.1, including the first land vertebrates, the ancestral tetrapods, which evolved 370 million years ago or so (Clack 2002). With amniotes, a group that appeared about 320 million years ago and includes all reptiles, birds, and mammals, the three-layered cortex known as the allocortex appeared. Parts of the allocortex have homologues in other vertebrates, but without its characteristic laminated structure (which was lost secondarily in birds).
Mammals evolved about 220 million years ago and have retained the allocortex as two substantial parts of their cerebral cortex, the piriform cortex and the hippocampus, along with some smaller, obscure allocortical areas. A different kind of cerebral cortex evolved in the earliest mammals or their immediate ancestors (Kaas 1995, 2008; Northcutt and Kaas 1995; Krubitzer and Kaas 2005). We can be certain of this conclusion because these areas occur only in mammals, and they can be found in all mammals, including monotremes and marsupials (see Figure 4.1). These mammalian innovations are often called the neocortex (meaning new cortex, which is true) or isocortex (meaning homogeneous cortex, which is false), but these terms are used inconsistently. Sometimes the terms neocortex and isocortex exclude mammalian innovations such as the so-called transition areas between the allocortex and the remainder of the neocortex. Here we use the term neocortex to include these transition areas. As noted above, rodent species, including rats, have their own set of adaptations and specializations, but the architecture of their frontal cortex represents the ancestral mammalian condition reasonably well. Rodents, like other mammals, possess several distinct neocortical fields: these include medial frontal areas, specifically the anterior cingulate (AC), infralimbic (IL), and prelimbic (PL) areas, as well as orbital frontal areas, specifically the agranular insular (Ia) and agranular orbitofrontal cortex. We use standard names and abbreviations for most of these areas, the ones noted in the previous sentence. We also use a new one, OFa, which stands for agranular orbitofrontal cortex. Some of these areas, specifically the medial frontal and orbitofrontal areas, go by the name “prefrontal cortex” in rodents and other nonprimate mammals (Kolb 2007), but their homologues in primates are not called prefrontal, so we use a different term. Regardless of their names, all of these specifically mammalian areas likely contribute to what mammalian brains can do with reward.
4.2.4. Primate Brains
Primates first appeared about 60 million years ago. Early in this long history, primate brains evolved a new kind of frontal cortex, as well as some additional sensory areas. Figure 4.2 shows the basic, architectonic types of cerebral cortex in two species of primate and a rodent (Carmichael and Price 1994; Öngür, Ferry, and Price 2003; Palomero-Gallagher and Zilles 2004). In the primate frontal lobe, the main types of cortex are the agranular cortex, which lacks an internal granular layer (layer 4), and areas that have either a conspicuous or subtle layer 4, collectively called the granular prefrontal cortex (PFg). The PFg occurs in all primates and only in primates, and therefore represents a primate innovation (Preuss 1995, 2007). In appreciation of this historical fact, we use another new abbreviation, PFg, for these areas collectively. Figure 4.2 illustrates the parsimonious idea that rodents and primates, among other mammals, share the agranular parts of the frontal cortex, but primates alone have the granular parts. This idea has been controversial, but cortical topology (the spatial relationships of the frontal areas to each other and to the allocortex) and corticostriatal connectivity (from the agranular frontal areas to and near the ventral striatum, in particular) point to the same conclusions (Wise 2008). Crucially, none of the arguments brought forward to dispute the idea that PFg is a primate innovation—dopamine inputs, inputs from the mediodorsal nucleus of the thalamus, behavioral effects of brain lesions—stand up to scrutiny (Wise 2008). The lack of granular prefrontal areas in rodents should not be surprising given the antiquity of the most recent common ancestor of primates and rodents (marked with a C in Figure 4.1). Some of the primate innovations, such as granular parts of the orbitofrontal cortex (PFo), surely influence what primate brains can do with reward.

FIGURE 4.2
Basic types of frontal cortex. The key indicates three basic types of cerebral cortex by grayscale code. (a) human, (b) macaque monkey, (c) rat. Architectonics from Öngür, Ferry, and Price (2003) (a), Carmichael and Price (1994) (b), and (more...)
4.3. WHAT CAN VERTEBRATE BRAINS DO WITH REWARD?
4.3.1. What Nonvertebrate Brains Can Do with Reward
The vertebrate brain evolved a dramatically new central nervous system, one not shared by nonvertebrate species. Nevertheless, nonvertebrates deal with reward effectively. They have the ability to seek specific rewards (e.g., salt and water) and demonstrate the ability to learn about rewards via both associative mechanisms (e.g., Pavlovian and instrumental conditioning) and nonassociative ones (e.g., habituation and sensitization). As in vertebrates, associative learning about rewards in protostomes concerns how the animal learns to predict and respond to different events in their environment, and it involves the formation of associations between representations of different stimuli (S), responses (R), and rewards (often referred to as outcomes, or O), as formalized by the concepts of Pavlovian and instrumental conditioning. The stored knowledge acquired via these mechanisms guides reward-seeking behavior in protostomes as it does in vertebrates, and it is likely that almost all animals have the capacity for Pavlovian and instrumental conditioning. In Pavlovian conditioning, rewards are referred to as unconditioned stimuli (USs). Through experience (such as temporal contiguity), an originally neutral stimulus, called a conditioned stimulus (CS), becomes associated with a US and therefore comes to elicit the same innate responses (unconditioned responses, or URs) that are produced in response to the US. It is generally accepted that underlying Pavlovian conditioning is the formation of CS-US (stimulus-outcome or S-O) associations. Activation of the US representation via this association triggers the associated URs, which become known as conditioned responses (CRs) when evoked by presentations of the CS. In addition, Pavlovian conditioning may lead to the formation of direct CS-CR associations (also known as S-R associations) under certain circumstances (such as extinction, as discussed in Section 4.4.2.3). Learning about the predictive nature of environmental events allows the animal to act in anticipation of the US. When the US is the reward, this process increases the likelihood of obtaining it.
In instrumental conditioning, associations develop when an animal learns about the effect of its actions on the environment. Therefore, unlike Pavlovian conditioning, in instrumental conditioning the availability of reward depends on an animal’s actions: its actions are instrumental in producing the reward. This type of learning involves the formation of associations between the instrumental response and reward (R-O and O-R associations). Guidance of instrumental behavior by R-O associations is conceptualized as reflecting goal-directed responding. If responding is followed by a reward in the presence of a particular stimulus, this stimulus comes to modulate the R-O association and is termed the discriminative stimulus. Furthermore, stimulus-response (S-R) associations also form between representations of the response and any stimuli present when the reward is delivered. Behavior predominantly guided by S-R associations is referred to as habitual. Chapter 14 explains these concepts in more detail.
Neither Pavlovian nor instrumental learning requires a vertebrate brain. Marine mollusks such as Aplysia, for example, demonstrate the ability to learn via both of these mechanisms (Carew, Walters, and kandel 1981; Carew and Sahley 1986; Brembs and Heisenberg 2000; Brembs et al. 2002). The garden slug, a terrestrial mollusk, shows evidence for more complex Pavlovian phenomena such as second-order conditioning, in which previously rewarded stimuli reinforce a new response. They also show the phenomena of blocking and extinction (Sahley, Gelperin, and Rudy 1981; Carew and Sahley 1986), which are described in more detail below. And nematodes (roundworms) can learn to discriminate between good and toxic food (Zhang, Lu, and Bargmann 2005). Given that nonvertebrates can do all that with reward, what is so special about what vertebrates can do with rewards?
4.3.2. Midbrain Dopamine Nucleus
The evolution of the midbrain dopamine system must have had a profound impact on what vertebrate brains can do with reward. Their function has been thoroughly reviewed (Schultz 2006, 2007) and so only selected aspects are mentioned here. In general, a reward acts as a reinforcer to strengthen associations between representations that are active in its presence. One consequence of this property is that initially neutral stimuli take on reward value, leading to the reinforcing value of secondary rewards, as mentioned in the previous section. Schultz showed in monkeys that as a stimulus becomes associated with reward, dopamine neurons increase their discharge rates when that stimulus appears. This finding explains why conditioned reinforcers work: dopamine cells respond to CSs much like they do to unconditioned reinforcers such as primary reward. Schultz also showed that dopamine-cell activity reflects an error signal that conveys the difference between predicted and received reward. The sources of the prediction and error remain unknown, but one possibility is that striatal GABAergic neurons provide a reward-prediction signal to the dopamine cells and that other inputs to the dopamine cells, such as those from the amygdala, hypothalamus, and peripeduncular nucleus or bed nucleus of the stria terminalis, provide the received reward signal. In this view, the dopamine cells compute the difference between these two inputs and their output serves as a teaching signal to its targets, among which the striatum figures especially prominently. Receipt of reward at an unexpected time or in larger-than-expected amounts leads to strengthening of associations between representations active at the time of the teaching signal, which makes it more likely that the action made in that context will be repeated when the context recurs. Failure to receive a reward at the expected time and in the expected amount leads to a form of extinction, and puts the system in a state that promotes exploration of alternative actions. Comparison of predicted and received reward is the fundamental mechanism by which vertebrate brains deal with reward.
4.3.3. Basal Ganglia
One of the most vexing problems in understanding the general role of the midbrain dopamine system, especially in relation to basal ganglia function, is that one line of research has focused on its role in motor control, while another has focused on its role in reward-seeking behavior, as exemplified by Pavlovian approach behavior (S-O associations), Pavlovian-to-instrumental transfer (PIT), and instrumental conditioning. The first line of research has, after a period of uncertainty, confirmed earlier opinions (Northcutt 1996) that the largely diencephalic group of dopamine neurons found in jawless fish (lamprey) is homologous to the midbrain dopamine system in tetrapods (Smeets, Marín, and Ganzález 2000). Therefore, it now appears even more likely that the dopamine system evolved in the earliest vertebrates (Figure 4.1). The relatively new evidence is that the same toxin that produces Parkinson’s disease (PD) in people causes similar symptoms in these jawless fish (Thompson et al. 2008). An equally ancient structure, the lateral habenula, appears to provide a signal to dopamine neurons that inhibits these neurons when rewards are not predicted (Matsumoto and Hikosaka 2007). It appears, then, that the evolution of this dopamine system in early vertebrates had profound implications for what their brains could do with reward, but it remains difficult to reconcile the motor functions of dopamine-basal ganglia interactions with those involving primary and secondary rewards. One approach toward this reconciliation has been to consider several different time courses for the actions of dopamine, extending several orders of magnitude from fractions of a second, as described above, to many hours (Schultz 2007). Alternatively, the dopamine system may compute the cost–benefit analyses that are fundamental to all decisions.
Recent work has pointed to a common function for both motor and nonmotor parts of the basal ganglia. According to Shadmehr and Krakauer (2008), the interaction between midbrain dopamine cells and the basal ganglia modifies the assessment of costs and benefits in a very general sense, one not limited to reward as traditionally construed. They based their view on a computational model and on recent psychophysical evidence that PD, which is caused by degeneration of the midbrain dopaminergic neurons, leads to a form of effort intolerance (Mazzoni, Hristova, and Krakauer 2007; Shadmehr and Krakauer 2008). Apart from tremor, two principal symptoms of PD are bradykinesia (slow movement) and akinesia (no movement). Shadmehr and Krakhauer have related these symptoms to a change in how the brain computes the value of a certain amount of energy expenditure. In a psychophysical experiment (Mazzoni, Hristova, and Krakauer 2007), human subjects made a series of voluntary reaching movements at varying speeds and distances. The investigators measured, among other things, the number of reaches needed to produce 20 reaches that fell within a given velocity range, such as 37–57 cm/s. Healthy people show a reluctance to make relatively fast reaching movements, measured by their need to attempt ~35 trials needed to make 20 within the fastest movement range. PD patients show even more reluctance to make fast movements than do healthy people, requiring ~45 trials to make the same number of correct ones. Yet their reaching movements maintain normal spatial accuracy. Similar conclusions come from the work of Desmurget and Turner (2008), who inactivated the internal segment of the globus pallidus, the output structure of the “motor” basal ganglia, in rhesus monkeys. This inactivation causes abnormally short movements (hypometria), which are slower than normal (bradykinetic), but have the normal trajectory, endpoint accuracy, and reaction times. Like Shadmehr and Krakhauer, Desmurget and Turner concluded that the basal ganglia “may regulate energetic expenditures during movement.”
Thus, the dopamine system—through its interaction with the basal ganglia—appears to lead to the minimization of a cost: the increased energy expenditure (effort) needed to make faster movements. In PD, the decrease in dopamine input to the striatum alters the sensitivity to such effort costs. According to Mazzoni, Hristova, and Krakauer (2007, 7105), “bradykinesia represents an implicit decision not to move fast because of a shift in the cost/benefit ratio of the energy expenditure needed to move at normal speed,” which is an exaggerated form of a similar shift that occurs in healthy people. The basal ganglia and its dopamine inputs thus play a key role in computing the cost of reaching movements, and this function likely generalizes to volitional, skeletal movements of many kinds, including those involved in seeking and consuming primary rewards.
This kind of cost–benefit analysis could link the well-known motor functions of parts of the dorsal basal ganglia to the standard psychological framework implicating the ventral basal ganglia in decisions based on predicted reward outcome (Schultz 2006). This computational view of reward generalizes its definition to include benefits other than typical primary and secondary reinforcers, including benefits that come in the form of reduced energy costs. As Mazzoni, Hristova, and Krakauer (2007, 7105) put it, a role for dopamine in minimizing effort cost is “analogous to the established role of dopamine in explicit reward-seeking behavior.” Note, however, that when Mazzoni et al. use the term “explicit” in this way, they do not do so in the sense usually meant in psychology, which implies conscious awareness and the ability to report on a decision and its basis. Instead, they mean to distinguish two levels of implicit decisions: one that leads to behaviors that have produced benefits in terms of primary and secondary rewards, and another that produces benefits in terms of reduced effort costs. It appears that the basal ganglia and its dopamine inputs play a key role in computing the costs and benefits in a very general sense, for use in neural functions that minimize costs and maximize benefits of many kinds.
We can only speculate about the common mechanisms between these two kinds of “reward” computations. One possibility is that dopamine cells do for effort costs more or less what they do for primary rewards. When vertebrates make a movement that uses more than the optimal amount of energy, i.e., they expend more effort than necessary to reach a movement target, the mismatch between predicted and actual effort would cause the dopamine cells to decrease their activity, and the behavior would be “extinguished.” Conversely, if they reached the target with less effort than expected, the dopamine cells would increase their activity and this would teach the system to make the movement requiring this lesser effort in the future, thus optimizing the amount of energy expended to reach a movement target.
4.3.4. Amygdala
As they learn the biological value of rewards, vertebrates tend to select the behavior that yields the highest reward value. Not only can vertebrates learn the value of particular rewards, they can rapidly update the valuation of a reward based on their current state. The reinforcer devaluation procedure assesses this updating ability. When an animal has recently consumed one kind of food to satiety, that food becomes less valued, and animals will reduce a behavior that produced the devalued food. This happens even when their behavioral choices no longer yield the devalued reward (i.e., under extinction), which implies that they can recall the reward (outcome) value in its updated form.
In contrast to intact animals, rats or macaque monkeys with amygdala lesions fail to adjust their responses after reinforcer devaluation (Hatfield et al. 1996; Malkova, Gaffan, and Murray 1997; Balleine, Killcross, and Dickinson 2003; Izquierdo and Murray 2007). Although the experiments in monkeys involved the whole amygdala, work in rodents indicates that the basolateral amygdala (BLA), not the central nucleus, subserves this ability (Hatfield et al. 1996; Balleine, Killcross, and Dickinson 2003). Importantly, rats and monkeys with amygdala lesions have intact satiety mechanisms and intact sensorimotor discrimination abilities. The deficit in shifting choices after amygdala lesions thus results from an impairment either in updating food value or in linking stimuli and responses with this new value to guide behavior.
Wellman, Gale, and Malkova (2005), also studying macaque monkeys, disrupted reinforcer-devaluation effects by temporarily inactivating the BLA with the GABAA agonist muscimol. They replicated the result described above for amygdala lesions, but only when the inactivation occurred during the selective satiation procedure, not when inactivation occurred afterwards. Similarly, Wang et al. (2005) demonstrated in rats that, for updating to occur, protein synthesis is needed in the BLA immediately following devaluation. These findings show that the amygdala updates reward value, but has little role in guiding choices after the value of the food has been updated.
In addition to devaluation experiments, amygdala lesions in rodents and monkeys impair behavior on a number of tasks that require associating a stimulus with value, including second-order conditioning, conditioned cue preference, conditioned reinforcement, delay discounting, and PIT, among others (Everitt et al. 2003; Murray 2007). For example, in delay discounting, a lever press produces a large reward at an increasingly longer delay, which devalues or discounts that reward. As the large reward is progressively delayed, rats gradually shift their choices to a lever that produces a smaller, more immediate reward. Rats with BLA lesions shift responses more quickly than intact rats, thereby exhibiting a steeper discounting function than intact rats (Winstanley et al. 2004). This relative intolerance for delay could result from difficulty in maintaining the representation of reward value in its absence (Winstanley et al. 2004), but the results of the next experiment suggest a different account.
Rudebeck and Murray (2008) found that monkeys with selective amygdala lesions use reward information better than controls in certain circumstances. Their monkeys chose between two stimuli: one choice produced positive feedback (reward) and the other produced negative feedback (effort cost with no benefit). Monkeys with amygdala lesions performed better than controls on the trial following a correctly performed trial, indicating they used reward feedback more efficiently than controls. Both groups used negative feedback equally well. As noted above, Winstanley et al. (2004) suggested the BLA lesions facilitated delay discounting because the rats could not represent the value of the large reward in its absence. The findings of Rudebeck and Murray (2008) suggest instead that the amygdala lesion disrupted an affective signal associated with the lever that produced large rewards. Indeed, the results of both Winstanley et al. (2004) and Rudebeck and Murray (2008), and perhaps others as well (Izquierdo and Murray 2005), could result from the lack of an affective signal that biases choices towards a previously rewarded stimulus. This account implies that the amygdala can impede reward-based decision making and, without its affective signals, animals can sometimes behave more “rationally,” as assessed in terms of costs and benefits.
We focused here on the BLA, but other parts of the amygdala might produce different reward-related signals. The BLA appears to link stimuli with the specific sensory properties of reward, whereas the central nucleus of the amygdala links them to reward’s general affective properties (see Balleine and Killcross 2006, for detailed evidence). As Balleine and Killcross noted, these two roles could contribute to different aspects of foraging, with general affect promoting the preparatory and approach phase, as in arousal, and signals related to specific rewards promoting their consumption.
In sum, the amygdala mediates several mechanisms linking stimuli and responses with the affective properties of rewards, and these mechanisms likely evolved in early vertebrates.
4.3.5. Hippocampus
There is ample evidence for a role of the hippocampus in spatial navigation and spatial memory in many vertebrate species, including animals in groups as diverse as reptiles, ray-finned fishes, birds, rodents, and primates. The activity of hippocampal pyramidal neurons in rats reflects spatially discrete locations (place fields) and collectively produces a map-like representation of the environment (O’Keefe and Nadel 1978). A growing body of evidence indicates that septal and temporal sectors of the hippocampus, often referred to in rats as the dorsal and ventral hippocampus, respectively, have dissociable functions. The septal region is essential for accurate spatial navigation and the temporal region subserves appropriate behavior in anxiety-inducing environments, as well as the contextual retrieval of internal cues based on motivational state (Kjelstrup et al. 2002; Bannerman et al. 2003). Anatomical relations of the septal and temporal portions of the hippocampus mirror their functional specializations. The temporal hippocampus, unlike the septal hippocampus, has extensive, reciprocal connections with the amygdala and hypothalamus, among other regions (Petrovich, Canteras, and Swanson 2001).
A recent finding places the septal-temporal dissociation of hippocampal function in a new perspective. Place fields within the hippocampus vary in size, with smaller place fields (<1 m) found septally and larger place fields (~10 m) located temporally (Kjelstrup et al. 2008). The large place fields in the temporal hippocampus may aid in identifying and representing familiar spatial contexts, as a form of generalization, and in identifying novel contexts. This ability to identify novel contexts could underlie both the reduction in exploratory behavior that occurs in anxiety-inducing environments and the reduction in eating that occurs in novel environments. These effects have been shown to depend on the temporal hippocampus (Kjelstrup et al. 2002; Bannerman et al. 2003; McHugh et al. 2004; Trivedi and Coover 2004) and likely reflect its interactions with the amygdala as well (Petrovich, Canteras, and Swanson 2001). Furthermore, the identification of familiar contexts is necessary for the contextual retrieval of cues based on internal signals regarding motivational state (Stouffer and White 2007). Thus, the functions of the temporal hippocampus may be an emergent property of its large place fields and specialized anatomical connections.
Table 4.1 summarizes the main points in Section 4.3, which outlines in broad terms what vertebrate brains can do with reward. Note that although Table 4.1 summarizes structure-function relationships in vertebrates, generally, it relies mainly on findings from mammals. Section 4.4 takes up the brain augmentations underlying what mammals can do with reward that their nonmammalian ancestors could not.
TABLE 4.1
Vertebrates.
4.4. WHAT CAN MAMMALIAN BRAINS DO WITH REWARD?
4.4.1. Mammal Brains: Basic Vertebrate Mechanisms Plus Neocortex
As mammals evolved from our amniote ancestors (Figure 4.1), we came equipped with a brain that had come to include some new structures, compared to those found in ancestral, nonmammalian species. As outlined in Section 4.2.3, the frontal, parietal, occipital, and temporal areas that make up the neocortex changed forever how our brains could deal with rewards. This fact does not imply, however, that the systems that had developed earlier in vertebrate evolution became redundant; reward processing in mammalian brains still relies heavily on the midbrain dopamine system, basal ganglia, amygdala, and hippocampus. The phylogenetically older systems not only continue to operate alongside the mammalian innovations, but interact with them in a manner that transforms their function to an extent. Here we consider the adaptive advantages that the neocortex provides for reward-seeking behavior, in the context of cost-benefit analysis and foraging.
As discussed earlier in this chapter (and throughout other chapters in “Part III” of this volume), the central nervous system provides the means for an animal to learn about the relationships between its own actions, different aspects of its environment (including discrete stimuli and contexts), and the occurrence of reward, along with costs. The formation of internal representations of these stimuli, contexts, responses, and outcomes, as well as the association of these representations with each other, underpins learning. Although nonmammalian vertebrate brains form complex representations too, the evolution of neocortex in mammals enabled the formation of richer, more versatile internal representations of sensory inputs to associate with reward, including the sensory aspects of reward, its motivational aspects, and its hedonic properties. For example, sensory areas of the neocortex improve, relative to nonmammalian ancestors, the processing of the sensory aspects of rewards and stimuli associated with rewards. As we discuss in more detail in Section 4.5.2, sensory neocortical areas such as the perirhinal cortex, a feature of all mammalian brains, process sensory information in advanced ways. Accordingly, the mammalian brain does what the brain of its nonmammalian ancestors did with reward but does it better in certain ways.
Although reward processing involves all of the neocortex directly or indirectly, the agranular frontal areas seem to play a particularly large and specific role. Accordingly, this section focuses on these frontal areas. As indicated earlier, rats and other modern rodents do not replicate the traits of early mammals. They, like the other mammalian lineages, have many specializations that emerged over tens of millions of years of independent evolution. However, the organization of the agranular frontal areas in rats appears to be reasonably representative of the ancestral mammalian condition, notwithstanding the many anatomical and behavioral specializations of rats. The long, separate evolutionary history of the agranular frontal cortex in each mammalian lineage makes it likely that differences in both structure and function occur in each order and species of mammal. Nevertheless, we know of no reason to think that the fundamental functions of the agranular frontal areas differ among the mammals.
Learning, along with innate mechanisms, drives reward-seeking behavior in all vertebrates and, if left unsupervised, the most dominant associations ultimately control behavior. The agranular frontal cortex changes this state of affairs. We propose that among the most important innovations of early mammals was an improved “executive function,” mediated by the agranular frontal cortex, including a top-down modulatory function that biases competition among different brain systems engaged in and competing for the control of behavior.
4.4.2. Agranular Frontal Cortex
The mammal innovations include the regions of the agranular frontal cortex called the anterior cingulate (AC), PL, IL, agranular orbital frontal (OFa), and agranular insular (Ia) cortex, which all mammals share (see Section 4.2.3). A growing body of evidence implicates these different parts of the frontal cortex in processes that, in the broadest sense, afford mammals a greater flexibility in their reward-seeking behavior and ultimately bestow upon them the ability to pick and choose between different behavioral options, selecting the one best suited for the exigencies of the moment. These areas have some unique inputs compared to other parts of the mammalian neocortex. The Ia cortex receives relatively direct inputs from parts of the gustatory cortex, located immediately caudal to it, as well as olfactory information from the piriform cortex and visceral related signals from the brainstem and thalamus (Ray and Price 1992). In addition, some “exotic” somatosensory inputs arrive there, leading to the view that it functions in interoception, including sensations conveying pain, itch, temperature, metabolic state (e.g., hypoxia or hypoglycemia), and inputs from the lungs, heart, baroreceptors, and digestive tract (Yaxley, Rolls, and Sienkiewicz 1990; Ray and Price 1992; Zhang, Dougherty, and Oppenheimer 1998; King et al. 1999; Craig 2002; Saper 2002; Drewes et al. 2006). In addition to these inputs, the agranular frontal cortex has reciprocal connections with the amygdala, hippocampus, and midbrain dopamine system, and projects to the basal ganglia. Through these and other pathways, the mammalian frontal cortex interacts with these phylogenetically older systems to promote flexibility in their function (see Sections 4.3.2–4.3.5). (See also Chapter 11 for an in-depth discussion of corticobasal ganglia circuitry and interactions.)
Aside from nonassociative processes such as sensitization and habituation, associative learning mechanisms account for much of a mammal’s ability to obtain rewards. Through Pavlovian and instrumental conditioning that occurs during reward-related episodes, an associative network builds up between internal representations of rewards, actions, and stimuli; each representation has the potential to enter into associations with other representations. This vast associative structure involves several “memory systems,” so called because different parts of the brain encode, represent, and store different kinds of information, which animals use to optimize their performance in terms of benefits and cost. Together with innate mechanisms, the associations mediated by these “memory systems” guide reward-seeking behavior, but do so in different ways. With no top-down modulation, the most dominant of these systems—perhaps based on the strength of its associations—will control behavior.
The behavior of nonmammalian vertebrates gives the impression of operating under the control of a dominant response system of the sort just described, sometimes called a prepotent response. Birds and reptiles often appear to have problems overriding their innate behavioral responses even when this prepotent response increases costs and reduces benefits. These relatively inflexible forms of behavior provide an advantage under many circumstances, especially routine ones. However, problems arise on the less frequent occasions when another behavioral choice would yield greater benefits or reduced costs. We present two examples of such inflexible behavior in nonmammals: one from a reptile, the other from a bird. Note from Figure 4.1 that both kinds of animals are crown species. Neither birds nor modern reptiles represent the ancestral vertebrate condition very well, but they do carry forward many traits from their most recent common ancestor and behavioral control by a dominant brain system may be one of them.
For instance, the green lizard Lacerta has an innate tendency to approach the color green, an instinctual response that presumably evolved to guide them towards leaves. Leaves are a good place for Lacerta to spend their time because they provide camouflage and house the insects and grubs that compose their diet. Wagner (1932) showed that it was almost impossible to train these lizards to override their innate response and reject green, even when in doing so they failed to obtain a palatable food reward. Lizards were trained on a task that required the choice of one of two panels: a red one with a palatable mealworm attached versus a green one with an unpalatable, saline-adulterated worm attached. The lizards consistently chose the green panel despite the cost of doing so: the loss of a tasty worm. Wagner (1932) reported that it took hundreds of trials for these lizards to override their innate response, if they could do so at all. Sensory and motor factors could not account for this result, as the lizards can easily distinguish the green and red panels and make the required choice. Instead, it appears that the prepotent response made it difficult or impossible for the brains of these lizards to use reward in a way that any mammal could easily do, at least any mammal that could see and discriminate red from green.
A frequently recounted study by Hershberger (1986) provides another clear demonstration of severe difficulty in using reward experience to override innate, prepotent responses, in this case from birds. Chicks (genus Gallus) were fed from a bowl with distinct visual characteristics and would subsequently approach the same food bowl if released at a distance from it. This consistent behavior shows that the bowl had acquired the properties of a Pavlovian CS, based on an association between the bowl and the reward outcome, which elicits an instinctive approach response. The chicks were then placed in a “looking-glass” scenario in which the bowl receded twice as fast as they ran towards it and approached them twice as fast as they ran away from it. They therefore had to inhibit their approach behavior in order to obtain the food. Even after a hundred minutes, the chicks continued to run toward the bowl. Apparently, they could not inhibit their conditioned approach response and alter their behavior, despite the fact that their actions cost them the reward every time. In short, the causal consequences of their behavior (i.e., the instrumental contingency) did not affect their behavior, either because they could not learn the experimentally contrived contingency or because they could not make use of it in the face of competition from the dominant response system, in this case one mediating an innate approach response.
Mammals, on the other hand, modify their reward-related behavior flexibly, adapting quickly to their changing needs and environment. This is not to say that instinct controls nonmammalian behavior but has no such control for mammalian behavior or that nonmammalian behavior lacks any flexibility. No such simplistic formulation could ever stand up to scrutiny. Rather, we suggest that mammals have brain areas—the agranular frontal areas—dedicated to modulating the balance among several behavioral-control systems. Nonmammals, lacking these areas, have more trouble doing that.
In rats, for example, the matching-to-position (MTP) task assesses the ability to override innate response tendencies. The MTP task pits a rat’s innate foraging tendency against a newly learned behavior. In order to maximize the likelihood of finding food when foraging, rats have a natural tendency to explore locations that they have not visited recently (if at all) rather than places recently explored and exploited. This foraging strategy means that when given the choice between two arms of a maze, each of which might contain food, rats usually choose the arm visited least recently. The MTP task requires rats to override this innate tendency and learn to select the most recently visited arm in order to obtain a food reward. Normal rats can learn this task easily, successfully overriding their propensity to spontaneously alternate, but rats with prior removals of the PL/IL region of the agranular frontal cortex cannot do so (Dias and Aggleton 2000). The same lesion has no effect on the acquisition of the nonmatching-to-position task, which requires behavior consistent with the rat’s innate tendencies.
The MTP task highlights the advantage of overriding innate responses, at least in a psychology laboratory. The ability to override this tendency must also have some value outside the laboratory. The tendency to vary foraging locations increases a rat’s likelihood of finding fluid or nutrients, as it would have for ancestral mammals. However, when a single location provides a large benefit at low costs, the ability to suppress the prepotent foraging strategy (“look somewhere else”) will likely reduce costs and increase benefits.
Sections 4.4.2.1–4.4.2.5 summarize some of the key research on subdivisions of the rat frontal cortex and their role in the flexible control of reward-seeking behavior, stressing two main themes: top-down biasing of behavioral control systems and flexible alterations of foraging strategies (Table 4.2).
TABLE 4.2
Mammals and Primates.
4.4.2.1. Anterior Cingulate
The AC cortex lies along the medial wall of the rat frontal cortex (mainly AC1 in Figure 4.2c). At first glance, the results of AC lesions on reward-based tasks seem contradictory: some studies report AC lesion-induced disruption across a range of Pavlovian and instrumental tasks, and others report no effect (e.g., Bussey, Everitt, and Robbins 1997a, 1997b; Cardinal et al. 2003; Haddon and Killcross 2005; de Wit et al. 2006). However, once the number of stimuli present at any one time is considered, a fairly consistent pattern of results emerges. Rats with AC lesions show deficits when two or more stimuli simultaneously vie for control of performance (Cardinal et al. 2002, 2003). The AC cortex seems to play the same role for both Pavlovian CSs and instrumental discriminative stimuli: biasing behavioral control towards one among multiple competing stimuli (and their associations). This bias has the effect of ensuring that the most beneficial stimulus maintains control of behavior.
The AC cortex also influences the cost-benefit calculations that influence reward-seeking behavior. The amount of energy required to obtain a reward represents a major cost factor and the AC cortex contributes to weighing whether the benefit of acquiring a reward merits the effort required to obtain it (Walton, Bannerman, and Rushworth 2002; Rushworth et al. 2007). This conclusion is based on research that employs a specially designed task employing a T-maze, one that requires rats to choose between two arms of the maze, each containing food. If a small barrier in one arm requires the rat to climb in order to obtain food, all other things being equal the rat will prefer the no-barrier arm. A larger quantity of food at the end of the barrier arm can induce the rats to climb for their food. Compared to normal rats, those with AC lesions show less tolerance for this increased effort (Walton, Bannerman, and Rushworth 2002; Schweimer and Hauber 2005; Rudebeck et al. 2006). Although AC lesions disrupt effort-based performance in the T-maze, other tasks that involve manipulating the level of effort fail to show such effects. Schweimer and Hauber (2005) reported that when the number of lever-presses required to produce a reward progressively increased in stages (called a progressive ratio schedule), the performance of rats with AC lesions did not differ from that of intact rats. Together, these findings suggest that the AC cortex may only be required for cost-benefit computations when rats must choose between two or more response options.
Based on these findings, we propose that the AC cortex contributes to optimizing behavior under conditions of multiple, competing stimuli and actions. The AC cortex may provide the mammalian brain with the means to weigh more behavioral options than our nonmammalian ancestors could.
4.4.2.2. Prelimbic Cortex
The PL cortex lies along the medial wall of the rat frontal cortex (Figure 4.2c). Like the AC cortex, it contributes to behavioral flexibility; however, unlike the AC cortex, the PL cortex appears to be important specifically in situations involving competition between associations guiding instrumental behavior. Evidence from PL lesions indicates that it is required for the encoding of response-outcome (R-O) associations during learning. This means that PL plays a vital part in maintaining control of instrumental behavior in favor of goal-directed R-O associations over competing habitual stimulus-response (S-R) associations, which are built up simultaneously during learning. If a behavior is goal-directed, and therefore under the control of R-O associations, an animal shows sensitivity to both the current value of the reward and to the contingency between its response and reward. If a behavior is habitual, and therefore under the control of competing S-R associations, these factors do not influence behavior (see Chapter 14).
The level of behavioral control by R-O associations can be assessed by the reinforcer devaluation procedure described earlier in Section 4.3.4, in which the reward outcome value is reduced. Alternatively, behavioral control by R-O associations can be assessed by a contingency degradation procedure, in which the causal relationship between a response and an outcome is degraded (so that the reinforcement is just as likely if the animal does not respond as if it does), while the probability of a rat receiving reinforcement following a response is kept the same. In this situation, rats are able to detect the loss in contingency (providing evidence for sensitivity to R-O correlations) and so reduce responding. Several studies using these techniques show that PL lesions lead to an insensitivity to reinforcer devaluation and contingency degradation procedures. Thus, rats with PL lesions are left with habitual behavior controlled purely by S-R associations; they are blind to the causal relationship between their responses and reward, as well as to the value of the outcome that their actions produce (Balleine and Dickinson 1998; Corbit and Balleine 2003; Killcross and Coutureau 2003). The role of PL is, however, time-limited; lesions of this region that occur after training do not disrupt the aforementioned devaluation procedure in rats, which indicates that PL is involved in the encoding (but not the storage or utilization) of R-O associations (Ostlund and Balleine 2005).
Through the use of inactivation techniques, the PL cortex has also been implicated in switching between strategies used to solve the problem posed by certain tasks. It is important to note that in these experiments the inactivation encompassed parts of both the PL and IL areas, although the inhibitory injections were centered on the PL. For example, Ragozzino et al. (1999, 2003) found that inactivation of the PL/IL cortex selectively disrupted switching between two strategies in a plus maze (see Ragozzino 2007 for review). In one experiment (Ragozzino et al. 2003), rats had learned to use odor cues (independently of spatial location) to guide responses. They were later required to use spatial location (independently of odor cues) to guide responses. Other rats were trained in the opposite order. PL/IL inactivation had no effect on initial performance of the task irrespective of which strategy was correct. However, relative to controls, the rats with PL/IL inactivation were slow to acquire the new strategy, regardless of which of the two strategies was new. The impairment was quite selective: PL/IL inactivation had no effect on reversal of the odor-reward contingencies, even when that task was matched for difficulty to the strategy-switching task. Although the precise role of PL in these strategy-switching tasks remains unknown, the data are consistent with the idea that PL plays a role in the formation of R-O associations. According to this model, PL inactivation would disrupt acquisition of R-O associations while leaving the formation of S-R associations unaffected. If so, PL inactivation during learning of the first strategy might not affect performance because, under these noncompetitive conditions, S-R associations would be sufficient to support behavior. However, PL inactivation during the switch—as the rats attempted to learn the second strategy— would disrupt the formation of the R-O associations encoding the new strategy. As a result, only the newly formed S-R associations would be available to compete with the older S-R and R-O associations, which encoded the first strategy.
One additional clue about the nature of PL’s contribution to strategy switching comes from another recent study (Rich and Shapiro 2007). Rats were required to switch between a response strategy (e.g., turn left) and a spatial or place strategy (e.g., turn north). Although PL/IL inactivation did not disrupt performance on the day of the switch, it did lead to increased use of the old strategy when rats were tested 24 hrs later. Interestingly, this effect was transient and did not persist over multiple switches between the two strategies, indicating that PL is not essential for switching between familiar, well-learned strategies. Thus, it appears that PL is not necessary for switching per se but rather for a mechanism that influences switches when the behaviors are not well learned. This conclusion makes sense if one considers that, across training, instrumental responding shifts from R-O to S-R control (Dickinson and Balleine 1994). In this view, early switches would rely heavily on R-O associations, whereas later switches would predominately involve S-R associations. Disruption of the formation of R-O associations by PL inactivation would therefore be expected to disrupt early switches more than later ones. Rich and Shapiro (personal communication) also examined the activity of neurons in the medial frontal cortex during a place-response strategy switching task. They found that the activity of PL neurons was correlated with the behavioral switch. In addition, although both PL and IL neurons were active during the switch phase, the changes in activity of PL neurons preceded those in IL neurons; the change in activity of IL neurons occurred when performance was close to being proficient once again. This finding probably reflects the passage of control of instrumental behavior from PL-dependent R-O associations to IL-dependent S-R associations (see Section 4.4.2.3). (Note that the neurophysiological data of Rich and Shapiro appeared in the Journal of Neuroscience after the completion of this chapter.)
In summary, a substantial body of work implicates PL in mediating goal-directed behavior by supporting the development of R-O associations. Recall our earlier discussion of the role played by the amygdala in updating the biological value of rewards (Section 4.3.4). In this context, PL could promote behavioral flexibility by allowing animals to take into account both the current value of a reward as well as the relationship between its response and the reward it produces. This role for PL cortex could also account for its contribution to strategy switching.
4.4.2.3. Infralimbic Cortex
The IL cortex lies along the medial wall of the frontal cortex and, in rats, is located immediately ventral to the PL cortex (Figure 4.2c). As in some of the rule-switching studies mentioned above, researchers have often considered the PL and IL cortex together. Lesions limited to the IL cortex, however, yield different behavioral effects than lesions limited to the PL cortex, especially for instrumental behavior and extinction.
Whereas the PL cortex supports the control of instrumental behavior by response-outcome (R-O) associations (see Section 4.4.2.2), the IL does the opposite, promoting behavioral control by S-R associations. Using satiety-specific devaluation of a reward associated with an instrumental response, Killcross and Coutureau (2003) demonstrated a double dissociation of function between the PL and IL cortex. As described in the previous section, PL (but not IL) lesions cause a rat’s behavior to become insensitive to devaluation of its associated reward, leaving them stimulus-bound, literally creatures of habit. By contrast, IL (but not PL) lesions result in behavior that remains under the control of a predicted reward outcome and its biological value, even after a lengthy training period. Put in positive terms, as opposed to the negative descriptions that arise from describing the effects of lesions or inactivations, these findings imply that whereas PL supports behavioral control of instrumental responding by outcome-dependent, goal-directed R-O associations, IL biases behavior toward outcome-independent, habitual S-R associations (Table 4.2). Given the well-documented shift in behavioral control from goal-directed to habitual responding that occurs with lengthy periods of instrumental training (Dickinson and Balleine 1994), such a transition presumably reflects a change in the balance between PL and IL influences, with IL ultimately prevailing for situations of high predictability. Consistent with this idea, temporary inactivation of IL following extensive training reinstates outcome-dependent, goal-directed performance after behavior becomes habitual (Coutureau and Killcross 2003).
The IL cortex also plays a role in extinction, during which the performance of behaviors that previously led to reward no longer do so. Extinction differs from forgetting or unlearning in that it depends on new learning that overrides the original knowledge, and evidence suggests that this learning takes the form of inhibitory S-R associations (Rescorla 1993). Like the kinds of behavioral competition mentioned earlier, extinction training establishes a situation in which the original learning and the new learning compete for behavioral control. Under certain conditions, such as the passage of time, the original learning can once again prevail, and this leads to a transient restoration of the original response referred to as spontaneous recovery. IL has been shown to play a vital role in the expression of extinction learning. Although most of the work has focused on extinction of Pavlovian conditioned fear (see Quirk and Mueller 2008 for a full review), IL also contributes to the extinction of appetitive Pavlovian learning (Rhodes and Killcross 2004). Rats with IL lesions have no problem in acquiring extinction; they show both the normal reduction in responding on the first day of extinction training, as well as faster extinction on the second day compared to the first (i.e., savings). Rats with IL lesions do, however, show a deficit in retrieving the extinction memory at the start of the second extinction session; they display a greater level of spontaneous recovery of the original CR compared to normal rats (Rhodes and Killcross 2004). This finding indicates that IL biases behavioral control towards the newer, weaker extinction learning and that, without IL, the more dominant, original learning maintains behavioral control. This idea is supported by the finding that the IL-lesion-induced increase in spontaneous recovery reduces across successive extinction sessions as the extinction learning strengthens (Lebron, Milad, and Quirk 2004).
Although the two lines of research summarized here for the IL cortex involve different learning mechanisms—one Pavlovian, the other instrumental—both represent situations in which two associations involving the same stimulus vie for control over behavior. In Pavlovian extinction, the inhibitory stimulus-response (S-R) association competes with previous learning regarding the same stimulus. By contrast, in instrumental behavior, the habitual S-R association competes with the goal-directed S-R-O associations, which are also under the control of the same stimulus. The IL cortex contributes in much the same way to other behaviors that involve competition between two associations involving the same stimulus, such as Pavlovian conditioned inhibition (Rhodes and Killcross 2007) and discrimination reversal learning (Chudasama and Robbins 2003).
The IL-dependent ability to bias the control by one kind of association over another would confer an adaptive advantage for mammals over nonmammalian ancestors that lacked an IL cortex. These nonmammalian ancestors would not have the behavioral flexibility provided by the ability to learn and bias competing and sometimes contradictory associations, a contribution that comes to the fore when an otherwise dominant behavior no longer yields a sufficient benefit.
Taken together, the medial frontal areas of mammals—AC, PL, and IL cortex—show similarities in their function. In their specialized ways, each appears to allow rapid adaptability to a changeable environment in which experiences lead to competing responses, any of which might be appropriate in a given circumstance (Table 4.2). Sections 4.4.2.4 and 4.4.2.5 take up the orbital frontal areas, OFa and Ia.
4.4.2.4. Orbitofrontal Cortex
Of the many parts of the agranular frontal cortex, the agranular orbitofrontal (OFa) cortex has perhaps attracted the most attention. A major problem with this literature involves the assumption that the regions called the “orbitofrontal” cortex in rats correspond to the regions with the same name in primates. As noted above, comparative neuroanatomy indicates, to the contrary, that the rostral, granular parts of the orbitofrontal cortex in primates have no homologue in nonprimate species, including rats (Figure 4.2). Accordingly, we distinguish between the granular parts of the orbitofrontal cortex in primates, which rodents lack, and the agranular orbitofrontal cortex, which is homologous in rodents and primates. For convenience, we call the former PFo, for orbital prefrontal cortex, and the latter OFa, for agranular orbitofrontal cortex.
There is little question that the rat OFa plays an important role in the control of reward-seeking behavior. The activity of neurons in OFa reflects reward expectation, especially the sensory-specific properties of reward (Schoenbaum, Chiba, and Gallagher 1998). In addition, OFa lesions impair the ability to make decisions on the basis of reward expectations (Gallagher, McMahan, and Schoenbaum 1999). Current views emphasize two interpretations of this kind of result: one posits a contribution to reward valuation per se and the other emphasizes the learning of predictive relationships between stimuli and rewards (i.e., Pavlovian S-O associations). Detailed reviews on this topic have been published (Holland and Gallagher 2004; Delamater 2007; Ostlund and Balleine 2007; Rushworth et al. 2007) and are not recapitulated here.
There is general agreement that lesions of the OFa cause deficits in choices based on reward value. Rats with OFa lesions are more likely than control rats to choose or approach stimuli associated with a devalued reward (Gallagher, McMahan, and Schoenbaum 1999; Pickens et al. 2003, 2005). These results could, however, reflect either a general deficit in the computation of reward values, which would disrupt behaviors driven by both instrumental R-O and Pavlovian S-O associations, or a specific deficit in some aspect of S-O associations. A recent study supports the latter interpretation; OFa lesions fail to disrupt the effect of reinforcer devaluation on an instrumental response, which relies on the integrity of R-O associations (Ostlund and Balleine 2007). This finding indicates that the OFa does not compute (or represent) current reward value in general and, by default, likely plays a more specific role in some aspect of the relationship between stimuli and the rewards that they predict. Findings from other behavioral paradigms that rely on S-O mechanisms, but do not involve any manipulation of outcome value, provide further evidence in support of this notion. These procedures include Pavlovian contingency degradation, PIT and the differential-outcomes effect (McDannald et al. 2005; Ostlund and Balleine 2007). These studies, among others, show outcome-selective effects of OFa lesions, suggesting that the OFa plays an important role in the association between a stimulus and the sensory aspects of reward (as opposed to its motivational or temporal aspects).
Burke et al. (2008) provided direct evidence for this idea using a trans-reinforcer blocking design to control those aspects of a reward representation that become associated with a stimulus. Rats were first trained to associate one stimulus (S1) with a particular reward (O1) and were subsequently presented with S1 as part of a compound stimulus (S1+S2). The compound stimulus was paired with a different reward (O2). Importantly, O1 and O2 were equally palatable food pellets that shared the same general motivational and hedonic characteristics but differed in their sensory-specific characteristics (taste). The associations built up between S1 and O1 in the first stage blocked the formation of associations between S2 and the general aspects of O2 in the second stage because they were already predicted by O1. However, associations between S2 and the sensory-specific properties of O2 are not blocked, and their presence was identified by testing the ability of S2 to act as a conditioned reinforcer (i.e., whether it had acquired reinforcing properties via its association with the food reward O2). When rats were given the opportunity to press a lever to bring about S2 presentations, S2 was able to support conditioned lever pressing (compared to a fully blocked control stimulus) and this lever pressing decreased following satiety-specific devaluation of O2. These findings from intact rats indicate that the conditioned responding was mediated by the associations between S2 and the specific taste of O2. Rats with OFa lesions, however, would not work for presentations of S2, demonstrating that behavioral control was unaffected by the relationship between a stimulus and the specific sensory properties of reward. The results thus indicate that OFa contributes more to learning associations between CSs and the sensory aspects of reward (e.g., taste, smell, visceral, tactile, and visual concomitants, etc.) than to a role in computing biological value per se.
Lesions of OFa also cause changes in delay tolerance. Relative to control rats, rats with OFa lesions have a greater tendency to choose larger, delayed rewards versus smaller, more immediate rewards (Winstanley et al. 2004; Kheramin et al. 2002). This result surprised many experts, who predicted that rats with OFa lesions would become “impulsive” based on a loss of “behavioral inhibition.” In that case, rats with OFa lesions should have become less tolerant of delay, the opposite of what was observed in the two papers cited above. Although we are aware that other experiments have yielded a different result, possibly because of differences in testing and training methods, let us assume for the sake of discussion that OFa lesions cause increased delay tolerance, as the two cited papers reported. Changes in delay tolerance probably reflect something about foraging strategies. For example, Stevens, Hallinan, and Hauser (2005) and Stevens, Rosati et al. (2005) have pointed to species-typical foraging strategies to account for different degrees of delay tolerance in two species of New World monkeys. Tamarins are more insectivorous than common marmosets, whereas marmosets eat more tree gum. Gum eating involves scratching tree bark and waiting for the sap to flow, whereas foraging for insects favors immediate acquisition of a widely dispersed, transiently available food source. Species that typically exploit lower value, but readily available, food resources tend to show considerable delay tolerance; they can wait a long time, their favored food will always be there. By contrast, species that favor high-value but less frequently available foods have little tolerance for delay; they must get what they want now or never. So how could S-O associations contribute to the increased delay tolerance shown by rats with OFa lesions? Perhaps, on the basis of environmental stimuli and their S-O associations, the OFa mediates a prediction of the specific sensory properties of a high-quality reward, such as its taste or smell. This prediction could lead to an affective response that biases rats toward seeking that higher-quality food source. Without this affective signal, the lower-value, more readily available reward predominates.
This view of OFa function stresses sensory processing and prediction, specifically about the sensory aspects of rewards, and complements the proposals put forward above for the medial frontal areas, AC, IL, and PL. Table 4.2 suggests that medial frontal areas bias behavioral control among competing stimuli, actions, and kinds of associations, such as R-O versus S-R associations. Perhaps the OFa, a more lateral part of the frontal cortex, instead biases behavioral control among competing foraging strategies.
4.4.2.5. Agranular Insular
The agranular insular (Ia) cortex comprises the lateral part of the rat’s agranular frontal cortex and is an elongated structure, extending more caudally than any other structures considered part of the agranular frontal cortex (see Figure 4.2). Probably as a direct result of this caudal extension, there is considerable variability in the lesions used to study this region; many lesions encroach into neighboring regions, such as the overlying granular insular cortex or the OFa, and many studies employ lesions that differ in rostrocaudal extent (cf. Balleine and Dickinson 2000; Boulougouris, Dalley, and Robbins 2007). Because Ia is near to and connected with the gustatory cortex in the rat, considerable research has focused on its involvement in food reward processing. The Ia cortex appears to be involved in retrieving the representation of a food reward’s incentive value when the food is absent. For example, Balleine and Dickinson (2000) used the reinforcer devaluation procedure to show that rats with lesions that probably included the caudal part of Ia, along with adjacent granular areas, have deficits when they need to activate the memory of a taste in order to access incentive-value information. In their task, the rats could press either of two levers to produce two different food rewards and subsequently underwent satiety-specific devaluation. When tested under conditions in which the lever presses resulted in the delivery of a reward, rats with Ia lesions, like controls, pressed the lever associated with the devalued reward less often. However, when tested under extinction (i.e., in the absence of reward), rats with Ia lesions pressed both levers equally, unlike controls. Balleine and Dickinson (2000) concluded that rats with Ia lesions could not recall the sensory properties of the food (e.g., its taste) in its absence and therefore could not activate a representation of the food’s value to guide behavior. More research needs to be done to test this hypothesis directly, but if the Ia cortex is involved in recalling the sensory properties of an instrumental reward in its absence (based on R-O associations), then it would play a role complementary to that of the OFa. As discussed above, the OFa may be involved in recalling the sensory properties of a reward associated with a Pavlovian stimulus (based on S-O associations). However, Holland (1998) has suggested that the Ia cortex might also contribute to the latter function. This idea is based on results by Kiefer and Orr (1992), who showed that rats with Ia lesions—a lesion that spared OFa—failed to show conditioned orofacial responses (a normal result of the decrease in palatability or hedonic value) to a flavored drink that had been associated with illness. This deficit occurred despite the fact that conditioning did take place, as indicated by a reduced consumption of the drink due to the decrease in its motivational value. This finding seems to argue against a distinction between OFa and Ia function in terms of instrumental (R-O) associations versus Pavlovian (S-O) associations. Nevertheless, it appears that like the OFa, the Ia plays some role in the associative activation of some aspect of reward representations.
The concept of accessing the representation of reward value in a food’s absence has explanatory value for other studies as well. Kesner and Gilbert (2007) found that rats with Ia lesions do not show the normal reduction in the consumption of a drink of lower-value reward (a 2% sucrose solution) when it is always followed by a drink of higher value (32% sucrose), a phenomenon called the anticipatory contrast effect. To perform this task well, rats need to recall the representation of the anticipated second solution when drinking the first, and based on their relative values defer consumption of the first solution pending the availability of the more beneficial reward. The Ia cortex could contribute to the process by which drinking the first solution leads to retrieval of the representation of the sensory characteristics of the second solution from memory. Once the representation of the second solution is activated in this way, its value can be accessed and compared to the value of the first solution to influence behavior.
According to the data summarized above, both Ia and OFa play a role in the associative activation of some aspect of reward, which enables the memory of that reward to guide behavior even when the reward is absent. Compared to nonmammalian ancestors that lack these frontal areas, the improved ability to remember and predict the specific sensations associated with each particular kind of reward would yield advantages when foraging choices need to be based on current needs. The results from Kesner and Gilbert (2007) indicate that Ia biases intact rats toward reducing their consumption of a lower-value reward when a higher-value one is expected later, in a sense promoting a more “patient” foraging strategy. The results from Winstanley et al. (2004) and Kheramin et al. (2002) indicate that the OFa biases rats toward obtaining lower-quality but readily available food rewards: in a sense, promoting a less “patient” foraging strategy aimed at rapid exploitation of a food resource (see Table 4.2).
Specific tests and refinement of these ideas remain for the future, but we propose that the core function—and adaptive advantage—conferred by the lateral parts of the rat’s agranular frontal cortex (OFa and Ia) involves the ability to have the best of both worlds, depending on circumstances. When cost-benefit calculations indicate an advantage in patient foraging for a highly reliable and available food source, Ia learns the context for this circumstance. Later, when this circumstance occurs again, Ia biases behavioral control toward high levels of delay tolerance. When cost-benefit calculations indicate an advantage in the rapid exploitation of an unreliable but highly beneficial food source, the OFa learns the context for this circumstance and later biases behavioral control toward “impatient” foraging, as reflected in steep delay discounting functions (Table 4.2). According to this scheme, medial frontal cortex mediates different influences over foraging, based more on spatial, motor, and associative factors, such as R-O, S-O, and S-R associations. For example, the results from Dias and Aggleton (2000), discussed in Section 4.4.2, indicate that the medial frontal cortex biases rats toward repeated exploitation of a given location’s resources, overcoming an innate tendency to avoid previously exploited foraging sites.
Stated generally, the idea presented here is that the agranular frontal areas, which evolved in early mammals, fine-tune the balance of behavioral control instantiated by phylogenetically older brain systems, which early mammals inherited from their amniote ancestors. Competing behavior-control systems all remain viable options, available simultaneously to contribute to the animal’s fitness when the appropriate context arises. This form of top-down control allows a choice among various courses of action, often promoting a less frequently practiced behavior if it is the more appropriate option for the current situation, taking into account both long-term and short-term benefits and costs. The ability to learn contradictory lessons from experience and to choose the behavioral-control system best suited to the exigencies of the moment provides mammals with a substantial advantage over ancestral species that had less proficiency in learning such contradictions.
4.5. WHAT CAN PRIMATE BRAINS DO WITH REWARD?
4.5.1. Primate Brains: Basic Mammalian Mechanisms Plus Granular Prefrontal Cortex
In Section 4.4, we noted that as mammals emerged from our amniote ancestors (Figure 4.1), the systems that had developed earlier in vertebrate evolution remained in operation, albeit with modifications. The midbrain dopamine system, basal ganglia, amygdala, and hippocampus continued to do more or less what they had done for hundreds of millions of years. Likewise for the emergence of primates, the ancestral systems, including the agranular frontal cortex, continued to play a central role in what primate brains can do with reward.
As explained in Section 4.2.4, primates and nonprimate mammals share the agranular frontal areas, but do not share a PFg (Figure 4.2), which evolved in primates. Among the prefrontal areas, the granular parts of the orbitofrontal cortex (PFo) play a large role in what primate brains can do with reward. These areas include area 11 as well as the light gray parts of areas 13 and 14 in Figure 4.2. In addition, primates also innovated certain sensory areas. Given the importance of vision in primates, the inferotemporal cortex (IT) is notable among these primate innovations. (Note that certain carnivores, such as domestic cats, also evolved high-order visual areas, but these likely arose independently, through parallel evolution (Preuss 2007).) Their long, separate evolutionary history means that the shared, agranular frontal areas will also have diverged in both structure and function in primates versus nonprimates.
Assuming that the emergence of the granular prefrontal areas provided primates with an advantage over the ancestral condition, what could that advantage be? Most answers to this question fall into one of two categories: (1) the new cortical areas functioned much like older parts, but performed those functions better in some way; and (2) the new areas permitted a novel function. However, there is no need to choose between the two and the emergence of a PFg probably led to both kinds of advances.
4.5.2. New Visual Areas in the Context of Feature Conjunctions
Cost-benefit analysis improves when the stimuli that predict rewards and costs do so with more fidelity, and also when those stimuli can be combined or categorized in useful ways. Some of the mechanisms in the PFg, generally, and the granular orbitofrontal cortex (PFo), in particular, seem to support such an advance.
The PFo of primates is in a pivotal position to permit novel kinds of S-O associations, if only because it receives information from visual areas, such as the IT, that other mammals lack (Figure 4.3). The PFo receives strong projections from the IT and other posterior sensory areas of cortex, including the auditory cortex. It also receives inputs from the agranular frontal areas, in particular areas specialized for gustatory, olfactory, and visceral inputs (Rolls, Yaxley, and Sienkiewicz 1990; Webster, Bachevalier, and Ungerleider 1994; Carmichael and Price 1995; Cavada et al. 2000; Kondo, Saleem, and Price 2005). By virtue of its receipt of all these kinds of information, PFo is one of the earliest sites for convergence of visual information with gustatory, olfactory, and visceral inputs. Furthermore, PFo receives inputs from the perioral and tongue representations of the primary somatosensory cortex, as well as strong projections from the perirhinal cortex, a multimodal area that has been shown to operate as an extension of the ventral visual stream or object-processing pathway ( Murray, Bussey, and Saksida 2007). Consistent with the anatomical evidence, individual neurons in the PFo of macaque monkeys respond to gustatory, olfactory, and visual stimuli presented separately or together (Rolls and Baylis 1994; Pritchard et al. 2008).
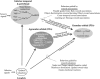
FIGURE 4.3
Convergent contributions to PFo function. PFo is depicted as receiving three inputs that influence what it can do with rewards: representations of visual feature conjunctions for objects and rewards; updated valuations of rewards; and conjunctions of (more...)
These findings suggest that primates may have a greater capacity to link visceral, olfactory, and gustatory inputs with high- and intermediate-order visual stimuli than our nonprimate ancestors. This new hypothesis originates, in part, from recent research on visual processing mechanisms in macaque monkeys. This work indicates that the perirhinal cortex represents complex conjunctions of visual features that promote object identification (Bussey and Saksida 2007; Murray, Bussey, and Saksida 2007). Other sensory modalities are also important to perirhinal cortex function, but given the predominance of vision in primates, we will focus on the visual aspects of scenes and objects. Bussey, Saksida, and Murray (2002, 2003) developed and tested this idea with a series of visual discrimination tasks in which combinations of features, rather than any individual feature, best predicted reward. In the context of discrimination learning, this objective can be attained by having individual features appear as part of both the correct (S+) and incorrect (S-) objects in a pair. In one experiment, for example, compound objects were comprised of two of four possible features: A, B, C, and D. Monkeys received rewards when they selected the compound stimuli AB and CD, but not when they selected stimuli CB and AD. This experimental design created a conflict, called feature ambiguity, because individual features were associated equally with both reward and non-reward. Accordingly, to obtain a reward, the monkeys were required to represent the combinations of features such as AB (depicted as VisAB in Figure 4.3, with the individual features depicted as VisA and VisB, respectively). Bussey, Saksida, and Murray (2002, 2003) showed that lesions of the perirhinal cortex disrupted performance in several tests based on this premise. Their results showed that the representation of feature conjunctions in the perirhinal cortex helped resolve feature ambiguity and promote object identification.
Homologues of the primate perirhinal cortex, along with lower-order visual areas such as the striate and near-striate cortex, can be found in rats and other mammals, but the evolution of additional areas such as the IT occurred in primates. What new capabilities could these new areas provide? Whereas perirhinal cortex is thought to represent the most complex (highest-order) visual features that compose objects, the IT cortex represents intermediate levels of feature conjunction. According to this view, the striate and near-striate cortex represent lower-order conjunctions, compared to the IT cortex, and the perirhinal cortex represents higher-order ones. Thus, it appears that during primate evolution, newly evolved areas emerged between the two extremes of conjunctive representations: mid-level conjunctions. Figure 4.3 depicts mid-level conjunctions as VisAB and VisCD, whereas high-level conjunctions are denoted as VisABCD. The existence of mid-level feature conjunctions changes what primate brains can do with reward, specifically by permitting categorization-based conjunctions that fall short of whole objects, but have greater complexity than their fundamental features.
4.5.3. Orbital Prefrontal Cortex
As illustrated in Figure 4.3, the PFo appears to be the earliest site at which the high- and mid-level feature conjunctions of an object (from perirhinal and IT cortex, respectively) converge with olfactory, gustatory, and visceral inputs (relayed via the agranular orbitofrontal areas). This anatomical convergence enables feature conjunctions of a particularly important kind. Comparison to mammals that lack a PFo, which is to say all nonprimate mammals, indicates that the PFo may provide the neural substrate for finer distinctions among food and other rewards based on a combination of their visual attributes with their chemosensory and visceral sensory properties (collectively depicted as “chemovisceral” feature conjunctions in Figure 4.3). In addition to representing finer distinctions among reward items, the PFo could also represent more generalized categories of foods. We propose that these visual-chemovisceral feature conjunctions promote an enhanced flexibility in responding to rewards. The sight of a particular food, a fruit at a particular stage of ripeness for example, might evoke its taste and smell, along with the pleasant sensations and positive affect that follow its consumption. Similarly, the fruit’s odor might evoke the whole, cross-modal configuration of features as well. This idea is much like that put forward in Section 4.4.2.4 for the OFa, but by making use of the richer analytical capacities of IT, for example, the PFo would provide an advantage over animals that lacked these areas.
So far, we have stressed ways in which the PFo functions much like the agranular frontal areas of rodents (and, presumably, the agranular frontal areas of primates as well). What about new functions? Some evidence points to a role for the PFo in representing value in a “common currency,” one that is independent of the specific kind of reward (benefit) or a particular kind of cost. In many neurophysiological studies in monkeys, a particular visual stimulus has been associated with a specific reward through learning, primarily via Pavlovian (S-O) associations. At the time the monkey views the stimulus, the activity of many PFo neurons reflects the value of the associated reward, even though the reward itself arrives much later. This property is by no means unique to the PFo: in this regard it resembles phylogenetically older structures, such as the midbrain dopamine neurons and many other parts of the frontal cortex. Yet, the reward-expectancy signal that the PFo generates when elicited by the sight of a particular food might differ in a subtle but important way from similar signals in these other structures. Recent neurophysiological studies in monkeys have found that the activity of PFo neurons reflects the value of rewards independent of reward type (Wallis and Miller 2003; Padoa-Schioppa and Assad 2006). In addition, neurons in the PFo (along with neurons in other parts of the cortex) encode at least three factors that enter into the calculation of reward value: the magnitude of reward, the probability of reward, and the effort required to obtain it (Kennerley et al. 2009). Thus, it appears that the PFo’s outcome representations extend beyond the particular substance (sucrose, quinine, juice) paired with a stimulus to signal a value that transcends particular types or amounts of reward (Padoa-Schioppa and Assad 2006).
To date, no neurophysiological study of the rodent frontal cortex has shown this property, although some have shown that neurons in the agranular frontal areas have activity that reflects specific expected reward outcomes and specific S-O associations (Schoenbaum, Chiba, and Gallagher 1998; Schoenbaum and Eichenbaum 1995). One possibility, then, is that the multiple levels of sensory convergence available in the PFo might enable the computation of both the “menu-independent” value signal observed by Padoa-Schioppa and Assad (2008), which indicates outcome values regardless of the reward options, and a drive-independent value signal (the “common currency”), which indicates values for rewards (and costs) of any kind (e.g., sex, food, fluid, exertion, etc.). This cost-benefit analysis can only be performed efficiently by reference to a common measure of value. It is possible that these features of PFo build on properties of the nearby agranular frontal areas, but do so in a particularly advanced way because of the extensive and varied visual inputs provided to the PFo by the perirhinal and IT cortex. Recall that the mid-order feature conjunctions provided by the IT permit a kind of generalization that would be much harder to come by in brains, such as rodent brains, that lack an IT cortex.
Autonomic signals or information about emotional state may also influence the calculation of value in the common currency described above. Experiments in monkeys have taken advantage of monkeys’ innate fear of fake or real snakes to provide findings consistent with this idea. In an experiment by Izquierdo et al. (2005), monkeys were given the opportunity to reach over either a neutral object or a fake snake to retrieve a food reward. Thus, the benefits of obtaining the food reward were directly pitted against the costs (in terms of risk) of approaching the snake. When required to reach over the fake snake, intact monkeys displayed long food-retrieval latencies or refused to take the food altogether. In contrast, monkeys with PFo lesions exhibited short food-retrieval latencies, similar to when they reached over neutral objects. Similar findings have been reported by Rushworth et al. (2007). It seems that monkeys with PFo lesions cannot integrate the sensory signals, in this case arising from visual signals about a potential predator, with food value to guide their behavior. The inability to compute value in a common currency for risks and reward could account for these findings. Figure 4.3 depicts the results of the snake test as depending on interactions between the amygdala and PFo.
Like amygdala lesions (see Section 4.3.4), PFo lesions cause a deficit on the reinforcer devaluation task (Izquierdo, Suda, and Murray 2004). Compared to controls, monkeys with bilateral removals of PFo more often choose an object that will produce the devalued reward. Accordingly, Figure 4.3 depicts this test as depending on interactions between the amygdala and PFo (Baxter and Murray 2002), with the PFo mediating the associations between the visually sensed objects, the sight of a particular food, and its updated value.
4.5.4. Other Granular Prefrontal Areas
So far, this section has concentrated on the PFo, but primate brains have many granular frontal areas, including the dorsal, dorsolateral, ventrolateral, medial, and polar prefrontal cortex (Figure 4.2). These areas function in higher-order aspects of cognitive control, including those involved in monitoring actions (Petrides 1991) and implementing rules and strategies (Murray, Bussey, and Wise 2000; Wallis, Anderson, and Miller 2001; Mansouri, Buckley, and Tanaka 2007). Rules and strategies allow animals to gain rewards when faced with novel stimuli and contexts. Rules and strategies have many similarities, but a few differences as well. Rules are input-output algorithms that can be applied to solve a behavioral problem for novel as well as familiar stimuli; strategies are either one of many solutions to a problem or a partial solution to a problem.
4.5.4.1. Rule-Outcome and Strategy-Outcome Associations
Monkeys can learn abstract rules and strategies, and it has been proposed that these abstract rules arise from reinforcement of rules rather than stimuli (Gaffan 1985). That is, much as the representation of a stimulus can be associated with reward outcome, as in Pavlovian conditioning (which forms S-O associations), the cognitive representation of a rule can be associated with reward outcome. For example, one widely used test of object-reward association—called win-stay by some (e.g., see Chapter 3), but called congruent recall by Gaffan—has two parts: acquisition and test. In the acquisition phase, a monkey displaces one object that yields a reward and a second object that does not. After a delay, the same two objects appear, and the monkey can choose only one. The monkey can obtain another food reward by displacing the object that had been paired with food in the acquisition phase. Orthodox animal learning theory predicts that the stimulus-outcome contingency controls the monkey’s choice. Gaffan’s results contradicted that prediction. For a different group of monkeys, he applied the opposite rule, which some might call win-shift, but Gaffan called incongruent recall. This response rule requires the monkey to avoid the object that had been associated with reward during the acquisition phase and choose the other one instead. Macaque monkeys not only acquired the incongruent-recall rule as quickly as they learned the congruent-recall rule, but also performed equally well on subsequent tests requiring the application of the rule to lists of novel stimuli. Thus, even though in one case (the win-shift, incongruent-recall rule) the monkeys had to overcome behavioral “control” by S-O associations, experienced monkeys efficiently did so. This finding shows that although monkeys learn the association of individual stimuli with rewards, they also learn the association of rules with rewards and when these two aspects of their experience come into competition they can bias behavioral control toward the brain systems that deal with choosing rules rather than choosing stimuli.
This idea relates to the discussion of foraging strategies developed in Section 4.4, as well as the concept of biases among competing mechanisms for behavioral control. As for the latter, a prediction arising from this idea is that monkeys without a PFo should be deficient in implementing rules (based on rule-reward associations), especially when the rule must override S-O associations. Consistent with this idea, macaques with lesions that include the PFo lost the ability to use preoperatively acquired performance rules (Rushworth et al. 1997; Bussey, Wise, and Murray 2001; Buckley et al. 2007).
Similarly, Murray and Gaffan (2006) proposed that monkeys can associate reward with problem-solving strategies. In traditional tests of object (or stimulus) discrimination learning set, a single pair of stimuli is used repeatedly for several if not dozens of trials. New stimulus discrimination problems are introduced periodically. When monkeys have had experience with many problems of this type, they exhibit faster learning. More rapid acquisition of later problems relative to earlier ones demonstrates the development of a learning set. Because discrimination problems typically involve massed trials (i.e., many consecutive trials with one and the same pair of stimuli), monkeys presumably retain a memory of the correct response from one trial to the next that permits the development of object discrimination learning set. To test this idea, Murray and Gaffan trained monkeys on a large set of discrimination problems involving long (24-hr) intertrial intervals. If the short-term memory of the outcome of the previous trial was important for developing learning set, monkeys given 24-hr intertrial intervals should fail to develop a learning set. In the Murray and Gaffan study, monkeys learned 20 stimulus discrimination problems concurrently. They received one trial per problem per day, with the 20 pairs used for the 20 trials per daily test session. The same stimulus pairs were presented for choice each day, until the monkeys reliably approached and displaced the rewarded object of each pair. After mastering one set of 20 problems, the monkeys learned another set, and so on, until they had learned 10 consecutive sets of discrimination problems. Despite their extensive experience—each monkey had learned 200 individual problems—the monkeys with 24-hr intertrial intervals failed to develop a learning set. Murray and Gaffan (2006) concluded that monkeys only acquire a learning set when intertrial intervals are short enough to allow the events of the preceding trial to be retained in short-term memory. Specifically, they proposed that the rule learning underlying learning set involves laying down a prospective memory of what stimulus to choose on the next trial. When the monkey succeeds, the reward reinforces the laying down of the prospective memory from each trial, gradually leading to the formation of a learning set. Thus, rather than the learning set resulting from improved response rules at the time of the choice test, a kind of learning-to-learn that would occur regardless of the length of intertrial intervals employed, learning set instead depends on learning what to remember. In short, what is reinforced is a strategy about what to remember over the intertrial interval. This idea likely applies to a wide range of rule learning (e.g., congruent and incongruent recall tasks, delayed nonmatching-to-sample, object reversal learning set, etc.). Recent evidence shows that both learning set and reversal learning set depend on the interaction of the PFg and IT cortex (Browning, Easton, and Gaffan 2007; Wilson and Gaffan 2008).
4.5.4.2. Value-Free Reward Representations
Monkeys can use reward information in other ways as well. One underappreciated aspect of reward-based learning is that animals can process positive reinforcement much like they would any other sensory signal. That is, the role of food reward in many instances can derive from its informational value, as opposed to its reinforcing or emotional value. Evidence in support of this idea comes from neuropsychological experiments showing a dissociation between the neural structures required to support learning about food value versus those required for learning about the occurrence of food (through object-reward associations). For example, the amygdala is essential for updating food value and linking that value with object-food associations, as explained in Section 4.3.4. The amygdala is not necessary, however, for learning which stimuli are associated with food reward when object-reward associations change, as they do in the object discrimination reversal task (Izquierdo and Murray 2007). (Recall that in this task, a previously rewarded object changes to become unrewarded and vice versa.) It appears that, in these tasks, the occurrence or nonoccurrence of food guides the selection of a performance rule. Once a performance rule has been learned, the role of food in such tasks is largely limited to its informational value, as opposed to its reinforcing or emotional value, and this informational processing does not depend on the amygdala. Accordingly, Figure 4.3 depicts this process as depending on interactions between visual areas (perirhinal cortex and IT) and the PFo, although other prefrontal areas also play a role.
To sum up this idea about value-free food representations, we propose that reward can operate independently of biological value in primates. When monkeys implement rules efficiently, as is the case for experienced monkeys performing object discrimination learning (learning set) or object discrimination reversal tasks (reversal set), the outcome serves primarily to provide feedback as to the success of that trial, which might be a visual representation of a food among other possible types of feedback, rather than a representation of food value itself.
4.5.5. What Can Human Brains Do with Reward?
The age-old question of what separates us from other animals is beyond the scope of this article, but a couple of comments seem pertinent because we humans can do things with reward that other animals cannot. For example, we can talk about reward. We also have the ability, called mental time travel, to remember past events and to imagine future ones as if they were current, incorporating a representation of ourselves into the event (Suddendorf and Corballis 2007; Corballis 2009). This ability probably contributes to overcoming the deep devaluation of rewards that occurs at a long delay. Mental time travel allows low-frequency and temporally distant events to guide behavior and to override choices prompted by high-frequency and more proximate events. Episodic memory can provide the substrate for a single event to guide behavior decades later. The human capacity to both remember and to plan over an extraordinarily long time horizon, therefore, has to count as one of the things that human brains—and therefore primate brains—can do with rewards.
Finally, we note that humans, at least, also have the capacity to develop second-order intentions about rewards. For example, people may not want to want food rewards. Second-order intentions are another novel way in which the primate brain deals with reward. It is extraordinarily doubtful that such a capacity exists in nonprimates and it is probably absent in nonhuman primates as well.
4.6. SUMMARY
With the early vertebrates came a long list of neural innovations, including the “midbrain” dopamine system and the telencephalon (Northcutt 1996; Thompson et al. 2008). The dopamine system plays a role in predicting the benefits called rewards and computing the differences between such predictions and what ultimately occurs (Schultz 2006, 2007). In the telencephalon, the amygdala updates neural representations of biological value—for example, the value of a food—based on the current state of an animal (Murray 2007). The striatum also arose in early vertebrates (Northcutt 1996, 2008) and it plays a major role in cost-benefit analysis. These functions all support the decisions needed for optimal foraging. The hippocampus appears to play a role in the navigation involved in foraging, including the affective correlates of places and behavioral contexts. These structure-function relationships (Table 4.1) determined what these ancient animals could do with reward and their legacy continues in modified form in descendant animals, including us (Figure 4.1).
With the origin of mammals came the neocortex, which includes the orbital and medial frontal areas (Kaas 1995; Northcutt and Kaas 1995; Krubitzer and Kaas 2005). These agranular frontal areas include the AC, PL, IL, OFa, and Ia cortex (Table 4.2), which permit finer distinctions among specific rewards (based on olfactory, gustatory, and visceral signals). The most important advance in the mammalian way of using reward, however, may be the ability to bias one behavioral control system over another when they compete to guide responses. This “executive function” can have the effect of potentiating a weaker behavioral-control system, which would otherwise not gain behavioral expression, and thus optimize the cost-benefit tradeoffs inherent in competing foraging strategies. Table 4.2 summarizes the possible contributions of each part of the mammalian agranular frontal cortex in terms of associative memory and foraging strategies. Through top-down, biased competition, mammals can take advantage of parallel “memory systems,” in which experience can lead to competing responses. This level of executive function allows mammals to explore and exploit a changeable environment, with sufficient behavioral flexibility to permit different systems to control behavior in different circumstances.
As for primates, we retain many of the traits of vertebrates and other mammals. But our primate ancestors evolved additional cortex areas collectively called the PFg (Figure 4.2) (Preuss 1995, 2007), which includes the PFo. These newly evolved prefrontal areas permit a primate way of using rewards, including the use of reward-specific sensory information that has been dissociated from its emotional, motivational, and affective attributes. This kind of value-free reward information could then be available for use in the same way that any other information is used by the primate brain, and it appears to be especially important for rule-guided behavior. Another primate way of using rewards is somewhat opposite to the first: the association of value with rules, strategies, and other cognitive constructs. In addition, the PFo is a site of convergence for visual feature conjunctions, which represent objects and categories of objects, and chemovisceral feature conjunctions, which represent the olfactory, gustatory, and visceral attributes of rewards. Finally, the brains of human primates use rewards in yet other ways, through mental time travel and second-order intentions. Mental time travel can overcome delay intolerance and produce behaviors aimed at both maximizing reward benefits and minimizing cost over the very long term. The ability to develop second-order intentions about reward, e.g., not wanting to want chocolate or wanting to pay the cost incurred by enervating exercise, probably depends on cognitive capacities that developed only during the evolution of modern humans.
REFERENCES
- Balleine B.W., Dickinson A. Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–19. [PubMed: 9704982]
- Balleine B.W., Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: Evidence for a role in incentive memory. JNeurosci. 2000;20:8954–64. [PMC free article: PMC6773086] [PubMed: 11102506]
- Balleine B.W., Killcross S. Parallel incentive processing: An integrated view of amygdala function. Trends Neurosci. 2006;29:272–79. [PubMed: 16545468]
- Balleine B.W., Killcross A.S., Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–75. [PMC free article: PMC6741878] [PubMed: 12533626]
- Bannerman D.M., Grubb M., Deacon R.M., Yee B.K., Feldon J., Rawlins J.N. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. [PubMed: 12642189]
- Barbas H., Pandya D.N. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–75. [PubMed: 2768563]
- Baxter M.G., Murray E.A. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–73. [PubMed: 12094212]
- Blood A.J., Zatorre R.J. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–23. [PMC free article: PMC58814] [PubMed: 11573015]
- Boulougouris V., Dalley J.W., Robbins T.W. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–28. [PubMed: 17337305]
- Brembs B., Heisenberg M. The operant and the classical in conditioned orientation of Drosophila melanogaster at the flight simulator. Learn Mem. 2000;7:104–15. [PMC free article: PMC311324] [PubMed: 10753977]
- Brembs B., Lorenzetti F.D., Reyes F.D., Baxter D.A., Byrne J.H. Operant reward learning in Aplysia: Neuronal correlates and mechanisms. Science. 2002;296:1706–9. [PubMed: 12040200]
- Browning P.G.F., Easton A., Gaffan D. Frontal-temporal disconnection abolishes object discrimination learning set in macaque monkeys. Cereb Cortex. 2007;17:859–64. [PubMed: 16707734]
- Buckley M.J., Tanaka K., Mahboubi M., Mansouri F.A. Double dissociations in the effects of prefrontal cortex lesions in macaque monkeys on a Wisconsin card sorting task analogue. In: Linking Affect to Action: Critical Contributions of the Orbitofrontal Cortex. Boston: Blackwell; 2007. pp. 657–58.
- Burke K.A., Franz T.M., Miller D.N., Schoenbaum G. The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature. 2008;454:340–44. [PMC free article: PMC2727745] [PubMed: 18563088]
- Bussey T.J., Everitt B.J., Robbins T.W. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: Implications for the neurobiology of emotion. Behav Neurosci. 1997a;111:908–19. [PubMed: 9383513]
- Bussey T.J., Everitt B.J., Robbins T.W. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997b;111:920–36. [PubMed: 9383514]
- Bussey T.J., Saksida L.M. Memory, perception, and the ventral visual-perihinal-hippocampal stream: Thinking outside of the boxes. Hippocampus. 2007;17:898–908. [PubMed: 17636546]
- Bussey T.J., Saksida L.M., Murray E.A. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci. 2002;15:365–74. [PubMed: 11849302]
- Bussey T.J., Saksida L.M., Murray E.A. Impairments in visual discrimination after perirhinal cortex lesions: Testing “declarative” vs. “perceptual-mnemonic” views of perirhinal cortex function. Eur J Neurosci. 2003;17:649–60. [PubMed: 12581183]
- Bussey T.J., Wise S.P., Murray E.A. The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatto). Behav Neurosci. 2001;115:971–82. [PubMed: 11584930]
- Butler A.B. Sensory system evolution at the origin of craniates. Phil Trans Roy Soc Lond B. 2000;355:1309–13. [PMC free article: PMC1692827] [PubMed: 11079421]
- Cardinal R.N., Parkinson J.A., Lachenal G., Halkerston K.M., Rudarakanchana N., Hall J., Morrison C.H., Howes S.R., Robbins T.W., Everitt B.J. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. 2002;116:553–67. [PubMed: 12148923]
- Cardinal R.N., Parkinson J.A., Marbini H.D., Toner A.J., Bussey T.J., Robbins T.W., Everitt B.J. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–87. [PubMed: 12802885]
- Carew T.J., Sahley C.L. Invertebrate learning and memory: From behavior to molecules. Annu Rev Neurosci. 1986;9:435–87. [PubMed: 2423010]
- Carew T.J., Walters E.T., Kandel E.R. Associative learning in Aplysia: Cellular correlates supporting a conditioned fear hypothesis. Science. 1981;211:501–4. [PubMed: 7455692]
- Carmichael S.T., Price J.L. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. [PubMed: 7527805]
- Carmichael S.T., Price J.L. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642–64. [PubMed: 8847422]
- Cavada C., Company T., Tejedor J., Cruz-Rizzolo R.J., Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–42. [PubMed: 10731218]
- Changizi M.A., McGehee R.M., Hall W.G. Evidence that appetitive responses for dehydration and food-deprivation are learned. Physiol Behav. 2002;75:295–304. [PubMed: 11897255]
- Chudasama Y., Robbins T.W. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–80. [PMC free article: PMC6740430] [PubMed: 14507977]
- Clack J.A. Gaining Ground: The Origin and Evolution of Tetrapods. Bloomington: Indiana University Press; 2002.
- Corballis M.C. Mental time travel and the shaping of language. Exp Brain Res. 2009;192:553–60. [PubMed: 18641975]
- Corbit L.H., Balleine B.W. Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. J Exp Psychol Anim Behav Process. 2003;29:99–106. [PubMed: 12735274]
- Coutureau E., Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res. 2003;146:167–74. [PubMed: 14643469]
- Craig A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. [PubMed: 12154366]
- Cummings J.L. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–80. [PubMed: 8352676]
- de Wit S., Kosaki Y., Balleine B.W., Dickinson A. Dorsomedial prefrontal cortex resolves response conflict in rats. J Neurosci. 2006;26:5224–29. [PMC free article: PMC6674252] [PubMed: 16687514]
- Delamater A.R. The role of the orbitofrontal cortex in sensory-specific encoding of associations in pavlovian and instrumental conditioning. Ann N Y Acad Sci. 2007;1121:152–73. [PubMed: 17872387]
- Desmurget M., Turner R.S. Testing basal ganglia motor functions through reversible inactivations in the posterior internal globus pallidus. J Neurophysiol. 2008;99:1057–76. [PMC free article: PMC2906399] [PubMed: 18077663]
- Dickinson A., Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18.
- Drewes A.M., Dimcevski G., Sami S.A., Funch-Jensen P., Huynh K.D., Le Pera D., Arendt-Nielsen L., Valeriani M. The “human visceral homunculus” to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Exp Brain Res. 2006;174:443–52. [PubMed: 16676165]
- Dunn C.W., Hejnol A., Matus D.Q., Pang K., Browne W.E., Smith S.A., Seaver E., Giribet G. et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–49. [PubMed: 18322464]
- Everitt B.J., Cardinal R.N., Parkinson J.A., Robbins T.W. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–50. [PubMed: 12724162]
- Gaffan D. Hippocampus: Memory, habit and voluntary movement. Phil Trans R Soc Lond B. 1985;308:87–99. [PubMed: 2858877]
- Gallagher M., McMahan R.W., Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–14. [PMC free article: PMC6782791] [PubMed: 10414988]
- Grimaldi D., Engel M.S. Evolution of the Insects. Cambridge: Cambridge University Press; 2005.
- Haddon J.E., Killcross A.S. Medial prefrontal cortex lesions abolish contextual control of competing responses. J Exp Anal Behav. 2005;84:485–504. [PMC free article: PMC1389777] [PubMed: 16596976]
- Hall W.G., Arnold H.M., Myers K.P. The acquisition of an appetite. Psychol Sci. 2000;11:101–5. [PubMed: 11273415]
- Hatfield T., Han J., Conley M., Gallagher M., Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–65. [PMC free article: PMC6579315] [PubMed: 8756453]
- Hershberger W.A. An approach through the looking-glass. Anim Learn Behav. 1986;14:443–51.
- Holland L.Z., Holland N.D. Chordate origins of the vertebrate central nervous system. Curr Opin Neurobiol. 1999;9:596–602. [PubMed: 10508734]
- Holland P. Amount of training affects associatively-activated event representation. Neuropharmacology. 1998;37:461–69. [PubMed: 9704987]
- Holland P.C., Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–55. [PubMed: 15082318]
- Izquierdo A., Murray E.A. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–46. [PubMed: 16262672]
- Izquierdo A., Murray E.A. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–62. [PMC free article: PMC6673199] [PubMed: 17267559]
- Izquierdo A., Suda R.K., Murray E.A. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–48. [PMC free article: PMC6729636] [PubMed: 15329401]
- Izquierdo A., Suda R.K., Murray E.A. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–42. [PMC free article: PMC6725674] [PubMed: 16162935]
- Jones E.G. The Thalamus. New York: Plenum Press; 1985.
- Kaas J.H. The evolution of isocortex. Brain Behav Evol. 1995;46:187–96. [PubMed: 8564462]
- Kaas J.H. The evolution of the complex sensory and motor systems of the human brain. Brain Res Bull. 2008;75:384–90. [PMC free article: PMC2349093] [PubMed: 18331903]
- Kennerley S.W., Dahmubed A.F., Lara A.H., Wallis J.D. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci. 2009;21:1162–78. [PMC free article: PMC2715848] [PubMed: 18752411]
- Kesner R.P., Gilbert P.E. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88:82–86. [PMC free article: PMC2095785] [PubMed: 17400484]
- Kheramin S., Body S., Mobini S., Ho M.Y., Velazquez-Martinez D.N., Bradshaw C.M., Szabadi E., Deakin J.F., Anderson I.M. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: A quantitative analysis. Psychopharmacology (Berl). 2002;165:9–17. [PubMed: 12474113]
- Kiefer S.W., Orr M.R. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci. 1992;106:140–46. [PubMed: 1313241]
- Killcross S., Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–8. [PubMed: 12631569]
- King A.B., Menon R.S., Hachinski V., Cechetto D.F. Human forebrain activation by visceral stimuli. J Comp Neurol. 1999;413:572–82. [PubMed: 10495443]
- Kjelstrup K.B., Solstad T., Bran V.H., Hafting T., Leutgeb S., Witter M.P., Moser E.I., Moser M.B. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–43. [PubMed: 18599792]
- Kjelstrup K.G., Tuvnes F.A., Steffenach H.A., Murison R., Moser E.I., Moser M.B. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99:10825–30. [PMC free article: PMC125057] [PubMed: 12149439]
- Kolb B. Do all mammals have a prefrontal cortex? In: The Evolution of Primate Nervous Systems. Amsterdam: Elsevier; 2007. pp. 443–50.
- Kondo H., Saleem K.S., Price J.L. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J. Comp Neurol. 2005;493:479–509. [PubMed: 16304624]
- Krubitzer L., Kaas J. The evolution of the neocortex in mammals: How is phenotypic diversity generated? Curr Opin Neurobiol. 2005;15:444–53. [PubMed: 16026978]
- Lebron K., Milad M.R., Quirk G.J. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–48. [PubMed: 15466306]
- MacLean P.D. Evolutionary psychiatry and the triune brain. Psychol Med. 1985;15:219–21. [PubMed: 4023126]
- Malkova L., Gaffan D., Murray E.A. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci. 1997;17:6011–20. [PMC free article: PMC6573210] [PubMed: 9221797]
- Mansouri F.A., Buckley M.J., Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–90. [PubMed: 17962523]
- Matsumoto M., Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–15. [PubMed: 17522629]
- Mazzoni P., Hristova A., Krakauer J.W. Why don’t we move faster? Parkinson’s Disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–16. [PMC free article: PMC6794577] [PubMed: 17611263]
- McDannald M.A., Saddoris M.P., Gallagher M., Holland P.C. Lesions of orbitofrontal cortex impair rats’ differential outcome expectancy learning but not conditioned stimulus-potentiated feeding. J Neurosci. 2005;25:4626–32. [PMC free article: PMC1201522] [PubMed: 15872110]
- McHugh S.B., Deacon R.M., Rawlins J.N., Bannerman D.M. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. [PubMed: 14979783]
- Medina L., Brox A., Legaz I., Garcia-Lopez M., Puelles L. Expression patterns of developmental regulatory genes show comparable divisions in the telencephalon of Xenopus and mouse: Insights into the evolution of the forebrain. Brain Res Bull. 2005;66:297–302. [PubMed: 16144605]
- Mesulam M.M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. [PubMed: 2260847]
- Mishkin M., Malamut B., Bachevalier J. Memories and habits: Two neural systems. In: Neurobiology of Learning and Memory. New York: Guilford; 1984. pp. 65–77.
- Moreno N., Gonzalez A. Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio-amniotic transition. J Anat. 2007;211:151–63. [PMC free article: PMC2375767] [PubMed: 17634058]
- Murray E.A. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–97. [PubMed: 17988930]
- Murray E.A., Bussey T.J., Saksida L.M. Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Ann Rev Neurosci. 2007;30:99–122. [PubMed: 17417938]
- Murray E.A., Bussey T.J., Wise S.P. Role of prefrontal cortex in a network for arbitrary visuomotor mapping. Exp Brain Res. 2000;133:114–29. [PubMed: 10933216]
- Murray E.A., Gaffan D. Prospective memory in the formation of learning sets by rhesus monkeys (Macaca mulatta). J Exp Psychol Anim Behav Process. 2006;32:87–90. [PubMed: 16435968]
- Neary T.J. The pallium of anuran amphibians. In: Cerebral Cortex: Comparative Structure and Evolution of Cerebral Cortex, Part I. New York: Plenum; 1990. pp. 107–38.
- Nieuwenhuys R. Deuterostome brains: Synopsis and commentary. Brain Res Bull. 2002;57:257–70. [PubMed: 11922969]
- Northcutt R.G. The agnathan ark: The origin of craniate brains. Brain Behav Evol. 1996;48:237–47. [PubMed: 8932865]
- Northcutt R.G. Changing views of brain evolution. Brain Res Bull. 2001;55:663–74. [PubMed: 11595351]
- Northcutt R.G. Forebrain evolution in bony fishes. Brain Res Bull. 2008;75:191–205. [PubMed: 18331871]
- Northcutt R.G., Kaas J.H. The emergence and evolution of mammalian neocortex. Trends Neurosci. 1995;18:373–79. [PubMed: 7482801]
- O’Keefe J., Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978.
- Öngür D., Ferry A.T., Price J.L. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp Neurol. 2003;460:425–49. [PubMed: 12692859]
- Ostlund S.B., Balleine B.W. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci. 2005;25:7763–70. [PMC free article: PMC6725247] [PubMed: 16120777]
- Ostlund S.B., Balleine B.W. Orbitofrontal cortex mediates outcome encoding in pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–25. [PMC free article: PMC6672090] [PubMed: 17475789]
- Padoa-Schioppa C., Assad J.A. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–26. [PMC free article: PMC2630027] [PubMed: 16633341]
- Padoa-Schioppa C., Assad J.A. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11:95–102. [PMC free article: PMC2646102] [PubMed: 18066060]
- Palomero-Gallagher N., Zilles K. Isocortex. In: The Rat Nervous System. San Diego, CA: Elsevier Academic Press; 2004. pp. 729–57.
- Petrides M. Monitoring of selections of visual stimuli and the primate frontal cortex. Proc R Soc Lond B Biol Sci. 1991;246:293–98. [PubMed: 1686095]
- Petrovich G.D., Canteras N.S., Swanson L.W. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–89. [PubMed: 11750934]
- Pickens C.L., Saddoris M.P., Gallagher M., Holland P.C. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav Neurosci. 2005;119:317–22. [PMC free article: PMC1201523] [PubMed: 15727536]
- Pickens C.L., Saddoris M.P., Setlow B., Gallagher M., Holland P.C., Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J. Neurosci. 2003;23:11078–84. [PMC free article: PMC6741041] [PubMed: 14657165]
- Preuss T.M. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cog Neurosci. 1995;7:1–24. [PubMed: 23961750]
- Preuss T.M. Primate brain evolution in phylogenetic context. In: Evolution of Nervous Systems. Oxford: Elsevier; 2007. pp. 2–34.
- Pritchard T.C., Nedderman E.N., Edwards E.M., Petticoffer A.C., Schwartz G.J., Scott T.R. Satiety-responsive neurons in the medial orbitofrontal cortex of the macaque. Behav Neurosci. 2008;122:174–82. [PubMed: 18298260]
- Quirk G.J., Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsycho-pharmacology. 2008;33:56–72. [PMC free article: PMC2668714] [PubMed: 17882236]
- Ragozzino M.E. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–75. [PubMed: 17698989]
- Ragozzino M.E., Kim J., Hassert D., Minniti N., Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–65. [PubMed: 14570554]
- Ragozzino M.E., Wilcox C., Raso M., Kesner R.P. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999;113:32–41. [PubMed: 10197904]
- Ray J.P., Price J.L. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol. 1992;323:167–97. [PubMed: 1401255]
- Rescorla R.A. Inhibitory associations between S and R in extinction. Learn Behav. 1993;21:327–36.
- Rhodes S.E., Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–16. [PMC free article: PMC523080] [PubMed: 15466316]
- Rhodes S.E., Killcross A.S. Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. Eur J Neurosci. 2007;26:2654–60. [PubMed: 17970744]
- Rich E.L., Shapiro M.L. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. J Neurosci. 2007;27:4747–55. [PMC free article: PMC6672999] [PubMed: 17460087]
- Rolls E.T., Baylis L.L. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci. 1994;14:5437–52. [PMC free article: PMC6577073] [PubMed: 8083747]
- Rolls E.T., Yaxley S., Sienkiewicz Z.J. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J Neurophysiol. 1990;64:1055–66. [PubMed: 2258734]
- Rudebeck P.H., Murray E.A. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338–43. [PMC free article: PMC2556079] [PubMed: 18701696]
- Rudebeck P.H., Walton M.E., Smyth A.N., Bannerman D.M., Rushworth M.F. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–68. [PubMed: 16921368]
- Rushworth M.E., Behrens T.E., Rudebeck P.H., Walton M.E. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–76. [PubMed: 17337237]
- Rushworth M.F., Nixon P.D., Eacott M.J., Passingham R.E. Ventral prefrontal cortex is not essential for working memory. J Neurosci. 1997;17:4829–38. [PMC free article: PMC6573332] [PubMed: 9169541]
- Sahley C., Gelperin A., Rudy J.W. One-trial associative learning modifies food odor preferences of a terrestrial mollusc. Proc Natl Acad Sci USA. 1981;78:640–42. [PMC free article: PMC319110] [PubMed: 16592960]
- Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: The Primate Brain. New York: Appleton-Century-Crofts; 1970. pp. 137–208.
- Saper C.B. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Ann Rev Neurosci. 2002;25:433–69. [PubMed: 12052916]
- Schmidt-Rhaesa A. The Evolution of Organ Systems. Oxford: Oxford University Press; 2007.
- Schoenbaum G., Chiba A.A., Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–59. [PubMed: 10195132]
- Schoenbaum G., Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–50. [PubMed: 7472378]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. [PubMed: 16318590]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–88. [PubMed: 17600522]
- Schweimer J., Hauber W. Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Mem. 2005;12:334–42. [PMC free article: PMC1142463] [PubMed: 15930509]
- Shadmehr R., Krakauer J.W. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–81. [PMC free article: PMC2553854] [PubMed: 18251019]
- Smeets W.J.A.J., Marín O., Ganzález A. Evolution of the basal ganglia: New perspectives through a comparative approach. J Anat. 2000;196:501–17. [PMC free article: PMC1468093] [PubMed: 10923983]
- Stevens J.R., Hallinan E.V., Hauser M.D. The ecology and evolution of patience in two New World monkeys. Biol Lett. 2005;1:223–26. [PMC free article: PMC1626214] [PubMed: 17148172]
- Stevens J.R., Rosati A.G., Ross K.R., Hauser M.D. Will travel for food: Spatial discounting in two new world monkeys. Curr Biol. 2005;15:1855–60. [PMC free article: PMC8049090] [PubMed: 16243033]
- Stouffer E.M., White N.M. Roles of learning and motivation in preference behavior: Mediation by entorhinal cortex, dorsal and ventral hippocampus. Hippocampus. 2007;17:147–60. [PubMed: 17183529]
- Striedter G.R. Principles of Brain Evolution. Sunderland, MA: Sinauer; 2005.
- Suddendorf T., Corballis M.C. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30:299–313. [PubMed: 17963565]
- Thompson R.H., Menard A., Pombal M., Grillner S. Forebrain dopamine depletion impairs motor behavior in lamprey. Eur J Neurosci. 2008;27:1452–60. [PubMed: 18336565]
- Trivedi M.A., Coover G.D. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem. 2004;81:172–84. [PubMed: 15082019]
- Ulinski P.S. The cerebral cortex of reptiles. In: Cerebral Cortex: Comparative Structure and Evolution of Cerebral Cortex, Part I. New York: Plenum; 1990. pp. 139–215.
- Valentine J.W. On the Origin of Phyla. Chicago: University of Chicago Press; 2004.
- Vermeij G.J. Animal origins. Science. 1996;274:525–26.
- Wagner H. Uber den Farbensinn der Eidechsen. Comp Physiol [A]: Neuroethol. 1932;18:378–92.
- Wallis J.D., Anderson K.C., Miller E.K. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–56. [PubMed: 11418860]
- Wallis J.D., Miller E.K. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–81. [PubMed: 14622240]
- Walton M.E., Bannerman D.M., Rushworth M.R. The role of rat medial frontal cortex in effortbased decision making. J Neurosci. 2003;22:10996–11003. [PMC free article: PMC6758435] [PubMed: 12486195]
- Wang S.H., Ostlund S.B., Nader K., Balleine B.W. Consolidation and reconsolidation of incentive learning in the amygdala. J Neurosci. 2005;25:830–35. [PMC free article: PMC6725620] [PubMed: 15673662]
- Webster M.J., Bachevalier J., Ungerleider L.G. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–83. [PubMed: 7530521]
- Wellman L.L., Gale K., Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25:4577–86. [PMC free article: PMC6725040] [PubMed: 15872105]
- Wicht H., Northcutt R.G. The forebrain of the Pacific hagfish: A cladistic reconstruction of the ancestral craniate forebrain. Brain Behav Evol. 1992;40:25–64. [PubMed: 1393518]
- Wicht H., Northcutt R.G. Telencephalic connections in the Pacific hagfish (Eptatretus stouti), with special reference to the thalamopallial system. J Comp Neurol. 1998;395:245–60. [PubMed: 9603376]
- Wilson C.R., Gaffan D. Prefrontal-inferotemporal interaction is not always necessary for reversal learning. J Neurosci. 2008;28:5529–38. [PMC free article: PMC6670622] [PubMed: 18495887]
- Winstanley C.A., Theobald D.E., Cardinal R.N., Robbins T.W. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–22. [PMC free article: PMC6729470] [PubMed: 15152031]
- Wise S.R. Forward frontal fields: Phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. [PMC free article: PMC2587508] [PubMed: 18835649]
- Wullimann M.R., Mueller T. Expression of Zash-1a in the postembryonic zebrafish brain allows comparison to mouse Mash1 domains. Brain Res Gene Expr Patterns. 2002;1:187–92. [PubMed: 12638130]
- Wullimann M.F., Rink E. The teleostean forebrain: A comparative and developmental view based on early proliferation, Pax6 activity and catecholaminergic organization. Brain Res Bull. 2002;57:363–70. [PubMed: 11922990]
- Yaxley S., Rolls E.T., Sienkiewicz Z.J. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700. [PubMed: 2341869]
- Zhang Y., Lu H., Bargmann C.I. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–84. [PubMed: 16281027]
- Zhang Z.H., Dougherty P.M., Oppenheimer S.M. Characterization of baroreceptor-related neurons in the monkey insular cortex. Brain Res. 1998;796:303–6. [PubMed: 9689483]
- Review [Evolution and development of dopaminergic neurotransmitter systems in vertebrates].[J Soc Biol. 2000]Review [Evolution and development of dopaminergic neurotransmitter systems in vertebrates].Kapsimali M, Dumond H, Le Crom S, Coudouel S, Vincent JD, Vernier P. J Soc Biol. 2000; 194(2):87-93.
- Ludwig Edinger: the vertebrate series and comparative neuroanatomy.[J Hist Neurosci. 2015]Ludwig Edinger: the vertebrate series and comparative neuroanatomy.Patton P. J Hist Neurosci. 2015; 24(1):26-57. Epub 2014 Sep 3.
- Review Engineering Aspects of Olfaction.[Neuromorphic Olfaction. 2013]Review Engineering Aspects of Olfaction.Persaud KC. Neuromorphic Olfaction. 2013
- Review [Origin and evolution of parasitism in mites of the infraorder Eleutherengona (Acari: Prostigmata). Report I. Lower Raphignathae].[Parazitologiia. 2008]Review [Origin and evolution of parasitism in mites of the infraorder Eleutherengona (Acari: Prostigmata). Report I. Lower Raphignathae].Bochkov AV. Parazitologiia. 2008 Sep-Oct; 42(5):337-59.
- Review The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis.[J Comp Neurol. 2011]Review The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis.O'Connell LA, Hofmann HA. J Comp Neurol. 2011 Dec 15; 519(18):3599-639.
- What Can Different Brains Do with Reward? - Neurobiology of Sensation and RewardWhat Can Different Brains Do with Reward? - Neurobiology of Sensation and Reward
Your browsing activity is empty.
Activity recording is turned off.
See more...
