31.1. INTRODUCTION
The use of computed tomography (CT) and magnetic resonance imaging (MRI) in mild traumatic brain injury (mTBI) is overviewed in this chapter. Although in the majority of mTBI cases no abnormality will be shown, the common neuropathological changes that may be identified on CT and/or MRI are highlighted with an emphasis that such abnormalities provide only a macroscopic perspective of the pathology that may be viewed. Emphasis is placed on understanding the subtle nature of neuropathology that may accompany mTBI, the potential for dynamic changes that vary with time post-injury and that detection depends on which neuroimaging method is used. The role of advanced neuroimaging techniques that provide quantitative information about potential network-level damage using diffusion tensor imaging (DTI) and resting state functional MRI is overviewed with numerous examples provided that illustrate neuroimaging techniques that detect mTBI abnormalities.
The common structural neuroimaging methods and findings in mTBI will be overviewed. The field of neuroimaging is expansive and the basics of neuroimaging will not be covered in this review. For the reader who would like additional background information in neuroimaging of TBI, Wilde et al. (2012) provide such a synopsis. The chapter will conclude with a section that relates the macroscopically identified pathologies using conventional and advanced neuroimaging techniques with the ultrastructure and underlying pathophysiology of mTBI.
The neuroimaging investigation by Yuh et al. (2013) represents a comprehensive investigation of the common, visibly detected abnormalities observed in mTBI using day-of-injury (DOI) computed tomography (CT) followed by magnetic resonance imaging (MRI) during the subacute timeframe. In the Yuh et al. study, 135 mTBI patients were evaluated for acute head injury in three separate level 1 trauma centers in the United States and all were enrolled through an emergency department (ED) for prospective 3-month neurobehavioral outcome, assessed by the Extended Glasgow Outcome Scale (GOS-E). Although DOI CT imaging was done acutely, MRI on average was performed within 2 weeks postinjury. The National Institutes of Health has established the TBI Common Data Elements (TBI-CDEs; Haacke et al., 2010; Yue et al., 2013) for classifying both acute as well as chronic abnormalities, with all scan abnormalities identified by CDE criteria. CDE guidelines for pathoanatomical TBI findings on DOI CT or early MRI include skull fracture, hematoma (either epidural and/or subdural), traumatic axonal injury (defined as one to three foci), and diffuse axonal injury (DAI; defined as at least four foci). DOI CT foci are typically characterized as visibly identified contusions or intraparenchymally identified petechia. On MRI, such foci may take the form of white matter (WM) signal abnormality (hyperintense) and/or characteristic signal changes (hypointense) that reflect prior hemorrhage, often at the gray matter (GM)-WM interface. All of these types of macroscopic pathologies will be depicted in this chapter. Importantly in the Yuh et al. investigation, TBI-CDE features of more severe TBI such as midline shift ≥5 mm and partial/complete basal cistern effacement were not observed in any of the mTBI patients as part of that study. This is understandable and highlights that the visible abnormalities in mTBI do not reach the threshold associated with more severe TBI; nonetheless, very significant parenchymal injury may accompany mTBI.
The 2013 Yuh et al. investigation was a subset of a much larger investigation (McMahon et al., 2013) that prospectively followed 375 mTBI patients at 3, 6, and 12 months. The McMahon et al. (2013) study found that by 1 year, 22.4% of mTBI patients were still below functional status as measured by the GOS-E. Although there was an association of positive CT findings with poorer 3-month outcome, by 1 year whether DOI CT was abnormal or not did not predict outcome. Clearly, mTBI results in lasting sequelae for some, but this is not necessarily predicted by DOI CT findings. As will be shown in this chapter, advanced neuroimaging studies provide additional information and insight into the neuropathological effects of mTBI potentially useful in better understanding mTBI sequelae as well as providing additional information in the assessment and treatment of mTBI.
Figure 31.1 summarizes the findings of Yuh et al., which show that 44% of all mTBIs in this cohort of ED assessed individuals with mTBI had at least an identifiable neuroimaging abnormality. Clearly MRI was superior to CT in identifying abnormalities, especially those neuroimaging markers that infer axonal pathology. In fact, 27% of mTBI patients with normal head CTs had abnormal MRIs that were otherwise “missed” by DOI CT imaging. Of the 135 mTBI patients assessed in the Yu et al. investigation, only one had a Glasgow Coma Scale (GCS) of 13, with 26/135 (19%) assessed with a GCS of 14 and 108/135 (80%) with a GCS of 15. As such, the majority had a classically defined maximum GCS score, yet almost half had some positive neuroimaging finding. This observation underscores the frequency with which MRI may identify structural pathology in mTBI, even with a GCS of 15. In terms of the frequency of CDE findings and their relation to outcome, presence of any type of CDE-identified TBI abnormality increased the likelihood of lower GOS-E at 3 months, supporting the importance of identifying neuroimaging-based MRI abnormalities because of increased sensitivity in detecting gross pathology (Bigler, 2013a,b). However, the majority scanned had negative conventional imaging. For those with DOI CT, presence of subarachnoid hemorrhage was associated with poorer 3-month GOS-E. For those with positive MRI findings in the subacute time frame, presence of contusion or DAI was found to predict lower GOS-E.

FIGURE 31.1
Incidence of CT versus MRI traumatic brain injury CDE abnormalities in 135 study participants. For MRI evidence of contusion and MRI evidence of hemorrhagic axonal injury, progressively darker shades of red indicate larger numbers of lesions. Study participants (more...)
Figure 31.2 from the Yuh et al. investigation depicts some common CT and MRI findings in mTBI during the acute to early subacute timeframe. What is depicted in this illustration shows many of the classic observable, macroscopic lesion types in mTBI, which will be discussed more fully throughout this chapter.
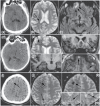
FIGURE 31.2
More extensive pathology demonstrated by MRI compared with CT in the study participants. (a–c) Fifty-year-old assaulted man. (a) Initial head CT was normal. MRI at 7 days postinjury demonstrated (b) hemorrhagic axonal injury along the right lateral (more...)
Impressively, the Yuh et al. study shows that greater than 40% of mTBI patients initially evaluated within the ED will have positive CDE-identified abnormalities. However, the CDE technique requires visual identification of the abnormality from conventional clinical imaging (CT and/or MRI) studies and does not incorporate advanced magnetic resonance (MR) techniques to be discussed in subsequent sections. Nonetheless, it is important to understand what constitutes early neuroimaging identified abnormalities and how such findings relate to underlying neuropathology.
Conventional CT and MRI clinical studies configure anatomical images with millimeter resolution, meaning they detect gross pathology at a similar level, although submillimeter MR resolution is now possible (Yassa et al., 2010; Heidemann et al., 2012). In contrast, the fundamental pathological changes that occur from TBI happen at the micron and nanometer cellular level (Bigler and Maxwell, 2011, 2012), with only the largest of lesions being visible with contemporary neuroimaging (Bigler, 2013b). This means for brain injuries in the mild range, with the subtlest of neural injury that the macroscopic lesions will not be observed. However, as will be discussed in this chapter, this does not mean that those with negative imaging have no underlying pathology.
31.2. CT AND MRI IN mTBI
Once the mTBI patient is prepared and in the scanner, CT imaging can be completed within seconds to minutes. Because CT imaging uses x-ray beam technology, objects with paramagnetic properties including life support and other medical assist devices are not precluded as in MRI. Likewise, metallic fragments from injury that may be paramagnetic can be imaged without concern about displacement by the strong magnetic fields generated by MRI, although the images will still be influenced by artifacts. Excellent contrast between bone and brain parenchyma can be achieved with CT, where CT clearly has the advantage over MRI in demonstrating presence and location of skull fractures, common sequelae with head injury, including mTBI. CT also provides methods for examining cerebrovasculature and inflammation in TBI, where potential perfusion technology may have application in identifying mTBI patients with more permanent sequelae (Metting et al., 2009, 2010, 2013).
It should be emphasized again that the clinical abnormalities as identified by CDE standards must be visible. To be visible on CT, there must be sufficient contrast between normal appearing parenchyma and damaged or abnormal tissue, typically observed in the form of hemorrhage or edema. On CT imaging, hemorrhage because of the quick clotting action of blood makes the lesion appear hyperdense in comparison to normal appearing parenchyma, whereas with edema the abnormality might appear less dense (i.e., more water content) or the typically distinct GM-WM boundaries are lost and/or there is loss of sulcal definition from swelling (Gean and Fischbein, 2010; Kim and Gean, 2011). However, these findings may not define where other shear/strain effects have occurred in the brain and likely reflect only a minimalist view of the pathology. To best understand TBI, the more advanced neuroimaging methods provide the tools to uncover additional neuropathology missed by the traditional “lesion analysis/identification” approach of conventional CT and MRI. Furthermore it is probably a fruitless endeavor to seek the prototypical “lesion” in mTBI because it likely does not exist. The macroscopic abnormalities identified in the CDE, especially with the DOI CT, may only indicate “tip-of-the-iceberg” phenomena and are not proportional to the total pathological effects of mTBI at the histological level. This is probably why the presence and location of a DOI CT abnormality does not necessarily predict outcome, because there is more than just the visibly identifiable lesion.
The common CT identified TBI-induced surface contusions typically occur at the brain–skull interface, whereas petechial hemorrhages often occur at the GM-WM interface (Gean and Fischbein, 2010; Kim and Gean, 2011). Both of these lesion patterns may be associated with focal or more diffuse patterns of edema. Presence of petechial hemorrhage in TBI is considered a marker of DAI, including in mTBI (Scheid et al., 2003, 2006); two examples are shown in Figure 31.3. Although skull fracture occurs externally to the brain, the biomechanical forces that resulted in the fracture do affect the brain. Skull fractures are associated with subdural, epidural, and subarachnoid hemorrhage. These hemorrhagic abnormalities may in turn be associated with brain displacement, mid-line shift, and associated edema. Interestingly, although hemorrhage and edema may evolve into a life-threatening medical emergency in mTBI, especially an epidural hematoma, once treated or removed, follow-up imaging may appear normal as shown in Figure 31.4. Figure 31.4 also demonstrates that dramatic acute abnormalities may occur in mTBI with subsequent “normal” appearing gross anatomy, which should not necessarily be interpreted as representing an entirely normal underlying microstructure, because it cannot actually be seen. Histological studies in TBI may show underlying pathology in what macroscopically appears to be normal tissue (Budde et al., 2011). This is also suggested by the fact that presence of extraaxial traumatic abnormalities such as skull fracture, epidural, subdural, and subarachnoid hemorrhages all increased the odds of poorer outcome in the Yuh et al. mTBI study, with simple skull fracture having the least influence and subarachnoid hemorrhage the most. As such, an identifiable intraparenchymal lesion does not have to be present in mTBI to be associated with residual impairments implicating pathology below visible detection.
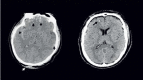
FIGURE 31.3
(Left) Hemorrhagic contusions as noted on DOI CT after a blow to the occiput, as indicated by soft-tissue swelling (white arrow) with characteristic contrecoup hemorrhagic contusions are seen in the inferior frontal and temporal lobes (black arrows). (more...)

FIGURE 31.4
The DOI CT revealed an epidural hematoma as shown in the middle. However, chronic imaging showed no distinct parenchymal abnormality, regardless of the imaging sequence used. This illustrates that the pathology observed in the DOI scan may not result (more...)
Presence of an abnormality on the DOI CT is the basis for the classification of “complicated mild TBI.” However, given contemporary advances that identify mTBI abnormalities that simply are not detected by CT imaging, this classification is mostly meaningless. As stated in the Yuh et al. investigation, close to one-third of mTBI patients who had no DOI CT abnormality in fact had underlying pathology identified with MRI. Figure 31.1 demonstrated this point as well as the case shown in Figure 31.15. Currently, the superior MRI method for detecting hemorrhagic shear lesions in mTBI is susceptibility-weighted imaging (SWI) (Spitz et al., 2013), although as shown in Figure 31.1, the T2*-weighted gradient echo readily detects hemosiderin as well. The fluid attenuated inversion recovery (FLAIR) sequence is best suited for visibly detecting WM abnormalities in mTBI (Benson et al., 2012), as will be shown in the cases highlighted in Figures 31.11 and 31.15. In both of these cases, the WM pathology detected during MRI follow-up was completely undetected in the DOI CT.
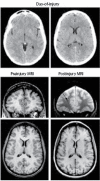
FIGURE 31.15
This young adult sustained a significant mTBI in an auto-pedestrian injury where she had positive LOC, but the DOI CT revealed no abnormality. However, as symptoms persisted, this patient was assessed with MRI that revealed hemosiderin and focal white (more...)

FIGURE 31.11
The DOI CT scan on the left revealed a small hemorrhage in the region of the globus pallidus-putamen as a solitary lesion. However, on follow-up MRI multiple other areas of prior hemorrhage are noted along with foci of abnormal hyperintense WM signal (more...)
31.3. EMPIRICALLY DERIVED QUANTITATIVE MRI ABNORMALITIES
The common anatomic images generated from MR technology, as with those shown in the various figures up to this point, are all derived from the underlying MR physics that constitute the image, which also form the basis for MR metrics. Diffusion tensor imaging (DTI) is one such metric related to assessing water anisotropy. As an example of how water diffusion metrics relate to axon integrity, and hence WM integrity, Figure 31.5 shows a DTI sequence with its associated color map in comparison to T1- and T2-weighted MR anatomical sequences. Two common metrics derived from DTI are referred to as fractional anisotropy (FA) and the apparent diffusion coefficient or ADC. Figure 31.5 provides a DTI schematic depicting the relationship of FA and ADC to axon integrity and putatively what happens with axon damage. These DTI metrics assess the microstructure of WM and are based on how water molecules are influenced by cell membranes and myelin characteristics including myelin sheath thickness. Healthy axonal membranes that are tightly compacted constrain the free movement and direction of water. Consequently, water molecules tend to move faster in parallel to nerve fibers rather than perpendicular to them. This characteristic, which is referred to as anisotropic diffusion, is measured by FA, which is influenced by the thickness of the axons, their myelin sheaths, aggregate compactness, and orientation with other axons. FA ranges from 0 to 1, where 0 represents maximal isotropic diffusion (e.g., free diffusion in perfect sphere) and 1 represents maximal anisotropic diffusion, i.e., diffusion in one direction (e.g., a long cylinder of minimal diameter). Diffusion anisotropy varies across WM regions, presumed to reflect differences in fiber myelination, fiber diameter, and directionality.
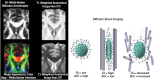
FIGURE 31.5
The images on the left show the comparison T1- and T2-weighted imaging sequence cropped at the level of the anterior corpus callosum (forceps minor) compared with the DTI sequence (upper left) and diffusion color map that reflects directionality of tracts (more...)
Using a technique referred to as tractography, the aggregate fiber tracts of an entire brain can be derived from DTI, as shown in Figure 31.6. In TBI, DTI may demonstrate a loss of fiber tract integrity, reflected as a thinning out of the number of aggregate tracts. In mTBI, this is typically not dramatic and as readily visible as that observed in severe TBI, shown in Figure 31.6. Nonetheless, if even a few of the aggregate pathways depicted in Figure 31.6 are affected in mTBI, DTI along with other advanced neuroimaging techniques provide methods for examining WM pathology associated with brain connectivity (Caeyenberghs et al., 2013; Vakhtin et al., 2013; Yeh et al., 2013). Although DTI tractography may indicate where tracts are damaged and even their absence, DTI metrics may also reflect inflammation and other findings about WM integrity (Filley, 2011; Voss and Schiff, 2009; Zappala et al., 2012). As such, some of the DTI metric is not so much a marker of a lesion in the traditional sense but an indication of WM health. Viewing network damage/disruption in the broad sense of WM integrity (and not just where a “lesion” may reside) provides an improved framework to better understand the effects of brain injury and its influence on cognition and behavior. Furthermore, these types of neuroimaging methods provide techniques for viewing the complexity of neural pathways in three-dimensional images, which assists in inspecting where lesion/abnormalities may be located within the network. Accordingly, DTI and other methods that provide network analyses will likely play a much more substantial role in the future in identifying abnormalities associated with mTBI.
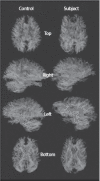
FIGURE 31.6
DTI tractography is shown in a control subject age-matched to a TBI case with severe injury showing the loss of tracts throughout the brain regardless of perspective. This illustration nicely demonstrates the complexity of the connections that create (more...)
There are numerous methods for analyzing DTI metrics (Shenton et al., 2012). One method, referred to as tract-based spatial statistics, is used to show where significant group differences may be observed by comparing the TBI group with a matched control sample. In Figure 31.7 from Wada et al. (2012), significant FA reductions associated with mTBI were found most prominently in the superior longitudinal fasciculus, region of the forceps minor of the corpus callosum, superior frontal gyrus, insula, and fornix during the chronic stage. These changes along with others also related to reduced cognitive performance. Although the areas of abnormal FA as shown in Wada et al. are unique to that sample, these areas of reported FA changes associated with mTBI are common regions consistent with what others have found in mTBI (Benson et al., 2012; Bigler, 2013a,b; Hellyer et al., 2012; Hulkower et al., 2013; Lipton et al., 2012; Messe et al., 2011). Furthermore, these are the brain regions assumed to classically experience the greatest stress/strain/shear and rotational forces during head trauma, including mTBI (Ropper and Gorson, 2007).
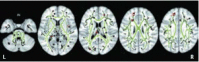
FIGURE 31.7
Tract-based spatial statistics analysis of the white matter skeleton. Voxels demonstrating significantly (p < 0.01) decreased FA values for the subjects with mTBI compared with the control group are shown in red-yellow. Voxels are thickened into (more...)
Trauma induced edematous reactions in the brain compress parenchyma, which in turn may influence water diffusion potentially detected by DTI. Using the FA metric, increases in FA beyond some normal baseline may signify edema, whereas low FA may occur when axon degradation, membrane abnormalities increase water diffusion, or actual degeneration has occurred, which increases extracellular water (Wilde et al., 2008). Accordingly, some aspects of DTI findings reflect different pathological features that are time dependent, such as early swelling with subsequent parenchymal loss (Rosenbaum and Lipton, 2012). Because TBI may induce dynamic changes over time, differences in FA over acute, subacute, and chronic time frames postinjury may differ as well. When axons degenerate, the increased space frees extracellular water, resulting in lower FA. Thus, in mTBI, low FA may reflect WM degeneration, whereas increased FA may reflect neuroinflammation. Rosenbaum and Lipton (2012) and Shenton et al. (2012) provide much more elaborate discussions concerning FA markers of pathology in mTBI as well as other DTI metrics. Sometimes in mTBI dilated perivascular, so-called Virchow-Robin spaces will be detected, especially on T2-weighted MR sequences (Inglese et al., 2005, 2006). How these findings relate to DTI in mTBI has not been examined.
31.4. MICROSTRUCTURE EFFECTS OF mTBI
Intuitively, mTBI must involve more subtle pathology than moderate-to-severe TBI and therefore fewer abnormalities including those visibly identified on conventional imaging (Bigler, 2013b). Diagrammatically, some of the potential differences on axon morphology between mild and severe TBI are depicted in Figure 31.8 from Smith et al. (2013). As shown in this illustration, there are not only morphological effects of shear/strain injury at the axonal level but ionic. The axon membrane that regulates ionic movement is but a few nanometers in thickness and similarly measured in nanometers is the cytoskeleton that provides the scaffolding for normal axon morphology (Maxwell, 2013). If the injury, even at the mild level, is sufficient to induce physiological change, it may be insufficient to produce morphological change, but may still produce changes in neural transmission and function. If the injury is time-limited and but transient disruption in neuronal cellular function recovery may be complete with no lasting effect. However, as shown in Figure 31.8, if a breakdown in axon integrity occurs, this has the potential to affect water diffusion properties because of membrane degradation or dissolution (Figure 31.5). If a sufficient number of axons becomes regionally affected, this may result in detectable differences with MRI techniques. In the classic chronic injury where loss of axonal fibers has occurred, this would typically result in reduced anisotropy, hence decreased FA and increased ADC.

FIGURE 31.8
Evolving pathophysiology of traumatic injury in myelinated axons as reproduced from Smith (2013). In this figure, the authors attempt, in an abbreviated fashion, to illustrate some of the key events believed to be involved in the pathobiology of traumatic (more...)
As already mentioned, finite element biomechanical modelling of mTBI over the past decade has refined our understanding of the most commonly vulnerable WM pathways involved in the stretch/shear mechanism associated with mTBI, including pathways coursing through the corona radiata, internal capsule, cerebral peduncle, and corpus callosum (Bayly et al., 2012; Ji et al., 2013; McAllister et al., 2012). In more severe injury, these regions also exhibit classic atrophic changes concomitant with DTI-identified abnormalities (Bigler, 2013b; Dinkel et al., 2013), so clearly these are particularly vulnerable areas in head injury. Accordingly, given the likelihood of significant stretch/strain influences occurring within these regions of interest in mTBI (Ropper and Gorson, 2007), it is also most likely that within these regions of interest is where the most frequent pathology will be detected with advanced MRI methods, including DTI. This is especially true in individuals younger than age 50 without prior neuropsychiatric disorder because WM abnormalities in these regions occur infrequently as just incidental findings (Bigler, 2013b).
What is also so important about where these maximal strain fields occur is that axon projection through densely compacted WM occurs with slightly different trajectory for each neuron depending on where in the trajectory the strain field influences the axon. No two neurons as adjacent partners in their origin will have an identical terminus, meaning that their respective axonal projections differ. Each axon contributes unique connectivity to the network. As such, a maximal strain field will not necessarily affect all axons equally, as shown in Figure 31.9 from Kraft et al. (2012). This in turn sets up an important dynamic in mTBI—a sufficient number of neural cells must be affected in aggregate for there to be detection with advanced MRI metrics and depending on the number of cells affected, neural networks will differentially be affected. But not all neurons may be affected, adding to the uniqueness of each injury.
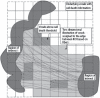
FIGURE 31.9
This schematic shows how different axon trajectories may or may not be vulnerable to injury. As can be seen, there are only certain sectors where the biomechanical deformation sufficiently alters brain parenchyma to damage axons. Lines represent hypothetical (more...)
As already pointed out, the CDE-identified abnormalities associated with mTBI are visibly recognized. If 500 small diameter (<5 microns) axons were damaged in a particular strain field that would not necessarily be detected by conventional MRI because the “lesion” physically would be less than 0.5 mm. As is well known from epilepsy research, a small number of aberrant pathways may wreak havoc with neural communication (Devinsky et al., 2013; Izhikevich and Edelman, 2008). Accordingly, when either conventional or advanced neuroimaging techniques are found, they likely represent significant aberrations in underlying neuronal integrity.
Advanced neuroimaging techniques provide a much more fruitful approach over just visible “lesion” detection in understanding mTBI. Microstructure aberration that affects membrane integrity, metabolic functioning including hemodynamic responsivity along with synaptic, and/or neurotransmitter dysfunction may all be the source of impaired neural function in mTBI, which would not be associated with an identifiable visible lesion. Whether an mTBI abnormality is structural, functional, or a combination ranging from a classic shear lesion to some physiological aberration associated with membrane or synaptic dysfunction, how such abnormalities disrupt neural networks is more important than where a “lesion” may reside. This leads to a discussion of network damage and how to use neuroimaging to identify where in the network damage/disruption may have occurred (Bigler, 2013b).
31.5. NETWORK DAMAGE AND DISRUPTION IN mTBI
Over the past two decades, neuroimaging techniques have provided a more refined view of the anatomical loci of certain neural networks that underlie behavior and cognition. Advanced neuroimaging methods, especially application of DTI and resting state (rs) functional connectivity (fc) mapping of the brain using functional MRI (fMRI) technology. Figure 31.10 is a simplified depiction of a network from the work of van den Heuvel and Sporns (2011). As depicted in this figure, there are both short and long coursing pathways within a hemisphere (intrahemispheric tracts) as well as across the two hemispheres (interhemispheric tracts) that integrate the brain via either gateway hubs that appear to be along main neural highways as signals pass from one region to another, or lesser hubs and nodes, with some of the smallest nodes out toward the periphery of the network. From this analogy, even a small lesion that affected a major hub could be very disruptive to the network, with lesser disruption if the injury only affected a peripheral node. Note that disruption to a hub could occur by damage to pathways remote to the hub but highly connected to it. Importantly, van de Heuvel and Sporns show that even minor hubs typically are but a single link away from a critical hub, which they refer to as a “rich club” (van den Heuvel and Sporns, 2013).
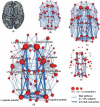
FIGURE 31.10
Rich-club regions and connections. The figure shows rich-club regions and connections of a group-averaged connectome (unweighted, k = 17; Figure 3a from the original article). The size of nodes reflects their number of connections, with bigger nodes representing (more...)
Figures 31.11 and 31.12 present two mTBI cases with different lesion patterns that show how dissimilar lesions very likely differentially influence distinctly different networks.

FIGURE 31.12
This child sustained an mTBI resulting in a left frontal contusion with residual hemosiderin deposition and an area of focal encephalomalacia that is back-filled with a pocket of CSF. In contrast to the mTBI case presented in Figure 31.11, note the entirely (more...)
In Figure 31.11, this individual with mTBI (at-the-scene GCS of 14, with a 15 at ED evaluation) had a solitary DOI CT finding of a small bleed within the basal ganglia on the left. This mTBI patient was observed overnight and the scan repeated, revealing no change, and discharged the next day. In the mTBI case (GCS never below 15) presented in Figure 31.12, a small left frontal surface contusion occurred, which resulted in a region of focal encephalomalacia. Based on the conventional imaging performed, both of these cases involved left hemisphere injury, but the network effects of what the mild injury induced in the case in Figure 31.11 would likely be greater that what occurred in Figure 31.12. Returning to the network diagram of van den Heuvel and Sporns, the patient in Figure 31.11 would most likely have central network disruption affecting a major hub, whereas the patient in Figure 31.12 would most likely have only a minor node affected.
The problem with the overly simplistic view just presented in these cases is that it does not take into account what is occurring below conventional lesion detection because it uses the old “lesion-localization” approach to image analysis. For example, the case in Figure 31.11 shows the distinct hemorrhage in the DOI CT scan, but SWI and FLAIR imaging during the chronic phase show much more widespread and frequent lesions than the DOI CT. The distribution and multifocal nature of the shear injuries demonstrated by the SWI and FLAIR imaging would reinforce the likelihood of greater key hub as well as nonspecific network disruption.
So beyond the lesion, how can network dysfunction be demonstrated in mTBI? As mentioned previously, one of the most common areas of WM injury arising from mTBI occurs within long-coursing pathways within the corona radiata like the superior longitudinal fasciculus. An extensively studied pathway associated with the superior longitudinal fasciculus is the default mode network (DMN). This network involves interconnectiveness of the parietal and frontal lobes as well as between each hemisphere that participate in regulation of attention and working memory, two common deficits associated with mTBI (Mayer et al., 2011). The likely pathological consequence of superior longitudinal fasciculus injury in mTBI would be to weaken network coherence. Interestingly, the inefficiency of the network connections may actually lead to increased recruitment within parts of the network or other networks to maintain functionality (McAllister et al., 2001; Perlstein et al., 2004). The recent rs-fcMRI study by Zhou et al. (2012) demonstrates some of these points where they found increased frontal connectivity and decreased posterior connectivity of the DMN in mTBI patients compared with controls. This is depicted in Figure 31.13. Importantly, these findings occurred in mTBI with no conventional abnormalities on clinical MRI. So, this aberration within the DMN occurred as a result of microstructural pathology below the threshold of conventional detection. Bigler and Maxwell (2011, 2012) have shown that a likely correlate of disrupted axonal integrity is axon membrane damage that would alter neural transmission. Because there were no conventional lesions or abnormalities visible in this cohort of mTBI patients, a network analysis using conventional imaging would have yielded no findings.
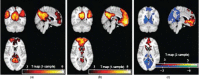
FIGURE 31.13
These fMRI activation plots are taken from Zhou et al. (2012). DMN templates obtained with the hybrid ICA seed method in patients and control subjects (corrected, p < 0.05; K ≥ 20). (a) Typical but enhanced connectivity pattern of the (more...)
The study by Johnson et al. (2012) provides a different approach in examining the DMN in concussed athletes during the subacute recovery phase. Also using fMRI-based functional connectivity mapping, as shown in Figure 31.14, they created maps akin to what was displayed in Figure 31.10. These investigators examined athletes who had sustained concussive head injuries from sports contact. mTBI resulted in less network complexity with the magnitude of connections significantly reduced.

FIGURE 31.14
The study by Johnson et al. (2012) shows posterior cingulate cortex (PCC) ROI-based connectivity maps for normal volunteers (left) and mTBI (right) subjects. The green dot designates the PCC ROI seed and red arrows and dots indicate significant positively (more...)
31.6. HETEROGENEITY OF THE LESION
As pointed out in the CDE and previously discussed, a variety of conventional abnormalities may occur in mTBI, some of which have been shown Figures 31.2 through 31.4, 31.11, and 31.12. What should be clearly evident in viewing these figures is the heterogeneity of the “lesion” patterns. In a study with 41 mTBI children with confirmed DOI abnormalities, there were no identical lesions or overlap in lesion distribution (Bigler, Abildskov, Petrie et al., 2013). At the macroscopic level, if lesions do not overlap, this would certainly be true for underlying microstructure changes. All of this would implicate heterogeneous outcome because as lesions/abnormalities vary with each patient, neurobehavioral and neurocognitive outcome would likely vary as well.
Heterogeneity in lesion type is also influenced by the metabolic cascade associated with concussion and a variety of pathophysiological events that may occur over time (Signorett et al., 2011). The interplay between structural and pathophysiological events associated with mTBI is dynamic in the first days to weeks postinjury, with individual time frames for recovery or persistence in defect (Bigler, 2013). For example, Figure 31.15 shows an mTBI case with “normal” DOI CT but because of persistence in symptoms, this patient underwent MRI scanning several weeks postinjury, which revealed significant findings of hemosiderin deposition within the frontal white matter along with associated FLAIR abnormalities.
Contrasting the case in Figure 31.15, in which DOI CT is negative but with follow-up MRI depicting abnormalities, with the case in Figure 31.4, in which DOI CT was distinctly abnormal but follow-up conventional MRI negative, shows the range of potential dynamic changes in neuroimaging of mTBI. Although not possible in human mTBI studies, animal investigations that model mTBI where acute neuroimaging abnormalities become nondetectable over time nonetheless may show histological pathology (Dewitt et al., 2013; Hylin et al., 2013). This has also been shown in a postmortem human mTBI case (Bigler, 2004). In this case study, the patient sustained a well-documented mTBI with positive loss of consciousness (LOC) and an initial GCS of 14, but DOI CT was negative. Several months postinjury, the patient died from a cardiac arrest, at which time an autopsy was performed and showed no gross pathology but microscopic hemosiderin deposition within the WM. This injury occurred in 2000, a time before improvements in MR gradient sequences that detect hemosiderin in vivo. As such, it is likely that cases where DOI CT demonstrates petechia but follow-up does not detect the prior hemorrhage may, in fact, have underlying microscopic residual hemosiderin, as shown in this case. Furthermore, this mTBI case showed macrophages within the WM, suggesting that even in the chronic stage of injury inflammatory reactions were still occurring (Bigler, 2013).
31.7. UNIQUENESS OF EACH MTBI “LESION” TO DISRUPT THE NETWORK
There is probably a simple explanation for the heterogeneity of abnormalities that may occur with mTBI: each injury is a unique circumstance of biomechanical forces affecting an individual with unique genetic and developmental environments that apply only to that individual. Although two brains may appear similar, neuronal development is, in part, experience-dependent; therefore, aspects of connectivity naturally will be unique for each. Furthermore, as shown in this chapter, lesion characteristics are diverse and change over time. As such, individual differences combined with heterogeneity in mechanism of injury and the unique nature of each brain to the effects of injury means no singular uniformity to outcome would occur across all aspects of mTBI.
As depicted in Figure 31.2, only certain WM tracts are actually damaged within a particular biomechanical strain field (Kraft et al., 2012). A neuron’s cell body is tightly held within the neuropil but axons intertwine and course is various directions within WM parenchyma, the direction of which may influence the strain field after impact. Watanabe et al. (2012) have shown how, with each individual impact injury, unique influences occur from the biomechanical movement of the brain within the cranium. These unique individual differences, when coupled with the fact that neural tissue has different elastic properties that are region- and structure-dependent (Feng et al., 2013; Mao et al., 2013), demonstrates that no two injuries from mTBI will ever produce identical pathology detectable by neuroimaging. Although a particular injury at the mild level may never be identical, some general aspects of brain morphology do result in certain WM regions (corpus callosum and corona radiata in particular and others as previously mentioned) that become especially vulnerable to stretch, strain, and tensile effects after the mechanical deformation that occurs with impact and/or acceleration/deceleration injury (Bayly et al., 2012; Feng et al., 2013).
31.8. TIME SEQUENCE OF NEUROPATHOLOGY ASSOCIATED WITH mTBI
There are primary and secondary effects associated with TBI. Some of the primary effects are immediate, the consequences of which play out within hours of the mTBI, whereas secondary effects have a slower time course (Bigler and Maxwell, 2011, 2012). In mTBI, peak symptoms may not occur for hours to days postinjury (Duhaime et al., 2012; Prichep et al., 2012). After the immediate effects of injury, there are complex pathophysiological effects that occur at the vascular and neuroinflammatory response level (Stahel et al., 1998) that may be partially detected with neuroimaging methods in mTBI (Metting et al., 2009, 2010, 2013).
Wilde et al. (2012) examined a group of mTBI patients repeatedly assessed with DTI metrics and neurocognitive examination over the first 8 days postinjury. Cognitive and neuroimaging findings fluctuated over time in mTBI. All of these mTBI patients had experienced an “uncomplicated” mTBI meaning that no abnormalities were identified in the DOI CT scan, almost all had an ED identified GCS of 15, and all were the result of some type of motor vehicle accident. The first assessment was completed within 2 days of injury, and serially at days 3–4, 5–6, and 7–8 post injury. Alternate forms of the Hopkins Verbal Learning Test were administered at each time point where memory performance typically dipped between days 3 and 6, suggesting the confluence of primary and secondary effects from mTBI reaching their apex on cognitive functioning at this time point in this cohort of mTBI patients. DTI metrics also fluctuated but not necessarily in concert with cognitive performance. Increased FA, which may reflect neuroinflammation (Wilde et al., 2008), was found in several mTBI patients with FA peaks observed between days 3–4 and 7–8. Decrease in FA may reflect axon damage, but without preinjury neuroimaging to know precisely each individual’s FA baseline, it is difficult to fully interpret these findings. However, from a memory performance perspective, almost all showed a decrease after days 1–2, with postconcussive symptoms reaching their peak around day 3. This does suggest that the variability in FA during this acute/subacute timeframe may reflect instability of WM microstructure associated with the injury.
Although a positive LOC in mTBI is abrupt and an obvious indicator of TBI, by definition for mTBI LOC has to be brief and transient or otherwise, the injury would no longer be considered “mild.” The evolution of symptoms/problems associated with the initial injury likely has much to do with complex cellular responses to the mechanical deformation of brain parenchyma following injury as overviewed by Stahel et al. (1998). Although mTBI is initiated by an event involving traumatic deformation of neural tissue, the event does not induce a singular pathological event that can be identified by neuroimaging methods at this time, but initiates a most complex array of structural and physiological changes in brain parenchyma that play out over time, potentially with permanent changes. If the biomechanical deformation is minimal, only transient disruption in neuron integrity occurs (Biasca and Maxwell, 2007; Magdesian et al., 2012), but as already shown in Figure 31.8 from Smith et al. (2013), various structural pathologies involving axon damage may occur and take time to develop.
31.9. CELLULAR BASIS OF mTBI NEUROPATHOLOGY
As repeatedly mentioned throughout this chapter, what is observed in mTBI with contemporary neuroimaging even with advanced MR techniques still represents a macro perspective of brain injury. The CDE definition of traumatic brain injury (TBI) is “… an alteration in brain function, or other evidence of brain pathology, caused by an external force” (Menon et al., 2010). External force induces brain injury via deformation of neural tissue that surpasses tolerance limits for normal displacement or strain that accompanies movement such as jumping, rapid turning of the head, simple bumps to the head, etc. So, at the most fundamental level of injury, cellular deformation disrupts anatomy and physiology sufficient to at least transiently impair function when the threshold for mTBI has been reached.
Too often, neural cells are viewed schematically as an artist’s rendition of what a neural cell looks like, typically with a large-appearing singular axon that looks sturdy. However, artistic schematics detract from the true complexity and delicate nature of what really constitutes neural tissue. For example, primary motor cortex-thalamic connections are depicted in two photomicrographs as shown in Figure 31.16 taken from the ventrolateral nucleus of the thalamus in a rhesus monkey (Kultas-Ilinsky et al., 2003). Disruption of corticothalamic and thalamocortical connections has been implicated in mTBI by DTI and rs-fcMRI studies (Little et al., 2010; Tang et al., 2011), including mild TBI (Zhou et al., 2013), so the axon elements depicted in this illustration are representative of neurons that could be affected by mechanical deformation in mTBI. Histological labeling was performed to track axons and their projections between cortex and within the thalamus. As viewed in Figure 31.16, axon projections are not straight-lined but complexly interdigitated. Note also the micron scaling indicating that most axons in this photomicrograph are <3 µ in diameter and that these views are merely two-dimensional of a three-dimensional structure. Given their size, axons are extremely delicate. Differences in angulation of the projecting axons would further influence which axons would be most affected depending on the orientation of the head at time of impact or angular acceleration/deceleration.
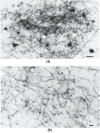
FIGURE 31.16
The photomicrograph from Kultas-Ilinsky et al. (2003) is taken from a study involving the histology of thalamocortical connection of a rhesus monkey (Kultas-Ilinsky et al., 2003). (a) Photomicrographs of dense plexuses (cell bodies dark) within the ventrolateral (more...)
Amid the complex intertwining of axons, all with multiple branches forming terminal boutons and synaptic connections, as shown in Figure 31.16, any misalignment from traumatically induced deformation would likely affect axonal function and synaptic integrity. Likewise, if the axon membrane is disrupted, membrane permeability will directly impact neuronal function and axon potential propagation. Only one strategic axon segment need be affected to disrupt neural transmission for the entire axon. A variety of finite element and various methods for re-creating the motion that displaces brain parenchyma in mTBI have been performed, mostly using sports concussion models. For example, Viano et al. (2005) showed on average in the typical sports-related concussion that the brain displaces between 4 and 8 mm in regions such as the corpus callosum, midbrain, medial temporal lobe, and fornix. Viewing Figure 31.16 from the perspective of this amount of deformation, noting that the photomicrograph depicts axons <0.5 mm in length, even deformation of a few millimeters would reflect a massive distortion of neurons this size and their axonal projections.
From what has been outlined in the previous paragraph and in Figure 31.16, looking back on the macroscopic pathology within the images presented in this chapter, essentially assessing tissue with millimeter-level resolution, even with all of the neuroimaging advancements, only the coarsest associations may be demonstrated via neuroimaging techniques with what may be occurring at the microstructure level with mTBI.
Blood vessels are just as delicate as neural tissue, especially at the capillary level. Each neuron is dependent on receiving a continuous source of glucose and oxygen with the smallest capillaries large enough for just a single red blood cell to traverse the capillary to deliver its oxygen and glucose (Bigler and Maxwell, 2011, 2012). As such, blood vessels are just as susceptible to the shear–strain biomechanics of head injury as are neurons (Madri, 2009). Subtle vascular changes in mTBI could also be the source for neural dysregulation (Pomschar et al., 2013), altering the hemodynamic response necessary for normal cellular function.
Understanding these cellular effects of mTBI and their potential relation to biomarkers of brain injury, combined with neuroimaging, will hopefully lead to furthering the diagnostic utility of advance neuroimaging methods, including DTI in assessing mTBI. It may be that blood biomarkers combined with tracking DTI metrics over time will reveal diagnostic features of mTBI (Fo et al., 2013; Kou et al., 2013).
31.10. VOLUMETRY FINDINGS IN mTBI
Based on the biomechanics of injury, as discussed earlier in this chapter, if atrophic changes associated with mTBI were to occur, they would most likely be found within those regions associated with the greatest likelihood for shear/strain and deformation injury. Indeed, several studies that have prospectively examined mTBI subjects have demonstrated this regional and whole brain atrophy (Benson et al., 2012; Lannsjo et al., 2013; MacKenzie et al., 2002; Ross et al., 2013; Tot et al., 2013; Zhou et al., 2013). For example, Zhou et al. demonstrated that by establishing a baseline in mTBI patients within the acute to early subacute time frame, when they were assessed with various volumetric techniques 1 year later, significant volume loss was observed in the anterior cingulum, cingulate gyrus, and scattered regions within the frontal lobes. Interestingly, they observed volume loss in the cuneus and precuneus regions as well. The volume loss within the cuneus and precuneus, both posterior brain regions, may actually be the result of Wallerian degeneration from the more focal frontal loss disrupting long coursing frontoparietal connections particularly vulnerable to stretch and shearing effects (Biasca and Maxwell, 2007, 2011, 2012).
31.11. CONCLUSION
Various neuroimaging methods provide a variety of techniques to detect underlying neuropathology that results from mTBI. The most common visible abnormalities are in the form of focal encephalomalacia, hemosiderin deposition, and/or white matter signal abnormalities. Several quantitative MRI methods have demonstrated techniques for the detection of underlying pathology associated with mTBI, which differ depending on the time postinjury that the scan is performed. Despite these advances neuroimaging remains but a coarse view of the microstructure pathology associated with mTBI.
REFERENCES
- Bayly P. V, Clayton E. H. et al. Quantitative imaging methods for the development and validation of brain biomechanics models. Annual Review of Biomedical Engineering. 2012;14:369–396. [PMC free article: PMC3711121] [PubMed: 22655600]
- Benson R. R, Gattu R. et al. Detection of hemorrhagic and axonal pathology in mild traumatic brain injury using advanced MRI: Implications for neurorehabilitation. NeuroRehabilitation. 2012;31(3):261–279. [PubMed: 23093454]
- Biasca N, Maxwell W. L. Minor traumatic brain injury in sports: A review in order to prevent neurological sequelae. Progress in Brain Research. 2007;161:263–291. [PubMed: 17618984]
- Bigler E. D. Neuroinflammation and the dynamic lesion in traumatic brain injury. Brain. 2013a;136(Pt 1):9–11. [PubMed: 23365089]
- Bigler E. D. Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychology Review. 2013b;23(3):169–209. [PubMed: 23974873]
- Bigler E. D, Abildskov T.J, Petrie J, Dennis M, Simic N, Taylor H.G. et al. Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology. 2013;27(4):438–451. [PubMed: 23876117]
- Bigler E. D, Maxwell W. L. Neuroimaging and neuropathology of TBI. NeuroRehabilitation. 2011;28(2):63–74. [PubMed: 21447905]
- Bigler E. D, Maxwell W. L. Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging and Behavior. 2012;6(2):108–136. [PubMed: 22434552]
- Bigler E. D. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. Journal of the International Neuropsychological Society. 2004;10(5):794–806. [PubMed: 15327725]
- Budde M. D, Janes L. et al. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134(Pt 8):2248–2260. [PMC free article: PMC3155707] [PubMed: 21764818]
- Caeyenberghs K, Leemans A. et al. Topological correlations of structural and functional networks in patients with traumatic brain injury. Frontiers in Human Neuroscience. 2013;7:726. [PMC free article: PMC3817367] [PubMed: 24204337]
- Devinsky O, Vezzani A. et al. Glia and epilepsy: Excitability and inflammation. Trends in Neurosciences. 2013;36(3):174–184. [PubMed: 23298414]
- Dewitt D. S, Perez-Polo R. et al. Challenges in the development of rodent models of mild traumatic brain injury. Journal of Neurotrauma. 2013;30(9):688–701. [PubMed: 23286417]
- Dinkel J, Drier A. et al. Long-term white matter changes after severe traumatic brain injury: A 5-year prospective cohort. AJNRAmerican Journal of Neuroradiology. 2013;35:23–29. [PMC free article: PMC7966468] [PubMed: 23846796]
- Duhaime A. C, Beckwith J. G. et al. Spectrum of acute clinical characteristics of diagnosed concussions in college athletes wearing instrumented helmets: Clinical article. Journal of Neurosurgery. 2012;117(6):1092–1099. [PMC free article: PMC3716254] [PubMed: 23030057]
- Feng Y, Clayton E. H. et al. Viscoelastic properties of the ferret brain measured in vivo at multiple frequencies by magnetic resonance elastography. Journal of Biomechanics. 2013;46(5):863–870. [PMC free article: PMC3616770] [PubMed: 23352648]
- Feng Y, Okamoto R. J. et al. Measurements of mechanical anisotropy in brain tissue and implications for transversely isotropic material models of white matter. Journal of the Mechanical Behavior of Biomedical Materials. 2013;23C:117–132. [PMC free article: PMC3752297] [PubMed: 23680651]
- Filley C. M. White matter: Beyond focal disconnection. Neurologic Clinics. 2011;29(1):81–97. viii. [PubMed: 21172572]
- Fox W. C, Park M. S. et al. Contemporary imaging of mild TBI: The journey toward diffusion tensor imaging to assess neuronal damage. Neurological Research. 2013;35(3):223–232. [PubMed: 23485049]
- Gean A. D, Fischbein N. J. Head trauma. Neuroimaging Clinics of North America. 2010;20(4):527–556. [PubMed: 20974375]
- Haacke E. M, Duhaime A. C. et al. Common data elements in radiologic imaging of traumatic brain injury. Journal of Magnetic Resonance Imaging. 2010;32(3):516–543. [PubMed: 20815050]
- Heidemann R. M, Ivanov D. et al. Isotropic submillimeter fMRI in the human brain at 7 T: Combining reduced field-of-view imaging and partially parallel acquisitions. Magnetic Resonance in Medicine. 2012;68(5):1506–1516. [PubMed: 22231859]
- Hellyer P. J, Leech R, Ham T. E, Sharp D. J. Individual prediction of white matter injury following traumatic brain injury. Annals of Neurology. 2012;73:489–499. [PubMed: 23426980]
- Hulkower M. B, Poliak D. B. et al. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR. American Journal of Neuroradiology. 2013;34(11):2064–2074. [PMC free article: PMC7964847] [PubMed: 23306011]
- Hylin M. J, Orsi S. A. et al. Behavioral and histopathological alterations resulting from mild fluid percussion injury. Journal of Neurotrauma. 2013;30(9):702–715. [PMC free article: PMC3941923] [PubMed: 23301501]
- Inglese M, Bomsztyk E. et al. Dilated perivascular spaces: Hallmarks of mild traumatic brain injury. AJNR. American Journal of Neuroradiology. 2005;26(4):719–724. [PMC free article: PMC7977096] [PubMed: 15814911]
- Inglese M, Grossman R. I. et al. Clinical significance of dilated Virchow-Robin spaces in mild traumatic brain injury. Brain Injury. 2006;20(1):15–21. [PubMed: 16403696]
- Izhikevich E. M, Edelman G. M. Large-scale model of mammalian thalamocortical systems. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3593–3598. [PMC free article: PMC2265160] [PubMed: 18292226]
- Ji S, Ghadyani H, Bolander R. P, Beckwith J. G, Ford J.C, McAlllister T. W. et al. Parametric comparisons of intracranial mechanical responses from three validated finite element models of the human head. Annals of Biomedical Engineering. 2013;42:11–24. [PMC free article: PMC4397967] [PubMed: 24077860]
- Johnson B, Zhang K. et al. Alteration of brain default network in subacute phase of injury in concussed individuals: Resting-state fMRI study. NeuroImage. 2012;59(1):511–518. [PMC free article: PMC3196274] [PubMed: 21846504]
- Kim J. J, Gean A. D. Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics. 2011;8(1):39–53. [PMC free article: PMC3026928] [PubMed: 21274684]
- Kou Z, Gattu R. et al. Combining biochemical and imaging markers to improve diagnosis and characterization of mild traumatic brain injury in the acute setting: Results from a pilot study. PloS One. 2013;8(11) e80296. [PMC free article: PMC3833898] [PubMed: 24260364]
- Kraft R. H, McKee P. J. et al. Combining the finite element method with structural connectome-based analysis for modeling neurotrauma: Connectome neurotrauma mechanics. PLoS Computational Biology. 2012;8(8) e1002619. [PMC free article: PMC3420926] [PubMed: 22915997]
- Kultas-Ilinsky K, Sivan-Loukianova E. et al. Reevaluation of the primary motor cortex connections with the thalamus in primates. The Journal of Comparative Neurology. 2003;457(2):133–158. [PubMed: 12541315]
- Lannsjo M, Raininko R. et al. Brain pathology after mild traumatic brain injury: An exploratory study by repeated magnetic resonance examination. Journal of Rehabilitation Medicine. 2013;45(8):721–728. [PubMed: 24002306]
- Lipton M. L, Kim N. et al. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: Intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging and Behavior. 2012;6(2):329–342. [PubMed: 22684769]
- Little D. M, Kraus M. F. et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74(7):558–564. [PMC free article: PMC2830915] [PubMed: 20089945]
- MacKenzie J. D, Siddiqi F. et al. Brain atrophy in mild or moderate traumatic brain injury: A longitudinal quantitative analysis. AJNR. American Journal of Neuroradiology. 2002;23(9):1509–1515. [PMC free article: PMC7976797] [PubMed: 12372740]
- Madri J. A. Modeling the neurovascular niche: Implications for recovery from CNS injury. Journal of Physiology and Pharmacology. 2009;60 Suppl 4:95–104. [PubMed: 20083857]
- Magdesian M. H, Sanchez F. S. et al. Atomic force microscopy reveals important differences in axonal resistance to injury. Biophysical Journal. 2012;103(3):405–414. [PMC free article: PMC3414878] [PubMed: 22947856]
- Mao H, Elkin B. S, Genthikatti V. V, Morrison B 3rd, Yang K. H. Why is CA3 more vulnerable than CA1 in experimental models of controlled cortical impact-induced brain injury? Journal of Neurotrauma. 2013;30:1521–1230. [PMC free article: PMC3751323] [PubMed: 23557208]
- Maxwell W. L. Damage to myelin and oligodendrocytes: A role in chronic outcomes following traumatic brain injury? Brain Sciences. 2013;3(3):1374–1394. [PMC free article: PMC4061868] [PubMed: 24961533]
- Mayer A. R, Mannell M. V. et al. Functional connectivity in mild traumatic brain injury. Human Brain Mapping. 2011;32(11):1825–1835. [PMC free article: PMC3204375] [PubMed: 21259381]
- McAllister T. W, Ford J. C. et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Annals of Biomedical Engineering. 2012;40(1):127–140. [PMC free article: PMC4388259] [PubMed: 21994062]
- McAllister T. W, Sparling M. B. et al. Differential working memory load effects after mild traumatic brain injury. NeuroImage. 2001;14(5):1004–1012. [PubMed: 11697932]
- McMahon P, Hricik A, Yue J. K, Puccio A. M, Inoue T, Lingsma H. F. et al. Symptomatology and functional outcome in mild traumatic brain injury: Results from the prospective TRACK-TBI Study. Journal of Neurotrauma. 2013;31:26–33. [PMC free article: PMC3880097] [PubMed: 23952719]
- Menon D. K, Schwab K. et al. Position statement: Definition of traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2010;91(11):1637–1640. [PubMed: 21044706]
- Messe A, Caplain S. et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Human Brain Mapping. 2011;32(6):999–1011. [PMC free article: PMC6870077] [PubMed: 20669166]
- Metting Z, Cerliani L. et al. Pathophysiological concepts in mild traumatic brain injury: Diffusion tensor imaging related to acute perfusion CT imaging. PloS One. 2013;8(5) e64461. [PMC free article: PMC3660324] [PubMed: 23704986]
- Metting Z, Rodiger L. A. et al. Acute cerebral perfusion CT abnormalities associated with posttraumatic amnesia in mild head injury. Journal of Neurotrauma. 2010;27(12):2183–2189. [PubMed: 20939700]
- Metting Z, Rodiger L. A. et al. Perfusion computed tomography in the acute phase of mild head injury: Regional dysfunction and prognostic value. Annals of Neurology. 2009;66(6):809–816. [PubMed: 20035508]
- Perlstein W. M, Cole M. A. et al. Parametric manipulation of working memory load in traumatic brain injury: Behavioral and neural correlates. Journal of the International Neuropsychological Society. 2004;10(5):724–741. [PubMed: 15327720]
- Pomschar A, Koerte I, Lee S, Laubender R. P, Straube A, Heinen F, Ertl-Wagner B, Alperin N. MRI evidence for altered venous drainage and intracranial compliance in mild traumatic brain injury. PloS One. 2013;8(2) e55447. [PMC free article: PMC3566196] [PubMed: 23405151]
- Prichep L. S, McCrea M, Barr W, Powell M, Chabot R. J. Time course of clinical and electrophysiological recovery after sport-related concussion. The Journal of Head Trauma Rehabilitation. 2012;28:266–273. [PubMed: 22588360]
- Ropper A. H, Gorson K. C. Clinical practice. Concussion. The New England Journal of Medicine. 2007;356(2):166–172. [PubMed: 17215534]
- Rosenbaum S. B, Lipton M. L. Embracing chaos: The scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging and Behavior. 2012;6(2):255–282. [PubMed: 22549452]
- Ross D. E, Castelvecchi C. et al. Brain MRI volumetry in a single patient with mild traumatic brain injury. Brain Injury. 2013;27(5):634–636. [PubMed: 23473341]
- Scheid R, Preul C. et al. Diffuse axonal injury associated with chronic traumatic brain injury: Evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR. American Journal of Neuroradiology. 2003;24(6):1049–1056. [PMC free article: PMC8149043] [PubMed: 12812926]
- Scheid R, Walther K. et al. Cognitive sequelae of diffuse axonal injury. Archives of Neurology. 2006;63(3):418–424. [PubMed: 16533969]
- Shenton M. E, Hamoda H. M. et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior. 2012;6(2):137–192. [PMC free article: PMC3803157] [PubMed: 22438191]
- Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM & R: The Journal of Injury, Function, and Rehabilitation. 2011;3 10 Suppl 2:S359–368. [PubMed: 22035678]
- Smith D. H, Hicks R. et al. Therapy development for diffuse axonal injury. Journal of Neurotrauma. 2013;30(5):307–323. [PMC free article: PMC3627407] [PubMed: 23252624]
- Spitz G, Maller J. J. et al. Detecting lesions after traumatic brain injury using susceptibility weighted imaging: A comparison with fluid-attenuated inversion recovery and correlation with clinical outcome. Journal of Neurotrauma. 2013;30(24):2038–2050. [PubMed: 23952803]
- Stahel P. F, Morganti-Kossmann M. C. et al. The role of the complement system in traumatic brain injury. Brain Research. Brain Research Reviews. 1998;27(3):243–256. [PubMed: 9729408]
- Tang L, Ge Y. et al. Thalamic resting-state functional networks: Disruption in patients with mild traumatic brain injury. Radiology. 2011;260(3):831–840. [PMC free article: PMC3157002] [PubMed: 21775670]
- Toth A, Kovacs N. et al. Multi-modal magnetic resonance imaging in the acute and sub-acute phase of mild traumatic brain injury: Can we see the difference? Journal of Neurotrauma. 2013;30(1):2–10. [PubMed: 22905918]
- Vakhtin A. A, Calhoun V. D. et al. Changes in intrinsic functional brain networks following blast-induced mild traumatic brain injury. Brain Injury. 2013;27(11):1304–1310. [PMC free article: PMC5075489] [PubMed: 24020442]
- van den Heuvel M. P, Sporns O. Rich-club organization of the human connectome. The Journal of Neuroscience. 2011;31(44):15775–15786. [PMC free article: PMC6623027] [PubMed: 22049421]
- van den Heuvel M. P, Sporns O. Network hubs in the human brain. Trends in Cognitive Sciences. 2013;17(12):683–696. [PubMed: 24231140]
- Viano D. C, Casson I. R. et al. Concussion in professional football: Brain responses by finite element analysis: Part 9. Neurosurgery. 2005;57(5):891–916. discussion 891–916. [PubMed: 16284560]
- Voss H. U, Schiff N. D. MRI of neuronal network structure, function, and plasticity. Progress in Brain Research. 2009;175:483–496. [PubMed: 19660675]
- Wada T, Asano Y. et al. Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. AJNR. American Journal of Neuroradiology. 2012;33(11):2117–2122. [PMC free article: PMC7965599] [PubMed: 22723057]
- Watanabe R, Katsuhara T. et al. Research of the relationship of pedestrian injury to collision speed, car-type, impact location and pedestrian sizes using human FE model (THUMS Version 4). Stapp Car Crash Journal. 2012;56:269–321. [PubMed: 23625564]
- Wilde E. A, Hunter J. V. et al. A primer of neuroimaging analysis in neurorehabilitation outcome research. Neuro Rehabilitation. 2012;31(3):227–242. [PubMed: 23093452]
- Wilde E. A, McCauley S. R. et al. Serial measurement of memory and diffusion tensor imaging changes within the first week following uncomplicated mild traumatic brain injury. Brain Imaging and Behavior. 2012;6(2):319–328. [PubMed: 22684768]
- Wilde E. A, McCauley S. R. et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–955. [PubMed: 18347317]
- Yassa M. A, Muftuler L. T. et al. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(28):12687–12691. [PMC free article: PMC2906542] [PubMed: 20616040]
- Yeh P. H, Wang B, Oakes T. R, French L. M, Paner H, Graner J. et al. Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Human Brain Mapping. 2013;35:2652–2672. [PMC free article: PMC6869078] [PubMed: 24038816]
- Yue J. K, Vassar M. J. et al. Transforming research and clinical knowledge in traumatic brain injury pilot: Multicenter implementation of the common data elements for traumatic brain injury. Journal of Neurotrauma. 2013;30(22):1831–1844. [PMC free article: PMC3814815] [PubMed: 23815563]
- Yuh E. L, Mukherjee P. et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Annals of Neurology. 2013;73(2):224–235. [PMC free article: PMC4060890] [PubMed: 23224915]
- Zappala G, Thiebaut de Schotten M. et al. Traumatic brain injury and the frontal lobes: What can we gain with diffusion tensor imaging? Cortex. 2012;48(2):156–165. [PubMed: 21813118]
- Zhou Y, Lui Y.W, Kierans A, Kenul D, Ge Y, Rath J, Reaume J. et al. Mild traumatic brain injury: Longitudinal regional brain volume changes. Radiology. 2013;267:880–890. [PMC free article: PMC3662902] [PubMed: 23481161]
- Zhou Y, Lui Y. W, Zuo X.N, Milham M.P, Reaume J, Grossman R.I. et al. Characterization of thalamo-cortical association using amplitude and connectivity of functional MRI in mild traumatic brain injury. Journal of Magnetic Resonance Imaging. 2013;39:1558–1568. [PMC free article: PMC3872273] [PubMed: 24014176]
- Zhou Y, Milham M. P. et al. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265(3):882–892. [PMC free article: PMC3504316] [PubMed: 23175546]
- INTRODUCTION
- CT AND MRI IN mTBI
- EMPIRICALLY DERIVED QUANTITATIVE MRI ABNORMALITIES
- MICROSTRUCTURE EFFECTS OF mTBI
- NETWORK DAMAGE AND DISRUPTION IN mTBI
- HETEROGENEITY OF THE LESION
- UNIQUENESS OF EACH MTBI “LESION” TO DISRUPT THE NETWORK
- TIME SEQUENCE OF NEUROPATHOLOGY ASSOCIATED WITH mTBI
- CELLULAR BASIS OF mTBI NEUROPATHOLOGY
- VOLUMETRY FINDINGS IN mTBI
- CONCLUSION
- REFERENCES
- Review Magnetic Resonance Imaging Application in the Area of Mild and Acute Traumatic Brain Injury: Implications for Diagnostic Markers?[Brain Neurotrauma: Molecular, ...]Review Magnetic Resonance Imaging Application in the Area of Mild and Acute Traumatic Brain Injury: Implications for Diagnostic Markers?Toth A. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. 2015
- Review Exploring Serum Biomarkers for Mild Traumatic Brain Injury.[Brain Neurotrauma: Molecular, ...]Review Exploring Serum Biomarkers for Mild Traumatic Brain Injury.Papa L, Edwards D, Ramia M. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. 2015
- Review Animal Models for Concussion: Molecular and Cognitive Assessments—Relevance to Sport and Military Concussions.[Brain Neurotrauma: Molecular, ...]Review Animal Models for Concussion: Molecular and Cognitive Assessments—Relevance to Sport and Military Concussions.Bolouri H, Zetterberg H. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. 2015
- Review Pathophysiology of Mild TBI: Implications for Altered Signaling Pathways.[Brain Neurotrauma: Molecular, ...]Review Pathophysiology of Mild TBI: Implications for Altered Signaling Pathways.Laskowski RA, Creed JA, Raghupathi R. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. 2015
- Review Prognostic Significance of Magnetic Resonance Imaging in Detecting Diffuse Axonal Injuries: Analysis of Outcomes and Review of Literature.[Neurol India. 2022]Review Prognostic Significance of Magnetic Resonance Imaging in Detecting Diffuse Axonal Injuries: Analysis of Outcomes and Review of Literature.Ravikanth R, Majumdar P. Neurol India. 2022 Nov-Dec; 70(6):2371-2377.
- Neuropathology of Mild Traumatic Brain Injury - Brain NeurotraumaNeuropathology of Mild Traumatic Brain Injury - Brain Neurotrauma
Your browsing activity is empty.
Activity recording is turned off.
See more...
