NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014.
4.1. INTRODUCTION
Pheromones, chemical signals for intraspecific communication (Karlson and Luescher 1959), are usually blends of chemical compounds in species-specific mixtures. Airborne pheromones of moths often consist of only two or three chemical components, each of which is perceived by a separate type of receptor neurons. Each of these neurons, called olfactory specialists (Boeckh et al. 1965) is tuned to one biologically significant compound; it responds to compounds other than the key compound only if presented at 10- to 10,000-fold higher stimulus concentrations. The composition of a pheromone blend is represented by the pattern of excitations across the types of specialists (Baker et al. 2004). Odor specialists are known also for compounds other than pheromones, such as plant volatiles or carbon dioxide. Less sharply tuned olfactory neurons have been called generalists. They may show varying and overlapping response spectra such as found in moths (Schneider et al. 1964) or in pine weevils (Mustaparta 1975).
Insect antennae—sense organs for various sensory modalities including the function of noses—provide simple and convenient subjects for morphological, electrophysiological, and biochemical studies. From the long hair sensilla of moths one may record the responses of individual olfactory neurons by extracellular electrodes. This review will cover various aspects of pheromone communication with an emphasis on reception and little on pheromone-controlled behavior. It will focus on two species of silk moths, Bombyx mori and the saturniid moth Antheraea polyphemus, with a few added remarks on other insects.
4.2. ASPECTS OF PHEROMONE COMMUNICATION
Olfaction in insects is described in numerous reviews and books (Blomquist and Vogt 2004; Cardé and Minks 1997; Field et al. 2000; Hansson 1999; Hildebrand and Shepherd 1997; Vogt 2005; Jacquin-Joly and Lucas 2005; Kaupp 2010; Mustaparta 2002; Pelosi 1996; Pelosi et al. 2006; Pernollet and Briandt 2004; Priesner 1979; Schneider 1984, 1992; Steinbrecht 1999; Tegoni et al. 2004). Studies of pheromone reception in insects enhance our general knowledge on chemoreception, on the control of behavior by chemical stimuli, and also provide a basis for insect pest control (Karg and Suckling 1999; Hummel and Hecker 2012).
Volatile insect pheromones are produced by a large variety of glands located at various places on the insect body. For instance, the sex attractant bombykol ((E,Z)-10, 12- hexadecadiene-1-ol) (Butenandt and Hecker 1961; Butenandt et al. 1959) is secreted by the abdominal sacculi laterales (Steinbrecht 1964) of the female silk moth B. mori (Figure 4.1a), together with traces of the (E,E)-isomer of the alcohol (Kasang et al. 1978a) and the analogous (E,Z)-aldehyde bombykal (Kasang et al. 1978b). Bombykol alone is able to elicit a pattern of sexual behavior of the male moth (Figure 4.1c), such as wing vibration, walking, and turning so that it is headed upwind. The excitatory effect of bombykol is partially blocked if bombykol is presented together with bombykal (Kaissling et al. 1978). In fact bombykal may elevate the threshold concentration for bombykol up to 1000-fold (Figure 4.5a later in the chapter). Extremely strong stimuli of bombykal would rapidly adapt the bombykal neuron, but elicit nerve impulses in the bombykol neuron (cf. Figures 4.8d, e, later in the chapter) and cause a behavioral response (Figure 4.5a).

FIGURE 4.1
(a) Female Bombyx mori in calling position, with everted abdominal pheromone glands. (b) Males of the milkweed or monarch butterfly Tirumala petiverana (Danainae, left hand) and the Asian arctiide moth Creatonotos gangis with expanded androconia. (Courtesy (more...)
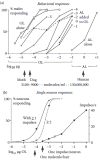
FIGURE 4.5
Bombyx mori. Responses of single male moths (a, c) and single neurons (b, c) under the same stimulus conditions. Abscissa (a, b): Loads of 1-cm2 filter papers with bombykol (OL) or bombykal (AL). For combined stimuli in (a) two filter papers were used. (more...)

FIGURE 4.8
Model of perireceptor and receptor events (model N). (a, b) Schematic view of the perireceptor space of a sensillum, with chemical reactions. (a) Simplified scheme with coefficients for two-dimensional diffusion (D2) on the hair surface, one-dimensional (more...)
The behavioral inhibition occurs by central processing of the excitatory responses of bombykol and bombykal receptor neurons. The biological function of the inhibition by bombykal is unknown. The behavioral dose-response curve obtained with the bombykol/bombykal blend released by the female increases over a range of stimulus intensities, which is wider than for bombykol alone (Figure 4.5a, dashed curve). In other species of moths the sexual behavior may be blocked by a pheromone component of a different but closely related moth species. The moth whose behavior is blocked may even possess a receptor-neuron type tuned to the behavioral inhibitor from the other species.
One example for behavioral inhibition between species is provided by the gypsy and nun moths (Lymantria dispar and L. monacha, respectively), which live sympatrically in parts of Europe and share (+)-disparlure as attractant (Hansen 1984). In addition to the attractant the female nun moth produces (–)-disparlure (2-methyl-7R,8S-epoxy-octadecane), which keeps the male gypsy moth from approaching her. The male gypsy moth has two receptor neurons, each specialized for one of the two enantiomers. The male nun moth, however, does not have receptor cells for the inhibitor and is attracted by females of both species.
Many pheromones of female moths are straight-chain unsaturated hydrocarbons with a terminal alcohol, aldehyde, or acetate function and are synthesized by abdominal glands (see database of pheromones and semiochemicals, El-Sayed 2012). When two or three components are attractive in a certain species-specific ratio, the relative amount of a synergistic component can be less than 0.1%. The same components mixed in a different ratio might represent the pheromone of a different species (Figure 4.4a later in the chapter).

FIGURE 4.4
(a) Relative trap catches of three species of moths in response to two obligatory pheromone components. Abscissa: Ratios of the two components. The average maximal numbers of moths per trap were (from left to right) 27.0, 23.8, 40.8, respectively (six (more...)
The female moths of Bombyx mori are not able to smell their own pheromone. Although the female sensilla trichodea look similar to those of the male with neurons for bombykol and bombykal, the two neurons innervating the female sensilla are insensitive to the pheromone components. Instead one neuron is tuned to benzoic acid (De Brito Sanchez 2000; De Brito Sanchez and Kaissling 2005; Heinbockel and Kaissling 1996; Priesner 1979), the other most sensitive to the norterpene 2,6-dimethyl-5-hepten-2-ol, and tenfold less to (+)-linalool and (–)-linalool (Barrozo and Kaissling 2002; Priesner 1979). While benzoic acid is emitted from the intestinal secretion (meconium) of freshly emerged moths, the natural origin of the norterpene so far is unknown, and its significance for Bombyx mori females is unexplored. Behavioral responses to both of these undoubtedly very sensitively perceived compounds (Ziesmann et al. 2000) have not been found.
In other species of moths the females smell their own pheromone (Schneider et al. 1998). Both sexes of the noctuid moth Spodoptera littoralis perceive the pheromone; they even are able to learn—like honeybees (Vareschi and Kaissling 1970)—to extend their proboscis upon pheromone stimuli (Hartlieb et al. 1999).
Males of several lepidopteran species (e.g., of the danaine butterflies or arctiide moths) release pheromones serving as aphrodisiacs (Boppré 1986). These odors are often distributed from special scent organs called androconia (Figure 4.1b). The male monarch butterfly for instance may present to the female brushes of cuticular hairs that produce fine (3–5 µm) particles impregnated with the pheromone (Figure 4.2a–c). This “love dust” sticks to the female antennae, providing a long-lasting source of the stimulus that makes the female receptive to copulation (Boppré and Vane-Wright 1989). The aphrodisiacs of Danainae originate from plant alkaloids, which are actively sought out, taken up, and metabolized by the male (Boppré 1990). Pheromonal compounds such as danaidone, hydroxydanaidal, and danaidal, frequently used by Danainae and Arctiidae and often occurring as blends of two components, are derived from pyrrolizidine alkaloids (PAs). PAs ingested by the larvae from their host plants regulate both scent-organ morphogenesis and pheromone biosynthesis in the arctiide moth genus Creatonotos (Boppré and Schneider 1989; Egelhaaf et al. 1992). Males of Bombyx mori possess small tiltable hair pencils hidden under the front legs as detected only recently (Anderson et al. 2009), with pending discovery of an aphrodisiac pheromone.
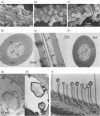
FIGURE 4.2
(a–c) “Love dust” particles on the hairs of the androconia of the male danaine butterflies Danaus formosa, Amauris tartarea, and Danaus sp. (Courtesy of M. Boppré.) (d–h) Olfactory hairs of a male moth of Antheraea (more...)
Communication by pheromones is highly developed in social insects such as honeybees, ants, or termites, which bear numerous pheromone glands on various body parts and produce a variety of chemicals. Correspondingly they possess a large number of types of specialist receptor cells (Dumpert 1972; Vareschi 1971). Pheromones of social insects are not only involved in sexual behavior, they also attract or repel conspecifics as markers of food, of trails, and of social groups, and have many other functions as well. For example, the honeybee queen (Apis mellifera L.) produces pheromones that attract a retinue of workers around her and drones on mating flights, that prevent workers from laying eggs and from swarming, and that regulate several other aspects of colony functioning (Keeling et al. 2003). The queen produces a synergistic, multiglandular pheromone blend of at least nine components. In termites a blend of cuticular hydrocarbons may play a key role in colony recognition (Kaib et al. 2004). Differences in the composition of cuticular hydrocarbons among colonies control variation in aggression between colonies.
4.3. INSECT ANTENNAE AND OLFACTORY SENSILLA
Insect pheromone receptor neurons innervate sensory hairs or plates, called sensilla, located on the antennae together with sensilla for stimuli of other sensory modalities. The antennae enormously differ in shape and size, often with a spectacular sexual dimorphism (Figure 4.3a). Male insects may have enlarged antennae with numerous sensilla designed for the most sensitive reception of the pheromone. Female saturniid moths are reported to attract their males via pheromones over distances of 1 km or more (Cardé and Charlton 1984; Priesner et al. 1986). The combed antennae of these moths may have an outline area of more than 1 cm2 (Boeckh et al. 1960), a size corresponding to the nostril of a human nose.
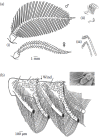
FIGURE 4.3
(a) Antennae of insects with sexual dimorphism: (i) Saturniid moth (Antheraea pernyi), (ii) scarabid beetle (Rhopaea sp.), (iii) honeybee (Apis mellifera L.). (Modified from Kaissling K.E. 1987. In R.H. Wright Lectures on Insect Olfaction, K. Colbow (ed.), (more...)
An important tool for quantitative studies on insect olfaction was tritium-labeled pheromone. Using 3H-pheromone the numbers of stimulus molecules released from the odor source and adsorbed on the antenna have been measured (Kanaujia and Kaissling 1985). From the 30% fraction of air actually passing the large combed antennae of silk moths (Kaissling 2009a; Vogel 1983), all pheromone molecules were caught. It can be calculated that a pheromone molecule—if it were reflected by the antennal surface—would hit the antenna about 100 times on its diffusional zigzag path through the lattice of the long hair sensilla (Figure 4.3b). Initially, 80% of the molecules adsorbed on the antenna were found on the hairs by measuring the radioactivity on hairs cut off immediately after stimulation with labeled pheromone (Kanaujia and Kaissling 1985; Steinbrecht and Kasang 1972). Due to the spacing of the hairs tuned to the diffusional movements of the pheromone in air and to the lipophilic surface of the hairs, the antenna serves as a kind of olfactory lens focusing the stimulus molecules to the receptor neurons. The size of a fruit fly antenna (Figure 4.3b, inset) is about 10,000-fold smaller, suggesting a much smaller absolute sensitivity to odors. Some insects with long flagellar antennae are able to recognize spatial odor patterns. Thus a topical representation of antennal areas was found in the central nervous system of cockroaches (Hoesl 1990).
In contrast to the vertebrate nasal epithelium, different types of olfactory neurons are not randomly intermingled but grouped in the sensilla, in morphologically and physiologically well-defined units (Keil 1984a, 1999, 2012; Keil and Steinbrecht 1984; Steinbrecht 1973, 1984). The pheromone-sensitive sensilla of moths consist of hollow cuticular hairs up to 400 µm long and 1 to 5 µm thick, innervated by one or several olfactory receptor neurons and furnished with three auxiliary cells (Figures 4.1d and 4.2d–f). The distal processes (dendrites, 0.1 to 0.5 mm in diameter) of the receptor neurons extend into the entire hair lumen. Interestingly the hair tip can be more sensitive than the hair base (Kaissling 2009b). The axons of each neuron connect to the antennal lobe of the central nervous system (CNS) (Ai and Kanzaki 2004; Anton and Homberg 1999; Boeckh and Boeckh 1979; Hansson and Christensen 1999; Hildebrand 1996), the insect equivalent of the vertebrate olfactory bulb. During ontogeny one of the auxiliary cells produces the cuticular wall of the hairs. Later the trichogen cell withdraws from the hair lumen and secretes the sensillum lymph. Finally the neuronal dendrites grow into the hair lumen (Ernst 1972; Keil and Steiner 1991; Kumar and Keil 1996).
The dendrites have a ciliary portion about 2 µm long that separates the inner segment from the outer segment. The outer segment contains no cellular organelles except microtubules. Mysteriously, in shorter hairs it often forms numerous parallel branches (>100, Steinbrecht 1999), with at least one microtubule per branch. The (waterproof) cuticle of olfactory hairs or plates is penetrated by so-called pore tubules (10 nm in diameter) extending into the hair lumen and sometimes contacting the neuron (Ernst 1972; Steinbrecht 1997; Steinbrecht and Stankiewicz 1999). Via these structures of unknown chemical composition the usually lipophilic odorant molecules are thought to reach the hair lumen (Figure 4.2g, h).
In cases of two or three pheromone components the respective sensilla are innervated by two or three specialist neurons, one for each of the components (Meng et al. 1989). Receptor neurons for pheromone components and behavioral inhibitors can occur in the same sensillum. With several thousands of such sensilla covering the antenna, a very fine spatial resolution of the pheromone distribution in air is feasible. It has been shown experimentally that the fine-scale distribution of pheromone components or of the pheromone blend and behavioral inhibitors influences the orientation of flying males approaching an odor source (Todd and Baker 1999).
The extracellular sensillum lymph bathing the olfactory dendrites (Figure 4.2i) corresponds to the mucus covering the vertebrate olfactory epithelium. Besides pheromone binding protein (PBP) (Steinbrecht et al. 1992) and pheromone-degrading enzymes, the sensillum lymph contains an unusual ion composition (200 mM K+, 40 mM Na+) (Figure 4.3c). Dispersive x-ray elementary analysis of sensillum lymph microdroplets revealed a lack of anions (Kaissling and Thorson 1980; Steinbrecht and Zierold 1989); only about half of the anions are covered by chloride. This is compensated by the PBP with 23 negative and 14 positive charges and its high concentration of 10 mM (Klein 1987; Vogt and Riddiford 1986a). Incidentally, the elementary analysis also revealed a high sulfur peak (Figure 4.3c), which indicates the sulfur content of the PBP (e.g., 12 sulfur atoms per BmorPBP1).
4.4. ELECTROPHYSIOLOGY
A simple method to investigate stimulus-response characteristics is the recording of the electroantennogram (EAG). The EAG represents summed fractions of receptor potentials of many olfactory sensilla located near both of the Ag/AgCl electrode capillaries inserted into tip and base of the antenna (Schneider 1957, 1992; Kaissling 1995). It is particularly useful for measuring the responses of the odor specialists, even if it does not show the contribution of each neuron type (Figure 4.7b later in the chapter). EAG recordings and also recordings from single sensilla combined with gas-chromatography have been employed to identify the effective components of blends, either of pheromones or of plant volatiles (Stranden et al. 2003).
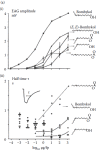
FIGURE 4.7
(a) Bombyx mori, EAG. Dose-response curves for bombykol ((E,Z)-10,12-hexadecadien-1-ol) and derivatives. Abscissae: Loads of the odor sources (filter papers). (i) EAG-amplitudes after 1-second stimuli. (ii) Half times of the declines, from the same experiments. (more...)
Single sensilla allow transepithelial recording of receptor potentials and nerve impulses from two or three identified receptor neurons. Electrical contact can be obtained by slipping the recording electrode capillary over the cut hair tip (Figure 4.3d). With a special way of squeezing (Kaissling 1995), the receptor-neuron dendrites are severed but immediately sealed, a method that avoids short-circuiting the membrane potential. Between sensillum lymph and hemolymph there is a transepithelial potential of +25 mV–+50 mV produced by an electrogenic potassium pump located in the folded apical membrane of auxiliary cells (Kaissling and Thorson 1980; Küppers and Bunse 1996). The latter pump is responsible for the high potassium concentration in the sensillum lymph (Figure 4.3c).
Pheromone stimuli elicit negative deflections of the transepithelial potential (receptor potentials) up to 30 mV, reflecting changes in membrane potential of the receptor neuron. The transepithelial resistance decreases by up to 20% (Zack 1979). Nerve impulses of a few millivolts amplitude show peak frequencies up to almost 300/s (Kaissling and Thorson 1980; Zack 1979). The opposite polarities in DC recordings of the receptor potential (negative) and the nerve impulses (positive) (Figure 4.4b) suggest that the potential is initiated by depolarization of the dendritic membrane, and that the impulses are generated in the soma region of the receptor neuron (De Kramer et al. 1984; Thurm and Küppers 1980). This is supported by experiments with selective cooling of the long hairs of moths (Kodadová and Kaissling 1996).
Pheromone sensilla with long hairs are convenient subjects for studying the electrical organization of the sensillum circuit (De Kramer and Hemberger 1987; Kaissling and Thorson 1980; Kodadová and Kaissling 1996; Redkozubov 2000; Vermeulen and Rospars 2001). Recording from the tip of a cut hair (Figure 4.3d) provides conditions equivalent to those for loose patch-clamp recordings; in this case, the entire dendrite represents the patch of the receptor-cell membrane. Typically the transepithelial resistance is around 200 MOhm, and the sensilla are electrically well isolated from each other. The analysis revealed a high specific resistance of the dendritic membrane (3000 Ohm cm2) providing a length constant of the dendrite large enough to conduct a distal membrane depolarization to the cell soma region with the generator region for the nerve impulses.
Recording from cut hairs allows some of the sensillum lymph to be replaced by the fluid from the capillary electrode. For instance, pheromone may be dissolved in the electrolyte and directly applied to the neuronal dendrites inside the hair (Kaissling et al. 1991; Pophof 2002, 2004; Van den Berg and Ziegelberger 1991). This method has been used for the long hairs of moths. Direct application of odorants by electrode penetration of the hair wall worked in Drosophila sensilla (Jones et al. 2011).
4.5. SENSITIVITY OF PHEROMONE RECEPTOR NEURONS AND BEHAVIORAL RESPONSES
Combined radiometric, electrophysiological and behavioral experiments (Kaissling 2009a; Kaissling and Priesner 1970) were employed to study the absolute sensitivity of the silk moth Bombyx mori, which will be briefly summarized here. An important tool was the radiolabeled pheromone bombykol (Kasang 1968). Although a high specific activity (31.7 Ci/g) was obtained by introducing one tritium atom per four bombykol molecules, the minimum amount detectable in a scintillation counter was 108 bombykol molecules, or 4 × 10−8 µg of bombykol. The load of the stimulus source (1 cm2 filter paper) at the behavioral 50% threshold of the male moths was about 2 × 10−5 µg of bombykol (Figure 4.5a). The release of bombykol from this source was determined by extrapolation from the release measured with 3H-bombykol at and above loads of 10−2 µg, assuming that the release below 10−2 µg was linearly proportional to the source load. This assumption was supported by the linear proportion of nerve impulses at loads below 10−2 µg. Loads of 10−3 µg, 10−4 µg, and 10−5 µg elicited 4.1, 0.31, and 0.03 nerve impulses, respectively, per neuron and per 1-s stimulus. The distribution of impulses conformed to a Poisson distribution for single random events such as expected for the arrival of single molecules (Figure 4.5b).
Stepwise determination (Figure 4.4d) revealed that about four bombykol molecules were adsorbed per hair sensillum when one nerve impulse was fired per neuron. At the behavioral 50% threshold about 24% of the antennal hairs received a molecule and about 6% of the neurons fired a nerve impulse; the antenna was exposed for 1 s to 3100 bombykol molecules per milliliter at an airstream velocity of 60 cm/s. About 52,000 molecules/ml were necessary to elicit one impulse per neuron. Our final conclusion was that one pheromone molecule is sufficient to elicit a nerve impulse.
A much larger number of nerve impulses, however, are needed in order to alert the moth (i.e., to overcome the noise of spontaneous firing of the receptor cells). Without pheromone a neuron fires a nerve impulse on average every 11.7 s, with a random distribution of intervals (Kaissling 1971). The 17,000 bombykol neurons per antenna (Schneider and Kaissling 1957; Steinbrecht 1970) permanently send an average of 1450 nerve impulses/s to the CNS. Because upon 1-s stimulation the behavioral and the impulse responses were distributed over 2 s after stimulus onset (Figure 4.5c), the noise may be calculated for 2900 spontaneous impulses; it amounts to sqrt 2900 = 54 impulses. At the behavioral 50% threshold, the 1-s bombykol stimulus induces 950 impulses fired during the 2-s time by the 17,000 neurons of one antenna (i.e., 5.6% of neurons fired an impulse). This reveals a signal-to-noise ratio of 950/54 = ~18.
The signal-to-noise ratio, however, would be smaller if the calculation is done for the time used by the CNS for integrating the nerve impulses. Half of the behavioral responses occurred within about 700 ms at weak stimuli (10−5 µg/fp) and within about 400 ms at stronger stimuli (10−2 µg/fp) (Figure 4.5c). The true integration time must be shorter than the behavioral reaction times because one has to subtract the time until a significant number of neurons fired a nerve impulse. A significant impulse signal (6% of receptor neurons firing one nerve impulse; see above) was reached at 10−3 µg after about 250 ms, and at 102 µg less than 200 ms after stimulus onset (Figure 4.5c). Half of the behavioral responses occurred after 450 and 400 ms, respectively. For the estimated true integration time of 200 ms the noise of spontaneous firing is sqrt 290 = 17 impulses, and the signal-to-noise ratio would be 95/17 = ~6. This value is little above the theoretical minimum signal-to-noise ratio of 3 for a significant signal. These calculations show that the moth CNS performs an astonishingly efficient signal-to-noise analysis of the input from the antennal neurons.
Olfactory receptor neurons of insects like those of vertebrates are primary sense cells. In insects they send their axons to glomeruli of the antennal lobe (Hildebrand and Shepherd 1997) where they terminate in the macroglomerular complex (MGC) of the antennal lobe, which has a subunit for every type of pheromone receptor cell (Sadek et al. 2002; Trona et al. 2013). The numerical convergence of 17,000 primary fibers onto the secondary 34 projection neurons in B. mori (Kanzaki et al. 2003) would be 500:1, not sufficient for integration over 17,000 neurons per antenna. Local interneurons and convergence of the projection neurons to higher neurons might be involved.
There are functional connections between the MGC and the ordinary glomeruli that receive input from receptor cells for general odors (Boeckh and Ernst 1987). The division of the antennal lobe into a region for the pheromone input and one for general odors (Hansson and Christensen 1999) resembles that of the olfactory system in many vertebrates, where the accessory and the main olfactory bulb are innervated from the vomeronasal organ and the main olfactory epithelium, respectively.
Comparing the insect antennae containing 10,000 up to 100,000 receptor neurons to the noses of vertebrates, it seems clear that the number of receptor neurons and the absolute sensitivity are not correlated. While the threshold for bombykol is at about 3000 molecules/ml (Figures 4.5a), one of the most effective odorants for humans (sec. butyl mercaptan) can be recognized at a concentration of 1.3 × 108 molecules/ml (Stuiver 1958) by the human nose with 6 million receptor neurons (Menco 1983). The dog’s nose (German shepherd) may have up to 500 million olfactory neurons; it may smell butyric acid at 9 × 103 molecules/ml (Neuhaus 1953), and alpha-ionone at 4 × 105 molecules/ml of air (Moulton 1977). Factors other than the number of receptor neurons may be important for a high sensitivity, such as a high effectiveness of molecule capture and conveyance to the sensitive structures, or a low background activity of the receptor neurons.
4.6. ELEMENTARY RECEPTOR POTENTIALS
By which mechanism would a single pheromone molecule elicit a nerve impulse? Extracellular DC recordings from single sensilla at stimulus loads of 10−3 µg/fp and below showed elementary receptor potentials (ERPs) preceding single nerve impulses (Figure 4.4c) (Kaissling 1974; Kaissling and Thorson 1980). ERPs also may occur without being followed by a nerve impulse. The ERPs appear as single “bumps” of a few milliseconds duration, or as bursts of a few bumps with amplitudes up to 0.5 mV. The amplitudes of the bumps from the bombykol and bombykal neuron differ (Figure 4.4c), they vary strongly (Redkozubov 2000). The temporal distribution of nerve impulses generated by bombykal (Figure 4.6aiii), however, is similar to those of bombykol-induced responses (compare with the impulse distribution at 10−4 µg of bombykol in Figure 4.5c). The average reaction times of the ERPs and those of the nerve impulses are within the range of 0.5 s.
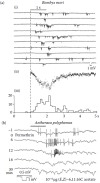
FIGURE 4.6
Extracellular DC recordings from single sensilla trichodea. (a) ERPs and nerve impulses from one sensillum. (i) 10 consecutive responses to 1-second stimuli of bombykal (1 ng/filter paper) with 1-minute intervals between stimuli. Single and superimposed (more...)
Minor and Kaissling (2003) assumed that each bump reflects an activation of a single receptor molecule and that a burst of bumps indicates repetitive activations of the same receptor molecule by the same pheromone molecule (assumption C in Kaissling 2001). According to an analysis of the electrical sensillum circuit, an average bump results from an increase of membrane conductance by 30 pS, which could be caused by opening of a single ion channel (Kaissling and Thorson 1980). Patch clamp experiments with extruded dendrites of receptor neurons of the saturniid moth A. polyphemus revealed pheromone-dependent channel openings of 56 pS, with an opening time of 1.2 ms (Zufall and Hatt 1991). These channels were observed with inside-out patches upon treatment with pheromone and cGMP (1 µM) or a membrane-permeable analog of diacylglycerol (1,2-dioctanoyl-sn-glycerol, 0.36 µM) in the presence of MgATP, but not by IP3 (1 µM) (see also Pophof and Van der Goes van Naters 2002).
Opening a single channel upon activation of a single receptor molecule would require tight functional coupling of receptor molecules and ion channels. This coupling appears to be realized in Drosophila and other insects (Sato et al. 2008; Wicher et al. 2008). These authors found that the olfactory receptor molecule is associated with a co-receptor molecule (Orco) forming an ion channel (Vosshall and Hansson 2011). This is supported by the recent finding that the Orco-agonist VUAA1 (found by screening over >100,000 compounds) opens channels with 22 pS conductance in outside-out membrane patches of HEK293 cells expressing AgOrco from Anopheles gambiae (calculated from Figure 3 of Jones et al. 2011). For the effects of VUAA1 and further Orco receptor activator molecules (OrcoRAMs), see Bohbot and Dickens 2012, Chen and Luetje 2012, and Nolte et al. 2013.
Bumps—so far observed only in B. mori, saturniid, and sphingid moths (Kaissling 2013)—seem to be an invention of the extremely sensitive pheromone neurons, which are able to fire one or a few nerve impulses upon the impact of a single stimulus molecule. Triggering a single nerve impulse requires a sufficiently large and long-lasting decrease of the membrane potential at the spike generator region located at the soma of the neuron (Kodadová and Kaissling 1996). The proper invention would be a receptor molecule, which—while activated by the pheromone molecule—keeps the associated ion channel open sufficiently long (for about 10 ms) to reaching a voltage change large enough for eliciting an action potential. The change of the membrane potential at the cell soma induced by the dendritic 30-pS conductance increase reaches about 1 mV, as expected from the electrical circuit analysis (Kaissling 1987). It should be noted that visual neurons of locusts and flies show bumps in response to light flashes containing single or a few quanta per neuron (Kirschfeld 1966; Scholes 1965).
Less sensitive receptor neurons such as those of the female B. mori responding to linalool or to benzoic acid do not show bumps even though they innervate sensilla trichodea similar to those innervated by the pheromone receptor neurons of the male. The benzoic acid neuron needs impacts of more than 1000 odorant molecules per second, which could open many ion channels but with smaller conductance per channel, in order to produce an increase in impulse firing. This neuron responds to 7 × 108 molecules of benzoic acid per milliliter of air, at an air speed of 60 cm/s (Ziesmann et al. 2000), not detected by the human nose. Its apparent spontaneous impulse firing in laboratory air was found to be partially due to the previously unknown contamination with benzoic acid.
With stronger stimuli, the bumps of the pheromone neurons superimpose and form a fluctuating receptor potential like that obtained by adding to each other many responses to smaller stimuli (Figure 4.6aii). A fluctuating response can be seen after selective blocking of the nerve impulses by the insecticide permethrin (Figure 4.6b) (Kaissling 1980), a compound known to interfere with nerve impulse formation (Vijverberg et al. 1982). Further increase of stimulus strength reduces the fluctuations, presumably by opening many ion channels but with reduced conductance per channel (Kaissling 1980). This effect may be responsible for the wider range of stimulus intensities covered by the pheromone receptor neurons compared with less sensitive neurons tuned to other odorants (Kaissling 2013).
Interestingly, responses to certain less effective pheromone derivatives do not show bumps; they produce smooth receptor potentials even at weak excitation, and their dose-response curves cover a smaller range of stimulus intensities (Figure 4.7ai). Most of the derivatives show a decline of the response after stimulus end faster than observed after bombykol stimuli (Figure 4.7aii). Modeling of the receptor potential kinetics suggests that the receptor activations by these compounds do not last as long as would be necessary for the formation of visible bumps (Kaissling 2013). Reducing duration of the activated state in the model also simulates other effects such as smaller maximum amplitudes of the dose-response curves, a shift of the curves along the abscissa to stronger stimuli, and faster declines of the response.
The mechanisms controlling the conductance per channel remain to be discovered. Impaired channel opening seems to be involved also in the reduction of sensitivity (adaptation) after strong stimuli (Kaissling 2013; Kaissling et al. 1987; Zack 1979). Adaptation may be induced locally by stimulation of small sections of a hair sensillum (Zack 1979; Zack-Strausfeld and Kaissling 1986).
4.7. INHIBITION OF PHEROMONE RECEPTOR NEURONS
The mechanism producing fluctuating responses may be selectively blocked. Brief (100-ms) exposure to the vapor of osmium tetroxide strongly reduced the responses of single neurons to bombykol but not to (Z)-10-tetradecenol, one of the derivatives producing smooth responses (Figure 16 in Kaissling 1974).
Responses of pheromone receptor neurons may be inhibited by the volatile decanoyl-thio-1,1,1-trifluoropropanone (DTFP), known as an inhibitor of the sensillar esterase, the sensillar enzyme that degrades a pheromone component of A. polyphemus (Vogt et al. 1985). If applied directly after a pheromone stimulus, DTFP rapidly repolarizes the transepithelial receptor potential in various moth species (Figure 4.6c) (Pophof 1998; Pophof et al. 2000). It did not, however, inhibit olfactory neurons tuned to compounds other than pheromones such as the benzoic acid receptor or the linalool-sensitive receptor of the Bombyx female.
The number of 3H-labeled DTFP molecules adsorbed per antenna necessary for inhibition was a little higher than the calculated number of pheromone receptor molecules but less than 0.1% of the number of PBPs. This protein occurs in extremely high concentrations (10 mM) in the sensillum lymph and apparently interacts—as a complex with the pheromone—with the receptor molecule (Kaissling 2013). Since DTFP strongly binds to various PBPs tested (Maida et al. 2003), it probably blocks the receptor molecule while bound to the PBP. The inhibitory effect (i.e., the repolarization of the receptor potential) is as rapid as the pheromone response, suggesting a competition between the DTFP-PBP complex and the pheromone-PBP complex formed by the preceding pheromone stimulus. A competitive inhibition might also occur between benzoic acid and the structurally related aniline (Figure 4.6d). Note that DTFP and other trifluoromethyl ketones interfere with behavioral responses to pheromone and may be suitable for insect pest control (Albajes et al. 2002; Hummel and Hecker 2012; Picimbon 2004; Karg and Suckling 1999; Levinson and Levinson 2002; Quero et al. 2004; Renou and Guerrero 2000; Renou et al. 2002).
Inhibition of pheromone receptor neurons has also been observed upon exposure to other volatiles, but it is not known whether this has a biological function. For instance, receptor potentials and the nerve impulse responses can be completely and reversibly abolished by terpenes, geraniol in A. polyphemus (Schneider et al. 1964), or (racemic) linalool in B. mori (Kaissling et al. 1989; Pophof and Van der Goes van Naters 2002). Linalool has been used to modulate long-lasting poststimulatory impulse firing of the bombykol neuron caused by (Z,E)-4,6-hexadecadiene (Kaissling et al. 1989). This bombykol derivative elicits anemotactic walk of Bombyx males, but only if the poststimulatory impulse firing is interrupted by 150-ms pulses of linalool stimuli given at a rate of 3/s (Kramer 1992). Clearly these terpenes are structurally more different than DTFP from the pheromones, whose excitatory action they inhibit. They are not, however, general inhibitors of olfactory receptor neurons, since for instance linalool effectively excites one of the Bombyx female neurons, as described above. Linalool does not affect the other neuron tuned to benzoic acid innervating the same sensillum.
Various compounds more generally inhibit but also irritate the cells if applied at high concentrations, including amines (Kaissling 1972, 1977). They inhibit pheromone receptor cells as well as other types of receptor cells. Often they inhibit the cell at lower concentrations and excite it at higher concentrations. Such compounds might interfere with the lipid structure of the plasma membrane so as to reduce membrane conductance at low doses. At high doses they cause increased conductance, probably destabilizing the membrane. After such stimuli recovery can be incomplete, indicating irreversible damage of the cell function. There are compounds that excite and inhibit at the same time. Often the inhibitory effect disappears more quickly than the excitatory one, leading to poststimulatory rebound effects (De Brito Sanchez 2000; De Brito Sanchez and Kaissling 2005; Pophof and Van der Goes van Naters 2002).
Inhibition and rebound was observed also after simultaneous exposure to pheromone and general anesthetics. When applied alone, general anesthetics may cause hyperpolarization and suppression of spontaneous impulse firing (Stange and Kaissling 1995). They also block the responses to pheromones or other key compounds. If applied during or directly after an excitatory stimulus, they rapidly repolarize the cell. If applied locally on the long sensilla trichodea, general anesthetics do not block the response to pheromone unless they are applied at the same locus as the pheromone. Thus, they might impair specifically the function of receptor molecules or ion channels in the receptor cell membrane either directly, or indirectly by interfering with the structure of the surrounding lipid matrix. Insecticides such as (+)-trans-Permethrin and DDT blocked the nerve impulses but not the receptor potential (Kaissling 1980).
4.8. CONCENTRATION DETECTORS AND FLUX DETECTORS
Concentration detectors and flux detectors are two types of chemoreceptors that differ in the velocity of adsorption and desorption of stimulus molecules (Kaissling 1998). In true concentration detectors the stimulus concentration at the receptor neuron equilibrates with the external stimulus concentration, limited by diffusion. Stimulus molecules would adsorb and desorb quickly, for micrometer distances to the neuron within a few milliseconds. In contrast, true flux detectors adsorb but do not desorb the stimulus molecules, as found for pheromone receptors of moths (Kanaujia and Kaissling 1985; Kasang 1971). Concentration and flux detectors might have fundamental chemical differences of the sensillum surface and the sensillum lymph composition.
During stimulation, flux detectors accumulate stimulus molecules. In order to provide the neuron at constant external stimulation with a constant concentration of stimulus molecules, and to avoid overstimulation, flux detectors need to deactivate the stimulus molecules about as fast as they are adsorbed. Stimulus deactivation must be an extracellular process, which could be relatively slow and rate-limiting for the neuronal response. Flux detectors might provide for higher sensitivity by keeping the stimulus molecule for a longer time in the neighborhood of the receptor neuron. One function of the odorant binding protein (OBP) could be to decelerate or prevent the desorption of the odorant. A rate-limiting pheromone deactivation is assumed for the quantitative model discussed below. Concentration detectors might not need odorant deactivation and odorant degradation. If these are absent other processes such as intracellular signaling may govern the response kinetics (reviewed in Gu and Rospars 2011).
Flux detectors respond to the product of stimulus concentration and the relative velocity of the medium, which reveals the adequate measure of stimulus intensity in molecules per area and per time. Concentration detectors are insensitive to airstream velocity. For a long time insect receptor neurons for carbon dioxide were the only examples known for olfactory concentration detectors, showing no response to changes in airstream velocity (Stange and Diesendorf 1973; Stange and Stowe 1999). A single CO2-sensitive neuron of the honeybee responds well to an increase of the background concentration of 350 ppm in air CO2 (9.4 × 1015 molecules/ml) by 5 × 1013 molecules/ml (Lacher 1964). Surprisingly true olfactory concentration detectors unaffected by airstream velocity have been found recently in Drosophila for three odorants (Zhou and Wilson 2012).
4.9. OLFACTORY TRANSDUCTION, EXTRACELLULAR
Extracellular transducer processes (perireceptor events) and pheromone-receptor interaction have been tentatively combined in a preliminary quantitative model (Figure 4.8a, b) (Kaissling 2001, 2009b), which has been discussed in detail recently (Kaissling 2013). Only a few aspects will be presented here. The model includes 12 chemical reactions:
- The adsorption of the pheromone (Figure 4.8a, reaction 1) and its diffusion from the adsorption site on the hair towards neuron
- The solubilization of the mostly lipophilic pheromone by binding to the PBP in the sensillum lymph (reactions 2–4)
- The activation of the receptor molecule in the plasma membrane of the receptor cell (reactions 5, 6)
- The deactivation of the pheromone within the sensillum lymph (Figure 4.8b, reactions 7, 8)
- The enzymatic degradation of the pheromone (Figure 4.8b, reactions 9–12)
4.10. DIFFUSION ON THE HAIRS
Radiolabeled pheromone was used to determine the velocity of pheromone transport to the receptor neuron. Because of the relatively small hair volume, the initial concentration of the 80% of pheromone adsorbed on the hairs is very high (cf. Figure 4.4d). Following the concentration gradient the molecules migrate from the hairs to the antennal body. By cutting hairs of male antennae at various time intervals after stimulation with 3H-labeled pheromone, the velocity of this migration was determined. The coefficient for longitudinal diffusion was D = 50 µm2/s for 3H-bombykol ((E,Z)-10,12-hexadecadienol) in B. mori (Steinbrecht 1973; Steinbrecht and Kasang 1972) and D = 30 µm2/s for 3H-(E,Z)-6,11-hexadecadienyl acetate, the major pheromone component of the saturniid moth, A. polyphemus (Kanaujia and Kaissling 1985). This velocity corresponds to the range expected for diffusion within the sensillum lymph of the PBP molecule with a molecular mass of 15 kD. Since the radioactivity was shown to enter the sensillum lymph, it was concluded that the longitudinal diffusion occurs inside the hairs while the pheromone is bound to the PBP.
The velocity of the diffusion from the adsorption site on the hair surface to the pore entrance, and through the pore tubules was determined from the migration along hairs of dried antennae (Kanaujia and Kaissling 1985). The sensillum lymph was evaporated and the hairs were filled with air so that the PBP was unable to move. On these hairs the longitudinal migration of the 3H-pheromone was about threefold faster than in intact antennae (D = 90 µm2/s). In the model we used D2 = D1 = 90 µm2/s for the two-dimensional diffusion along the hair surface and the one-dimensional diffusion through the tubules, and D3 = 30 µm2/s for the diffusion within the lymph (Figure 4.8a). The mean time between adsorption on the hair surface and the arrival at the neuron is in the 10-ms range (Kaissling 1987; Steinbrecht 1973). The diffusional delay is independent on stimulus intensity; it becomes visible at strong stimulus intensities, where the receptor potential starts after about 10 ms (Figure 4.8e). The first nerve impulse is elicited after another 5 ms. With infinitely fast diffusion the model response starts practically without a delay (Figure 4.8e) (Kaissling 2001). Note that the average reaction time of the nerve impulses at weak stimulation (in the range of half a second, Figures 4.5c, 4.6a) must be due to processes other than diffusion, as e.g. the pheromone-receptor association (Figure 4.8b, reaction 5).
4.11. KINETIC MODEL
Since the tertiary structure of the bombykol binding protein was analyzed by x-ray crystallography (Sandler et al. 2000) and by nuclear magnetic resonance (NMR) (Horst et al. 2001; Klusák et al. 2003), further insect binding proteins were studied. The preliminary model presented here (Figure 4.8a, b) is restricted to biochemical and electrophysiological data obtained from two species of moths, B. mori and A. polyphemus by various labs. Rate constants for most reactions and the initial concentrations of the reaction partners (reviewed in Kaissling 2009b, 2013) allow to calculate fictive half-lives of the latter if one reaction only were to occur (Figure 4.8b). For instance, the half-life of F would be ln2/(k2 [A]) = 2.7 ms due to binding of F and the PBP form A alone (reaction 2, with the association rate constant k2 = 0.068/(s µM) and [A] = 3.8 mM). Due to the enzymatic degradation alone (reaction 9 = binding to the pheromone degrading enzyme E, and reaction 10 = catalytic step), the half-life of F would be 13 ms. The half-life of F is 2.1 ms if both binding to A and degradation are considered.
As mentioned above it is tentatively assumed that the decline of the receptor potential is governed by the pheromone deactivation (Figure 4.8b, reactions 7, 8). From the decline after stimulus end at medium stimulus intensities, and considering the dose-response relationship of the receptor potential amplitude, a half-life of 0.8 s was estimated for the activated pheromone-PBP-receptor complex FAR′. At high intensities the decline was delayed due to overloading of the deactivation process (Figures 4.7aii, b, and 4.8c). In the favored model version presented here the deactivation is catalyzed by the hypothetical enzyme N (binding of FB to N, reaction 7, and the catalytical step forming FB, reaction 8). Alternatively, it was assumed that the receptor molecules themselves could act as enzymes catalyzing the deactivation, or that this process occurs spontaneously (Kaissling 2009b).
When entering the hair lumen the pheromone F encounters one of two reaction partners dissolved in the sensillum lymph, either the PBP form A (reactions 2 and 3) or the pheromone-degrading enzyme E (reaction 9, followed by the catalytic reaction 10). Without the ligand the PBP exists in its A form independent on pH (Lautenschlager et al. 2005) with the inner binding cavity of the protein occupied by the C terminus in a helical conformation (Figure 4.1e). Within less than 3 ms, 17% of the pheromone F is metabolized to M by the enzyme E, whereas 83% of F is bound to the PBP and thereby protected from enzymatic degradation (Vogt and Riddiford 1986a).
When F binds at neutral pH to the A form, mainly the complex FB is produced (reaction 2); that is, the conformation of the PBP changes from A to B (Figure 4.1e). Now the pheromone is bound inside the inner cavity, and the C terminus forms a flexible, mainly hydrophobic tail. At low pH the binding of F and A forms the complex FA (reaction 3), with the pheromone presumably attached to the periphery of the protein. This reaction may be neglected here because the formation of FA via FB is about 10-fold faster. Upon pH changes, the complex FB may be rapidly converted into FA, and back to FB (reaction 4), with rate constants obtained from stop-flow experiments by Leal et al. (2005). The change of FB to FA is thought to occur at low pH via protonation of histidines (Horst et al. 2001; Nemoto et al. 2002; Wojtasek and Leal 1999), in vivo within a zone of negative charges fixed at the cell membrane of the receptor neuron (Figure 4.2g, h) (Keil 1984b). The complex FA is assumed to be the only species in vivo binding to the receptor molecule (R) (reaction 5) (see below). According to the model the association of FA and R (reaction 5) is the slowest one of the processes leading to receptor activation, with a half-life of FA of more than 400 ms (Figure 4.8b). Therefore the formation of FAR is largely responsible for the reaction times of the responses to single stimulus molecules at very low stimulus intensities (Figures 4.5c and 4.6a).
The ternary complex FAR may change one or several times to the activated state FAR′ (reaction 6), triggering a “bump” upon each change, as described above. The lumped lifetime of the states FAR and FAR′ is also considered as the residence time of the pheromone-PBP complex at the receptor molecule (Tc, Figure 4.7c).
In parallel to the reactions leading to the receptor activation, the pheromone molecules are deactivated (reactions 7, 8). Finally, while bound to the scavenger PBP form B’, they are enzymatically degraded (reactions 11, 12), but 20,000-fold more slowly than the free pheromone (see below). Only Q3 = 25% of the molecules adsorbed on the hair sensilla have the chance to activate a receptor molecule (see Figure 4.4d).
4.12. FIVE FUNCTIONS OF THE PHEROMONE BINDING PROTEIN
In vitro the free pheromone might be able to activate the receptors if applied in unphysiological concentrations. In vivo, however, the pheromone-PBP complex is thought to interact with the receptor molecule. The idea that the complex FA, rather than the free pheromone, interacts with the receptor molecule is supported by experimental evidence (a–c, below) and by model calculations (d, e).
- Infusion of the sensillum lymph space with pheromone-PBP mixtures revealed that besides the pheromone also the PBP is involved in the receptor activation (Pophof 2002). Furthermore, infusion of one PBP of A. polyphemus (ApolPBP1) elicited nerve impulses of the bombykol receptor neuron of B. mori even in the absence of pheromone (Pophof 2004).
- In Drosophila the PBP “Lush” activated the receptor directly via a pheromone-induced conformation of the protein, again without the pheromone (Ha and Smith 2009; Laughlin et al. 2008; Xu et al. 2005).
- The sensitivity of HEK293 cells expressing one of the three receptors of A. polyphemus (ApolOR1) was about 300-fold increased for one of the three pheromone components ((E,Z)-6,11-headecadienal) when a specific PBP (ApolPBP2) was added (Forstner et al. 2009; Grosse-Wilde et al. 2006).
- According to the model the concentration of the complex FA is—during stimulation—about 60-fold higher than that of the free pheromone F.
- Finally the lifetime of the free pheromone is much shorter (a few milliseconds) than the time necessary for association with the receptor (several hundred milliseconds; see Figure 4.8b).
Modeling showed that the PBP serves several, at first glance contradictory, functions (Kaissling 2001, 2009b):
- The protein binds and solubilizes the hydrophobic pheromone and serves as a transporter towards the receptor neuron.
- Binding to the PBP protects the pheromone from enzymatic degradation (Vogt and Riddiford 1986a). Without protection, 93% of the pheromone would be degraded before binding to the receptor (Kaissling 2009b).
- There is evidence that the PBP is involved in the pheromone-receptor interaction, as described above.
- The PBP performs the postulated pheromone deactivation (see below).
- The PBP serves as an organic anion compensating the partial lack of chloride ions found by electron microprobe analysis of the sensillum lymph (Figure 4.3c).
A typical PBP, 3 nm wide, has a molecular mass of 15 kD and 142 amino acids, and possesses six highly conserved cysteines forming three disulfide bridges (Leal et al. 1999; Maida et al. 2005; Scaloni et al. 1999; Vogt et al. 1999). The amino acid sequence is known for many of these proteins. Species with a larger number of pheromone components possess a diversity of PBPs with different binding specificities (Nagnan-Le Meillour and Jacquin-Joly 2003). Nonpheromone sensilla contain so-called general odorant binding proteins (GOBPs), which are related to the PBPs and share the six conserved cysteines (He et al. 2010; Pelosi and Maida 1995; Steinbrecht et al. 1995; Zhang et al. 2001; Zhou et al. 2009). Besides these OBPs further proteins of lower homology with possible chemosensory function (chemosensory proteins [CSPs]) have been found in several insect orders (Dani et al. 2011; Picimbon 2003; Tegoni et al. 2004; Vogt 2003). These are similar in size to or smaller than OBPs but differ in amino acid sequence and cysteine content. Drosophila melanogaster has about 40 PBP-related proteins (Graham and Davies 2002).
The PBP was detected using gel electrophoresis and 3H-labeled (E,Z)-6,11-hexadecadienyl acetate (Ac1), the main pheromone component of A. polyphemus (Vogt and Riddiford 1981). The binding survived the electrophoresis. The dissociation constant of purified PBP and the pheromone component Ac1 from A. polyphemus antennae was 60 nM (Kaissling et al. 1985), from an assay where PBP dissolved in Ringer solubilized the pheromone initially bound to a glass surface. A different assay revealed 640 nM (Du et al. 1994). The values for bombykol and PBP A form and B form of B. mori were Kd3 = 1.6 µM and Kd2 = 105 nM, respectively (Leal et al. 2005). The solubilization of pheromone by PBP was also shown by means of electrophysiological recording during direct application of the polyphemus pheromone and the PBP to the sensillum lymph via the recording glass capillary (Van den Berg and Ziegelberger 1991). In these experiments bovine serum albumin (BSA) solubilized the pheromone equally well.
PBP binding may contribute to the specificity of the neuron response (Grosse-Wilde et al. 2006; He et al. 2010; Hooper et al. 2009; Steinbrecht 1996). For instance in A. polyphemus (E,Z)-6,11-hexadecadienol bound to the purified PBP 1000-fold less strongly than the pheromone (E,Z)-6,11-hexadecadienyl acetate, and was 1000-fold less effective as a stimulus for the neuron (Du and Prestwich 1995; Du et al. 1994). However, the saturated acetate bound to the PBP only 10-fold weaker than the pheromone, whereas its effect on the neuron response was 1 million times weaker than that of the pheromone (De Kramer and Hemberger 1987). The dissociation constants of (+)- and (–)-disparlure and two recombinant PBPs in the gypsy moth differed by about two- to fourfold (Plettner et al. 2000). In contrast, the sensitivities of both types of receptor neurons for the two enantiomers differed by factors of more than 100 (Hansen 1984).
The same sensillum may contain several PBPs with different binding specificities and in very different amounts. Thus the same three types of PBPs occur in the sensilla trichodea of A. polyphemus and A. pernyi together with three receptor neurons, each tuned to one of the three pheromone components (Maida et al. 2003). Each of the PBPs preferentially binds one of these components. The binding results agree with the finding of Mohanty et al. (2004) that a more bulky amino acid joins in the pheromone-binding cavity of the PBP preferring the shorter pheromone molecule.
Bombykal bound to the bombykol binding protein (BmorPBP1) similarly as bombykol (Graeter et al. 2006; He et al. 2010). However, bombykal in combination with BmorPBP1 failed to activate the bombykal neuron, but it did activate it in combination with ApolPBP1 (Pophof 2004). So far no PBP for bombykal has been found (Forstner et al. 2006). Surprisingly, BmorGOBP2 bound bombykol as well as BmorPBP1, and even discriminated it from bombykal (He et al. 2010).
Since the structure of BmorPBP became known, many other antennal PBPs of insects have been analyzed (Dani et al. 2011; Fan et al. 2011). The ApolPBP1 of the moth A. polyphemus is similar in secondary and tertiary structure to BmorPBP (Mohanty et al. 2004). BmorPBP and ApolPBP1 have 5 histidines. The PBPs of the cockroach Leucophaea madera (LmadPBP, binding a pheromone component [Lartigue et al. 2003]), the honeybee Apis mellifera (Amel-ASP1, binding two major pheromone components [Lartigue et al. 2004]), and the fly D. melanogaster (LUSH, binding short-chain n-alcohols [Kruse et al. 2003] and the pheromone (Z)-11 vaccenyl acetate [Xu et al. 2005]) show interesting differences from BmorPBP (e.g., only two histidines in LUSH, one in Amel-ASP1, and none in LmadPBP). The C-terminus of Amel-ASP1 is placed against the “body” of the protein along the wall of the internal cavity. LmadPBP has a C-terminus shortened by 24 amino acids. Thus pH-dependent changes such as found in BmorPBP are not expected for all PBPs.
Besides PBPs and the related GOBPs there are various smaller chemosensory proteins of unknown function (Dani et al. 2011). Finally it should be noted that PBP-related proteins also occur in insect taste receptors (Nagnan Le Meillour et al. 2000), possibly involved in the transport and perception of noxious taste substances (Ozaki et al. 2003).
Interestingly, the principle of a double-walled nanocapsule has been implemented at least twice in evolution. OBPs of mammals belong to the lipocalin family with a size and function similar to those of insect OBPs, also called encapsulins (Leal 2003). However lipocalins, serving as OBPs in vertebrates (Pernollet and Briand 2004), have a different structure characterized by antiparallel beta-sheet folding and in addition comprise two alpha helices near the N terminal. The sheets, held together by hydrogen bridges, form a container-like structure called the beta barrel.
4.13. PHEROMONE DEGRADATION AND DEACTIVATION
Degradation of pheromone was first found in the silk moth B. mori by Kasang (1971). Living antennae exposed in air for 10 seconds to 3H-bombykol were subsequently eluted for 10 minutes by pentane and for another 10 minutes by a chloroform-methanol mixture, and the amounts of bombykol and its metabolites in the resulting solutions were checked by thin-layer chromatography. Bombykol had been turned into aldehyde and acid, and later into esters, with a half-life of 4 to 5 minutes. The degradation was sensitive to temperature, suggesting catalysis by an enzyme, probably a dehydrogenase. Interestingly, pheromone degradation was also found in female Bombyx antennae lacking pheromone receptor neurons and on other body parts such as the wings or legs of both sexes (Kasang and Kaissling 1972; Kasang et al. 1988, 1989a, 1989b). While these body parts are tightly covered with scales, the pheromone degradation also occurs on these dry cuticular structures devoid of cellular elements. Vogt and Riddiford (1986b) isolated an enzyme from body scales, an interesting case of enzymatic reactions in nonaqueous material. The degradation on the entire body surface prevents the generation of secondary pheromone sources that could interfere with the mating behavior of the males.
Pheromone-degrading enzymes (esterases, aldehyde oxidases) were isolated from moth antennae (Rybczynski et al. 1990; Vogt et al. 1985), besides enzymes belonging to the cytochrome P450 family (Maibeche-Coisne et al. 2002). Kinetic studies with the enriched pheromone esterase of A. polyphemus revealed a Km of 2.2 µM, a catalytic rate constant in the range of 98/s, and an estimated concentration in vivo of 1 µM (Vogt et al. 1985). The respective values for the cloned enzyme were 1.2 µM, 127/s, and 0.5 µM (Ishida and Leal 2005). With these values—and without protection—the half-life of the pheromone in vivo would be 15 or 13 ms, respectively, in contrast to the 4.5 minutes found by Kasang et al. (Kasang et al. 1988, 1989a, 1989b). In fact Kasang’s curves show a twofold time course, with an initial very rapid decrease of the intact pheromone by 17% (Kaissling 2009b, 2013). Modeling revealed that this fraction of the pheromone is degraded within the first few milliseconds when the pheromone enters the hair lumen, before most of it is bound to the PBP and thereby protected from the enzyme. That the degradation is not responsible for the decline of the receptor potential is supported by recordings from single antennae with very little enzyme activity but normal decline (Maida et al. 1995).
Stimulus deactivation was postulated by Kaissling (1972) in order to explain why the receptor potential declines within seconds after cessation of a brief external stimulus although intact pheromone molecules remain for minutes on the antenna, and for tenths of seconds on and even inside the hairs (Kanaujia and Kaissling 1985). The receptor potential, however, is not adapted and responds to a new stimulus. The process of deactivation seems to be saturable since after strong stimuli the response declines more slowly (Figure 4.7aii), and after extremely strong stimuli the response does not decline but continues for a time interval depending on the amount of odorant loaded onto the antenna (Figures 4.7b and 4.8c). Since the saturation for pheromone derivatives may occur at a submaximal excitation level (Figure 4.7b), the deactivation is likely an extracellular rather than an intracellular process.
In the modeled deactivation the PBP carrying a pheromone molecule undergoes a hypothetical structural change from B to B’ (Figure 4.8b, reactions 7, 8), which blocks the release of the pheromone from the PBP and renders it “invisible” for the receptor. As a result of a discussion with F. Damberger and W. Leal, we proposed that a hypothetical enzyme N might be able to recognize the pheromone-carrying B-form by the exposed hydrophobic C-terminal tail, and to discriminate it from the empty A-form with the exposed hydrophilic N-terminal tail (Figure 4.1e). The enzyme N could block or even remove the C-terminal tail of FB, and thus prevent the stimulatory complexes FA, FAR, and finally FAR′ from being formed.
The latter idea is supported by the finding that experimental removal of the C-terminus eliminated the FB → FA transformation at low pH (Figure 4.8b, reaction 4). The pheromone was irreversibly locked inside the binding cavity of the truncated PBP (Leal et al. 2005; Michel et al. 2011). Furthermore, the pheromone binding of the PBP at low pH was retained by one-point mutation of the C terminus (Xu and Leal 2008). Apparently the formation of a C-terminal alpha helix—necessary for the ejection of the pheromone from the inner binding cavity—was blocked.
The deactivated pheromone is not completely protected from degradation, although the velocity of the latter is reduced by a factor of 20,000 (Kaissling 2009b). Enzymatic degradation may have a useful function in removing traces of the adsorbed pheromone. This is important to guarantee full recovery of the receptor neurons from previous stimulation and to reduce the nerve impulse discharge to the level of spontaneous activity.
The idea of two processes—a deactivation followed by a much slower degradation—is supported by the response the bombykol neuron to the derivative (Z,E)-4,6-hexadecadiene (Kaissling et al. 1989). A 1-s stimulus by this compound produces a response, which—after stimulus offset—declines like a response to bombykol. Since the initial decline is incomplete (see tailing in Figure 4.8c), it is followed by a prolonged (15-minute) firing of nerve impulses of the bombykol receptor neuron. The initial decline could be due to deactivation whereas the poststimulatory firing may indicate that the hexadecadiene cannot be degraded by the dehydrogenase postulated by Kasang (see above).
Besides pheromones other odorants can be metabolized on antennae of moths. Thus benzoic acid, the most effective compound for the B neuron of the female sensilla trichodea was conjugated with serine to N-benzoylserine 10 s after exposure of fresh (but not of heat-treated) antennae to benzoic acid. While pheromone degradation occurs also on the body scales of the moth, no derivatization of benzoic acid was found on body parts other than the antennae (Oldenburg et al. 2001).
4.14. RECEPTOR MOLECULES, ION CHANNELS, AND SENSORY NEURON MEMBRANE PROTEIN
In D. melanogaster more than 60 types of candidate odorant receptor molecules have been identified, each having seven transmembrane domains activating G-proteins. Each receptor cell expresses only one type of receptor protein (Clyne et al. 1999; Dobritsa et al. 2003; Vosshall 2001). Molecules belonging to the seven-transmembrane-domain category were also identified and localized by in situ hybridization in antennae of the moth Heliothis virescens (Krieger et al. 2002, 2003). Receptor molecules for bombykol and bombykal were identified by Sakurai et al. (2004) and Krieger et al. (2005). An impressive overview and evolutionary tree of the receptor molecules of males and females of B. mori is given by Anderson et al. (2009). For further receptor molecules in moths see Grosse-Wilde et al. (2010).
Nakagawa et al. (2005) determined an EC50 = 1.5 µM for bombykol and the bombykol receptor molecule expressed in Xenopus oocytes. For the complex FA and the receptor molecule our model revealed EC50 model = 6.8 µM (Kaissling 2009b). Considering the different experimental conditions, no closer agreement with the results of Nakagawa et al. would be expected. Comparing these EC50 values with the above dissociation constants of pheromone and PBP (between 60 nM and 1.6 µM), the pheromone seems to be bound more strongly to the PBP than the pheromone-PBP complex to the receptor molecule. Using oocyte expression of the bombykol receptor Xu et al. (2012) found an EC50 for bombykol of 0.99 µM, but of 9.6 µM for bombykal, for the same receptor. This result does not reflect the in vivo situation: The bombykol neuron does not respond to bombykal unless the stimulus intensity is at least 10,000-fold higher than for bombykol (Kaissling et al. 1978).
In principle, processes such as the postulated pheromone deactivation or the binding of the odorant to extracellular binding proteins (see Section 4.12) may contribute to the response specificity. However, the receptor-neuron specificity seems mainly bound to the neuron, and is most likely determined by the interaction of stimulus molecules with receptor molecules since the specificity of other processes such as binding to PBPs or deactivation seems much less sharp than the specificity of the cell response. It should be noted that the bombykol receptor molecule was functionally expressed in an “empty” olfactory neuron of D. melanogaster (Syed et al. 2006) which then responded to bombykol. Similarly, a receptor for (Z)-11-hexadecenal from the diamondback moth Plutella xylostella was expressed in the bombykol neuron of B. mori males, which then responded behaviorally to the xylostella pheromone almost as well as to bombykol (Sakurai et al. 2011).
A preliminary calculation reveals densities of olfactory receptor molecules at the neuronal membrane of about 4000/µm2 for B. mori and 6000/µm2 for A. polyphemus (Figure 4.7c). The average density of repetitive structures—putative receptor molecules—found by negative staining in isolated membrane vesicles obtained from isolated sensilla in A. polyphemus was 10,000 units/µm2 (maximally 30,000 units/µm2) (Klein and Keil 1984). The number of receptor molecules estimated per neuron amounts to 260,000 for B. mori, and to 2.6 million for A. polyphemus. The large numbers of receptor molecules are required for a wide working range of stimulus intensities covered by the neuronal response. This applies especially to flux detectors like insect pheromone sensilla where the number of receptor molecules occupied by stimulus molecules linearly depends on the stimulus uptake (Kaissling 1998).
The calculated densities of olfactory receptor molecules are far higher than the presumed minimum density of ion channels in the plasma membrane of the olfactory receptor neuron. The analysis of the electrical sensillum circuit revealed that opening of 10,000 ion channels per receptor neuron of A. polyphemus with a conductance per channel of 30 pS would suffice for full depolarization of the receptor neuron (Kaissling 2013; Kaissling and Thorson 1980). This number would correspond to a minimum channel density of 23 per µm2. A much higher number of channels, however, are expected if each receptor molecule together with a co-receptor molecule forms an ion channel (see Section 4.6). This would mean that the real number of ion channels is close or equal to the number of receptor molecules as determined here for model N.
Of particular interest is a membrane protein known as the sensory neuron membrane protein (SNMP), a member of the so-called CD36 protein family (Rogers et al. 1997, 2001a, 2001b). Its members are characterized by two terminal transmembrane domains and a large extracellular domain; they function as docking sites, where extracellular protein molecules can become coupled to the cell membrane. In Drosophila the receptor molecules are associated with the SNMP (Benton et al. 2007), and this protein is required for pheromone sensitivity (Jin et al. 2008). The density of SNMP molecules may be roughly estimated from impressive electron micrographs showing gold-labeled antibodies against SNMP associated with the neuronal cell membrane of A. polyphemus (Figure 4.2d, e). The density of SNMP could well be equal to that of the receptors if only a few percent of the SNMP molecules carried a gold particle. A different type of SNMP was found in the auxiliary cells of pheromone sensilla (Forstner et al. 2008).
Assuming a ternary association of receptor, co-receptor, and SNMP we arrive at 18,000 protein units per µm2. This is a minimum estimate because the pheromone uptake U (molecules per hair volume and per s) has been related to the entire hair volume of 2.6 pl (Keil 1984a). If the pheromone molecules stay within the hair lumen only, the pheromone uptake U including Usat, and, consequently, the receptor number calculated (Figure 4.7c) would be even higher. This means the protein density of the olfactory neuron is close to the density of rhodopsin in the outer disc membrane of vertebrate visual cells with 40,000 units/µm2 (Dratz and Hargrave 1983).
4.15. OLFACTORY TRANSDUCTION, INTRACELLULAR
After the activation of receptor molecules by the odorant, a variety of intracellular signal compounds seems to be involved in the transduction process; among them are diacyl glycerol, cGMP, and Ca++ (Gu and Rospars 2011; Krieger and Breer 2003; Stengl et al. 1999). The function of 1,4,5 inositol trisphosphate (IP3) seems questionable (Kaissling 1994; Kaissling and Boekhoff 1993). Various constituents of intracellular pathways have been identified and immunolocalized (Jacquin-Joly et al. 2002; Laue et al. 1997; Maida et al. 2000).
Often it is thought that high sensitivity of olfactory receptor neurons requires amplification by intracellular signaling processes (Gu et al. 2009; Nakagawa and Vosshall 2009; Stengl 2010; Wicher et al. 2008). At least for the extremely sensitive pheromone receptors of moths—producing bumps eliciting nerve impulses upon a conductance increase of 30 pS—it seems clear that amplification is performed solely by the electrical organization of the sensillum. Intracellular messengers, however, may play a role as modulators and for adaptation after strong stimulation, for instance for the supposed reduction of ion channel opening (see Section 4.6).
Intracellular signaling processes are more likely rate-limiting for the neuronal response in concentration detectors, which do not need extracellular odorant degradation and deactivation (see Section 4.8). Thus olfactory response characteristics like long-lasting impulse firing upon brief stimuli, such as observed in Drosophila (Montague et al. 2011) and in moths (Kaissling et al. 1989), could have different origins (e.g., from intracellular or extracellular processes, respectively).
The model of perireceptor- and receptor events (Section 4.9 ff.) satisfactorily simulates the rise and fall of the receptor potential except that the fall measured at high stimulus intensities proceeds much more slowly than the simulated one. A simplified version of the model of Kaissling (2001) was combined with a model of intracellular signaling (Rospars et al. 2007). When square wave pulses of the concentration of activated receptor molecules (FAR′, Figure 4.8a,b) were applied in the combined model, the intracellular processes turned out to be relatively fast and contributed relatively little to the receptor potential kinetics (Gu et al. 2009). Considering intracellular processes, however, can improve the simulation at high stimulus intensities (Gu and Rospars 2011). Adaptation phenomena such as diminished ERPs (Section 4.6) still await modeling.
More than one type of ion channel appears to contribute to the receptor potential, and further channels must be involved in the generation of nerve impulses in the soma region of the receptor cell (Stengl et al. 1999). In A. polyphemus the initial burst of nerve impulses observed at relatively high stimulus intensities might be induced by opening of a Ca++-activated nonspecific ion (CAN) channel located in the soma region of the receptor cell (Zufall et al. 1991). This phasic response adapts very quickly, possibly because this type of channel is blocked by cGMP. The cloned cDNA of a cyclic nucleotide and voltage-activated ion channel from the antennae of the moth Heliothis virescens was heterologously expressed and analyzed by patch clamp recordings and in situ hybridization (Krieger et al. 1999). It was suggested that this channel plays a role in regulating the responsiveness of the cell via intracellular cAMP-levels, possibly controlled by the neuromodulator octopamine (Pophof 2000; Von Nickisch-Rosenegk et al. 1996).
4.16. TEMPORAL CODING
With stronger stimulation, the elementary receptor potentials add up to an overall receptor potential that can reach 30 mV. While the average latency of the responses to single pheromone molecules is about 0.5 seconds, at high stimulus intensities the onset of the overall receptor potential may be delayed by 10 ms only (Figure 4.8e). At high stimulus intensities, insect olfactory receptor neurons and also higher-order neurons within the antennal lobe (Christensen and Hildebrand 1988; Lei et al. 2002) resolve repetitive stimulus pulses up to frequencies of 10 pulses per second (Almaas et al. 1991; Barrozo and Kaissling 2002; Kaissling 1986; Rumbo and Kaissling 1989). The time resolution of the nerve impulse response depends on the type of receptor neuron and on temperature (Kodadová 1996) (Figure 4.9a). The astonishing resolution is restricted to higher stimulus intensities where the response latency is short. It was first shown by Kramer (1986) that the anemotactic walk of a male moth of B. mori near the odor source consists of several pheromone-elicited turns per second into the upwind direction (Kaissling 1997; Kaissling and Kramer 1990; Kramer 1996; Todd and Baker 1999). Each turn was elicited by a brief odor pulse such as a male encounters due to turbulence within a pheromone plume. A single upwind turn of a flying almond moth elicited by a 10-ms pheromone stimulus has been marvelously demonstrated by Mafra-Neto and Cardé (1994) (Figure 4.9b). It should be noted that the upwind orientation during flight requires visual reference to the ground. For temporal coding see also M. Renou (Chapter 2, this volume).

FIGURE 4.9
(a) Responses of a single pheromone receptor neuron repetitively stimulated at various frequencies by 20-ms pulses of the main pheromone component (E,Z)-6,11-hexadecadienal, at 8°C. (Modified from Kodadová B. 1996. J Comp Physiol A 179:301–310.) (more...)
ACKNOWLEDGMENTS
The author thanks A. Krikellis and his team for librarian help, A.M. Biederman-Thorson for linguistic improvements, and C. Mucignat for generous editorial support.
REFERENCES
- Ai H, Kanzaki R. Modular organization of the silkmoth Antennal lobe macroglomerular complex revealed by voltage-sensitive dye imaging. J Exp Biol. 2004;207:633–644. [PubMed: 14718506]
- Albajes R, Konstantopoulou M, Etchepare O, Eizaguirre M, Frerot B, Sans A, Krokos F, Ameline A, Mazomenos B. Mating disruption of the corn borer Sesamia nonagrioides (Lepidoptera: Noctuidae) using sprayable formulations of pheromone. Crop Protection. 2002;21:217–225.
- Almaas T.J, Christensen T.A, Mustaparta H. Chemical communication in Heliothine moths I. Antennal receptor neurons encode several features of intra- and interspecific odorants in the male corn earworm moth Helicoverpa zea. J Comp Physiol A. 1991;169:249–258.
- Anderson A.R, Wanner K.W, Trowell S.C, Warr C.G, Jaquin-Joly E, Zagatti P, Robertson H, Newcomb R.D. Functional analysis of female-biased odorant receptors from the silkworm, Bombyx mori. Insect Biochem Molec Biol. 2009;39:189–197. [PubMed: 19100833]
- Anton S, Homberg U. Antennal lobe structure. In: Hansson B.S, editor. In Insect Olfaction. Berlin: Springer Verlag; 1999. pp. 98–124.
- Baker T.C, Ochieng S.A, Cossé A.A, Lee S.G, Todd J.L, Quero C, Vickers N.J. A comparison of responses from olfactory receptor neurons of Heliothis subflexa and Heliothis virescens to components of their sex pheromone. J Comp Physiol A. 2004;190:155–165. [PubMed: 14689220]
- Barrozo R.B, Kaissling K.E. Repetitive stimulation of olfactory receptor cells in female silkmoths Bombyx mori L. J Insect Physiol. 2002;48:825–834. [PubMed: 12770060]
- Benton R, Vannice K.S, Vosshall L. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450:289–203. [PubMed: 17943085]
- Blomquist G.J, Vogt R.G, editors. Insect Pheromone Biochemistry and Molecular Biology. London: Elsevier Academic Press; 2003.
- Boeckh J, Kaissling K.E, Schneider D. Sensillen und Bau der Antennengeißel von Telea polyphemus. Zool Jahrb Anat Ontog. 1960;78:559–584.
- Boeckh J, Kaissling K.E, Schneider D. Insect olfactory receptors. Cold Spring Harbor Symp Quant Biol. 1965;30:1263–1280.
- Boeckh J, Boeckh V. Threshold and odor specificity of pheromone-sensitive neurons in the deutocerebrum of Antheraea pernyi and A. polyphemus (Saturnidae). J Comp Physiol. 1979;132:235–242.
- Boeckh J, Ernst K.D. Contribution of single unit analysis in insects to an understanding of olfactory function. J Comp Physiol. 1987;161:549–565.
- Bohbot J.D, Dickens J.C. Odorant receptor modulation: Ternary paradigm for mode of action of insect repellents. Neuropharmacology. 2012;62:2086–2095. [PubMed: 22269900]
- Boppré M. Insects pharmacophageously utilizing defensive plant chemicals (pyrrolizidine alkaloids). Naturwissenschaften. 1986;73:17–26.
- Boppré M. Lepidoptera and pyrrolizidine alkaloids, exemplification of complexity in chemical ecology. J Chem Ecol. 1990;16:165–185. [PubMed: 24264905]
- Boppré M, Schneider D. The biology of Creatonotos (Lepidoptera: Arctiidae) with special reference to the androconial system. Zool J Linnean Society. 1989;96:339–356.
- Boppré M, Vane-Wright R.I. Androconial system in Danainae (Lepidoptera): Functional morphology of Amauris, Danaus, Tirumala and Euploea. Zool J Linn Soc. 1989;97:101–133.
- Butenandt A, Beckmann R, Stamm D, Hecker E. Über den Sexuallockstoff des Seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z Naturforschung. 1959;14b:283–284.
- Butenandt A, Hecker E. Synthese des Bombykols, des Sexual-Lockstoffes des Seidenspinners, und seiner geometrischen Isomeren. Angew Chemie. 1961;73:349–353.
- Cardé R.T, Charlton R.E. Olfactory sexual communication in Lepidoptera: Strategy, sensitivity and selectivity. In: Lewis T, editor. In Insect Communication. New York: Academic Press; 1984. pp. 241–265.
- Cardé R.T, Minks A.K, editors. Insect Pheromone Research: New Directions. New York: Chapman & Hall; 1997.
- Chen S, Luetje C.W. Identification of new agonists of the insect odorant receptor co-receptor subunit. PLoS One. 2012;7(5):e36784. [PMC free article: PMC3348135] [PubMed: 22590607]
- Christensen T.A, Hildebrand J.G. Frequency coding of central olfactory neurons in the sphinx moth Manduca sexta. Chem Senses. 1988;13:123–130.
- Clyne P.J, Warr C.G, Freeman M.R, Lessing D, Kim J, Carlson J.R. A novel family of divergent seven-transmembrane proteins: Candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. [PubMed: 10069338]
- Dani F.R, Michelucci E, Francese S, Mastrobuoni G, Cappellozza S, La Marca G, Niccolini A, Felicioli A, Moneti G, Pelosi P. Odorant-binding proteins and chemosensory proteins in pheromone detection and release in the silkmoth Bombyx mori. Chem Senses. 2011;36:335–347. [PubMed: 21220518]
- De Brito Sanchez M.G. Argentina: University of Buenos Aires; 2000. Estudios electrofisiológicos de estructura-actividad de la cèlula antenal receptora del Ácido benzoico de la hembra de Bombyx mori L. PhD thesis.
- De Brito Sanchez M.G, Kaissling K.E. Inhibitory and excitatory effects of iodobenzene on the antennal benzoic acid receptor cell of the female silk moth Bombyx mori L. Chem Senses. 2005;30:435–442. [PubMed: 15901657]
- De Kramer J.J, Kaissling K.E, Keil T. Passive electrical properties of insect sensilla may produce the biphasic shape of spikes. Chem Senses. 1984;8:289–295.
- De Kramer J.J, Hemberger K. The neurobiology of pheromone reception. In: Prestwich G.D, Blomquist G.J, editors. In Pheromone Biochemistry. New York: Academic Press; 1987. pp. 433–472.
- Dobritsa A.A, Van der Goes van Naters W, Warr C, Steinbrecht R.A, Carlson J.R. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. [PubMed: 12628173]
- Dratz E.A, Hargrave P.A. The structure of rhodopsin and the rod outer segment disk membrane. Trends Biochem Sci. 1983;8:128–131.
- Du G, Ng C.S, Prestwich G.D. Odorant binding by a pheromone binding protein: Active site mapping by photoaffinity labeling. Biochemistry. 1994;33:4812–4819. [PubMed: 8161541]
- Du G, Prestwich G.D. Protein structure encodes the ligand binding specificity in pheromone binding proteins. Biochemistry. 1995;34:8726–8732. [PubMed: 7612612]
- Dumpert K. Alarmstoffrezeptoren auf der Antenne von Lasius fuliginosus (Latr.) (Hymenoptera, Formicidae). Z Vergl Physiol. 1972;76:403–425.
- Egelhaaf A, Rick-Wagner S, Schneider D. Development of the male scent organ of Creatonotos transiens (Lepidoptera, Arctiidae) during metamorphosis. Zoomorphology. 1992;111:125–139.
- El-Sayed A. M. 2012. The Pherobase: Data base of pheromones and semiochemicals, < http://www
.pherobase.com>. - Ernst K.D. Die Ontogenie der basiconischen Riechsensillen auf der Antenne von Necrophorus (Coleoptera). Z Zellforsch Mikrosk Anat. 1972;129:217–236. [PubMed: 5042771]
- Fan J, Francis F, Liu Y, Chen J.L, Cheng D.F. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet Mol Res. 2011;10:3056–3069. [PubMed: 22180039]
- Field L.M, Pickett J.A, Wadhams L.J. Molecular studies in insect olfaction. Insect Molec Biol. 2000;9:545–551. [PubMed: 11122463]
- Forstner M, Gohl T, Breer H, Krieger J. Candidate pheromone binding proteins of the silkmoth Bombyx mori. Invert Neurosci. 2006;6:177–187. [PubMed: 17082917]
- Forstner M, Gohl T, Gondesen I, Raming K, Breer H, Krieger J. Differential expression of SNMP-1 and SNMP-2 proteins in pheromone-sensitive hairs of moths. Chem Senses. 2008;33:291–299. [PubMed: 18209018]
- Forstner M, Breer H, Krieger J. A receptor and binding protein interplay in the detection of a distinct pheromone component in the silkmoth Antheraea polyphemus. Int J Biol Sci. 2009;5:745–757. [PMC free article: PMC2793307] [PubMed: 20011135]
- Graeter F, Xu W, Leal W, Grubmueller H. Pheromone discrimination by the pheromone-binding protein of Bombyx mori. Structure. 2006;14:1577–1586. [PubMed: 17027506]
- Graham L.A, Davies P.L. The odorant-binding proteins of Drosophila melanogaster: annotation and characterization of a divergent gene family. Gene. 2002;292:43–55. [PubMed: 12119098]
- Grosse-Wilde E, Svatos A, Krieger J. A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem Senses. 2006;31:547–555. [PubMed: 16679489]
- Grosse-Wilde E, Stieber R, Forstner M, Krieger J, Wicher D, Hansson B. Sex-specific odorant receptors of the tobacco hornworm Manduca sexta. Front Cell Neurosci. 2010;4:22. [PMC free article: PMC2922936] [PubMed: 20725598]
- Gu Y, Lucas P, Rospars J.-P. Computational model of the insect pheromone transduction cascade. Plos Comput Biol. 2009;5(3):e1000321. [PMC free article: PMC2649447] [PubMed: 19300479]
- Gu Y, Rospars J.-P. Dynamical modeling of the moth pheromone-sensitive olfactory receptor neuron within its sensillar environment. PLoS ONE. 2011;6(3):e17422. [PMC free article: PMC3047557] [PubMed: 21399691]
- Ha T.S, Smith D.P. Odorant and pheromone receptors in insects. Front Cell Neurosci. 2009;3:10–15. [PMC free article: PMC2759369] [PubMed: 19826623]
- Hansen K. Discrimination and production of disparlure enantiomers by the gypsy moth and the nun moth. Physiol Entomol. 1984;9:9–18.
- Hansson B.S, editor. Insect Olfaction. Berlin: Springer Verlag; 1999.
- Hansson B.S, Christensen T.A. Functional characteristics of the antennal lobe. In: Hansson B.S, editor. In Insect Olfaction. Berlin: Springer Verlag; 1999.
- Hartlieb E, Anderson P, Hansson B.S. Appetitive learning of odours with different behavioural meaning in moths. Physiol Behav. 1999;67:671–677. [PubMed: 10604836]
- He X, Tzotzos G, Woodcock C, Pickett J.A, Hooper T, Field L.M, Zhou J.J. Binding of the general odorant binding protein of Bombyx mori BmorGOBP2 to the moth sex pheromone components. J Chem Ecol. 2010;36:1293–1305. [PubMed: 20981477]
- Heinbockel T, Kaissling K.E. Variability of olfactory receptor neuron responses of female silkmoths (Bombyx mori L.) to benzoic acid and (+)-linalool. J Insect Physiol. 1996;42:565–578.
- Hildebrand J.G. Olfactory control of behavior in moths: Central processing of odor information and the functional significance of olfactory glomeruli. J Comp Physiol A. 1996;178:5–19. [PubMed: 8568724]
- Hildebrand J.G, Shepherd G.M. Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. [PubMed: 9056726]
- Hoesl M. Pheromone-sensitive neurons in the deutocerebrum of Periplaneta americana: Receptive fields on the antenna. J Comp Physiol. 1990;167:321–327.
- Hooper A.M, Dufour S, He X, Muck A, Zhou J.J, Almeida R, Field L.M, Svatos A, Pickett J.A. High-throughput ESI-MS analysis of binding between the Bombyx mori pheromone-binding protein BmorPBP, its pheromone components and some analogues. Chem Commun. 2009;2009:5725–5727. [PubMed: 19774249]
- Horst R, Damberger F, Luginbühl P, Güntert P, Peng G, Nikonova L, Leal W.S, Wüthrich K. NMR structure reveals intramolecular regulation mechanism for pheromone binding and release. Proc Natl Acad Sci U S A. 2001;98:14374–14379. [PMC free article: PMC64689] [PubMed: 11724947]
- Hummel H.E, Hecker E. Half a century of pheromones—Their contributions to sustainable insect pest management (IPM). Mitt Dtsch Ges Allg Angew Ent. 2012;18:451–460.
- Ishida Y, Leal W.S. Rapid inactivation of a moth pheromone. Proc Natl Acad Sci U S A. 2005;102:14075–14079. [PMC free article: PMC1216831] [PubMed: 16172410]
- Jacquin-Joly E, Francois M.C, Burnet M, Lucas P, Bourrat F, Maida R. Expression pattern in the antennae of the newly isolated lepidopteran Gq protein alpha subunit cDNA. Eur J Biochem. 2002;269:2133–2142. [PubMed: 11985591]
- Jacquin-Joly E, Lucas P. Pheromone reception and transduction: Mammals and insects illustrate converging mechanisms across phyla. Curr Top Neurochem. 2005;4:75–105.
- Jin X, Ha T.S, Smith D.P. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci USA. 2008;105:10995–11000. [PMC free article: PMC2504837] [PubMed: 18653762]
- Jones P.L, Pask G.M, Rinker D.C, Zwiebel L.J. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci U S A. 2011;108:8821–8825. [PMC free article: PMC3102409] [PubMed: 21555561]
- Kaib M, Jmhasly P, Wilfert L, Durka W, Franke S, Francke W, Leuthold R.H, Brandl R. Cuticular hydrocarbons and aggression in the termite Macrotermes subhyalinus. J Chem Ecol. 2004;30:365–385. [PubMed: 15112730]
- Kaissling K.E. Kinetic studies of transduction in olfactory receptors of Bombyx mori. In: Schneider D, editor. In Int Symp Olfaction and Taste IV. Stuttgart: Wiss Verlagsgesellsch; 1972. pp. 207–21.
- Kaissling K.E. Sensory transduction in insect olfactory receptors. In: Jaenicke L, editor. In Biochemistry of sensory functions. Mosbacher Coll Ges Biolog Chemie. Vol. 25. Berlin: Springer; 1974. pp. 243–273.
- Kaissling K.E. Structures of odour molecules and multiple activities of receptor cells. In: Le Magnen J, MacLeod P, editors. In Olfaction and Taste VI. London: Inf Retrieval; 1977. pp. 9–16.
- Kaissling K.E. In Insect Neurobiology and Pesticide Action (Neurotox 79). London: Society of Chemical Industry; 1980. Action of chemicals, including (+)trans Permethrin and DDT, on insect olfactory receptors; pp. 351–358.
- Kaissling K.E. Chemo-electrical transduction in insect olfactory receptors. Annu Rev Neurosci. 1986;9:21–45. [PubMed: 3518584]
- Kaissling K.E. Burnaby B.C. R.H. Wright Lectures on Insect Olfaction. Colbow K, editor; Canada: Simon Fraser University; 1987. pp. 1–190.
- Kaissling K.E. IP3 effects in moth pheromone receptors: Calculations. In: Elsner N, Breer H, editors. In Sensory Transduction Proceedings of the 22nd Göttingen Neurobiological Conference. Vol. 1. Stuttgart: Thieme Verlag; 1994. p. 94.
- Kaissling K.E. Single unit and electroantennogram recordings in insect olfactory organs. In: Spielman A.L, Brand J.G, editors. In Experimental Cell Biology of Taste and Olfaction: Current Techniques and Protocols. Boca Raton, FL: CRC Press; 1995. pp. 361–386.
- Kaissling K.E. Pheromone-controlled anemotaxis in moths. In: Lehrer M, editor. In Orientation and Communication in Arthropods. Basel: Birkhäuser; 1997. pp. 343–374.
- Kaissling K.E. Flux detectors versus concentration detectors: Two types of chemoreceptors. Chem Senses. 1998;23:99–111. [PubMed: 9530975]
- Kaissling K.E. Olfactory perireceptor and receptor events in moths: A kinetic model. Chem Senses. 2001;26:125–150. [PubMed: 11238244]
- Kaissling K.E. The sensitivity of the insect nose: The example of Bombyx mori. In: Gutiérrez A, Marco S, editors. In Biologically Inspired Signal Processing, SCI. Vol. 188. Berlin: Heidelberg: Springer Verlag; 2009a. pp. 45–52.
- Kaissling K.E. Olfactory perireceptor and receptor events in moths: A kinetic model revised. J Comp Physiol A. 2009b;195:895–922. [PMC free article: PMC2749182] [PubMed: 19697043]
- Kaissling K.E. Kinetics of olfactory responses might largely depend on the odorant-receptor interaction and the odorant deactivation postulated for flux detectors. J Comp Physiol A. 2013;199:879–896. [PubMed: 23563709]
- Kaissling K.E, Priesner E. Die Riechschwelle des Seidenspinners. Naturwissenschaften. 1970;57:23–28. [PubMed: 5417282]
- Kaissling K.E, Kasang G, Bestmann H.J, Stransky W, Vostrowsky O. A new pheromone of the silkworm moth Bombyx mori. Naturwissenschaften. 1978;65:382–384.
- Kaissling K.E, Thorson J. Insect olfactory sensilla: Structural, chemical and electrical aspects of the functional organisation. In: Sattelle D.B, Hall L.M, Hildebrand J.G, editors. In Receptors for Neurotransmitters, Hormones and Pheromones in Insects. Amsterdam, New York: Elsevier/North-Holland Biomedical Press; 1980. pp. 261–282.
- Kaissling K.E, Klein U, de Kramer J.J, Keil T.A, Kanaujia S, Hemberger J. Insect olfactory cells: Electrophysiological and biochemical studies. In: Changeux J.P, Hucho F, Maelicke A, Neumann E, editors. In Molecular Basis of Nerve Activity. Proceedings of the International Symposium in Memory of David Nachmansohn. Berlin: W. de Gruyter; 1985. pp. 173–183.
- Kaissling K.E, Zack Strausfeld C, Rumbo E.R. Adaptation processes in insect olfactory receptors. Mechanisms and behavioral significance. Ann N Y Acad Sci. 1987;510:104–112. [PubMed: 3324874]
- Kaissling K.E, Meng L.Z, Bestmann H.J. Responses of bombykol receptor cells to (Z,E)-4,6-hexadecadiene and linalool. J Comp Physiol A. 1989;165:147–154.
- Kaissling K.E, Kramer E. Sensory basis of pheromone-mediated orientation in moths. Verh Dtsch Zool Ges. 1990;83:109–131.
- Kaissling K.E, Keil T.A, Williams L. Pheromone stimulation in perfused olfactory hairs of Antheraea polyphemus. J Insect Physiol. 1991;37:71–78.
- Kaissling K.E, Boekhoff I. Transduction and intracellular messengers in pheromone receptor cells of the moth Antheraea polyphemus. In: Wiese K, Gribakin F.G, Popov A.V, Renninger G, editors. In Arthropod Sensory Systems. Basel: Birkhäuser Verlag; 1993. pp. 489–502.
- Kanaujia S, Kaissling K.E. Interactions of pheromone with moth antennae: Adsorption, desorption and transport. J Insect Physiol. 1985;31:71–81.
- Kanzaki R, Soo K, Seki Y, Wada S. Projections to higher olfactory centres from subdividions of the antennal lobe macroglomerular complex of the male silkmoth. Chem Senses. 2003;28:113–130. [PubMed: 12588734]
- Karg G, Suckling M. Applied aspects of insect olfaction. In: Hansson B.S, editor. In Insect Olfaction. Berlin: Springer Verlag; 1999. pp. 351–377.
- Karlson P, Lüscher M. “Pheromones”: A new term for a class of biologically active substances. Nature (Lond.). 1959;183:55–56. [PubMed: 13622694]
- Kasang G. Tritium labeling of the sex attractant Bombykol. Z Naturforschung. 1968;23b:1331–1335. [PubMed: 4387310]
- Kasang G. Bombykol reception and metabolism on the antennae of the silkmoth Bombyx mori. In: Ohloff G, Thomas A.F, editors. In Gustation and Olfaction. London/New York: Academic Press; 1971. pp. 245–250.
- Kasang G, Kaissling K.E. Specificity of primary and secondary olfactory processes in Bombyx antennae. In: Schneider D, editor. In Intern Symp Olfaction and Taste IV. Stuttgart: Wissensch Verlagsgesellsch; 1972. pp. 200–206.
- Kasang G, Schneider D, Schäfer W. The silkworm moth Bombyx mori. Presence of the (E,E)-stereoisomer of bombykol in the female pheromone gland. Naturwissenschaften. 1978a;65:337–338.
- Kasang G, Kaissling K.E, Vostrowsky O, Bestmann H.J. Bombykal, a second pheromone component of the silkworm moth Bombyx mori. L. Angew Chemie. 1978b;90:74–75. Angew Chemie, Int ed Engl 17:60.
- Kasang G, von Proff L, Nicholls M. Enzymatic conversion and degradation of sex pheromones in antennae of the male silkworm moth Antheraea polyphemus. Z Naturforschung. 1988;43c:275–284.
- Kasang G, Nicholls M, von Proff L. Sex pheromone conversion and degradation in antennae of the silkworm moth Bombyx mori L. Experientia. 1989a;45:81–87.
- Kasang G, Nicholls M, Keil T, Kanaujia S. Enzymatic conversion of sex pheromones in olfactory hairs of the male silkworm moth Antheraea polyphemus. Z Naturforschung. 1989b;44c:920–926.
- Kaupp U.B. Olfactory signalling in vertebrates and insects: Differences and commonalities. Nat Rev Neurosci. 2010;11:188–200. [PubMed: 20145624]
- Keeling C.I, Slessor K.N, Higo H.A, Winston M.L. New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc Natl Acad Sci U S A. 2003;100:4486–4491. [PMC free article: PMC153582] [PubMed: 12676987]
- Keil T.A. Reconstruction and morphometry of silkmoth olfactory hairs: a comparative study of sensilla trichodea on the antennae of male Antheraea polyphemus and Antheraea pernyi (Insecta, Lepidoptera). Zoomorphology. 1984a;104:147–156.
- Keil T.A. Surface coats of pore tubules and olfactory sensory dendrites of a silkmoth revealed by cationic markers. Tissue Cell. 1984b;16:705–717. [PubMed: 6515640]
- Keil T.A. Morphology and development of the peripheral olfactory organs. In: Hansson B.S, editor. In Insect Olfaction. Berlin: Springer Verlag; 1999. pp. 5–47.
- Keil T.A. Sensory cilia in arthropods. Arth Struct & Dev. 2012;41:515–534. [PubMed: 22814269]
- Keil T.A, Steinbrecht R.A. Mechanosensitive and olfactory sensilla of insects. In: King R.C, Akai H, editors. In Insect Ultrastructure. Vol. 2. New York: Plenum Publishers; 1984. pp. 477–516.
- Keil T.A, Steiner C. Morphogenesis of the antenna of the male silkmoth, Antheraea polyphemus III. Development of olfactory sensilla and the properties of hair-forming cells. Tissue Cell. 1991;23:821–851. [PubMed: 18621189]
- Kirschfeld K. Oxford: Pergamon Press; 1966. Discrete and graded receptor potentials in the compound eye of the fly (Musca). Proceedings of the International Symposium on the Functional Organization of the Compound Eye; pp. 291–307.
- Klein U. Sensillum-lymph proteins from antennal olfactory hairs of the moth Antheraea polyphemus (Saturniidae). J Insect Biochem. 1987;17:1193–1204.
- Klein U, Keil T.A. Dendritic membrane from insect olfactory hairs: Isolation method and electron microscopic observations. Cell Mol Neurobiol. 1984;4:385–396. [PubMed: 6532523]
- Klusák V, Havlas Z, Rulísek L, Vondrásek J, Svatos A. Sexual attraction in the silkworm moth: Nature of binding of bombykol in pheromone binding protein—An ab initio study. Chem Biol. 2003;10:331–340. [PubMed: 12725861]
- Kodadová B. Resolution of pheromone pulses in receptor cells of Antheraea polyphemus at different temperatures. J Comp Physiol A. 1996;179:301–310.
- Kodadová B, Kaissling K.E. Effects of temperature on responses of silkmoth olfactory receptor neurones to pheromone can be simulated by modulation of resting cell membrane resistances. J Comp Physiol. 1996;179:15–27.
- Kramer E. Turbulent diffusion and pheromone triggered anemotaxis. In: Payne T.L, Birch M.C, Kennedy C.E.J, editors. In Mechanisms in Insect Olfaction. Oxford: Oxford University Press; 1986. pp. 58–67.
- Kramer E. Attractivity of pheromone surpassed by time-patterned application of two nonpheromone compounds. J Insect Behav. 1992;5:83–97.
- Kramer E. A tentative intercausal nexus and its computer model on insect orientation in windborne pheromone plumes. In: Cardé R.T, Minks A, editors. In Pheromone Research: New Directions. New York: Chapman & Hall; 1996. pp. 232–247.
- Krieger J, Strobel J, Vogl A, Hanke W, Breer H. Identification of a cyclic nucleotide and voltage-activated ion channel from insect antennae. Insect Biochem Mol Biol. 1999;29:255–267. [PubMed: 10319439]
- Krieger J, Raming K, Dewer Y.M.E, Bette S, Conzelmann S, Breer H. A divergent gene family encoding candidate olfactory receptors of the moth Heliothis virescens. Eur J Neurosci. 2002;16:619–628. [PubMed: 12270037]
- Krieger J, Breer H. Transduction mechanisms of olfactory sensory neurons. In: Blomquist G.J, Vogt R.G, editors. In Insect Pheromone Biochemistry and Molecular Biology. London: Elsevier Academic Press; 2003. pp. 593–607.
- Krieger J, Klink O, Mohl C, Raming K, Breer H. A candidate olfactory receptor subtype highly conserved across insect orders. J Comp Physiol A. 2003;189:519–526. [PubMed: 12827420]
- Krieger J, Grosse-Wilde E, Gohl T, Breer H. Candidate pheromone receptors of the silkmoth Bombyx mori. Eur J Neurosci. 2005;21:2167–2176. [PubMed: 15869513]
- Kruse S.W, Zhao R, Smith D, Jones N. Structure of a specific alcohol-binding site defined by the odorant binding protein LUSH from Drosophila melanogaster. Nat Struct Biol. 2003;10:694–700. [PMC free article: PMC4397894] [PubMed: 12881720]
- Kumar G.L, Keil T.A. Pheromone stimulation induces cytoskeletal changes in olfactory dendrites of male silkmoths (Lepidoptera, Saturniidae, Bombycidae). Naturwissenschaften. 1996;83:476–478.
- Küppers J, Bunse I. A primary cation transport by a V-type ATPase of low specificity. J Exp Biol. 1996;199:1327–1334. [PubMed: 9319211]
- Lacher V. Elektrophysiologische Untersuchungen an einzelnen Rezeptoren für Geruch, Kohlendioxyd, Luftfeuchtigkeit und Temperatur auf den Antennen der Arbeitsbiene und der Drohne (Apis mellifica L.). Z Vergl Physiol. 1964;48:587–623.
- Lartigue A, Gruez A, Spinelli S, Riviere S, Brossut B, Tegoni M, Cambillau C. The crystal structure of a cockroach pheromone-binding protein suggests a new ligand binding and release mechanism. J Biol Chem. 2003;278:30213–30218. [PubMed: 12766173]
- Lartigue A, Gruez A, Briand L, Blon F, Bézirard V, Walsh M, Pernollet J.C, Tegoni M, Cambillau C. Sulfur single-wavelength anomalous diffraction crystal structure of a pheromone-binding protein from the honeybee Apis mellifera L. J Biol Chem. 2004;279:4459–4464. [PubMed: 14594955]
- Laue M, Maida R, Redkozubov A. G-protein activation, identification and immunolocalization in pheromone-sensitive sensilla trichodea of moths. Cell Tissue Res. 1997;288:149–158. [PubMed: 9042782]
- Laughlin J.D, Ha T.S, Jones D.N.M, Smith D.P. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. [PMC free article: PMC4397981] [PubMed: 18585358]
- Lautenschlager C, Leal W.S, Clardy J. Coil-to-helix and ligand release of Bombyx mori pheromone-binding protein. Biochem Biophysic Res Commun. 2005;335:1044–1050. [PubMed: 16111659]
- Leal W.S. Proteins that make sense. In: Blomquist G.J, Vogt R.G, editors. In Insect Pheromone Biochemistry and Molecular Biology: The Biosynthesis and Detection of Pheromones and Plant Volatiles. London: Elsevier Academic Press; 2003. pp. 447–476.
- Leal W.S, Nikonova L, Peng G. Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 1999;464:85–90. [PubMed: 10611489]
- Leal W.S, Chen A.M, Ishida Y, Chiang V.P, Erickson M.L, Morgan T.I, Tsuruda J.M. Kinetics and molecular properties of pheromone binding and release. Proc Natl Acad Sci U S A. 2005;102:5386–5391. [PMC free article: PMC555038] [PubMed: 15784736]
- Lei H, Christensen T.A, Hildebrand J.G. Local inhibition evoked synchronization of glomerulus-specific output neurons. Nat Neurosci. 2002;5:557–565. [PubMed: 12006983]
- Levinson H, Levinson A. In Encyclopedia of Pest Management. New York: Marcel Dekker; 2002. Insectistasis as a means of controlling pest populations in the storage environment; pp. 402–406.
- Mafra-Neto A, Cardé R.T. Dissection of the pheromone-modulated flight of moths using single-pulse response as a template. Experientia. 1996;52:373–379.
- Maibeche-Coisne M, Jacquin-Joly E, Francois M.C, Nagnan Le Meillour P. cDNA cloning of biotransformation enzymes belonging to the cytochrome P450 family in the antennae of the noctuid moth Mamestra brassicae. Insect Mol Biol. 2002;11:273–281. [PubMed: 12000647]
- Maida R, Ziegelberger G, Kaissling K.E. Esterase activity in the olfactory sensilla of the silkmoth Antheraea polyphemus. NeuroReport. 1995;6:822–824. [PubMed: 7605955]
- Maida R, Redkozubov A, Ziegelberger G. Identification of PLCß and PKC in pheromone receptor neurons of Antheraea polyphemus. Neuroreport. 2000;11:1773–1776. [PubMed: 10852242]
- Maida R, Ziegelberger G, Kaissling K.E. Ligand binding to six recombinant pheromone-binding proteins of Antheraea polyphemus and Antheraea pernyi. J Comp Physiol B. 2003;173:565–573. [PubMed: 12879348]
- Maida R, Mameli M, Mueller B, Krieger J, Steinbrecht R.A. The expression pattern of four odorant-binding proteins in male and female silk moths, Bombyx mori. J Neurocytol. 2005;34:149–163. [PubMed: 16374716]
- Menco B.P. The ultrastructure of olfactory and nasal respiratory epithelium surfaces. In: Reznik G, Stinson S.F, editors. In Nasal Tumors in Animals and Man, Vol 1 Anatomy, Physiology, and Epidemiology. Boca Raton, FL: CRC Press; 1983. pp. 45–102.
- Meng L.Z, Wu C.H, Wicklein M, Kaissling K.E, Bestmann H.J. Number and sensitivity of three types of pheromone receptor cells in Antheraea pernyi and A. polyphemus. J Comp Physiol A. 1989;165:139–146.
- Michel E, Damberger F.F, Ishida Y, Fiorito F, Lee D, Leal W.S, Wüthrich K. Dynamic conformational equilibria in the physiological function of the Bombyx mori pheromone-binding protein. J Mol Biol. 2011;408:922–931. [PubMed: 21396939]
- Minor A.V, Kaissling K.E. Cell responses to single pheromone molecules may reflect the activation kinetics of olfactory receptor molecules. J Comp Physiol A. 2003;189:221–230. [PubMed: 12664098]
- Mohanty S, Zubkov S, Gronenborn A.M. The solution NMR structure of Antheraea polyphemus PBP provides new insight into pheromone recognition by pheromone-binding proteins. J Mol Biol. 2004;337:443–451. [PubMed: 15003458]
- Montague S.A, Mathew D, Carlson J.R. Similar odorants elicit different behavioral and physiological responses, some supersustained. J Neurosci. 2011;31:7891–7899. [PMC free article: PMC3116233] [PubMed: 21613503]
- Moulton D.G. Minimum odorant concentrations detectable by the dog and their implications for olfactory receptor sensitivity. In: Müller Schwarze D, Mozell M.M, editors. In Chemical Signals in Vertebrates. New York: Plenum Press; 1977. pp. 455–464.
- Mustaparta H. Responses of single olfactory cells in the pine weevil Hylobius abietes L. (Col.: Curculionidae). J Comp Physiol. 1975;97:271–290.
- Mustaparta H. Encoding of plant odour information in insects: Peripheral and central processing. Entomologia Experimentalis et Applicata. 2002;104:1–13.
- Nagnan Le Meillour P, Cain A.H, Jacquin-Joly E, Francois M.C, Ramachandran S, Maida R, Steinbrecht R.A. Chemosensory proteins from the proboscis of Mamestra brassicae. Chemical Senses. 2000;25:541–553. [PubMed: 11015326]
- Nagnan-Le Meillour P, Jacquin-Joly E. Biochemistry and diversity of insect odorant-binding proteins. In: Blomquist G.J, Vogt R.G, editors. In Insect Pheromone Biochemistry and Molecular Biology. London: Elsevier Academic Press; 2003. pp. 509–537.
- Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–1642. [PubMed: 15692016]
- Nakagawa T, Vosshall L.B. Controversy and consensus: Noncanonical signaling mechanisms in the insect olfactory system. Curr Opin Neurobiol. 2009;19:284–292. [PMC free article: PMC2752668] [PubMed: 19660933]
- Nemoto T, Uebayashi M, Komeiji Y. Flexibility of a loop in a pheromone binding protein from Bombyx mori: A molecular dynamics simulation. Chem-Bio Info J. 2002;2:32–37.
- Neuhaus W. Über die Riechschärfe des Hundes für Fettsäuren. Z vergl Physiol. 1953;35:527–552.
- Nolte A, Funk N.W, Mukunda L, Gawalek P, Werckenthin A, et al. In situ tip-recordings found no evidence for an Orco-based ionotropic mechanism of pheromone-transduction in Manduca sexta. PLoS ONE. 2013;8(5):e62648. [PMC free article: PMC3643954] [PubMed: 23671617]
- Oldenburg C, Kanaujia S, Spiteller D, Oldham N.J, Boland W, Kaissling K.E. Benzoic acid, a stimulant of odorant receptors of Bombyx mori, is rapidly metabolized to N-benzoylserine on the antennae. Chemoecology. 2001;11:183–190.
- Ozaki M, Takahara T, Kawahara Y, Wada-Katsumata A, Seno K, Amakawa T, Yamaoka R, Nakamura T. Perception of noxious compounds by contact chemoreceptors of the blowfly, Phormia regina: Putative role of an odorant-binding protein. Chem Senses. 2003;28:349–359. [PubMed: 12771021]
- Pelosi P. Perireceptor events in olfaction. J Neurobiol. 1996;30:3–19. [PubMed: 8727979]
- Pelosi P, Maida R. Odorant-binding proteins in insects. Comp Biochem Physiol. 1995;111B:503–514. [PubMed: 7613772]
- Pelosi P, Zhou J.J, Ban L.P, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63:1658–1676. [PubMed: 16786224]
- Pernollet J.C, Briand L. Structural recognition between odorants, olfactory-binding proteins and olfactory receptors, primary events in odor coding. In: Taylor A, Roberts D, editors. In Flavor Perception. Oxford: Blackwell Publishing; 2004. pp. 86–150.
- Picimbon J.F. Biochemistry and evolution of OBP and CSP proteins. In: Blomquist G.J, Vogt R.G, editors. In Insect Pheromone Biochemistry and Molecular Biology. London: Elsevier Academic Press; 2003. pp. 539–566.
- Picimbon J.F. Synthesis of odorant reception-suppressing agents: Odorant binding proteins (OBPs) and chemosensory proteins (CSPs) as molecular targets for pest management. In: Philogène B, Regnault-Roger C, Vincent C, editors. In Phytoprotection. Paris: Lavoisier Tech and Doc; 2004. pp. 245–266.
- Plettner E, Lazar J, Prestwich E.G, Prestwich G.D. Discrimination of pheromone enantiomers by two pheromone binding proteins from the gypsy moth Lymantria dispar. Biochemistry. 2000;39:8953–8962. [PubMed: 10913308]
- Pophof B. Inhibitors of sensillar esterase reversibly block the responses of moth pheromone receptor cells. J Comp Physiol A. 1998;183:153–164. [PubMed: 10049224]
- Pophof B. Octopamine modulates the sensitivity of silkmoth pheromone receptor neurons. J Comp Physiol A. 2000;186:307–313. [PubMed: 10757246]
- Pophof B. Moth pheromone binding proteins contribute to the excitation of olfactory receptor cells. Naturwissenschaften. 2002;89:515–518. [PubMed: 12451455]
- Pophof B. Pheromone-binding proteins contribute to the activation of olfactory receptor neurons in the silkmoths Antheraea polyphemus and Bombyx mori. Chem Senses. 2004;29:117–126. [PubMed: 14977808]
- Pophof B, Gebauer T, Ziegelberger G. Decyl-thio-trifluoropropanone, a competitive inhibitor of moth pheromone receptors. J Comp Physiol A. 2000;186:315–323. [PubMed: 10757247]
- Pophof B, Van der Goes van Naters W. Activation and inhibition of the transduction process in silkmoth olfactory receptor neurons. Chem Senses. 2002;27:435–443. [PubMed: 12052780]
- Priesner E. Progress in the analysis of pheromone receptor systems. Ann Zool Ecol Anim. 1979;11:533–546.
- Priesner E. Sex attractant system in Polia pisi (Lepidoptera: Noctuidae). Z Naturforsch. 1980;35c:990–994.
- Priesner E. The pheromone receptor system of male Eulia ministrana L., with notes on other Cnephasiini moths. Z Naturforsch. 1984;39c:849–852.
- Priesner E, Schroth M. Supplementary data on the sex attractant system of Panolis flammea. Z Naturforsch. 1983;38c:870–873.
- Priesner E, Witzgall P, Voerman S. Field attraction response of raspberry clearwing moths, Pennisetia hyleiformis Lasp. (Lepidoptera: Sesiidae), to candidate pheromone chemicals. J Appl Ent. 1986;102:195–210.
- Quero C, Bau J, Guerrero A, Renou M. Responses of the olfactory receptor neurons of the corn stalk borer Sesamia nonagrioides to components of the pheromone blend and their inhibition by trifluoromethyl ketone analogue. Pest Manag Sci. 2004;60:719–726. [PubMed: 15260305]
- Redkozubov A. Elementary receptor currents elicited by a single pheromone molecule exhibit quantal composition. Pfluegers Arch: Eur J Physiol. 2000;440:896–901. [PubMed: 11041556]
- Renou M, Guerrero A. Insect parapheromones in olfaction research and semiochemicalbased pest control strategies. Annu Rev Entomol. 2000;45:605–630. [PubMed: 10761591]
- Renou M, Berthier A, Guerrero A. Disruption of responses to pheromone by (Z)-11-hexadecenyl trifluoromethyl ketone, an analogue of the pheromone, in the cabbage armyworm Mamestra brassicae. Pest Manag Sci. 2002;58:839–844. [PubMed: 12192910]
- Rogers M, Sun M, Lerner M.R, Vogt R.G. Snmp-1, a novel membrane protein of olfactory neurons of the silk moth Antheraea polyphemus with homology to the CD36 family of membrane proteins. J Biol Chem. 1997;272:14792–14804. [PubMed: 9169446]
- Rogers M.E, Steinbrecht R.A, Vogt R.G. Expression of SNMP-1in olfactory neurons and sensilla of male and female antennae of the silkmoth Antheraea polyphemus. Cell Tissue Res. 2001a;303:433–446. [PubMed: 11320659]
- Rogers M.E, Krieger J, Vogt R.G. Antennal SNMPs (sensory neuron membrane proteins) of Lepidoptera define a unique family of invertebrate CD36-like proteins. J Neurobiol. 2001b;49:47–61. [PubMed: 11536197]
- Rospars J.P, Lucas P, Coppey M. Modelling the early steps of transduction in insect olfactory receptor neurons. Biosystems. 2007;89:101–109. [PubMed: 17284344]
- Rumbo E.R, Kaissling K.E. Temporal resolution of odour pulses by three types of pheromone receptor cells in Antheraea polyphemus. J Comp Physiol A. 1989;165:281–291.
- Rybczynski R, Vogt R.G, Lerner M.R. Antennal-specific pheromone-degrading aldehyde oxidases from the moths Antheraea polyphemus and Bombyx mori. J Biol Chem. 1990;32:19712–19715. [PubMed: 2246254]
- Sadek M.M, Hansson B.S, Rospars J.P, Anton S. Glomerular representation of plant volatiles and sex pheromone components in the antennal lobe of Spodoptera littoralis. J Exp Biol. 2002;205:1363–1376. [PubMed: 11976349]
- Sakurai T, Nakagawa T, Mitsuno H, Mori H, Endo Y, Tanoue S, Yasukochi Y, Touhura K, Nishioka T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc Natl Acad Sci U S A. 2004;101:16653–16658. [PMC free article: PMC528734] [PubMed: 15545611]
- Sakurai T, Mitsuno H, Haupt S.S, Uchino K, Yokohari F, Nishioka T, Kobayashi I, Sezutsu H, Tamura T, Kanzaki R. A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoSGenet. 2011;7(6):e1002115. [PMC free article: PMC3128102] [PubMed: 21738481]
- Sandler B.H, Nikonova L, Leal W.S, Clardy J. Sexual attraction in the silkworm moth: structure of the pheromone-binding-protein-bombykol complex. Chem Biol. 2000;7:143–151. [PubMed: 10662696]
- Sato K, Pellegrino M, Nakagawa T, Vosshall L.B, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. [PubMed: 18408712]
- Scaloni A, Monti M, Angeli S, Pelosi P. Structural analysis and disulfide-bridge pairing of two odorant-binding proteins from Bombyx mori. Biochem Biophys Res Commun. 1999;266:386–391. [PubMed: 10600513]
- Schneider D. Electrophysiological investigation of the antennal receptors of the silk moth during chemical and mechanical stimulation. Experientia. 1957;13:89.
- Schneider D. Insect olfaction—Our research endeavour. In: Dawson W.W, Enoch J.M, editors. In Foundations of Sensory Science. Berlin, Heidelberg: Springer-Verlag; 1984. pp. 381–481.
- Schneider D. 100 years of pheromone research, an essay on Lepidoptera. Naturwissenschaften. 1992;79:241–250.
- Schneider D, Kaissling K.E. Der Bau der Antenne des Seidenspinners Bombyx mori L. II. Sensillen, cuticulare Bildungen und innerer Bau. Zool Jahrb Anat Ontog. 1957;76:224–250.
- Schneider D, Lacher V, Kaissling K.E. Die Reaktionsweise und das Reaktionsspektrum von Riechzellen bei Antheraea pernyi (Lepidoptera, Saturniidae). Z vergl Physiol. 1964;48:632–662.
- Schneider D, Schulz S, Priesner E, Ziesmann J, Francke W. Autodetection and chemistry of female and male pheromone in both sexes of the tiger moth Panaxia quadripunctaria. J Comp Physiol A. 1988;182:153–161.
- Scholes J. Discontinuity of the excitation process in locust visual cells. Cold Spring Harbour Symp Quant Biol. 1965;30:517–527. [PubMed: 5219500]
- Stange G, Diesendorf M. The response of the honey bee antennal CO2-receptors to N2O and Xe. J Comp Physiol. 1973;86:139–158.
- Stange G, Kaissling K.E. The site of action of general anaesthetics in insect olfactory receptor neurons. Chem Senses. 1995;20:421–432. [PubMed: 8590027]
- Stange G, Stowe S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc Res Tech. 1999;47:416–427. [PubMed: 10607381]
- Steinbrecht R.A. Feinstruktur und Histochemie der Sexualduftdrüse des Seidenspinners Bombyx mori. Z Zellforsch. 1964;64:227–261.
- Steinbrecht R.A. Zur Morphometrie der Antenne des Seidenspinners, Bombyx mori L.: Zahl und Verteilung der Riechsensillen (Insecta, Lepidoptera). Z Morph Tiere. 1970;66:93–126.
- Steinbrecht R.A. Der Feinbau olfaktorischer Sensillen des Seidenspinners (Insecta, Lepidoptera). Rezeptorfortsätze und reizleitender Apparat. Z Zellforsch. 1973;139:533–565. [PubMed: 4724530]
- Steinbrecht R.A. Chemo-, hygro-, and thermoreceptors. In: Bereiter-Hahn J, Matoltsy A.G, Richards K.S, editors. In Biology of the Integument. Vol. 1. Berlin, Heidelberg: Springer Verlag; 1984. pp. 523–553.
- Steinbrecht R.A. Are odorant-binding proteins involved in odorant discrimination? Chem Senses. 1996;21:719–727. [PubMed: 8985600]
- Steinbrecht R.A. Pore structures in insect olfactory sensilla—A review of data and concepts. Int J Insect Morphol Embryol. 1997;26:229–245.
- Steinbrecht R.A. Olfactory receptors. In: Eguchi E, Tominaga Y, editors. In Atlas of Arthropod Sensory Receptors: Dynamic Morphology in Relation to Function. Tokyo: Springer Verlag; 1999. pp. 155–176.
- Steinbrecht R.A, Kasang G. Capture and conveyance of odour molecules in an insect olfactory receptor. In: Schneider D, editor. In Int Symp Olfaction and Taste IV. Stuttgart: Wiss Verlagsgesellsch; 1972. pp. 193–199.
- Steinbrecht R.A, Zierold K. Electron probe X-ray microanalysis in the silkmoth antenna—Problems with quantification in ultrathin cryosections. In: Zierold K, Hagler H.K, editors. In Electron Probe Microanalysis—Applications in Biology and Medicine. Berlin, Heidelberg: Springer Verlag; 1989. pp. 87–97.
- Steinbrecht R.A, Ozaki M, Ziegelberger G. Immunocytochemical localization of pheromone-binding protein in moth antennae. Cell Tissue Res. 1992;270:287–302.
- Steinbrecht R.A, Laue M, Ziegelberger G. Immunolocalization of pheromone-binding protein and general odorant-binding protein in olfactory sensilla of the silk moths Antheraea and Bombyx. Cell Tissue Res. 1995;282:203–217.
- Steinbrecht R.A, Stankiewicz B.A. Molecular composition of the wall of insect olfactory sensilla—The chitin question. J Insect Physiol. 1999;45:785–790. [PubMed: 12770310]
- Stengl M. Pheromone transduction in moths. Front Cell Neurosci. 2010;4:1–15. [PMC free article: PMC3018772] [PubMed: 21228914]
- Stengl M, Ziegelberger G, Boekhoff I. Perireceptor events and transduction mechanisms in insect olfaction. In: Hansson B.S, editor. In Insect Olfaction. Berlin: Springer Verlag; 1999. pp. 49–66.
- Stranden M, Røstelien T, Liblikas I, Almaas T.J, Borg-Karlson A.K, Mustaparta H. Receptor neurones in three heliothine moths responding to floral and inducible plant volatiles. Chemoecology. 2003;13:143–154.
- Stuiver M. Groningen: 1958. Biophysics of the sense of smell. Thesis Rijksuniversiteit.
- Syed Z, Ishida Y, Taylor K, Kimbrell D.A, Leal W.S. Pheromone reception in fruit flies expressing a moth’s odorant receptor. Proc Natl Acad Sci U S A. 2006;103:16538–16543. [PMC free article: PMC1621046] [PubMed: 17060610]
- Tegoni M, Campanacci V, Cambillau C. Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem Sci. 2004;29:257–264. [PubMed: 15130562]
- Thurm U, Küppers J. Epithelial physiology of insect sensilla. In: Locke M, Smith D.S, editors. In Insect Biology in the Future. New York: Academic Press; 1980. pp. 735–763.
- Todd J.L, Baker T.C. Function of peripheral olfactory organs. In: Hansson B.S, editor. In Insect Olfaction. Berlin: Springer Verlag; 1999. pp. 67–96.
- Trona F, Anfora G, Balkenius A, Bengtsson M, Tasin M, Knight A, Janz N, Witzgall P, Ignell R. Neural coding merges sex and habitat chemosensory signals in an insect herbivore. Proc R Soc B. 2013;280:1760. [PMC free article: PMC3652457] [PubMed: 23595270]
- Van den Berg M.J, Ziegelberger G. On the function of the pheromone-binding protein in the olfactory hairs of Antheraea polyphemus. J Insect Physiol. 1991;37:79–85.
- Vareschi E. Duftunterscheidung bei der Honigbiene—Einzelzellableitungen und Verhaltensreaktionen. Z Vergl Physiol. 1971;75:143–173.
- Vareschi E, Kaissling K.E. Dressur von Bienenarbeiterinnen und Drohnen auf Pheromone und andere Duftstoffe. Z vergl Physiol. 1970;66:22–26.
- Vermeulen A, Rospars J.P. Electrical circuitry of an insect olfactory sensillum. Neurocomputing. 2001;29:587–596. [PubMed: 11288834]
- Vijverberg H.P.M, van der Zalm J.M, van den Bercken J. Similar mode of action of pyrethroids and DDT on sodium channel gating in myelinated nerves. Nature (Lond.). 1982;295:601–603. [PubMed: 6276777]
- Vogel S. How much air passes through a silkmoth antenna? J Insect Physiol. 1983;29:597–602.
- Vogt R.G. Biochemical diversity of odor detection: OBPs, ODEs and SNMPs. In: Blomquist G.J, Vogt R.G, editors. In Insect Pheromone Biochemistry and Molecular Biology. London: Elsevier Academic Press; 2003. pp. 391–446.
- Vogt R.G. Molecular basis of pheromone detection in insects. In: Gilbert L.I, Iatro K, Gill S, editors. In Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology Volume 3. Endocrinology. London: Elsevier; 2005. pp. 753–804.
- Vogt R.G, Riddiford L.M. Pheromone binding and inactivation by moth antennae. Nature (London). 1981;293:161–163. [PubMed: 18074618]
- Vogt R.G, Riddiford L.M. Pheromone reception: A kinetic equilibrium. In: Payne T.L, Birch M, Kennedy C.E.J, editors. In Mechanisms in Insect Olfaction. Oxford: Clarendon Press; 1986a. pp. 201–208.
- Vogt R.G, Riddiford L.M. Scale esterase: A pheromone degrading enzyme from the wing scales of the silk moth Antheraea polyphemus. J Chem Ecol. 1986b;12:469–482. [PubMed: 24306791]
- Vogt R.G, Riddiford L.M, Prestwich GD. Kinetic properties of a pheromone degrading enzyme: The sensillar esterase of Antheraea polyphemus. Proc Natl Acad Sci U S A. 1985;82:8827–8831. [PMC free article: PMC391531] [PubMed: 3001718]
- Vogt R.G, Callahan F.E, Rogers M.E, Dickens J.C. Odorant binding protein diversity and distribution among the insect orders, as indicated by LAP, an OBP-related protein of the true bug Lygus lineolaris (Hemiptera, Heteroptera). Chem Senses. 1999;24:481–495. [PubMed: 10576256]
- Von Nickisch-Rosenegk E, Krieger J, Kubick S, Laage R, Strobel J, Strotmann J, Breer H. Cloning of biogenic amine receptors from moth (Bombyx mori and Heliothis virescens). Insect Biochem Mol Biol. 1996;26:817–827. [PubMed: 9014328]
- Vosshall L.B. The molecular logic of olfaction in Drosophila. Chem Senses. 2001;26:207–213. [PubMed: 11238253]
- Vosshall L.B, Hansson S.B. A unified nomenclature system for the insect olfactory coreceptor. Chem Senses. 2011;36:497–498. [PubMed: 21441366]
- Wicher D, Schaefer R, Bauernfeind R, Stensmyr M.C, Heller R, Heinemann S.H, Hansson B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. [PubMed: 18408711]
- Wojtasek H, Leal W.S. Conformational change in the pheromone-binding protein from Bombyx mori induced by pH and by interaction with membranes. J Biol Chem. 1999;274:30950–30956. [PubMed: 10521490]
- Xu P.X, Atkinson R, Jones D.N.M, Smith D.P. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45:193–200. [PubMed: 15664171]
- Xu P.X, Hooper A.M, Pickett J.A, Leal W.S. Specificity determinants of the silkworm moth sex pheromone. PLoS ONE. 2012;7(9):e44190. [PMC free article: PMC3434217] [PubMed: 22957053]
- Xu W, Leal W.S. Molecular switches for pheromone release from a moth pheromone-binding protein. Biochem Biophys Res Commun. 2008;372:559–564. [PubMed: 18503757]
- Zack C.W.M. Sensory adaptation in the sex pheromone receptor cells of saturniid moths. 1979 Diss Fak Biol LMU München.
- Zack-Strausfeld C, Kaissling K.E. Localized adaptation processes in olfactory sensilla of Saturniid moths. Chem Senses. 1986;11:499–512.
- Zhang S.G, Maida R, Steinbrecht R.A. Immunolocalization of odorant-binding proteins in noctuid moths (Insecta, Lepidoptera). Chem Senses. 2001;26:885–896. [PubMed: 11555483]
- Zhou J.J, Robertson G, He X, Dufour S, Hooper A.M, Pickett J.A, Keep N.H, Field L.M. Characterisation of Bombyx mori odorant-binding proteins reveals that a general odorant-binding protein discriminates between sex pheromone components. J Mol Biol. 2009;389:529–545. [PubMed: 19371749]
- Zhou Y, Wilson R.I. Transduction in Drosophila olfactory receptor neurons is invariant to air speed. J Neurophysiol. 2012;108:2051–2059. [PMC free article: PMC3544999] [PubMed: 22815404]
- Ziesmann J, Valterova I, Haberkorn K, De Brito Sanchez M.G, Kaissling K.E. Chemicals in laboratory room air stimulate olfactory neurons of female Bombyx mori. Chem Senses. 2000;25:31–37. [PubMed: 10667991]
- Zufall F, Hatt H. Dual activation of sex pheromone-dependent ion channel from insect olfactory dendrites by protein kinase C activators and cyclic GMP. Proc Natl Acad Sci U S A. 1991;88:8520–8524. [PMC free article: PMC52540] [PubMed: 1717980]
- Zufall F, Hatt H, Keil T.A. A calcium-activated nonspecific cation channel from olfactory receptor neurons of the silkmoth Antheraea polyphemus. J Exp Biol. 1991;161:455–468. [PubMed: 22141156]
- INTRODUCTION
- ASPECTS OF PHEROMONE COMMUNICATION
- INSECT ANTENNAE AND OLFACTORY SENSILLA
- ELECTROPHYSIOLOGY
- SENSITIVITY OF PHEROMONE RECEPTOR NEURONS AND BEHAVIORAL RESPONSES
- ELEMENTARY RECEPTOR POTENTIALS
- INHIBITION OF PHEROMONE RECEPTOR NEURONS
- CONCENTRATION DETECTORS AND FLUX DETECTORS
- OLFACTORY TRANSDUCTION, EXTRACELLULAR
- DIFFUSION ON THE HAIRS
- KINETIC MODEL
- FIVE FUNCTIONS OF THE PHEROMONE BINDING PROTEIN
- PHEROMONE DEGRADATION AND DEACTIVATION
- RECEPTOR MOLECULES, ION CHANNELS, AND SENSORY NEURON MEMBRANE PROTEIN
- OLFACTORY TRANSDUCTION, INTRACELLULAR
- TEMPORAL CODING
- ACKNOWLEDGMENTS
- REFERENCES
- Review Introduction to Chemical Signaling in Vertebrates and Invertebrates.[Neurobiology of Chemical Commu...]Review Introduction to Chemical Signaling in Vertebrates and Invertebrates.Wyatt TD. Neurobiology of Chemical Communication. 2014
- Pheromone-binding proteins contribute to the activation of olfactory receptor neurons in the silkmoths antheraea polyphemus and Bombyx mori.[Chem Senses. 2004]Pheromone-binding proteins contribute to the activation of olfactory receptor neurons in the silkmoths antheraea polyphemus and Bombyx mori.Pophof B. Chem Senses. 2004 Feb; 29(2):117-25.
- Antennal-specific pheromone-degrading aldehyde oxidases from the moths Antheraea polyphemus and Bombyx mori.[J Biol Chem. 1990]Antennal-specific pheromone-degrading aldehyde oxidases from the moths Antheraea polyphemus and Bombyx mori.Rybczynski R, Vogt RG, Lerner MR. J Biol Chem. 1990 Nov 15; 265(32):19712-5.
- Review Pheromones and General Odor Perception in Insects.[Neurobiology of Chemical Commu...]Review Pheromones and General Odor Perception in Insects.Renou M. Neurobiology of Chemical Communication. 2014
- Review The Synthetic Moth: A Neuromorphic Approach toward Artificial Olfaction in Robots.[Neuromorphic Olfaction. 2013]Review The Synthetic Moth: A Neuromorphic Approach toward Artificial Olfaction in Robots.Vouloutsi V, Lopez-Serrano LL, Mathews Z, Escuredo Chimeno A, Ziyatdinov A, Perera i Lluna A, Bermúdez i Badia S, Verschure PFMJ. Neuromorphic Olfaction. 2013
- Pheromone Reception in Insects - Neurobiology of Chemical CommunicationPheromone Reception in Insects - Neurobiology of Chemical Communication
Your browsing activity is empty.
Activity recording is turned off.
See more...
