NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Ca2+ is an important signal ion in photoreceptors for recovery after excitation and light adaptation. It enters the outer segment as a minor fraction of the dark current through cGMP-gated channels and is extruded in the same cell compartment by Na/Ca2+, K exchange. Channel and exchanger are located exclusively in the plasma membrane, but not in the cytoplasmic membrane stack, the discs, which contain the visual pigment rhodopsin. The channel consists presumably of two α-subunits and two β-subunits, whereas the exchanger is a monomeric protein. Recently, considerable evidence has been accumulated indicating that both proteins form a complex which is bound to peripherin/Rds, an integral protein of the disc rim. This review focuses on the complex of cGMP-gated channel and Na/Ca2+, K exchanger. The possibility of direct functional interaction between channel and exchanger is discussed. Furthermore, the consequences of different subunit arrangements of the channel for the channel-exchanger complex are considered. Finally, a Ca2+ diffusion model is presented which examines the possibility that Ca2+ currents are locally restricted to the close vicinity of the channel.
Introduction
Vertebrate photoreceptors constitute an impressive paradigm of ultimate functional performance: the sensitivity of these cells reaches the physical limit of single photon detection.1,2 A key feature to understand this amazing performance appears to be the high degree of structural organization: outer segment, inner segment, nerve axon, and synaptic terminal are distinctly separated parts of these cells (Fig. 1). The primary task of photon detection and conversion into a voltage signal is exclusively located in the outer segment, whereas the rest of the cell is basically a normal nerve cell.
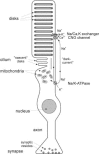
Figure 1
Rod photoreceptor, schematic representation.
The vertebrate retina comprises two different types of photoreceptors, rods and cones. There is general agreement that the basic molecular mechanism of excitation of both classes of photoreceptors is similar. We concentrate on rod photoreceptors, the prevalent class of photoreceptors which was most thoroughly investigated. The rod outer segment consists of a stack of several hundred flattened sacks, the so-called discs, surrounded by the cell membrane (Fig. 1). The discs are physically separated from the cell membrane and contain the light-absorbing visual pigment, rhodopsin.
The ion conductance of the cell membrane of the rod outer segment is conspicuously low. The only ion channels are cGMP-gated channels which mediate a standing cation influx in the nonexcited photoreceptor. This so-called dark-current consists mainly of Na ions, and to a lesser extent of Ca2+ ions. Na ions diffuse through the rod outer segment before they are exported by the Na/K pump in the inner segment. In contrast, Ca2+ ions are exported in the rod outer segment by Na/Ca2+, K exchange. Therefore, the Ca2+ flux controlling the intracellular Ca2+ of the rod outer segment is restricted to this cell compartment.
Light stimulation of the photoreceptor leads to hydrolysis of cGMP resulting in deactivation of cGMP-gated channels and abatement of the dark current.3-5 Because Ca2+ extrusion by Na/Ca2+, K exchange continues, the cytoplasmic Ca2+ decreases. This light-induced drop of intracellular Ca2+ constitutes a major signal for recovery and adaptation following light exposure.6 At maximal light excitation, all channels are closed and the cytoplasmic Ca2+ concentration decreases to about 10-50 nM.7-11
Several Ca2+-dependent responses of rod photoreceptors have been described; however, the underlying molecular mechanism has not been identified for all of them.6,12 Most Ca2+ signaling mechanisms in photoreceptors are indirect, mediated by the Ca2+ binding proteins calmodulin, recoverin, and guanylate cyclase activating proteins (GCAPs).13-16 While only a small influence of calmodulin has been observed in the electrophysiological response of rod photoreceptors, recoverin and GCAPs play important roles in Ca2+ signaling.17-21 Both proteins are involved in the recovery of the resting state after light excitation: GCAPs regulate guanylate cyclases and recoverin appears to control the activity of the rhodopsin kinase. However, the physiological relevance involving recoverin is still controversial.22 Calmodulin, recoverin and GCAPs are members of a large family of Ca2+ binding proteins containing one or more EF-hand Ca2+ binding sites.23
Generally, Ca2+-dependent pathways in the outer segment of the rod photoreceptor are considered to be controlled by the overall intracellular Ca2+ concentration. This view is supported by the fact that there is a steady Ca2+ ion influx in the dark which is reduced upon light excitation, being fully shut off upon maximal excitation. For this reason, there is sufficient time for thermal equilibration to take place. This does not necessarily mean that the cytoplasmic Ca2+ concentration is spatially constant. In the presence of several concurrent Ca2+ regulatory steps, these regulatory mechanisms would be coupled if there would be only a constant intracellular Ca2+ concentration.
However, different Ca2+ signaling processes are often uncoupled in cells by local changes of the Ca2+ concentration. This may also be the case for photoreceptors. Ca2+ ions entering the rod outer segment in the resting phase through the cGMP-gated channel may create a local elevation of the Ca2+ concentration. Similarly, Ca2+-dependent processes may experience a different Ca2+ concentration at the plasma membrane than at the disc membrane. Even the Ca2+ concentration at the plasma membrane may not be homogeneous, changing with the distance to the channel and the Na/Ca2+, K exchanger.
These considerations indicate that not only the molecular components must be identified to understand Ca2+ signaling in photoreceptors, but the ultrastructure must be understood, as well. It is the purpose of this chapter to address this issue, and to review the characteristics of disc and plasma membrane. In particular, ultrastructural aspects of the cGMP-gated channel and the Na/Ca2+, K exchanger, which constitute source and sink of the intracellular Ca2+ in the rod outer segment, will be considered. Finally, the relevance of the occurrence of local Ca2+ phenomena will be discussed.
Channel and Na/Ca2+, K Exchanger Reside Only in the Plasma Membrane
In rod photoreceptors, discs are continually renewed at the base (the cilium) and are shed at the tip where they become phagocytosed in the pigment epithelium.24 The discs are initially contiguous with the plasma membrane (nascent discs) and during maturation become eventually separated from the plasma membrane.25 The major integral protein of both membranes is rhodopsin which makes up 90 percent of the disc and 60 percent of the plasma membrane proteins. Rhodopsin is structurally and functionally identical in both membranes.26
In spite of this common feature, there are distinct differences between these two membranes. Disc membranes consist of a flat lamellar region of two opposed membranes circumscribed by a more bulgy rim region. Rhodopsin is highly concentrated in the lamellar region of the disc but not in the rim region.27 This special region of the discs contains rim-specific proteins including peripherin/Rds and Rom-1 which serve structural purposes,28-30 and the 220-kDa glycoprotein ABCR, a member of the ATP binding cassette transporters.31-34 Deep infoldings of the disc rim, incisures, constitute a characteristic feature which is particularly distinct in the large photoreceptors of amphibians. Immunohistochemically, these incisures can be labeled with antibodies against the glutamic acid-rich protein (GARP) and with antibodies against tubulin.35,36 Moreover, the lipid composition of the two membranes is markedly different: disc membranes contain 35 percent of a lipid with highly unsaturated fatty acid side chains, whereas the plasma membrane contains only 5 percent of this lipid.37 The presence of highly unsaturated lipids in the disc membranes results in high lateral and rotational diffusion constants of rhodopsin, and presumably also in specific hydrophobic interactions of membrane associated proteins.38-40 Recently, it has been found in transgenic mice that the high rhodopsin concentration in disc membranes actually leads to a reduction of the rhodopsin diffusion due to protein collisions.41
The plasma membrane also contains several nonrhodopsin molecules which are not present in the disc membranes.42 Most remarkably, the ion permeability of disc and plasma membrane is completely different. Initially, this became clear from Ca2+ flux studies of bovine ROS membrane vesicles which indicated that only about 6 percent of the vesicle contained cGMP-gated channels and Na/Ca2+, K exchangers whereas the remaining vesicle fraction didn't contain either of these proteins.43 One estimates that in bovine rod outer segments, about 6 percent of the total membrane area is plasma membrane (based on the fraction 2·d/D where d denotes the interdisc spacing and D the diameter of the bovine ROS with D = 1 μm, d = 0.03 μm). Since the plasma membrane contains cGMP-gated channels and Na/Ca2+, K exchanger, this finding indicates that these proteins are virtually absent in disc membranes.43
This conclusion was corroborated further by immunohistochemical identification of cGMP-gated channel in the plasma membranes but not in disc membranes of lysed ROS membranes.44 Moreover, cGMP-induced Ca2+ fluxes were only observed with purified plasma but not with disc membrane vesicles. Similarly, the Na/Ca2+, K exchanger was identified as the main ricin-binding protein of the plasma membrane which permitted the biochemical separation of the plasma membrane from the disc membrane.45 Fluorescein-conjugated cGMP also indicated a high density of cGMP binding sites at the cell membrane but not in the disc space of toad rod photoreceptors.46
Together, these results indicate that there is a clear functional organization in the rod outer segment. Cation fluxes occur only through the cGMP-gated channels located in the plasma membrane, but there is no evidence for ion fluxes through the disc membrane. Disc membranes contain most of the light absorbing pigment rhodopsin and provide the appropriate membrane surface for association of the proteins of the visual transduction cascade. Regarding the steady renewal of disc and plasma membranes at the base of the ROS, experimental evidence has been reported that the disc rim protein peripherin/Rds undergoes light-dependent phosphorylation and may be involved in membrane fusion.47 However, the efficient sorting process which leads to the generation of these two completely different membranes is still largely unknown and remains to be clarified further.
The Density of cGMP-Gated Channels
Several attempts have been undertaken to estimate the density of cGMP-gated channels localized in the plasma membrane of rod outer segments. Initially, noise analysis of the dark current of amphibian rod photoreceptors was extensively carried out.48-51 The dark current of the outer segment exhibits considerable high frequency noise which decreases upon light absorption. Assuming that this noise is generated only by one class of channels which fluctuate independently of each other between an open and a closed state it was estimated that 5000 to 10,000 channels are simultaneously open in the dark. Because only about 5 percent or less of the channels are activated under these conditions, a channel density of at least 100-200 channels per μm2 was estimated. These studies were carried out in the presence of Ca2+ ions which partially block the cGMP-gated channel by binding to the pore region.52 Therefore, the unitary conductance of the channel was in the range of about 100 fS, far too small to be detectable as single channel conductance.
In the absence of divalent ions, the single channel conductance increases to 25 pS, i.e., by more than two orders of magnitude and can readily be determined in patch clamp experiments.53 The number of channels in a patch was calculated directly as the ratio of the maximal ion current and the single channel current. The channel density of excised patches of rod outer segment membrane was determined to be 200-600 channels/μm2, in reasonable agreement with the noise analysis data.53-55 Remarkably, a wide variation of channel densities was observed in these studies which was inconsistent with a random distribution of channels over the plasma membrane.55 No evidence for a distinct channel clustering was detected; however, some physical stress during membrane excision could not be excluded if the channels interacted with the cytoskeleton or directly with the disc membrane. In fact, recent evidence clearly indicates that the GARP domain of the cyclic GMP-gated channel binds to peripherin/Rds, an integral membrane protein of the disc rim.56
When compared to amphibians, warm-blooded vertebrates have very tiny photoreceptors which limit the possibility of electrophysiological studies considerably. Fortunately, the channel density of bovine rod photoreceptors could be estimated biochemically.57 Starting with purified rod outer segments, the membrane proteins were solubilized in the presence of Ca2+ and functionally reconstituted into artificial lipid vesicles after the detergent was removed by dialysis. The Ca2+ flux assay indicated that only a small fraction of lipid vesicles contained cGMP-gated channels suggesting that this fraction contained only one channel per vesicle. From the volume fraction of lipid vesicles and the rhodopsin concentration, a channel density of 600 channels/μm2 was calculated based on the assumption that 3 percent of the membrane area was plasma membrane. If this percentage was assumed to be about 6 percent,43 then the channel density would be estimated to be about 300 channels/μm2, assuming that all channels were still functionally active after solubilization and reconstitution.
The channel density of bovine ROS plasma membranes was also estimated without solubilization of the membranes. Ca2+ flux experiments indicated that only a small fraction of ROS membrane vesicles contained cGMP-gated channels.43 However, if these membranes were subjected to a single freeze-thaw cycle before preparation of membrane vesicles, the channel-containing fraction increased almost ten-fold.58 Because freeze-thawing often leads to membrane fusion, this observation suggested that both disc and plasma membranes became fused and the cGMP-gated channels, which were initially only in the plasma membrane, became distributed over a much larger membrane area. If small vesicles were prepared by prolonged sonication of the fused ROS membranes, there were more vesicles than channel molecules. Assuming that the channels were randomly distributed over the vesicles (i.e., not clustered), a mean density of 0.37 channels per vesicle was obtained for vesicles with a mean diameter of 0.12 nm.58 These data yielded a channel density of about 16 channels per μm2 for fused membranes, corresponding to about 270 channels per μm2 in the plasma membrane, in excellent agreement with the channel density estimated by Cook et al.57
Together, these very different experimental approaches led to conspicuously similar values of the channel density between 200 and 600 channels per μm2 of plasma membrane, irrespective of the animal species. This is rather remarkable, considering that the volumes of the amphibian and the bovine ROS differ by more than two orders of magnitude.
The Density of Na/Ca2+, K Exchangers: An Intriguing Problem
Similar to the cardiac Na/Ca2+ exchanger, the retinal Na/Ca2+, K exchanger is electrogenic, and therefore, can be investigated electrophysiologically.59-62 In bright light, all cGMP-dependent channels of the photoreceptor are closed, and the exchange current can be recorded in the absence of any other interfering ion current. Using this approach, salamander rod photoreceptors were loaded in the dark with various amounts of Ca2+, and the kinetics of Ca2+ export studied after closing the light-sensitive channels.60-62 The maximal exchange current was proportional to the total number of exchangers present. A rigorous quantitative analysis of the Ca2+ transport kinetics, based on a Michaelis-type Ca2+-binding isotherm in the presence of a low-affinity Ca2+ buffer60, yielded a maximal turnover rate of 10Ca2+/s/exchanger and a very high exchanger density of 35,000 μm−2. However, the latter value which is even greater than the rhodopsin density, seems unlikely. Moreover, the maximal turnover rate seems too low. Nevertheless, this analysis is instructive because it suggests that a simple kinetic model based on overall Ca2+ concentrations does not appropriately describe the molecular transport mechanism.
Purification of the exchanger protein yielded a single polypeptide with an apparent molecular mass of 220 kDa which made up about 0.1 percent of the total protein mass of ROS membranes.63,64 Assuming that the exchanger was only localized in the plasma membrane, an exchanger density of 600-1000 μm−2 was estimated from these data. This value is somewhat greater than the channel density and in agreement with Ca2+ flux data from proteoliposomes.63
Moreover, we found that upon reconstitution of the ROS membrane proteins in the presence of increasing amounts of lipid, the ratio of the maximal Na-induced and the cGMP-induced Ca2+ release increased in parallel up to about four (see Fig. 1, ref. 58). Assuming random orientation, only half of the channels could be activated with cGMP, whereas the exchanger would mediate Ca2+ flux regardless of orientation. We concluded from these findings that the exchanger outnumbered the channel by a factor of two.
Surprisingly, channel and exchanger appeared to be associated after reconstitution of ROS membrane proteins into lipid vesicles.58 This was suggested by the outcome of a competition experiment, where in the same sample cGMP-dependent and Na-dependent Ca2+ releases were induced consecutively. If channel and exchanger would be on different vesicle fractions, then the amplitudes of the Ca2+ release should not depend on the order of addition of cGMP and Na. However, this was not observed experimentally (Fig. 2). When cGMP was added first and then Na, both agents elicited a distinct Ca2+ release, but when cGMP was added after a saturating Na induced Ca2+ release, little or no cGMP-induced Ca2+ release was observed. This finding indicated that vesicles containing a channel also contained at least one exchanger molecule. Because channel and exchanger constitute only minor protein components in the ROS membranes, this finding suggested that both proteins form a complex in the plasma membrane. This suggestion will be further examined in the following sections.
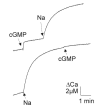
Figure 2
Ca2+ flux from ROS proteoliposomes. Na (100 mM) and cGMP (100 μM) was added at the indicated times. Ca2+ was monitored photometrically at 652nm with the Ca2+ indicator arsenazo III.
Interaction Between Solubilized Channel and Exchanger
The cGMP-gated channels of rod photoreceptors bind Ca2+-calmodulin with very high affinity.13,14 Based on this characteristic property, the cGMP-gated channel could be purified in a single step using calmodulin affinity chromatography13. The nonbound fraction contained rhodopsin and other membrane proteins, but not the channel. The bound fraction, which was eluted at zero free Ca2+, contained the channel, but some exchanger co-eluted with the channel, as well.65 Even a second calmodulin affinity chromatographic run did not fully remove the exchanger, suggesting that the exchanger was specifically bound to the channel. In agreement with this finding, it was recently reported that purification of the cGMP-gated channel by immuno-affinity chromatography co-purified the exchanger under some conditions.66
In contrast to the cardiac-type Na/Ca2+ exchanger, the retinal Na/Ca2+, K exchanger does not contain a putative calmodulin binding site and, therefore, cannot bind to a calmodulin affinity column.67-69 In fact, we did not observe any binding of the purified retinal Na/Ca2+, K exchanger to a calmodulin affinity column. The finding that purification of the channel either by calmodulin affinity or immunoaffinity chromatography co-purified part of the Na/Ca2+, K exchanger indicated that the channel has a specific binding affinity for the exchanger. This idea was examined more directly in a Western blot of the purified channel (Fig. 3). After incubation of this blot with purified exchanger protein, monoclonal antibodies against the exchanger identified distinctly the band of the α-subunit, but not of the β-subunit of the channel. To enhance the detection sensitivity, the exchanger was biotinylated to enable its detection also by streptavidin binding. Horseradish peroxidase conjugated streptavidin detected also a distinct band at the location of the channel α-subunit, indicating that the exchanger specifically bound to the α-subunit, but not to the β-subunit of the channel.
Association of Channel and Exchanger in the Plasma Membrane
The preceding section indicated that the solubilized exchanger binds to the α-subunit of the cGMP-gated channel. However, this does not necessarily mean that this is also the case in vivo. In fact, many membrane proteins, including rhodopsin and the cGMP-gated channel, tend to aggregate when solubilized. Therefore, we addressed the questions: does the exchanger bind to the channel in the plasma membrane? If so, how many exchangers bind to one channel?
A suitable technique to address these questions is covalent cross-linking of adjacent proteins. Thiol-specific cross-linking appeared to be particularly suited because both channel and exchanger contain only a few cysteines. Therefore, the probability of accidental cross-links with non-associated proteins, or intramolecular cross-links was small.
Covalent modification of ROS membranes with thiol-specific bivalent reagents yielded cross-links of the exchanger as well as cross-links of the β-subunit, but no cross-links of the α-subunit of the channel. We investigated thoroughly the cross-links of the exchanger.70 The main cross-link band of the exchanger had an apparent molecular mass of 490 kDa, which was about twice the molecular mass of the monomeric exchanger. Under certain conditions, the exchanger could be almost completely cross-linked to the 490 kDa band. Purification of the cross-linked exchanger and subsequent cleavage of the cross-link indicated that the exchanger existed as a homodimer. The purified cross-linked exchanger indicated no accidental cross-linking between exchanger and rhodopsin, the latter being by far the prevalent protein in the plasma membrane. These findings led us to conclude that in the plasma membrane the exchanger molecules exist as dimers.70
In the absence of cGMP, thiol-specific reagents did not yield any cross-link between channel and exchanger. Realizing that both α- and β-subunit of the channel contain three cysteines in or near the cGMP-binding domains, we carried out thiol-specific cross-linking in the presence of cGMP. Figure 4 shows that cross-linking with dimaleimides in the presence of cGMP yielded indeed a strong dimer band of the α-subunit at about 130 kDa, whereas in the absence of cGMP only the monomer band at 63kDa was detected.65
Cross-linking in the presence of cGMP also yielded additional bands of the exchanger.65 These additional bands were observed at about 60 kDa greater molecular masses than the monomer and the dimer band of the exchanger, suggesting that these bands were due to cross-links between the α-subunit of the channel and the exchanger. In agreement with this suggestion purification of the cross-linked channel led to an enrichment of these cross-link bands of the exchanger whereas the other cross-link bands and the monomer band disappeared. Furthermore, chemical cleavage of the cross-links in the polyacrylamide gel and analysis by electrophoresis in a second polyacrylamide gel identified these bands as cross-links between the α-subunit and the exchanger.65
The β-subunit of the cGMP-gated channel was almost completely cross-linked with thiol-specific reagents to a host of adduct bands with apparent molecular masses up to about 700 kDa. However, we detected no cross-links between β-subunit and exchanger. No obvious difference was discernible between the cross-link band patterns of the β-subunit observed in the absence and in the presence of cGMP.65 Presumably, most of these bands originate from cross-links of the β-subunit with peripherin/Rds and/or cytoskeletal proteins.56
Different Subunit Arrangements of the Channel Entail Different Quaternary Structures
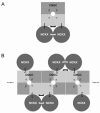
Summarizing this cross-linking study, we found that exchanger and channel are associated in the plasma membrane. Moreover, both the α-subunits of the channel and the exchanger can be cross-linked to homodimers. The simplest picture compatible with these findings is depicted in Figure 5A: an exchanger dimer is associated with a dimer of α-subunits of the channel. The cGMP-gated channel is assumed to consist of a dimer of α-subunits and a dimer of β-subunits, as suggested recently.71,72
It should be stressed that there is direct evidence of (at least) two α-subunits in the natural cGMP-gated channel of the rod photoreceptor; however, the evidence of two β-subunits is indirect. The β-subunit binds Ca2+-calmodulin with a Hill coefficient of 1.36, suggesting binding of two molecules of Ca2+-calmodulin per channel.14 However, this finding does not necessarily indicate the presence of two β-subunits in the channel, because two Ca2+-calmodulin binding sites have been identified on the β-subunit.73 A more direct indication of a β-subunit dimer derives from the recent finding that the GARP domain of the β-subunit interacts with peripherin/Rds of the disc membrane.56 Peripherin/Rds has a strong tendency to dimerize and to associate to higher oligomers.74 Therefore, it seems likely that the cGMP-gated channel of rod photoreceptors contains two β-subunits which are dimerized.
A different subunit arrangement of the cGMP-gated channel has recently been suggested by He et al.75 These authors conclude that like subunits of the channel are diagonally opposed. Assuming that only adjacent proteins can be cross-linked, our result would only be compatible with an αβαβ subunit arrangement if the channels were linearly assembled, as depicted in Figure 5B. Although channel clustering is a common phenomenon, clustering of cGMP-gated channels in photoreceptors has not been detected electrophysiologically in "loose-patch" recordings.55 Clustering of cGMP-gated channels was also not observed when the channel/exchanger complex was allowed to diffuse in a much larger membrane area by fusion of disc and plasma membrane:58 as pointed out above (see: "The density of cGMP-gated channels"), the results of this study indicated a random distribution of the channels in the membrane. Together, there is little evidence of clustering of the cGMP-gated channels in the plasma membrane of the photoreceptor.
What is the physiological meaning of the association of channel and exchanger? Two mutually non-exclusive hypotheses will be addressed in the following sections: (1), channel and exchanger influence each other functionally, and/or (2), local Ca2+ signaling occurs near the channel-exchanger complex.
Self-Inhibition of the Exchanger: An Allosteric Regulatory Mechanism?
The exchanger can only be studied functionally when the channel is closed. The question whether activation of the channel influences the exchanger activity cannot be examined experimentally. Nevertheless, activation of the channel might entail a small change in distance of the associated exchanger dimer influencing the exchanger activity.
We examined this hypothesis by studying the exchanger activity after cross-linking with thiol-specific reagents which differed in the length of the spacer arm. If cross-linking was carried out with dimaleimides there was only little variation in the cross-linking efficiency between different compounds. Surprisingly, cross-linking generally enhanced the exchanger activity.76
The enhancement of the activity correlated with the length of the spacer arm of the cross-linking reagent. Considering that only about 50 percent of the exchangers were cross-linked, the maximal enhancement of the exchange rate was estimated to be almost a factor of two. Only cross-linking by disulfide formation led to a reduction of the exchanger activity. These findings suggest that the contact sites of the exchanger dimer are inhibitory.
Local Ca2+ Signaling in Photoreceptors
Generally, microdomains of elevated Ca2+ are known to be generated for a short period of time in the close vicinity of Ca2+ channels during activation.77 However, the cGMP-gated channels of photoreceptors are continuously activated in the dark. The close apposition of Ca2+ source (the channel) and Ca2+ sink (the exchanger) hints at a novel mechanism which restricts part of the Ca2+ spatially to the close vicinity of the channel. In the following, the possibility of local Ca2+ currents in photoreceptors will be considered on the basis of a simple Ca2+ flux model.
To restrict a continuous Ca2+ current to a microdomain, two necessary conditions must be met: (1) the Ca2+ efflux must balance the Ca2+ influx (mass conservation), and (2), the diffusion kinetics of the fluxes be consistent with such a mechanism.
The following calculation shows that the first condition is met in amphibian rod photoreceptors. The plasma membrane area is about 1200 μm2 and the dark current is 25 pA, or 1.3·105 positive charges/μm2/s.78 Using the mean channel density of 650 channels/μm2 reported for the rod photoreceptor of salamander,55 this value corresponds to an ion flux of 200 positive charges/channel/s. Considering that only 15 percent of the dark current is carried by Ca2+ ions,79 this means that only 30 positive charges/channel/s, i.e., 15 Ca2+ ions/channel/s enter the rod outer segment. This value is considerably smaller than the maximal Ca2+ export rate of the exchanger of amphibians.60 For mammalian photoreceptors, the Ca2+ influx through the cGMP-gated channel is likely to be similar to that of amphibian photoreceptors. Therefore, the Ca2+ export by the two exchangers associated with the channel occurs far from saturation and can readily keep up with the Ca2+ influx through the channel.
Are the kinetics of intracellular Ca2+ diffusion compatible with the idea of a local Ca2+ current? To address this question, two Ca2+ concentration profiles will be considered: first, the stationary Ca2+ concentration near a point source alone, and second, near a point source coupled with a point sink (Fig. 6). The total Ca2+ content of a hemisphere centered at the point source, J1, and at a point source plus a point sink, J2, can be calculated.80 The difference of the two values divided by J1 yields the fraction of Ca2+ which does not leave the close vicinity of the channel.

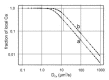
Figure 6
Schematic representation of ion fluxes through the cGMP-gated channel alone (left), and when associated with the exchanger (right) to estimate the fraction of local Ca2+ flux.
It should be noted that the combined effect of source and sink can be obtained by linear superposition of the solutions of the source and the sink alone because the differential equations of Ca2+ diffusion are linear.81 The rate of Ca2+ extrusion by the exchanger is given by a Michaelis relation with a Ca2+ binding constant of 1.6 μM.82
The most important parameter in this consideration is the intracellular Ca2+ diffusion constant. Therefore, the fraction of local Ca2+ was calculated for different diffusion constants (Fig. 7). In the cytoplasm, the Ca2+ diffusion constant is about 220 μm2/s at high Ca2+ concentrations (i.e., 1mM); however, it strongly depends on the Ca2+ concentration, and drops under physiological conditions to about 13 μm2/s due to buffered diffusion.83 Figure 7, curve a, shows the result if a distance of 5 nm between the channel pore and the Ca2+ binding sites of the exchangers is assumed, and the maximal exchange rate of the two exchangers is taken to be 120 Ca2+/s.84 If, as discussed above, the exchange rate would increase by a factor of two upon channel activation, the result would be that depicted in curve b of Figure 7. The grey line marks the diffusion constant of 13 μm2/s determined by Allbritton et al for cytoplasmic Ca2+ diffusion under physiological conditions.83 The main result of this estimation is that the association of channel and exchanger presumably results in a considerable fraction of localized Ca2+ of at least 30%, assuming a Ca2+ diffusion constant of 13 μm2/s (Fig. 7, red line). This Ca2+ diffusion constant can also vary locally. For the following reasons it is likely that in the close vicinity of channel-exchanger-complex this constant is in fact smaller than 13 μm2/s. The β-subunit of the channel has a very acidic stretch on the carboxyl terminus, the GARP domain. The amino terminus of the β-subunit is very acidic, as well. Furthermore, the intracellular loop of the Na/Ca2+, K exchanger is highly acidic. Interestingly, the carboxyl terminus of the α-subunit is highly basic, so that possible electrostatic interaction between positive and negative domains may occur. Nevertheless, the acidic amino acids prevail by far, so that the local Ca2+ diffusion constant is likely to be even below 13 μm2/s. Consequently, the fraction of local Ca2+ should be even higher than the values calculated with the diffusion constant 13 μm2/s (see Fig. 7).
Interestingly, the fraction of local Ca2+ current turns out to be almost independent of the value assumed for the Ca2+ influx as long as the Ca2+ influx is much smaller than the maximal exchange rate. The reason for this result is that, e.g., an increase in the Ca2+ influx entails a corresponding increase of the rate of Na/Ca2+, K exchange, resulting in an almost identical fraction of local Ca2+.
In summary, the necessary conditions for a local Ca2+ current are met. At least 30 percent of the Ca2+ entering the outer segment of the rod photoreceptor are presumably confined to the microdomain of the channel exchanger complex.
Conclusion and Outlook
In the plasma membrane of photoreceptors, cGMP-gated channel and Na/Ca2+,K exchanger form a complex of two exchangers binding to one channel. Binding occurs to the α-subunit, but not to the β-subunit of the channel. Both, α-subunit and exchanger form dimers. Assuming that the channel has a tetrameric structure, the channel exchanger complex consists presumably of a stoichiometry of x2 α2β2 where "x" denotes the exchanger, "α" the α-subunit, and "β" the β-subunit of the channel (Fig. 5).
The association of channel and exchanger hints to a novel mechanism to create a local Ca2+ microdomain which is realized in rod photoreceptors. As opposed to the "classical" mechanism, where Ca2+ microdomains at Ca2+ channels are shaped by mobile Ca2+ buffers,85 restriction of the Ca2+ current to a microdomain is achieved by local Ca2+ extrusion.86 Immobile Ca2+ buffering domains increase the fraction of localized Ca2+ flux. At least part of the Ca2+ signaling in rod photoreceptors appears to be based on such a mechanism of localization of the Ca2+ flux. The characteristics of local Ca2+ domains is fast signaling and the avoidance of cross coupling.77 Furthermore, the complex of channel and exchanger enables an allosteric regulation by direct interaction of these proteins.76
Na/Ca2+, K exchanger and cGMP-gated channels are only part of a greater protein assembly. Recent evidence strongly indicates that the GARP domains of the cGMP-gated channel interact with peripherin/Rds, a major disc rim protein.56 Therefore, the channel-exchanger complex is apparently tethered to the disc rim. It seems reasonable to assume that the guanylate cyclase, a major effector protein for Ca2+ signaling in rod photoreceptors, is close to the channel-exchanger complex. However, although the presence of guanylate cyclase could be demonstrated by immunolabeling in the outer segments of rod and cone photoreceptors, the reported results do not allow to map this protein definitely in the membrane stack.87-89 Moreover, a protein tyrosine kinase has recently been suggested to be associated with the α-subunit of the channel, as well.90 Further knowledge of the ultrastructure of the proteins assembled with the cyclic GMP-gated channel is required to understand the molecular basis of the signaling processes in photoreceptors.
Acknowledgment
I thank Dr. W. Baehr, Dr. K. Palczewski and Dr. D. Schulze for comments on the manuscript and Dr. R.S. Molday for generously providing the monoclonal antibodies PMc 2G11 and PMe 1B3 against the α-subunit of the cGMP-gated channel and the Na/Ca2+, K exchanger, respectively. This work was supported by a grant of the Deutsche Forschungsgemeinschaft (Ba721/1-3).
References
- 1.
- Hecht S, Shlaer S, Pirenne MH. Energy, quanta, and vision. J Gen Physiol. 1942;25:819–40. [PMC free article: PMC2142545] [PubMed: 19873316]
- 2.
- Rieke F, Baylor DA. Single photon detection by rod cells of the retina. Reviews of Modern Physics. 1998;70:1027–36.
- 3.
- Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. [PubMed: 11520918]
- 4.
- Pugh EN Jr, Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr Opin Neurobiol. 1999;9:410–8. [PubMed: 10448166]
- 5.
- Yau KW. Phototransduction mechanism in retinal rods and cones. Invest Ophthalmol Vis Sci. 1994;35:9–32. [PubMed: 7507907]
- 6.
- Detwiler PB, Gray-Keller MP. The mechanisms of vertebrate light adaptation: speeded recovery versus slowed activation. Curr Opin Neurobiol. 1996;6:440–4. [PubMed: 8794098]
- 7.
- Ratto GM, Payne R, Owen WG. et al. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with Fura-2. J Neurosci. 1988;8:3240–6. [PMC free article: PMC6569449] [PubMed: 2459322]
- 8.
- Gray-Keller MP, Detwiler PB. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–61. [PubMed: 7524559]
- 9.
- McCarthy ST, Younger JP, Owen WG. Free calcium concentrations on bullfrog rods determined in the presence of multiple forms of Fura-2. Biophys J. 1994;67:2076–89. [PMC free article: PMC1225583] [PubMed: 7858145]
- 10.
- Sampath AP, Matthews HR, Cornwall MC. et al. Bleached pigment produces a maintained decrease in outer segment Ca2+ in salamander rods. J Gen Physiol. 1998;111:53–64. [PMC free article: PMC1887770] [PubMed: 9417134]
- 11.
- Detwiler PB, Gray-Keller MP. Measurement of light-evoked changes in cytoplasmic calcium in functionally intact isolated rod outer segments. Methods Enzymol. 2000;316:133–46. [PubMed: 10800673]
- 12.
- Koutalos Y, Yau KW. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. [PubMed: 8820871]
- 13.
- Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–9. [PubMed: 7678445]
- 14.
- Bauer PJ. Cyclic GMP-gated channels of bovine rod photoreceptors: affinity, density and stoichiometry of Ca2+calmodulin binding sites. J Physiol. 1996;494:675–85. [PMC free article: PMC1160668] [PubMed: 8865065]
- 15.
- Ray S, Zozulya S, Niemi GA, Flaherty KM, Brolley D, Dizhoor AM. et al. Cloning, expression, and crystallization of recoverin, a calcium sensor in vision. Proc Natl Acad Sci USA. 1992;89:5705–9. [PMC free article: PMC49365] [PubMed: 1385864]
- 16.
- Gorczyca WA, Gray-Keller MP, Detwiler PB. et al. Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc Natl Acad Sci USA. 1994;91:4014–8. [PMC free article: PMC43713] [PubMed: 7909609]
- 17.
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993;362:855–7. [PubMed: 8386803]
- 18.
- Koutalos Y, Nakatani K, Tamura T. et al. Characterization of guanylate cyclase activity in single retinal rod outer segments. J Gen Physiol. 1995;106:863–90. [PMC free article: PMC2229293] [PubMed: 8648296]
- 19.
- Koutalos Y, Nakatani K, Yau KW. The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J Gen Physiol. 1995;106:8919–21. [PMC free article: PMC2229286] [PubMed: 8648297]
- 20.
- Koch KW. Control of photoreceptor proteins by Ca2+ Cell Calcium. 1995;18:314–21. [PubMed: 8556770]
- 21.
- Senin II, Zargarov AA, Alekseev AM. et al. N-Myristoylation of recoverin enhances its efficiency as an inhibitor of rhodopsin kinase. FEBS Lett. 1995;376:87–90. [PubMed: 8521974]
- 22.
- Otto-Bruc AE, Fariss RN, Van HooserJP. et al. Phosphorylation of photolyzed rhodopsin is calcium-insensitive in retina permeabilized by alpha-toxin. Proc Natl Acad Sci USA. 1998;95:15014–9. [PMC free article: PMC24567] [PubMed: 9844007]
- 23.
- Ikura M. Calcium binding and conformational response in EF-hand proteins. Trends Biochem Sci. 1996;21:14–7. [PubMed: 8848832]
- 24.
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. [PMC free article: PMC2107286] [PubMed: 6033942]
- 25.
- Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–518. [PubMed: 6771304]
- 26.
- Hsu YT, Wong SYC, Connell GJ, Molday RS. Structural and functional properties of rhodopsin from rod outer segment disk and plasma membrane. Biochim Biophys Acta. 1993;1145:85–92. [PubMed: 8422414]
- 27.
- Molday RS, Hicks D, Molday LL. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthal Vis Sci. 1987;28:50–61. [PubMed: 2433249]
- 28.
- Arikawa K, Molday LL, Molday RS. et al. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol. 1992;116:659–67. [PMC free article: PMC2289304] [PubMed: 1730772]
- 29.
- Moritz OL, Molday RS. Molecular cloning, membrane topology, and localization of bovine Rom-1 in rod and cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1996;37:352–62. [PubMed: 8603840]
- 30.
- Goldberg AFX, Molday RS. Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: Hydrodynamic evidence for a tetrameric quaternary structure. Biochemistry. 1996;35:6144–9. [PubMed: 8634257]
- 31.
- Papermaster DS, Schneider BG, Zorn MA. et al. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Cell Biol. 1978;78:415–25. [PMC free article: PMC2110123] [PubMed: 690173]
- 32.
- Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272:10303–10. [PubMed: 9092582]
- 33.
- Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–81. [PubMed: 10075733]
- 34.
- Bungert S, Molday LL, Molday RS. Membrane topology of the ATP binding cassette transporter ABCR and its relationship to ABC1 and related ABCA transporters. Identification of N-linked glycosylation sites. J Biol Chem. 2001;276:23539–46. [PubMed: 11320094]
- 35.
- Körschen HG, Beyermann M, Müller F. et al. Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. Nature. 1999;400:761–6. [PubMed: 10466724]
- 36.
- Eckmiller MS. Microtubules in a rod-specific cytoskeleton associated with outer segment incisures. Vis Neurosci. 2000;17:711–22. [PubMed: 11153651]
- 37.
- Boesze-Battaglia K, Albert AD. Fatty acid composition of bovine rod outer segment plasma membrane. Exp Eye Res. 1989;49:699–701. [PubMed: 2806432]
- 38.
- Cone RA. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972;236:39–43. [PubMed: 4537062]
- 39.
- Liebman PA, Entine G. Lateral diffusion of visual pigment in photoreceptor disk membranes. Science. 1974;185:457–9. [PubMed: 4546260]
- 40.
- Poo M, Cone RA. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974;247:438–41. [PubMed: 4818543]
- 41.
- Calvert PD, Govardovskii VI, Krasnoperova N. et al. Membrane protein diffusion sets the speed of rod phototransduction. Nature. 2001;411:90–4. [PubMed: 11333983]
- 42.
- Molday RS, Molday LL. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by ricin-gold-dextran density perturbation method. J Cell Biol. 1987;105:2589–601. [PMC free article: PMC2114690] [PubMed: 2447095]
- 43.
- Bauer PJ. Evidence for two functionally different membrane fractions in bovine retinal rod outer segments. J Physiol. 1988;401:309–327. [PMC free article: PMC1191851] [PubMed: 2845062]
- 44.
- Cook NJ, Molday LL, Reid D. et al. The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J Biol Chem. 1989;264:6996–9. [PubMed: 2468664]
- 45.
- Reid DM, Friedel U, Molday RS. et al. Identification of the sodium-calcium exchanger as the major ricin-binding glycoprotein of bovine rod outer segments and its localization to the plasma membrane. Biochemistry. 1990;29:1601–1607. [PubMed: 2334719]
- 46.
- Caretta A, Saibil HR. Visualization of cyclic nucleotide binding sites in the vertebrate retina by fluorescence microscopy. J Cell Biol. 1989;108:1517–22. [PMC free article: PMC2115528] [PubMed: 2538481]
- 47.
- Boesze-Battaglia K, Kong F, Lamba OP. et al. Purification and light-dependent phosphorylation of a candidate fusion protein, the photoreceptor cell peripherin/rds. Biochemistry. 1997;36:6835–6846. [PubMed: 9184167]
- 48.
- Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985;313:310–3. [PubMed: 2578616]
- 49.
- Bodoia RD, Detwiler PB. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985;367:183–216. [PMC free article: PMC1193059] [PubMed: 3877161]
- 50.
- Gray P, Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Sci. 1985;223:379–88. [PubMed: 2579400]
- 51.
- Zimmerman AL, Baylor DA. Electrical properties of the light-sensitive conductance of salamander retinal rods. Biophys J. 1985;47:357a.
- 52.
- Eismann E, Müller F, Heinemann SH. et al. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+blockage, and ionic selectivity. Proc Natl Acad Sci USA. 1994;91:1109–13. [PMC free article: PMC521463] [PubMed: 7508120]
- 53.
- Haynes LW, Kay AR, Yau KW. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986;321:66–70. [PubMed: 2422558]
- 54.
- Zimmerman AL, Baylor DA. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986;321:70–2. [PubMed: 2422559]
- 55.
- Karpen JW, Loney DA, Baylor DA. Cyclic GMP-activated channels of salamander retinal rods: spatial distribution and variation of responsiveness. J Physiol. 1992;448:257–74. [PMC free article: PMC1176198] [PubMed: 1375637]
- 56.
- Poetsch A, Molday LL, Molday RS. The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem. 2001;276:48009–16. [PubMed: 11641407]
- 57.
- Cook NJ, Zeilinger C, Koch KW. et al. Solubilization and functional reconstitution of the cGMP-dependent cation channel from bovine rod outer segments. J Biol Chem. 1986;261:17033–9. [PubMed: 2430972]
- 58.
- Bauer PJ, Drechsler M. Association of cyclic GMP-gated channels and NaCa2+ K exchangers in bovine retinal rod outer segment plasma membranes. J Physiol. 1992;451:109–31. [PMC free article: PMC1176153] [PubMed: 1328615]
- 59.
- Yau KW, Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984;311:661–3. [PubMed: 6434995]
- 60.
- Hodgkin AL, McNaughton PA, Nunn BJ. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987;391:347–70. [PMC free article: PMC1192218] [PubMed: 2451008]
- 61.
- Hodgkin AL, Nunn BJ. The effect of ions on sodium-calcium exchange in salamander rods. J Physiol. 1987;391:371–98. [PMC free article: PMC1192219] [PubMed: 2451009]
- 62.
- Lagnado L, Cervetto L, McNaughton PA. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proc Natl Acad Sci USA. 1988;85:4548–52. [PMC free article: PMC280468] [PubMed: 3380806]
- 63.
- Cook NJ, Kaupp UB. Solubilization, purification, and reconstitution of the sodium-calcium exchanger from bovine retinal rod outer segments. J Biol Chem. 1988;263:11382–8. [PubMed: 2841326]
- 64.
- Nicoll DA, Applebury ML. Purification of the bovine rod outer segment Na/Ca2+ exchanger. J Biol Chem. 1989;264:16207–13. [PubMed: 2777786]
- 65.
- Schwarzer A, Schauf H, Bauer PJ. Binding of the cGMP-gated channel to the Na/Ca-K exchanger in rod photoreceptors. J Biol Chem. 2000;275:13448–54. [PubMed: 10788457]
- 66.
- Molday RS, Molday LL. Molecular properties of the cGMP-gated channel of rod photoreceptors. Vision Res. 1998;38:1315–23. [PubMed: 9666999]
- 67.
- Li Z, Nicoll DA, Collins A. et al. Identification of a peptide inhibitor of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem. 1991;266:1014–20. [PubMed: 1985930]
- 68.
- He Z, Petesch N, Voges KP. et al. Identification of important amino acid residues of the Na+-Ca2+ exchanger inhibitory peptide, XIP. J Membr Biol. 1997;156:149–56. [PubMed: 9075646]
- 69.
- Hale CC, Bliler S, Quinn TP. et al. Localization of an exchange inhibitory peptide (XIP) binding site on the cardiac soidum-calcium exchanger. Biochem Biophys Res Commun. 1997;236:113–117. [PubMed: 9223436]
- 70.
- Schwarzer A, Kim TSY, Hagen V. et al. The Na/Ca-K exchanger of rod photoreceptor exists as dimer in the plasma membrane. Biochemistry. 1997;36:13667–76. [PubMed: 9354636]
- 71.
- Shammat IM, Gordon SE. Stoichiometry and arrangement of subunits in rod cyclic nucleotide-gated channels. Neuron. 1999;23:809–19. [PubMed: 10482246]
- 72.
- Liu DT, Tibbs GR, Paoletti P. et al. Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron. 1998;21:235–48. [PubMed: 9697867]
- 73.
- Weitz D, Zoche M, Müller F. et al. Calmodulin controls the rod photoreceptor CNG channel through an unconventional binding site in the N-terminus of the β-subunit. EMBO J. 1998;17:2273–84. [PMC free article: PMC1170571] [PubMed: 9545240]
- 74.
- Loewen CJR, Molday RS. Disulfide-mediated ologomerization of peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J Biol Chem. 2000;275:5370–8. [PubMed: 10681511]
- 75.
- He Y, Ruiz ML, Karpen JW. Constraining the subunit order of rod cyclic nucleotidegated channels reveals a diagonal arrangement of like subunits. Proc Natl Acad Sci U S A. 2000;97:895–900. [PMC free article: PMC15427] [PubMed: 10639176]
- 76.
- Bauer PJ, Schauf H. Mutual inhibition of the dimerized Na/Ca-K exchanger in rod photoreceptors. Biochim Biophys Acta. 2002;1559:121–34. [PubMed: 11853679]
- 77.
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–99. [PubMed: 9539117]
- 78.
- Stryer L. Cyclic GMP cascade of vision. Ann Rev Neurosci. 1986;9:87–119. [PubMed: 2423011]
- 79.
- Nakatani K, Yau KW. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988;395:695–729. [PMC free article: PMC1192017] [PubMed: 2457685]
- 80.
- Bauer PJ. Binding of the retinal rod Na/Ca2+-K exchanger to the cGMP-gated channel indicates local Ca2+signaling in vertebrate photoreceptors. Ann NY Acad Sci. 2002;976:325–334. [PubMed: 12502575]
- 81.
- Neher E. Usefulness and limitations of linear approximations to the understanding of Ca signals. Cell Calcium. 1998;24:345–57. [PubMed: 10091004]
- 82.
- Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992;455:111–42. [PMC free article: PMC1175636] [PubMed: 1282928]
- 83.
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphophate. Science. 1992;258:1812–5. [PubMed: 1465619]
- 84.
- Huppertz B, Bauer PJ. NaCa2+,K exchange in bovine retinal rod outer segments: quantitative characterization of normal and reversed mode. Biochim Biophys Acta. 1994;1189:119–26. [PubMed: 8292615]
- 85.
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implication for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci. 1997;17:6961–73. [PMC free article: PMC6573285] [PubMed: 9278532]
- 86.
- Bauer PJ. The local Ca concentration profile in the vicinity of a Ca channel. Cell Biochem Biophys. 2001;35:49–61. [PubMed: 11898855]
- 87.
- Liu X, Seno K, Nishizawa Y. et al. Ultrastructural localization of retinal guanylate cyclase in human and monkey retinas. Exp Eye Res. 1994;59:761–8. [PubMed: 7698269]
- 88.
- Cooper N, Liu L, Yoshida A. et al. The bovine rod outer segment guanylate cyclase, ROS-GC, is present in both outer segment and synaptic layers of the retina. J Mol Neurosci. 1996;6:211–22. [PubMed: 8672403]
- 89.
- Yang RB, Garbers DL. Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem. 1997;272:13738–42. [PubMed: 9153227]
- 90.
- Molokanova E, Savchenko A, Kramer RH. Interactions of cyclic nucleotide-gated channel subunits and protein tyrosine kinase probed with genistein. J Gen Physiol. 2000;115:685–96. [PMC free article: PMC2232887] [PubMed: 10828243]
- Introduction
- Channel and Na/Ca2+, K Exchanger Reside Only in the Plasma Membrane
- The Density of cGMP-Gated Channels
- The Density of Na/Ca2+, K Exchangers: An Intriguing Problem
- Interaction Between Solubilized Channel and Exchanger
- Association of Channel and Exchanger in the Plasma Membrane
- Different Subunit Arrangements of the Channel Entail Different Quaternary Structures
- Self-Inhibition of the Exchanger: An Allosteric Regulatory Mechanism?
- Local Ca2+ Signaling in Photoreceptors
- Conclusion and Outlook
- Acknowledgment
- References
- The Complex of cGMP-gated Channel and Na/Ca2+, K Exchanger in Rod Photoreceptors...The Complex of cGMP-gated Channel and Na/Ca2+, K Exchanger in Rod Photoreceptors - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...