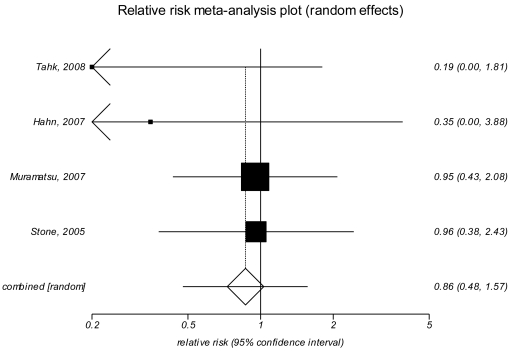
From: Appendix I, Forest Plots for Results of Final Health Outcomes Analyzed at Individual Time Points

Adjunctive Devices for Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention [Internet].
Comparative Effectiveness Reviews, No. 42.
Sobieraj DM, White CM, Kluger J, et al.
Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Dec.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.