NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ratko TA, Vats V, Brock J, et al. Local Nonsurgical Therapies for Stage I and Symptomatic Obstructive Non–Small-Cell Lung Cancer [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Jun. (Comparative Effectiveness Reviews, No. 112.)
This publication is provided for historical reference only and the information may be out of date.

Local Nonsurgical Therapies for Stage I and Symptomatic Obstructive Non–Small-Cell Lung Cancer [Internet].
Show detailsIntroduction
Overview
This chapter presents the results of this comparative effectiveness review (CER) on local nonsurgical interventions for patients with non–small-cell lung cancer (NSCLC) in three distinct settings. Key Question 1 addresses interventions in patients with stage I disease who are deemed medically inoperable due to comorbidities that preclude definitive resection. Key Question 2 addresses local nonsurgical intervention in patients with stage I disease who are deemed medically operable but refuse surgery. Key Question 3 addresses evidence for the use of local nonsurgical interventions in patients with symptoms secondary to an inoperable obstructive endoluminal NSCLC.
The results from the electronic literature search enumerate studies that were included and excluded from the review based on full-text examination. The excluded studies are shown in Appendix B. We did not perform a quantitative data synthesis for any Key Question.
Results of Literature Searches
Electronic Search
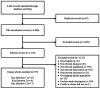
Of the 4,648 unique titles identified, we screened 1,178 in full-text. Of these, 55 met the CER inclusion criteria: 35 were relevant to Key Question 1, six were relevant to Key Question 2 and 17 were relevant to Key Question 3. Three studies47–49 addressed both Key Questions 1 and 2. Details are given in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)38 diagram (Figure 4). All studies relevant to Key Questions 1 and 2 were single-arm design, prospective (n=15), retrospective (n=21) or not specified (n=2). Among 17 papers included for Key Question 3, five were randomized controlled trials (RCTs), one was a nonrandomized comparative study and 11 were single-arm studies.
Grey Literature (Publication Bias)
Following review of 759 potentially relevant abstracts in the American Society of Clinical Oncology, and the American Society and American Society for Radiation Oncology proceedings over the past two years, and other sources including Clinicaltrials.gov, we identified one RCT that met the criteria for inclusion based on our protocol. This study (NCT00020709) is a phase 3 RCT of surgery versus stereotactic body radiotherapy (SBRT) in patients with Stage IA NSCLC who were fit to undergo primary resection. This study was terminated due to poor recruitment. After a MEDLINE search of the NCT number and title, we did not find any published results; it is unknown if any data have been reported. In examination of the U.S. Food and Drug Administration website and the Scientific Information Packets received from device manufacturers, we identified no additional RCTs that were relevant to this CER.
Key Question 1. Comparative Effectiveness of Local Nonsurgical Interventions for Stage I NSCLC in Medically Inoperable Patients
Description of Included Studies
Table 2 provides a summary of characteristics of 35 single-arm studies that met our selection criteria for Key Question 1. Fourteen studies were prospective.17,47,49–59 Interventions included SBRT (24 studies, total n=1665 patients), 17,48,49,53,56–75 three-dimensional radiotherapy (3DRT) (7 studies, total n=240 patients),50,51,54,55,76–78 proton beam radiotherapy (PBRT) (three studies, total n=144 patients)47,52,79 and radiofrequency ablation (RFA) (1 study, n=19 patients).80 More detailed information on the interventions is provided in Appendix C. Patients included in these studies were typically in their 70s, with median ages ranging from 67–81 years, and an overall range from 31–93 years. Common reasons for medical inoperability included presence of pulmonary disease (chronic obstructive pulmonary disease), insufficient predicted post-therapy lung function, cardiovascular disease, and other comorbidities that in total preclude surgical resection. Sex distribution was uneven, with proportions of females ranging from 9–80 percent across studies. Karnofsky performance status (KPS) of enrollees ranged from 40–100 in 11 studies,51,52,54,56,60,61,63–66,71,74 Eastern Cooperative Oncology Group (ECOG),50,57,69,70,72,74,76,77 World Health Organization (WHO)48,49,53,75, or European Organization for Research and Treatment of Cancer (EORTC)79 KPS ranged from 0–3 across 11 studies. Perfomance status was not reported in 11 studies.17,47,55,58,59,62,67,68,73,78,80 Sixteen studies (46 percent) reported histological confirmation of NSCLC cell types in 100 percent of patients.47,49,50,52–54,56,60,61,66,67,71,76–78 The remaining nineteen (54 percent) studies17,48,54,56–58,61–64,67–69,71–75,79,80 included patients without histological confimation of NSCLC. In 18 of such studies, a median 26 percent of patients did not have histologically confirmed NSCLC. Such studies used the rate of tumor growth in sucessive computed tomography (CT) scans and presence of 18F-fluorodeoxyglucose activity as a diagnostic marker of NSCLC rather than histological confirmation of NSCLC.
Table 2
Summary of characteristics for studies that address Key Question 1.
Key Points
- All evidence included in this report for Key Question 1 is from single-arm studies. No evidence is available from any type of direct comparative study of one intervention versus another.
- Evidence compiled from 35 single-arm studies is insufficient to form conclusions about the comparative benefits or harms of SBRT (24 studies), 3DRT (seven studies), PBRT (three studies) and RFA (one study) in medically inoperable patients with stage I NSCLC.
- The evidence comprises direct outcomes overall survival and cancer-specific survival; an indirect outcome, local control; and radiation-associated toxicities.
- Overall, post-treatment toxicities were reported across studies, but no relative trend was detected among interventions.
- We are uncertain whether the limited evidence on adverse events (AEs) reflects their absence, or that the investigators did not systematically collect those data or report them.
Detailed Synthesis
All survival outcomes abstracted for this CER are compiled in Appendix C. In Table 3, we have aggregated evidence from studies with the longest followup period.
Table 3
Survival and local control outcomes for local nonsurgical interventions in medically inoperable patients with stage I NSCLC.
The evidence summarized in Table 3 reflects single-arm studies that report direct outcomes- overall survival and cancer-specific survival and an indirect outcome- local control. Rates for overall survival, cancer-specific survival, and local control for SBRT at 3-years followup suggest a possible trend toward exceeding those reported with 3DRT. However, the reported ranges overlap. Furthermore, as this evidence comprises single-arm studies with no direct comparisons, conclusions are precluded. The nature of the evidence – no RCTs- does not support making indirect comparisons among interventions.
Intervention-Associated Adverse Events
Intervention-related toxicities reported in at least 2 percent of the study population are shown in Appendix C. The reported toxicities are grade 2 or greater (moderate) on a standardized crietria such as Common Toxicity Criteria for Adverse Events (CTCAE), or the WHO scale. They are all similar with respect to their grades and definitions. For Key Questions 1 and 2, these included radiation-associated pneumonitis and pulmonary toxicity, dyspnea, esophagitis, thoracic wall pain, pericardial or pleural effusion, bronchial stricture, and rib fracture. Rib fractures were reported in nine (41 percent) SBRT studies17,53,56,57,60–62,65,66 and one PBRT study.79 One death was attributed to grade 5 pericardial effusion at 3 months post-treatment in a 3DRT study.54 A second death was attributed to grade 5 hemoptysis in an SBRT study.70 Complications associated with RFA included pneumothorax and prolonged air leak from the lung.
As shown in Table 4, no relative difference in the proportion of studies reporting toxicities is evident among or across interventions, with the possible exception of rib fractures mentioned above.
Table 4
Percentage of studies reporting intervention-associated toxicities in stage I medically inoperable NSCLC patients.
Overall, post-treatment toxicities were not commonly reported across studies in the body of evidence. We are uncertain whether the limited evidence on AEs reflects absence, or that the investigators did not systematically collect data or report them.
Key Question 2. Comparative Effectiveness of Local Nonsurgical Interventions for Stage I NSCLC in Medically Operable Patients
Description of Included Studies
Table 5 provides a summary of characteristics of six single-arm studies that address Key Question 2. Three studies were prospective.47,49,81 Three studies81–83 used SBRT and enrolled only operable patients and two studies48,49 used SBRT and enrolled inoperable and operable patients but reported outcomes separately. One study47 used PBRT. Overall, patients were typically in their mid-70s, with median ages ranging from 7483 to 7848 years, and overall range from 43–91 years. Sex distribution was uneven, with proportions of females ranging from 40 percent81 to 72 percent83 across studies. ECOG and WHO performance status ranged from 0–3 across studies. Four studies reported 100 percent histological confirmation of NSCLC cell type.47,49,81,83 Details on the included studies are provided in Appendix C.
Table 5
Summary of characteristics for studies that address Key Question 2.
Key Points
- All evidence included in this report for Key Question 2 is from single-arm studies. No evidence is available from any type of direct comparative study of one intervention versus another.
- Evidence compiled from six single-arm studies is insufficient to form conclusions about the comparative benefits or harms of SBRT (five studies) or PBRT (one study) in medically operable patients with stage I NSCLC.
- The results of interest for this report comprise direct outcomes overall survival and cancer-specific survival; an indirect outcome, local control; and radiation-associated toxicities as shown in Figure A.
- Post-treatment toxicities were not common across studies. No relative trend was detected among interventions.
- We are uncertain whether the limited evidence on AEs reflects absence, or that the investigators did not systematically collect data or report them
Detailed Synthesis
Appendix C shows survival and local control outcomes with PBRT or SBRT in four studies relevant to Key Question 2. Survival outcomes were not reported in these the studies. Table 6 shows survival and local control outcomes for each intervention.
Table 6
Survival and local control outcomes for local nonsurgical interventions in medically operable patients with stage I NSCLC.
The evidence summarized in Table 6 above comprises single-arm studies that report direct outcomes overall survival and cancer-specific survival and an indirect outcome, local control. No direct comparative evidence is available to suggest any relative difference between the technologies in overall survival, cancer-specific survival or local control rates.
Intervention-Associated Adverse Events
Appendix C shows intervention-related grade 2 or greater toxicities reported in at least 2 percent of the study population. Toxicities enumerated included radiation-associated pneumonitis and pulmonary toxicity, dermatitis, and rib fracture. Rib fractures were reported in two (67 percent) SBRT studies82,83 and in the PBRT study.47 The toxicity reporting criteria for each study (when provided by the authors) are shown in Appendix C. Definitions used to grade toxicities vary, which further complicates any possible assessment. Table 7 shows the distribution of reporting post-treatment toxicities across studies.
Table 7
Percentage of studies reporting intervention-associated toxicities in stage I medically operable NSCLC patients.
No relative trend in reporting toxicities was discerned among interventions. We are uncertain whether the limited evidence on AEs reflects absence, or that the investigators did not systematically collect data or report them.
Risk of Bias for Individual Studies Addressing Key Question 1 and Key Question 2
We used the convention described by Carey and Boden42 to assess the risk of bias of individual single-arm studies included to address Key Question 1 and Key Question 2 (see Methods chapter). Our ratings of good, fair and poor are shown in Table 8. Studies that met 8 of 8 criteria were classified as good, studies that met 7 of 8 criteria were classified as fair and studies that met fewer than 7 criteria were classified as poor.
Table 8
Carey and Boden study quality rating summaries for Key Questions 1 and 2.
Among 38 unique single-arm studies, all reported the use of validated outcomes. Study quality was most often downgraded because authors did not acknowledge the funding source in 23 studies (60 percent).48,51,54,56,57,59,62–69,72–74,76–79,81,82 In eleven studies (29 percent)17,54,55,57,59,60,67,75,78,79,81 it was unclear whether or not the conclusions and discussion were supported by the data. Eight studies (23 percent)17,50,54,55,58,59,67,73 did not adequately describe the study population. Six (16 percent)54,55,59,78,79,81 did not describe results well. Four (10 percent)50,75,79,82 did not adequately describe the intervention. Three (9 percent)54,60,64 did not report the use of appropriate statistical analysis. The only consistent reason for which a study was downgraded was “failure to report the funding source.”
Key Question 3. Comparative Effectiveness of Local Nonsurgical Therapies for Symptoms Secondary to an Inoperable Obstructive Endoluminal NSCLC
Overview
This section describes the literature that evaluates the efficacy and safety of local nonsurgical therapies for palliation or treatment of endobronchial NSCLC. After an overview of the literature, the results are described for outcomes in three categories: outcomes related to obstructive symptom resolution, survival outcomes, and safety outcomes. Improvement in obstructive symptoms was the primary outcome of interest because palliative interventions are most proximately expected to have an impact on obstructive symptoms. We specifically looked for resolution or improvement in dyspnea, cough, hemoptysis, and pneumonitis and abstracted all other symptoms in the “other” category. In addition, we also abstracted survival outcomes that included overall survival (reported as both median overall survival and time specific survival), disease specific survival and local control. Among the outcomes related to treatment-related toxicities, we focused on hemoptysis, pneumothorax and radiation bronchitis. We only abstracted toxicities that were grade 2 or greater or necessitated an active intervention or considered serious by the authors.
Overall, 17 studies were abstracted for this review. The evidence base consisted of six comparative studies85–90 and 11 noncomparative studies.91–101 Overall data for these studies is presented in Appendix C. Table 9 and Table 22 summarize the comparative and noncomparative studies reviewed for Key Question 3, respectively.
Table 9
Overview of comparative studies that address Key Question 3.
Study Characteristics of Comparative Studies
Among the six comparative studies that address Key Question 3, five were RCTs and one was a retrospective nonrandomized comparative study. Three hundred and forty-two patients were randomized in these six studies85–90 that compared six distinct treatment combinations. Additionally, we did not report data for one RCT102 as it reported outcome data for three different endobronchial treatments cumulatively. The detailed outcomes related to symptom improvement, survival and AEs for all six comparative studies are presented in Appendix C. All six studies included patients with a histologically confirmed NSCLC. Four studies85,86,89,90 reported staging of lung cancer patients but only one study86 reported the criteria used for staging NSCLC. The duration of the study enrollment period was reported by four studies85,87,88,90 and ranged from 2 to 4 years. Three studies85,87,88 were conducted in an outpatient setting; one90 was conducted in the inpatient setting and for two studies86,89 study setting was not reported. Four85,88–90 were single-center studies, the remaining two86,87 were multicenter studies. Four studies87–90 did not state whether there existed a conflict of interest or not, the remaining two studies85,86 stated no conflict of interest. Three studies87,88,90 did not state the source of funding, one each was manufacturer sponsored,89 professional scientific society sponsored86 and investigator initiated.85
All six85–90 comparative studies were rated as poor quality, five studies85–88,90 reported data on symptom relief, five studies85–88,90 reported survival data, two studies reported quality of life (QOL) data85,87 and six studies85–90 reported data related to treatment-related toxicity. Detailed characteristics of patients included in the six studies are summarized in Table 10.
Table 10
Study characteristics of comparative studies that address Key Question 3.
Key Points
- All RCTs included in this report were of poor quality according to the U.S. Preventive Services Task Force (USPSTF) rating criteria.
- Evidence from six comparative studies is insufficient to draw conclusions about relative benefits and harms of six unique treatment comparisons (brachytherapy plus external-beam radiotherapy (EBRT) versus brachytherapy alone; brachytherapy plus EBRT versus EBRT alone; brachytherapy versus EBRT; laser plus brachytherapy versus laser alone; laser versus electrocautery or photodynamic therapy (PDT) for local nonsurgical therapies in symptomatic inoperable patients with obstructive endoluminal NSCLC.
- None of the six comparative studies included interventions related to debridement and stenting and RFA. These interventions are addressed in three single-arm studies.
- The evidence comprises direct outcomes (overall survival), symptom relief and treatment-related toxicities.
- Overall, treatment-related toxicities varied according to type of intervention. Hemoptysis was the most common toxicity reported across studies. There may be underreporting of treatment-related toxicities, as three comparative studies did not describe the frequency, process of data collection, or assessment of severity of treatment-related toxicities.
Description of Comparative Studies According to Intervention(s)
Brachytherapy Plus EBRT Versus Brachytherapy Alone
One RCT85 compared brachytherapy plus EBRT versus brachytherapy alone and included a total of 45 patients (Table 11 and 12). These 45 patients were randomized equally across three treatment groups; EBRT plus brachytherapy (16 Grays [Gy]), EBRT plus brachytherapy (10Gy) and brachytherapy (15Gy) alone. All patients in the first two treatment arms received the same dose of EBRT (30 Gy in 10 fractions over 2 weeks). The authors assessed treatment harms at a predefined periods (at weekly intervals for acute toxicities) using a standardized Radiation Therapy Oncology Group (RTOG) morbidity-scoring criterion. This was the strength of this trial. Weaknesses of the trial included lack of sample size calculation, small number of patients per treatment group, and lack of defined statistical adjustments for multiple comparisons. These weaknesses affected the USPSTF domain of “appropriate analysis of results” (Table 13). Further, no details were provided on randomization or allocation concealment, which adversely affected the USPSTF domain of “assembled comparable groups.” Therefore, we judged this trial to have a poor USPSTF quality rating.
Table 11
Comparative effect of brachytherapy plus EBRT versus brachytherapy alone on obstructive symptoms in the Mallick trial.
Table 12
Comparative effect of brachytherapy plus EBRT versus brachytherapy alone on quality of life outcomes in the Mallick trial.
Table 13
USPSTF study quality ratings of the Mallick trial.
There was no statistical difference in the response rate of dyspnea, cough, hemoptysis and obstructive pneumonia among the 3 treatment groups. The authors did not provide clear definitions of what constituted a partial or complete response for obstructive symptoms. Though the authors reported significant improvement in obstruction scores as well as multiple QOL scores (including sub-domains) within treatment groups, they did not report the results of between treatment groups. Survival data was not reported. Using the RTOG morbidity scoring criteria, the authors did not observe any grade II-grade IV acute toxicities. One patient died due to hemoptysis in the treatment group that received brachytherapy alone.
Brachytherapy Plus EBRT Versus EBRT Alone
One RCT86 compared brachytherapy plus EBRT versus EBRT alone. The trial was planned to recruit a total of 160 patients with an 80 percent power to detect a 25 percent decrease in the rate of palliation of dyspnea with 0.05 type-I error (Table 14). However, the trial was discontinued prematurely due to lack of patient accrual. The authors reported results of 95 evaluable patients who were randomized to brachytherapy plus EBRT (n=47) or EBRT alone (n=48) using a central randomization process. The USPSTF trial quality rating was poor (Table 15). The analysis with 95 patients was underpowered to detect a prespecified difference in the rate of dyspnea (a primary outcome) and therefore adversely affected the USPSTF domain of “appropriate analysis of results.” The authors did not report the frequency, the process or the method of assessing severity of treatment-related toxicity. This negatively affected the USPSTF domain of “valid measurement.”
Table 14
Comparative effect of brachytherapy plus EBRT versus EBRT alone on obstructive symptoms in the Langendijk trial.
Table 15
USPSTF study quality ratings of the Langendijk trial.
The results did not show any difference in the response rate of dyspnea in patients treated with EBRT plus brachytherapy versus EBRT alone (46 percent and 37 percent respectively). The median overall survival was similar across both groups; 7.0 (95% confidence interval (CI): 5.3 to 8.9) and 8.5 (95% CI: 5.4 to 11.6) months respectively. The authors assessed QOL scores (Dutch version of the EORTC Quality of Life Questionnaire (EORTC QLQ-C30) and lung cancer module QLQ-LC13) both before and after therapy with a 90 percent compliance rate, but the results were not reported in the paper. We were unable to find a citation in subsequent years. The proportion of patients with death due to hemoptysis was similar across the two treatment groups (15 percent and 13 percent in the EBRT plus brachytherapy versus EBRT alone group respectively).
Brachytherapy Versus EBRT
One controlled trial87 randomly allocated 108 inoperable NSCLC patients with endobronchial tumors to two treatment arms: brachytherapy (n=49) or EBRT (n=50) (Table 16). Nine patients were excluded from the analysis. The primary aim of the trial was to evaluate symptom relief, treatment-related toxicities and impact on QOL. The strength of the trial was that it assessed treatment harms adequately at predefined periods using patient questionnaires but did not use standardized scoring criteria to rate severity of treatment-related toxicities. The trial was judged to have a poor quality on USPSTF rating for failing to appropriately take into account potential confounding—here fundamentally important for estimating an unbiased effect estimated owing to its time-dependent nature. Fifty-one percent in the brachytherapy arm received EBRT if the symptoms persisted or deteriorated or if the symptoms recurred. Similarly, 28 percent in the EBRT arm received brachytherapy. In the absence of taking into account this time-dependent confounding (a per-protocol analysis with appropriate censoring), it is impossible to judge the magnitude or even direction of potential bias. This fatal flaw negatively affected all three domains of USPSTF quality rating: “maintained comparable groups,” “measurements valid” and “appropriate analysis of results” (Table 17) Further, lack of details about randomization and allocation concealment adversely affected the domain of “assembled comparable groups.”
Table 16
Comparative effect of brachytherapy versus EBRT on obstructive symptoms in the Stout trial.
Table 17
USPSTF study quality ratings of the Stout trial.
The response to treatment measured as positive symptom (improvement or no change in symptom severity from baseline to 4 and 8 weeks after treatment) by the physician was similar across two treatment arms. Though survival was not a planned endpoint, the EBRT treatment arm had a statistically significant higher survival than the brachytherapy arm (287 versus 250 days at 1 year, p=0.04). The authors did not report the treatment-related toxicities in detail except that they were similar across two treatment groups. Four (8 percent) and three (6 percent) patients died due to hemoptysis in the brachytherapy and EBRT group, respectively.
EBRT Versus Endobronchial Treatments (Brachytherapy, Laser or Cryotherapy)
One RCT102 randomly allocated patients to EBRT or endobronchial treatment (clinician choice of any one endobronchial treatment: brachytherapy, laser therapy or cryotherapy). This trial was designed to have a 90 percent power to detect a difference of 15 percent in the relief of breathlessness at 0.05 significance level with 400 patients randomized across four treatment arms. The trial102 was discontinued before completion due to lack of patient accrual. The authors presented data for only 75 patients, of whom 16 patients did not receive the allocated treatment. As a result, the interpretation of available data for 59 patients distributed across four treatment arms poses significant limitations, namely small number per group and uncertainty about the preservation of randomization sequence. Further, the data for three different endobronchial treatment groups is reported cumulatively which does not allow comparison of treatment effects. Therefore, we did not report the data for this trial in this report. Details of this trial are provided in the abstraction tables in Appendix C.
Laser Plus Brachytherapy Versus Laser Alone
One RCT88 compares combination treatment of laser plus brachytherapy versus laser therapy only (Table 18). This trial by Chella el al.,88 randomized 29 patients across two treatment arms: laser plus brachytherapy (n=14) versus laser (n=15) alone. This small trial lacked details on randomization and allocation concealment. It did not report the NSCLC staging of patients, which is an important prognostic factor. These factors adversely affected the USPSTF domain of “assembled comparable groups.” The authors did not report the frequency, the process or the method of assessing severity of treatment-related toxicity. This negatively affected the USPSTF domain of “measurements valid” (Table 19) We therefore rated this trial to have a poor USPSTF quality rating.
Table 18
Comparative effect of laser plus brachytherapy versus laser alone on obstructive symptoms in the Chella trial.
Table 19
USPSTF study quality ratings of the Chella trial.
The reported median overall survival in the two treatment groups was not statistically significant different between the two treatment arms (10.3 months and 7.4 months respectively). Speiser’s index (a semi-quantitative score in which a higher score indicates severe obstruction) was reduced by 4.2 and 3.4 points in the combined versus single treatment arms respectively. This reduction in score was not statistically different between the two arms. The authors also reported the pretreatment and post-treatment values of lung function tests but showed no statistically significant differences between the treatment arms. One patient died due to hemoptysis 12 months after treatment in the laser plus brachytherapy arm.
Laser Versus Photodynamic Therapy
One controlled trial89 randomized 31 NSCLC patients with airway obstruction to either PDT (n=14) versus laser therapy (n=17). The trial assessed treatment harms at predefined and regular periods and assessed causality but the authors did not report using standardized criteria to assess the severity of treatment-related toxicities. This small trial lacked details on randomization and allocation concealment. At the baseline, the proportion of patients with stage III–IV cancer in the PDT group and laser group was 57% (8 of 14) and 88% (15 of 17) respectively. The authors did not explain the imbalance in tumor stage distribution even though it was a randomized trial. Further, the authors did not report whether they adjusted for the baseline differences in the outcomes. This negatively affected the USPSTF domain of “assembled comparable groups” and was considered a fatal flaw in the USPSTF quality rating (Table 20). We therefore judged this trial to have a poor USPSTF quality rating.
Table 20
USPSTF study quality ratings of the Jimenez trial.
Median survival was reported to be longer in the PDT versus laser group (265 versus 95 days, p=0.007). Though quantitative symptom relief was not reported, the authors described amelioration of symptoms to be similar in both treatment groups. Two patients (one in each group) died from hemoptysis, and there was one probable death due to treatment in the PDT-treated group.
Laser Versus Electrocautery
One nonrandomized retrospective study90 conducted with 29 patients compared the effects of treatment with laser (n=14) versus electrocautery (n=17) on dyspnea relief in NSCLC patients with tracheobronchial obstruction due to an endobronchial tumor. The study was judged to have poor quality on USPSTF quality rating because of lack of adjustment for any potential confounders given that it was a nonrandomized retrospective study with imbalanced distribution of prognostic factors at the baseline. A disproportionate number of patients had received previous treatment in the laser treated group (93 percent) as compared with the electrocautery group (53 percent). Further, the mean time from diagnosis to study treatment was different in the two groups (4.7 versus 7.5 months in laser versus electrocautery group). These factors negatively affected the USPSTF domain of “assembled comparable groups” (Table 21).
Table 21
USPSTF study quality ratings of the Boxem study.
The reported mean survival and percent improvement of symptoms was similar in both groups. The mean survival was 8.0±2.5 and 11.5±3.5 months in the laser and electrocautery treated groups respectively. The proportion of patients with symptom improvement (rated on a dichotomous scale by the treating clinician) was 10 (71 percent) and 13 (76 percent) in the laser and electrocautery treated groups respectively.
Study Characteristics of Noncomparative Studies
A total of 11 studies91–101 included 858 patients given eight distinct treatment modalities (three single intervention: brachytherapy, PDT, RFA; five multiple interventions: brachytherapy plus EBRT, brachytherapy plus PDT plus chemotherapy, EBRT plus chemotherapy, stenting plus brachytherapy and stenting plus laser therapy). Data were abstracted from a single arm of three otherwise comparative studies.91,93,96 In the latter, the comparator arms were not considered relevant and not abstracted, for reasons summarized in the section, “Description of Noncomparative Studies” below. Three studies (27 percent) originated in the United States, seven were from Europe (64 percent), and one (9 percent) was from former Yugoslavia. An overview of the noncomparative studies is given in Table 22.
Table 22
Overview of noncomparative studies of local nonsurgical endobronchial therapies.
Key Points
- Of the total 11 noncomparative studies that addressed Key Question 3, we focused on three studies that cover two unique interventions (RFA and debridement and stenting) for which comparative data was not available.
- All three non-comparative studies were of poor quality according to Carey and Boden quality ratings.
Description of Noncomparative Studies
We do not present detailed study data (study characteristics and outcomes) of eight94–100 noncomparative studies that utilize interventions for which comparative studies exists. These interventions include brachytherapy, EBRT, laser, electrocautery and PDT. Instead, we focused on three noncomparative studies91–93 that cover two unique interventions (RFA and debridement and stenting) for which comparative data was not available. Table 23 provides a summary of patient characteristics of three single-arm studies that covers RFA and debridement and stenting. It includes two studies on combination treatment with stenting and one study on RFA.
Table 23
Study characteristics of noncomparative studies that address Key Question 3.
Among these three noncomparative studies, two were prospective91,92 and one was retrospective.93 Ninety-five patients were included in these three studies. The Lencioni and Chhajed studies included patients which were not relevant to Key Question 3. The Lencioni study included 73 patients with non-NSCLC malignancy; the Chhajed study included 92 NSCLC patients that did not have endobronchial obstruction and received chemotherapy. The data presented in this report for these two studies exclude data of such non-relevant patient population. All but one study93 included patients with a histologically non-confirmed NSCLC. One study92 included only recurrent patients, one study91 included recurrent and stage I patient, and the third study93 did not report on tumor stage of patients. None of the three studies reported the criteria used for staging NSCLC. The duration of the study enrollment period was reported by only one study.91 One study92 was conducted in an outpatient setting; the remaining two studies91,93 did not describe the study setting. Two92,93 were single-center studies, the third91 was a multicenter study. All three studies91–93 stated no conflict of interest. Two studies92,93 did not state the source of funding, the remaining study91 was manufacturer sponsored. All three noncomparative studies were rated as poor quality. All three studies reported data on survival data. The Lencioni study91 reported 1 and 2-year survival rates; Chhajed93 reported 3, 9, and 12-month survival rates and median survival. However, of the 52 patients assessed in the Chhajed study, 13 each received laser and stenting alone respectively and remaining 26 patients received both laser and stenting. However, the authors did not present data of patients stratified by the treatment they received. This severely limited meaningful interpretation of the data. Mean survival for 10 patients included in the Allison study92 was not reported in the published paper but calculated for this report. Among the miscellaneous outcomes related to symptom relief and quality of life, the Lencioni study91 reported results of lung function tests and QOL and the Allison study 92 reported results on performance status. The detailed outcomes related to survival, symptom improvement and quality of life are presented in Table 24.
Table 24
Survival and local control outcomes for noncomparative studies that address Key Question 3.
Intervention-Associated Adverse Events
As per Agency for Healthcare Research and Quality (AHRQ) guidance on comparing harms about medical interventions,103 data about harms from observational studies should always be assessed. This is because quantity and quality of harms reporting in clinical trials is frequently inadequate and hypotheses are usually designed to evaluate benefits than harms. Further, clinical trials usually are not large enough to capture rare adverse events nor are they long enough to capture late adverse events. Moreover, clinical trials tend to include homogenous and healthier subjects who are less likely to have AEs than the general population.103 Therefore, we report the treatment-related toxicities data from all 11 noncomparative studies. These data are compiled in Table 25. In the largest prospective study98 of 320 patients who were treated with a combination of brachytherapy and EBRT, radiation bronchitis was the most common treatment-related toxictiy observed. The incidence of grade 2, 3 and 4 radiation bronchitis was 7, 10 and 8 percent respectively. In the second largest single arm study by Guilcher,95 226 patients with endobronchial NSCLC treated with brachytherapy alone were analysed retrospectively. The incidence of radiation bronchitis was 12 percent. Six percent (n=13) of patients died due to complication (10 hemoptysis, 2 of necrosis and 1 of radiation stenosis). The authors of the study did not specify if these were treatment-related complications or not. There was only one study91 that reported incidence of pneumothorax with use of RFA. Pneumothorax occurred in 13 percent of patients included in the study. The incidence of hemoptysis in more than 2 percent of study subjects was observed in four studies93,95,98,99 and ranged from 2 to 7 percent. The toxicity reporting criteria for each study (when provided by the authors) are shown in Appendix C.
Table 25
Treatment-related toxicities in noncomparative studies that address Key Question 3.
Risk of Bias for Noncomparative Studies Addressing Key Question 3
We used the convention described by Carey and Boden42 to assess the risk of bias of individual noncomparative studies included to address Key Question 3 (see Methods chapter). We rated the quality of only three noncomparative studies that utilize interventions for which no comparative data was available. Our ratings of good, fair and poor are shown in Table 26. Studies that met 8 of 8 criteria were classified as good, studies that met 7 of 8 criteria were classified as fair and studies that met fewer than 7 criteria were classified as poor.
Table 26
Carey and Boden quality rating summary.
The reasons for not fullfilling Carey and Boden crietria were as follows: the Chhajed study 93 did not clearly define a research question, did not well describe the study population or the intervention used in the study nor did they describe the results well. One study91 did not use valdiated outcome measure, two studies91,92 did not use appropraite statistical analysis, discussion and conclusion was not supported by data for two studies 91,93 and two studies92,93 did not describe their funding source.
- Introduction
- Results of Literature Searches
- Comparative Effectiveness of Local Nonsurgical Interventions for Stage I NSCLC in Medically Inoperable Patients
- Comparative Effectiveness of Local Nonsurgical Interventions for Stage I NSCLC in Medically Operable Patients
- Risk of Bias for Individual Studies Addressing Key Question 1 and Key Question 2
- Comparative Effectiveness of Local Nonsurgical Therapies for Symptoms Secondary to an Inoperable Obstructive Endoluminal NSCLC
- Risk of Bias for Noncomparative Studies Addressing Key Question 3
- Results - Local Nonsurgical Therapies for Stage I and Symptomatic Obstructive No...Results - Local Nonsurgical Therapies for Stage I and Symptomatic Obstructive Non–Small-Cell Lung Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...