FGF signalling is one among a handful of cell signalling pathways that orchestrate embryonic development. Integration of these pathways is central to the correct patterning, cell specification, and tissue differentiation that occur during normal development; many examples of the interplay of FGF and other signalling pathways are presented in this review.
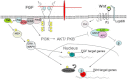
The canonical Wnt signalling pathway, for instance, has several points where cross-talk can take place with the FGF pathway (see Figure 4). Stabilisation of β-catenin is the major result of the canonical Wnt pathway (recent reviews of Wnt signalling (Kikuchi et al., 2009; van Amerongen and Nusse, 2009). In the absence of Wnt signals, β-catenin is phosphorylated by a destruction complex including Axin, APC, and GSK3, which primes it for degradation by the ubiquitin pathway. When Wnt signalling is activated, GSK3 is inhibited by Dishevelled (Dvl) and β-catenin is stabilised, accumulates, and moves to the nucleus to promote transcription by associating with the transcription factor TCF/LEF. In the absence of Wnt signalling, TCF is often associated with the co-repressor Groucho so that TCF-dependent transcription is inhibited. One point where FGF signalling feeds into this pathway is by phosphorylating GSK3 (Dailey et al., 2005). Neural progenitor cells derived from mice were treated with FGF protein and found to have higher levels of phospho-GSK3 and β-catenin (Israsena et al., 2004). This is most likely mediated by FGF activation of AKT (Hashimoto et al., 2002; Jope and Johnson, 2004; Katoh, 2006). However, in other contexts, AKT is not involved in mediating interactions between the FGF and Wnt signalling pathways (Keenan et al., 2006).
Another level of integration occurs in the nucleus: MAPK phosphorylation sites have been identified in the co-repressor Groucho and FGF signalling has been shown to inhibit Groucho’s repressor activity in vivo (Cinnamon et al., 2008; Murai et al., 2007). In addition, the ability of FGF to phosphorylate Groucho has been shown to affect transcription downstream of Wnt in cells expressing a TCF-dependent reporter (Burks et al., 2009). One model is that MAPK phosphorylation of Groucho allows it to dissociate with TCF, thus promoting the β-catenin/TCF partnership that drives Wnt dependent transcription. The effects of MAPK phosphorylation of Groucho could facilitate the integration of FGF signalling with many signalling pathways as Groucho is known to operate downstream of Notch, BMPs, TGFb, and Wnts (Hasson and Paroush, 2006).
Integration of Wnt and FGF signalling can also occur at the level of the individual gene promoter. The Xenopus Cdx4 gene contains functional binding sites for the transcriptional effectors of both the FGF and Wnt signalling pathways (Haremaki et al., 2003).
β-catenin also functions at the cell membrane by associating with cell adhesion proteins including E-cadherin (Willert and Nusse, 1998). Thus, the presence of large amounts of E-cadherin could influence levels of β-catenin available to mediate Wnt signalling by sequestering it at the cell membrane. In FGFR1 mutant mice, high levels of E-cadherin are expressed and β-catenin does not accumulate in the cytoplasm (Ciruna and Rossant, 2001) suggesting another way where FGF can promote Wnt signalling by inhibiting the expression of E-cadherin.
FGF directly interacts with the BMP signal transduction pathway by phosphorylating and inhibiting the activity of SMAD1 (Pera et al., 2003). FGF also impacts BMP signalling through transcriptional regulation: by repressing the expression of BMP ligands (Furthauer et al., 2004) and activating the expression of secreted BMP antagonists (Branney et al., 2009; Fletcher and Harland, 2008). A fully active FGF signalling pathway is essential for activin (a TGFβ signalling molecule, often used to mimic nodal signalling in experiments using Xenopus explants) to induce mesoderm (Cornell and Kimelman, 1994), and this dependence could involve the rapid, activin-dependent expression of FGF ligands, such as FGF4 (Fisher, 2002). The interplay of FGF signalling with the other signalling pathways important for embryonic development, including the Wnt, BMP, nodal, and sonic hedgehog pathways, will be evident in the diverse examples discussed in this review.
Publication Details
Copyright
Publisher
Morgan & Claypool Life Sciences, San Rafael (CA)
NLM Citation
Pownall ME, Isaacs HV. FGF Signalling in Vertebrate Development. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Integration with Other Signalling Pathways.