NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Henriksen K, Battles JB, Marks ES, et al., editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005 Feb.

Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology).
Show detailsAbstract
Clinical inertia is defined as lack of treatment intensification in a patient not at evidence-based goals for care. Clinical inertia is a major factor that contributes to inadequate chronic disease care in patients with diabetes mellitus, hypertension, dyslipidemias, depression, coronary heart disease, and other conditions. Recent work suggests that clinical inertia related to the management of diabetes, hypertension, and lipid disorders may contribute to up to 80 percent of heart attacks and strokes. Clinical inertia is, therefore, a leading cause of potentially preventable adverse events, disability, death, and excess medical care costs. This paper addresses three specific objectives: (1) to present a conceptual model of clinical inertia that takes into account recent developments in human factors research, cognitive science, and organizational behavior; (2) to operationally define clinical inertia and propose simple clinical protocols that can be used to identify and map its incidence across populations of patients and physicians; and (3) to propose future research to reduce clinical inertia by specifically targeting the root causes of the problem. Ultimately, a better understanding of clinical inertia and the development of specific interventions to reduce it may be a productive strategy to reduce passive errors that contribute to hundreds of thousands of adverse events and tens of thousands of premature deaths annually in the United States.
Introduction
The contribution of medical errors to adverse clinical outcomes is well documented in recent reports from the Institute of Medicine (IOM) and other sources. 1, 2 Most reports of medical errors thus far have focused on errors related to inappropriate use or misuse of various therapies. However, it is likely that in chronic disease care, errors related to underuse of potentially efficacious therapy are very common and often lead to serious adverse events. 3 Clinical inertia is a major factor that contributes to inadequate chronic disease care in patients with diabetes mellitus (DM), hypertension (HT), dyslipidemia, depression, coronary heart disease (CHD), and other conditions.
Failure to intensify therapy in patients with elevated blood glucose, blood lipids, or blood pressure fits the definition of medical errors given by the IOM. Clinical inertia leads to adverse events just as surely as erroneous injections of certain medications can rapidly lead to death in a fragile hospitalized patient. 4, 5 The principal substantive distinction between the adverse events caused by overuse or misuse of therapies, and adverse events attributable to clinical inertia in chronic disease care, is the time frame over which the adverse event occurs. In hospital settings misuse of a therapy may lead to adverse events in minutes to hours. In the context of outpatient chronic disease care, clinical inertia will inexorably lead to adverse events in a high proportion of patients, but it may take years or even decades for the consequent adverse event to declare itself.
Operational definition of clinical inertia
In instances of clinical inertia, both of the following must occur: (a) The patient fails to achieve major evidence-based clinical goals, and (b) the patient fails to receive appropriate intensification of pharmacotherapy in a defined period of time. Figures 1–3 show simple algorithms that operationally define clinical inertia in the care of patients with diabetes, hypertension, or lipid disorders. These algorithms can identify specific instances of clinical inertia using routinely available clinical data, including diagnostic, laboratory, and pharmacy data.
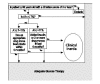
Figure 1
Algorithm to identify clinical inertia related to glucose therapy.
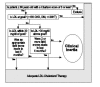
Figure 3
Algorithm to identify clinical inertia related to treatment of lipid disorders.
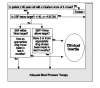
Figure 2
Algorithm to identify clinical inertia related to blood pressure control in patients with hypertension.
To operationally define clinical inertia, several decisions must be made. First, the clinical goals of care must be selected. For chronic diseases such as hypertension, diabetes, and lipid disorders, these goals are not solidly evidence based. Second, the therapy of the disease must be defined in such a way that it can be measured. With data now available, it is easier to recognize and measure drug therapy than it is to measure lifestyle interventions that are an important part of chronic disease care. This represents a limitation of the algorithms in Figures 1–3. Finally, one must define a time window from the date of a visit, test, or other clinical event within which intensification of therapy is designated as timely.
The measured patterns of clinical inertia will vary depending on how treatment goals, therapies, and time windows are selected or defined. Flexibility in how clinical inertia is defined could be seen by some as a limitation. However, from the point of view of care improvement, this sort of flexibility may often be an advantage because it allows local tailoring of initiative and interventions. Thus, the algorithms in Figures 1–3 are presented as examples rather than as concrete definitions of how to measure clinical inertia.
Prevalence and cost of clinical inertia
Prevalence
It is clear from numerous clinical trials that the final common pathway leading to glycemic, lipid, and blood pressure (BP) control is intensified pharmacotherapy. 6 – 8 Failure to appropriately intensify pharmacologic therapy is also a fundamental cause of inadequate chronic disease control in routine office practice. Although nonpharmacologic strategies have beneficial impact on BP, lipids, and glycemic control, 9 the magnitude of these effects are substantially less, on average, than the effects of pharmacologic interventions. For example, salt restriction, dietary approaches to stop hypertension (DASH), or alcohol restriction may lower systolic blood pressure (SBP) about 5–10 mm Hg on average, while drug combinations routinely lower SBP more than 10 mm Hg in many clinical trials. 10 – 12
Despite evidence that intensified therapy is usually needed to achieve and maintain evidence-based chronic disease care goals, a number of studies document high levels of clinical inertia in patients with diabetes or lipid disorders 13, 14 and demonstrate that more active clinical management improves absolute glycated hemoglobin (A1c), low-density lipoprotein-cholesterol (LDL), and SBP control. 12, 15
Cost
The frequent occurrence of serious adverse events as a direct result of clinical inertia has been well documented by both epidemiological studies and randomized clinical trials. Recent metanalyses suggest that for approximately every 20 adults with type 2 diabetes with an A1c value 1 percent over the current goal of 7 percent, 1 patient will have suffered a potentially avertable microvascular complication over a 5-year time frame. For every 20 diabetes patients with LDL 30 mg/dl above goal, there will be 1 excess myocardial infarction or stroke over 5 years. For every 20 patients with SBP 10 mm Hg above 150 mm Hg, there will be 1 additional heart attack or stroke, plus 1 additional occurrence or worsening of a microvascular complication over 5 years. 6, 8, 12, 16 – 19 More than 12 million adults are under treatment for type 2 diabetes in the United States, and at best only 20 percent simultaneously have their A1c, SBP, and LDL at goal. Thus, clinical inertia in diabetes care may lead to several hundred thousand serious adverse events, billions of dollars of excess health care charges for these events, and tens of thousands of excess deaths per year in the United States alone. 20, 21
What are the causes of clinical inertia?
Figure 4 shows a conceptual model of the causes of clinical inertia. We postulate that clinical inertia has three principal sources: physician factors, patient factors, and office system factors. If, as we hypothesize, clinical inertia has several sources that may interact in complex ways, then the development of interventions to reduce clinical inertia may best be multifactorial in nature to optimize their effectiveness.
Physician factors that contribute to clinical inertia
In a pioneering paper on clinical inertia, Phillips et al. 22 enumerates three physician factors that contribute to the problem. First, physicians overrate the quality of the care they already deliver and substantially underestimate the number of patients in need of intensified pharmacotherapy. There is substantial evidence to support this argument. 23 – 27
Second, physicians make “soft excuses” to avoid intensifying care. These include blaming patients for nonadherence to previous recommendations, citing lack of time at office visits, or suggesting that the physician can tell (without asking) that the patient will resist any suggestion to intensify therapy. While there is a certain level of nonadherence within the diabetes patient population, it should not be used as an excuse for inaction in specific cases without strong supporting evidence for its existence, such as not filling prescriptions. Both qualitative and quantitative studies support this argument. 28 – 31
Third, physicians lack the relevant knowledge, tools, training, and office systems to support active care of those with chronic diseases. There is substantial evidence to support this third point as well. 32 – 38
While the clinical points made by Phillips et al. are highly relevant, there are additional considerations related to the physician's role in clinical inertia that can be gleaned from a cognitive science analysis of the processes involved in chronic disease care. In chronic disease management, the task faced by health care providers (and the patient) is one in which decisions are made to control a process that is manifest in varying states of patient health. In this setting the provider must make a series of decisions over time, where each decision is dependent on past as well as future choices. The setting is subject to change, both autonomously and as a result of past decisions.
Under these conditions, decisions can be organized as a strategy comprised of three parts: (1) setting clinical goals, (2) initiating appropriate treatment, and (3) titrating (adjusting) treatment over time to reach clinical goals. Implementation of such a strategy must also recognize and manage conditions that interfere with achieving goals.
The presence of clinical inertia in the management of chronic disease can be represented as patterns of care based on the use of a decision strategy (as above) to achieve particular states of patient health (e.g., level of BP). Such patterns of care reflect failure of one or more parts of the decision strategy (i.e., failure to set appropriate goals, failure to initiate appropriate treatment, failure to titrate to goal). To assess the failures of thinking underlying these patterns, we draw on research conducted to explain failures of decisionmaking more generally. In such work there is evidence to suggest that failures of decisionmaking of the sort that we have identified as clinical inertia can be grouped into three categories:
- 1.
In the first category, decisions are made based on goal-related pathologies of two types. In one type, goals continually shift over time, so that decisions are never consistent and final goal states are never achieved. (This type of pathology is sometimes referred to as thematic vagabonding.) 39 A second type of goal pathology for this category of clinical inertia is one in which decisions are made based on goals with which the decisionmaker is most familiar (and most comfortable), even though they may be inappropriate. This type of pathology is often referred to as goal fixation, since it typically occurs in the face of evidence that the current decision strategy is unsuccessful. 40
- 2.
A second category of clinical inertia is based on use of a faulty control strategy. To control a time-varying process, decisions must reflect not only current states, but future states as well. The use of information to take action based on anticipated system states is referred to as feed forward process control. 41 An alternative strategy is feedback control. In feedback control, decisions are based solely on current information. The mental model employed with feedback control typically fails to reflect both time-dependent as well as positive feedback processes. 42, 43 Because information regarding the appropriateness of a given mental model is often obscure, the decision agent fails to realize that the mental model being used is disconnected from reality. 44 As a result, control of patient states is never achieved.
- 3.
The third category of clinical inertia is based on the use of faulty control actions. 45 There are three types in this category. In the first type, the threshold for taking action is either inaccurate or missing. In either case, the initial action is incorrect and the system response is not as expected. Subsequent actions are also in error, since knowledge of appropriate thresholds fails to cover future states as well as the initial state. The second type of failure is based on choice of response taken as the control action. 46 Here the issue is lack of knowledge (or faulty knowledge) regarding the kind or amount of action taken (e.g., the type and dose of medication). A related issue is failure to understand how multiple actions should be coordinated over time so as to achieve a desired effect (titration to goal). The third type of failure in this category of clinical inertia is based on lack of understanding of the side effects or consequences of actions taken. 40, 46 Many of the pathologies of thinking comprising clinical inertia stem from failure to understand how positive and negative feedback processes are linked in the system under consideration. As a result, the process of interest (e.g., patient state) may deteriorate (or become unpredictable) as a consequence of actions intended to correct it.
Patient factors that contribute to clinical inertia
Patient factors that contribute to the problem of clinical inertia may include patient denial of a disease, the belief that a particular disease is not dangerous, medication nonadherance, or resistance to adopting lifestyles that support chronic disease care. 30 Several studies suggest that about one-third of the problem of inadequate diabetes care is related to patient factors such as those listed in Figure 4. 47, 48
Most patient-related factors that contribute to clinical inertia are familiar to physicians, but a few comments may be called for. Clinical inertia may derive in part from the patient's mental model of the disease process. A patient who does not really believe they have diabetes, or believes that diabetes is not a serious disease, may be unwilling to make the lifestyle adjustments needed to care for diabetes. It is possible that interventions targeted directly to such a patient's mental model of diabetes may reduce clinical inertia by increasing the patient's willingness to intensify treatment. The power of mental models and their susceptibility to influence using marketing approaches is illustrated by successful direct-to-consumer pharmaceutical advertisements that encourage treatment of conditions such as erectile dysfunction, facial wrinkles, and tinea unguis. Similar strategies that encourage greater use of statins and certain antihypertensive or glucose-lowering therapies have also created substantial market demand for specific newer therapies. This approach would be expected to reduce clinical inertia, if flexibility in selection of specific pharmacologic agents is emphasized.
Office system factors that contribute to clinical inertia
A number of office system factors that may contribute to the problem of clinical inertia are listed in Figure 4. These include a reactive style of practice in which the delivery of clinical care is triggered by a patient-initiated visit, with the patient setting the clinical agenda for the visit. In many settings, primary care physicians, who are variably skilled in the science and art of chronic disease care, deliver care. Moreover, clinical practice guidelines, which seek to reduce the amount of variation in the delivery of chronic disease care, may lead to loss of appropriate customization of care to the clinical status and behavioral readiness of individual patients.
Promising approaches to reduce clinical inertia
Despite the need for effective strategies to reduce clinical inertia and promote a more active approach to chronic disease care, few researchers have developed interventions based on learning theory or office systems theory to reduce clinical inertia in chronic disease care. We postulate that interventions that address the principal factors contributing to clinical inertia—physician factors, patient factors, and office system factors—will be powerful enough to reduce the rate of clinical inertia related to chronic disease care in primary care settings.
A variety of practical approaches to reduce clinical inertia are possible. While it seems reasonable to direct intervention strategies to specific root causes of inertia, quality improvement theory suggests that multiple interventions that simultaneously target multiple factors tend to be more powerful than interventions that limit their focus to a single factor. We ask the reader to keep this guiding principle in mind when considering the following list of promising strategies to reduce clinical inertia in chronic disease care.
Feedback to physicians and/or patients on quality of chronic disease care
One strategy that has been somewhat successful as a way to reduce clinical inertia is monitoring and providing feedback of quality of care to physicians. Typically, patients with selected chronic diseases are identified using diagnostic codes, and information on selected aspects of their care is systematically given as feedback to provider teams. The information is specific enough to support patient-specific clinical actions to remedy deficits in care. These active interventions with patients may be prioritized on the basis of factors such as resource availability, patient readiness to change, or degree of risk of complications.
Feedback of information on care directly to patients has also been done with positive results in some settings. Such a strategy could be as simple as mailing a patient their A1c or LDL lab results with advice on what to do in response to the value. More sophisticated feedback, including providing graphs indicating trends in A1c or LDL values over time, with indicators of evidence-based goal, and with suggestions for both lifestyle changes and the likely need to intensify or change pharmacotherapy, have been shown to be acceptable to most patients in some recent studies. 49, 50 Such strategies may serve the synergistic purposes of activating both the patient and the patient's physician to focus on selected aspects of care where intensification of care is likely to provide significant benefit to the patient.
Cognitive interventions targeting specific decision pathologies
Diagnostic tools that assess a given physician's decisionmaking pathologies have recently been developed by others and us, and are reported elsewhere in this publication. 51 One type of diagnostic tool relies on simulated cases presented to individual physicians in an interactive format. It takes about 60 minutes of monitoring a physician's performance on such cases to identify specific errors related to the physician's goal setting, control strategies, control actions, or deficiencies in necessary knowledge or skills related to chronic disease care. A second approach is to use algorithms to search automated clinical databases and passively identify errors of omission or commission that actually occur in the care a particular physician provides to his or her real patients. The accuracy of this method of identifying decisionmaking pathologies also appears to be acceptable and is described elsewhere in these volumes.
Once a diagnosis has been established, specific learning interventions may be applied to address the decision pathology. Many types of learning interventions to either physicians or patients may be considered. The efficacy of customized case-based learning, with selection of teaching cases to focus on the identified decision pathology, has been successful in some fields of inquiry, and its application to reduce clinical inertia and medical errors appears promising. We are currently investigating one customized approach to case-based learning. 51 Case-based learning approaches are currently being applied by several medical schools and by the National Board of Medial Examiners to improve the training of medical students and residents.
Enhanced primary care—frequent office visits
Clinical trials are designed to provide planned care that achieves specified clinical objectives. In contrast, usual primary care practice is too often chaotic and unplanned. It has been argued that incorporating key design features of clinical trials into routine chronic disease care may dramatically improve care and reduce clinical inertia. 33, 52, 53 However, clinical trial protocols are notoriously expensive and too resource-intensive to provide a practical template for usual care. As primary care physicians with experience in both routine primary care practice and in clinical trials, we have identified three particular features of clinical trial protocols that may be transferable to routine primary care practice. One feature is frequent, carefully planned office visits. The second feature is timely and tailored decision support for providers, to prompt appropriate initiation and adjustment of medications until specified clinical goals are achieved. The third feature is emphasis on physician accountability by introducing visit resolution tools that systematically record whether recommended intensifications were actually implemented.
The recommended visit interval of 3–6 months for those with diabetes, hypertension, and some other chronic diseases is based on expert opinion rather than empiric data. This visit interval may be appropriate for patients who have already achieved acceptable levels of BP, A1c, or lipid control. However, patients who have not achieved recommended clinical goals may, if they exhibit motivation to improve their care, benefit from more frequent visits to sustain focus on specific domains of care. 54, 55 The argument for more frequent chronic disease care visits for those not in control is reinforced by experience in the various clinical trials. For example, in some clinical trials, an initial “pulse” of four consecutive monthly visits improved both SBP and A1c substantially. These improved levels of care were subsequently maintained by visits every 4 months for more than 3 years. From these observations we conclude that (a) a pulse of four consecutive monthly visits enables effective and timely intensification of therapy and leads to sustained improvement in chronic disease care, and (b) after such a pulse of visits, return to an every-4-month visit frequency is sufficient to maintain improved care for many patients.
There are many theoretical reasons why increased frequency of office visits may reduce clinical inertia in routine office practice:
- The physician has more opportunities to intensify care.
- Frequent visits may send the patient the message that intensified care is important and reduce patient resistance to initiation or up-titration of pharmacotherapy.
- More frequent assessment of response to the previous medication adjustments allows more rapid titration to clinical goal.
- The physician and patient increase their familiarity and presumably their trust of one another.
- The physician and patient learn that frequent medication adjustments are a predictable part of excellent chronic disease care, rather than a sign of failed therapy.
The marginal direct cost of a sequence of four visits at monthly intervals includes the cost of only two extra office visits (relative to an average of five clinic visits per patient per year), plus the cost of any additional medications or tests done at the “extra” visits. This marginal cost may compare quite favorably with the cost of other interventions to improve chronic disease care, some of which involve use of expensive home-monitoring equipment or elaborately structured patient education programs. For cost comparison, case management programs offered by outside vendors are typically implemented for a longer period of time than 4 months and often cost from $75 to $150 per patient per month, or $900 to $1,800 per patient per year, exclusive of increased medication costs.
Enhanced primary care—clinical decision support
Decision support is a critically important component of chronic disease care, especially for patients with complex chronic diseases, such as hypertension. 52, 56 – 59 Decision support is defined as timely information made available to providers, which prompts appropriate intensification of therapy to reach evidence-based hypertension care goals. Three recent randomized trials illustrate both the potential and the limitations of currently available decision support systems for chronic disease care. 35, 36, 60
In 2003, Meigs et al. 36 reported that an electronic medical record (EMR) that prompted physicians to intensify therapy, but did not provide tailored or specific decision support (what drug, what dose), failed to improve A1c levels. In a 2002 Mayo Clinic study, a similar rudimentary decision support delivered through an EMR system increased frequency of A1c testing but failed to improve A1c levels. 35 A recent report from Tierney et al., in Indiana demonstrated that even more sophisticated clinical decision support interventions did not improve the care of heart disease patients in office settings. 60 From these and other studies we conclude that (a) decision support of a general nature does lead to changes in physician behavior, and (b) to improve A1c levels, more specific and tailored decision support based on and tailored to specific physicians' cognitive processes may be needed.
An alternative to EMR-based decision support interventions is paper-based, tailored clinical support and simple treatment algorithms to guide chronic disease care. In a clinical trial we are currently participating in, paper-based clinical decision support tools have led to very low rates of clinical inertia and correspondingly high rates of clinical improvement for SBP, A1c, and lipids. From this we conclude that (a) decision support does not have to be EMR-based to be effective, and (b) tailored information based on simple treatment protocols (what drug, what dose) can lead to improved SBP, A1c, and lipid control.
Enhanced primary care—visit resolution and accountability tools
A key strategy used to change physician's behavior in large clinical trials is routine documentation, after each office visit or telephone contact, of whether protocol-determined changes in therapy were actually made. If recommended changes in therapy were not made, the physician must report why not. Not only does such documentation provide the opportunity to enumerate and classify physician-reported reasons for clinical inertia, but it also provides a strong element of accountability to providers who must justify a specific occurrence of clinical inertia. Simple visit resolution and accountability tools direct physician attention to proper drug intensification and provide useful documentation of physician-reported reasons (Phillips' “soft reasons”) why therapy was not intensified. This information on common reasons for clinical inertia—even when the physician knows that clinical goals are not being met—facilitates the evolution of ongoing efforts to reduce clinical inertia.
Financial incentives
Positive incentives to physicians to focus on certain clinical goals may also be effective ways to reduce clinical inertia. For example, providing cash payments to medical groups that achieve targeted levels of A1c, LDL, or SBP in certain groups of patients, such as those with diabetes or heart disease, has often led to more effective care through more intensive pharmacotherapy. 61 Factors that amplify or dampen the effect of various types of incentives offered at various points in the care delivery system need further investigation. 62
Barriers to improving clinical inertia
A discussion of strategies to reduce clinical inertia would not be complete without mentioning some of the real-world factors that may impede application of potentially effective interventions. Monitoring and feedback of clinical information, which is the heart of most improvement strategies, requires vigilance to assure that patient privacy or confidentiality is not violated. This problem is mitigated to some degree if the information is developed, stored, and applied locally, for example, at the level of the patient's clinic. The Health Insurance Portability and Accountability Act of 1996 (HIPAA) regulations and some State laws related to privacy or confidentiality may make centralized approaches to patient-level data impractical, unethical, or illegal.
Another fundamental obstacle to overcoming clinical inertia is physician disagreement on the evidence supporting a care recommendation or disagreement with clinical goals, even when supported by evidence. One of the potential benefits of clinical guidelines is to specify clinical goals. Conversely, when clinical guidelines advocate discordant goals, the resultant confusion among practitioners makes efforts to reduce clinical inertia more difficult. 63
Often, physicians appropriately tailor clinical goals—for example, BP, A1c, or LDL goals—to specific patient circumstances. 29 Thus, patients who are elderly, have serious comorbidities, or have affective or substance-abuse problems are often not treated as aggressively as other patients. 64 Unfortunately, physician judgments about patient desires, motivation, and readiness to change may be erroneous. Moreover, numerous studies have shown that more appropriate lipid treatment is provided for men than women. 14 These considerations suggest that “tailoring” of care is often appropriate, but may sometimes be done in a way that does not optimize well-defined clinical benefits.
Methodological challenges in clinical inertia research
To advance research on clinical inertia, a number of methodological points will need refinement and further conceptual or practical development. First, the working definition of clinical inertia is evolving and will likely need modification as it is applied across a broad clinical spectrum.
Second, the operational definition of clinical inertia is necessarily time-dependent and can be measured from the time of an office visit, test, or other assessment until a later point in time. The time window should be selected to allow sufficient time for an appropriate medication change to impact its target measure, yet represent a clinically reasonable outer bound for the clinically acceptable time interval between data collected at a visit or test, and clinical action.
Third, the adequacy of therapy is a concept that requires reflection and clear conceptual definition. If adequate therapy is being provided, but the patient has yet to reach evidence-based clinical goals, the problem is not clinical inertia. The definition of “adequate therapy” can become operationally quite complex.
Conclusion
Clinical inertia contributes to many medical errors and contributes to widespread failure to achieve evidence-based goals related to blood pressure control, glucose control, lipid control, and other clinical domains. 65, 66 There is little doubt that clinical inertia contributes enormously to the burden of potentially preventable adverse events, deaths, and excess long-term health care costs caused by inadequate chronic disease control. 16, 17, 67
Clinical inertia derives from a number of sources and is influenced by physician, patient, and office system factors. Physician factors include specific decisionmaking pathologies, overestimation of care actually delivered, disagreement with evidence-based goals of care, and “soft reasons” to avoid the efforts required to intensify therapy. Patient factors include unawareness of the need to intensify therapy, denial or overly optimistic views of the risks presented by chronic diseases, and avoidance of increased expenses and side effects associated with more intensive therapy. Office system factors include lack of data to monitor the quality of care and routinely identify patients in need of more intensive care, lack of visit planning, lack of active outreach to patients in need of care, and failure to implement decision support strategies—especially those that provide actionable information at the time of an office visit.
Fortunately, advances in behavior change science 34, 40, 43, 68 – 72 and the increasing availability of EMRs and other office systems to better support chronic disease care 57, 73 – 75 may support conceptually cogent and practical interventions to reduce clinical inertia. On the basis of available evidence, we recommend three major avenues to reduce clinical inertia: (a) cognitive behavioral interventions directed to physicians, (b) information systems redesign interventions, and (c) patient-direct interventions to increase demand for more intensive chronic disease care. Further work is needed to assess the comparative effectiveness and return on investment of various interventions to reduce the problem of clinical inertia.
References
- 1.
- Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
- 2.
- Kohn LT, Corrigan JM, Donaldson MS, editors. To err is human: building a safer health system. A report of the Committee on Quality of Health Care in America, Institute of Medicine. Washington, DC: National Academy Press; 2000. [PubMed: 25077248]
- 3.
- Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003 Jun 28;326(7404):1419. [PMC free article: PMC162259] [PubMed: 12829553]
- 4.
- Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003 Jun 23;163(12):1409–16. [PubMed: 12824090]
- 5.
- Barker KN, Flynn EA, Pepper GA. et al. Medication errors observed in 36 health care facilities. Arch Intern Med. 2002 Sep 9;162(16):1897–1903. [PubMed: 12196090]
- 6.
- Systolic Hypertension in the Elderly Program. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: Final results of the Systolic Hypertension in the Elder Program (SHEP). JAMA. 1991;265:3255–64. [PubMed: 2046107]
- 7.
- Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002 Dec 18;288(23):2981–97. [PubMed: 12479763]
- 8.
- Hansson L, Zanchetti A, Carruthers SG. et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998 Jun 13;351(9118):1755–62. [PubMed: 9635947]
- 9.
- Egede LE. Lifestyle modification to improve blood pressure control in individuals with diabetes: is physician advice effective? Diabetes Care. 2003 Mar;26(3):602–7. [PubMed: 12610008]
- 10.
- Chobanian AV, Bakris GL, Black HR. et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–72. [PubMed: 12748199]
- 11.
- Gaede P, Vedel P, Larsen N. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003 Jan 30;348(5):383–93. [PubMed: 12556541]
- 12.
- Pyorala K, Pedersen TR, Kjekshus J. et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997 Apr;20(4):614–20. [PubMed: 9096989]
- 13.
- O'Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003 Dec 8;163(22):2677–78. [PubMed: 14662620]
- 14.
- O'Connor PJ, Rush WA, Trence DL. Relative effectiveness of niacin and lovastatin for treatment of dyslipidemias in a health maintenance organization. J Fam Pract. 1997 May;44(5):462–7. [PubMed: 9152263]
- 15.
- O'Connor PJ, Quiter ES, Rush WA. et al. Impact of hypertension guideline implementation on blood pressure control and drug use in primary care clinics. Jt Comm J Qual Improv. 1999;25(2):68–77. [PubMed: 10027112]
- 16.
- Barton S. Clinical evidence. London: BMJ Publishing Group; 2002.
- 17.
- O'Connor PJ, Spann SJ, Woolf SH. Care of type 2 diabetes mellitus: a review of the evidence. J Fam Pract. 1998;47(5 Suppl):S13–22. [PubMed: 9834750]
- 18.
- UK Prospective Diabetes Study (UKPDS) Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ. 1998 Sep 12;317(7160):713–20. [PMC free article: PMC28660] [PubMed: 9732338]
- 19.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. UKPDS 34. Lancet. 1998 Sep 12;352(9131):854–65. [PubMed: 9742977]
- 20.
- O'Connor PJ, Gilmer T, Rush WA. The cost of poor glycemic control. Paper presented at the American Diabetes Association's 57th Annual Meeting and Scientific Sessions; 1997 Jun 21–24; Boston, MA.
- 21.
- Wagner EH, Sandhu N, Newton KM. et al. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285(2):182–9. [PubMed: 11176811]
- 22.
- Phillips LS, Branch WT, Cook CB. et al. Clinical inertia. Ann Intern Med. 2001 Nov 6;135(9):825–34. [PubMed: 11694107]
- 23.
- Kuykendall DH, Ashton CM, Johnson ML. et al. Identifying complications and low provider adherence to normative practices using administrative data. Health Serv Res. 1995 Oct;30(4):531–54. [PMC free article: PMC1070074] [PubMed: 7591780]
- 24.
- Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ. 2003 Jan 25;326(7382):196. [PMC free article: PMC140276] [PubMed: 12543836]
- 25.
- Ely JW, Osheroff JA, Ebell MH. et al. Obstacles to answering doctors' questions about patient care with evidence: qualitative study. BMJ. 2002 Mar 23;324(7339):710. [PMC free article: PMC99056] [PubMed: 11909789]
- 26.
- Brown JB, Harris SB, Webster-Bogaert S. et al. The role of patient, physician and systemic factors in the management of type 2 diabetes mellitus. Fam Pract. 2002 Aug;19(4):344–9. [PubMed: 12110552]
- 27.
- Kirk JK, Poirier JE, Mattox MG. et al. Compliance with national guidelines in patients with diabetes in a family practice clinic. Pharmacotherapy. 2002 Dec;22(12):1541–6. [PubMed: 12495165]
- 28.
- Ziemer DC, Christopher D, Doyle JP, et al. Differences in clinical decision-making underlie differences in diabetes control in a primary care site compared to a specialized diabetes clinic. Paper presented at the 60th Scientific Session of the American Diabetes Association; 2000; San Antonio, TX.
- 29.
- Helseth L, Sussman S, Crabtree B. et al. Primary care physicians' perceptions of diabetes management. A balancing act. J Fam Pract. 1999;48(1):37–42. [PubMed: 9934381]
- 30.
- O'Connor PJ, Crabtree BF, Yanoshik MK. Differences between diabetic patients who do and do not respond to a diabetes care intervention: a qualitative analysis. Fam Med. 1997 Jun;29(6):424–8. [PubMed: 9193915]
- 31.
- Stolar MW. Clinical management of the NIDDM patient. Impact of the American Diabetes Association practice guidelines, 1985-1993. Endocrine Fellows Foundation Study Group. Diabetes Care. 1995 May;18(5):701–7. [PubMed: 8586012]
- 32.
- Casalino L, Gillies RR, Shortell SM. et al. External incentives, information technology, and organized processes to improve health care quality for patients with chronic diseases. JAMA. 2003 Jan 22-29;289(4):434–41. [PubMed: 12533122]
- 33.
- Wagner EH, Davis C, Schaefer J. et al. A survey of leading chronic disease management programs: are they consistent with the literature? Manag Care Q. 1999;7(3):56–66. [PubMed: 10620960]
- 34.
- O'Connor PJ, Solberg LI, Baird M. The future of primary care. The enhanced primary care model. J Fam Pract. 1998 Jul;47(1):62–7. [PubMed: 9673610]
- 35.
- Montori VM, Dinneen SF, Gorman CA. et al. The impact of planned care and a diabetes electronic management system on community-based diabetes care: the Mayo Health System Diabetes Translation Project. Diabetes Care. 2002 Nov;25(11):1952–7. [PubMed: 12401738]
- 36.
- Meigs JB, Cagliero E, Dubey A. et al. A controlled trial of Web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care. 2003 Mar;26(3):750–7. [PubMed: 12610033]
- 37.
- Sanders KM, Satyvavolu A. Improving blood pressure control in diabetes: limitations of a clinical reminder in influencing physician behavior. J Contin Educ Health Prof. 2002 Winter;22(1):23–32. [PubMed: 12004637]
- 38.
- Mazzi CP, Kidd M. A framework for the evaluation of Internet-based diabetes management. J Med Internet Res. 2002 Jan-Mar;4(1):e1. [PMC free article: PMC1761926] [PubMed: 11956033]
- 39.
- Brehmer B. Dynamic decision-making: human control in complex systems. Acta Psychol (Amst). 1992;81(3):211–41. [PubMed: 1462786]
- 40.
- Dorner D. The logic of failure. Reading, MA: Addison-Wesley; 1997.
- 41.
- Brehmer B. Strategies in real-time, dynamic decision making. In: Hogarth RM, editor. Insights in decision making. Chicago, IL: University of Chicago Press; 1990. pp. 263–79.
- 42.
- Sterman JD. Misperceptions of feedback in dynamic decision-making. Organ Behav Hum Decis Process. 1989;43(3):301–35.
- 43.
- Bainbridge L. The process controller. In: Singleton WT, editor. The anlaysis of practical skills. Baltimore, MD: University Park Press; 1978. pp. 236–63.
- 44.
- Weick KE. Technology as equivoque: sensemaking in new technologies. In: Goodman PS, Sproull LS, Associates, editors. Technology and organizations. San Francisco: Jossey-Bass; 1990.
- 45.
- Umbers IG. Models of the process operator. Int J Man-Machine Stud. 1979;11:263–84.
- 46.
- Brehmer B, Allard R. Dynamic decision making: the effects of task complexity and feedback delay. In: Rasmussen J, Brehmer B, Leplat J, editors. Distributed decision making: cognitive models for cooperative work. Chichester, West Sussex, England: John Wiley & Sons Ltd.; 1991. pp. 319–33.
- 47.
- Pellegrini F, Belfiglio M, De Berardis G. et al. Role of organizational factors in poor blood pressure control in patients with type 2 diabetes: the QuED Study Group—quality of care and outcomes in type 2 diabetes. Arch Intern Med. 2003 Feb 24;163(4):473–80. [PubMed: 12588208]
- 48.
- Heisler M, Bouknight RR, Hayward RA. et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002 Apr;17(4):243–52. [PMC free article: PMC1495033] [PubMed: 11972720]
- 49.
- Sperl-Hillen J, O'Connor PJ, Carlson RR. et al. Improving diabetes care in a large health care system: an enhanced primary care approach. Jt Comm J Qual Improv. 2000 Nov;26(11):615–22. [PubMed: 11098424]
- 50.
- Peterson KA, Hughes M. Readiness to change and clinical success in a diabetes educational program. J Am Board Fam Pract. 2002 Jul-Aug;15(4):266–71. [PubMed: 12150458]
- 51.
- Dutta P, Biltz GR, Johnson PE, et al. SimCare: a simulation model to investigate physician decision-making in the care of patients with type 2 diabetes. In: Henriksen K, Battles J, Marks E, Lewin DI, editors. Advances in patient safety: from research to implementation. Vol. 4, Programs, tools and products. Rockville, MD: Agency for Healthcare Research and Quality; 2005. [PubMed: 21250021]
- 52.
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998 Aug-Sep;1(1):2–4. [PubMed: 10345255]
- 53.
- Diabetes Control and Complications Trial Research Group. Resource utilization and costs of care in the diabetes control and complications trial. Diabetes Care. 1995;18(11):1468–78. [PubMed: 8722072]
- 54.
- American Diabetes Association. Clinical practice recommendations 2003. Diabetes Care. 2003;26(Suppl 1):S1–S156. [PubMed: 9028710]
- 55.
- Petitti DB, Grumbach K. Variation in physicians' recommendations about revisit interval for three common conditions. J Fam Pract. 1993;37(3):235–40. [PubMed: 8409873]
- 56.
- Von Korff M, Gruman J, Schaefer J. et al. Collaborative management of chronic illness. Ann Intern Med. 1997 Dec 15;127(12):1097–102. [PubMed: 9412313]
- 57.
- Wagner EH, Austin BT, Korff MV. Organizing care for patients with chronic illness. Milbank Mem Fund Q. 1996;74(4):511–44. [PubMed: 8941260]
- 58.
- De Jaegher K, Jegers M. The physician-patient relationship as a game of strategic information transmission. Health Econ. 2001 Oct;10(7):651–68. [PubMed: 11747047]
- 59.
- Young RJ, Khong CK, Vaughan NJ. et al. The evolution of diabetes information systems. Diabet Med. 2002 Jul;19(Suppl 4):6–12. [PubMed: 12121331]
- 60.
- Tierney WM, Overhage JM, Murray MD. et al. Effects of computerized guidelines for managing heart disease in primary care. J Gen Intern Med. 2003 Dec;18(12):967–76. [PMC free article: PMC1494965] [PubMed: 14687254]
- 61.
- O'Connor PJ, Solberg LI, Whitebird RR, et al. The impact of clinic and medical group characteristics on diabetes care outcomes in primary care clinics. Paper presented at the American Diabetes Association's 64th Scientific Sessions; 2004; Orlando, FL.
- 62.
- Hellinger F. Regulating the financial incentives facing physicians in managed care plans. Am J Manag Care. 1998;4(5):663–74. [PubMed: 10179920]
- 63.
- Solberg L, Brekke M, Fazio C. et al. Lessons from experienced guideline implementers: attend to many factors and use multiple strategies. Jt Comm J Qual Improv. 2000;26(4):171–88. [PubMed: 10749003]
- 64.
- Brown AF, Mangione CM, Saliba D. et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003 May;51(5 Suppl Guidelines):S265–80. [PubMed: 12694461]
- 65.
- Avorn J. Improving drug use in elderly patients: getting to the next level. JAMA. 2001 Dec 12;286(22):2866–8. [PubMed: 11735764]
- 66.
- Shekelle PG, Kahan JP, Bernstein SJ. et al. The reproducibility of a method to identify the overuse and underuse of medical procedures. N Engl J Med. 1998;338(26):1888–95. [PubMed: 9637810]
- 67.
- Gilmer TP, O'Connor PJ, Manning WG. et al. The cost to health plans of poor glycemic control. Diabetes Care. 1997;20(12):1847–53. [PubMed: 9405905]
- 68.
- O'Connor PJ, Rush WA, Solberg LI, et al. Variation in diabetes care outcomes across patients, providers, and clinics: an hierarchical analysis. Paper presented at the ADA National Committee Meeting; Mar 17, 2002; Alexandria, VA.
- 69.
- Pine BJI. Mass customization. Boston: Harvard Business School Press; 1999. pp.171–212.
- 70.
- Glasgow RE, Toobert DJ, Hampson SE. et al. Behavioral research on diabetes at the Oregon Research Institute. Ann Behav Med. 1995;17:32–40. [PubMed: 24203500]
- 71.
- Glasgow R, Wagner E, Kaplan R. et al. If diabetes is a public health problem, why not treat it as one? A population-based approach to chronic illness. Ann Behav Med. 1999;21(2):159–70. [PubMed: 10499137]
- 72.
- Glaser BG, Strauss AL. Discovery of grounded theory: strategies for qualitative research. London: Wiedenfeld and Nicholson; 1967.
- 73.
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002 Oct 9;288(14):1775–9. [PubMed: 12365965]
- 74.
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002 Oct 16;288(15):1909–14. [PubMed: 12377092]
- 75.
- Boult C, Kane RL, Pacala JT. et al. Innovative healthcare for chronically ill older persons: results of a national survey. Am J Manag Care. 1999 Sep;5(9):1162–72. [PubMed: 10621082]
- Abstract
- Introduction
- Operational definition of clinical inertia
- Prevalence and cost of clinical inertia
- What are the causes of clinical inertia?
- Promising approaches to reduce clinical inertia
- Barriers to improving clinical inertia
- Methodological challenges in clinical inertia research
- Conclusion
- References
- Review Clinical inertia is the enemy of therapeutic success in the management of diabetes and its complications: a narrative literature review.[Diabetol Metab Syndr. 2020]Review Clinical inertia is the enemy of therapeutic success in the management of diabetes and its complications: a narrative literature review.Andreozzi F, Candido R, Corrao S, Fornengo R, Giancaterini A, Ponzani P, Ponziani MC, Tuccinardi F, Mannino D. Diabetol Metab Syndr. 2020; 12:52. Epub 2020 Jun 17.
- [Drawing up guidelines for the attendance of physical health of patients with severe mental illness].[Encephale. 2009][Drawing up guidelines for the attendance of physical health of patients with severe mental illness].Saravane D, Feve B, Frances Y, Corruble E, Lancon C, Chanson P, Maison P, Terra JL, Azorin JM, avec le soutien institutionnel du laboratoire Lilly. Encephale. 2009 Sep; 35(4):330-9. Epub 2009 Jul 9.
- Clinical inertia in diagnosis and treatment of hypertension in primary care: quantification and associated factors.[Blood Press. 2010]Clinical inertia in diagnosis and treatment of hypertension in primary care: quantification and associated factors.Gil-Guillén V, Orozco-Beltrán D, Pérez RP, Alfonso JL, Redón J, Pertusa-Martínez S, Navarro J, Cea-Calvo L, Quirce-Andrés F, Merino-Sánchez J, et al. Blood Press. 2010 Feb; 19(1):3-10.
- Decalogue of the Spanish Society of Arteriosclerosis to reduce therapeutic inertia.[Clin Investig Arterioscler. 2017]Decalogue of the Spanish Society of Arteriosclerosis to reduce therapeutic inertia.Blasco M, Pérez-Martínez P, Lahoz C. Clin Investig Arterioscler. 2017 Sep-Oct; 29(5):218-223. Epub 2017 Aug 26.
- Review Therapeutic inertia.[Aust Prescr. 2024]Review Therapeutic inertia.Usherwood T. Aust Prescr. 2024 Feb; 47(1):15-19.
- Clinical Inertia and Outpatient Medical Errors - Advances in Patient Safety: Fro...Clinical Inertia and Outpatient Medical Errors - Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology)
Your browsing activity is empty.
Activity recording is turned off.
See more...

