NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The development of a multicellular organism is mostly controlled at the transcriptional level but it has also been shown to require the transport of membrane and proteins through the exocytic pathway to the plasma membrane and the extracellular medium. As they are transported in the different compartments making up this pathway, newly synthesized proteins are modified and dispatched to their final destinations. In this review, we will first outline how mutations in genes encoding key proteins of this pathway, such as components of the COPII coat, tethers, components of the SNARE machinery, glycosylation enzymes, etc, lead to severe developmental defects. In the second part, we will describe how specific steps of epithelial development, such as epithelial cell formation, establishment of polarity, junction formation and morphogen secretion, are controlled or regulated by the exocytic machinery.
Introduction
The developmental journey from a single cell to an adult organism requires its proliferation followed by the differentiation of its progenitors. This is essential to shape a wide range of organs and structures that sustain the many functions a body performs. Cell proliferation and differentiation followed by organogenesis is mostly controlled at the transcriptional level, but it is clear that other cellular events and pathways are critical. Among these are membrane and protein traffic in the secretory and endocytic/lysosomal pathways. The role of endocytosis in certain aspects of development has recently been well (reviewed by Dudu et al (2004) and Emery and Knoblish, (2006) refs. 1,2), so we will focus here on the exocytic pathway. We first will introduce different molecular components, outlining the functional organisation of this pathway, and we will pinpoint developmental disorders brought about by mutations in the genes encoding key components of this pathway. This will shed a new light on how proteins fulfil specific functions in a multicellular organism. Some of this has been summarised a few years ago by Aridor and Hannan (2000, 2002),3,4 but exciting and unexpected recent discoveries have been made that we review here. In the second part, we will describe how certain steps of epithelial development depend on the exocytic pathway for their completion.
Alterations of the Exocytic Pathway Lead to Severe Development Defects
Proteins destined for secretion to the extracellular space or to the plasma membrane are synthesized and transported through a series of membrane bound organelles, making up the exocytic pathway.5 The proteins enter the exocytic pathway at the endoplasmic reticulum (ER) as newly synthesized proteins where they are glycosylated, folded and oligomerised before exiting the ER to be transported toward the Golgi apparatus from which they are sorted to their final destination.
Exit from the ER
Proteins exit the ER at specific sites called the ER exit sites, or tER sites, characterized by the presence of COPII coated vesicles. The COPII coat machinery includes the small GTPase Sar1 and its GEF (guanidine exchange factor), the transmembrane protein Sec12 as well as the Sec23/24 complex and the Sec13/31 scaffold 6 (see chapter by Pagant et al).
Sec23A and Craniofacial Diseases
Mutations in the human and zebrafish sec23a gene have recently been shown to lead to bone malformations (especially the cranio-facial bones in human) due to the seemingly specific retention of collagen in the ER that shows a large expansion.7,8 The cranial bones are seemingly more affected than other tissues due to the low Sec23B level that can therefore not compensate for the loss of function of Sec23A.
The mutation in sec23A leading to craniofacial diseases is subtle (replacement from a phenylalanine to a leucine). It does not affect binding to Sec24 and does not change its intrinsic GAP activity. However, the formation of COPII vesicles is inhibited, as the Sec13/31 complex is not recruited. Surprisingly, the inhibition is much more prominent when the mutant Sec23A protein is combined with Sar1B than with Sar1A. When combined to Sar1A, COPII vesicles can still form, at least in vitro. This modulation could be explained by the higher affinity of Sec13/31 complex for Sar1A than for Sar1B, indicating that their binding might involve at least one differential aminoacid.9
The lower affinity between Sec13/31 and Sar1B could lessen the constraints within the COPII cage and increase its flexibility, perhaps allowing the formation of COPII coated ER derived carriers larger than the typical 60-70nm COPII vesicles (discussed in detail in Hughes and Stephens, 2007).10 Sar1b could therefore be implicated in the ER exit of larger cargo.
Sar1B and Chylomicron Retention
In this context, it is interesting to point out that mutations in the human gene encoding Sar1B leads to clinically important defects in lipoprotein metabolism11 such as the Anderson's disease, in which the retention of chylomicron-like particle in membrane bound compartments is observed.11,12 Mutations in Sar1B either cause truncation or significant changes in the immediate vicinity of the GTP binding site of the protein. Chylomicrons are large lipoprotein particles that once secreted into the bloodstream, transport exogenous lipids to the liver, cardiac and skeletal muscle tissue. In patients carrying mutations in Sar1B, these particles are not released and seem to be retained in the ER. Sar1A and B have almost identical sequences and probably tightly overlapping distribution but Sar1B has at least one function that is nonredundant with Sar1A. As mentioned above, Sar1B could form a more flexible cage required for the packaging of large chylomicron lipoprotein particles. Alternatively, this difference could lie in coupling to a specific packaging receptor for lipoprotein particles, or involve the mobilization of lipid rather than protein. This remains to be investigated as the packaging of other large cargo do not seem to be affected in these patients.
In a Drosophila sar1 mutant, a block in anterograde transport from the dendrite Golgi outpost results in a specific reduction of dendrite outgrowth without affecting axon development as well as a complete dispersion of these outposts,13 in agreement with data in tissue cell cultures (CR personal communication).
Cargo Receptors
Cargo selection is a crucial step in the protein export out of the ER. The COPII subunit Sec24 plays a role in this selection,14 but several other cargo receptors also have been identified. One of them is the mannose binding transmembrane protein ERGIC53.15,16 Mutations in the gene encoding this protein results specifically in a combined deficiency of factor V and factor VIII (F5F8D) causing an autosomal recessive bleeding disorder characterized by coordinate reduction of the secretion of both clotting proteins.17,18
The Erv protein family19 also displays cargo sorting properties. In Drosophila, mutation in cornichon, which encodes the homologue of S. cerevisiae Erv14p, an integral membrane protein involved in sorting of Axl2,20 leads to a strong ventralization of the Drosophila egg. This is due to the lack of the ER export of the TGFα-like growth factor Gurken, which causes the deficient release of the Gurken bioactive peptide that normally signals to the adjacent follicle cells to adopt a dorsal fate.21,22
Vesicle Tethering, Docking and Fusion
Upon exiting the ER via COPII vesicles, the newly synthesized proteins reach the Golgi. A consensual view is that the COPII vesicles uncoat, fuse together or with a pre-existing intermediate compartment to reach the cis Golgi. Transport through the Golgi might occur through cisternal maturation, anterograde transport mediated by COPI vesicles or by tubular connection of cisternae within the same stack. In the cisternal maturation model, the COPI vesicles would mediate the retrograde movement of resident Golgi enzymes.23
Tethering
In any case, the net forward movement of newly synthesized proteins is mediated by fusion of vesicles to their cognate acceptor compartment. This is preceded by their tethering and docking, respectively. The small GTPase Rab family has been clearly involved in tethering. A key characteristic of the Rab proteins is that they undergo a cycle of GTP hydrolysis that controls their membrane association and often their effector binding. In a GTP loaded form, they are membrane bound and are able to deliver their effectors to this target membrane. These effectors might then bind factors also present in the target membrane and therefore mediate vesicle tethering.24 One class of factors recruited by Rab proteins are the Golgins, long coiled coil proteins that are involved in the functional organisation of the Golgi apparatus.25 How-ever, so far, no developmental phenotypes have yet been linked to mutations in the Golgins.
On the other hand, the Golgi localised Rab6 that is involved in several steps of intracellular trafficking has been shown to be triply required during Drosophila oogenesis.26,27 First, it is needed for the general organization and growth of the egg chamber. Indeed, in rab6 null eggchambers, exocytosis is greatly affected, especially in the nurse cells, in agreement with a role for Rab6 in TGN to plasma membrane transport.28 Second, Rab6 is required for the polarization of the oocyte microtubule cytoskeleton and localization of the polarity determinant, oskar mRNA, an effect that is mediated by the formation of a complex with Bicaudal-D.29 Third, this complex is also required for the properly delivery of a second polarity determinant, the TGFα homolog Gurken.
Recently, two other types of tethering complexes have been identified30 (see Chapter by Lupashin et al), namely the TRAPP I and II complexes,31,32 and the COG complex.33 Mutations in the genes encoding the COG subunits lead to metabolic disorders and developmental defects (see below).
The SNARE Machinery
Fusion of vesicles is mediated by SNAREs (soluble N-ethylmaleimide-sensitive attachment protein receptors), a family of type II membrane proteins all related to three different neuronal proteins, Synaptobrevin, Syntaxin1, and SNAP-2534-36 (see Chapter by Xu et al). The specific role of the SNAREs in membrane fusion is still to be precisely defined and seems difficult to resolve due to the redundancy and promiscuity of SNAREs. Hence, clear phenotypes from deletions/siRNA are usually unclear.
What is clear, however, is that they do not only play a crucial role in synaptic transmission37 but also in other steps of development.38,39 For instance, Drosophila carrying thermosensitive null alleles of SNAP-25 die at the pharate adult stage due to the inhibition in fusion of synaptic vesicles at the synapse.40 Furthermore, proper formation of the Drosophila embryo exoskeleton, the cuticle, requires the plasma membrane t-SNARE Syntaxin 1A. Syntaxin 1A is required for the fusion of secretory vesicles with the apical plasma membrane in the polarized cells of the epidermis.41-43 Syntaxin 1A seems therefore necessary for the bundle formation and secretion of chitin microfibrils in cuticle laminae.44
SNAP and Hydrocephaly
An interesting twist in the role of the SNARE fusion machinery in development comes from SNAP (the Soluble NSF attachment protein), normally involved in SNARE priming. The hyh (hydrocephalus with hop gait) phenotype in mice has been mapped to a mutation in SNAP, in which the methionine 105 is changed to an isoleucine. However, this methionine mutation does not change the structure and the function of the protein in its ability to bind and dissociate SNARE pairs, at least in vitro. The mRNA only seems slightly more unstable. Nevertheless, the fate of cells within the cerebral cortex is compromised in the mutant mice due to the reduced polarity of apical markers, leading to precocious neurogenesis. For instance, the localization of Vamp7, a vesicle SNARE typically involved in apical membrane transport in epithelial cells and neurons, was strongly apical in normal neuroepithelial cells and profoundly disrupted in hyh mutants. This suggests that a partial loss of SNAP disrupts its apical targeting (as well as that of many markers) without disrupting general transport or fusion, thus highlighting a novel function of SNAP.45-47
Bitesize and Epithelial Integrity
Another unexpected result comes from studying the synaptotagmin-like protein Bitesize. Synaptotagmins regulate SNARE complex formation.48,49 But recently Bitesize has been shown to be critical in epithelial integrity and in the stabilization of the adherens junction,50 functioning seemingly independently from SNAREs. Bitesize binds Moesin, a cytoplasmic protein that is believed to mediate membrane-cytoskeletal interactions at the apical domain of polarized epithelial cells51 and also apical F-actin assembly at the adherens junction.50 In bitesize embryo mutants, the integrity of the epithelium was disrupted due to the instability of adherens junctions.
Protein Glycosylation
In the Golgi apparatus, proteins en route to the cell surface and the extracellular medium are further modified, proteolytically cleaved and sorted. One important Golgi-based modification is the maturation/completion of complex oligosaccharides moieties attached by these proteins either through N- or O-linked glycosylation.
Congenital Disorders of Glycosylation
Years of research have led to the understanding that glycosylation is critical in the biological function of the large number of secreted proteins or plasma membrane receptors, and an emerging family of developmental disorders, the Congenital Disorders of Glycosylation (CDG) exemplifies this importance.52 The CDG are characterised by mutations in genes encoding proteins affecting O- and N-linked glycosylation53,54 (http://www.euroglycanet.org/). Very recently, mutations in genes encoding 4 of the 8 subunits of the COG complex have been shown to result in a CDG.55 The COG complex is a tethering complex involved in retrograde transport.33 One hypothesis is that mutation in the COG complex would alter its function in this transport step that in turn would lead to the loss of Golgi structural integrity. Protein glycosylation would, as a result, be affected, leading to serious developmental defects.
The importance of glycosylation in development has also been shown by the generation of knockout mice for genes with crucial functions in N-linked glycosylation, such as the gene encoding Mannosidase II56,57 leading to auto-immune disease.58,59 Furthermore, N-acetylglucosamine transferase I (NAGT1/Magt1)60 and NAGT5/Mgat561 have also been associated to diseases. The particularity of this latter enzyme is that its activity is increased in carcinomas and this could be a primary cause of cancer as it is able, through specific interactions with pTEN and/or galectin, to influence tumor formation and progression.62,63
Fringe
O-linked glycosylation has also been linked to developmental defects in Drosophila and mammals. Wing development requires that the dorsoventral margin is properly defined and Notch has been shown to be involved. Notch is a transmembrane protein localised at the plasma membrane of all cells across the dorsoventral margin and it acts as a receptor for proteins on the surface of neighbouring cells. The ligands for Notch on the cells on the ventral side of the margin is Delta and that on dorsal cells is Serrate. Crucially, Delta only activates Notch on cells on the dorsal side of the margin and Serrate only activates Notch on cells on the ventral side of the margin. The mechanism behind this specificity depends on Fringe.
Fringe is a Golgi resident N-acetylglusosamine transferase to O-linked fucose residues, and Notch is a substrate of Fringe in Drosophila.64,65 Once modified through this single sugar addition, Notch has a greater affinity for Delta than for Serrate, and this differential affinity is critical for the formation and maintenance of the dorsoventral wing margin. In fringe mutants, this margin is not maintained and the wings fail to develop properly.64,65
Notch is also a substrate of Fringe in mammals.66 The role of Lunatic Fringe (one of the three mammalian homologues of Fringe in mice) in Notch signalling has also been studied in the formation of the short whiskers that develop on the upper lip of the mouse (the vibrissae).67 Here, Lunatic Fringe seems also lead to differential substrate modifications, one in conjunction with Notch2 and Delta1 in the formation of dermal papilla, and the other in conjunction with Notch1 and Serrate2 in the segregation of the hair placodes (both being zones within the hair shaft that are involved in the formation of vibrissae in the embryo).
Furthermore, mice carrying homozygous disruptions of the genes that encode Notch1, Delta1 and Lunatic Fringe have been shown to die in mid-gestation with severe defects in the repetitive segmented structures of the somites, the precursors for the vertebrae.68 Recently elegant work provided evidence for the existence of a segmentation clock working in synchrony with the formation of each somite. This clock comprises the cyclical transcription of most of the genes involved in the Notch signaling pathway69 including the transcriptional oscillation of lunatic fringe.70 This supports the idea that cyclic activations of Notch signalling by lunatic fringe are essential for somite formation and patterning.
The GRASP65/55 Protein Family
Mirroring the complex and multiple functions that it carries out, the Golgi apparatus has a unique and remarkable architecture71 (see Chapter by Hua et al). It is characterized by stacks of flattened membrane bound compartments, the Golgi cisternae. In mammalian cells, the Golgi stacks are connected laterally by tubules to form the Golgi ribbon or reticulum capping the nucleus. In Drosophila, the stacks have been shown to be paired and are always found associated to tER sites, thus forming the tER-Golgi units.72
The stack architecture is a unique feature of the Golgi apparatus and the Golgi localized peripherally associated GRASP55 and 65 have been shown to mediate this stacking in vitro.73,74 However, depletion of these proteins from mammalian or Drosophila cells does not lead to significant disruption in stacking75,76 leading to the notion that this family of proteins could have additional, perhaps unrelated functions.
This has been investigated in Dictyostelium that has a single gene encoding a GRASP protein, GrhA, the removal of which does not cause lethality. However, the spores from the fruiting bodies are not fully viable. The analysis of this defect has revealed that GrhA is required at the plasma membrane of the spores to mediate the nonconventional secretion of a cellular nonmembrane associated factor, AcbA. AcbA is produced in the cytoplasm of the spore cells and released in the extracellular medium, where it binds to a specific spore receptor and elicits signaling leading to spore development.77,78
Drosophila GRASP (dGRASP) has also recently been shown to sustain a nonconventional secretion, but of a seemingly different kind. Drosophila mutants for dGRASP show a strong epithelial disorganization in the wing and the follicular epithelium covering the oocyte. This is due to the fact that the alpha integrin subunit PS1 is not transported properly to the plasma membrane at very specific stages of development, though anterograde transport as a whole is not affected. This specific integrin deposition requires dGRASP to adopt a plasma membrane localization and seems to bypass the Golgi as it is insensitive to BFA and to the loss of Syntaxin 5.79 Although different in the nature of its substrate and the type of secretion, it is remarkable that GRASP, a bonafide Golgi protein, exhibits an additional function at the plasma membrane, both in Dictyostelium and Drosophila epithelium.
The Exocyst
At the exit face of the Golgi (the Trans Golgi Network), the modified proteins are dispatched toward their correct final destination. A great deal is known about the sorting of proteins destined to the endosomal system, particularly the trafficking of the mannose-6-phosphate receptors and their ligands, a process that requires Clathrin and GGA.80,81
The formation and transport of vesicles carrying proteins destined to the plasma membrane has recently been characterized at a molecular level.82 PKD is clearly involved in the formation/fission of TGN to cell surface transport carriers.83 Developmental defects are associated with overexpression, mutation or silencing of PKD in Drosophila,84 but it is unclear that it relates to defects in membrane trafficking as PKD is involved in many different pathways.85
What is established, however, is the composition and function of a machinery tethering the incoming vesicles to specific sites of the plasma membrane, called the exocyst,86 which is an octameric complex comprising Sec3p, Sec5p, Sec6p, Sec8p Sec10p, Sec15p, Exo70p and Exo84p. Sec15 can bind Sec4-GTP.87 Sec2 is the GEF for Sec4 and this activation is necessary for the polarized delivery of vesicles88 before the SNARE complex assembly.89
The exocyst complex plays a role in a wide variety of cells types. In polarized epithelial cells, it is required for transport of vesicles to the lateral membrane.90 Disruption of Sec6/8 function in MDCK cells causes mis-sorting of basal-lateral membrane proteins.91 Expression of a mutant form of Sec8 or Sec10 subunits blocks neurite outgrowth in PC12 cells,92 and expression of a mutant form of Exo70 blocks insulin-dependent GLUT-4 translocation to the plasma membrane of adipocytes.93 Furthermore, the study of a truncated form of Sec5 in Drosophila has revealed a role in endocytosis, at least in the oocyte, possibly by tethering recycling vesicles from endosomes to the plasma membrane.94
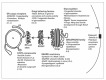
All together, this data suggests that a large number of genetic diseases and developmental defects are caused by mutations in the genes encoding keys proteins functioning in the exocytic pathway (summarized in Figure 1). This is likely the tip of the iceberg. Study of developmental disorders in a multicellular organism will continue to reveal specific proteins functions that so far have been missed while studying them in tissue culture cells, as well as revealing details of crucial aminoacid sequences and critical folding required for a wild type function.
Epithelial Development Depends on the Exocytic Pathway
As mentioned in the introduction, development requires cell proliferation and differentiation, leading to the formation of four major classes of tissues, connective, neuronal, the muscular and the epithelial tissues. Although this latter class comprises a large variety of cell types, they share a number of characteristics. In this part, we describe some these common features and how the exocytic pathway is involved in their development.
The Formation of Epithelial Cells
Model organisms such as Drosophila have been very useful to study the biology of epithelial cells. In this model organism, they emerge from the blastoderm embryo after a process called cellularization95 (see below) and lead to the formation of primary epithelia, such as the larval and adult epidermis, the fore and hindgut, the Malphigian tubules, the trachea and the salivary glands.
Cellularisation in Drosophila Embryo
Cellularization is a process by which 6000 cells are formed in a synchronous fashion. Two hours after egg fertilization, the egg nucleus undergoes 13 synchronous divisions within a single cytoplasm, yielding approximately 6000 nuclei that are found positioned very close to the plasma membrane of the so-called syncytial embryo. The cellularization process starts by the formation of shallow invaginations of the plasma membrane, called furrows, between the adjacent nuclei. After reaching a length of about 5 microns, the furrow recruits components, such as F-actin, Myosin II, aniline, cofilin, spectrins, septins, formins/diaphanous, at its tip, to form a donut shaped structure called the furrow canal that represents the leading edge of the invaginating membrane.96-105
The Exocytic Pathway and Cellularisation
During invagination, the furrow canal is pulled inwards by an actin-myosin based mechanism, and a very large amount of membrane is needed to make the newly formed plasma membrane. From elegant studies in live embryos, membrane delivery has been shown to be mediated by Golgi derived membrane, or post-Golgi vesicles.106
The furrow canal progression is inhibited upon injection of potent inhibitor of ER to Golgi transport, Brefeldin A.99,107 One of the proteins involved in cellularization is the Golgi peripheral protein Lava Lamp (Lva).99 There are no mutations known for lva but the protein function was assessed by injection of inactivating antibodies. This inhibited furrow progression and the Golgi seemed fragmented.99 Lva was also shown to interact with microtubules, which suggests that Golgi derived membrane vesicle transport is a key mechanism in cellularization.99 Accordingly, depolymerization of microtubules at the beginning of cellularization blocks the post-Golgi transport of the transmembrane protein neurotactin to the plasma membrane, indicating again the requirement for microtubules in this process.106
At the beginning of cellularization, the incorporation of new membrane occurs primarily toward the apical site of the forming cell. Later, membrane delivery is targeted closer to the furrow canal.106 This targeted delivery is mediated largely by the concentration of the exocytic machinery (ER and Golgi) near the site of membrane insertions.108 The fusion of vesicles delivering new membrane needed for the invaginating plasma membrane requires Syntaxin 1 function. syntaxin1 mutant embryos fail to cellularize109 and Syntaxin 1 staining was found localized both at the progressing furrow and at the entire lateral membrane.
The Establishment of Epithelial Cell Polarity
General Principles
One of the characteristics of the epithelial cells is their apical/basal polarity. The primary epithelia that form from the cellularization process described above are likely to be inherently polarized by the formation of physical junctions (see part II.3 below).
There are, however, so-called secondary epithelia, such as the midgut, the heart and the follicular epithelia. These are formed from mesenchymal-epithelial transitions during which nonpolarized mesenchymal cells receive cues from their environment that results in the establishment of an initial polarity. Ultimately, these cells will form adhesive contacts and form a tight polarized epithelium110 (see part II.3).
The initiation of polarity starts at cortical landmarks, which serve to orient the cytoskeleton, and to target vesicle traffic pathways.111,112 This initial asymmetry is reinforced by the localisation and the fine interplay of at least three complexes that leads to the establishment of polarity. The Bazooka (PAR3) (Bazooka/PAR6/aPKC) localizes to the sub apical region (just apically of where will adherens junction will form, see below, Fig. 2), and acts first in the hierarchy to specify the apical domain;113,114 the Scribble complex (comprising the neoplastic tumor suppressor genes products Scribble, Disc large, and Lethal giant larvae) is found just basolateral of the adherens junction and functions as basolateral determinant by repressing the apicalizing activity of the Bazooka complex. Finally, the Bazooka complex recruits the Crumbs complex (comprising the transmembrane protein Crumbs and two scaffolding proteins Disc-lost and Stardust that antagonizes the activity of the Scribble complex.115 Most of these gene products display an asymmetric localization within epithelial cells.116,117
Polarised Exocytosis
This now established polarity reinforces, in turn, the cytoskeleton rearrangement, and the polarized delivery of transmembrane (or secreted) proteins to different plasma membrane domains, referred to as polarised exocytosis. Evidence for this model includes the observations that yeast (Sro7 and 77) and mammalian Lethal giant larvae (Lgl) interact with SNAREs, Sec9 and Syntaxin 4. Analysis of cold sensitive sro7 and sro77 yeast mutants show a secretion pheno-type identical to that of sec9 mutants, that is, a block in the docking and fusion of post-Golgi secretory vesicles to the cell surface, leading to an accumulation of vesicles primarily in the bud.118 A similar situation is observed in polarised mammalian cells. MDCK cells achieve and maintain their polarity by direct targeting of apical and basolateral proteins in separate exocytic carriers to their respective surface domains.119 The presence of specific t-SNAREs at the different plasma membrane domains defines distinct membrane fusion events. MDCK cells express the post-Golgi SNARE Syntaxin 3 at the apical surface and Syntaxin 4 at the basolateral surface.120 The homologue of Lgl, Mlgl, has been shown to interact with Syntaxin 4 and therefore becomes associated with the basolateral membrane when the MDCK cells polarize, suggesting a role for Mlgl in regulating basolateral exocytosis in epithelial cells.121 The neuronal cell Lgl-related protein, Tomosyn, is found in a complex with the plasma membrane t-SNARE Syntaxin1, and antibodies to Tomosyn inhibit the exocytosis of dense core vesicles from PC12 cells in vitro.122 This process, though, does not seem to be polarised. So far, mutations in these SNAREs have not been described to be related to developmental defects.
On the other hand, PAR1, the serine/threonine protein kinase of the MARK/KIN family, is also localized on the membrane of the exocytic pathway.123 The S. cerevisiae Par1 orthologues Kin1 and Kin2 are proposed to act downstream of the Rab-GTPase Sec4, its GEF Sec2, and several other components of the exocyst (see introduction). Furthermore, Kin1 and Kin2 phosphorylates the t-SNARE Sec9, and binds Sro7, which itself binds to Sec9,124 indicating that PAR1 modulates the function of the exocytic pathway via phosphorylation of SNAREs.
The Formation of Epithelial Cell Junctions
The second feature that characterizes differentiated epithelial cells is that they exhibit physical junctions, the adherens and tight junctions that are critical for carrying out their barrier property and maintaining their apical/basal polarity.
The Junctions
The first cell-cell junction are the adherens junctions that in addition to the membrane associated Bazooka and Crumbs polarity complexes (see above) require E-Cadherin and Armadillo/β-Catenin for their formation and the maintenance of apical-basal polarity. This has been shown in a series of Drosophila mutants. The DE-Cadherin, β-catenin, bazooka, stardust, crumbs and discs lost mutants all show striking phenotypes in which the adherens junction formation is disrupted and cell polarity is often lost.125-130 In the Drosophila embryo, formation of adherens junctions starts during the process of cellularization (see above). When the furrows have formed and progressed, junctional proteins such as Discs-lost are also recruited to the membrane invaginations and are essential for furrow formation.130,131 Upon completion of the cellularization process, the typical apical adherens junction will form to connect adjacent cells in the newly formed epithelium and maintain the integrity of the tissue.
The second type of junctions is the tight/septate junctions. In mammalian cells, tight junctions are more apical than the adherens junctions, but their Drosophila equivalent, the septate junctions are more baso-lateral132 (Fig. 2). The tight junctions are formed by a series of integral membrane proteins, such as the occludins, claudins and junctional adhesion molecules (JAMs).133-136 In Drosophila the septate junction proteins Megatrachea137 and Sinuous138 are the homologues of vertebrate claudins, but the other integral septate junction membrane proteins, such as Neurexin IV, Gliotactin, Contactin, Neuroglian, Fasciclin III and NaK-ATPase139-146 show no overall structure similarities with those in tight junctions.
Intracellular Trafficking and Junctions
Not much is known about the transport and specific incorporation of these integral membrane junctional proteins, especially the regulation of their deposition, whether they form complexes earlier in the exocytic pathway and what happens to the junctions if these complexes cannot form. However slowly, investigations on the regulation of E-Cadherin trafficking are starting to shed light on how exocytosis contributes to the steady state distribution of functional proteins in polarized cells.
Newly synthesized E-cadherin has been shown to traffic with β-catenin as a complex.147-149 Recent data showed that E-Cadherin positive post-Golgi carriers emerge from the TGN as pleiomorphic tubulo-vesicular structures.150 This exit seems to be mediated by golgin-97 that belongs to a group of large coil-coiled membrane associated tethering proteins localised to the Golgi (see part I.2). siRNA mediated knockdown of golgin-97 leads to the accumulation of E-Cadherin in an intracellular pool, demonstrating a role for this class of proteins in post-Golgi transport.150
Furthermore, in MDCK cells, the E-Cadherin/βcatenin complex is sorted to the basolateral plasma membrane through the recognition of a dileucine motif in the cytoplasmic tail of E-Cadherin.149,151 This motif is highly conserved and is an essential sorting signal, though its cognate receptor is yet to be identified. In the absence of this motif E-cadherin/β-catenin containing post Golgi carriers are missorted to the apical surface resulting in the loss of epithelial polarity and integrity.149,151
The exocyst complex (see introduction) is also clearly involved in the maintenance of the junctions. Mutation of the Exo84 homologue in Drosophila results not only in the accumulation of Crumbs, but also of the scaffolding proteins Bazooka, aPKC and Discs lost, in large aggregates along the apical-basal axis152 away from where they are normally deposited near the adherens junctions. Disruption of the Drosophila Sec5, Sec6, or Sec15 leads to the accumulation of E-Cadherin, Armadillo and α-catenin in enlarged Rab11 positive recycling endosomes,153 indicating, as mentioned in the introduction, that the exocyst mediates the tethering/docking of vesicles coming from recycling endosomes.94 This suggests that the transmembrane and scaffolding proteins are not only delivered through the exocytic pathway, but that they can be recycled or stored in the endocytic pathway and used for the maintenance and development of epithelial junctions.
Epithelium Dynamics and the Exocytic Pathway
A very important aspect of epithelial biology is the remodeling and rearrangement of epithelia to create new tissues and make organs. In many cases, such as germ band extension, this requires the cell-cell interactions to be disrupted to allow cells to dramatically change shape in a coordinated fashion.154,155 Similar events take place during tube formation156,157 and many other processes. Junction remodeling involves in principle the degradation of the junctional components after endocytosis, followed by recycling, but until recently, a role for exocytosis was limited to the requirement of the exocyst in the tethering of vesicles coming from the Rab11 positive endosomes. So far, no clear involvement of the exocytic pathway has been clearly exemplified.
Organogenesis and morphogenesis also requires that adhesion of the epithelia to the extra-cellular matrix is altered. Adhesion largely relies on integrins that interact with extracellular matrix on one side of the cell, and recruit many cytoplasmic components to form focal adhesions.158 Modulation of adhesion is largely mediated by (de)-phosphorylation of focal adhesion components,159 the endocytosis of integrins,160 but could also be modulated by the exocytosis of newly synthesised integrins when adhesion needs to be upregulated. Recently, a novel pathway for the delivery of integrins in the Drosophila follicular epithelium required during epithelial remodelling has been identified. This pathway differs from the typical exocytic pathway in that it requires the Golgi protein dGRASP, but seems to be independent of the Golgi apparatus79 (see above, I.4).
Planar Polarity
Other aspects relate to the epithelial planar polarity and asymmetric divisions but again, these two events have been shown to require proteins playing a role in endocytosis, not in exocytosis. This has been reviewed in Le Borgne et al (2005) for the planar polarity,161 and in Knoblich, (2006); and Somers and Chia, (2005) for the asymmetric division.162,163
The Secretion of Morphogens
Within a continuous epithelium, cells can acquire differential properties, such as growing a hair on a surface. These differential properties are manifestations of cell fate which, in many cases, is specified by morphogens. Morphogens are signaling molecules produced and secreted from a restricted region of a developing tissue that spread to form a concentration gradient that provides the receiving cells with positional information. One response of cells to these gradients is differential gene expression, leading to differential developmental programmes.
By far, the epithelia forming the Drosophila larval wing imaginal discs is the best understood model for tissues that produce, and respond to morphogens, in particular Wnt and Hedgehog (Hh). Members of the Wnt family are secreted glycoproteins implicated in a variety of developmental processes and in tumorgenesis, as regulators of cell proliferation, migration, and differentiation.164 The founding of the family is the Drosophila Wingless. The members of the Hedgehog family are essential secreted signaling molecules controlling growth and patterning in both vertebrates and invertebrates.165 Unlike Drosophila, which has only one member of the Hedgehog family, mammals have three hedgehog genes sonic, desert, and Indian, of which Sonic Hedgehog is the best studied. A large effort in the community has been focused on how the receiving cells respond to these morphogens, that is, how they bind to specific receptors to elicit signaling pathways and subsequent transcriptional events. It is now clear that they are endocytosed upon binding to their receptors, leading to a very important downregulation of the signaling cascade.166
A more recent effort, however, has been made to identify components involved in the secretion of these morphogens by the producing cells. As mentioned above, Wnt and Hh are secreted by different subsets of wing disc cells but they are both synthesized in the ER and lipid-modified.167,168
Lipid Modifications of Wnt and Hh: an ER Based Event?
Wingless harbors two lipid modifications. The first one is the addition of a palmitate group to Cys 93.169,170 A second addition of an unsaturated fatty acid (palmitoleic acid) as seen for the murine Wnt3a has not been reported for Wingless. However these modifications are thought to be conserved on all mature mammalian Wnt molecules since the lipidated aminoacid and the surrounding residues are conserved (Cys 77 and Ser209 in murine Wnt3a). These lipid modifications seem to serve two important functions. Ser209 acylation is required for correct intracellular targeting and secretion.171 Mutation of Ser209 to Ala results in a retention of Wnt3A in the ER, showing that this lipid modification is crucial to its intracellular transport. Cys 77 acylation, on the other hand, seems to be required for the signaling activity of the secreted Wnt protein.169 Indeed Cys77 to Ala mutant Wnt molecule is still secreted but has little to no signaling activity, at least in vitro. The acyltransferase Porcupine has been proposed to be the enzyme that catalyses the addition of an acyl groups to Ser209.169,171 Porcupine, a putative multipass transmembrane protein belongs to the membrane bound O-acyltransferase superfamily and localizes to the ER.172,173 In the absence of porcupine Wnt secretion is blocked.174,175
The mature Drosophila Hh is synthesized as precursor protein that undergoes a series of posttranslational modifications, probably in the ER, leading to the covalent attachment of a cholesterol moiety at its C-terminus and a palmitic acid at its N-terminus. The understanding of the role of palmitoylation in Hh signaling came from the identification of the Drosophila sightless/skinny hedgehog/central missing/rasp gene (ski).176-179 Fly mutants deficient in both maternal and zygotic Ski function have strong developmental defects. They die during embryogenesis with aberrant patterning resembling that observed in other mutants defective in Hh signaling. Drosophila Ski encodes a putative acyltransferase, presumably catalyzing the transfer of a palmitoyl moiety to the Hh N-terminus. Mosaic analysis indicates that Ski is required in Hh-producing cells, and thus likely plays an essential role in the maturation or secretion of the biologically active Hh.176-179
Lipoprotein Particle Binding
As secreted proteins, Wnt and Hh should in principle follow the exocytic pathway for their delivery to the extracellular medium. However, their lipid modifications might alter their trafficking. For instance, their lipids could mediate binding to lipoprotein particles as it has been shown for the Drosophila Wnt protein, Wingless.180,181 It is proposed the binding to these particles could help secretion though the exocytic pathway, or that they could extract Wingless from the plasma membrane and help establish the gradient. However, the involvement of these particles has not yet been extended to other organisms.
Wnt, Evi and the Retromer
To identify novel components involved in the secretion of Wnt and Hh proteins, RNAi screens in Drosophila S2 cells have been performed. Using such screens, the protein Evi was identified to be essential for Wingless secretion.182 At the same time, a genetic screen for the Wnt gain-of-function phenotype in the Drosophila eye was performed and identified Wntless, identical to Evi.183 Evi/Wntless is a conserved transmembrane protein comprising 7 trans-membrane domains that bind Wingless. However, its localisation remains elusive as it has been reported to be on the ER, the Golgi and at the cell surface.
Evi/Wntless is reminiscent of the multipass transmembrane protein Dispatched (Disp), a protein needed for Hh release from producing cells.184 In disp mutants, lipid-modified Hh accumulates intracellularly. Although the mechanism of Hh release is not known, it has been suggested that Disp contains a sterol-sensing domain that recognizes lipid-modified Hh and subsequently is involved in its packaging into freely diffusing aggregates.185
Recently, a novel component involved in Wnt secretion has been identified in C.elegans. This novel component is a subunit of the retromer complex170,186,187 that has a clear role in the retrograde movement of proteins from the endosomes to the TGN. Loss of the core protein of the retromer complex, Vps35, blocks Wnt signaling in C.elegans,186,187 and the knockdown of Vps35 in mammalian cells and Xenopus eggs inhibits Wnt target gene expression,186 probably because Wnt is not produced. Based on the role of the retromer complex in endosome to TGN trafficking, the retromer is proposed to recycle a molecule/factor critical for Wnt secretion. This candidate factor has recently been identified as Evi/Wntless by five independent labs.188-192 The consensus model so far in Drosophila and C.elegans is that Evi binds Wingless in the TGN. The complex then travels to the plasma membrane where Wingless is released in the extracellular space, whereas Evi is retrieved back to endosomes where the retromer takes care of its retrograde movement to the TGN for another round of Wingless transport to the plasma membrane.193
Concluding Remarks/Perspectives
Mutations in key components of the exocytic pathway lead to severe developmental disorders shedding light on specific functions of proteins in cells within tissue (see part I). Conversely, the regulation of the transport of key proteins involved in critical development steps depends on (and sometimes modifies) the proper functioning of the exocytic pathway. Here, we have focused on certain aspects of epithelial development that depends on a coordinated and regulated transport of a myriad of transmembrane proteins whose deposition needs to be regulated in time and space. We have reviewed what we know, but again, this is only the tip of the iceberg. In reality, an integrated picture of the regulation of these transport events within the exocytic pathway is missing. This picture is now slowly emerging for endocytosis and we can only hope that in the near future, we will have a broader view on how both transport pathways cooperate to bring about the development of such a complex tissue, the epithelia.
The study of protein traffic during development is becoming a field on its own as a part of the “Cell biology of developing tissues” aimed at elucidating developmental processes at the cellular level within a tissue. This creates yet a new bridge between cell and developmental biology allowing us to move from gene to protein function but also to study protein function in a living organism. Studying processes in model organisms for which the genome is sequenced and annotated is now not only possible but offers many exciting prospects for the merging of genetics, developmental biology and cell biology.
Acknowledgments
We thank all our colleagues in the department and in the field for helpful discussions. HS is supported by a Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) ZonMw grant (912-04-009) to CR.
Literature cited
- 1.
- Dudu V, Pantazis P, Gonzalez-Gaitan M. Membrane traffic during embryonic development: Epithelial formation, cell fate decisions and differentiation. Curr Opi Cell Biol. 2004;16:407–414. [PubMed: 15261673]
- 2.
- Emery G, Knoblich JA. Endosome dynamics during development. Curr Opi Cell Biol. 2006;18:407–415. [PubMed: 16806877]
- 3.
- Aridor M, Hannan LA. Traffic jam: A compendium of human diseases that affect intracellular transport processes. Traffic. 2000;1:836–851. [PubMed: 11208074]
- 4.
- Aridor M, Hannan LA. Traffic jams II: An update of diseases of intracellular transport. Traffic. 2002;3:781–790. [PubMed: 12383344]
- 5.
- Mellman I, Warren G. The road taken: Past and future foundations of membrane traffic. Cell. 2000;100:99–112. [PubMed: 10647935]
- 6.
- Aridor M. Visiting the ER: The endoplasmic reticulum as a target for therapeutics in traffic related diseases. Adv Drug Del Rev. 2007;59:759–781. [PubMed: 17681635]
- 7.
- Boyadjiev SA, Fromme JC, Ben J. et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–1197. [PubMed: 16980979]
- 8.
- Lang MR, Lapierre LA, Frotscher M. et al. Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat Genet. 2006;38:1198–1203. [PubMed: 16980978]
- 9.
- Fromme JC, Ravazzola M, Hamamoto S. et al. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–34. [PMC free article: PMC2262049] [PubMed: 17981132]
- 10.
- Hughes H, Stephens DJ. Assembly, organization, and function of the COPII coat Histochem Cell Biol 2007, (In press) [PMC free article: PMC2228377] [PubMed: 18060556]
- 11.
- Jones B, Jones EL, Bonney SA. et al. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003;34:29–31. [PubMed: 12692552]
- 12.
- Shoulders CC, Stephens DJ, Jones B. The intracellular transport of chylomicrons requires the small GTPase, Sar1b. Curr Opi Lipid. 2004;15:191–197. [PubMed: 15017362]
- 13.
- Ye B, Zhang Y, Song W. et al. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. [PMC free article: PMC2020851] [PubMed: 17719548]
- 14.
- Miller EA, Beilharz TH, Malkus PN. et al. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. [PubMed: 12941277]
- 15.
- Appenzeller C, Andersson H, Kappeler F. et al. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat Cell Biol. 1999;1:330–334. [PubMed: 10559958]
- 16.
- Hauri HP, Kappeler F, Andersson H. et al. ERGIC-53 and traffic in the secretory pathway. J Cell Sci. 2000;113:587–596. [PubMed: 10652252]
- 17.
- Nichols WC, Seligsohn U, Zivelin A. et al. Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell. 1998;93:61–70. [PubMed: 9546392]
- 18.
- Zhang B, Cunningham MA, Nichols WC. et al. Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat Genet. 2003;34:220–225. [PubMed: 12717434]
- 19.
- Barlowe C. Signals for COPII-dependent export from the ER: What's the ticket out? Trend Cell Biol. 2003;13:295–300. [PubMed: 12791295]
- 20.
- Powers J, Barlowe C. Transport of axl2p depends on erv14p, an ER-vesicle protein related to the Drosophila cornichon gene product. J Cell Biol. 1998;142:1209–1222. [PMC free article: PMC2149358] [PubMed: 9732282]
- 21.
- Roth S, Neuman-Silberberg FS, Barcelo G. et al. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. [PubMed: 7540118]
- 22.
- Bokel C, Dass S, Wilsch-Brauninger M. et al. Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development. 2006;133:459–470. [PubMed: 16396907]
- 23.
- Rabouille C, Klumperman J. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol. 2005;6:812–817. [PubMed: 16167055]
- 24.
- Gillingham AK, Munro S. The small G proteins of the ARF family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. [PubMed: 17506703]
- 25.
- Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744:383–395. [PubMed: 15979508]
- 26.
- Coutelis JB, Ephrussi A. Rab6 mediates membrane organization and determinant localization during Drosophila oogenesis. Development. 2007;134:1419–30. [PubMed: 17329360]
- 27.
- Januschke J, Nicolas E, Compagnon J. et al. Rab6 and the secretory pathway affect oocyte polarity in Drosophila. Development. 2007;134:3419–3425. [PubMed: 17827179]
- 28.
- Grigoriev I, Splinter D, Keijzer N. et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13:305–14. [PubMed: 17681140]
- 29.
- Matanis T, Akhmanova A, Wulf P. et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4:986–992. [PubMed: 12447383]
- 30.
- Oka T, Krieger M. Multi-component protein complexes and Golgi membrane trafficking. J Biochem. 2005;137:109–114. [PubMed: 15749823]
- 31.
- Haas AK, Barr FA. COP sets TRAPP for vesicles. Dev Cell. 2007;12:326–327. [PubMed: 17336899]
- 32.
- Morozova N, Liang Y, Tokarev AA. et al. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol. 2006;8:1263–1269. [PubMed: 17041589]
- 33.
- Ungar D, Oka T, Krieger M. et al. Retrograde transport on the COG railway. Trend Cell Biol. 2006;16:113–120. [PubMed: 16406524]
- 34.
- Sollner T, Whiteheart SW, Brunner M. et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. [PubMed: 8455717]
- 35.
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. [PubMed: 7969419]
- 36.
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nature Rev Mol Cell Biol. 2006;7:631–643. [PubMed: 16912714]
- 37.
- Wu MN, Bellen HJ. Genetic dissection of synaptic transmission in Drosophila. Curr Opi Neurobiol. 1997;7:624–630. [PubMed: 9384538]
- 38.
- Hepp R, Langley K. SNAREs during development. Cell Tissue Res. 2001;305:247–253. [PubMed: 11545262]
- 39.
- Stewart BA. Membrane trafficking in Drosophila wing and eye development. Sem Cell Dev Biol. 2002;13:91–97. [PubMed: 12127141]
- 40.
- Rao SS, Stewart BA, Rivlin PK. et al. Two distinct effects on neurotransmission in a temperature-sensitive SNAP-25 mutant. EMBO J. 2001;20:6761–6771. [PMC free article: PMC125330] [PubMed: 11726512]
- 41.
- Littleton JT. A genomic analysis of membrane trafficking and neurotransmitter release in Drosophila. J Cell Biol. 2000;150:F77–82. [PubMed: 10908590]
- 42.
- Schulze KL, Broadie K, Perin MS. et al. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. [PubMed: 7834751]
- 43.
- Sharma N, Low SH, Misra S. et al. Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J Biol Chem. 2006;173:937–948. [PMC free article: PMC2063918] [PubMed: 16785322]
- 44.
- Moussian B, Veerkamp J, Muller U. et al. Assembly of the Drosophila larval exoskeleton requires controlled secretion and shaping of the apical plasma membrane. Matrix Biol. 2007;26:337–347. [PubMed: 17360167]
- 45.
- Chae TH, Kim S, Marz KE. et al. The hyh mutation uncovers roles for alpha Snap in apical protein localization and control of neural cell fate. Nat Genet. 2004;36:264–70. [PubMed: 14758363]
- 46.
- Hong HK, Chakravarti A, Takahashi JS. The gene for soluble N-ethylmaleimide sensitive factor attachment protein alpha is mutated in hydrocephaly with hop gait (hyh) mice. Proc Natl Acad Sci (USA). 2004;101:1748–53. [PMC free article: PMC341847] [PubMed: 14755058]
- 47.
- Bajjalieh S. Trafficking in cell fate. Nat Genet. 2004;36:216–7. [PubMed: 14988718]
- 48.
- Sudhof TC, De Camilli P, Niemann H. et al. Membrane fusion machinery: Insights from synaptic proteins. Cell. 1993;75:1–4. [PubMed: 8402889]
- 49.
- Littleton JT, Bellen HJ. Presynaptic proteins involved in exocytosis in Drosophila melanogaster: A genetic analysis. Invert Neurosci. 1995;1:3–13. [PubMed: 9372128]
- 50.
- Pilot F, Philippe JM, Lemmers C. et al. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature. 2006;442:580–584. [PubMed: 16862128]
- 51.
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: Integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. [PubMed: 12154370]
- 52.
- Grunewald S. Congenital disorders of glycosylation: Rapidly enlarging group of (neuro)metabolic disorders. Early Hum Dev. 2007;83:825–830. [PubMed: 17959325]
- 53.
- Leroy JG. Congenital disorders of N-glycosylation including diseases associated with O- as well as N-glycosylation defects. Pediatric Res. 2006;60:643–656. [PubMed: 17065563]
- 54.
- Freeze HH. Congenital disorders of glycosylation: CDG-I, CDG-II, and beyond. Curr Mol Med. 2007;7:389–396. [PubMed: 17584079]
- 55.
- Zeevaert R, Foulquier F, Jaeken J. et al. Deficiencies in subunits of the Conserved Oligomeric Golgi (COG) complex define a novel group of Congenital Disorders of Glycosylation. Mol Genet Metab. 2007;93:15–21. [PubMed: 17904886]
- 56.
- Chui D, Oh-Eda M, Liao YF. et al. Alpha-mannosidase-II deficiency results in dyserythropoiesis and unveils an alternate pathway in oligosaccharide biosynthesis. Cell. 1997;90:157–167. [PubMed: 9230311]
- 57.
- Akama TO, Nakagawa H, Wong NK. et al. Essential and mutually compensatory roles of α-mannosidase II and α-mannosidase IIx in N-glycan processing in vivo in mice. PNAS USA. 2006;103:8983–8988. [PMC free article: PMC1474017] [PubMed: 16754854]
- 58.
- Chui D, Sellakumar G, Green R. et al. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. PNAS USA. 2001;98:1142–1147. [PMC free article: PMC14722] [PubMed: 11158608]
- 59.
- Green RS, Stone EL, Tenno M. et al. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity. 2007;27:308–320. [PubMed: 17681821]
- 60.
- Campbell RM, Metzler M, Granovsky M. et al. Complex asparagine-linked oligosaccharides in Mgat1-null embryos. Glycobiology. 1995;5:535–543. [PubMed: 8563140]
- 61.
- Mendelsohn R, Cheung P, Berger L. et al. Complex N-glycan and metabolic control in tumor cells. Cancer Res. 2007;67:9771–9780. [PubMed: 17942907]
- 62.
- Lagana A, Goetz JG, Cheung P. et al. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol. 2006;26:3181–3193. [PMC free article: PMC1446937] [PubMed: 16581792]
- 63.
- Cheung P, Dennis JW. et al. Mgat5 and Pten interact to regulate cell growth and polarity. Glycobiology. 2007;17:767–773. [PubMed: 17400585]
- 64.
- Bruckner K, Perez L, Clausen H. et al. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406:411–415. [PubMed: 10935637]
- 65.
- Munro S, Freeman M. et al. The notch signalling regulator fringe acts in the Golgi apparatus and requires the glycosyltransferase signature motif DXD. Curr Biol. 2000;10:813–820. [PubMed: 10899003]
- 66.
- Rampal R, Li AS, Moloney DJ. et al. Lunatic fringe, manic fringe, and radical fringe recognize similar specificity determinants in O-fucosylated epidermal growth factor-like repeats. J Biol Chem. 2005;280:42454–63. [PubMed: 16221665]
- 67.
- Favier B, Fliniaux I, Thélu J. et al. Localisation of members of the notch system and the differentiation of vibrissa hair follicles: Receptors, ligands, and fringe modulators. Dev Dyn. 2000;218:426–437. [PubMed: 10878608]
- 68.
- Moloney DJ, Panin VM, Johnston SH. et al. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406:369–375. [PubMed: 10935626]
- 69.
- Aulehla A, Herrmann BG. Segmentation in vertebrates: Clock and gradient finally joined. Genes Dev. 2004;18:2060–2067. [PubMed: 15342488]
- 70.
- Serth K, Schuster-Gossler K, Cordes R. et al. Transcriptional oscillation of lunatic fringe is essential for somitogenesis. Genes Dev. 2003;17:912–925. [PMC free article: PMC196028] [PubMed: 12670869]
- 71.
- Rabouille C, Warren G. The changes in the architecture of the Golgi apparatus during mitosis. In: Berger EG, Roth, eds. The Golgi Apparatus. Basel/Switzerland: Birkhäuser Verlag. 1997
- 72.
- Kondylis V, van Nispen tot Pannerden HE, Herpers B. et al. The Golgi comprises a paired stack that is separated at G2 by modulation of the actin cytoskeleton through Abi and Scar/WAVE. Dev Cell. 2007;12:901–915. [PubMed: 17543863]
- 73.
- Barr FA, Puype M, Vandekerckhove J. et al. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. [PubMed: 9346242]
- 74.
- Shorter J, Watson R, Giannakou ME. et al. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999;18:4949–4960. [PMC free article: PMC1171566] [PubMed: 10487747]
- 75.
- Kondylis V, Spoorendonk KM, Rabouille C. dGRASP localization and function in the early exocytic pathway in Drosophila S2 cells. Mol Biol Cell. 2005;16:4061–4072. [PMC free article: PMC1196319] [PubMed: 15975913]
- 76.
- Sütterlin C, Polishchuk RS, Pecot M. et al. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol Biol Cell. 2005;16:3211–3222. [PMC free article: PMC1165405] [PubMed: 15888544]
- 77.
- Kinseth MA, Anjard C, Fuller D. et al. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell. 2007;130:524–534. [PubMed: 17655921]
- 78.
- Levi SK, Glick BS. GRASPing unconventional secretion. Cell. 2007;130:407–409. [PubMed: 17693251]
- 79.
- Schotman H, Karhinen L, Rabouille C. The dGRASP mediated noncanonical integrin secretion is required for Drosophila epithelial remodelling. Dev Cell. 2008;14:171–182. [PubMed: 18267086]
- 80.
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Bioch. 2003;72:395–447. [PubMed: 12651740]
- 81.
- Bonifacino JS. The GGA proteins: Adaptors on the move. Nat Rev Mol Cell Biol. 2004;5:23–32. [PubMed: 14708007]
- 82.
- Ponnambalam S, Baldwin SA. Constitutive protein secretion from the trans-Golgi network to the plasma membrane. Mol Memb Biol. 2003;20:129–139. [PubMed: 12851070]
- 83.
- Bossard C, Bresson D, Polishchuk RS. et al. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol. 2007;179:1123–1131. [PMC free article: PMC2140039] [PubMed: 18086912]
- 84.
- Maier D, Nagel AC, Gloc H. et al. Protein kinase D regulates several aspects of development in Drosophila melanogaster. BMC Dev Biol. 2007;7:74–81. [PMC free article: PMC1933421] [PubMed: 17592635]
- 85.
- Van Lint J, Rykx A, Maeda Y. et al. Protein kinase D: An intracellular traffic regulator on the move. Trends Cell Biol. 2002;12:193–200. [PubMed: 11978539]
- 86.
- Hsu SC, TerBush D, Abraham M. et al. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. [PubMed: 15037366]
- 87.
- Guo W, Roth D, Walch-Solimena C. et al. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. [PMC free article: PMC1171198] [PubMed: 10022848]
- 88.
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. [PMC free article: PMC2137815] [PubMed: 9199166]
- 89.
- Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–452. [PMC free article: PMC2192655] [PubMed: 11038189]
- 90.
- Grindstaff KK, Yeaman C, Anandasabapathy N. et al. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. [PubMed: 9630218]
- 91.
- Moskalenko S, Henry DO, Rosse C. et al. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. [PubMed: 11740492]
- 92.
- Vega IE,, Hsu SC. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J Neurosci. 2001;21:3839–3848. [PMC free article: PMC3674029] [PubMed: 11356872]
- 93.
- Inoue M, Chang L, Hwang J. et al. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. [PubMed: 12687004]
- 94.
- Sommer B, Oprins A, Rabouille C. et al. The exocyst component Sec5 is present on endocytic vesicles in the oocyte of Drosophila melanogaster. J Cell Biol. 2005;169:953–963. [PMC free article: PMC2171629] [PubMed: 15955846]
- 95.
- Foe VE, Odell GM, Edgar BA. Mitosis and morphogenesis in the Drosophila embryo In: Bate M, Martinez-Arias A, eds. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory 1993:149–300.
- 96.
- Warn RM, Robert-Nicoud M. F-actin organization during the cellularization of the Drosophila embryo as revealed with a confocal laser scanning microscope. J Cell Sci. 1990;96:35–42. [PubMed: 2373743]
- 97.
- Lecuit T, Samanta R, Wieschaus E. slam encodes a developmental regulator of polarized membrane growth during cleavage of the Drosophila embryo. Dev Cell. 2002;2:425–436. [PubMed: 11970893]
- 98.
- Royou A, Field C, Sisson JC. et al. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol Biol Cell. 2004;15:838–850. [PMC free article: PMC329397] [PubMed: 14657248]
- 99.
- Sisson JC, Field C, Ventura R. et al. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. [PMC free article: PMC2169433] [PubMed: 11076973]
- 100.
- Young PE, Pesacreta TC, Kiehart DP. Dynamic changes in the distribution of cytoplasmic myosin during Drosophila embryogenesis. Development. 1991;111:1–14. [PubMed: 1901784]
- 101.
- Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. [PMC free article: PMC2120607] [PubMed: 7559773]
- 102.
- Thomas GH, Williams JA. Dynamic rearrangement of the spectrin membrane skeleton during the generation of epithelial polarity in Drosophila. J Cell Sci. 1999;112:2843–2852. [PubMed: 10444379]
- 103.
- Adam JC, Pringle JR, Peifer M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol Biol Cell. 2000;11:3123–3135. [PMC free article: PMC14980] [PubMed: 10982405]
- 104.
- Fares H, Peifer M, Pringle JR. Localization and possible functions of Drosophila septins. Mol Biol Cell. 1995;6:1843–1859. [PMC free article: PMC301337] [PubMed: 8590810]
- 105.
- Afshar K, Stuart B, Wasserman SA. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 2000;127:1887–1897. [PubMed: 10751177]
- 106.
- Lecuit T. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J Cell Biol. 2000;150:849–860. [PMC free article: PMC2175274] [PubMed: 10953008]
- 107.
- Chardin P, McCormick F, Brefeldin A. The advantage of being uncompetitive. Cell. 1999;97:153–155. [PubMed: 10219235]
- 108.
- Frescas D, Mavrakis M, Lorenz H. et al. The secretory membrane system in the Drosophila syncytial blastoderm embryo exists as functionally compartmentalized units around individual nuclei. J Cell Biol. 2006;173:219–230. [PMC free article: PMC2063813] [PubMed: 16636144]
- 109.
- Burgess RW, Deitcher DL, Schwarz TL. The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos. J Cell Biol. 1997;138:861–875. [PMC free article: PMC2138053] [PubMed: 9265652]
- 110.
- Fremion F, Astier M, Zaffran S. et al. The heterotrimeric protein Go is required for the formation of heart epithelium in Drosophila. J Cell Biol. 1999;145:1063–1076. [PMC free article: PMC2133120] [PubMed: 10352022]
- 111.
- Nelson WJ. Cytoskeleton functions in membrane traffic in polarized epithelial cells. Semin Cell Biol. 1991;2:375–385. [PubMed: 1813027]
- 112.
- Mays RW, Beck KA, Nelson WJ. Organization and function of the cytoskeleton in polarized epithelial cells: A component of the protein sorting machinery. Curr Opin Cell Biol. 1994;6:16–24. [PubMed: 8167021]
- 113.
- Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol. 2003;5:46–52. [PubMed: 12510193]
- 114.
- Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. [PubMed: 12510194]
- 115.
- Horne-Badovinac S, Bilder D. Mass transit: Epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232:559–574. [PubMed: 15704134]
- 116.
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. [PMC free article: PMC3373010] [PubMed: 12700771]
- 117.
- Bilder D. Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. [PubMed: 15314019]
- 118.
- Lehman K, Rossi G, Adamo JE. et al. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J Cell Biol. 1999;146:125–140. [PMC free article: PMC2199738] [PubMed: 10402465]
- 119.
- Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opi Cell Biol. 2000;12:483–490. [PubMed: 10873817]
- 120.
- Low SH, Chapin SJ, Weimbs T. et al. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. [PMC free article: PMC276046] [PubMed: 8970161]
- 121.
- Musch A, Cohen D, Yeaman C. et al. Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin-Darby canine kidney cells. Mol Biol Cell. 2002;13:158–168. [PMC free article: PMC65098] [PubMed: 11809830]
- 122.
- Fujita Y, Shirataki H, Sakisaka T. et al. Tomosyn: A syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. [PubMed: 9620695]
- 123.
- Vaccari T, Rabouille C, Ephrussi A. The Drosophila PAR-1 spacer domain is required for lateral membrane association and for polarization of follicular epithelial cells. Curr Biol. 2005;15:255–261. [PubMed: 15694310]
- 124.
- Elbert M, Rossi G, Brennwald P. The yeast par-1 homologs kin1 and kin2 show genetic and physical interactions with components of the exocytic machinery. Mol Biol Cell. 2005;16:532–549. [PMC free article: PMC545889] [PubMed: 15563607]
- 125.
- Tepass U, Gruszynski-DeFeo E, Haag TA. et al. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes and Development. 1996;10:672–685. [PubMed: 8598295]
- 126.
- Uemura T, Oda H, Kraut R. et al. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Gene Dev. 1996;10:659–671. [PubMed: 8598294]
- 127.
- Muller HA, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134:149–163. [PMC free article: PMC2120925] [PubMed: 8698811]
- 128.
- Tepass U, Theres C, Knust E. Crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. [PubMed: 2344615]
- 129.
- Wodarz A, Hinz U, Engelbert M. et al. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. [PubMed: 7606787]
- 130.
- Bhat MA, Izaddoost S, Lu Y. et al. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. [PubMed: 10102271]
- 131.
- Pielage J, Stork T, Bunse I. et al. The Drosophila cell survival gene discs lost encodes a cytoplasmic Codanin-1-like protein, not a homolog of tight junction PDZ protein Patj. Dev Cell. 2003;5:841–851. [PubMed: 14667407]
- 132.
- Tepass U, Tanentzapf G, Ward R. et al. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. [PubMed: 11700298]
- 133.
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. [PubMed: 11283726]
- 134.
- Ebnet K, Suzuki A, Ohno S. et al. Junctional adhesion molecules (JAMs): More molecules with dual functions? J Cell Sci. 2004;117:19–29. [PubMed: 14657270]
- 135.
- Hirabayashi S, Tajima M, Yao I. et al. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol. 2003;23:4267–4282. [PMC free article: PMC156145] [PubMed: 12773569]
- 136.
- Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology. 2004;19:331–338. [PubMed: 15546850]
- 137.
- Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5:611–620. [PubMed: 14536062]
- 138.
- Wu VM, Schulte J, Hirschi A. et al. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164:313–323. [PMC free article: PMC2172325] [PubMed: 14734539]
- 139.
- Baumgartner S, Littleton JT, Broadie K. et al. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. [PubMed: 8978610]
- 140.
- Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol. 2003;161:979–989. [PMC free article: PMC2172966] [PubMed: 12782686]
- 141.
- Faivre-Sarrailh C, Banerjee S, Li J. et al. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. [PubMed: 15459097]
- 142.
- Auld VJ, Fetter RD, Broadie K. et al. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell. 1995;81:757–767. [PubMed: 7539719]
- 143.
- Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J Cell Biol. 2003;161:991–1000. [PMC free article: PMC2172969] [PubMed: 12782681]
- 144.
- Snow PM, Bieber AJ, Goodman CS. Fasciclin III: A novel homophilic adhesion molecule in Drosophila. Cell. 1989;59:313–323. [PubMed: 2509076]
- 145.
- Knust, Bossinger. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. [PubMed: 12471248]
- 146.
- Paul SM, Ternet M, Salvaterra PM. et al. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130:4963–4974. [PubMed: 12930776]
- 147.
- Huber AH, Stewart DB, Laurents DV. et al. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin: A possible mechanism for regulating cadherin turnover. J Biol Chem. 2001;276:12301–12309. [PubMed: 11121423]
- 148.
- Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Bio. 1999;144:687–699. [PMC free article: PMC2132940] [PubMed: 10037790]
- 149.
- Miranda KC, Joseph SR, Yap AS. et al. Contextual binding of p120ctn to E-cadherin at the basolateral plasma membrane in polarized epithelia. J Biol Chem. 2003;278:43480–43488. [PubMed: 12923199]
- 150.
- Lock JG, Hammond LA, Houghton F. et al. E-cadherin transport from the trans-Golgi network in tubulovesicular carriers is selectively regulated by golgin-97. Traffic. 2005;6:1142–1156. [PubMed: 16262725]
- 151.
- Miranda KC, Khromykh T, Christy P. et al. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem. 2001;276:22565–22572. [PubMed: 11312273]
- 152.
- Blankenship JT, Fuller MT, Zallen JA. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J Cell Sci. 2007;120:3099–3110. [PubMed: 17698923]
- 153.
- Langevin J, Morgan MJ, Sibarita JB. et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell. 2005;9:355–376. [PubMed: 16224820]
- 154.
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Developmental Cell. 2004;6:343–355. [PubMed: 15030758]
- 155.
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. [PubMed: 15190355]
- 156.
- Lubarsky B, Krasnow MA. Tube morphogenesis: Making and shaping biological tubes. Cell. 2003;112:19–28. [PubMed: 12526790]
- 157.
- Neumann M, Affolter M. Remodelling epithelial tubes through cell rearrangements: From cells to molecules. EMBO Rep. 2006;7:36–40. [PMC free article: PMC1369232] [PubMed: 16391535]
- 158.
- Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. [PubMed: 1555235]
- 159.
- Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–43. [PubMed: 16375667]
- 160.
- Caswell PT, Norman JC. Integrin trafficking and the control of cell Migration. Traffic. 2006;7:14–21. [PubMed: 16445683]
- 161.
- Le Borgne R, Bardin A, Schweisguth F. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 2005;132:1751–1762. [PubMed: 15790962]
- 162.
- Knoblich JA. Sara splits the signal. Science. 2006;314:1094–1096. [PubMed: 17110561]
- 163.
- Somers WG, Chia W. Recycling polarity. Dev Cell. 2005;9:312–313. [PubMed: 16139221]
- 164.
- Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. [PubMed: 15473860]
- 165.
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. [PubMed: 11731473]
- 166.
- Vincent JP, Dubois L. Morphogen transport along epithelia, an integrated trafficking problem. Dev Cell. 2002;3:615–623. [PubMed: 12431369]
- 167.
- Hausmann G, Banziger C, Basler K. Helping Wingless take flight: How WNT proteins are secreted. Nat Rev Mol Cell Biol. 2007;8:331–336. [PubMed: 17342185]
- 168.
- Guerrero I, Chiang C. A conserved mechanism of Hedgehog gradient formation by lipid modifications. Trend Cell Biol. 2006;17:1–5. [PubMed: 17126548]
- 169.
- Willert K, Brown JD, Danenberg E. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. [PubMed: 12717451]
- 170.
- Coudreuse D, Korswagen HC. The making of Wnt: New insights into Wnt maturation, sorting and secretion. Development. 2007;134:3–12. [PubMed: 17138665]
- 171.
- Takada R, Satomi Y, Kurata T. et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11:791–801. [PubMed: 17141155]
- 172.
- Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trend Biochem Sci. 2000;25:111–112. [PubMed: 10694878]
- 173.
- Kadowaki T, Wilder E, Klingensmith J. et al. The segment polarity gene porcupine encodes a putative ultitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. [PubMed: 8985181]
- 174.
- van den Heuvel M, Harryman-Samos C, Klingensmith J. et al. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J. 1993;12:5293–5302. [PMC free article: PMC413795] [PubMed: 8262072]
- 175.
- van den Heuvel M, Nusse R, Johnston P. et al. Distribution of the wingless gene product in Drosophila embryos: A protein involved in cell-cell communication. Cell. 1989;59:739–749. [PubMed: 2582493]
- 176.
- Amanai K, Jiang J. Distinct roles of Central missing and Dispatched in sending the Hedgehog signal. Development. 2001;128:5119–5127. [PubMed: 11748147]
- 177.
- Chamoun Z, Mann RK, Nellen D. et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. [PubMed: 11486055]
- 178.
- Lee JD, Treisman JE. Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr Biol. 2001;11:1147–1152. [PubMed: 11509241]
- 179.
- Micchelli CA, The I, Selva E. et al. Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development. 2002;129:843–851. [PubMed: 11861468]
- 180.
- Panakova D, Sprong H, Marois E. et al. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. [PubMed: 15875013]
- 181.
- Eaton S. Release and trafficking of lipid-linked morphogens. Curr Opi Gen Dev. 2006;16:17–22. [PubMed: 16364628]
- 182.
- Bartscherer K, Pelte N, Ingelfinger D. et al. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. [PubMed: 16678096]
- 183.
- Banziger C, Soldini D, Schutt C. et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. [PubMed: 16678095]
- 184.
- Burke R, Nellen D, Bellotto M. et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–815. [PubMed: 10619433]
- 185.
- Zeng X, Goetz JA, Suber LM. et al. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. [PubMed: 11395778]
- 186.
- Coudreuse DY, Roe G, Betist MC. et al. Wnt gradient formation requires retromer function inWnt-producing cells. Science. 2006;312:921–924. [PubMed: 16645052]
- 187.
- Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. [PubMed: 16571624]
- 188.
- Belenkaya TY, Wu Y, Tang X. et al. The retromer complex influences wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. [PubMed: 18160348]
- 189.
- Franch-Marro X, Wendler F, Guidato S. et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. [PMC free article: PMC7611556] [PubMed: 18193037]
- 190.
- Pan CL, Baum PD, Gu M. et al. C. elegans AP-2 and Retromer Control Wnt Signaling by Regulating MIG-14/Wntless. Dev Cell. 2008;14:132–139. [PMC free article: PMC2709403] [PubMed: 18160346]
- 191.
- Port F, Kuster M, Herr P. et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Cell Biol. 2008;10:178–185. [PubMed: 18193032]
- 192.
- Yang PT, Lorenowicz M, Silhankova M. et al. Wnt Signaling Requires Retromer-Dependent Recycling of MIG-14/Wntless in Wnt-Producing Cells. Dev Cell. 2008;14:140–147. [PubMed: 18160347]
- 193.
- Eaton S. Retromer retrieves Wntless. Dev Cell. 2008;14:4–6. [PubMed: 18194646]
- The Exocytic Pathway and Development - Madame Curie Bioscience DatabaseThe Exocytic Pathway and Development - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...