From: Regulation of the Rod Photoreceptor Cyclic Nucleotide-Gated Channel

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
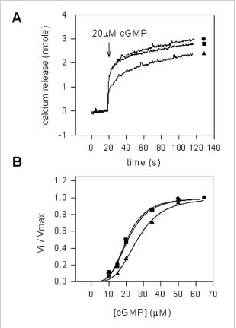
Bovine rod CNG channel is modulated by endogenous CaM, but not other Ca2+-binding proteins. CHAPS-solubilized ROS membrane proteins were reconstituted into Ca2+containing lipid vesicle. Potential soluble channel modulators (HEPES buffer), ROS soluble fraction containing endogenous CaM (+CaM), or ROS soluble fraction depleted of endogenous CaM (CaM) were added to the vesicles, and cGMP-dependent Ca2+ efflux was measured using the Arsenazo III assay. A. Time course and B. normalized rate as a function of cGMP concentration for cGMP-dependent Ca2+ efflux from vesicles treated with 100 ml of HEPES buffer (l), ROS soluble fraction (+CaM) (s), and ROS soluble fraction (CaM) (n). The data in B was analyzed with a Sigma Plot curve fit program of using the Hill equation V/Vmax = [cGMP]n/([cGMP]n + Kmn) where Km and n are the Michaelis constant and the Hill coefficient, respectively. The cGMP-gated channel incubated with HEPES (l) had a Km = 20.5 μM and n = 3.7, the channel incubated with a ROS lysate containing calmodulin(s) had a Km = 26.5 μM and n = 3.7 and the channel incubated with a ROS lysate depleted of calmodulin (n) had a Km = 21.1 μM and n = 3.6. These results indicate that endogenous CaM, but not other soluble proteins, modulates the activity of the channel.
From: Regulation of the Rod Photoreceptor Cyclic Nucleotide-Gated Channel

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.