The content of this book is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 Unported license. To view the terms and conditions of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 4th edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2022. doi: 10.1101/glycobiology.4e.56
Knowledge of the cellular pathways of glycosylation across phylogeny provides opportunities for designing glycans via genetic engineering in a wide variety of cell types including bacteria, fungi, plant cells, and mammalian cells. The commercial demand for glycosylation engineering is broad, including production of biological therapeutics with defined glycosylation (Chapter 57). This chapter describes how knowledge of glycan structures and their metabolism (Chapters 2–27) has led to the current state of glycosylation engineering in different cell types. Perspectives for rapid advances in this area using precise gene editing technologies are also described.
GOALS FOR GLYCOENGINEERING OF CELLS
There is a long history of engineering glycosylation in mammalian cells, plants, fungi (yeast), and bacteria using genetic strategies, and many well-characterized glycosylation mutants are available (Chapters 20–27 and 49). This chapter focuses on approaches and methods for designing glycosylation in cells, whereas other active areas of engineering glycans to produce structural bioproducts, foods, and fuels are not covered. Today, cellular glycoengineering is often used to produce recombinant therapeutic glycoproteins that require glycosylation for their efficacy and at the same time must have human-compatible glycosylation to avoid immune responses to nonhuman glycans. Glycosylation can alter the size, charge, and solubility of therapeutic glycoproteins to prevent rapid clearance from the circulation. In addition, glycoengineering has been used to improve or develop new therapeutic modalities (Chapter 57). Glycans can also serve as ligands for lectin receptors that target therapeutics to certain cells. Of particular importance is the role of N-glycosylation for effector functions of IgG antibodies; therapeutic IgG antibodies with N-glycosylation designed to improve their cytotoxic properties are in clinical use. In the past decade, new methods have emerged to precisely engineer glycosylation by gene editing, and with increased knowledge the field seems to be limited only by imagination.
Cell lines are widely used as factories to produce recombinant glycoproteins from introduced gene constructs. The most common factories for glycoproteins include yeast, plants, insect cells, nonhuman mammalian cells, and, more rarely, human cells. More recently, bacteria are also being engineered to accommodate production of glycoproteins. The glycosylation capabilities of different species vary substantially in terms of both the sites of glycan attachment and the glycans attached (Figure 56.1 and Chapters 9–27). A first step in glycoengineering strategies is therefore to consider which cell type to use. This decision requires detailed knowledge of glycosylation pathways and genes. Historically, the mammalian Chinese hamster ovary (CHO) cell line has played a dominant role, and today most biologics are produced in CHO cells (Chapter 49). The CHO line was selected for human therapeutic production because its glycosylation capacities are relatively simple and resemble those of humans. The CHO cell line produces a comparatively narrow repertoire of glycans that are not immunogenic in humans; glycoengineering can expand their native glycosylation capabilities and provide optimization of glycoforms. Alternate host species in which native glycosylation (or lack thereof) provides a simpler starting point for engineering can also be selected. For example, glycoproteins for enzyme replacement therapies have been produced in yeast and glycan vaccines in bacteria.

FIGURE 56.1.
Overview of species-specific glycosylation features. The figure presents the different classes of glycoconjugates present in mammalian, plant, insect, and yeast cells with a representative glycan from each class. Structures to the right of the break are (more...)
There have been major achievements in glycoengineering of cells from bacteria to yeast and “higher” eukaryotes (Table 56.1 and Chapter 49). New precise gene editing technologies described below enable glycoengineering in a wide variety of species and open opportunities for selection of host cells based on optimal production efficiency and production of human-like glycans. Common principles in glycoengineering via gene editing are described below, followed by more detailed descriptions of progress in cells from various species.
TABLE 56.1.
Examples of major achievements in glycoengineering of cells
KNOWLEDGE OF GLYCOSYLATION PATHWAYS ENABLES GLYCOENGINEERING
Although the glycomes of different species have distinct features (Figure 56.1), the basic biosynthetic machinery and pathways are remarkably conserved in eukaryotes, and there are even similarities with glycosylation pathways in some bacteria and Archaea. Most enzymes involved in glycosylation in eukaryotes are highly conserved in fungi, plants, and animals, facilitating the design and execution of glycoengineering strategies in these organisms. Nevertheless, current knowledge is far from complete, and glycoengineering across species is still in its infancy. Whereas expression of a particular protein in a heterologous host may require only introduction of the single gene for that protein, precise glycosylation engineering of that protein may require introduction of a suite of genes, including those required for the biosynthesis and transport of appropriate activated nucleotide sugar donors, as well as multiple glycosyltransferases.
Successful glycoengineering requires knowledge of the glycosyltransferase genes and substrates required to direct synthesis of a particular glycan. Certain genes may need to be removed and others inserted to create biosynthetic pathways that produce the glycans of interest. Four decades of glycogene discoveries have resulted in the identification of many genes encoding glycosyltransferases, hydrolases, and other enzymes involved in synthesizing and metabolizing the glycans of eukaryotic cells and the biosynthetic pathways involved (Chapters 8–19). Different glycosylation pathways may function independently using different sets of enzymes or, in some cases, may share enzymes. Enzymes working in consecutive order to assemble mature glycans generally work independently, although there may also be cooperative effects. In principle, there is sufficient knowledge to predict the role of individual enzymes and assign them to specific pathways, allowing prediction of the enzyme repertoire required to generate a particular glycan on a particular glycoconjugate. An excellent resource in this regard is the classification of homologous gene families from diverse species in the “Carbohydrate-Active enZYmes” (CAZy) database (Chapter 8).
Among the prerequisites for glycoengineering a desired glycan in a chosen host is that the appropriate repertoire of activated sugar donors and their transporters are present (Chapter 5). This is especially important when engineering glycosylation in prokaryotes or nonmammalian eukaryotes in which the nucleotide sugar donors required to synthesize therapeutics with human glycosylation may not be present. For example, yeast does not produce UDP-GalNAc, and many organisms do not produce CMP-sialic acids.
THE IN/OUT STRATEGIES OF GENETIC GLYCOENGINEERING IN EUKARYOTES
Different genetic strategies may be used to alter the glycosylation capabilities of cells. Knockdown and nontargeted overexpression in eukaryotes have been used for many years, and precisely targeted gene editing strategies are now well-established.
Knockdown
Reducing undesirable glycosyltransferase activities in cells has been achieved by gene silencing strategies. Whereas this has been particularly successful in plants and Drosophila, silencing has not gained wide use in glycoengineering mammalian cell lines because the low efficiency of knockdown often leaves undesirable levels of target glycosyltransferase activity remaining.
Overexpression
Adding desirable glycosyltransferase activities to eukaryotic cells is achieved by transfection of glycogenes from any organism, random integration of plasmid DNA, and antibiotic selection of stable clones. Although this strategy is successful, it provides no control over site(s) of genomic integration (unless specific strategies are used), gene copy number, or gene expression levels. Overexpression of enzymes can lead to disruption of normal glycosylation patterns and unpredictable glycosylation. Instability of the introduced glycosylation genes and the use of antibiotics for selection have also been problematic for the long-term use of such engineered cells for production of therapeutic glycoproteins.
Knockout and Knock-In by Precision Genome Editing
Knockout of glycosylation genes to eliminate unwanted glycans has long been a simple task in bacteria and yeast. Although powerful, knockout or knock-in strategies have been time-consuming and difficult to use in “higher” eukaryotic cells (see Fut8 knockout below). However, these difficulties were substantially reduced with the introduction of nuclease-based precise gene editing tools including zinc-finger nucleases, transcription activator–like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat/targeted Cas9 endonucleases (CRISPR/Cas9), which enable highly specific gene manipulation in all cell types (Figure 56.2; Chapters 27 and 49). These tools can also be used to activate endogenous silent genes, edit gene sequences to mimic hypomorphic disease mutations, and insert foreign genes at specific genomic sites.
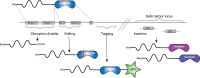
FIGURE 56.2.
Precise gene editing modalities. Glycosyltransferases are represented with their catalytic domains and amino-terminal membrane spanning domains. Examples of gene editing for gene disruption (knockout), mutation, tagging, and insertion of heterologous (more...)
Precise gene editing can insert foreign genes at “safe harbor” sites in the genome to ensure stable expression and avoid interfering with endogenous gene expression. One such safe harbor in human cells is the AAVS1 site on chromosome 19 known to enable stable expression of transgenes without adverse effects. However, precise genetic engineering allows insertion of one or more foreign genes at any position in the genome. For example, precise knock-in of glycogenes can be combined with knockout by inserting an exogenous gene in place of an unwanted endogenous gene. Precise knock-in strategies also enable control of the number of copies inserted and can be used to insert entire landing platforms of multiple genes inserted consecutively.
Successful expression of an enzyme or transporter for glycoengineering requires that the expressed protein finds its way to the correct subcellular compartment. Heterologous expression of type 2 Golgi transmembrane glycosyltransferases, for example, often requires testing different Golgi retention sequences. Although design guidance is available, this is often a trial and error exercise, and in some cases a combinatorial screening is required to identify the optimal construct.
GLYCOENGINEERING IN BACTERIA
The most common bacterium used for heterologous protein production, Escherichia coli, does not have the native capacity to glycosylate proteins. However, research in the last two decades identified N- and O-glycoproteins, and the glycosylation pathways responsible for their biosynthesis, in the pathogenic proteobacterium Campylobacter jejuni and other bacterial species. Moreover, several bacterial toxins have glycosyltransferase domains that exert their pathogenicity by glycosylating highly specific amino acids in key host proteins to interfere with essential cellular functions. Chapter 21 discusses glycosylation in Eubacteria. Another unique feature of bacteria is that sugar nucleotide donors are synthesized and remain in the cytosol, so that engineered glycan assembly on a lipid carrier (for systems based on en bloc glycosylation) or on proteins directly (for systems based on processive glycosylation) must take place in the cytosol unless nucleotide sugar transporters are introduced to the periplasmic membrane.
Engineering Free and Lipid-Linked Oligosaccharides
Bacteria efficiently produce free and lipid-linked oligo- and polysaccharides including capsular polysaccharides (CPSs) and lipopolysaccharides (LPSs) (Chapter 21), and these pathways have been engineered to produce a variety of complex human-like glycans. In particular, the LPS pathway has been used to engineer and display glycans in E. coli. LPS consists of a base lipid (lipid A) linked to a core oligosaccharide followed by highly diverse O-polysaccharides (O-antigens). Genetically interrupting biosynthesis of the lipid A core oligosaccharide prevents coupling of the O-antigen, thereby allowing engineering of novel glycans on lipid A for display on the cell surface. This strategy has been used to engineer the synthesis of a variety of human glycan epitopes, including blood group antigens (Chapter 14) and cancer-associated glycolipid glycans (Chapter 11).
In a complex example of engineering large free complex glycans in bacteria, 4-O-sulfated chondroitin sulfate, a sulfated glycosaminoglycan (Chapter 17), was produced in E. coli by deleting a fructosyltransferase (kfoE), introducing bacterial UDP-Glc/-GlcNAc 4-epimerase and chondroitin synthase genes, and introducing a mutated human chondroitin-4-O-sulfotransferase gene, opening the way for glycosaminoglycan production in bacteria.
Engineering N-Glycosylation
Two types of native protein N-glycosylation occur in some bacteria, although not in E. coli. One type is similar to eukaryotic cell N-glycosylation with production of a lipid-linked oligosaccharide in the cytosol, which is then transferred en bloc to Asn by an oligosaccharyltransferase (OST) in the periplasm. The OST of C. jejuni, PglB, is a single polypeptide related to the catalytic STT3 subunits of the multiprotein OST complex of eukaryotes (Chapter 9). PglB shows a more restricted acceptor sequence motif than the eukaryotic N-X-S/T, with requirement of an acidic residue (D/EXNXS/T, in which X cannot be P) (Chapter 21). This places some restrictions on the usefulness of engineering human-like N-glycans, because most N-glycan sites in mammalian proteins do not conform to this extended consensus sequence. PglBs from other species, or mutants evolved by adaptive evolution, have been identified to address this problem, but further improvements are needed. Importantly, PglB has rather relaxed donor substrate specificity. Although bacterial lipid-linked oligosaccharides are distinct from those in eukaryotes, PglB can use mammalian-type lipid-linked oligosaccharides as donors.
An important feature for using prokaryotes for glycoengineering is the arrangement of entire glycosylation machineries in multigene operons. This enables the transfer of large genetic elements of 10–20 kb between species. A major achievement was the successful transfer of the entire N-glycosylation operon from C. jejuni to E. coli, producing N-linked glycoproteins in E. coli. Production of glycoproteins carrying a Man3GlcNAc2 core N-glycan has been achieved by introduction of eukaryotic enzymes (Table 56.1). Bacterial N-linked glycosylation is being exploited as an alternative method for glycoconjugate vaccine production, and vaccines against both Gram-negative and Gram-positive bacteria have been developed.
Another type of N-glycosylation found in γ-proteobacteria involves a cytosolic N-glycosyltransferase (NGT) that targets the N-X-S/T acceptor sequence motif recognized by mammalian OST (Chapter 9). NGT transfers a single monosaccharide (e.g., Glc) from an activated sugar nucleotide donor, with loose donor substrate specificity that includes both UDP and GDP sugar nucleotides. This provides an entirely new approach to engineering alternate types of N-glycosylation. Engineering this pathway in bacteria resulted in the assembly of glycan motifs including α1-3-galactose epitopes as well as fucosylated and sialylated lactose or poly-N-acetyllactosamine (LacNAc) units primed by Glc residues on glycoproteins (Table 56.1).
Although most bacteria do not have the capacity for sialylation, there are exceptions (Chapters 15 and 21). Bacterial genes for CMP-sialic acid synthesis and for sialyltransferases with specificities similar to those in mammals have been introduced with plasmids or integrated into the genome of host bacteria cells, enabling the production of sialylated N- and O-glycoproteins.
Engineering O-Glycosylation
Some bacteria have processive O-glycosylation pathways controlled by glycosyltransferases using activated sugar donors. These pathways inspired the engineering of human O-glycosylation reactions in E. coli. By introducing mammalian polypeptide GalNAc-transferase genes and a UDP-Glc/GlcNAc 4-epimerase, O-GalNAc protein glycosylation (Chapter 10) has been achieved. Further introduction of a β1-3-galactosyltransferase enabled biosynthesis of core 1 O-glycans (T antigen) on cytoplasmic acceptor proteins. Introduction of GalNAc residues has been used for postexpression enzymatic addition of polyethylene glycol (PEG)-derivatized sialic acids to enhance the therapeutic properties of protein drugs.
Other bacteria possess a protein O-glycosylation mechanism that is unlike the stepwise biosynthesis of O-glycans in eukaryotes (Chapter 10), in that preassembled undecaprenol-PP-linked oligosaccharides are transferred en bloc to proteins by several OSTs with relaxed donor substrate specificities and poorly understood acceptor substrate specificities. Engineering of this endogenous glycosylation machinery has been used for en bloc transfer of human O-GalNAc glycans (Tn, T, sialyl-Tn, and sialyl-T antigens) onto acceptor proteins (Table 56.1).
GLYCOENGINEERING IN YEAST
Yeast natively produce N-glycans and O-mannosyl glycans on diverse glycoproteins. The general features of the biosynthetic pathways for initial glycan transfer are common in eukaryotes from yeast to human, and the enzymes involved are highly homologous. However, subsequent glycan processing in yeast generally results in polymannosylated glycans instead of the complex N- and O-glycans found in “higher” eukaryotes (Figure 56.1). Yeast have similar systems for protein folding, quality control, and posttranslational modifications to other eukaryotic cells, in contrast to bacteria. Because genetic engineering in yeast has long been rapid and easy, experience with glycoengineering is more advanced in this organism compared with most others. Several commercial ventures have been based on engineering “humanized” N-glycosylation in yeast; the Pichia GlycoSwitch platform uses engineered yeast to add simple human N-glycans to expressed proteins.
Engineering N-Glycosylation
N-glycans on yeast glycoproteins differ from those in vertebrates (Chapter 9), comprising large polymannosyl glycans on a poly(Manα1-6)n backbone, which are highly immunogenic in mammals (Chapter 23). A key α1-6-mannosyltransferase, Och1p largely initiates polymannosylation. Knockout of the och1 gene, however, does not completely abrogate polymannosylation, and additional knockouts of mannosyl- and phosphomannosyltransferases, depending on yeast strain, are needed to achieve a homogenous Man8GlcNAc2 N-glycan suitable for further engineering. Reducing Man8GlcNAc2 to Man5GlcNAc2 is achieved by expressing an α1-2-mannosidase in the endoplasmic reticulum (ER), creating a convenient platform for generating complex N-glycans. Introduction of GlcNAcT-I (MGAT1) in the Golgi initiates complex N-glycan synthesis, and further addition of α3/6-mannosidase II (MAN2A1) and GlcNAcT-II (MGAT2) results in the biantennary GlcNAc2Man3GlcNAc2 N-glycan suitable for appending galactose and sialic acid by further engineering. Some yeast species, including Pichia pastoris, do not contain UDP-Gal, and all yeast lack the native ability to synthesize CMP-Neu5Ac, so considerable engineering with introduction of multiple genes is required to obtain mature complex N-glycans. Although the engineering appears simple in silico, considerable efforts have been devoted to identifying optimal chimeric gene constructs with respect to both catalytic efficiency and ER/Golgi targeting.
Engineering O-Glycosylation
Yeast perform extensive co- and posttranslational ER protein O-mannosylation (Chapter 23) using several polypeptide mannosyltransferases (PMTs). Saccharomyces cerevisiae has six PMTs, and only a subset can be knocked out without reducing viability. Protein O-Man residues undergo polymannosylation in the Golgi. Muticellular eukaryotes also perform O-mannosylation and express two PMT orthologs, POMT1 and POMT2 (Chapter 13), but these have narrower acceptor substrate specificities. However, multicellular eukaryotes perform several other types of O-glycosylation (Figure 56.1) (Chapters 10, 13, and 14), and their O-GalNAc glycans tend to be located in similar regions and protein sites as O-Man glycans in yeast. This means that expression of human O-glycoproteins in yeast may result in O-mannosylation at sites that carry O-GalNAc in mammals. Examples of this include the hinge region of IgA and mucin sequences. Because it is still difficult to predict types of O-glycosylation, human proteins expressed in yeast must be tested to determine if they are O-mannosylated.
Human O-GalNAc glycans have been successfully engineered into yeast by introducing human polypeptide GalNAc-transferases (Chapter 10) along with UDP-Glc/GlcNAc C4-epimerase and a UDP-Gal/GalNAc Golgi transporter. The entire biosynthetic machinery for CMP-Neu5Ac synthesis and transport has also been introduced together with a human sialyltransferase, and sialylated O-glycans have been produced in yeast. The problem with competing endogenous O-mannosylation can be partly eliminated by including a mannosyltransferase inhibitor (rhodanine-3-acetic acid). A deeper understanding of the yeast O-Man and human O-GalNAc glycosylation pathways is needed to provide new strategies to circumvent competition between the two systems and enhance O-glycan engineering in yeast.
GLYCOENGINEERING IN PLANT CELLS
Plants offer a simpler starting point than yeast for N-glycan humanization because the predominant native N-glycans of plants are paucimannose (Man3GlcNAc2) and biantennary terminating in GlcNAc (GlcNAc2Man3GlcNAc2). The abundance of paucimannose N-glycans appears to be due to a β-hexosaminidase that removes attached GlcNAc residues in competition with GlcNAc-transferases, a feature also found in insect cells. Two plant-specific N-glycan modifications include core α1-3-Fuc (instead of mammalian core α1-6-Fuc) and β1-2-Xyl linked to the β-Man in the N-glycan core. Both modifications are potentially immunogenic in humans. Plants also produce unique types of O-glycosylation not found in other species that pose potential problems for the generation of therapeutic glycoproteins.
Engineering N-Glycosylation
Great advances in engineering plants for human-like N-glycosylation have been achieved. Knockdown or knockout of the β-hexosaminidase that inhibits complex N-glycan formation, as well as the α1-3 fucosyltransferase and β1-2 xylosyltransferase, have been achieved in different plants, including Arabidopsis thaliana and Nicotiana benthamiana. Nearly homogeneous biantennary GlcNAc2Man3GlcNAc2 N-glycans were produced. These were further engineered by the introduction of Gal (using B4GALT1) and sialic acid (using ST6GAL1 along with the enzymes needed to synthesize and transport CMP-Neu5Ac) in an engineering design using up to six gene constructs. Such humanized plants produced α2-6-Neu5Ac capped biantennary N-glycans without core fucose on a variety of recombinant glycoproteins. These achievements depended on combinatorial screening strategies to identify appropriate chimeric constructs of exogenous enzymes to drive the engineered glycosylation toward homogeneity.
Glycoproteins produced in plants carrying native paucimannose N-glycosylation are in use as approved drugs. For enzyme replacement therapy, the terminal mannose N-glycans of glucocerebrosidase (taliglucerase alfa) produced in carrots is beneficial for targeting to endogenous human mannose receptors, despite α1-3-Fuc and β1-2-Xyl modifications, and is in clinical use. Moreover, glycoengineered N. benthamiana cells without α1-3-Fuc and β1-2-Xyl have been used to produce a triple-antibody cocktail used to treat Ebola virus infections.
Engineering O-Glycosylation
Plants do not have the types of O-glycosylation found in other eukaryotes but produce extensins and arabinogalactan proteins with two unique O-glycans. A family of prolyl-4-hydroxylases (P4H) converts selected Pro residues to hydroxyproline that may be arabinosylated by a series of enzymes. In addition, Ser residues may be O-glycosylated by the addition of Gal residues. Although a number of the P4Hs and glycosyltransferases have been knocked out in different plants, it is unclear whether these modifications can be completely eliminated without affecting viability. Nonetheless, the human machinery for O-GalNAc glycosylation has been engineered into plants by introducing the necessary polypeptide GalNAc-transferases and elongation enzymes, whereas UDP-Glc/-GlcNAc 4-epimerase and a UDP-GalNAc transporter may not be required. Human core 1 O-glycan biosynthesis and sialylation machinery including ST3GalI sialyltransferase have also been successfully introduced into plants. If issues related to hydroxyproline can be resolved, plants offer a valuable system in which different types of mammalian O-glycosylation could be engineered and exploited. A clear highlight of glycoengineering in plants was the combined introduction of 14 genes for production of the major human therapeutic glycoprotein erythropoietin with human sialylated biantennary N-glycans and core 1 O-glycans in tobacco cells (Table 56.1).
GLYCOENGINEERING IN INSECT CELLS
Engineering in insect cells involves multiple strategies. Two different platforms are generally used for recombinant expression of proteins—transient expression in the baculovirus-insect cell system and constitutive expression in Sf9 Spodoptera frugiperda or S2 Drosophila melanogaster cells. The baculovirus-insect cell platform can be glycoengineered by including glycosylation genes in either the recombinant baculovirus vector genome or the insect cell line host genome. Engineering host insect cell lines has been the more common strategy, but remarkable success has been achieved by incorporating up to nine glycogenes in a baculovirus vector (Table 56.1). CRISPR/Cas gene targeting of Sf9 insect cells has been established, and their use for glycoengineered baculovirus protein expression is feasible.
Engineering N-Glycosylation
Insect cells produce mostly high-mannose and paucimannose N-glycans despite having the genetic capacity to produce complex sialylated N-glycans (Figure 56.1). This is due in part to the action of a processing β-hexosaminidase, FDL, which removes attached GlcNAc residues from the α1-3-Man branch, and in part to low levels of GlcNAcT-II (MGAT2) activity. Like plants, some insect cells may add a potentially immunogenic core α1-3-Fuc and do not typically add terminal sialic acids. However, sialylation has been engineered by introducing genes encoding a CMP-sialic acid synthase and an N-acetylglucosamine-6-phosphate 2′-epimerase into insect cells. For efficient sialylation, a dedicated CMP-sialic acid transporter appears to be needed as well. Using different strategies, production of glycoproteins carrying biantennary N-glycans with galactosylation and sialic acid capping has been achieved. Precision gene editing was used to knock out fdl in Sf9 and S2 cells to greatly improve complex N-glycan formation.
Engineering O-Glycosylation
Insect cells perform the same range of O-glycosylation reactions as mammalian cells (Figure 56.1), although the extent to which O-GalNAc glycans are attached at the same sites as in mammals is unexplored. Moreover, processing of O-glycans is limited to mainly truncated core 1 structures (Tn and T). Although insect cells offer a straightforward host for production of glycoproteins with human O-glycans, little has been investigated in this regard.
GLYCOENGINEERING IN MAMMALIAN CELLS
The cores of all types of glycoprotein glycans (Figure 56.1) are highly conserved among mammals, although there are terminal glycan variations (Chapter 14). At least 16 different glycosylation pathways have been delineated in mammalian cells; maps of the predicted genetic regulation of biosynthetic steps by more than 170 distinct glycosyltransferases have been generated. The most popular mammalian cell line used for glycoengineering is the CHO cell line established more than 60 years ago. The success of the CHO cell line is partly due to the ease with which glycosylation mutants could be isolated (Chapter 49), and it was the first cell used to manufacture a recombinant therapeutic with relatively simple human-type terminal glycans without expression of antigenic nonhuman glycans or unusual modifications of the glycans. As discussed in Chapter 49, the CHO cell line has an important place in glycoengineering history, exemplified by the Lec mutant lines generated by lectin selection. These cell lines with distinct mutations in glycosylation genes have provided tools for the scientific community for more than three decades and illustrate the importance of access to recombinant proteins with particular glycoforms for discovery of biological functions of glycans.
CHO cells can be considered as Glycobiology's gift to Biopharma. Major successes have been achieved in engineering CHO and other mammalian cell lines for production of human therapeutics (Table 56.1 and Chapters 49 and 57). The field is, however, undergoing a revolution with the new methods for facile, targeted, precise gene editing that allow the design of almost any conceivable glycosylation capacity in any mammalian cell by combining knockout and knock-in events.
Engineering N-Glycosylation
The first major feat in gene editing of mammalian cells was elimination of the core α1-6-Fuc for production of recombinant IgG antibodies with enhanced binding to the Fcγ-IIIa receptor (Table 56.1). Overexpression of bisecting GlcNAcT-III (MGAT3) resulted in stable CHO cells with highly limited capacity for core fucosylation (commercialized by Roche). A second strategy involved a tour-de-force approach using homologous recombination (HR) to knock out the two Fut8 alleles in CHO cells. More than 10,000 CHO clones were screened to identify the final knockout cell. Although this was an impressive achievement, such laborious random selection limits options for selecting cell clones that retain the attributes needed for optimal bioprocessing. Using precise gene editing, the same engineering was rapidly replicated, providing ample clones for selection of those with optimal properties. Glycoengineered CHO lines optimized for antibody production are now commercially available (Potelligent CHOK1SV, Lonza/Kyowa Kirin BioWa). Another elegant strategy introduced GDP-6-deoxy-D-lyxo-4-hexulose reductase to deflect the endogenous production of GDP-Fuc and enable fine-tuning of fucosylation by exogenous addition of fucose.
Engineering N-glycan sialylation has been another focus in the field. CHO cells produce only α2-3-linked sialic acids on N-glycans, whereas human HEK293-T cells (for example) produce a mixture of α2-3- and α2-6-linked sialic acids. Most soluble glycoproteins in human blood (including IgG) have α2-6-linked sialic acids on N-glycans, and reports have suggested that the sialic acid linkage may influence immunomodulatory functions as well as circulatory half-life. It has therefore been of interest to engineer more homogeneous α2-6-sialylation in cells. These efforts have mainly been limited to the overexpression of α2-6-sialyltransferases to override endogenous α2-3-sialylation with variable results, illustrating the complexity of engineering glycosylation in cells with competing pathways.
An innovative glycoengineering strategy (GlycoDelete) reduced the inherent heterogeneity of mammalian N-glycan structures. Human HEK293-T cells lacking MGAT1 were stably transfected to express a fungal endo-N-acetylglucosaminidase (EndoT) that efficiently truncated N-glycans to a single GlcNAc, which was an acceptor for galactosylation and sialylation. Recombinant antibodies with truncated N-glycans had lower affinity for Fcγ receptors, suggesting that this glycoengineering strategy may be suitable for use with neutralizing antibodies.
Deconstruction of the N-glycosylation pathway in CHO cells was performed by precise gene editing to knock out 19 glycosyltransferases, including all four α2-3-sialyltransferases that function on N-glycans (Figure 56.3). Combining knockout of St3gal4 and St3gal6 with site-specific knock-in of St6gal1 resulted in homogeneous α2-6-sialylation. Combinatorial knockout of all isoenzymes involved in N-glycan sialylation, galactosylation/LacNAc formation, branching, and core fucosylation has provided a design matrix for improving the homogeneity of N-glycans in CHO cells. A combination of five gene knockouts and the knock-in of St6gal1 created the glycoprotein therapeutic erythropoietin having homogeneous biantennary N-glycans with terminal α2-6-Neu5Ac. Wider engineering of almost all genes involved in N-glycosylation in CHO cells has shown that there are few limitations for engineering of glycosylation. For example, the GlcNAc-1-phosphate transferase (Gnptab) that tags select oligomannose N-glycans on glycoproteins destined for lysosomal targeting was knocked out to produce lysosomal enzymes bearing complex-type sialylated glycans with extended blood circulation and improved biodistribution.

FIGURE 56.3.
(A) A complex N-glycan with glycosyltransferases responsible for each reaction. Combinatorial knockout of the glycosyltransferase isoenzyme genes indicated led to the identification of the primary genes (highlighted in bold) controlling N-glycan branching (more...)
Therapeutic glycoprotein production still suffers from heterogeneity, including variations in which Asn residues are glycosylated (site occupancy, macroheterogeneity) and/or the diversity of mature glycan structures at any one site (microheterogeneity). This is currently addressed by ensuring reproducibility in batch-to-batch production through the use of highly standardized bioprocessing protocols, but this strategy is far from optimal. For example, incompletely sialylated therapeutic glycoproteins may be cleared by the hepatic asialoglycoprotein receptor (Ashwell–Morell receptor), resulting in inconsistent circulatory half-lives of therapeutic glycoproteins (Chapter 34). Considerable efforts have been devoted to improving sialylation by overexpressing relevant sialyltransferases as well as inhibiting or knocking out endogenous sialidases in host cells. Protein-specific glycosylation patterns and heterogeneity are more difficult to control.
Nonhuman mammalian cell lines can produce two immunogenic nonhuman glycans: α1-3-Gal added to N-acetyllactosamine and Neu5Gc added to Gal or GalNAc (Chapters 14 and 15). The α1-3-galactosyltransferase and CMP-N-acetylneuraminic acid hydrolase genes responsible are inactive in humans. Although α1-3-Gal and Neu5Gc are not produced in CHO cells, both genes have been knocked out as a precaution. Even so, Neu5Gc scavenged from animal glycoproteins used in cell culture can appear in expressed glycoproteins, so use of defined media lacking nonhuman glycoproteins is also necessary. In engineering mammalian cell lines, it is important to consider that the glycosylation capacity is driven by the expression of a subset of available enzyme genes, but unexpressed genes can become activated. Thus, cell-specific glycosylation features are generally controlled by transcriptional regulation rather than mutations or gene aberrations. Analysis of all known glycosylation genes in five distinct CHO production cell lines derived from the original CHO-K1 cell line found no apparent deleterious mutations or loss of genes, despite severe chromosomal alterations. This suggests that one must consider all the known glycogenes in a mammalian cell line for glycoengineering strategies.
Engineering the glycosylation capacity of CHO cells has also enabled more homogeneous bioconjugation of therapeutic drugs. For example, therapeutic drugs may be chemically conjugated with PEG chains to enhance circulatory half-life, but chemical conjugation is difficult to direct to specific sites in glycoproteins. A strategy for enzymatic modification of glycans postproduction has been developed that involves desialylation of recombinant glycoproteins, followed by in vitro transfer of a modified (PEGylated) Neu5Ac (as its CMP analog) to exposed Gal/GalNAc residues by a sialyltransferase. The process is in use with approved drugs, although heterogeneous modifications occur when multiantennary N-glycans are targeted. CHO cells have now been engineered to produce monoantennary, unsialylated N-glycans, which circumvent heterogeneity while retaining multiple exposed Gal acceptor sites for sialo-PEGylation.
Engineering O-Glycosylation
Mammalian cells perform many different types of O-glycosylation (Figure 56.1), and although these exert diverse and important biological functions, the interest in O-glycans for recombinant therapeutics has been limited. Nevertheless, recombinant coagulation factors in clinical use carry O-GalNAc, O-Fuc, and/or O-Glc glycans, and many other approved drugs including erythropoietin and Enbrel have O-GalNAc glycans. O-GalNAc glycans are also used for site-specific bioconjugation.
Engineering O-GalNAc glycans involves a new level of complexity because up to 20 isoenzymes (polypeptide GalNAc-transferases) direct the initiation of O-GalNAc glycans. It may therefore be important to consider the repertoire of these enzymes in a cell line. In theory, a protein that is naturally found with an O-glycan may not be O-glycosylated when expressed in a specific production cell line, and vice versa. An illuminating case is the important phosphaturic factor FGF23, a potential drug for patients with a congenital deficiency associated with hyperphosphatemia, which requires an O-GalNAc glycan for activity. The repertoire of polypeptide GalNAc-transferases in CHO and HEK293 cells has been extensively engineered by knockout and also knock-in of GALNT genes, revealing adaptation of mammalian cells to loss of O-glycosylation capacities.
GLYCOENGINEERING IN GLYCOSCIENCE
Glycoengineering of cell lines has vast potential to address a number of unmet needs in the glycosciences. As already mentioned, the CHO Lec, Ldl, Pgs, and Pig mutant cell lines (Chapters 12 and 49) served the research community for decades by providing defined alterations in glycosylation that enable studies of the functional roles of glycans. For example, CHO and HEK293-T mutant cells with MGAT1 deficiency have been widely used to produce recombinant proteins with homogeneous N-glycans suitable for crystallization studies.
Moreover, targeting glycosylation genes in whole organisms has provided immense insight into the importance of glycosylation and has revealed biological functions of specific glycosylation genes (Chapter 41). However, discovery of distinct biological functions of specific glycans, and the molecular mechanism(s) involved in multicellular organisms, is complicated by cell-type regulation of glycosylation and the cellular heterogeneity of tissues. Cell lines help to answer certain specific questions and complement whole organism studies.
Precise gene editing provides vast opportunities for glycoengineering cell lines and designing new strategies to probe glycan functions. Truncation of O-glycan elongation was used to produce homogeneous simple O-glycoproteomes (SimpleCell strategy), which enabled enrichment and sensitive mapping of the O-GalNAc and O-Man glycoproteomes of human cell lines. The development of isogenic cell lines differing in only one glycosyltransferase gene allows comparative studies to explore the function of a particular glycan or glycosylation pathway. For example, truncation of O-GalNAc glycans by targeting of the COSMC chaperone (Chapter 10) induced oncogenic features (proliferation, growth, and invasive behavior) of human nontransformed keratinocytes, an interesting finding in light of the frequent overexpression of truncated O-glycans (Tn, sialyl-Tn) in cancer. Large libraries of isogenic cells with comprehensive engineered glycosylation are used for cell-based glycan arrays and are useful for studying glycan binding in the natural context of the cell surface. The strategy has been expanded to organotypic tissue models used to address the more complex functions of distinct types of glycoconjugates (glycolipids, N-glycans, O-GalNAc, O-Fuc, O-Glc glycans) in human tissue formation.
A related approach is to use glycoengineering for discovery of host glycans required for microbial and viral infectivity. In one remarkable study using binding of Lassa virus to a haploid cell line, the large number of glycogenes required for synthesis of the extended O-Man glycan termed matriglycan (Chapter 13) that are bound by Lassa virus were identified and validated using a combination of selection for virus resistance and TALEN-mediated gene knockout.
FUTURE PERSPECTIVES
Glycoengineering of cells has entered a phase that may be described as “LEGO toying” because of the efficiency of precise gene editing. Entire glycosylation machineries can be deconstructed and rebuilt in various cell types. Cells show remarkable plasticity for engineering glycosylation pathways, with only a few glycosylation enzymes essential for cell growth in vitro. The essential functions for viability of mammalian cells are the initial steps of N-glycosylation (Chapter 9) and nuclear/cytosolic O-GlcNAc modifications (Chapter 19). These aside, essentially all glycosylation pathways in cells can be genetically deconstructed. Combining engineering of glycosylation pathways in creative ways by introducing completely foreign enzymes can be used to produce novel glycans for study.
Large-scale glycosylation screening and discovery strategies are possible. The CRISPR/Cas9 editing tool is particularly suited for multiplexed screening strategies, and whole-genome lentiviral-based knockout libraries have already been used for screening mutations that result in altered biological function. Although knockdown strategies have been successful for screening biological functions of glycogenes in multiple organisms (worms, flies, frogs, and zebrafish), these have generally not been effective in mammalian cells because of the low efficiency of knockdown. It is now possible to apply whole-glycogenome screening strategies in cell lines to probe and dissect the roles of glycosylation. These tools are dramatically improving options for dissection of structure–function relationships in the field.
Glycoengineered cell lines and validated targeting constructs (knockout, knock-in, and mutated) including libraries for screening will become important community resources that will advance the glycoscience field and help disseminate and integrate glycosciences more broadly in biology. Large libraries of engineered isogenic cells with subtle differences of all types of glycosylation are available for dissection of glycan functions using different assays not limited to binding. Expanding these to organotypic tissue models is providing deeper insights. The ability to produce glycoproteins with a large variety of glycans opens up for unbiased testing of different glycoforms and determines the optimal design for therapeutic uses. Glycans can be custom designed, for example, to improve homogeneity, circulation time, targeting to select organs, or stimulating immunity.
A word of caution, however: Whereas glycoengineering by knockout is fairly straightforward, there is still considerable work needed to establish methods to build robust complex glycosylation capabilities requiring multiple gene insertions in cells. Here, activation of endogenous genes in mammalian cells may provide a solution. Gene editing technologies caused a revolution in the glycoscience field, and we have only begun to see the new possibilities for manipulating glycosylation in cells and organisms and for exploiting glycoengineering in therapeutic glycoproteins and biologics.
Finally, the well-established power and cost efficiency of using bacteria as reactors for production of proteins is now being harnessed to generate designer glycoproteins. There remains much to be explored in this rapidly developing area, but the potential is enormous.
ACKNOWLEDGMENTS
The authors acknowledge contributions to previous versions of this chapter by Catherina Steenhoft and appreciate helpful comments and suggestions from Jenny Mortimer.
FURTHER READING
- Wacker M, Linton D, Hitchen PG, Nita Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW, et al. 2002. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 298: 1790–1793. doi:10.1126/science.298.5599.1790 [PubMed: 12459590] [CrossRef]
- Hamilton SR, Gerngross TU. 2007. Glycosylation engineering in yeast: the advent of fully humanized yeast. Curr Opin Biotechnol 18: 387–392. doi:10.1016/j.copbio.2007.09.001 [PubMed: 17951046] [CrossRef]
- Malphettes L, Freyvert Y, Chang J, Liu PQ, Chan E, Miller JC, Zhou Z, Nguyen T, Tsai C, Snowden AW, et al. 2010. Highly efficient deletion of FUT8 in CHO cell lines using zinc-finger nucleases yields cells that produce completely nonfucosylated antibodies. Biotechnol Bioeng 106: 774–783. doi:10.1002/bit.22751 [PubMed: 20564614] [CrossRef]
- North SJ, Huang HH, Sundaram S, Jang-Lee J, Etienne AT, Trollope A, Chalabi S, Dell A, Stanley P, Haslam SM. 2010. Glycomics profiling of Chinese hamster ovary cell glycosylation mutants reveals N-glycans of a novel size and complexity. J Biol Chem 285: 5759–5775. doi:10.1074/jbc.m109.068353 [PMC free article: PMC2820803] [PubMed: 19951948] [CrossRef]
- Baker JL, Celik E, DeLisa MP. 2013. Expanding the glycoengineering toolbox: the rise of bacterial N-linked protein glycosylation. Trends Biotechnol 31: 313–323. doi:10.1016/j.tibtech.2013.03.003 [PubMed: 23582719] [CrossRef]
- Bosch D, Castilho A, Loos A, Schots A, Steinkellner H. 2013. N-glycosylation of plant-produced recombinant proteins. Curr Pharm Des 19: 5503–5512. doi:10.2174/1381612811319310006 [PubMed: 23394562] [CrossRef]
- Merritt JH, Ollis AA, Fisher AC, DeLisa MP. 2013. Glycans-by-design: engineering bacteria for the biosynthesis of complex glycans and glycoconjugates. Biotechnol Bioeng 110: 1550–1564. doi:10.1002/bit.24885 [PubMed: 23456823] [CrossRef]
- Strasser R, Altmann F, Steinkellner H. 2014. Controlled glycosylation of plant-produced recombinant proteins. Curr Opin Biotechnol 30: 95–100. doi:10.1016/j.copbio.2014.06.008 [PubMed: 25000187] [CrossRef]
- Castilho A. 2015. Glyco-engineering. Preface. Methods Mol Biol 1321: v–vii. doi:10.1007/978-1-4939-2760-9 [PubMed: 26280044] [CrossRef]
- Laukens B, De Visscher C, Callewaert N. 2015. Engineering yeast for producing human glycoproteins: where are we now? Future Microbiol 10: 21–34. doi:10.2217/fmb.14.104 [PubMed: 25598335] [CrossRef]
- Laukens B, De Wachter C, Callewaert N. 2015. Engineering the Pichia pastoris N-glycosylation pathway using the GlycoSwitch technology. Methods Mol Biol 1321: 103–122. doi:10.1007/978-1-4939-2760-9_8 [PubMed: 26082218] [CrossRef]
- Yang Z, Wang S, Halim A, Schulz MA, Frodin M, Rahman SH, Vester-Christensen MB, Behrens C, Kristensen C, Vakhrushev SY, et al. 2015. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat Biotechnol 33: 842–844. doi:10.1038/nbt.3280 [PubMed: 26192319] [CrossRef]
- Dabelsteen S, Pallesen EMH, Marinova IN, Nielsen MI, Adamopoulou M, Rømer TB, Levann A, Andersen MM, Ye Z, Thein D, et al. 2020. Essential functions of glycans in human epithelia dissected by a CRISPR-Cas9-engineered human organotypic skin model. Dev Cell 54: 669−684. doi:10.1016/j.devcel.2020.06.039 [PMC free article: PMC7497784] [PubMed: 32710848] [CrossRef]
- Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H. 2020. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol 21: 729−749. doi:10.1038/s41580-020-00294-x [PubMed: 33087899] [CrossRef]
- Narimatsu Y, Büll C, Chen YH, Wandall HH, Yang Z, Clausen H. 2021. Genetic glycoengineering in mammalian cells. J Biol Chem 296: 100448. doi:10.1016/j.jbc.2021.100448 [PMC free article: PMC8042171] [PubMed: 33617880] [CrossRef]
- GOALS FOR GLYCOENGINEERING OF CELLS
- KNOWLEDGE OF GLYCOSYLATION PATHWAYS ENABLES GLYCOENGINEERING
- THE IN/OUT STRATEGIES OF GENETIC GLYCOENGINEERING IN EUKARYOTES
- GLYCOENGINEERING IN BACTERIA
- GLYCOENGINEERING IN YEAST
- GLYCOENGINEERING IN PLANT CELLS
- GLYCOENGINEERING IN INSECT CELLS
- GLYCOENGINEERING IN MAMMALIAN CELLS
- GLYCOENGINEERING IN GLYCOSCIENCE
- FUTURE PERSPECTIVES
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Glycosylation Engineering.[Essentials of Glycobiology. 2015]Review Glycosylation Engineering.Clausen H, Wandall HH, Steentoft C, Stanley P, Schnaar RL. Essentials of Glycobiology. 2015
- Review Genetic glycoengineering in mammalian cells.[J Biol Chem. 2021]Review Genetic glycoengineering in mammalian cells.Narimatsu Y, Büll C, Chen YH, Wandall HH, Yang Z, Clausen H. J Biol Chem. 2021 Jan-Jun; 296:100448. Epub 2021 Feb 20.
- Rapid assays for lectin toxicity and binding changes that reflect altered glycosylation in mammalian cells.[Curr Protoc Chem Biol. 2014]Rapid assays for lectin toxicity and binding changes that reflect altered glycosylation in mammalian cells.Stanley P, Sundaram S. Curr Protoc Chem Biol. 2014 Jun 3; 6(2):117-133. Epub 2014 Jun 3.
- Glycoengineering of Mammalian Expression Systems on a Cellular Level.[Adv Biochem Eng Biotechnol. 2021]Glycoengineering of Mammalian Expression Systems on a Cellular Level.Heffner KM, Wang Q, Hizal DB, Can Ö, Betenbaugh MJ. Adv Biochem Eng Biotechnol. 2021; 175:37-69.
- Review Engineering of glycosylation in yeast and other fungi: current state and perspectives.[Appl Microbiol Biotechnol. 2010]Review Engineering of glycosylation in yeast and other fungi: current state and perspectives.De Pourcq K, De Schutter K, Callewaert N. Appl Microbiol Biotechnol. 2010 Aug; 87(5):1617-31. Epub 2010 Jun 29.
- Glycosylation Engineering - Essentials of GlycobiologyGlycosylation Engineering - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
