This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Show detailsContinuing Education Activity
Odontogenic tumors are quite uncommon lesions. It can be either derived from odontogenic ectoderm or ectomesenchyme. It should always be considered as a differential diagnosis for any new growth in the jaws. They have a great variety in their presentation, either clinically or radiographically. This activity will review the evaluation and management of different odontogenic tumors by an interprofessional team.
Objectives:
- Review the pathophysiology of odontogenic tumors of the jaw.
- Outline the clinical presentation of the most common odontogenic tumors of the jaw.
- Describe the imaging findings associated with each tumor.
- Summarize the available treatment options when dealing with different odontogenic tumors.
Introduction
Odontogenic tumors of the jaw are a group of lesions that originate from remnants of epithelium or ectomesenchyme associated with teeth development. It ranges from hamartomas to true tumors. The fourth edition of WHO histological typing of odontogenic tumors classifies them as benign and malignant. Then, they are subdivided according to the cell of origin to epithelial, ectomesenchymal, or mixed.[1]
Etiology
The genetic mutation affects the cell cycle, especially the G1- S checkpoint. This stimulates the formation of odontogenic cysts or tumors. Studies suggest that mutations in ameloblastin, KRAS, FHIT, and P53 genes are related to the development of odontogenic tumors.[2][3][4]
Understanding the etiology of odontogenic tumors went through many phases. Initially, it was thought to be related to trauma, nutritional deficiencies, and chronic irritation from dental caries. This concept changed over time as studies have shown that odontogenic tumors express proteins similar to the developing tooth-like cytokeratin and vimentin.[5] Other studies linking them to the absence of the stratum intermedium layer.[6]
At the genetic level, a different expression of certain genes has been noticed in odontogenic tumor patients, especially ameloblastoma cases. Some of them are overexpressed, and others are underexpressed compared with the normal level. Examples of the over-expressed genes are c-fos proto-oncogene (FOS), tumor necrosis factor receptor 1A (TNFRSF1A), cyclin-dependent kinase inhibitor 1A (CDKN1A), collagen type VIII alpha 1 (COL8A1), matrix metalloproteinase 12 (macrophage elastase) (MMP-12) and WNT5A and WNT-1. While the under-expressed genes include sonic hedgehog (SHH), cadherin 12 (CDH12), cadherin 13 (CDH13), transforming growth factor beta 1 (TGFB1), patch (PTCH), and others.[7]
The molecular pathogenesis of ameloblastoma is now considered to be related to the dysregulation of the mitogen-activated protein kinase (MAPK) signaling pathway. Mutation in the BRAF, a protein kinase responsible for activating the MAPK signaling pathway, has been seen in around 63% of ameloblastoma cases.[8][9][10] Moreover, recent studies showed that mutation of other non MAPK genes especially smoothened (SMO), a signaling effector in the SHH pathway, is also associated with ameloblastoma development.[11]
Epidemiology
The incidence of odontogenic tumors varies significantly between different geographical regions. For example, while they represent 1% of all oral pathologies in North America, this figure has been reported as high as 19% in African nations.[12][13]
Pathophysiology
Odontogenic Tumors That Arise Only From Odontogenic Epithelium Without Odontogenic Ectomesenchyme
They include ameloblastoma, calcifying epithelial odontogenic tumor, squamous odontogenic tumor, and adenomatoid odontogenic tumor. Ameloblastoma is the second most common odontogenic tumor after odontoma. It can originate from any of the following: remnants of the dental lamina, enamel organ, odontogenic cyst lining, or oral mucosa basal cells. It can be either central (intra-osseous), which includes multicystic or solid and unicystic or peripheral (extra-osseous). The majority of cases are solid or multicystic 92%, while unicystic and peripheral represent 6% and 2%, respectively.[14] Recurrent and aggressive ameloblastoma are associated with a high number of PCNA-positive cells, overexpression of BCL2, BCLX, IL1, and IL6.[15] Malignant ameloblastoma has been removed from the malignant category and added to the ameloblastoma group in the most recent edition of the WHO classification due to its bland histological features.
Other types in this category include calcifying epithelial odontogenic tumors (Pindborg tumor), which usually arises from the remnants of the dental lamina or reduced enamel epithelium, and squamous odontogenic tumors that usually originate from the remnants of the dental lamina.[16] The adenomatoid odontogenic tumor usually arises from odontogenic epithelium around the crowns of unerupted teeth. It is considered by many as a hamartoma. It was previously considered as a variant of ameloblastoma named adenoameloblastoma. However, a better understanding of the biological behavior of this tumor changed this concept. It is biologically non-aggressive.[17][18]
Odontogenic Tumors That Arise From Both Odontogenic Epithelium and Odontogenic Ectomesenchyme, With or Without Dental Hard Tissue Formation
This includes odontoma, odontogenic ghost cell tumor, ameloblastic fibroma Primordial odontogenic tumor. Odontoma is the most common odontogenic tumor. Its prevalence exceeds all other tumors combined. It is considered to be hamartoma rather than an actual tumor. It can present in one of two forms: compound type, which is composed of multiple teethlike structures, and complex type, in which irregular masses are present that do not resemble dental structures.[19] Odontogenic ghost cell tumor (calcifying odontogenic cyst) is another lesion that belongs to the same category. It is an uncommon lesion and has a highly variable presentation in both clinical and histopathological features. It can be either solid or cystic. Although it was previously considered being cyst, new evidence suggested its subclassification as a tumor.[20][21][22]
Another true neoplasm is ameloblastic fibroma, in which both epithelial and ectomesenchymal components are neoplastic. This differentiates it from ameloblastic fibro odontoma and odontoma, which are considered to be hamartomatous lesions.[23] Ameloblastic fibro-odontoma and ameloblastic fibrodentinoma have been excluded from the 4th edition of WHO classification for odontogenic lesions. Evidence suggests that once hard dental tissue is formed, these two lesions usually progress to odontomas.[1]
Odontogenic Tumors That Arise From Ectomesenchyme With or Without Included Odontogenic Epithelium
This includes odontogenic fibroma, odontogenic myxoma (myxofibroma), cementoblastoma, and cemento-ossifying fibroma. Odontogenic fibroma can be either central (intra-osseous type) or peripheral (extra-osseous type).[19] Odontogenic myxoma is an aggressive intra-osseous lesion that is derived from ectomesenchyme. It is believed to be originating from periodontal ligaments. Its aggressive behavior is usually linked to the over-expression of BCL2 and BCLX.[24][25] Cementoblastoma is another rare benign tumor that belongs to this category. It usually grows in continuity with the apical cemental layers of molar and premolar teeth and may lead to cortical expansion and pain.[26]
Malignant Odontogenic Tumors
This includes ameloblastic carcinoma, clear cell odontogenic carcinoma, primary intraosseous carcinoma, Non otherwise specified (NOS) sclerosing odontogenic carcinoma, Ghost cell odontogenic carcinoma, Odontogenic carcinosarcoma, and Odontogenic sarcomas. All these tumors have the typical microscopic features of malignancy and can metastasize to regional lymph nodes or distant metastases.
The majority of ameloblastic carcinoma cases arise de novo and less commonly arise from pre-existing ameloblastoma. It is a rare tumor that represents less than 1% of all odontogenic tumors.[27][28] Primary intraosseous carcinoma is another entity that can arise de novo or as a malignant transformation of an odontogenic cyst. Clear cell odontogenic carcinoma is another malignant intra-osseous lesion of odontogenic origin previously thought to be distant metastasis from renal carcinoma.[29]
Non otherwise specified (NOS) sclerosing odontogenic carcinoma has been recently added in the 4th edition of WHO classification for odontogenic tumors. It is a very rare tumor, as only 15 cases have been reported in the literature. It is considered a locally aggressive tumor with low potential for metastatic spread due to marked sclerosis in the surrounding stroma.[30][31][30] Its origin is still unclear. The two potential sources are either de novo from the odontogenic rests or as a malignant transformation of an odontogenic cyst.[32] Ghost cell odontogenic carcinoma is thought to originate from calcifying odontogenic cysts with some features of odontogenic ghost cells or calcifying odontogenic tumors. Odontogenic sarcoma and carcinosarcoma includes ameloblastic fibrosarcoma, fibro-odontosarcoma, and fibro-dentisarcoma.[33][34]
Histopathology
Odontogenic Tumors That Arise Only From Odontogenic Epithelium Without Odontogenic Ectomesenchyme
Ameloblastoma: It may present as multicystic/ solid type, uni-cystic type, peripheral type, and metastasizing type, which has been recently removed from the malignant group.
Multi-cystic or solid type: It usually originates de novo, and it has many variants, including follicular subtype in which follicles of epithelial cells are present in connective tissue stroma. It is composed of loosely arranged stellate cells in the center and columnar cells in the periphery. These follicles are like the enamel organ seen in the early stages of tooth development. Cystic changes may be seen as well. Plexiform subtype: arranged in long anastomosing sheets of odontogenic epithelium. It may lack reverse polarization and may not resemble any stages of tooth development. It is called a “fishnet pattern.” Cystic changes are uncommon.
Acanthomatous subtype in which extensive squamous metaplasia of the central core is present. It is often associated with keratin formation. It may be confused as squamous cell carcinoma. Granular cell type is a clinically aggressive type in which tumor cells' cytoplasm shows abundant eosinophilic granules. The desmoplastic type is characterized by densely collagenized stroma. It is clinically more aggressive with a higher rate of recurrence. Finally, the basal cell type is the least common type with a nest of basaloid cells, which resemble basal cell carcinoma.
Uni-cystic type: It usually arises from the lining of a dentigerous cyst, and it can be associated with impacted molar.[35][36] It has three different types—luminal type in which all changes are confined to the luminal surface of the cyst. The epithelial lining changes to ameloblastic columnar epithelium with hyperchromatic nuclei and reversed polarity. Secondly, the intra-luminal type in which nodules of the ameloblastic cells project from cyst lining into the lumen of the cyst. If it is large enough to fill the cystic lumen, a plexiform pattern like plexiform ameloblastoma can be seen. This will be referred to as plexiform unicystic ameloblastoma. Lastly, the mural type in which the connective tissue wall of the cyst is infiltrated by ameloblastic cells. The most aggressive of all three types.[15]
Peripheral type: It usually arises from the remnants of the dental lamina or the basal layer of the surface epithelium.[37] It is extra-osseous and usually occupies the lamina propria just below the surface epithelium and outside the bone. It is like intra-osseous ameloblastoma with follicular histological type is the most common variant.[38]
Malignant ameloblastoma: The tumor cells have the typical features of benign ameloblastoma both in the primary lesion and secondaries. The 4th edition of the WHO classification removed this lesion from the malignant subcategory due to its bland histological features.
Other lesions are adenomatoid odontogenic tumor which is a well-defined lesion with a thick fibrous capsule. The center of the lesion is usually solid but may show areas of cystic changes. The epithelial cells are either a spindle or columnar and are arranged in sheets, whorled or ductal patterns. The latter pattern is characteristic of these tumors.[39][40]
A calcifying epithelial odontogenic tumor (Pindborg tumor) also arises mainly from odontogenic epithelium. It usually originates from the remnants of the dental lamina or reduced enamel epithelium. Histologically, it appears as sheets of polygonal cells surrounded by fibrous stroma with areas of eosinophilic amyloid-like materials. Calcification is a distinctive feature and usually develops within the amyloid-like material and forms rings, known as Liesegang’s rings. The exact nature of the eosinophilic material is controversial as it does not stain as amyloid when exposed to Congo red. However, it exhibits apple-green birefringence when seen by polarized light. This material may represent enamel protein that is secreted by the tumor cells.[41]
The last item in this category is squamous odontogenic tumors that usually show islands of stratified squamous epithelium with microcyst formation at the center. Spherical calcification can be seen as well.[16]
Odontogenic tumors that arise from both odontogenic epithelium and odontogenic ectomesenchyme, with or without dental hard tissue formation: Odontogenic ghost cell tumor (calcifying odontogenic cyst) epithelium usually resembles ameloblastoma with an outer layer of palisaded columnar cells and an inner layer of stellate reticulum. Spherical calcification and hyalinized materials can also be seen. Some of the eosinophilic epithelial cells may lack nuclei, which are called “ghost cells.”[21][20]
While ameloblastic fibroma contains thin strands of odontogenic epithelium in the background of embryonic connective tissue, it may also show focal areas of hyalinization and calcification.[23] Finally, odontoma can be either compound or complex. Compound odontoma consists of enamel, dentin, and pulp surrounded by follicular connective tissue. They are usually arranged in an orderly manner, similar to normal teeth. While in complex odontoma, tissue arrangement is totally different from normal teeth.[19]
Odontogenic tumors that arise from ectomesenchyme with or without included odontogenic epithelium:
Histological features of odontogenic fibroma show stellate fibroblast with islands of odontogenic epithelium. They may also show spherical or diffuse calcification. It may be central or peripheral.[19][42] While odontogenic myxoma shows abundant myxoid stroma with haphazardly arranged spindle cells. Abundant collagen fibers can be seen in some patients, and these lesions are considered to be fibromyxoma.[24]
Lastly, cementoblastoma resembles osteoblastoma and osteoid osteoma, except that it is usually in continuity with the cemental layer of the roots. Eosinophilic matrix rimmed by cementoblast cells. It is relatively acellular in the periphery with a more vascular and mineralized center. The center usually shows multinucleated giant cells with reversal lines which indicates extensive remodeling.[26]
Malignant odontogenic tumors: In ameloblastic carcinoma, both primary lesion and metastases show cytological malignancy features, which is the main difference from metastasizing (malignant) ameloblastoma.[27][28] While clear cell odontogenic carcinoma may appear in two forms, either monophasic. Only clear cells are present or biphasic, in which clear cells are present along with cells containing eosinophilic cytoplasm. It may also show some ameloblastoma features, such as central cystic changes with squamous proliferation and peripheral palisading appearance.[29]
Primary intraosseous carcinoma usually has the features of high-grade squamous cell carcinoma. Ghost cell odontogenic carcinoma also shows features of ameloblastoma such as peripheral palisading and reverse polarization along with the presence of ghost cells. NOS Sclerosing odontogenic carcinoma shows extensive sclerosis within connective tissue stroma and bland epithelial cell that does not show keratin differentiation. Mitoses and nuclear pleomorphism are rare. However, perineural and perivascular invasion and the invasion of the surrounding musculature and cortical bones are quite common.[43][44]
Diagnosis is challenging in small biopsies as it can mimic other head and neck tumors. Finally, odontogenic sarcoma and carcinosarcoma in which epithelial component is similar to ameloblastoma, or ameloblastic fibroma. However, the mesenchymal component shows malignant features. With recurrence, the stromal component increases, and the epithelial component diminishes. If dysplastic dentin is present in ameloblastic fibrosarcoma, then it is called ameloblastic fibrodentinosarcoma. While if it additionally shows foci of dysplastic enamel protein, it is called ameloblastic fibro-odontosarcoma. Moreover, if both carcinomatous and malignant spindle cell components are present, it is then odontogenic carcinosarcoma.[45]
History and Physical
Odontogenic Tumors That Arise Only From Odontogenic Epithelium Without Odontogenic Ectomesenchyme
Ameloblastoma
The multi-cystic or solid type is the most common (92% of cases). It can be seen in a wide age range with an average of 40.2.[14] It usually presents as a slowly growing painless expansion of the molar region of the mandible (85% of cases). Nevertheless, 15% of cases occur in the posterior maxilla. Large tumors can present with a significant facial deformity. On assessment, the diagnostic sign of eggshell cracking may be elicited on palpation due to the thin expanded outer cortex. Most cases report no neurosensory changes.[46]
The second most common type is the Uni-cystic ameloblastoma (6% of cases). It mainly affects younger patients with an average age of 22.1. More than 90% of cases are found in the molar part of the mandible. This is followed by the peripheral type (2% of cases). It may occur over a wide age range with an average of 52. Clinically, it presents as a small (less than 1.5cm), non-ulcerated sessile or pedunculated lesion. Most cases arise from the posterior gingival mucosa.[38] Finally, Malignant ameloblastoma behaves like the solid or multicystic type.
Other tumors include adenomatoid odontogenic tumors, an uncommon tumor representing 3% to 7% of all odontogenic tumors. Most cases are diagnosed in the second decade of life and show female sex predilection. It often affects the anterior maxilla.[47][48]
Clinically, it appears as a small, well-circumscribed lesion (less than 3 cm) around an unerupted tooth. A calcifying epithelial odontogenic tumor (Pindborg tumor) is a rare tumor that accounts for less than 1% of the total number of odontogenic tumor cases. It mainly affects patients between the age of 30 and 50 years of age. However, fewer cases have been reported with a wider age range.[49] Mandible seems to be the most affected area, with around 66% of cases.[50]
The most common clinical feature is painless, slowly growing mandibular mass. Squamous odontogenic tumor is also another rare benign odontogenic tumor that may be clinically aggressive. The affected patients are usually in their third decade of life. The most common presentation is a slowly growing painless swelling and loosening of teeth.[16]
Odontogenic Tumors That Arise From Both Odontogenic Epithelium and Odontogenic Ectomesenchyme, With or Without Dental Hard Tissue Formation
Odontogenic ghost cell tumor (calcifying odontogenic cyst) is a rare well-circumscribed solid or cystic lesion. It mainly affects areas anterior to the molars with slightly higher incidence on the maxilla compared to the mandible, 38% and 27%, respectively. It is more common in the second decade of life with no sex predilection, and the most common presentation is a painless expansion of the cortical plates.[21][20]
Ameloblastic fibroma tends to affect the younger population with an average age of 14. The posterior mandible is the most affected site in 70% of cases. It may be asymptomatic if small lesions, while larger lesions usually cause painless swelling.[23] Finally, odontoma is the most common odontogenic tumor. The affected patients are usually in their first or second decade of life. The majority of cases are asymptomatic. Compound odontoma usually affects the anterior maxilla, while complex odontoma has a greater tendency to affect the posterior maxilla and mandible.[19]
Odontogenic Tumors That Arise From Ectomesenchyme With or Without Included Odontogenic Epithelium
Odontogenic fibroma is an uncommon tumor with female sex predilection. The male to female ratio is 2.2 to 1. It affects a wide age range from 4 to 80 years of age. It presents as a painless swelling. While it mainly affects the mandible molar region, it tends to affect anterior teeth in the maxilla.[19][42] While odontogenic myxoma is a rare mesenchymal tumor that commonly affects children as early as the age of 10. It does not show any sex or jaw predilection. Painless swelling is the most common presentation.[24] Cementoblastoma usually presents as a painful swelling of the jaw is the most common symptom in two-third of cases. The mandible tends to be the most affected area with no sex predilection.[26]
Malignant Odontogenic Tumors
The most common presentations of all malignant tumors are progressive painful swelling of the jaw along with tooth loosening, paresthesia (involvement of the inferior alveolar nerve), and cervical lymph node metastases. Distant metastases are uncommon. Ameloblastic carcinoma usually presents as rapid painful growth in the posterior mandible with 5 years survival of 70%.[27][28]
Clear cell odontogenic carcinoma is another uncommon malignant tumor that usually affects elderly females in their 5th to 7th decades of life. It usually manifested as a painful swelling of either the mandible or maxilla, and it is frequently associated with the loosening of teeth. It is potentially aggressive with the potential of recurrence.[29] Primary intraosseous carcinoma mainly affects men (70% of cases), and the mean age is 52 years. The mandible is the most common site to be affected (92% of cases). It has an aggressive behavior with a 4-year survival of 40%.[51]
NOS sclerosing odontogenic carcinoma affects a wide range of ages between 30 to 70 years of age with no sex predilection. Premolar and molar areas of the mandible are the most common sites.[52] It is the diagnosis of exclusion as it can mimic other head and neck tumors.
Accurate histological assessment is difficult in small biopsies. Ghost cell odontogenic carcinoma is also a rare tumor as only 40 cases were reported worldwide, with more than half of them from Asia. It is more common in males, and the maxilla is usually the primary site—the 5-year survival rate of about 70%. Odontogenic sarcoma and carcinosarcoma are regarded as low-grade tumors that rarely metastasize compared with odontogenic carcinomas.[53]
Evaluation
Odontogenic Tumors That Arise Only From Odontogenic Epithelium Without Odontogenic Ectomesenchyme
Ameloblastoma
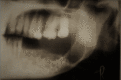
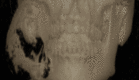
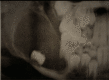
Multi-cystic or Solid type appears as multi-locular radiolucency that resembles honeycomb or soap bubble appearance. Expansion of the buccal and lingual cortices can be seen as well (Fig. 1 and 2). At the same time, the uni-cystic type shows Well demarcated Uni-locular translucency that mimics dentigerous cysts. An impacted tooth may be present, and the peripheral type may show superficial erosion of the alveolar bone in few cases (Fig. 3). Malignant type is usually similar to multicystic type. In these cases, the use of functional PET/ CT scans is extremely helpful in determining the extent of soft tissue involvement and distant metastases.[54]
Other tumors include adenomatoid odontogenic tumor that usually appears as a well-circumscribed unilocular translucency around an unerupted tooth, usually a canine.[47][48] A calcifying epithelial odontogenic tumor (Pindborg tumor) can be either unilocular or multi-locular. It usually appears as a radiolucent area with flecks of radio-opacities with an indistinct line of demarcation from the unaffected bone. It may be associated with an impacted tooth.[55] (Fig. 4) Finally, squamous odontogenic tumors that are usually unilocular in small lesions and multilocular in large ones and they may displace the affected tooth.[16]
Odontogenic Tumors That Arise From Both Odontogenic Epithelium and Odontogenic Ectomesenchyme, With or Without Dental Hard Tissue Formation
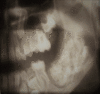
Odontogenic ghost cell tumor (calcifying odontogenic cyst) usually appears as well-circumscribed unilocular translucency with flecks of radio-opacity and may be associated with unerupted canine.[20] While ameloblastic fibroma can be either a unilocular or multilocular lesion. Compound odontoma is usually a unilocular lesion with multiple radiopaque miniature teeth-like structures. Complex odontoma is usually a solid radiopaque mass surrounded by a zone of translucency (Fig. 5). Developing odontoma may be translucent and mostly associated with unerupted teeth.[19]
Odontogenic Tumors That Arise From Ectomesenchyme With or Without Included Odontogenic Epithelium
Odontogenic fibroma usually appears as well-circumscribed unilocular translucency with sclerotic borders and flecks of radio-opaque areas. Larger lesions may be multilocular and may be associated with root resorption.[19][42] (Fig. 6) While odontogenic myxoma appears as either unilocular or multilocular radiolucency and is usually may be associated with teeth resorption. Thin bone trabeculae can be seen in the radiolucency, usually arranged at the right angle. This gives these tumors the characteristic “step ladder” pattern. However, it is not pathognomonic.[24] (Fig. 7) Lastly, cementoblastoma usually presents as a radiopaque lesion surrounded by a thin rim of translucency which represents the periodontal ligament space. It often leads to resorption of the root of the affected teeth.[26](Fig. 8)
Malignant Odontogenic Tumors
Cone beam CT scan and PET scan may be beneficial to assess the extent of the lesion and the potential metastases. Ameloblastic carcinoma appears to be an ill-defined radiolucent lesion with significant cortical destruction.[27][28] Clear cell odontogenic carcinoma also exhibits an ill-defined radiolucency with a honeycomb appearance.[29] Primary intraosseous carcinoma has a radiolucent cystic-like pattern of bone destruction but with well-defined margins. NOS Sclerosing odontogenic carcinoma has a mixed appearance of radiolucent- radio-opaque lesions that mimic fibro-osseous lesions such as fibrous dysplasia. It is locally aggressive and causes cortical bone thinning and root resorption, and it may extend to the surrounding soft tissue.[32] Ghost cell odontogenic carcinoma and odontogenic sarcomas commonly present as mixed radiolucency and radiopacity with ill-defined margins.
Treatment / Management
Surgery is the mainstay of treatment.
Odontogenic Tumors That Arise Only From Odontogenic Epithelium Without Odontogenic Ectomesenchyme
Ameloblastoma
There is no consensus on the biological behavior of multicystic ameloblastoma and the best way to treat them. However, most agree that aggressive management is essential to achieve a cure. Many modalities have been described ranging from simple enucleation and curettage with a recurrence rate of around 50% to wider resection.[56][57][58][59] Most surgeons advocate resection of the tumor with a 1 cm bony margin which should be confirmed via intraoperative specimen radiography. In terms of the soft tissue, it is recommended to excise one uninvolved anatomic barrier on the periphery of the tumor. The recurrence rate is around 15%. Non-resectable ameloblastoma can be offered radiotherapy.[60][61]
Most cases of unicystic ameloblastoma are misdiagnosed as a dentigerous cyst. For that reason, surgeons should always open any cystic lesions to look for any luminal proliferation. It is also advisable to get the frozen section done. This should include multiple sections throughout the whole specimen as this will help with the subclassification of uni-cystic ameloblastoma to its three different categories. If it is luminal or intra-luminal variants, enucleation with curettage would be sufficient with a recurrence rate of 10% to 20%.[62] While in the case of luminal variant, resection is advisable as this type has a higher recurrence rate. In the peripheral type, wide local excision is the most acceptable treatment modality to achieve negative margins.[23] Some studies suggest using TBRAF inhibitors as a neoadjuvant treatment to reduce the lesion size and decrease the possibility of recurrence, especially in a locally advanced stage. These patients should be tested for BRAF mutation first.[63] Finally, malignant ameloblastoma treatment is the same as the solid type along with management of distant metastases.
Owing to the well-circumscribed nature of the adenomatoid odontogenic tumor lesion and it is being encapsulated, enucleation and curettage are considered to be curative in the majority of cases.[64] There is no consensus on the ideal treatment of calcifying epithelial odontogenic tumor (Pindborg tumor) cases. This is due to the small number of reported cases and lack of prolonged follow-up. Most surgeons will treat it like ameloblastoma with 1 cm bony linear margin resection and removing the clinically unaffected soft tissue layer as an anatomical barrier. This is due to the highly infiltrative and destructive capacity of these tumors.[50] Finally, for squamous odontogenic tumors cases, most surgeons will treat them by local excision.[16]
Odontogenic Tumors That Arise From Both Odontogenic Epithelium and Odontogenic Ectomesenchyme, With or Without Dental Hard Tissue Formation
In most cases of odontogenic ghost cell tumors (calcifying odontogenic cyst), enucleation and curettage will be the treatment of choice unless there is any malignant potential on histological evaluation.[21][20] Ameloblastic fibroma cases are also usually treated by enucleation and curettage as recurrence is rare. In cases of recurrent cases, resection should be performed as 45% of ameloblastic fibrosarcoma develop in the context of recurrent ameloblastic fibroma.[23] Odontoma can also be treated by enucleation, and curettage is the treatment of choice as not known to recur.[19]
Odontogenic Tumors That Arise From Ectomesenchyme With or Without Included Odontogenic Epithelium
The main treatment of odontogenic fibroma is enucleation and curettage unless extremely large, and then local excision will be the treatment of choice.[19][42] This is not sufficient for cases of odontogenic myxoma. As these tumors are not encapsulated with great potential of invasion of the surrounding structures, they should be treated with 1 cm bony linear resection with removal of one uninvolved anatomical soft tissue barrier as a safety margin. Moreover, the resected bony specimen should be examined under radiography to confirm clearance.[24] Finally, in cementoblastoma cases, the affected teeth should be removed along with the tumor, and this should be followed by curettage or peripheral osteotomy to decrease the possibility of recurrence.[26]
Malignant Odontogenic Tumors
Resection of the lesion remains the mainstay treatment along with dealing with metastases such as neck dissection for cervical lymph node metastases. The role of chemotherapy and radiotherapy is still controversial and usually reserved for recurrent or unresectable cases.[27][28]
Differential Diagnosis
The differential diagnosis of jaw lesions can be divided into cystic, neoplastic, and vascular anomalies (least common). Proper clinical assessment along with radiographic and histological evaluation is the mainstay to differentiate between different lesions. A well-defined unilocular appearance is more suggestive of the cystic lesion.
Examples of These Lesions
- Dentigerous cyst: It usually presents as a unilocular translucent lesion that causes painless bony expansion around the impacted third molar tooth.[65]
- Traumatic bone cyst: It usually presents as a unilocular translucent lesion affecting the mandibular molar and premolar region in young adults. It always spares the inferior alveolar canal.[66]
- Aneurysmal bone cyst: It mainly affects the long bones and vertebral column of young adults rather than jaws. It can either present as unilocular or multilocular translucent lesions with marked cortical expansion and teeth displacement.[67]
Other Rare Conditions
- Intraosseous mucoepidermoid carcinoma: The most common salivary gland tumor that may arise centrally within the jaw. It mainly affects the mandible of middle-aged patients. It can be either unilocular or multilocular.[68]
- Hyperparathyroidism (brown tumor): These patients usually present with bone pains, abdominal groans, and renal stones. Renal dysfunction is the main cause of secondary hyperparathyroidism.[69]
- Arteriovenous vascular malformations: These patients are usually aged between 10 and 20 years of age, and the mandible is the most commonly affected site. Loose teeth, gingival bleeding, and audible bruit are the most common symptoms. It usually presents as a multilocular translucent lesion. Aspiration of all undiagnosed jaw lesions is mandatory to rule out the presence of arteriovenous malformation as it may lead to fatal hemorrhage.[70]
Radiation Oncology
Radiotherapy can be considered in patients who are medically unfit for surgery. Different modalities have been used, including tomotherapy, proton beam therapy, imaging-guided radiotherapy, and intensity-modulated radiotherapy. Some of them have been used as a combined treatment with surgery or chemotherapy. Their use is recommended when treating ameloblastic carcinoma or recurrent ameloblastoma after multiple surgical excision. However, studies show mixed responses when using them as an adjuvant treatment with or without chemotherapy. Risks should be discussed with the patient, including the potential of life-threatening malignant transformations.[71][72]
Medical Oncology
Chemotherapy
The efficacy of chemotherapy in the management of primary and recurrent ameloblastoma is still controversial. Many drug regimens have been used with surgery and/ or radiotherapy. This includes the following combinations: adriamycin, cisplatin, and cyclophosphamide; vinblastine, cisplatin, and bleomycin; gemcitabine and carboplatin; and doxorubicin and cisplatin. However, more high-quality randomized control studies are needed to validate their use.[73][74]
Targeted Therapy
Recent understanding of the molecular basis of the development of odontogenic tumors, especially ameloblastoma, led to the development of targeted therapies.[75] Many MAPK specific drugs have been introduced. This includes BRAF inhibitors such as vemurafenib and dabrafenib, MEK inhibitors like trametinib, and FGFR2 inhibitors such as ponatinib and regorafenib. In general, MEK inhibitors are better when compared with BRAF inhibitors as the latter’s effect may be blunted by the compensatory activation of the MAPK pathway by epidermal growth factor receptors.[9][76]
Similarly, different drugs have been manufactured to target the SMO mutation associated with ameloblastoma. This include vismodegib and itraconazole. However, some resistance mechanism against these drugs has been reported.[10] Moreover, several drugs have been made to antagonize SHH signaling pathway. The most widely used one is cyclopamine, but its main side effect is impairment of bone healing as it suppresses osteoblastic proliferation and differentiation.[77][78]
Prognosis
When appropriately treated with surgical excision, benign tumors have a low recurrence rate except for ameloblastoma, which has a recurrence rate of 10 to 20% according to histological subtypes.[62] While the majority of malignant odontogenic tumors are either malignant ameloblastoma or odontogenic carcinoma. The improved survival rate is associated with an age of under 57 years, lack of comorbidities, surgical resection, and absence of nodal disease. The 5- year overall survival for surgical excision is around 88% compared with 26.6% for non-surgical management. The presence of lymph node metastases is always associated with a poor prognosis.[79]
Complications
The complications can be either related to the tumor or surgical management. Gradual expansion of the tumor may lead to mandibular maxillary distortion leading to facial asymmetry, pain, teeth displacement, malocclusion, or recurrence. Distant metastases can be seen in cases of malignant tumors. Lungs and cervical lymph nodes are the most affected sites.
At the same time, surgical complications may be in the form of postoperative bleeding, which may be quite significant as it will endanger the airway patency, infection, neurosensory disturbance due to inadvertent injury of the inferior alveolar nerve. Other general complications may also occur, including aspiration, deep venous thrombosis (DVT), and pulmonary embolism. All necessary postoperative measures should be taken to avoid the occurrence of these complications.[34]
Deterrence and Patient Education
It is important to educate patients on the natural history of different odontogenic tumors and highlight the red flags to look for, such as rapid growth, neurosensory changes, pain, and neck masses. In addition, educating the patients on how to examine themselves and keeping regular follow-up is crucial for early detection of any possible recurrence or malignant transformation.
Enhancing Healthcare Team Outcomes
It is mandatory to treat odontogenic tumors in an interprofessional team approach to ensure proper treatment and outcome. The team should include an oral surgeon and head and neck surgeon with proper expertise in neck dissection. Having a plastic surgeon in the team is also important in cases when possible reconstruction might be needed. Postoperative care is crucial for optimal outcomes. Nursing staff should look after the airway make sure it is clear and patent by frequent suctioning to prevent aspiration and maintain good oxygenation.
Prophylactic measures should also be taken to avoid potential risks of deep venous thrombosis (DVT) or stress ulcers. Pain team involvement in the early postoperative period can significantly improve the overall patient treatment experience. Dietitian may be needed for early postoperative care for patients struggling with oral feeding or who have been instructed not to eat to heal the surgical site. In these cases, parenteral nutrition may be considered. Speech and language therapist plays a great role the rehabilitation of surgical patients to improve their long-term outcome.
When an operation involves major resection with significant cosmetic consequences, care should be taken to involve mental health nurses to counsel these patients and provide the needed support. Although the surgical option is the main treatment in most cases, few cases may benefit from radiotherapy or chemotherapy. In such cases, the involvement of the oncology team is mandatory. All team members should keep good communication to ensure that the best possible outcome could be achieved.[34] [Level 5]
Figure
Fig. 1: Orthopantomogram of solid ameloblastoma of the mandible Contributed by Amir Labib
Figure
Fig. 2: CT of solid ameloblastoma of the mandible Contributed by Amir Labib
Figure
Fig. 3: OPG of unicystic ameloblastoma of the mandible Contributed by Amir Labib

Figure
Fig. 4: OPG of Pindborg tumor of the mandible Contributed by Amir Labib
Figure
Fig. 5: OPG of complex odontoma Contributed by Amir Labib
References
- 1.
- Soluk-Tekkeşin M, Wright JM. The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2017 (4th) Edition. Turk Patoloji Derg. 2018;34(1) [PubMed: 28984343]
- 2.
- Malcić A, Jukić S, Anić I, Pavelić B, Kapitanović S, Kruslin B, Pavelić K. Alterations of FHIT and P53 genes in keratocystic odontogenic tumor, dentigerous and radicular cyst. J Oral Pathol Med. 2008 May;37(5):294-301. [PubMed: 18221322]
- 3.
- Perdigão PF, Carvalho VM, DE Marco L, Gomez RS. Mutation of ameloblastin gene in calcifying epithelial odontogenic tumor. Anticancer Res. 2009 Aug;29(8):3065-7. [PubMed: 19661317]
- 4.
- Coura BP, Bernardes VF, de Sousa SF, França JA, Pereira NB, Pontes HAR, Batista AC, da Cruz Perez DE, Albuquerque Junior RLC, de Souza LB, Martins MD, Diniz MG, Gomez RS, Gomes CC. KRAS mutations drive adenomatoid odontogenic tumor and are independent of clinicopathological features. Mod Pathol. 2019 Jun;32(6):799-806. [PubMed: 30643167]
- 5.
- Brown NA, Betz BL. Ameloblastoma: A Review of Recent Molecular Pathogenetic Discoveries. Biomark Cancer. 2015;7(Suppl 2):19-24. [PMC free article: PMC4597444] [PubMed: 26483612]
- 6.
- Jussila M, Thesleff I. Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb Perspect Biol. 2012 Apr 01;4(4):a008425. [PMC free article: PMC3312678] [PubMed: 22415375]
- 7.
- Heikinheimo K, Jee KJ, Niini T, Aalto Y, Happonen RP, Leivo I, Knuutila S. Gene expression profiling of ameloblastoma and human tooth germ by means of a cDNA microarray. J Dent Res. 2002 Aug;81(8):525-30. [PubMed: 12147741]
- 8.
- Brown NA, Rolland D, McHugh JB, Weigelin HC, Zhao L, Lim MS, Elenitoba-Johnson KS, Betz BL. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014 Nov 01;20(21):5517-26. [PubMed: 24993163]
- 9.
- Kurppa KJ, Catón J, Morgan PR, Ristimäki A, Ruhin B, Kellokoski J, Elenius K, Heikinheimo K. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol. 2014 Apr;232(5):492-8. [PMC free article: PMC4255689] [PubMed: 24374844]
- 10.
- Sweeney RT, McClary AC, Myers BR, Biscocho J, Neahring L, Kwei KA, Qu K, Gong X, Ng T, Jones CD, Varma S, Odegaard JI, Sugiyama T, Koyota S, Rubin BP, Troxell ML, Pelham RJ, Zehnder JL, Beachy PA, Pollack JR, West RB. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet. 2014 Jul;46(7):722-5. [PMC free article: PMC4418232] [PubMed: 24859340]
- 11.
- Mishra P, Panda A, Bandyopadhyay A, Kumar H, Mohiddin G. Sonic Hedgehog Signalling Pathway and Ameloblastoma - A Review. J Clin Diagn Res. 2015 Nov;9(11):ZE10-3. [PMC free article: PMC4668540] [PubMed: 26674664]
- 12.
- Regezi JA, Kerr DA, Courtney RM. Odontogenic tumors: analysis of 706 cases. J Oral Surg. 1978 Oct;36(10):771-8. [PubMed: 280645]
- 13.
- Olaitan AA, Adeola DS, Adekeye EO. Ameloblastoma: clinical features and management of 315 cases from Kaduna, Nigeria. J Craniomaxillofac Surg. 1993 Dec;21(8):351-5. [PubMed: 8113429]
- 14.
- Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995 Mar;31B(2):86-99. [PubMed: 7633291]
- 15.
- Piattelli A, Fioroni M, Santinelli A, Rubini C. Expression of proliferating cell nuclear antigen in ameloblastomas and odontogenic cysts. Oral Oncol. 1998 Sep;34(5):408-12. [PubMed: 9861350]
- 16.
- Leventon GS, Happonen RP, Newland JR. Squamous odontogenic tumor. Am J Surg Pathol. 1981 Oct;5(7):671-7. [PubMed: 7337159]
- 17.
- Dhirawani RB, Pathak S, Mallikaarjuna K, Sharma A. An adenomatoid odontogenic tumor in disguise. J Indian Soc Pedod Prev Dent. 2016 Jul-Sep;34(3):291-3. [PubMed: 27461816]
- 18.
- Halperin V, Carr RF, Peltier JR. Follow-up of adenoameloblastomas. Review of thirty-five cases from the literature and report of two additional cases. Oral Surg Oral Med Oral Pathol. 1967 Nov;24(5):642-7. [PubMed: 5234281]
- 19.
- Wright JM, Soluk Tekkesin M. Odontogenic tumors: where are we in 2017 ? J Istanb Univ Fac Dent. 2017;51(3 Suppl 1):S10-S30. [PMC free article: PMC5750825] [PubMed: 29354306]
- 20.
- Praetorius F, Hjørting-Hansen E, Gorlin RJ, Vickers RA. Calcifying odontogenic cyst. Range, variations and neoplastic potential. Acta Odontol Scand. 1981;39(4):227-40. [PubMed: 6948493]
- 21.
- Freedman PD, Lumerman H, Gee JK. Calcifying odontogenic cyst. A review and analysis of seventy cases. Oral Surg Oral Med Oral Pathol. 1975 Jul;40(1):93-106. [PubMed: 1057143]
- 22.
- Buchner A. The central (intraosseous) calcifying odontogenic cyst: an analysis of 215 cases. J Oral Maxillofac Surg. 1991 Apr;49(4):330-9. [PubMed: 2005490]
- 23.
- Slootweg PJ. An analysis of the interrelationship of the mixed odontogenic tumors--ameloblastic fibroma, ameloblastic fibro-odontoma, and the odontomas. Oral Surg Oral Med Oral Pathol. 1981 Mar;51(3):266-76. [PubMed: 6938886]
- 24.
- Regezi JA. Odontogenic cysts, odontogenic tumors, fibroosseous, and giant cell lesions of the jaws. Mod Pathol. 2002 Mar;15(3):331-41. [PubMed: 11904346]
- 25.
- Barker BF. Odontogenic myxoma. Semin Diagn Pathol. 1999 Nov;16(4):297-301. [PubMed: 10587272]
- 26.
- Brannon RB, Fowler CB, Carpenter WM, Corio RL. Cementoblastoma: an innocuous neoplasm? A clinicopathologic study of 44 cases and review of the literature with special emphasis on recurrence. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002 Mar;93(3):311-20. [PubMed: 11925541]
- 27.
- Rizzitelli A, Smoll NR, Chae MP, Rozen WM, Hunter-Smith DJ. Incidence and overall survival of malignant ameloblastoma. PLoS One. 2015;10(2):e0117789. [PMC free article: PMC4333213] [PubMed: 25692490]
- 28.
- Dissanayake RK, Jayasooriya PR, Siriwardena DJ, Tilakaratne WM. Review of metastasizing (malignant) ameloblastoma (METAM): pattern of metastasis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011 Jun;111(6):734-41. [PubMed: 21459020]
- 29.
- Iezzi G, Rubini C, Fioroni M, Piattelli A. Clear cell odontogenic carcinoma. Oral Oncol. 2002 Feb;38(2):209-13. [PubMed: 11854070]
- 30.
- Irié T, Ogawa I, Takata T, Toyosawa S, Saito N, Akiba M, Isobe T, Hokazono C, Tachikawa T, Suzuki Y. Sclerosing odontogenic carcinoma with benign fibro-osseous lesion of the mandible: an extremely rare case report. Pathol Int. 2010 Oct;60(10):694-700. [PubMed: 20846269]
- 31.
- El-Naggar AK. Editor's perspective on the 4th edition of the WHO head and neck tumor classification. J Egypt Natl Canc Inst. 2017 Jun;29(2):65-66. [PubMed: 28455004]
- 32.
- Wood A, Young F, Morrison J, Conn BI. Sclerosing odontogenic carcinoma presenting on the hard palate of a 43-year-old female: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 Dec;122(6):e204-e208. [PubMed: 27743835]
- 33.
- Mosqueda Taylor A, Meneses García A, Ruíz Godoy Rivera LM, Suárez Roa Mde L, Luna Ortiz K. Malignant odontogenic tumors. A retrospective and collaborative study of seven cases. Med Oral. 2003 Mar-Apr;8(2):110-21. [PubMed: 12618671]
- 34.
- Goldenberg D, Sciubba J, Koch W, Tufano RP. Malignant odontogenic tumors: a 22-year experience. Laryngoscope. 2004 Oct;114(10):1770-4. [PubMed: 15454770]
- 35.
- Robinson L, Martinez MG. Unicystic ameloblastoma: a prognostically distinct entity. Cancer. 1977 Nov;40(5):2278-85. [PubMed: 922668]
- 36.
- Gardner DG. Plexiform unicystic ameloblastoma: a diagnostic problem in dentigerous cysts. Cancer. 1981 Mar 15;47(6):1358-63. [PubMed: 7226059]
- 37.
- Woo SB, Smith-Williams JE, Sciubba JJ, Lipper S. Peripheral ameloblastoma of the buccal mucosa: case report and review of the English literature. Oral Surg Oral Med Oral Pathol. 1987 Jan;63(1):78-84. [PubMed: 3468468]
- 38.
- Tajima Y, Kuroda-Kawasaki M, Ohno J, Yi J, Kusama K, Tanaka H, Fukunaga S, Shimada J, Yamamoto Y. Peripheral ameloblastoma with potentially malignant features: report of a case with special regard to its keratin profile. J Oral Pathol Med. 2001 Sep;30(8):494-8. [PubMed: 11545241]
- 39.
- Smith RR, Olson JL, Hutchins GM, Crawley WA, Levin LS. Adenomatoid odontogenic tumor: ultrastructural demonstration of two cell types and amyloid. Cancer. 1979 Feb;43(2):505-11. [PubMed: 421178]
- 40.
- el-Labban NG. The nature of the eosinophilic and laminated masses in the adenomatoid odontogenic tumor: a histochemical and ultrastructural study. J Oral Pathol Med. 1992 Feb;21(2):75-81. [PubMed: 1556665]
- 41.
- More CB, Vijayvargiya R. Intraosseous calcifying epithelial odontogenic (Pindborg) tumor: A rare entity. J Oral Maxillofac Pathol. 2015 May-Aug;19(2):269. [PMC free article: PMC4611947] [PubMed: 26604515]
- 42.
- Santoro A, Pannone G, Ramaglia L, Bufo P, Lo Muzio L, Saviano R. Central odontogenic fibroma of the mandible: A case report with diagnostic considerations. Ann Med Surg (Lond). 2016 Feb;5:14-8. [PMC free article: PMC4687461] [PubMed: 26793312]
- 43.
- Hussain O, Rendon AT, Orr RL, Speight PM. Sclerosing odontogenic carcinoma in the maxilla: a rare primary intraosseous carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Oct;116(4):e283-6. [PubMed: 23567261]
- 44.
- Koutlas IG, Allen CM, Warnock GR, Manivel JC. Sclerosing odontogenic carcinoma: a previously unreported variant of a locally aggressive odontogenic neoplasm without apparent metastatic potential. Am J Surg Pathol. 2008 Nov;32(11):1613-9. [PubMed: 18753945]
- 45.
- Slater LJ. Odontogenic sarcoma and carcinosarcoma. Semin Diagn Pathol. 1999 Nov;16(4):325-32. [PubMed: 10587276]
- 46.
- Jackson IT, Callan PP, Forté RA. An anatomical classification of maxillary ameloblastoma as an aid to surgical treatment. J Craniomaxillofac Surg. 1996 Aug;24(4):230-6. [PubMed: 8880449]
- 47.
- Poulson TC, Greer RO. Adenomatoid odontogenic tumor: clinicopathologic and ultrastructural concepts. J Oral Maxillofac Surg. 1983 Dec;41(12):818-24. [PubMed: 6581285]
- 48.
- Mendis BR, MacDonald DG. Adenomatoid odontogenic tumour. A survey of 21 cases from Sri Lanka. Int J Oral Maxillofac Surg. 1990 Jun;19(3):141-3. [PubMed: 2114455]
- 49.
- Franklin CD, Pindborg JJ. The calcifying epithelial odontogenic tumor. A review and analysis of 113 cases. Oral Surg Oral Med Oral Pathol. 1976 Dec;42(6):753-65. [PubMed: 792760]
- 50.
- Veness MJ, Morgan G, Collins AP, Walker DM. Calcifying epithelial odontogenic (Pindborg) tumor with malignant transformation and metastatic spread. Head Neck. 2001 Aug;23(8):692-6. [PubMed: 11443753]
- 51.
- Thomas G, Pandey M, Mathew A, Abraham EK, Francis A, Somanathan T, Iype M, Sebastian P, Nair MK. Primary intraosseous carcinoma of the jaw: pooled analysis of world literature and report of two new cases. Int J Oral Maxillofac Surg. 2001 Aug;30(4):349-55. [PubMed: 11518362]
- 52.
- Hanisch M, Baumhoer D, Elges S, Fröhlich LF, Kleinheinz J, Jung S. Sclerosing odontogenic carcinoma: current diagnostic and management considerations concerning a most unusual neoplasm. Int J Oral Maxillofac Surg. 2017 Dec;46(12):1641-1649. [PubMed: 28641898]
- 53.
- Jundt G, Reichart PA. [Malignant odontogenic tumors]. Pathologe. 2008 May;29(3):205-13. [PubMed: 18392827]
- 54.
- Effiom OA, Ogundana OM, Akinshipo AO, Akintoye SO. Ameloblastoma: current etiopathological concepts and management. Oral Dis. 2018 Apr;24(3):307-316. [PubMed: 28142213]
- 55.
- Kamboj M, Yadav AB, Narwal A, J N. Unusual Cystic Variant of Calcifying Epithelial Odontogenic Tumor. J Dent (Shiraz). 2020 Jun;21(2):147-152. [PMC free article: PMC7280540] [PubMed: 32582831]
- 56.
- Feinberg SE, Steinberg B. Surgical management of ameloblastoma. Current status of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996 Apr;81(4):383-8. [PubMed: 8705582]
- 57.
- Laino L, Cicciù M, Russo D, Cervino G. Surgical Strategies for Multicystic Ameloblastoma. J Craniofac Surg. 2020 Mar/Apr;31(2):e116-e119. [PubMed: 31842085]
- 58.
- Sampson DE, Pogrel MA. Management of mandibular ameloblastoma: the clinical basis for a treatment algorithm. J Oral Maxillofac Surg. 1999 Sep;57(9):1074-7; discussion 1078-9. [PubMed: 10484108]
- 59.
- Gardner DG. A pathologist's approach to the treatment of ameloblastoma. J Oral Maxillofac Surg. 1984 Mar;42(3):161-6. [PubMed: 6583361]
- 60.
- Atkinson CH, Harwood AR, Cummings BJ. Ameloblastoma of the jaw. A reappraisal of the role of megavoltage irradiation. Cancer. 1984 Feb 15;53(4):869-73. [PubMed: 6420036]
- 61.
- Aoki T, Akiba T, Kondo Y, Sasaki M, Kajiwara H, Ota Y. The use of radiation therapy in the definitive management of ameloblastic carcinoma: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019 Feb;127(2):e56-e60. [PubMed: 30393089]
- 62.
- Gardner DG, Corio RL. Plexiform unicystic ameloblastoma. A variant of ameloblastoma with a low-recurrence rate after enucleation. Cancer. 1984 Apr 15;53(8):1730-5. [PubMed: 6697311]
- 63.
- Tan S, Pollack JR, Kaplan MJ, Colevas AD, West RB. BRAF inhibitor treatment of primary BRAF-mutant ameloblastoma with pathologic assessment of response. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 Jul;122(1):e5-7. [PubMed: 27209484]
- 64.
- Philipsen HP, Reichart PA, Zhang KH, Nikai H, Yu QX. Adenomatoid odontogenic tumor: biologic profile based on 499 cases. J Oral Pathol Med. 1991 Apr;20(4):149-58. [PubMed: 2061853]
- 65.
- Daley TD, Wysocki GP. The small dentigerous cyst. A diagnostic dilemma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995 Jan;79(1):77-81. [PubMed: 7614167]
- 66.
- Cortell-Ballester I, Figueiredo R, Berini-Aytés L, Gay-Escoda C. Traumatic bone cyst: a retrospective study of 21 cases. Med Oral Patol Oral Cir Bucal. 2009 May 01;14(5):E239-43. [PubMed: 19218905]
- 67.
- López-Arcas Calleja JM, Cebrián Carretero JL, González Martín J, Burgueño M. Aneurysmal bone cyst of the mandible: case presentation and review of the literature. Med Oral Patol Oral Cir Bucal. 2007 Sep 01;12(5):E401-3. [PubMed: 17767108]
- 68.
- Simon D, Somanathan T, Ramdas K, Pandey M. Central Mucoepidermoid carcinoma of mandible - A case report and review of the literature. World J Surg Oncol. 2003 Feb 25;1(1):1. [PMC free article: PMC156026] [PubMed: 12773198]
- 69.
- Keyser JS, Postma GN. Brown tumor of the mandible. Am J Otolaryngol. 1996 Nov-Dec;17(6):407-10. [PubMed: 8944301]
- 70.
- Kriwalsky MS, Papadimas D, Maurer P, Brinkmann M, Jackowski J, Kunkel M. Life-threatening bleeding after tooth extraction due to vascular malformation: a case report and literature review. Oral Maxillofac Surg. 2014 Sep;18(3):279-82. [PubMed: 24756853]
- 71.
- Huang CM, Chen JY, Chen CH, Huang CJ. Radiotherapy for a repeatedly recurrent ameloblastoma with malignant transformation. Head Neck. 2014 Jan;36(1):E1-3. [PubMed: 23633444]
- 72.
- Kennedy WR, Werning JW, Kaye FJ, Mendenhall WM. Treatment of ameloblastoma and ameloblastic carcinoma with radiotherapy. Eur Arch Otorhinolaryngol. 2016 Oct;273(10):3293-7. [PubMed: 26796877]
- 73.
- Van Dam SD, Unni KK, Keller EE. Metastasizing (malignant) ameloblastoma: review of a unique histopathologic entity and report of Mayo Clinic experience. J Oral Maxillofac Surg. 2010 Dec;68(12):2962-74. [PubMed: 20970910]
- 74.
- Amzerin M, Fadoukhair Z, Belbaraka R, Iraqui M, Boutayeb S, M'rabti H, Kebdani T, Hassouni K, Benjaafar N, El Gueddari BK, Errihani H. Metastatic ameloblastoma responding to combination chemotherapy: case report and review of the literature. J Med Case Rep. 2011 Oct 03;5:491. [PMC free article: PMC3198715] [PubMed: 21968082]
- 75.
- Sauk JJ, Nikitakis NG, Scheper MA. Are we on the brink of nonsurgical treatment for ameloblastoma? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 Jul;110(1):68-78. [PubMed: 20418126]
- 76.
- Heikinheimo K, Kurppa KJ, Elenius K. Novel targets for the treatment of ameloblastoma. J Dent Res. 2015 Feb;94(2):237-40. [PMC free article: PMC4438731] [PubMed: 25425580]
- 77.
- Stanton BZ, Peng LF. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst. 2010 Jan;6(1):44-54. [PubMed: 20024066]
- 78.
- Schaefer GI, Perez JR, Duvall JR, Stanton BZ, Shamji AF, Schreiber SL. Discovery of small-molecule modulators of the Sonic Hedgehog pathway. J Am Chem Soc. 2013 Jul 03;135(26):9675-80. [PMC free article: PMC3703668] [PubMed: 23725514]
- 79.
- Rooney MK, Korpics MC, Turchan WT, Callahan N, Koshy M, Spiotto MT. Patterns of Care and Survival Outcomes for Odontogenic Cancers. Laryngoscope. 2021 May;131(5):E1496-E1502. [PMC free article: PMC8689582] [PubMed: 33135786]
Disclosure: Amir Labib declares no relevant financial relationships with ineligible companies.
Disclosure: Roger Adlard declares no relevant financial relationships with ineligible companies.
- Continuing Education Activity
- Introduction
- Etiology
- Epidemiology
- Pathophysiology
- Histopathology
- History and Physical
- Evaluation
- Treatment / Management
- Differential Diagnosis
- Radiation Oncology
- Medical Oncology
- Prognosis
- Complications
- Deterrence and Patient Education
- Enhancing Healthcare Team Outcomes
- Review Questions
- References
- Review Odontogenic tumors: where are we in 2017 ?[J Istanb Univ Fac Dent. 2017]Review Odontogenic tumors: where are we in 2017 ?Wright JM, Soluk Tekkesin M. J Istanb Univ Fac Dent. 2017; 51(3 Suppl 1):S10-S30. Epub 2017 Dec 2.
- Review [Odontogenic tumors and neoplastic-like changes of the jaw bone. Clinical study and evaluation of treatment results].[Folia Med Cracov. 1998]Review [Odontogenic tumors and neoplastic-like changes of the jaw bone. Clinical study and evaluation of treatment results].Stypułkowska J. Folia Med Cracov. 1998; 39(1-2):35-141.
- Non-Odontogenic Tumors of the Jaws.[StatPearls. 2024]Non-Odontogenic Tumors of the Jaws.Shaw SE, Chan CH. StatPearls. 2024 Jan
- [Mixed odontogenic tumors in children and adolescents].[Fogorv Sz. 2007][Mixed odontogenic tumors in children and adolescents].Gyulai-Gaál S, Takács D, Barabás J, Tarján I, Martonffy K, Szabó G, Suba Z. Fogorv Sz. 2007 Apr; 100(2):65-9.
- Review [Benign "mixed" odontogenic tumors].[Pathologe. 2008]Review [Benign "mixed" odontogenic tumors].Reichart PA, Jundt G. Pathologe. 2008 May; 29(3):189-98. Epub 2008 Mar 29.
- Odontogenic Tumors of the Jaws - StatPearlsOdontogenic Tumors of the Jaws - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...