NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
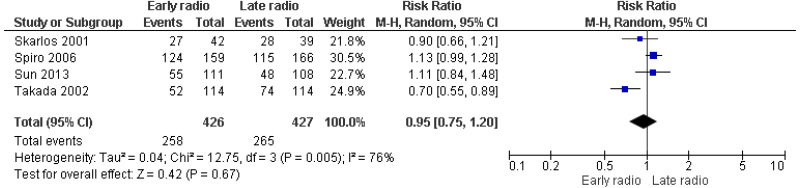
Evidence reviews for the most clinically and cost-effective regimen of chemoradiotherapy for people with limited-stage small cell lung cancer (SCLC)
Review questions
RQ3.4: What is the most clinically and cost effective regimen of chemoradiotherapy for people with limited-stage SCLC?
Introduction
New evidence on chemoradiotherapy dosing for people with limited-stage SCLC has become available. Therefore, the aim of this review is to review all evidence from randomised controlled trials (RCTs) in this area to provide clearer guidance regarding the optimal regimen.
Table 1PICO table
| Population | People with stage limited-stage SCLC |
|---|---|
| Interventions |
Drug regimens, number of cycles and duration of treatment. The timing of radiotherapy in relation to chemotherapy (early/late), the fractionation of radiotherapy, the radiotherapy regiment (e.g. once/twice daily |
| Comparators |
|
| Outcomes |
|
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual (2014). Methods specific to this review question are described in the review protocol in appendix A, and the methods section in appendix B. In particular, the minimally important differences (MIDs) used in this review are summarised in appendix B.
During screening of potential papers it was noted that a large amount of the evidence came from studies taking place before 2000, with the likely potential for the treatment used in these studies to now be outdated. The protocol specified no date limit for searches, however upon discussion with the committee it was agreed that there have been considerable advancements in the treatment of lung cancer over recent decades. As a result, the protocol was changed: all studies that took place prior to 1999 were excluded from the evidence review. The limit of 1999 was agreed upon as to include the Turrisi (1999) paper for which current practice is guided by regarding the usage of twice-daily radiotherapy. Those studies taking place in 1999 were included but marked down for indirectness. This is because the committee agreed that higher doses of radiotherapy are now used.
Declarations of interest were recorded according to NICE’s 2018 conflicts of interest policy.
Clinical evidence
Included studies
This review was conducted as part of a larger update of the NICE Lung cancer: diagnosis and management guideline (CG 121). A systematic literature search for RCTs and systematic reviews with a no date limit yielded 2,145 references.
Papers returned by the literature search were screened on title and abstract, with 44 full-text papers ordered as potentially relevant systematic reviews or RCTs.
Nineteen papers representing 16 unique RCTs were included after full text screening. Following application of the 1999-present date limit, an additional seven papers were excluded, leaving 12 papers representing ten unique RCTs. The RCTs were:
- Faivre-Finn 2017: CONVERT trial, N=547, follow-up period median 45 months.
- Turrisi 1999: N=417, follow-up 5 years.
- Bonner 1999: Also reported in Schild 2004, N=262, follow-up median 8 years.
- Gronberg 2016: Also reported in Halvorsen 2016, N=157, follow-up median 81 months.
- Spiro 2006: N=325, follow-up 5 years.
- Skarlos 2001: N=219, follow-up median 3 years.
- Sun 2013: N= 219, follow-up 5 years.
- Takada 2002: N=224, follow-up 5 years (minimum)
- Blackstock 2005: N=224, follow-up 10 years (minimum)
- Lebeau 1999: N= 156, follow up median 66 months
For the search strategy, please see appendix C. For the clinical evidence study selection flowchart, see appendix D. For the full evidence tables and full GRADE profiles for included studies, please see appendices E and G.
Excluded studies
Details of the studies excluded at full-text review are given in appendix H along with a reason for their exclusion.
Summary of clinical studies included in the evidence review
Study locations
One randomised controlled trial was from the UK (Spiro 2006), 1 was from Greece (Skarlos 2001), 3 were from the USA (Blackstock 2005, Bonner 1999, Turrisi 1999), 1 was from Norway (Gronberg 2016), 1 was from France (Lebeau 1999), 1 was from South Korea (Sun 2013), and 1 was from Japan (Takada 2002). The CONVERT trial took place across Belgium, the UK, The Netherlands, France, Spain, Canada, Poland and Slovenia.
Outcomes and sample sizes
The reported outcomes with extractable data were mortality, adverse events and quality of life. The sample sizes ranged from 64 participants to 547 across studies.
See full evidence tables and Grade profiles in appendices E and G.
Quality assessment of clinical studies included in the evidence review
See appendix E for full GRADE tables.
Economic evidence
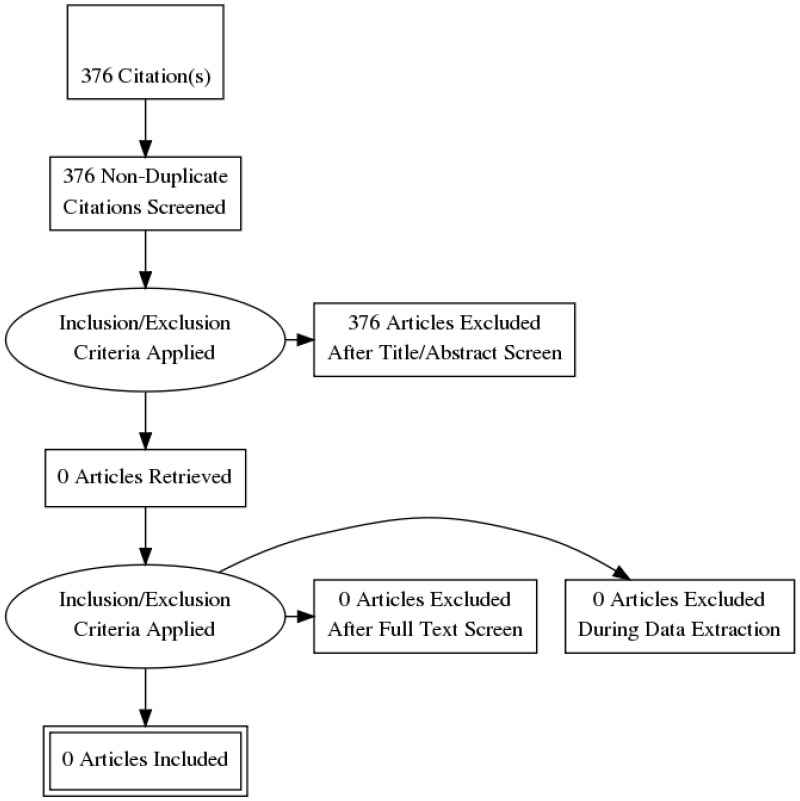
Standard health economic filters were applied to the clinical search for this question, and a total of 376 citations were returned. Following review of titles and abstracts, no full text studies were retrieved for detailed consideration. Therefore, no relevant cost–utility analyses were identified for this question.
Evidence statements
Once-daily versus twice-daily radiotherapy (with concomitant chemotherapy in both arms)
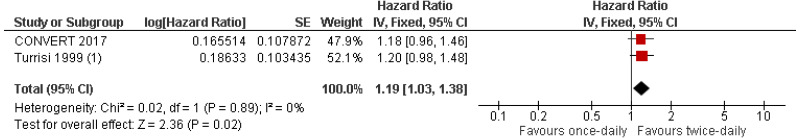
Moderate quality evidence from two RCTs reporting data on 906 people with limited-stage small cell lung cancer found a greater length of time to any-cause mortality in people given twice-daily radiotherapy than those given once-daily radiotherapy.
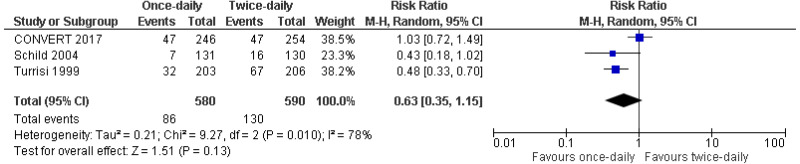
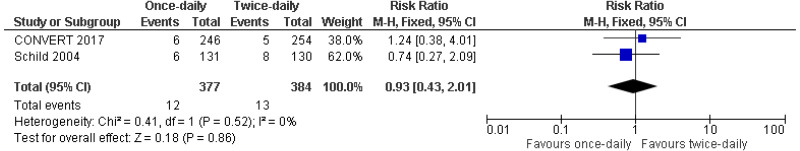
Very-low- to low-quality evidence from up to 3 RCTs reporting data on up to 1,170 people with limited-disease small cell lung cancer could not differentiate rates of grade 3 or above adverse events (oesophagitis, pneumonitis or cardiac toxicity) or rates of mortality (2, 3 or 5-years) between people given twice-daily radiotherapy and those given once-daily radiotherapy.
Once-daily hypofractionated radiotherapy versus twice-daily hypofractonated radiotherapy (with concomitant chemotherapy in both arms)
Low- to moderate-quality evidence from 1 RCT reporting data on 157 people with limited-stage small cell lung cancer could not differentiate time to any-cause mortality or rates of grade 3 or above adverse events (oesophagitis and pneumonitis) between those give once-daily hypofractionated radiotherapy and those given twice-daily hyperfractionated radiotherapy.
Early versus late radiotherapy (with concomitant chemotherapy in both arms)
Early radiotherapy began on weeks 1 to 3. Late radiotherapy began on weeks 9 to 15.
Very low- to low-quality evidence from up to 4 RCTs reporting data on up to 853 people with limited-stage small-cell lung cancer could not differentiate rates of mortality at 12 months, 24 months, 36 months or 60 months, or rates of grade 3 or above adverse events (oesophagitis, cardiac, pneumonitis) between people given early and those given late radiotherapy.
Continuous versus alternating radiotherapy (with concomitant chemotherapy in both arms)
Moderate quality evidence from up to 2 RCTs reporting data on up to 266 people could not differentiate rates of mortality (2, 3 or 5 years) or grade 3 or above adverse events (oesophagitis) between those people receiving continuous radiotherapy and those receiving alternating radiotherapy.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
Overall survival is particularly important due to the low survival rates associated with small cell lung cancer. In addition, adverse events (toxicity) are of importance due to the impact these have on quality of life and the ability of patients to complete treatment following radiotherapy, including the remainder of the chemotherapy course and prophylactic cranial irradiation. Quality of life is also an important outcome and the lack of quality of life evidence available for this review question was noted by the committee.
The quality of the evidence
The evidence available for this review was of moderate to very-low quality. All studies were likely to have been non-blinded because of the nature of the interventions. This is unlikely to have had a major impact on the reporting of overall survival but may have created bias in the reporting of adverse events.
The committee noted that the survival data from the Turrisi 1999 study and the CONVERT 2017 trial was homogeneous (0% heterogeneity), which provides sufficient support to recommend twice daily over once daily radiotherapy.
There was a very high level of heterogeneity in many of the analyses. This was likely a result of the large time gaps between studies and differences in radiotherapy dosages and dose frequency. In particular, rates of adverse events were difficult to interpret due to high levels of heterogeneity in results, despite the relatively large sample sizes for rates of grade 3 or above oesophagitis. All included RCTs had sample sizes of at least 100 participants.
Benefits and harms
A recommendation of twice-daily radiotherapy was made because the survival data favours twice-daily radiotherapy over once-daily. The adverse events data could not differentiate between the two. In the committee’s experience, people who are not well enough to tolerate twice-daily radiotherapy should be offered once-daily radiotherapy.
Cost effectiveness and resource use
The committee discussed the suggestion made in the CONVERT trial that twice-daily radiotherapy is potentially cost saving due to patients requiring less travel time to treatment because the total number of days attending hospital would be lower. They felt, however, that there was insufficient evidence for the cost saving potential of twice-daily therapy and noted that people may require overnight stays due to longer hospital time per session, which could incur costs to the system. They also noted that the overall number of fractions, and therefore the radiotherapy costs, would be similar between the two options. Although there were some clinical benefits associated with twice daily treatment, the committee felt that the potential for symptomatic burden and associated downstream consequences from more intense treatment meant it was highly uncertain which schedule was the more cost-effective. Additionally, they felt it was not possible to select a subgroup (based on patient fitness, for example) in which this could be determined.
Other factors the committee took into account
Although evidence shows a survival benefit from twice-daily radiotherapy compared with once daily, the committee noted that some patients would find the practicalities of the twice-daily treatment schedule and the associated side effects and travel burdensome. The committee agreed that it was important for patients to be able to complete chemotherapy and be fit enough to undergo subsequent prophylactic cranial irradiation.
The committee noted that higher doses of radiotherapy are now used compared to doses reported in most of the pre-1999 trials. In addition, the radiotherapy techniques used for small cell lung cancer have changed dramatically since 1999. Therefore, it was agreed that a pre-1999 cap be applied to the inclusion criteria, to remove older studies but keep the Turrisi (1999) paper, which was seen as the first clinically relevant study using treatment methods relevant to current practice. Additionally, papers reported in 1999 were likely to have used outdated procedures and were rated down for indirectness.
Appendix A. Review protocols
Review protocol for the most clinically and cost-effective regimen of chemoradiotherapy for people with limited-stage SCLC?
What is the most clinically and cost-effective regimen of chemoradiotherapy for people with limited-stage SCLC?
| Field (based on PRISMA-P | Content |
|---|---|
| Review question | This question was identified as requiring updating through the 2016 surveillance review. The review will aim to address the most clinical and cost effective chemoradiotherapy regimen for people with limited-stage SCLC. |
| Type of review question | Intervention |
| Objective of the review | To provide clearer guidance regarding the treatment of limited-stage SCLC. |
| Eligibility criteria – population/ disease/ condition/ issue/ domain | People with limited- stage SCLC. |
| Eligibility criteria – intervention(s)/ exposure(s)/ prognostic factor(s) |
Consider drug regimens and number of cycles and duration of treatment. Timing and fractionation. For example: Concurrent once-daily versus twice-daily chemoradiotherapy (either 45 Gy radiotherapy in 30 twice-daily fractions of 1·5 Gy over 19 days, or 66 Gy in 33 once-daily fractions of 2 Gy over 45 days, starting on day 22 after commencing cisplatin–etoposide chemotherapy (given as four to six cycles every 3 weeks) |
| Eligibility criteria – comparator(s)/ control or reference (gold) standard | Each regimen with the other (once daily versus twice daily regimen of chemo-radiotherapy) |
| Outcomes and prioritisation |
|
| Eligibility criteria – study design |
|
| Other inclusion exclusion criteria |
|
| Proposed sensitivity/sub-group analysis, or meta-regression | Pre-existing performance status defined by ECOG and Karnofsky performance status scale |
| Selection process – duplicate screening/select ion/analysis | |
| Data management (software) | See appendix B. |
| Information sources – databases and dates |
No date limit. See appendix C. Main Searches:
The search will not be date limited because this is a new review question. Note. There was a post-hoc amendment to the protocol to exclude studies prior to 1999 |
| Identify if an update | New question. |
| Author contacts | Guideline update |
| Highlight if amendment to previous protocol | For details please see section 4.5 of Developing NICE guidelines: the manual |
| Search strategy – for one database | For details please see appendix C |
| Data collection process – forms/ duplicate | A standardised evidence table format will be used, and published as appendix H (clinical evidence tables) or I (economic evidence tables) of the full guideline. |
| Data items – define all variables to be collected | For details please see evidence tables in appendix H (clinical evidence tables) or I (economic evidence tables) of the full guideline. |
| Methods for assessing bias at outcome/study level |
Standard study checklists were used to critically appraise individual studies. For details please see section 6.2 of Developing NICE guidelines: the manual The risk of bias across all available evidence was evaluated for each outcome using an adaptation of the ‘Grading of Recommendations Assessment, Development and Evaluation (GRADE) toolbox’ developed by the international GRADE working group http://www For further detail see Appendix B. |
| Criteria for quantitative synthesis (where suitable) | For details please see section 6.4 of Developing NICE guidelines: the manual |
| Methods for analysis – combining studies and exploring (in)consistency |
For details please see the methods chapter of the full guideline. See appendix B. |
| Meta-bias assessment – publication bias, selective reporting bias |
For details please see section 6.2 of Developing NICE guidelines: the manual. See appendix B. |
| Assessment of confidence in cumulative evidence |
For details please see sections 6.4 and 9.1 of Developing NICE guidelines: the manual See appendix B. |
| Rationale/ context – Current management | For details please see the introduction to the evidence review in the full guideline. |
| Describe contributions of authors and guarantor |
A multidisciplinary committee developed the guideline. The committee was convened by NICE Guideline Updates Team and chaired by Gary McVeigh in line with section 3 of Developing NICE guidelines: the manual. Staff from NICE Guideline Updates Team undertook systematic literature searches, appraised the evidence, conducted meta-analysis and cost-effectiveness analysis where appropriate, and drafted the guideline in collaboration with the committee. For details please see the methods chapter of the full guideline. |
| Sources of funding/support | The NICE Guideline Updates Team is an internal team within NICE. |
| Name of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| Roles of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| PROSPERO registration number | N/A |
Appendix B. Methods
Priority screening
The reviews undertaken for this guideline all made use of the priority screening functionality with the EPPI-reviewer systematic reviewing software. This uses a machine learning algorithm (specifically, an SGD classifier) to take information on features (1, 2 and 3 word blocks) in the titles and abstract of papers marked as being ‘includes’ or ‘excludes’ during the title and abstract screening process, and re-orders the remaining records from most likely to least likely to be an include, based on that algorithm. This re-ordering of the remaining records occurs every time 25 additional records have been screened.
Research is currently ongoing as to what are the appropriate thresholds where reviewing of abstract can be stopped, assuming a defined threshold for the proportion of relevant papers it is acceptable to miss on primary screening. As a conservative approach until that research has been completed, the following rules were adopted during the production of this guideline:
- In every review, at least 50% of the identified abstract (or 1,000 records, if that is a greater number) were always screened.
- After this point, screening was only terminated when the threshold was reached for a number of abstracts being screened without a single new include being identified. This threshold was set according to the expected proportion of includes in the review (with reviews with a lower proportion of includes needing a higher number of papers without an identified study to justify termination), and was always a minimum of 250.
- A random 10% sample of the studies remaining in the database when the threshold were additionally screened, to check if a substantial number of relevant studies were not being correctly classified by the algorithm, with the full database being screened if concerns were identified.
As an additional check to ensure this approach did not miss relevant studies, the included studies lists of included systematic reviews were searched to identify any papers not identified through the primary search.
Evidence synthesis and meta-analyses
Where possible, meta-analyses were conducted to combine the results of studies for each outcome. For mean differences, where change from baseline data were reported in the studies and were accompanied by a measure of spread (for example standard deviation), these were extracted and used in the meta-analysis. Where measures of spread for change from baseline values were not reported, the corresponding values at study end were used and were combined with change from baseline values to produce summary estimates of effect. All studies were assessed to ensure that baseline values were balanced across the treatment/comparison groups; if there were significant differences in important confounding variables at baseline these studies were not included in any meta-analysis and were reported separately.
When averages were given as medians, no meta-analysis of the data were performed.
Evidence of effectiveness of interventions
Quality assessment
Individual RCTs were quality assessed using the Cochrane Risk of Bias Tool. Each individual study was classified into one of the following three groups:
- Low risk of bias – The true effect size for the study is likely to be close to the estimated effect size.
- Moderate risk of bias – There is a possibility the true effect size for the study is substantially different to the estimated effect size.
- High risk of bias – It is likely the true effect size for the study is substantially different to the estimated effect size.
Each individual study was also classified into one of three groups for directness, based on if there were concerns about the population, intervention, comparator and/or outcomes in the study and how directly these variables could address the specified review question. Studies were rated as follows:
- Direct – No important deviations from the protocol in population, intervention, comparator and/or outcomes.
- Partially indirect – Important deviations from the protocol in one of the population, intervention, comparator and/or outcomes.
- Indirect – Important deviations from the protocol in at least two of the following areas: population, intervention, comparator and/or outcomes.
Methods for combining intervention evidence
Meta-analyses of interventional data were conducted with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al. 2011).
Where different studies presented continuous data measuring the same outcome but using different numerical scales (e.g. a 0-10 and a 0-100 visual analogue scale), these outcomes were all converted to the same scale before meta-analysis was conducted on the mean differences. Where outcomes measured the same underlying construct but used different instruments/metrics, data were analysed using standardised mean differences (Hedges’ g).
A pooled relative risk was calculated for dichotomous outcomes (using the Mantel–Haenszel method). Both relative and absolute risks were presented, with absolute risks calculated by applying the relative risk to the pooled risk in the comparator arm of the meta-analysis.
Fixed- and random-effects models (der Simonian and Laird) were fitted for all syntheses, with the presented analysis dependent on the degree of heterogeneity in the assembled evidence. Fixed-effects models were the preferred choice to report, but in situations where the assumption of a shared mean for fixed-effects model were clearly not met, even after appropriate pre-specified subgroup analyses were conducted, random-effects results are presented. Fixed-effects models were deemed to be inappropriate if one or both of the following conditions was met:
- Significant between study heterogeneity in methodology, population, intervention or comparator was identified by the reviewer in advance of data analysis. This decision was made and recorded before any data analysis was undertaken.
- The presence of significant statistical heterogeneity in the meta-analysis, defined as I2≥50%.
In any meta-analyses where some (but not all) of the data came from studies at high risk of bias, a sensitivity analysis was conducted, excluding those studies from the analysis. Results from both the full and restricted meta-analyses are reported. Similarly, in any meta-analyses where some (but not all) of the data came from indirect studies, a sensitivity analysis was conducted, excluding those studies from the analysis.
Meta-analyses were performed in Cochrane Review Manager v 5.3.
Minimal clinically important differences (MIDs)
The Core Outcome Measures in Effectiveness Trials (COMET) database was searched to identify published minimal clinically important difference thresholds relevant to this guideline. However, no relevant MIDs were found. In addition, the Guideline Committee were asked to specify any outcomes where they felt a consensus MID could be defined from their experience. In particular, any questions looking to evaluate non-inferiority (that one intervention is not meaningfully worse than another) required an MID to be defined to act as a non-inferiority margin. However, the committee agreed that in their experience, they could not define any MIDs. This is because the committee agreed that the protocol outcomes were objective rather than subjective measures and the committee were not aware of evidence supporting the use of MIDs for the protocol’s outcomes. Therefore, for pooled mean differences, a MID of 0.2 SD was used because this corresponds to the threshold for a small effect size initially suggested by Cohen et al. (1988). The line of no effect was used as a MID for risk ratios and hazard ratios.
GRADE for pairwise meta-analyses of interventional evidence
GRADE was used to assess the quality of evidence for the selected outcomes as specified in ‘Developing NICE guidelines: the manual (2014)’. Data from RCTs was initially rated as high quality and the quality of the evidence for each outcome was downgraded or not from this initial point.
Table 2. Rationale for downgrading quality of evidence for intervention studies
Publication bias
Publication bias was assessed in two ways. First, if evidence of conducted but unpublished studies was identified during the review (e.g. conference abstracts, trial protocols or trial records without accompanying published data), available information on these unpublished studies was reported as part of the review. Secondly, where 10 or more studies were included as part of a single meta-analysis, a funnel plot was produced to graphically assess the potential for publication bias.
Evidence statements
Evidence statements for pairwise intervention data are classified in to one of four categories:
- Situations where the data are only consistent, at a 95% confidence level, with an effect in one direction (i.e. one that is ‘statistically significant’), and the magnitude of that effect is most likely to meet or exceed the MID (i.e. the point estimate is not in the zone of equivalence). In such cases, we state that the evidence showed that there is an effect.
- Situations where the data are only consistent, at a 95% confidence level, with an effect in one direction (i.e. one that is ‘statistically significant’), but the magnitude of that effect is most likely to be less than the MID (i.e. the point estimate is in the zone of equivalence). In such cases, we state that the evidence could not demonstrate a meaningful difference.
- Situations where the data are consistent, at a 95% confidence level, with an effect in either direction (i.e. one that is not ‘statistically significant’) but the confidence limits are smaller than the MIDs in both directions. In such cases, we state that the evidence demonstrates that there is no difference.
- In all other cases, we state that the evidence could not differentiate between the comparators.
Appendix C. Literature search strategies
Scoping search strategies
Scoping searches Scoping searches were undertaken on the following websites and databases (listed in alphabetical order) in April 2017 to provide information for scope development and project planning. Browsing or simple search strategies were employed.
| Guidelines/website |
|---|
| American Cancer Society |
| American College of Chest Physicians |
| American Society for Radiation Oncology |
| American Thoracic Society |
| Association for Molecular Pathology |
| British Lung Foundation |
| British Thoracic Society |
| Canadian Medical Association Infobase |
| Canadian Task Force on Preventive Health Care |
| Cancer Australia |
| Cancer Care Ontario |
| Cancer Control Alberta |
| Cancer Research UK |
| Care Quality Commission |
| College of American Pathologists |
| Core Outcome Measures in Effectiveness Trials (COMET) |
| Department of Health & Social Care |
| European Respiratory Society |
| European Society for Medical Oncology |
| European Society of Gastrointestinal Endoscopy |
| European Society of Thoracic Surgery |
| General Medical Council |
| Guidelines & Audit Implementation Network (GAIN) |
| Guidelines International Network (GIN) |
| Healthtalk Online |
| International Association for the Study of Lung Cancer |
| MacMillan Cancer Support |
| Medicines and Products Regulatory Agency (MHRA) |
| National Audit Office |
| National Cancer Intelligence Network |
| National Clinical Audit and Patient Outcomes Programme |
| National Health and Medical Research Council - Australia |
| National Institute for Health and Care Excellence (NICE) - published & in development guidelines |
| National Institute for Health and Care Excellence (NICE) - Topic Selection |
| NHS Choices |
| NHS Digital |
| NHS England |
| NICE Clinical Knowledge Summaries (CKS) |
| NICE Evidence Search |
| Office for National Statistics |
| Patient UK |
| PatientVoices |
| Public Health England |
| Quality Health |
| Royal College of Anaesthetists |
| Royal College of General Practitioners |
| Royal College of Midwives |
| Royal College of Nursing |
| Royal College of Pathologists |
| Royal College of Physicians |
| Royal College of Radiologists |
| Royal College of Surgeons |
| Scottish Government |
| Scottish Intercollegiate Guidelines Network (SIGN) |
| UK Data Service |
| US National Guideline Clearinghouse |
| Walsall community Health NHS Trust |
| Welsh Government |
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between October 2017 and April 2018 for 9 review questions (RQ).
Searches were re-run in May 2018.
Where appropriate, in-house study design filters were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 3.
Search strategy
|
Medline Strategy, searched 8th March 2018 Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Strategy: |
|---|
| 1 Small Cell Lung Carcinoma/ |
| 2 Carcinoma, Small Cell/ |
| 3 SCLC.tw. |
| 4 ((pancoast* or superior sulcus or pulmonary sulcus) adj4 (tumo?r* or syndrome*)).tw. |
| 5 or/1-4 |
| 6 ((small or oat or reserve or round) adj1 cell adj1 (lung* or pulmonary or bronch*) adj3 (cancer* or neoplasm* or carcinoma* or tumo?r* or lymphoma* or metast* or malignan* or blastoma* or carcinogen* or adenocarcinoma* or angiosarcoma* or chrondosarcoma* or sarcoma* or teratoma* or microcytic*)).tw. |
| 7 (non adj1 small adj1 cell adj1 (lung* or pulmonary or bronch*) adj3 (cancer* or neoplasm* or carcinoma* or tumo?r* or lymphoma* or metast* or malignan* or blastoma* or carcinogen* or adenocarcinoma* or angiosarcoma* or chrondosarcoma* or sarcoma* or teratoma* or microcytic*)).tw. |
| 8 6 not 7 |
| 9 5 or 8 |
| 10 exp Chemoradiotherapy/ |
| 11 (chemoradiotherap* or radiochemotherap* or chemoradiation*).tw. |
| 12 (chemo adj1 (radiotherap* or radiation)).tw. |
| 13 ((chemotherap* or antineoplastic* or anti-neoplastic* or polychemotherap* or CTX) adj4 combin* adj4 (radiotherap* or radiotreat* or irradiat* or RT or RTx or XRT or TRT or TCRT)).tw. |
| 14 Combined Modality Therapy/ |
| 15 (combine* adj4 modal* adj4 (treat* or therap* or regimen* or manag* or intervention*)).tw. |
| 16 ((tri-modal* or trimodal* or multi-modal* or multimodal*) adj4 (treat* or therap* or regimen* or manag* or intervention*)).tw. |
| 17 TMT.tw. |
| 18 or/10-17 |
| 19 Drug Therapy/ |
| 20 exp Drug Therapy, Combination/ |
| 21 exp Antineoplastic Protocols/ |
| 22 exp Antineoplastic Agents/ |
| 23 Chemotherapy, Adjuvant/ |
| 24 (chemotherap* or antineoplastic* or anti-neoplastic* or polychemotherap* or CTX).tw. |
| 25 ((anticancer* or anti-cancer* or antitumo?r or anti-tumo?r or anticarcinogen* or anticarcinogen*) adj4 (drug* or agent* or therap* or treat* or medicat* or protocol*)).tw. |
| 26 or/19-25 |
| 27 (concurrent* or follow* or after* or with or consecutiv* or alongside or synchroni?ed or parallel or coexisting or concomitant or accompan*).tw. |
| 28 exp Radiotherapy/ |
| 29 Radiation Oncology/ |
| 30 exp Radiography, Thoracic/ |
| 31 radiotherapy.fs. |
| 32 (radiotherap* or radiotreat* or roentgentherap* or radiosurg*).tw. |
| 33 ((radiat* or radio* or irradiat* or roentgen or x-ray or xray) adj4 (therap* or treat* or repair* or oncolog* or surg*)).tw. |
| 34 (RT or RTx or XRT or TRT or TCRT).tw. |
| 35 ((chest* or thorac* or thorax) adj4 irradiat*).tw. |
| 36 or/28-35 |
| 37 26 and 27 and 36 |
| 38 18 or 37 |
| 39 9 and 38 |
| 40 Animals/ not Humans/ |
| 41 39 not 40 |
| 42 limit 41 to english language |
Note: In-house RCT and systematic review filters were appended. No date limit was used as this was a new question.
Study Design Filters
| The MEDLINE SR, RCT, and observational studies filters are presented below. |
|---|
| Systematic Review |
| 1. Meta-Analysis.pt. |
| 2. Meta-Analysis as Topic/ |
| 3. Review.pt. |
| 4. exp Review Literature as Topic/ |
| 5. (metaanaly$ or metanaly$ or (meta adj3 analy$)).tw. |
| 6. (review$ or overview$).ti. |
| 7. (systematic$ adj5 (review$ or overview$)).tw. |
| 8. ((quantitative$ or qualitative$) adj5 (review$ or overview$)).tw. |
| 9. ((studies or trial$) adj2 (review$ or overview$)).tw. |
| 10. (integrat$ adj3 (research or review$ or literature)).tw. |
| 11. (pool$ adj2 (analy$ or data)).tw. |
| 12. (handsearch$ or (hand adj3 search$)).tw. |
| 13. (manual$ adj3 search$).tw. |
| 14. or/1-13 |
| 15. animals/ not humans/ |
| 16. 14 not 15 |
| RCT |
| 1 Randomized Controlled Trial.pt. |
| 2 Controlled Clinical Trial.pt. |
| 3 Clinical Trial.pt. |
| 4 exp Clinical Trials as Topic/ |
| 5 Placebos/ |
| 6 Random Allocation/ |
| 7 Double-Blind Method/ |
| 8 Single-Blind Method/ |
| 9 Cross-Over Studies/ |
| 10 ((random$ or control$ or clinical$) adj3 (trial$ or stud$)).tw. |
| 11 (random$ adj3 allocat$).tw. |
| 12 placebo$.tw. |
| 13 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw. |
| 14 (crossover$ or (cross adj over$)).tw. |
| 15 or/1-14 |
| 16 animals/ not humans/ |
| 17 15 not 16 |
| Observational |
| 1 Observational Studies as Topic/ |
| 2 Observational Study/ |
| 3 Epidemiologic Studies/ |
| 4 exp Case-Control Studies/ |
| 5 exp Cohort Studies/ |
| 6 Cross-Sectional Studies/ |
| 7 Controlled Before-After Studies/ |
| 8 Historically Controlled Study/ |
| 9 Interrupted Time Series Analysis/ |
| 10 Comparative Study.pt. |
| 11 case control$.tw. |
| 12 case series.tw. |
| 13 (cohort adj (study or studies)).tw. |
| 14 cohort analy$.tw. |
| 15 (follow up adj (study or studies)).tw. |
| 16 (observational adj (study or studies)).tw. |
| 17 longitudinal.tw. |
| 18 prospective.tw. |
| 19 retrospective.tw. |
| 20 cross sectional.tw. |
| 21 or/1-20 |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Apr 2015
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the review question search strategies. For some health economics strategies additional terms were added to the original review question search strategies (see sections 4.2, 4.3 and 4.4) The searches were conducted between October 2017 and April 2018 for 9 review questions (RQ).
Searches were re-run in May 2018.
Searches were limited to those in the English language. Animal studies were removed from results.
Economic evaluation and quality of life filters
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom$.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov$.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree$ or analys$)).tw. |
| 20 (cost or costs or costing$ or costly or costed).tw. |
| 21 (price$ or pricing$).tw. |
| 22 budget$.tw. |
| 23 expenditure$.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic$ or (pharmaco adj economic$)).tw. |
| 26 or/1-25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly$ or qald$ or qale$ or qtime$).tw. |
| 7 disability adjusted life.tw. |
| 8 daly$.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health$ year$ equivalent$.tw. |
| 19 utilit$.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili$.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble$.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1-30 |
Appendix D. Evidence study selection
Appendix E. Clinical evidence tables
Download PDF (312K)
Appendix F. Forest plots
Once- versus twice-daily radiotherapy with concomitant chemotherapy for the treatment of limited-disease small cell lung cancer
Early versus late radiotherapy with concomitant chemotherapy for the treatment of limited-disease small cell lung cancer
Appendix G. GRADE tables
Once- versus twice-daily radiotherapy with concomitant chemotherapy for the treatment of limited-disease small cell lung cancer
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Once-daily | Twice-daily | Summary of results (95% CI) | |
| Mortality: all-cause hazard ratio (values greater than 1 favour twice-daily) | |||||||||
| 2 studies (CONVERT 2017; Turrisi 1999*) | RCT | Not serious | Serious5 | Not serious | Not serious | 446 | 460 | HR 1.19 (1.03, 1.38) | Moderate |
| Mortality: risk ratio for mortality at 2 years (values greater than 1 favour twice-daily) | |||||||||
| 1 study Bonner 1999 | RCT | Not serious | Serious1 | N/A | Serious2 | 132 | 130 | RR 0.95 (0.76, 1.19) | Low |
| Mortality: risk ratio for mortality at 3 years (values greater than 1 favour twice-daily) | |||||||||
| 1 study Bonner 1999 | RCT | Not serious | Serious1 | N/A | Serious2 | 132 | 130 | RR 0.93 (0.79, 1.10) | Low |
| Mortality: risk ratio for mortality at 5 years (values greater than 1 favour twice-daily) | |||||||||
| 1 study Schild 2004 | RCT | Not serious | Serious1 | N/A | Serious2 | 132 | 130 | RR 1.01 (0.89, 1.15) | Low |
| Adverse events grade 3 or above: Risk ratio for oesophagitis (values greater than 1 favour twice-daily) | |||||||||
| 3 studies Turrisi 1999 Schildd 2004 Convert 2017 | RCT | Serious4 | Serious5 | Very serious3 | Serious2 | 580 | 590 | RR 0.68 (0.35, 1.15) | Very low |
| Adverse events grade 3 or above: Risk ratio for pneumonitis (values greater than 1 favour twice-daily) | |||||||||
| 2 studies Schildd 2004 Convert 2017 | RCT | Serious4 | Serious5 | Not serious | Serious2 | 377 | 384 | RR 0.93 (0.43, 2.01) | Very low |
| Adverse events grade 3 or above: Risk ratio for cardiac toxicity (values greater than 1 favour twice-daily) | |||||||||
| 1 study Schild | RCT | Serious4 | Serious1 | N/A | Serious2 | 131 | 130 | RR 0.20 (0.02, 1.68) | Very low |
- 1
Partially directly applicable: Participants were delayed in being randomized to and receiving radiotherapy until after 3 cycles of chemotherapy.
- 2
95% CI of the effect size crosses the line of no effect.
- 3
I2 >66.6%.
- 4
Studies were not blinded and this had the potential to bias reporting of outcome.
- 5
Long length of time difference between the studies resulting in differences in standard of care.
- *
Hazard ratio data taken from De Ruysscher 2016 meta-analysis as Estimate in original paper is inconsistent with confidence intervals
Once-daily hypofractionated versus twice-daily hyperfractionated radiotherapy for the treatment of limited-disease small cell-lung cancer
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Once-daily | Twice-daily | Summary of results (95% CI) | |
| Mortality: Any-cause hazard ratio (values greater than 1 favour twice-daily) | |||||||||
| 1 study Halvorsen 2016 | RCT | Not serious | Not serious | N/A | Serious2 | 84 | 73 | RR 1.19 (0.79, 1.79) | Moderate |
| Adverse events grade 3 or above: Risk ratio for oesophagitis (values greater than 1 favour twice-daily) | |||||||||
| 1 study Gronberg 2016 | RCT | Serious1 | Not serious | N/A | Serious2 | 84 | 73 | RR 0.94 (0.60, 1.49) | Low |
| Adverse events grade 3 or above: Risk ratio for Pneumonitis (values greater than 1 favour twice-daily) | |||||||||
| 1 study Gronberg 2016 | RCT | Serious1 | Not serious | N/A | Serious2 | 84 | 73 | RR 1.45 (0.36, 5.85) | Low |
- 1
Studies were not blinded and this had the potential to bias reporting of outcome.
- 2
95% CI of the effect size crosses the line of no effect.
Early versus late radiotherapy with concomitant chemotherapy for the treatment of limited-disease small cell lung cancer
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Early | Late | Summary of results (95% CI) | |
| Mortality: risk ratio for mortality at 12 months (values greater than 1 favour late) | |||||||||
| 1 study Spiro 2006 | RCT | Not serious | Serious5 | N/A | Serious3 | 159 | 166 | RR 1.12 (0.87, 1.46) | Low |
| Mortality: risk ratio for mortality at 24 months (values greater than 1 favour late) | |||||||||
| 4 studies Skarlos 2001 Spiro 2006 Sun 2013 Takada 2002 | RCT | Not serious | Serious1 | Very serious2 | Serious2 | 426 | 427 | RR 0.95 (0.75, 1.20) | Very low |
| Mortality: risk ratio for mortality at 36 months (values greater than 1 favour late) | |||||||||
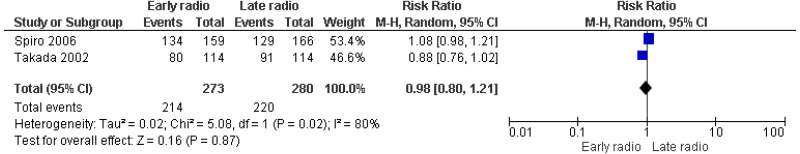
| 2 studies Spiro 2006 Takada 2002 | RCT | Not serious | Serious5 | Very serious2 | Serious2 | 273 | 280 | RR 0.98 (0.80, 1.21) | Very low |
| Mortality: risk ratio for mortality at 60 months (values greater than 1 favour late) | |||||||||
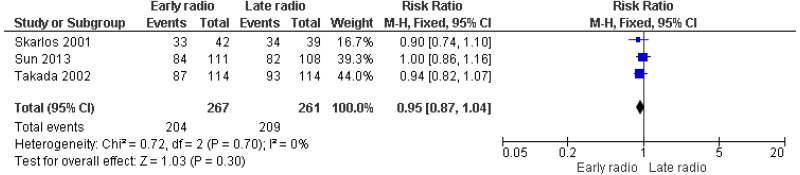
| 3 studies Skarlos 2001 Sun 2013 Takada 2002 | RCT | Not serious | Serious1 | Not serious | Serious3 | 267 | 261 | RR 0.95 (0.87, 1.04) | Low |
| Adverse events grade 3 or above: Oesophagitis (values greater than 1 favour late) | |||||||||
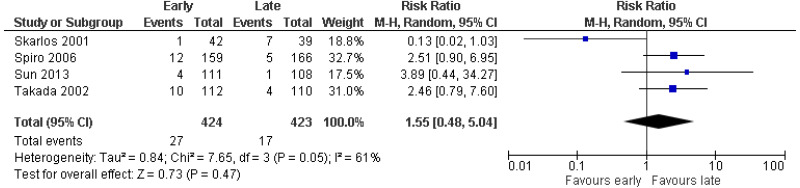
| 4 studies Skarlos 2001 Spiro 2006 Sun 2013 Takada 2002 | RCT | Serious4 | Serious1 | Serious6 | Serious3 | 424 | 423 | RR 1.55 (0.48, 5.04) | Very low |
| Adverse events grade 3 or above: Pneumonitis (values greater than 1 favour late) | |||||||||
| 1 study Sun 2013 | RCT | Serious4 | Serious5 | N/A | Serious3 | 111 | 108 | RR 1.62 (0.40, 6.62) | Very low |
| Adverse events grade 3 or above: Cardiac (values greater than 1 favour late) | |||||||||
| 1 study Spiro 2006 | RCT | Serious4 | Serious5 | N/A | Serious3 | 159 | 166 | RR 9.39 (0.51, 173.08) | Very low |
- 1
Partially directly applicable: Two or more studies used a once-daily, very high dose-per-fraction regimen.
- 2
I2 >66%.
- 3
95% CI of the effect size crosses the line of no effect.
- 4
Non-blinded and this had the potential to bias reporting of outcome.
- 5
Partially directly applicable: Study used a once-daily regimen.
- 6
I2 >33%.
Continuous versus alternating radiotherapy for the treatment of limited-disease small cell lung cancer
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Continuous | Alternating | Summary of results (95% CI) | |
| Mortality: risk ratio for staying mortality at 2 years (values greater than 1 favour alternating) | |||||||||
| 2 studies Blackstock 2005 Lebeau 1999 | RCT | Not serious | Not serious | Not serious | Serious2 | 138 | 128 | RR 1.01 (0.89, 1.15) | Moderate |
| Mortality: risk ratio for mortality at 3 years (values greater than 1 favour alternating) | |||||||||
| 1 study Lebeau 1999 | RCT | Not serious | Not serious | N/A | Serious2 | 82 | 74 | RR 1.05 (0.96, 1.16) | Moderate |
| Mortality: risk ratio for mortality at 5 years (values greater than 1 favour alternating) | |||||||||
| 1 study Blackstock 2005 | RCT | Not serious | Not serious | N/A | Serious2 | 56 | 54 | RR 1.03 (0.86, 1.24) | Moderate |
| Adverse events grade 3 or above: Risk ratio for oesophagitis (values greater than 1 favour alternating) | |||||||||
| 1 study Blackstock 2005 | RCT | Serious1 | Not serious | N/A | Serious2 | 56 | 54 | RR 2.41 (0.49, 11.90) | Low |
- 1
Study was not blinded and this had the potential to bias reporting of outcome.
- 2
95% CI of the effect size crosses the line of no effect.
Appendix H. Excluded Studies
| Study | Title | Reason for exclusion |
|---|---|---|
| Anonymous (1983) | Cytotoxic chemotherapy before and after radiotherapy compared with radiotherapy followed by chemotherapy in the treatment of small-cell carcinoma of the bronchus: the results up to 36 months |
|
| Choi (1998) | Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer |
|
| De Ruysscher (2006) | Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer |
|
| De Ruysscher (2012) | Radiation-induced oesophagitis in lung cancer patients. Is susceptibility for neutropenia a risk factor? |
|
| De Ruysscher (2016) | Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis |
|
| Fried (2004) | Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer |
|
| Gregor (1995) | Acute toxicity of alternating schedule of chemotherapy and irradiation in limited small-cell lung cancer in a pilot study (08877) of the EORTC Lung Cancer Cooperative Group |
|
| Gregor (1997) | Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small-cell lung cancer: a European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study |
|
| Hackshaw (2007) | The timing of radiotherapy when given with chemotherapy in patients with limited-disease small cell lung cancer |
|
| Halvorsen (2016) | Tumour size reduction after the first chemotherapy-course and outcomes of chemoradiotherapy in limited disease small-cell lung cancer |
|
| Hu (2010) | A prospective randomized study of the radiotherapy volume for limited-stage small cell lung cancer: a preliminary report |
|
| Huncharek (2004) | A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer |
|
| Jeremic (1997) | Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: a randomized study |
|
| Kraft (1990) | Role of thoracic radiotherapy combined with chemotherapy in limited stage small cell lung cancer (SCLC). A randomized multicenter phase III trial |
|
| Le Chevalier (1988) | Combination of chemotherapy and radiotherapy in limited small cell lung carcinoma: Results of alternating schedule in 109 patients |
|
| Lee (2002) | Randomized Trial of Early Versus Late Alternating Radiotherapy/ Chemotherapy in Limited-Disease Patients with Small Cell Lung Cancer |
|
| Liu (2010) | Whole brain radiotherapy concomitant or sequential Vm26/DDP in treating small cell lung cancer patients with brain metastases |
|
| Lu (2014) | A meta-analysis of randomized controlled trials comparing early and late concurrent thoracic radiotherapy with etoposide and cisplatin/carboplatin chemotherapy for limited-disease small-cell lung cancer |
|
| Lueza (2014) | Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer |
|
| Murray (1993) | Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group |
|
| Park (1996) | The effects according to the timing of thoracic radiotherapy in limited stage small cell lung cancer |
|
| Perez (1981) | Thoracic and elective brain irradiation with concomitant or delayed multiagent chemotherapy in the treatment of localized small cell carcinoma of the lung: a randomized prospective study by the Southeastern Cancer Study Group |
|
| Pijls-Johannesma (2004) | Early versus late chest radiotherapy in patients with limited-stage small cell lung cancer |
|
| Pijls-Johannesma (2007) | Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials |
|
| Qiao (2004) | Concurrent radiotherapy combined with carboplatin and etoposide in limited stage small cell lung cancer |
|
| Samson (2007) | Evidence for management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) |
|
| Sculier (2008) | A phase III randomised study of concomitant induction radiochemotherapy testing two modalities of radiosensitisation by cisplatin (standard versus daily) for limited small-cell lung cancer |
|
| Seidenfeld (2006) | Management of small cell lung cancer |
|
| Sheikh (2011) | Use of G-CSF during concurrent chemotherapy and thoracic radiotherapy in patients with limited-stage small-cell lung cancer safety data from a phase II trial |
|
| Work (1997) | Randomized study of initial versus late chest irradiation combined with chemotherapy in limited-stage small-cell lung cancer. Aarhus Lung Cancer Group |
|
| Ye (2011) | Three-dimensional conformal radiotherapy or intensity-modulated radiotherapy combined with concurrent sequential chemotherapy for limited stage small cell lung cancer |
|
Appendix I. References
Clinical Studies - Included
- Blackstock A W, Bogart J A, Matthews C, Lovato J F, McCoy T, Livengood K, Ho C, White D, Atkins J N, and Miller A A (2005) Split-course versus continuous thoracic radiation therapy for limited-stage small-cell lung cancer: final report of a randomized phase III trial. Clinical Lung Cancer 6(5), 287–92 [PubMed: 15845179]
- Bonner J A, Sloan J A, Shanahan T G, Brooks B J, Marks R S, Krook J E, Gerstner J B, Maksymiuk A, Levitt R, Mailliard J A, Tazelaar H D, Hillman S, and Jett J R (1999) Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. Journal of Clinical Oncology 17(9), 2681–91 [PubMed: 10561342]
- Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, Bezjak A, Cardenal F, Fournel P, Harden S, Le Pechoux, C, McMenemin R, Mohammed N, O’Brien M, Pantarotto J, Surmont V, Van Meerbeeck, J P, Woll P J, Lorigan P, Blackhall F, and Team Convert Study (2017) Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncology 18(8), 1116–1125 [PMC free article: PMC5555437] [PubMed: 28642008]
- Gronberg B H, Halvorsen T O, Flotten O, Brustugun O T, Brunsvig P F, Aasebo U, Bremnes R M, Tollali T, Hornslien K, Aksnessaether B Y, Liaaen E D, Sundstrom S, Norwegian Lung Cancer Study, and Group (2016) Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncologica 55(5), 591–7 [PubMed: 26494411]
- Lebeau B, Urban T, Brechot J M, Paillotin D, Vincent J, Leclerc P, Meekel P, L’Her P, Lebas F X, and Chastang C (1999) A randomized clinical trial comparing concurrent and alternating thoracic irradiation for patients with limited small cell lung carcinoma. “Petites Cellules” Group. Cancer 86(8), 1480–7 [PubMed: 10526276]
- Skarlos D V, Samantas E, Briassoulis E, Panoussaki E, Pavlidis N, Kalofonos H P, Kardamakis D, Tsiakopoulos E, Kosmidis P, Tsavdaridis D, Tzitzikas J, Tsekeris P, Kouvatseas G, Zamboglou N, and Fountzilas G (2001) Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small-cell lung cancer: a randomized phase II study of the Hellenic Cooperative Oncology Group (HeCOG). Annals of Oncology 12(9), 1231–8 [PubMed: 11697833]
- Spiro S G, James L E, Rudd R M, Trask C W, Tobias J S, Snee M, Gilligan D, Murray P A, Ruiz de Elvira, M C, O’Donnell K M, Gower N H, Harper P G, Hackshaw A K, London Lung Cancer, and Group (2006) Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. Journal of Clinical Oncology 24(24), 3823–30 [PubMed: 16921033]
- Sun J M, Ahn Y C, Choi E K, Ahn M J, Ahn J S, Lee S H, Lee D H, Pyo H, Song S Y, Jung S H, Jo J S, Jo J, Sohn H J, Suh C, Lee J S, Kim S W, and Park K (2013) Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer.[Erratum appears in Ann Oncol. 2014 Aug;25(8):1672]. Annals of Oncology 24(8), 2088–92 [PubMed: 23592701]
- Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T, Fukuda H, and Saijo N (2002) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. Journal of Clinical Oncology 20(14), 3054–60 [PubMed: 12118018]
- Turrisi A T, 3rd, Kim K, Blum R, Sause W T, Livingston R B, Komaki R, Wagner H, Aisner S, and Johnson D H (1999) Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. New England Journal of Medicine 340(4), 265–71 [PubMed: 9920950]
Clinical studies – Excluded
- Anonymous (1983) Cytotoxic chemotherapy before and after radiotherapy compared with radiotherapy followed by chemotherapy in the treatment of small-cell carcinoma of the bronchus: the results up to 36 months. British Journal of Cancer 48(6), 755–61 [PMC free article: PMC2011559] [PubMed: 6197081]
- Choi N C, Herndon J E, 2nd, Rosenman J, Carey R W, Chung C T, Bernard S, Leone L, Seagren S, and Green M (1998) Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. Journal of Clinical Oncology 16(11), 3528–36 [PubMed: 9817271]
- De Ruysscher, D, Pijls-Johannesma M, Bentzen S M, Minken A, Wanders R, Lutgens L, Hochstenbag M, Boersma L, Wouters B, Lammering G, Vansteenkiste J, and Lambin P (2006) Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. Journal of Clinical Oncology 24(7), 1057–63 [PubMed: 16505424]
- De Ruysscher, D, Van Meerbeeck, J, Vandecasteele K, Oberije C, Pijls M, Dingemans A M, Reymen B, van Baardwijk, A, Wanders R, Lammering G, Lambin P, De Neve, and W (2012) Radiation-induced oesophagitis in lung cancer patients. Is susceptibility for neutropenia a risk factor?. Strahlentherapie und Onkologie 188(7), 564–7 [PubMed: 22543884]
- De Ruysscher, D, Lueza B, Le Pechoux, C, Johnson D H, O’Brien M, Murray N, Spiro S, Wang X, Takada M, Lebeau B, Blackstock W, Skarlos D, Baas P, Choy H, Price A, Seymour L, Arriagada R, Pignon J P, and Group Rtt-Sclc Collaborative (2016) Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Annals of Oncology 27(10), 1818–28 [PMC free article: PMC5035783] [PubMed: 27436850]
- Fried D B, Morris D E, Poole C, Rosenman J G, Halle J S, Detterbeck F C, Hensing T A, and Socinski M A (2004) Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. Journal of Clinical Oncology 22(23), 4837–45 [PubMed: 15570087]
- Gregor A, Drings P, Rinaldi M, Schuster L, Burghouts J, Postmus P E, Dalesio O, Kirkpatrick A, Hoctin Boes, G, Van Zandwijk, and N (1995) Acute toxicity of alternating schedule of chemotherapy and irradiation in limited small-cell lung cancer in a pilot study (08877) of the EORTC Lung Cancer Cooperative Group. Annals of Oncology 6(4), 403–5 [PubMed: 7619758]
- Gregor A, Drings P, Burghouts J, Postmus P E, Morgan D, Sahmoud T, Kirkpatrick A, Dalesio O, and Giaccone G (1997) Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small-cell lung cancer: a European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study. Journal of Clinical Oncology 15(8), 2840–9 [PubMed: 9256127]
- Hackshaw A, and Spiro S (2007) The timing of radiotherapy when given with chemotherapy in patients with limited-disease small cell lung cancer. American Journal of Hematology/ Oncology 6(2), 74–78
- Halvorsen T O, Herje M, Levin N, Bremnes R M, Brustugun O T, Flotten O, Kaasa S, Sundstrom S, and Gronberg B H (2016) Tumour size reduction after the first chemotherapy-course and outcomes of chemoradiotherapy in limited disease small-cell lung cancer. Lung Cancer 102, 9–14 [PubMed: 27987595]
- Hu X, Bao Y, Zhang L, Cheng Y, Li K, Wang W, Liu Y, He H, Sun Z, Zhuang T, Wang Y, Chen J, Liang Y, Zhang Y, Zhao H, Wang F, and Chen M (2010) A prospective randomized study of the radiotherapy volume for limited-stage small cell lung cancer: a preliminary report. Zhongguo fei ai za zhi [Chinese journal of lung cancer] 13(7), 691–699 [PMC free article: PMC6000379] [PubMed: 20673485]
- Huncharek M, and McGarry R (2004) A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist 9(6), 665–72 [PubMed: 15561810]
- Jeremic B, Shibamoto Y, Acimovic L, and Milisavljevic S (1997) Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: a randomized study. Journal of Clinical Oncology 15(3), 893–900 [PubMed: 9060525]
- Kraft A, Arnold H, Zwingers T, Bodemann H, von Bultzingslowen, F, Hinkelbein W, and Wannenmacher M (1990) Role of thoracic radiotherapy combined with chemotherapy in limited stage small cell lung cancer (SCLC). A randomized multicenter phase III trial. Onkologie 13(4), 253–8 [PubMed: 2172884]
- Le Chevalier, T, Arriagada R, De The, H, De Cremoux, H, Martin M, Baldeyrou P, Ruffie P, Benna F, Cerrina M L, Sancho-Garnier H, and Hayat M (1988) Combination of chemotherapy and radiotherapy in limited small cell lung carcinoma: Results of alternating schedule in 109 patients. NCI Monographs (6), 335–338 [PubMed: 2832769]
- Lee Cg, Kim Jh, Kim Sk, Kim Sk, Kim Ge, and Suh Co (2002) Randomized Trial of Early Versus Late Alternating Radiotherapy/ Chemotherapy in Limited-Disease Patients with Small Cell Lung Cancer. The journal of the korean society for therapeutic radiology and oncology 20(2), 116–122
- Liu M, Zhou Y, Han Q, Gao T, Luo Z, and Wang W (2010) Whole brain radiotherapy concomitant or sequential Vm26/DDP in treating small cell lung cancer patients with brain metastases. Chinese-German Journal of Clinical Oncology 9(1), 17–21
- Lu H, Fang L, Wang X, Cai J, and Mao W (2014) A meta-analysis of randomized controlled trials comparing early and late concurrent thoracic radiotherapy with etoposide and cisplatin/carboplatin chemotherapy for limited-disease small-cell lung cancer. Molecular and Clinical Oncology 2(5), 805–810 [PMC free article: PMC4106745] [PubMed: 25054049]
- Lueza B, Le Pechoux, C, and Pignon J P (2014) Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Annals of Oncology 25(9), 1865–6 [PubMed: 24920790]
- Murray N, Coy P, Pater J L, Hodson I, Arnold A, Zee B C, Payne D, Kostashuk E C, Evans W K, Dixon P, and et al (1993) Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology 11(2), 336–44 [PubMed: 8381164]
- Park Sk, Kim Gh, Jeong Ss, Shin Ks, Kim Ak, Cho Hj, Suhr Jw, Kim Js, Cho Mj, Kim Jo, and Kim Sy (1996) The effects according to the timing of thoracic radiotherapy in limited stage small cell lung cancer. Tuberculosis and respiratory diseases 43(6), 903–915
- Perez C A, Krauss S, Bartolucci A A, Durant J R, Lowenbraun S, Salter M M, Storaalsi J, Kellermeyer R, and Comas F (1981) Thoracic and elective brain irradiation with concomitant or delayed multiagent chemotherapy in the treatment of localized small cell carcinoma of the lung: a randomized prospective study by the Southeastern Cancer Study Group. Cancer 47(10), 2407–13 [PubMed: 6268269]
- Pijls-Johannesma Madelon, De Ruysscher Dirk K M, Lambin Philippe, Houben Ruud, Rutten Isabelle, and Vansteenkiste Johan F (2004) Early versus late chest radiotherapy in patients with limited-stage small cell lung cancer. Cochrane Database of Systematic Reviews (4),
- Pijls-Johannesma M, De Ruysscher, D, Vansteenkiste J, Kester A, Rutten I, and Lambin P (2007) Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treatment Reviews 33(5), 461–73 [PubMed: 17513057]
- Qiao Tk, Zhou Da, Xin L, Shu L, and Wu W (2004) Concurrent radiotherapy combined with carboplatin and etoposide in limited stage small cell lung cancer. Zhonghua jie he he hu xi za zhi [Chinese journal of tuberculosis and respiratory diseases] 27(4), 237–239 [PubMed: 15144613]
- Samson D J, Seidenfeld J, Simon G R, Turrisi A T, 3rd, Bonnell C, Ziegler K M, Aronson N, American College of Chest, and Physicians (2007) Evidence for management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132(3 Suppl), 314S–323S [PubMed: 17873177]
- Sculier J P, Lafitte J J, Efremidis A, Florin M C, Lecomte J, Berchier M C, Richez M, Berghmans T, Scherpereel A, Meert A P, Koumakis G, Leclercq N, Paesmans M, Van Houtte, P, European Lung Cancer Working, and Party (2008) A phase III randomised study of concomitant induction radiochemotherapy testing two modalities of radiosensitisation by cisplatin (standard versus daily) for limited small-cell lung cancer. Annals of Oncology 19(10), 1691–7 [PubMed: 18504252]
- Seidenfeld J, Samson D J, Bonnell C J, Ziegler K M, and Aronson N (2006) Management of small cell lung cancer. Evidence Report/Technology Assessment (143), 1–154 [PMC free article: PMC4781398] [PubMed: 17764204]
- Sheikh H, Colaco R, Lorigan P, Blackhall F, Califano R, Ashcroft L, Taylor P, Thatcher N, and Faivre-Finn C (2011) Use of G-CSF during concurrent chemotherapy and thoracic radiotherapy in patients with limited-stage small-cell lung cancer safety data from a phase II trial. Lung Cancer 74(1), 75–9 [PubMed: 21353720]
- Work E, Nielsen O S, Bentzen S M, Fode K, and Palshof T (1997) Randomized study of initial versus late chest irradiation combined with chemotherapy in limited-stage small-cell lung cancer. Aarhus Lung Cancer Group. Journal of Clinical Oncology 15(9), 3030–7 [PubMed: 9294465]
- Ye T, Geng C, Chen H-L, Wang Q, and Zhang X-G (2011) Three-dimensional conformal radiotherapy or intensity-modulated radiotherapy combined with concurrent sequential chemotherapy for limited stage small cell lung cancer. Chinese journal of cancer prevention and treatment 18(15), 1195–1197+1203
Health Economic studies – Included
None
Health Economic studies – Excluded
None
Final
Evidence reviews
These evidence reviews were developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Review Surgery for limited-stage small-cell lung cancer.[Cochrane Database Syst Rev. 2017]Review Surgery for limited-stage small-cell lung cancer.Barnes H, See K, Barnett S, Manser R. Cochrane Database Syst Rev. 2017 Apr 21; 4(4):CD011917. Epub 2017 Apr 21.
- Guideline for the Initial Management of Small Cell Lung Cancer (Limited and Extensive Stage) and the Role of Thoracic Radiotherapy and First-line Chemotherapy.[Clin Oncol (R Coll Radiol). 2018]Guideline for the Initial Management of Small Cell Lung Cancer (Limited and Extensive Stage) and the Role of Thoracic Radiotherapy and First-line Chemotherapy.Sun A, Durocher-Allen LD, Ellis PM, Ung YC, Goffin JR, Ramchandar K, Darling G. Clin Oncol (R Coll Radiol). 2018 Oct; 30(10):658-666. Epub 2018 Jul 11.
- Review Chemotherapy versus best supportive care for extensive small cell lung cancer.[Cochrane Database Syst Rev. 2013]Review Chemotherapy versus best supportive care for extensive small cell lung cancer.Pelayo Alvarez M, Westeel V, Cortés-Jofré M, Bonfill Cosp X. Cochrane Database Syst Rev. 2013 Nov 27; (11):CD001990. Epub 2013 Nov 27.
- Review Primaquine for preventing relapse in people with Plasmodium vivax malaria treated with chloroquine.[Cochrane Database Syst Rev. 2013]Review Primaquine for preventing relapse in people with Plasmodium vivax malaria treated with chloroquine.Galappaththy GN, Tharyan P, Kirubakaran R. Cochrane Database Syst Rev. 2013 Oct 26; 2013(10):CD004389. Epub 2013 Oct 26.
- Review Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care.[Health Technol Assess. 2001]Review Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care.Marshall M, Crowther R, Almaraz-Serrano A, Creed F, Sledge W, Kluiter H, Roberts C, Hill E, Wiersma D, Bond GR, et al. Health Technol Assess. 2001; 5(21):1-75.
- Evidence reviews for the most clinically and cost-effective regimen of chemoradi...Evidence reviews for the most clinically and cost-effective regimen of chemoradiotherapy for people with limited-stage SCLC
Your browsing activity is empty.
Activity recording is turned off.
See more...