Guiding Question 1. 1a. For which cancers has stereotactic body radiation therapy been used?
SBRT can be used as a primary therapy for early stage cancer or as a targeted treatment for metastatic disease. In the latter setting, SBRT is intended to be an adjuvant cytoreductive treatment in concert with ongoing systemic therapy.18 Based on our literature search, SBRT has been used for tumors located in the lung/thorax, pancreas, liver, colon, uterus, pelvis, sacrum, kidney, prostate, and thyroid. The bulk of the studies identified in our searches were for tumors of the lung/thorax (k = 68).19–86 Details for these studies can be found in Appendix L, Results for Guiding Question 3.
1b. What are the theoretical advantages and disadvantages of stereotactic body radiation therapy compared to other radiation therapies that are currently used for cancer treatment?
Standard Fractionated Radiotherapy
The goal of external beam radiation therapy (EBRT) is to deliver the prescribed amount of radiation to the targeted tumor and minimize the amount of radiation received by surrounding normal tissues. EBRT can be used as a therapeutic or palliative treatment and is delivered using linacs and various conformal techniques. Technology for the delivery of external radiation therapy includes two-dimensional (2D) conformal radiation therapy (RT), three-dimensional (3D) conformal RT, intensity modulated RT (IMRT), SBRT, proton therapy, carbon ion therapy, and electron therapy87 (proton, electron, and carbon ion therapy are outside the scope of this Technical Brief.) AHRQ has recently commissioned a Technical Brief on proton therapy.88
Two-dimensional radiation therapy(2D -CRT), which uses images from plain x-rays and fluoroscopy for planning purposes, delivers radiation beams of uniform intensity from one to six directions or arcs to the tumor.89 Anatomical landmarks or fiducials help determine the location of the tumor. Three-dimensional conformal radiation therapy(3D -CRT), on the other hand uses three dimensional images from computed tomography, positron emission tomography or magnetic resonance imaging for treatment planning. 3D-CRT uses computer software and 3D imaging techniques (from a CT simulator) to display the size, shape, and location of the tumor.90 The treatment planning team can determine the size and shape of the radiation beam to fit the targeted tumor by using a multi-leaf collimator (MLC) or custom fabricated field-shaping blocks.90 IMRT is a type of therapy in which the leaves of the MLC can be moved while the radiation beam is “on” variably blocking parts of the field to increase the intensity of some of the beamlets and decrease the intensity of others.91 IMRT involves advanced treatment planning algorithms which allow the physician to input the desired radiation treatment dose constraints for the targeted tumor and the surrounding normal tissue into a computer. The computer software is used to develop a detailed treatment plan of the radiation beams required to deliver the prescribed radiation dose. Multiple iterations may be necessary to optimize the treatment plan. IMRT systems can shape the photon (x-ray) beam through step and shoot and/or dynamic MLCs (computer controlled). As a result, the beam intensity more closely matches the thickness of the tumor. The EBRT treatment plan is reviewed and agreed upon by members of the treatment team (radiation oncologist, medical physicist, etc.) before the procedure can begin.
Differences Between Standard Fractionated Radiotherapy and SBRT
EBRT is a noninvasive procedure for patients undergoing cancer treatment. Patients are allowed to return to daily activities after the completion of the procedure, and most patients do not require any type of sedation to aid in immobilization during treatment. Patients are advised to complete scheduled follow-up procedures, as results of the treatment may not be visible during first follow-up visits. The importance of imaging techniques, accurate planning techniques, and accurate dose distribution are relevant for all forms of EBRT delivery. Minimizing the exposure of surrounding normal tissues from the radiation dose is also important for all forms of EBRT delivery. However, the ability to shape the radiation beam to the targeted tumor varies among EBRT delivery technologies.
Table 1
Radiation delivery techniques.
With advances in technology and dose planning, the prescribed dose can be more closely tailored to the tumor volume with greater sparing of surrounding anatomy. The complexity of the treatment plan will depend on the tumor characteristics, surrounding tissue and goal of the treatment (palliative or therapeutic). 2D-CRT and 3D-CRT tend to include larger margins of surrounding normal tissue because of treatment-planning limitations. This may limit the total radiation dose that can be delivered to the target and may decrease the ability to treat the targeted tumor. Treatment planning for IMRT takes into account the dose constraints of the targeted tumor and the surrounding normal tissues with the goal of varying intensities across the treatment field. SBRT uses orthogonal x-ray beams to locate the targeted tumor, and several radiation beams that are finely collimated and that intersect to deliver a conformal, single, high dose of radiation.
When the treatment team determines that a patient is not a candidate for a single high dose treatment based on tumor location and size, tumor motion, and radiosensitivity, fractionated treatment is an option. The number of treatment fractions and overall length of treatment depends on the ability to conform the radiation beam to the shape of the tumor and to protect surrounding normal tissue and organs at risk from the radiation dose. As the number of fractions increase, the dose per fraction decreases. 2D-CRT, 3D-CRT, and IMRT are typically delivered in many more fractions than SBRT. Typical treatment fractions for 2D-CRT, 3D-CRT, and IMRT are 25–50 fractions delivered five days per week for approximately 5–10 weeks. A typical daily dose is approximately 2Gy per fraction. When these small doses are given repeatedly, the cumulative dose may not be as potent as an equivalent single fraction dose, so a higher overall dose is delivered. IMRT can also be used to deliver SBRT(1 –5 fractions of a high dose). The two IMRT delivery methods are differentiated by the terms “conventional fraction IMRT” versus “SBRT-based IMRT.” Because SBRT delivers a high dose of radiation (20–60Gy), treatment can be completed in 1–5 fractions delivered in a few days (e.g., 1–5 days).
SBRT’s most important features and theoretical advantages compared to other forms of EBRT are the high degree of dose conformality, the use of high -dose radiation, the delivery of a single or very few fractions (thus decreasing the overall length of treatment), and an improved treatment response.5 However, SBRT can also be difficult to administer because of the high level of accuracy required, interfraction or intrafraction movements within the body (e.g., respiratory movements) and movements of the body.5 Similar to other forms of EBRT, SBRT can be used in combination with chemotherapy, and sometimes after other radiation therapy (RT) interventions.92 As with other radiation treatments, geographic misses of the targeted tumor causes damage to surrounding healthy tissues. However, because each SBRT radiation fraction is a higher dose compared to other forms of EBRT, there is greater potential for radiation injury.9
1c. What are the potential safety issues and harms of the use of stereotactic body radiation therapy?
Quality Assurance and Quality Control of SBRT Treatment
SBRT is a high-dose radiation treatment. For high-dose radiation treatments, errors in radiation dose and spatial positioning must be minimized.10 SBRT treatments can be difficult to plan because tumors located within the body may move periodically (e.g., respiratory movement), irregularly (e.g., peristalsis), or with shrinkage of the tumor between fractionated treatments.
The quality assurance (QA) for SBRT must go beyond physical measurements and include a proper review of individual patient data (e.g., results of treatment).93 An essential part of SBRT is the strict quality control of the tumor images and the regular verification of the image sets to maintain the accurate delivery of the prescribed dose. SBRT requires tight conformity of the prescription dose to the tumor volume, with rapid dose fall off.16 During treatment the targeted tumor can be tracked by methods such as respiratory gating or target tracking (monitoring the motion of the tumor). Before a patient is treated, phantoms can be used as part of the QA process to make sure these tracking techniques are measuring the tumor location and movement correctly.93
ACR/American Society for Radiation Oncology SBRT Guideline
In 2004, the American College of Radiology (ACR) and the American Society for Radiation Oncology (ASTRO) developed a practice guideline for the performance of SBRT. This guideline was revised in 2009. The purpose of the guideline is to provide guidance to practitioners considering using SBRT and to define quality criteria for the delivery of SBRT.1 The advanced training of personnel and the careful management of patients are the key aspects for performing SBRT safely. Appendix D lists the qualifications and responsibilities of the personnel, and Appendix E provides a snapshot of the suggestions within the guideline for procedure specifications, quality control of accessories, quality control of images, quality control for the treatment-planning system, simulation and treatment, and followup. This guideline does not specify physician specialties (e.g., surgeons) for SBRT applications. However, specialists (e.g., surgeons) may play an integral role in the treatment process.
Guiding Question 2. 2a. What specialized instrumentation is needed for stereotactic body radiation therapy and what is the FDA status of this instrumentation?
Linacs
SBRT can be delivered by dedicated and nondedicated linacs. These systems may require patient immobilization and/or a method to account for any organ motion during treatment. Nondedicated systems are capable of performing conventional radiation therapy, IMRT, along with SBRT, while dedicated systems are for SBRT treatments alone. Advanced patient positioning, patient immobilization, x-ray tracking (stereotactic), advanced control systems, and treatment-planning software are other requirements for linac modification when performing an SBRT treatment. SBRT can be delivered via a step and shoot method or by dynamic delivery.5 Step and shoot delivery turns the radiation beam off when the gantry rotates to the next planned delivery angle. The use of dynamic delivery enables continuous delivery of the radiation beam by adjusting the MLC as the gantry rotates. Advantages of dynamic delivery include a decrease in treatment time, less organ movement during the treatment session, and an increase in patient throughput.5,10
A listing of 12 commercially available systems with identifiable features can be found in Appendix F, Currently Marketed Devices for SBRT. Accessories sold with or incorporated into linacs (nondedicated) include multi-leaf collimators (MLC) and micro-MLCs. MLCs consist of individual leaves usually made of tungsten alloy, which may be mounted to or integrated in to the linac. MLC leaf widths typically range from 5 mm to 10 mm. Micro-MLCs have leaf widths ranging in size from 1 mm to 4 mm94 and generally use smaller treatment fields than MLCs (see listing of available linac-based SBRT accessories including MLC sizes in Appendix G, Linac-based SBRT Accessories. In Appendix M, we provide the details on the energy source, beam angles, collimation techniques, body immobilization systems, imaging used for treatment planning, treatment planning systems, tumor tracking, respiratory tracking and image guidance during treatment as reported in the studies included for Guiding Question 3. Various manufacturers were contacted to provide further detail on the devices that are capable of performing an SBRT treatment and accessories used with those devices. The information provided included treatment-planning and treatment-delivery techniques and necessary equipment and software. For more information, see Appendix N, Responses from Device Manufacturers on Device Specifications and Compatible Accessories (January 2010).
FDA Status of SBRT Equipment
SBRT devices are regulated by the FDA under the 510(k) process. Most of these devices are generally cleared for marketing for treatment of lesions, tumors, and conditions anywhere in the body. Indications currently approved by the FDA’s Center for Devices and Radiological Health (CDRH) as well as marketing clearance information including 510(k) applicant/number, product code, and approval dates are provided in Appendix H, Applicant’s FDA 510(k) Information. Devices and accessories used for the administration of SBRT can be accessed by searching the following CDRH codes: IXI, IYE, and MUJ. Information was captured by a search of the manufacturers Web sites (Appendix I, Manufacturer Web sites)and a search of the FDA’s CDRH (http://www.fda.gov/cdrh/).
2b. What is an estimate of the number of hospitals that currently have the capability for stereotactic body radiation therapy in the United States?
According to the 2009 Edition of the American Hospital Association (AHA) Guide,95 approximately 700 facilities claim to administer SRS in the United States. Of these 700, we identified 384 facilities describing capability to perform SBRT. This information was accessed by visiting the Web sites provided in the AHA guide and through manufacturer Websites (see Appendix I, Manufacturer Web sites). An overall listing of these 384 facilities, including specific body sites treated and devices employed can be found in Appendix J, Facilities Performing SBRT for Solid Tumors. Information from Web sites was updated in September 2009.
2c. What instrumentation technologies are in development?
The Gyro Knife, manufactured by GammaStar Medical Group Ltd., is commercially available in the European Union having recently received the CE certification for European Union, medical devices.11 The device, featuring a Cobalt 60 radioactive source and two vertical rotating gyros, currently awaits clearance by FDA.
Guiding Question 3. Evidence Base
The goal of this systematic literature scan was to provide an overview of the studies of SBRT, not to evaluate the quality of the studies or to perform analysis of the data reported by the studies. We have screened the titles of 5,585 citations to determine if the abstract should be reviewed. A total of 1,588 abstracts were screened, and 550 full-text articles were ordered for further review. In total, 124 studies were relevant to the topic and data extraction was performed (see Figure 1). The included studies can be found in Appendix B. The excluded studies, along with reason for exclusion, can be found in Appendix C.
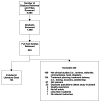
Figure 1
Study selection process.
The included studies have been organized into two tables by whether they were prospective or retrospective(see Appendix L, Results for Guiding Question 3). These tables are organized by year of publication (most recent year first), and then alphabetically by author. The study details covered within the tables include author; year; cancer type; instrumentation; algorithms; study design; study size; prior and/or concurrent treatment; length of followup in months; outcomes measured; and adverse events. Patient inclusion criteria have not been included in these tables, but are presented in Table 2 in section 3.a.
Table 2
Patient inclusion criteria summary.
3a. Type of cancer and patient inclusion criteria
Our search results identified studies of the use of SBRT for tumors located in the lung/thorax, pancreas, liver, colon, uterus, pelvis, sacrum, kidney, prostate, and thyroid. The bulk of the studies were for tumors of the lung/thorax (k = 68).19–86 We found 27 studies for tumors located in the pancreas, liver, colon,96–122 and fewer than 10 studies each for sites within uterus, pelvis, sacrum,123–127 kidney,128–133 prostate, 134–140 and thyroid.141 There were 10 studies that included multiple treatment sites within the study.142–151
Patient inclusion criteria for SBRT treatment varied based on cancer types and individual studies. Criteria commonly used regardless of cancer type include inoperable tumors or patients refusing surgery; biopsy-proven disease; minimum life expectancy; no prior RT or prior RT received at a minimum length of time before SBRT; and a minimum level of performance on the Karnofsky or World Health Organization (WHO)/Eastern Cooperative Oncology Group (ECOG) scales. The Karnofsky Performance Status (KPS) scoring system measures the cancer patient’s abilities to perform ordinary tasks. The scoring system ranges from 0 to 100, with a higher score indicating a better ability to perform tasks.152 The retrieved studies often reported KPS scores of at least 40. The WHO/ECOG performance status assesses a patient’s functional and/or physical performance. There are six codes used to evaluate a patient, and the codes seen within the retrieved studies were between 0 (fully active) and 2 (ambulatory, capable of self-care but unable to carry out work activities).153 Since the level of detail of patient inclusion criteria varied with each study, we have provided an overview of the criteria frequently reported within the studies for each cancer type. Table 2 below also lists the number of studies retrieved and total number of patients.
Based on our search results, the majority of studies (k = 49) performed since 2000 were in the United States for tumors located in the lung/thorax. Germany and Japan have also performed several lung studies in the past 10 years (see Table 3).
Table 3
Country and number of cancer types.
3b. Type of radiation and instrumentation and algorithms used
Photon radiation was used in all included studies for SBRT treatment. The instrumentation reported in all studies included modified linacs (k = 47), CyberKnife(k = 39), Novalis Shaped Beam or Clinac (k = 16), Body GammaKnife (k =1), Tomotherapy Hi-Art (k= 2), FOCAL unit (k = 1), and Synergy systems(k = 6). Algorithms are used to plan and deliver treatment. The studies reported inverse treatment planning algorithms, pencil beam algorithms for dose calculation, and tissue maximum ratio calculation algorithms. Most of the studies described the device and photon energy, radiation beam angles, collimation technique, body immobilization technique, treatment planning imaging, treatment planning system/algorithm, tumor tracking, respiratory tracking/control, and image guidance during treatment (Appendix M, Literature Results Device Specifications). The number and type of radiation beams delivered during treatments included 1–12 conformal and/or nonconformal beams. The studies reported various body immobilization techniques including Smithers Medical Alpha Cradle (k = 16) and Elekta’s stereotactic body frame (k = 26). CT, MRI, and PET imaging scans were often used to plan treatment. Treatment planning was conducted on software systems typically specific to the device used during treatment. Elekta’s Render 3D, Varian’s CadPlan and Eclipse, BrainLABs BrainScan systems, CyberKnife planning system, Philips Medical Systems Pinnacle Treatment Planning System (TPS), CMS Focus or Xio, and MDS Nordion Helax were the treatment planning systems most often reported. Studies reported breath-holding, respiratory gating (radiation beam turns on/off during respiratory cycle), and abdominal compression techniques to control respiratory movement. Lastly, the type of image guidance (MV or kV) utilized during treatment (e.g., just before treatment begins) included CT, cone-beam CT, and orthogonal x-rays For more information, see Appendix M, Literature Results Device Specifications.
SBRT doses and fractions varied based on factors such as the type of cancer and location of tumor. According to ACR/ASTRO’s SBRT definition1 and the AMA CPT codes,8 SBRT is categorized as a treatment delivered in 1–5 fractions. Typically, doses were delivered in one to five fractions. Fourteen studies delivered treatment in more than five fractions, and also considered this to be SBRT. Table 4 lists the 14 studies delivering hypofractionated (more than five fractions) SBRT (also located in Appendix L, Results for Guiding Question 3) alphabetically by author, and details the cancer type, study design, study size (n), instrumentation/algorithms, and total dose (Gy)/number of fractions.
Table 4
Hypofractionated stereotactic body radiation therapy.
3c. Study design and study size
Study designs for SBRT include prospective, single-group studies and retrospective studies. Patient populations were heterogeneous across the cancer types. Study populations included as few as three patients for a prospective, single-group study and as many as 398 for a retrospective study. Table 5, below, lists the smallest and largest patient populations for the studies within each cancer type, and the type of studies conducted for each cancer type. We have also calculated an overall mean and median age for patients in the studies within each cancer type (see Table 6).
Table 5
Study designs and sizes.
Table 6
Overall Mean and Median (Range) for Age.
3d. Comparator used in comparative studies
There were no included studies that compared SBRT to another form of radiation treatment. To date, the largest literature base for SBRT is treatment in the lung/thorax, but these were all single-group studies. We searched www.clinicaltrials.gov and identified 50 ongoing SBRT trials (see Appendix K, Ongoing Clinical Trials). The trials include metastatic breast cancer, biliary tract cancer, kidney cancer, liver cancer, lung cancers(principally non -small cell lung cancer), pancreatic cancer, prostate cancer, and unspecified treatment sites.
Only one of these ongoing trials involves a direct comparison of SBRT to a different form of radiation therapy. This trial commenced in April 2009 in France (NCT00870116), and is a nonrandomized comparison of SBRT delivered by CyberKnife vs. SBRT delivered by linac vs. conformational RT for treatment of NSCLC. The primary outcome measure is local control, and planned enrollment is 120 patients. There are three other comparative trials which plan to use historical controls, one for metastatic breast cancer (NCT00167414), one in NSCLC (NCT00727350) and one in pancreatic cancer (NCT00350142). One of the lung cancer trials based in the Netherlands (NCT00687986) is a randomized study comparing SBRT to primary resection. The primary outcomes include local control, regional control, quality of life (QoL), and treatment costs. The estimated enrollment is 960 patients and is set for completion in December 2013. Another trial being conducted in China (NCT00840749) will compare SBRT to surgical resection in NSCLC. The enrollment target is 1030 patients, with planned completion in 2013. Another trial (NCT00843726) being conducted in Roswell, NY, will randomize 98 patients to either one or three fractions of SBRT for treatment of NSCLC.
3e. Concurrent and/or prior treatments used
The prior and concurrent treatments used varied with each study based on the population evaluated, and on inclusion and exclusion criteria (see the tables in Appendix L, Results for Guiding Question 3). Some studies included patients with prior and/or concurrent treatment, while other studies excluded patients with prior or concurrent treatment. Prior treatments reported include surgery, radiation therapy (e.g., IMRT, brachytherapy), pharmaceuticals (e.g., tamoxifen), and/or chemotherapy. Some studies specified that prior radiation therapy or chemotherapy had to be completed within a certain timeframe before SBRT (e.g., at least 12 weeks prior to SBRT). Chemotherapy was the concurrent treatment most often reported within the studies.
3f. Length of followup
The individual study length of followup was reported as a mean, median, and/or range. We have calculated an overall mean and median for the length of followup for each cancer type. The shortest mean and median followup was within the multiple site category (12.9 and 8.2 months [1–95 months] respectively). Studies of the tumors involving the pelvis, sacrum, and uterus had the longest mean/median followup (31 and 33 months [range 2–77 months]). Table 7 lists the cancer types and the calculated overall mean, median, and range of followup across studies within each cancer type.
Table 7
Overall mean and median followup.
3g. Outcomes measured
The outcomes measured typically included tumor control or tumor response, toxicity, and overall survival. Overall cause-specific survival rates (chances of death due to cancer at a defined time point), overall survival rates (chances of death due to cancer and/or other complications at a defined time point), and disease-free survival rates were typically calculated using the Kaplan-Meier method. Most studies used the following four criteria to measure tumor control or tumor response: complete response (disappearance of tumor), partial response (percentage of decrease in tumor size), stable disease (smaller percentage change than with partial response), and progression of disease (increase in tumor size). The percentages of tumor response varied with each study. Table 8 provides a summary of the types of outcomes measured within each cancer type.
Table 8
Summary of outcomes measured.
Evaluating the extent of cell destruction caused by SBRT can be a difficult task, as older calculation methods(e.g., linear quadratic model [LQ]) were developed for use with conventional radiation therapy. The LQ model assumes there are two components of radiation-induced cell destruction—one component proportional to dose and one component proportional to the square of the dose.16 The application of the LQ model for low dose conventional fractions may not have the same consequences as the use of the model with SBRT. The LQ model possibly overestimates cell destruction, and it may not describe the cell survival curve for the high doses of SBRT properly.16 Making comparisons between studies for SBRT can also be challenging. Studies may report equivalent prescription doses; however, differences in fractionation schedules can result in a substantial difference in the biologically effective dose (BED).16 The BED is an index that can serve as a useful parameter for comparing the potency of two different fractionation schedules.18
3h. Adverse events, harms, safety issues reported
Radiation Therapy Oncology Group criteria and Common Toxicity Criteria version 2.0 (RTOG/CTC) were typically used to grade acute and late toxicity at each followup. In general, Grade 1 toxicities require no treatment, Grade 2 toxicities require medication or a simple intervention, Grade 3 toxicities have more severe symptoms and require more complex interventions, and Grade 4 toxicities can be life threatening.135 Some studies reported acute versus late complications; however, they did not always specify complications related to individual patients. Some of the most frequently reported adverse events include pain, fatigue, nausea, bleeding, and diarrhea. Some of the patients in these studies had prior cancer treatment and received SBRT for recurring cancers, and some patients had comorbid conditions. Toxicities for large radiation doses are predominantly late occurrences which take more time to observe. Therefore, longer followup will be required for wider acceptance of SBRT.9
A total of 11 studies did not report any adverse events for tumors located at the following sites: one kidney,131 six lung,29,35,52,61,77,157 two multiple sites,148,150 and two GI (colon, liver, pancreas).100,103 Also, some studies did not report adverse events using the RTOG/CTC scale, or stated that investigators and/or patients observed or reported no adverse events. One study of SBRT for renal cell cancer stated that there were no adverse effects of treatment.131 Table 9 summarizes the reported adverse events for the studies within each cancer type.
Table 9
Summary of adverse events.
Publication Details
Copyright
Publisher
Agency for Healthcare Research and Quality (US), Rockville (MD)
NLM Citation
Tipton KN, Sullivan N, Bruening W, et al. Stereotactic Body Radiation Therapy [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 May. (Comparative Effectiveness Technical Briefs, No. 6.) Results.
