NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | 177Lu-Labeled ethylenediamine tetramethylene phosphonic acid |
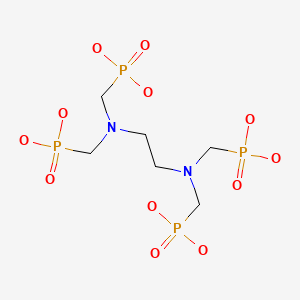
|
| Abbreviated name: | [177Lu]-EDTMP | |
| Synonym: | (ethylenedinitrilo)-tetramethylenephosphonic acid | |
| Agent Category: | Compound | |
| Target: | Bone (hydroxyapatite) at low concentration; farnesyl disphosphate (pyrophosphate) synthase (molecular target) at high concentration | |
| Target Category: | Enzyme | |
| Method of detection: | Single-photon emission computed tomography (SPECT); gamma planar imaging | |
| Source of signal / contrast: | 177Lu | |
| Activation: | No | |
| Studies: |
| Click on the above structure of EDTMP for additional information in PubChem. |
Background
[PubMed]
Most patients with malignancies of the breast, prostate, lungs, thyroid, or kidneys suffer from severe bone pain due to metastases of the cancer to the skeletal tissue (2, 3). Although several interventions such as analgesics, bisphosphonates, hormone therapy, and systemic radionuclide therapy are available to manage the pain, these treatments are known to have many undesirable secondary effects on the patient (3). Radiopharmaceuticals containing nuclides such as strontium-89 (as 89SrCl2) and samarium-153 (administered as 153Sm-labeled ethylenediamine tetramethylene phosphonic acid (EDTMP)), which have been approved by the United States Food and Drug Administration for the treatment of bone pain due to metastases, are commonly used for palliative care of pain in the bones of cancer patients (3). However, these are not ideal agents to treat bone pain because the radionuclide either has a long half-life and generates high-energy β– particles (89Sr has a half-life of ~50 days; Eβ(max) = 1.49 MeV) or is short-lived and has to be produced in close vicinity to the treatment center (153Sm has a half-life of ~47 h; Eβ(max) = 0.81 MeV; Eγ = 103 keV (28%)) (2). A major limitation of using these bone pain palliative agents is that they produce myelotoxicity in some patients (4). Between the two labeled compounds, 89SrCl2 appears to be the agent of choice for clinical applications because its longer half-life allows some flexibility to develop a suitable treatment regimen for the patient. There is great interest in the development of alternative radiolabeled compounds that can be used to treat pain resulting from osseous metastases (3). An important characteristic of this labeled compound is that it must have the ability to be targeted specifically to the cancerous lesions on the skeleton and should be minimally toxic to the bone marrow as discussed elsewhere (4-6).
In an earlier study with healthy rats, it was reported that EDTMP labeled with lutetium-177 ([177Lu]-EDTMP) was cleared rapidly from blood circulation, showed little uptake in the soft tissues, and accumulated primarily in the bones of these animals (7). Chakraborty et al. made similar observations when they investigated the biodistribution of [177Lu]-EDTMP in rats (8). A freeze-dried kit for the preparation of [177Lu]-EDTMP was developed subsequently by Garnuszek et al. (9). On the basis of these observations, there is a renewed interest to use 177Lu (half-life, ~7 days; Eβ(max) = 497 keV; Eγ = 113 keV (6.4%); 208 keV (11%)) as an alternative nuclide to those currently in use (89Sr and 153Sm) in the development of a palliative care agent for pain due to the metastases of cancer to the skeletal tissue (5, 6). The main advantages of using 177Lu are that it can be easily transported to places where it is not available and the low-energy gamma photons emitted by the nuclide allow detection of the bone lesions with scintigraphy. The biodistribution of [177Lu]-EDTMP was studied recently in mice, rats, and rabbits, and scintigraphic imaging was performed on rodents, rabbits, and dogs (5, 6). In addition, the International Atomic Energy Agency has initiated projects to develop 177Lu-labeled compounds as palliative care agents for bone pain (6).
Other Sources of Information
Related chapters in MICAD
FDA-approved clinical trials with EDTMP, 89SrCl2, and 153Sm complexes
Clinical trials with other-bone imaging agents
Protein and mRNA sequence of human farnesyl diphosphate synthase (also known as geranylgeranyl diphosphate synthase 1)
Gene information regarding human farnesyl diphosphate synthase (GeneID: 2224)
Farnesyl diphosphate synthase in Online Mendelian Inheritance in Man (OMIM)
Structure of farnesyl diphosphate synthase complexed with a bisphosphonate
Farnesyl diphosphate synthase in Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways
Other publications regarding EDTMP in PubMed
Synthesis
[PubMed]
The synthesis of [177Lu]-EDTMP has been described by Chakraborty et al. (5, 8). The radiochemical yield and purity of the labeled compound were reported to be >98% and >99.0%, respectively, as determined with paper chromatography (Rf of [177Lu]-EDTMP was ~1, whereas 177LuCl3, the labeling agent, stayed at the origin) and paper electrophoresis (only [177Lu]-EDTMP moved toward the anode). The specific activity of the radiochemical was not reported.
The development of a freeze-dried kit containing sodium EDTMP, stannous chloride, and ascorbic acid for the preparation of [177Lu]-EDTMP was reported by Garnuszek et al. (9). The yield of the final labeled product prepared from this kit was >99% as determined with paper chromatography and paper electrophoresis. The specific activity of the radiolabeled compound varied from 3.2 GBq/mg (86 mCi/mg) to 390 GBq/mg (10.5 Ci/mg) depending on the specific activity of the 177LuCl3 used to prepare the tracer.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The [177Lu]-EDTMP complex was reported to retain its stability at room temperature for at least 14 days (8). Investigators have also reported that [177Lu]-EDTMP prepared from a freeze-dried kit had a purity of >99% over a period of 10 days (9). Chakraborty et al. reported that [177Lu]-EDTMP maintained a purity of >99% at room temperature for at least 30 days under in vitro conditions (5). In another study, incubation of [177Lu]-EDTMP in the presence of 0.1 M hydrochloric acid was shown to reduce its purity to <70% within 24 h (10).
Animal Studies
Rodents
[PubMed]
In a biodistribution study with normal rats (n = 3–5 animals/time point), radioactivity from [177Lu]-EDTMP was reported to clear rapidly from blood circulation and selectively accumulate in the skeletal tissue of the animals (7). This observation was confirmed by several investigators (2, 5, 8-10). In all these studies, only small amounts of radioactivity were detected in the soft tissue of the animals. In addition, a similar biodistribution pattern was observed in rats injected with other 177Lu-labeled phosphonates (diethylenetriamine pentamethylene phosphonate, triethylene tetraamine hexamethylene phosphonate, and 1,4,7,10-azacyclododecane-1,4,7,10-tetramethylene phosphonic acid) (8, 10).
Single-photon emission computed tomography (SPECT) imaging of rats injected with [177Lu]-EDTMP showed that the tracer was clearly present in all bones and that greater amounts of the label were visible in bones undergoing remodeling (e.g., the back bone and the knee joints of the animals) (10). Similar observations were reported by Mathe et al. (6).
Approximately 41% of the injected radioactivity from [177Lu]-EDTMP was shown to accumulate in the bones of mice (n = 5 animals/time point) at 2 h postinjection (p.i.) (6). In this study, little radioactivity was found in soft tissues of the animals compared to the bones. In another study, mice (n = 3 animals) were injected with [177Lu]-EDTMP through the tail vein, euthanized 3 days later, and frozen in liquid nitrogen (6). Whole-body autoradiography of cryostat sagittal sections prepared from the frozen animals showed that radioactivity was present primarily on the surface and remodeling areas of the bone. Negligible uptake of the label was observed in the internal spongiosa areas of the skeletal tissue.
Other Non-Primate Mammals
[PubMed]
The biodistribution of [177Lu]-EDTMP was investigated in healthy white New Zealand rabbits (n = 3–5 animals/time point) after an injection of the tracer through the ear vein (6, 10). Accumulation of the radiochemical was observed primarily in the bones of the animals, and only small amounts of radioactivity were detected in other tissues, similar to observations from the studies with the mice and rats (see above). Scintigraphy of rabbits injected with [177Lu]-EDTMP showed that uptake of the tracer in the skeleton was apparent within 1 h p.i., and the complete skeleton was visible by 3 h p.i (5). Radioactivity in the bones of the rabbits was reported to remain visible for at least 4 weeks p.i (6).
Healthy Beagle dogs were injected intravenously with [177Lu]-EDTMP, and whole-body SPECT imaging from 30 min up to 28 days p.i. showed that the label was visible in the skeleton within 3 h p.i., and no accumulation of radioactivity was observed in other major organs or tissues of the animals (5). Similar results were reported by Mathe et al., who performed SPECT imaging studies with Beagle dogs (6).
On the basis of the results presented above, [177Lu]-EDTMP can be considered a suitable tracer for the possible initiation of a multicenter human clinical trial coordinated by the International Atomic Energy Agency (6).
References
- 1.
- Slavicek J.M., Hayes-Plazolles N. The Lymantria dispar nucleopolyhedrovirus contains the capsid-associated p24 protein gene. Virus Genes. 2003;26(1):15–8. [PubMed: 12680688]
- 2.
- Das T. et al. (170)Tm-EDTMP: a potential cost-effective alternative to (89)SrCl(2) for bone pain palliation. Nucl Med Biol. 2009;36(5):561–8. [PubMed: 19520297]
- 3.
- Paes F.M., Serafini A.N. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Semin Nucl Med. 2010;40(2):89–104. [PubMed: 20113678]
- 4.
- Jansen D.R. et al. Targeted radiotherapy of bone malignancies. Curr Drug Discov Technol. 2010;7(4):233–46. [PubMed: 21034411]
- 5.
- Chakraborty S. et al. 177Lu-EDTMP: a viable bone pain palliative in skeletal metastasis. Cancer Biother Radiopharm. 2008;23(2):202–13. [PubMed: 18454689]
- 6.
- Mathe D. et al. Multispecies animal investigation on biodistribution, pharmacokinetics and toxicity of 177Lu-EDTMP, a potential bone pain palliation agent. Nucl Med Biol. 2010;37(2):215–26. [PubMed: 20152721]
- 7.
- Ando A. et al. 177Lu-EDTMP: a potential therapeutic bone agent. Nucl Med Commun. 1998;19(6):587–91. [PubMed: 10234664]
- 8.
- Chakraborty S. et al. 177Lu labelled polyaminophosphonates as potential agents for bone pain palliation. Nucl Med Commun. 2002;23(1):67–74. [PubMed: 11748440]
- 9.
- Garnuszek P. et al. Evaluation of a freeze-dried kit for EDTMP-based bone-seeking radiopharmaceuticals. Appl Radiat Isot. 2003;58(4):481–8. [PubMed: 12672628]
- 10.
- Chakraborty S. et al. Comparative studies of 177Lu-EDTMP and 177Lu-DOTMP as potential agents for palliative radiotherapy of bone metastasis. Appl Radiat Isot. 2008;66(9):1196–205. [PubMed: 18372188]
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review (177)Lu-Labeled methylene diphosphonate.[Molecular Imaging and Contrast...]Review (177)Lu-Labeled methylene diphosphonate.Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(170)Tm]-Labeled ethylenediamine tetramethylene phosphonic acid.[Molecular Imaging and Contrast...]Review [(170)Tm]-Labeled ethylenediamine tetramethylene phosphonic acid.Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Comparative studies on the potential use of (177)Lu-based radiopharmaceuticals for the palliative therapy of bone metastases.[Int J Radiat Biol. 2020]Comparative studies on the potential use of (177)Lu-based radiopharmaceuticals for the palliative therapy of bone metastases.Zakaly HMH, Mostafa MYA, Deryabina D, Zhukovsky M. Int J Radiat Biol. 2020 Jun; 96(6):779-789. Epub 2020 Mar 3.
- Formulation, preclinical evaluation, and preliminary clinical investigation of an in-house freeze-dried EDTMP kit suitable for the preparation of 177Lu-EDTMP.[Cancer Biother Radiopharm. 2014]Formulation, preclinical evaluation, and preliminary clinical investigation of an in-house freeze-dried EDTMP kit suitable for the preparation of 177Lu-EDTMP.Das T, Sarma HD, Shinto A, Kamaleshwaran KK, Banerjee S. Cancer Biother Radiopharm. 2014 Dec; 29(10):412-21.
- Multispecies animal investigation on biodistribution, pharmacokinetics and toxicity of 177Lu-EDTMP, a potential bone pain palliation agent.[Nucl Med Biol. 2010]Multispecies animal investigation on biodistribution, pharmacokinetics and toxicity of 177Lu-EDTMP, a potential bone pain palliation agent.Máthé D, Balogh L, Polyák A, Király R, Márián T, Pawlak D, Zaknun JJ, Pillai MR, Jánoki GA. Nucl Med Biol. 2010 Feb; 37(2):215-26. Epub 2009 Nov 12.
- 177Lu-Labeled ethylenediamine tetramethylene phosphonic acid - Molecular Imaging...177Lu-Labeled ethylenediamine tetramethylene phosphonic acid - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro