OVERVIEW
Introduction
Cannabidiol (CBD) is a non-psychoactive component of Cannabis sativa (marijuana), one of more than 80 cannabinoids identified in the plant, and the second most common constituent after Δ9-tetrahydrocannabinol (THC), the major psychoactive component. Cannabidiol is available as an FDA approved prescription medication (Epidiolex) used in moderately high doses for severe forms of epilepsy, but it is also widely available as over-the-counter CBD oils, gummies, topical creams or vaping solutions. High daily doses of cannabidiol are associated with frequent serum enzyme elevations during therapy, but has not been linked to cases of clinically apparent liver injury with jaundice. The lower doses of cannabidiol found typically in over-the-counter CBD products are generally well tolerated without evidence of liver injury.
Background
Cannabidiol (kan" a bi dye' ol) is a natural cannabinoid, the second most common component in Cannabis sativa (marijuana). Unlike the most abundant cannabinoid, delta-9-tetrahydrocannabinol (THC), the psychoactive ingredient of marijuana, cannabidiol has minimal psychoactive properties and may actually decrease the risk of psychotic symptoms and impaired cognition after cannabis use. The mechanism of action of cannabidiol is unknown, but it may be a partial agonist of cannabinoid receptors. Cannabidiol in moderately high doses was found to reduce the frequency of seizures in treatment-resistant epilepsy, particularly in patients with Lennox-Gastaut or Dravet syndrome, two rare but severe childhood onset forms of epilepsy. Cannabidiol was approved for use in the United States in 2018. Current indications are limited to treatment of seizures associated with Lennox-Gastaut or Dravet syndrome in adults and in children above the age of two. Cannabidiol is available as an oral solution of 100 mg/mL under the brand name Epidiolex. The typical dose is 2.5 mg/kg twice daily, which can be increased based upon tolerance and effect to 5 mg/kg twice daily and to a maximum of 10 mg/kg twice daily. The dose should be reduced in patients with preexisting hepatic impairment. Side effects are mostly dose related and can include fatigue, somnolence, dizziness, sleep disturbance, insomnia, anorexia, weight loss, diarrhea, infections and rash. Rare, but potentially severe side effects include marked sedation and somnolence, suicidal behavior and ideation and hypersensitivity reactions. Cannabidiol is generally used in combination with other anticonvulsants and is prone to drug-drug interactions that may affect drug levels and side effects of those agents, particularly valproate and clobazam.
Cannabidiol as found in over-the-counter products such as CBD oil, gummies or vaping solutions is largely unregulated and often of uncertain concentration and purity. Cannabidiol can be derived from hemp, a Cannabis sativa species with low levels of THC, typically <0.3%, compared to 15% in marijuana. Various forms of cannabidiol have been marketed as having analgesic, antiinflammatory, anxiolytic, antipsychotic and antiarthritic effects, but none of these have been definitely proven in prospective, properly designed studies. Furthermore, the concentration of CBD in most products is not known and varies widely as do low or trace amounts of THC and other contaminants. As a consequence, the purported activities of CBD may actually be effects of THC, accounting for the anecdotal reports of its effects on pain, anxiety, and sleep. The concentration of CBD in these nonprescription forms has generally been less than those in the FDA approved form used in epilepsy (Epidiolex) and daily doses are in the range of 25 to 300 mg daily. Prospective studies of CBD in daily doses of less than 400 mg have generally found few beneficial effects, but also few adverse events except for mild somnolence and dizziness.
Hepatotoxicity
In prelicensure studies, serum aminotransferase elevations arose during cannabidiol therapy for epilepsy in 34% to 47% of patients compared to 18% of controls who were receiving other anticonvulsant medications. Elevations above 3 times ULN occurred in 13% of cannabidiol treated compared to 1% on placebo. ALT and AST elevations were more frequent with higher doses and were particularly common (and sometimes delayed) in patients who were receiving valproate and clobazam. The aminotransferase elevations typically arose within the first two months of treatment and were transient, mild-to-moderate in severity, and not associated with symptoms or jaundice. There have been no convincing reports of clinically apparent liver injury with jaundice attributable to prescription forms of cannabidiol, but it has had very limited general use.
There have been few studies of liver test abnormalities during therapy with lower doses of CBD or with commercially available, over-the-counter CBD products. In doses between 200 and 400 mg daily, mild-to-moderate serum aminotransferase elevations have occasionally been reported, but there have been no reports of clinically apparent liver injury. Furthermore, liver injury arising in persons taking supplements with CBD must also consider the possibility of contaminants in the products or other herbal and dietary supplement use.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury, particularly with high doses).
Mechanism of Injury
The cause of serum aminotransferase elevations during cannabidiol therapy in doses used to treat epilepsy is not known, but may represent direct toxicity and be due to the molecule itself or to the production of a toxic intermediate in its metabolism. Cannabidiol is metabolized by the liver in large part by CYP 3A4 and is susceptible to drug-drug interactions with agents that induce or inhibit CYP 3A4 activity.
Outcome and Management
High dose cannabidiol therapy can be associated with serum ALT and AST elevations that generally arise within the first 2 months of treatment and are mild-to-moderate in severity. The frequency of elevations is dose related and more frequent when given in combination with valproate and clobazam. The elevations, however, are generally asymptomatic, self-limited in course and not associated with jaundice. Nevertheless, routine monitoring of liver tests is recommended before starting and at 1, 3 and 6 months during treatment as well as periodically thereafter, particularly in patients who are also receiving valproate. Cannabidiol should be discontinued if there are ALT elevations accompanied by symptoms of jaundice or if the levels rise and persist at more than 5 times ULN.
Drug Class: Anticonvulsants; Marijuana
Other Related Cannabinoid Agents: Dronabinol, Nabilone
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cannabidiol – Epidiolex®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
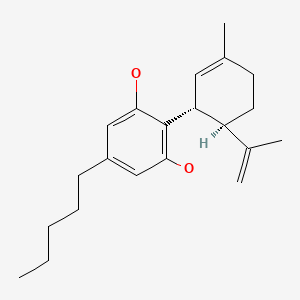
CHEMICAL FORMULA AND STRUCTURE
ANNOTATED BIBLIOGRAPHY
References updated: 16 February 2023
Abbreviations used: AIDS, acquired immune deficiency syndrome; CB, cannabinoids; CBD, cannabidiol; THC, Δ9 tetrahydrocannabinol.
- Zimmerman HJ. Antiemetic and prokinetic compounds. Miscellaneous drugs and diagnostic chemicals. In, Hepatotoxicity: The adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams and Wilkins, 1999, p. 721.(Expert review of hepatotoxicity published in 1999 does not discuss cannabidiol).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacotherapy of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2018/210365Orig1s000MedR.pdf. (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; cannabidiol caused dose related elevations in ALT arising in 52% of patients and to above 5 times ULN in 6%, occurring most frequently when given with valproate and sometimes with delayed onset, but no case was associated with concurrent significant bilirubin elevations). - Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–8. [PubMed: 26724101](Among 162 patients [ages 1 to 30 years] with intractable, childhood-onset epilepsy who were treated with cannabidiol for at least 12 weeks, the frequency of seizures decreased by 37% while adverse events included somnolence [25%], anorexia [19%] diarrhea [19%], fatigue [13%] and elevated liver tests [7%] which led to early discontinuation in patients on valproate).
- McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225–31. [PubMed: 29241357](Among 88 patients with schizophrenia in a placebo controlled trial for 6 weeks, clinical improvements were more frequent with cannabidiol [1000 mg daily] than placebo, as were adverse events including diarrhea [9% vs 4%] and nausea [7% vs 0%]).
- Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, et al. GWPCARE4 Study Group. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085–96. [PubMed: 29395273](Among 171 patients with treatment resistant seizures due to Lennox-Gastaut syndrome in a 14 week placebo controlled trial, seizure frequency decreased by 44% with cannabidiol vs 22% in controls and side effects included diarrhea [19% vs 8%], somnolence [15% vs 9%], fatigue [6% vs 2%] and ALT elevations [9% vs 2%]).
- Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, Greenwood S, et al. GWPCARE1 Part A Study Group. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–e1211. [PMC free article: PMC5890607] [PubMed: 29540584](Among 34 patients with refractory seizures due to Dravet syndrome treated with cannabidiol [5, 10 or 20 m/kg/day] or placebo, 6 of 27 [22%] on cannabidiol developed ALT elevations above 3 times ULN [all on valproate], which resolved in all but led to early drug discontinuation in one subject).
- Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, et al. GWPCARE3 Study Group. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888–97. [PubMed: 29768152](Among 225 patients [ages 2 to 55 years] with refractory drop seizures due to Lennox-Gastaut syndrome enrolled in a 14 week placebo controlled trial, seizure frequency decreased more with cannabidiol [-37% at 10 mg and -42% at 20 mg per kg daily] than placebo [-17%], while adverse events were more frequent including somnolence [21-30% vs 5%], anorexia [16-26% vs 8%] and diarrhea [10-15% vs 8%], and ALT elevations were the most common reason for drug discontinuation, although no patient developed clinically apparent liver injury or jaundice).
- Szaflarski JP, Bebin EM, Comi AM, Patel AD, Joshi C, Checketts D, Beal JC, et al. CBD EAP study group. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia. 2018;59:1540–8. [PMC free article: PMC6175436] [PubMed: 29998598](Among 607 patients [ages <1 to 62 years] with treatment-resistant epilepsy treated with cannabidiol in an expanded access program with median treatment of 48 weeks, seizure frequency decreased by 51% and common adverse events were diarrhea [29%], somnolence [22%], anorexia [12%] and fatigue [11%]; ALT or AST elevations above 3 times ULN arose in 61 subjects [10%], most of whom [n=46] were also receiving valproate, but there were no instances of clinically apparent liver injury).
- Hazekamp A. The trouble with CBD oil. Med Cannabis Cannabinoids. 2018;1:65–72. [PMC free article: PMC8489347] [PubMed: 34676324](Commentary on the uncertainties of the efficacy, safety and purity of cannabidiol marketed products).
- Bar-Lev Schleider L, Mechoulam R, Saban N, Meiri G, Novack V. Real life experience of medical cannabis treatment in autism: analysis of safety and efficacy. Sci Rep. 2019;9:200. [PMC free article: PMC6336869] [PubMed: 30655581](Among 188 patients with autism spectrum disorders treated with miscellaneous cannabis products [usually consisting of 30% CBD and 1.5% THC, given 3 times daily] between 2015 and 2017 in Israel, 49% reported significant improvement, 31% moderate improvement, 6% side effects and 14% no change; no mention of ALT elevations or hepatotoxicity).
- Thiele E, Marsh E, Mazurkiewicz-Beldzinska M, Halford JJ, Gunning B, Devinsky O, Checketts D, et al. Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia. 2019;60:419–28. [PMC free article: PMC6850399] [PubMed: 30740695](Among 366 patients who completed controlled trials of cannabidiol for Lennox-Gastaut syndrome and continued therapy for an average of 38 weeks, adverse events included diarrhea [27%] and somnolence [24%], while ALT or AST elevations above 3 times ULN arose in 21% of patients also receiving valproate but only 3.5% of those who were not, and no patient developed clinically apparent liver injury with jaundice although 1.5% stopped therapy because of ALT or AST elevations).
- Schmitz SM, Lopez HL, Marinotti O. Post marketing safety of Plus CBD™ Products, a full spectrum hemp extract: a 2-year experience. J Diet Suppl. 2020;17:587–598. [PubMed: 32449632](Among 1334 spontaneous reports of adverse events arising during taking a commercial cannabidiol over a 2 year period, the most common adverse events were abdominal discomfort, headache, and hypersensitivity reactions; while only one report mentioned elevated liver enzymes).
- Sholler DJ, Schoene L, Spindle TR. Therapeutic efficacy of cannabidiol (CBD): a review of the evidence from clinical trials and human laboratory studies. Curr Addict Rep. 2020;7:405–412. [PMC free article: PMC7880228] [PubMed: 33585159](Review of trials of CBD for efficacy in various conditions concludes that the evidence is lacking for many disease conditions except for epilepsy; no discussion of adverse events).
- Chesney E, McGuire P, Freeman TP, Strang J, Englund A. Lack of evidence for the effectiveness or safety of over-the-counter cannabidiol products. Ther Adv Psychopharmacol. 2020;10:2045125320954992. [PMC free article: PMC7491225] [PubMed: 32973998](Extensive review of the efficacy and safety of cannabidiol compares pharmaceutical grade to over-the-counter grade products, most of which are of uncertain purity and concentration and are used in lower doses [<300 mg/day], which are unlikely to achieve plasma levels associated with engagement of cannabinoid receptors and which have not consistently shown the effects of the higher doses of pharmaceutical grade cannabidiol that has been shown to reduce anxiety and psychiatric symptoms but also cause liver enzyme elevations, particularly when given with clobazam or valproate).
- Ballotin VR, Bigarella LG, Brandão ABM, Balbinot RA, Balbinot SS, Soldera J. Herb-induced liver injury: systematic review and meta-analysis. World J Clin Cases. 2021;9:5490–5513. [PMC free article: PMC8281430] [PubMed: 34307603](Systematic review of the literature on herb induced liver injury identified 446 references describing 936 cases of liver injury due to 79 different herbal products, the most common being He Shou Wu [n=91], green tea [90], Herbalife products [64], kava kava [62], and greater celandine [48]; cannabis and marijuana were not listed).
- Kanjanarangsichai A, Mitarnun W, Mitarnun W, Pangwong W, Laoharattanahirun N, Kajornrith W, Junlaor P, et al. Cannabidiol-enriched cannabis extraction product in Parkinson's disease: a randomized, double-blind, and placebo-controlled trial in Buriram Hospital. J Neurosci Rural Pract. 2022;13:663–668. [PMC free article: PMC9894020] [PubMed: 36743777](Among 40 patients with Parkinson disease treated with a sublingual cannabinoid product [16 mg of cannabidiol and 0.6 mg of THC daily] for 8 weeks, there were no differences in measures of Parkinson disease symptoms and signs and no differences in serum ALT, AST, Alk P and bilirubin levels between the two groups).
- Bessone F, García-Cortés M, Medina-Caliz I, Hernandez N, Parana R, Mendizabal M, Schinoni MI, et al. Herbal and dietary supplements-induced liver injury in Latin America: experience from the LATINDILI Network. Clin Gastroenterol Hepatol. 2022;20:e548–e563. [PubMed: 33434654](Among 367 cases of hepatotoxicity enrolled in the Latin American DILI Network between 2011 and 2019, 29 [8%] were attributed to herbal products, the most frequent being green tea [n=7], Herbalife products [n=5] and garcinia [n=3], while marijuana and cannabis were not implicated in any cases).
- Nathan R, Mupamombe CT, Elibol J, Case AA, Smith D, Hyland A, Attwood K, et al. Assessing efficacy and use patterns of medical cannabis for symptom management in elderly cancer patients. Am J Hosp Palliat Care. 2023;40(4):368–373. [PubMed: 35749740](Among 85 cancer patients ages 65 years and above who were treated with medical cannabis products [containing both THC and CBD] who filled out symptom questionnaires before and during therapy there were slight decreases but no statistically significant changes in pain, nausea, anorexia or anxiety; no mention of adverse events, ALT elevations or hepatotoxicity).
- Souza JDR, Pacheco JC, Rossi GN, de-Paulo BO, Zuardi AW, Guimarães FS, Hallak JEC, et al. Adverse effects of oral cannabidiol: an updated systematic review of randomized controlled trials (2020-2022). Pharmaceutics. 2022;14:2598. [PMC free article: PMC9782576] [PubMed: 36559092](Systematic review of trials of CBD for safety and efficacy published in 2020 with data from 12 studies in 745 patients, reported adverse events of gastrointestinal symptoms in 60%, somnolence and loss of appetite in 17%, elevations in ALT or AST in 13% [above 3 times ULN in 6% all of whom were on other anticonvulsants], and rash in 1%).
- Mboumba Bouassa RS, Needham J, Nohynek D, Singer J, Lee T, Bobeuf F, Samarani S, et al. Safety and tolerability of oral cannabinoids in people living with HIV on long-term ART: a randomized, open-label, interventional pilot clinical trial (CTNPT 028). Biomedicines. 2022;10:3168. [PMC free article: PMC9775551] [PubMed: 36551926](A trial of 3 different cannabinoid products in only 10 patients, 2 of whom developed ALT elevations when the CBD dose was increased from 200 to 800 mg daily; to ~90 U/L in one resolving upon stopping and to >1000 U/L in the second who subsequently was found to have pancreatic cancer).
- Erridge S, Holvey C, Coomber R, Hoare J, Khan S, Platt M, Rucker J, et al. Clinical outcome data of children treated with cannabis based medicinal products for treatment resistant epilepsy – analysis from the UK Medical Cannabis Registry. Neuropediatrics. Neuropediatrics. 2023 Jan 30; Epub ahead of print. [PMC free article: PMC10166640] [PubMed: 36539215](Among 35 children with resistant epilepsy treated with medical cannabis based products, improved control of seizures was achieved in 66% and adverse events arose in 46% but were largely self-limited and mild; no mention of ALT levels or hepatotoxicity).
- Englund A, Oliver D, Chesney E, Chester L, Wilson J, Sovi S, De Micheli A, et al. Does cannabidiol make cannabis safer? A randomised, double-blind, cross-over trial of cannabis with four different CBD:THC ratios. Neuropsychopharmacology. 2022 Nov 16; Epub ahead of print. [PMC free article: PMC10156730] [PubMed: 36380220](Among 47 healthy volunteers given granulated cannabis after pretreatment with one of 3 doses of cannabidiol [20, 30 or 40 mg] or placebo, serum THC concentrations and its cognitive and psychological effects were not affected by CBD pretreatment).
- Johnson E, Kilgore M, Babalonis S. Cannabidiol (CBD) product contamination: quantitative analysis of Δ9 -tetrahydrocannabinol (Δ9-THC) concentrations found in commercially available CBD products. Drug Alcohol Depend. 2022;237:109522. [PMC free article: PMC9899037] [PubMed: 35690015](Among 80 samples of commercially purchased CBD [from local or online retailers, some labelled as THC-free], 51 [64%] were found to have detectable levels of THC ranging in concentration from 0.1 to 2.1 mg/mL).
- EFSA Panel on Nutrition. Statement on safety of cannabidiol as a novel food: data gaps and uncertainties. EFSA J. 2022;20:e07322. Novel Foods and Food Allergens (NDA), Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Maciuk A, Mangelsdorf I, et al. [PMC free article: PMC9172591] [PubMed: 35686177](Major review of the mechanisms of action, clinical effects and toxicities of cannabidiol [CBD] used as a “novel food” in the European Union, difficulties being the variable purity and concentration of commercial CBD products, its uncertain mechanism of action, the lack of rigorous clinical studies of its efficacy and poor description of potential toxicities leading to the conclusion that more and better studies are needed; in animal models CBD increases the relative liver weight and has variably been found to result in transient elevations in ALT, Alk P and bilirubin; similar results have been found in healthy human volunteers, but largely in higher doses and without instances of clinically apparent liver injury).
- Stolar O, Hazan A, Vissoker RE, Kishk IA, Barchel D, Lezinger M, Dagan A, et al. Medical cannabis for the treatment of comorbid symptoms in children with autism spectrum disorder: An interim analysis of biochemical safety. Front Pharmacol. 2022 Sep 29;13:977484. [PMC free article: PMC9559854] [PubMed: 36249785](Among 59 children with autistic spectrum disorder treated with medium chain triglyceride oil with cannabidiol in escalating doses for an average of 18 weeks, there were no clinical or statistically significant changes in complete blood counts or chemistry tests including ALT, AST, Alk P).
- Arnold JC, McCartney D, Suraev A, McGregor IS. The safety and efficacy of low oral doses of cannabidiol: An evaluation of the evidence. Clin Transl Sci. 2023;16:10–30. [PMC free article: PMC9841308] [PubMed: 36259271](Review of literature on the effects and tolerability of low doses of cannabidiol [less than 400 mg daily] as are found in widely available over-the-counter CBD products concludes that evidence for effects on anxiety, pain, lipids, sleep and muscle damage is weak, but adverse events are few and similar in incidence to those with placebo).
- Grimison P, Mersiades A, Kirby A, Lintzeris N, Morton R, Haber P, Olver I, et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Ann Oncol. 2020;31:1553–1560. [PubMed: 32801017](Among 81 patients with refractory chemotherapy induced nausea and vomiting were treated with 1 to 4 oral cannabis extract tablets [THC 2.5 mg/CBD 2.5 mg] or placebo three times daily 1 day before and 5 days after chemotherapy in a crossover design, a complete response [no nausea or vomiting] occurred in 25% of THC/CBD treated compared to 14% of placebo treated courses; adverse events more frequent with THC/CBD included sedation, dizziness, and disorientation, but there were no cannabinoid-related serious adverse events and no mention of ALT elevations or hepatotoxicity).
- Park YD, Linder DF, Pope J, Flamini JR, Moretz K, Diamond MP, Long SA. Long-term efficacy and safety of cannabidiol (CBD) in children with treatment-resistant epilepsy: Results from a state-based expanded access program. Epilepsy Behav. 2020;112:107474. [PubMed: 33181893](Among 45 children with treatment resistant epilepsy treated with cannabidiol in an expanded access program found a reduction in seizure frequency of 61-70% compared to baseline, but with frequent adverse events including 11% with ALT or AST elevations but none were scored as serious adverse events).
- Scheffer IE, Halford JJ, Miller I, Nabbout R, Sanchez-Carpintero R, Shiloh-Malawsky Y, Wong M, et al. Add-on cannabidiol in patients with Dravet syndrome: Results of a long-term open-label extension trial. Epilepsia. 2021;62:2505–2517. [PubMed: 34406656](Among 315 patients with Dravet syndrome [97% children] treated with cannabidiol in controlled trials who were then followed in a long term, open label extension trial for a median duration of 1.2 years and dose of 22 mg/kg daily, seizure frequency decreased by 49-84% and adverse events with frequent, ALT elevations arose in 11% most of whom were also receiving valproate, but there were no instances of liver injury with jaundice or deaths from liver disease).
- Patel AD, Mazurkiewicz-Bełdzińska M, Chin RF, Gil-Nagel A, Gunning B, Halford JJ, Mitchell W, et al. Long-term safety and efficacy of add-on cannabidiol in patients with Lennox-Gastaut syndrome: results of a long-term open-label extension trial. Epilepsia. 2021;62:2228–2239. [PubMed: 34287833](Among 366 patients with Lennox-Gastaut syndrome who participated in randomized controlled trials of cannabidiol and were continued on therapy in an extension trial with a median follow up of 3 years, median decreases in the frequency of seizures was 48-68%, while serious adverse events arose in 42% including 55 [15%] with ALT or AST elevations above 3 times ULN, leading to discontinuation in 15 [27%], but resolved in most patients spontaneously or after reduction in doses of concomitant valproate or clobazam).
- Bouquet E, Pain S, Eiden C, Jouanjus E, Richard N, Fauconneau B, Pérault-Pochat MC., French Addictovigilance Network. Adverse events of recreational cannabis use reported to the French Addictovigilance Network (2012-2017). Br J Clin Pharmacol. 2021;87:3925–3937. [PubMed: 34282851](Among 2217 patients enrolled in a French vigilance registry of addiction involving cannabis use between 2012 and 2017, most were men [76%], and 18 to 34 years old [57%], the most frequent adverse events were psychiatric [51%], neurological [37%], cardiac [8%], and cannabinoid hyperemesis syndrome [3%], with only 3 being hepatobiliary events [abscess, sclerosing cholangitis and “cytolysis”], with no unexpected hepatic events, or deaths from liver disease).
- Iannone LF, Arena G, Battaglia D, Bisulli F, Bonanni P, Boni A, Canevini MP, et al. CBD LICE Italy Study Group. Results from an Italian expanded access program on cannabidiol treatment in highly refractory Dravet syndrome and Lennox-Gastaut syndrome. Front Neurol. 2021;12:673135. [PMC free article: PMC8173151] [PubMed: 34093420](Among 93 Italian patients with Dravet or Lennox-Gastaut syndrome enrolled in an open label study of cannabidiol, 40% had a 50% or greater decrease in seizures but adverse events arose in 52%, most commonly somnolence [23%] and diarrhea [12%] and ALT or AST elevations above 3 times ULN [11%], and hyperammonemia [8%] usually in patients who were also taking valproate; no episodes of clinically apparent liver injury).
- Watkins PB, Church RJ, Li J, Knappertz V. Cannabidiol and abnormal liver chemistries in healthy adults: results of a phase I clinical trial. Clin Pharmacol Ther. 2021;109:1224–1231. [PMC free article: PMC8246741] [PubMed: 33022751](Among 16 healthy volunteers treated with cannabidiol in doses of 1500 mg daily for 3 to 4 weeks, 7 [44%] developed ALT elevations which were greater than 5 times ULN in 5 [31%] beginning with 2-4 weeks and resolving rapidly with discontinuation; no mention of bilirubin elevations but alkaline phosphatase elevations occurred as well and some patients developed symptoms or signs of hepatitis including fever, nausea, abdominal discomfort, and eosinophilia).
- Yan K, Forman L. Cannabinoid use among liver transplant recipients. Liver Transpl. 2021;27:1623–1632. [PubMed: 34018308](Among 1227 liver transplant recipients, 538 responded to an exploratory survey on cannabinoid use, 24% were using marijuana [72% smoking, 47% daily use] and 21% were using CBD, usually for relieving pain [85%] and anxiety [31%]; the effects of liver tests and transplant outcomes could not be assessed).
- Szaflarski JP, Devinsky O, Lopez M, Park YD, Zentil PP, Patel AD, Thiele EA, et al. Long-term efficacy and safety of cannabidiol in patients with treatment-resistant epilepsies: four-year results from the expanded access program. Epilepsia. 2023;64:619–629. [PubMed: 36537757](Among 840 patients in a long term open labelled extension study of cannabidiol for refractory seizures treated for a median duration of 1.9 years and dose of 25 mg/kg daily, the reduction in seizure frequency was 50-66% and adverse events arose in 88% resulting in discontinuation in 7% of patients, the most common events being diarrhea, seizures, somnolence with ALT elevations above 5 times ULN in 3%, resolving in all spontaneously or with dose reduction or discontinuation and without jaundice; among 20 deaths none were due to liver failure).
- Pillai M, Erridge S, Bapir L, Nicholas M, Dalavaye N, Holvey C, Coomber R, et al. Assessment of clinical outcomes in patients with post-traumatic stress disorder: analysis from the UK Medical Cannabis Registry. Expert Rev Neurother. 2022;22(11-12):1009–1018. [PubMed: 36503404](Among 162 patients enrolled in the UK Medical Cannabis Registry who were treated for posttraumatic stress disorder, improvements in PTSD symptoms occurred with therapy and adverse events were reported in 20% of patients including fatigue, insomnia and dizziness but with no reports of liver injury).
- Vickery AW, Roth S, Ernenwein T, Kennedy J, Washer P. A large Australian longitudinal cohort registry demonstrates sustained safety and efficacy of oral medicinal cannabis for at least two years. PLoS One. 2022;17:e0272241. [PMC free article: PMC9674134] [PubMed: 36399463](Among 3961 patients in an Australian longitudinal registry of patients treated with medicinal cannabis for at least 2 years, improvements in symptoms were rapid and sustained for two, during which 37% had at least one adverse event including somnolence [11%], dry mouth [9%], lethargy [6%], dizziness [6%] and nausea [5%], without mention of liver related adverse events or ALT elevations).
- Thiele EA, Bebin EM, Filloux F, Kwan P, Loftus R, Sahebkar F, Sparagana S, et al. Long-term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: An open-label extension trial. Epilepsia. 2022;63:426–439. [PMC free article: PMC9305454] [PubMed: 34957550](Among 199 patients with the tuberous sclerosis complex and refractory seizures enrolled in a long term open label extension trial of cannabidiol therapy, the median reduction in seizure frequency ranged from 54-68% and common adverse events included diarrhea [42%], decreased appetite [20%], and ALT or AST elevations above 3 times ULN [9%] that usually arose within 3 months and resolved without jaundice either spontaneously, with dose reduction, or stopping other anticonvulsants, requiring discontinuation in only 1 patient).
- Gelow K, Chalasani S, Green K, Lammert C. Utilization and impact of complementary and alternative medicines in symptomatic autoimmune hepatitis patients. Dig Dis Sci. 2022;67:2891–2898. [PMC free article: PMC9236966] [PubMed: 34160734](Among patients with autoimmune hepatitis who completed an online questionnaire, 45% reported using CBD oil after the diagnosis was made usually for fatigue, pain, disturbed sleep, or itching).
- Olsson F, Erridge S, Tait J, Holvey C, Coomber R, Beri S, Hoare J, et al. An observational study of safety and clinical outcome measures across patient groups in the United Kingdom Medical Cannabis Registry. Expert Rev Clin Pharmacol. 2023;16:257–266. [PubMed: 36848456](Among 2833 persons enrolled in the UK Medical Cannabis Registry between 2019 and 2022, indications for treatment included non-cancer pain [32%], anxiety [11%], fibromyalgia [11%], neuropathy [8%]. PTSD [6%] and depression [5%], health related quality of life improved after 1 month and was improved in all subscales except self-care at 12 months, the most common therapies were THC oil [20 mg/mL] and CBD oil [50 mg/mL]; adverse events being reported by 17% of patients which were usually mild or moderate [79%], most commonly fatigue [14%], dry mouth [12%], somnolence [11%] insomnia [11%] headache [10%], impaired concentration [10%], nausea [9%], and dizziness [8%]; no mention of ALT elevations or liver injury).
- Morris M, Chye R, Liu Z, Agar M, Razmovski-Naumovski V. A retrospective medical record review of adults with non-cancer diagnoses prescribed medicinal cannabis. J Clin Med. 2023;12:1483. [PMC free article: PMC9965412] [PubMed: 36836018](Among 157 adults treated with medicinal cannabis [typically balanced THC/CBD oil] at a single specialty clinic after its approval in Australia, the major reasons were for pain [87%], muscle spasm [12%] and sleep [6%] and its was considered beneficial by the patient in 54%, most frequently for neuropathy [67%], Parkinson disease [61%], multiple sclerosis [60%], migraine [44%], chronic pain syndrome [42% and spondylosis [40%] and specifically for sleep [80%], fatigue [52%], and pain [50%]; side effects were reported in 43% of patients and were generally mild including somnolence, dry mouth and confusion; no mention of ALT elevations or liver injury).
- Kühne F, Becker LL, Bast T, Bertsche A, Borggraefe I, Boßelmann CM, Fahrbach J, et al. Real world data on cannabidiol treatment of various epilepsy subtypes: a retrospective, multicenter study. Epilepsia Open. 2023 Jan 24; Epub ahead of print. [PMC free article: PMC10235575] [PubMed: 36693811](Among 311 patients with various forms of epilepsy followed at 16 European epilepsy centers and treated with cannabidiol [2.5-45 mg/kg/day], response rates were similar across different forms of epilepsy [not just Dravet and Lennox-Gastaut syndrome], and adverse events were reported by 47% of patients, leading to discontinuation in 9%, and described as fatigue in 24%, gastrointestinal 21%, psychiatric 6%, and liver test elevations in 5 subjects, 4 of whom were also receiving valproate; positive side effects included improved mood, sleep, concentration and appetite).
Publication Details
Publication History
Last Update: February 16, 2023.
Copyright
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
NLM Citation
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Cannabidiol. [Updated 2023 Feb 16].