OVERVIEW
Introduction
Foscarnet a simple pyrophosphate molecule which has antiviral activity against many viruses and is used intravenously in therapy of serious cytomegalovirus infections, largely in immunocompromised patients. Foscarnet has been associated with mild-to-moderate serum aminotransferase elevations during intravenous therapy, but not with episodes of clinically apparent liver injury.
Background
Foscarnet (fos kar' net) is a pyrophosphate (phosphonoformate) that has antiviral activity against several DNA viruses, including cytomegalovirus (CMV) and hepatitis B virus. Foscarnet appears to act by inhibition of the pyrophosphate binding sites of viral DNA polymerases. Foscarnet is poorly absorbed orally and must be given intravenously. Foscarnet was approved for use in the United States in 1991, but has had limited use. Current indications include therapy of CMV retinitis and acyclovir-resistant herpes simplex infections in immunocompromised individuals. Foscarnet is available as an intravenous formulation of 24 mg/mL generically and previously under the brand name of Foscavir. The recommended dose in adults is 40 to 60 mg/kg intravenously every 8 to 12 hours for 2 to 4 weeks, which must be carefully monitored and dose adjusted for renal insufficiency. Side effects include headache, dizziness, confusion, fever, fatigue, abdominal pain, bone marrow suppression, renal dysfunction and rash. Uncommon but potentially severe adverse events include renal failure, seizures, hypersensitivity reactions, Stevens Johnson syndrome and rhabdomyolysis.
Hepatotoxicity
Intravenous foscarnet therapy is associated with mild-to-moderate serum ALT elevations in a proportion of patients, but the drug is usually given to patients with multiorgan disease and conditions that may be associated with some degree of hepatic injury. The ALT elevations are usually asymptomatic and resolve even with continuation of foscarnet. Single case reports of clinically apparent liver injury that were possibly attributable to foscarnet have been reported. The pattern of injury was cholestatic arising within weeks of starting foscarnet but the clinical features of the liver injury have not been described in any detail.
Likelihood score: D (possible cause of clinically apparent liver injjry).
Mechanism of Injury
Foscarnet is rapidly excreted in the urine and has little hepatic metabolism, which may account for its relative lack of hepatotoxicity. Acute liver injury during foscarnet therapy might be a result of a hypersensitivity reaction to the drug or of a complication of the condition for which foscarnet was used.
Outcome and Management
The serum aminotransferase elevations during foscarnet therapy are usually self-limited and do not require dose adjustment. Foscarnet is usually given to severely immunocompromised patients who are frequently on multiple other medications, many of which may be hepatotoxic and attribution of hepatic injury in such individuals can be difficult.
Drug Class: Antiviral Agents
Other Antiviral Agents for Herpes Virus Infections: Acyclovir, Cidofovir, Famciclovir, Ganciclovir, Letermovir, Valacyclovir, Valganciclovir
PRODUCT INFORMATION
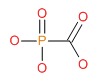
CHEMICAL FORMULA AND STRUCTURE
ANNOTATED BIBLIOGRAPHY
References updated: 06 February 2018
- Zimmerman HJ. Antiviral agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 621-3.(Expert review of antiviral agents and liver injury published in 1999; mentions that foscarnet "...has not been reported to lead to hepatic injury").
- Núñez M. Hepatotoxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 513.(Review of hepatotoxicity of antiviral agents states that ALT and AST elevations occurred in 1-5% of patients treated with foscarnet in clinical trials).
- Acosta EP, Flexner C. Antiviral agents (nonretroviral). In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1602-3.(Textbook of pharmacology and therapeutics; foscarnet must be given parenterally to achieve adequate drug levels for its antiviral action; dose limiting side effects are nephrotoxicity and hypocalcemia).
- Ringdén O, Lönnqvist B, Paulin T, Ahlmén J, Klintmalm G, Wahren B, Lernestedt JO. Pharmacokinetics, safety and preliminary clinical experiences using foscarnet in the treatment of cytomegalovirus infections in bone marrow and renal transplant recipients. J Antimicrob Chemother 1986; 17: 373-87. [PubMed: 3009383](Experience in 57 episodes of severe CMV infection in 46 patients given intravenous foscarnet for 2 to 46 days; elevations in serum enzymes were frequen, but usually attributed to underlying conditions; one patient developed jaundice [bilirubin 0.7 rising to 7.1 mg/dL] with mild rise in Alk P; another had tremor, hallucinations and ALT elevations with high levels of foscarnet and renal failure).
- Cacoub P, Deray G, Baumelou A, Le Hoang P, Rozenbaum W, Gentilini M, Soubrie C, et al. Acute renal failure induced by foscarnet: 4 cases. Clin Nephrol 1988; 29: 315-8. [PubMed: 2840226](Four case reports of acute renal failure in patients after 5-15 days of high dose foscarnet [5-12 g/day] for CMV retinitis, improving with stopping; liver tests were either normal or not mentioned).
- Palestine AG, Polis MA, De Smet MD, Baird BF, Falloon J, Kovacs JA, Davey RT, et al. A randomized, controlled trial of foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. Ann Intern Med 1991; 115: 665-73. [PubMed: 1656826](Controlled trial of foscarnet vs delayed therapy in 24 patients with CMV retinitis and HIV infection; one patient on foscarnet and zidovudine "developed cholestatic jaundice that resolved after both drugs were withdrawn").
- Chrisp P, Clissold SP. Foscarnet: a review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs 1991; 41: 104-29. [PubMed: 1706982](Thorough review of antiviral activity, pharmacokinetics, efficacy and safety of foscarnet; "Foscarnet has negligible effects on hepatic function, only occasionally causing slight elevations in serum transaminase levels").
- Styrt B, Freiman JP. Hepatotoxicity of antiviral agents. Gastroenterol Clin North Am 1995; 24: 839-52. [PubMed: 8749901](Review of liver toxicity of antiviral agents; mentions that foscarnet has caused alterations in liver tests uncommonly [<1%]).
- Drugs for non-HIV viral infections. Treat Guidel Med Lett 2007; 5: 59-70. [PubMed: 17565338](Review of status of non-antiretroviral antiviral agents for prevention and treatment of herpes, varicella-zoster, cytomegalovirus, influenza A and B, and hepatitis B and C; no mention of liver related side effects for foscarnet).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 8 were attributed to antiviral agents including one due to valacyclovir, but none to foscarnet).
- Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, Nakao S. Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant 2011; 46: 863-9. [PubMed: 20838386](Among 15 patients receiving hematopoietic cell transplantation, 8 received preemptive therapy with foscarnet for human herpesvirus-6 infection, among whom only 1 had liver enzyme elevations compared to 3 of 7 who did not receive foscarnet and none developed clinically apparent liver injury).
- Antiviral drugs. Treat Guidel Med Lett 2013; 11 (127): 19-30. [PubMed: 23459414](Review of status of non-antiretroviral antiviral agents; foscarnet is used for acyclovir resistant varicella-zoster and herpes virus infections and for CMV retinitis in patients with AIDs; adverse effects include renal dysfunction, gastointestinal upset, CNS disturbances hypocalcemia and seizures; no mention of liver toxicity or ALT elevations).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 [1.3%] were attributed to antiviral agents, but none to foscarnet).
- Pierce B, Richardson CL, Lacloche L, Allen A, Ison MG. Safety and efficacy of foscarnet for the management of ganciclovir-resistant or refractory cytomegalovirus infections: a single center study. Transpl Infect Dis 2018 Jan 30. [Epub ahead of print] [PubMed: 29380479](Among 31 post-solid organ transplant patients who received foscarnet, side effects were common but related mostly to renal dysfunction; no mention of ALT elevations or hepatotoxicity and none of 10 deaths were due to liver failure).
Publication Details
Publication History
Last Update: February 6, 2018.
Copyright
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
NLM Citation
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Foscarnet. [Updated 2018 Feb 6].