NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
OVERVIEW
Introduction
Primidone is an aromatic anticonvulsant used to treat complex, partial and generalized seizures. Therapy with primidone can be associated with increases in gamma glutamyltranspeptidase levels, but is not associated with serum aminotransferase elevations, and despite its similarity in structure to phenobarbital and phenytoin, clinically apparent liver injury from primidone has not been reported and must be quite rare if it occurs at all.
Background
Primidone (prim' i done) is a pyrimidinedione anticonvulsant and is partially metabolized to phenobarbital. Primidone is effective in suppressing seizure activity, but its mechanism of action is not well defined. It is believed to work centrally via interactions with voltage-gated sodium channels inhibiting repetitive firing of action potentials. Primidone was approved for use in epilepsy in the United States in 1954. For many years, primidone was considered a first line anticonvulsant agent, but it has been largely replaced by more recently developed anticonvulsants that are better tolerated, less sedative and have fewer long term adverse side effects. Primidone has also been used to treat essential tremor. Primidone is available as tablets of 50 and 250 mg in several generic formulations and under the brand names Mysoline, Myidone, Sertan and Apo-Primidone. The recommended initial dose for adults is 100 to 125 mg daily, increasing slowly to a maintenance dose of 250 mg three times daily. The most common side effects are dose related and include drowsiness, ataxia, diplopia, and headache. The initial dose may be associated with an acute toxic reaction with nausea, malaise, sedation, ataxia and confusion. Long term therapy may be associated with suicidal ideation and behaviors, megaloblastic anemias and birth defects.
Hepatotoxicity
In clinical trials in epilepsy, therapy with primidone was not associated with an increased frequency of serum aminotransferase elevations or liver toxicity. Primidone therapy can lead to increases in gamma glutamyltranspeptidase (GGT) levels. Elevations in alkaline phosphatase levels were largely due to bone isoforms of the enzyme. There have been no convincing reports of hepatotoxicity due to primidone in humans and no reports of its association with acute liver failure. Interestingly, primidone appears to cause cirrhosis in dogs. Because of its similarity in structure to phenytoin and phenobarbital (aromatic anticonvulsant), it has been suspected to cross react with those agents in causing anticonvulsant hypersensitivity syndrome, but convincing case reports have not been published.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
Primidone, like phenobarbital, is extensively metabolized by the liver and can induce CYP 450 enzyme activities. Primidone can interfere with porphyrin metabolism and like phenobarbital and phenytoin can cause worsening of porphyria.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Primidone – Generic, Mysoline®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
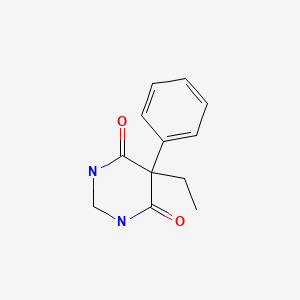
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Primidone | 125-33-7 | C12-H14-N2-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 July 2020
Abbreviations used: SJS/TEN, Stevens-Johnson syndrome and toxic epidermal necrolysis.
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; mentions that there have been no reports of hepatotoxicity from primidone).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-41.(Review of anticonvulsant induced liver injury does not specifically discuss primidone).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacology of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- Tschudy DP, Valsamis M, Magnussen CR. Acute intermittent porphyria: clinical and selected research aspects. Ann Intern Med. 1975;83:851–64. [PubMed: 1106284](Review article on porphyria; drugs that can induce an acute exacerbation include the aromatic anticonvulsants).
- Reynolds NC Jr, Miska RM. Safety of anticonvulsants in hepatic porphyrias. Neurology. 1981;31:480–4. [PubMed: 7194443](In vitro studies showing that aromatic anticonvulsants increased intracellular hepatic delta-aminolevulinic acid [ALA] levels).
- Bunch SE, Castleman WL, Hornbuckle WE, Tennant BC. Hepatic cirrhosis associated with long-term anticonvulsant drug therapy in dogs. J Am Vet Med Assoc. 1982;181:357–62. [PubMed: 7118708](Case reports of autopsies showing cirrhosis in dogs who had received long term primidone therapy).
- Fichsel H, Spiess S. Klin Padiatr. 1982;194:332–4. [Serum alkaline phosphatase izoenzymes in childhood during long-term primidone therapy for convulsions] German. [PubMed: 7144052](Among 69 children on primidone for >1 year and 98 controls, Alk P levels were higher [~30%] in primidone treated children, but elevations were largely in bone isoenzyme levels).
- Deutsch J, Fritsch G, Gölles J, Semmelrock HJ. Effects of anticonvulsive drugs on the activity of gammaglutamyltransferase and aminotransferases in serum. J Pediatr Gastroenterol Nutr. 1986;5:542–8. [PubMed: 2874203](Among 198 children on anticonvulsants in a cross sectional study, GGT and ALT were higher in children on phenytoin; primidone was associated with mild increases in GGT but not ALT).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996;15:378–93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine and not reported to be higher on tiagabine or gabapentin; no mention of hepatotoxicity of primidone, although case of systemic lupus related to primidone is discussed).
- Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf. 1999;21:489–501. [PubMed: 10612272](Review of anticonvulsant hypersensitivity syndrome; triad of fever, rash and internal organ injury occurring 1-8 weeks after exposure to anticonvulsant; liver being most common internal organ involved. Occurs in 1:1000-1:10,000 initial exposures to phenytoin, carbamazepine, phenobarbital or lamotrigine, unrelated to dose, perhaps predisposed by valproate; mentions that primidone may be a cause but gives no publications documenting it; nevertheless, cross reactivity among the agents can be assumed).
- Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Cleve Clin J Med. 1999;66:239–45. [PubMed: 10199060](Clinical review of anticonvulsant hypersensitivity syndrome, which occurs in 1-5/10,000 users, higher risk in African Americans and affected siblings; liver involvement common, but most cases anicteric; other manifestations include facial edema, lymphadenopathy, bone marrow aplasia, pseudolymphoma, thyroiditis, interstitial nephritis: states that the syndrome occurs with primidone but without documentation).
- Newell BD, Moinfar M, Mancini AJ, Nopper AJ. Retrospective analysis of 32 pediatric patients with anticonvulsant hypersensitivity syndrome (ACHSS). Pediatr Dermatol. 2009;26:536–46. [PubMed: 19840307](Among 32 children with anticonvulsant hypersensitivity syndrome the suspected cause was carbamazepine in 13, phenytoin in 12, lamotrigine in 5 and phenobarbital in 5, one patient also received primidone; hepatic involvement occurred in 90%, jaundice 18%, eosinophilia 56%, atypical lymphocytes 72%, none died).
- Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–61. [PubMed: 21256454](Review of therapy of essential tremor states that primidone is effective in reducing tremor in half of patients, but side effects of drowsiness and sedation are common and can be dose limiting; no mention of hepatotoxicity or ALT elevations).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013;11:9–18. Erratum in Treat Guidel Med Lett 2013; 11: 112. [PubMed: 23348233](Concise review of drugs of choice for epilepsy; primidone is not discussed, but is mentioned in passing with phenobarbital as effective for partial seizures, but having a higher rate of sedation compared to other drugs).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, of which only 1 case was attributed to an anticonvulsant [phenytoin], none were attributed to primidone).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 7 [4%] of which were attributed to anticonvulsants including 3 due to phenytoin, 3 to valproate, and 1 to carbamazepine, but none to primidone).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were due to anticonvulsants including 12 due to phenytoin, 9 lamotrigine, 7 valproate, 4 carbamazepine, 3 gabapentin, 2 topiramate and 1 each for ethosuximide, fosphenytoin, and pregabalin; but none to primidone).
- Drugs for epilepsy. Med Lett Drugs Ther. 2017;59(1526):121–30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy lists primidone as an effective anticonvulsant that is now rarely used because of a high incidence of sedation).
- Borrelli EP, Lee EY, Descoteaux AM, Kogut SJ, Caffrey AR. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;59:2318–24. [PMC free article: PMC6420776] [PubMed: 30395352](Review of adverse event reports to the FDA between 2014 and 2018 identified ~2.9 million reports, 1034 for SJS/TEN, the most common class of drugs being anticonvulsants with 17 of 34 currently available anticonvulsants having at least one report, those most frequently linked being lamotrigine [n=106], carbamazepine [22], levetiracetam [14], phenytoin [14], valproate [9], clonazepam [8], zonisamide [7]; pregabalin [4], gabapentin [4] and oxcarbazepine [3], but primidone not listed; no mention of accompanying liver injury or whether attribution was as a single agent or one of several).
- Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M. Epidemiology of drug-induced liver injury in a University Hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann Hepatol. 2019;18:501–7. [PubMed: 31053545](Among 286 patients with liver test abnormalities seen in a single hospital in Colombia over a 1 year period, 17 were diagnosed with drug induced liver injury, the most common cause being antituberculosis therapy [n=6] followed by anticonvulsants [n=3, 1 each due to phenytoin, gabapentin and valproate]).
- Wang YH, Chen CB, Tassaneeyakul W, Saito Y, Aihara M, Choon SE, Lee HY, et al. Asian Severe Cutaneous Adverse Reaction Consortium. The medication risk of Stevens-Johnson Syndrome and toxic epidermal necrolysis in Asians: the major drug causality and comparison with the US FDA label. Clin Pharmacol Ther. 2019;105:112–20. [PubMed: 29569740](Among 1028 cases of SJS/TEN reported to registries in 8 Asian countries, the most frequently implicated class of drugs was anticonvulsants including carbamazepine [26%], phenytoin [13%], lamotrigine [10%], phenobarbital [2%] and oxcarbazepine [1.7%]; no cases were attributed to primidone).