NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The orally available cephalosporins are widely used as broad spectrum antibiotics for mild-to-moderate infections with susceptible organisms. Despite their widescale use, cases of drug induced liver disease from the cephalosporins are very rare, with only isolated case reports having been published.
Background
The oral cephalosporins are available in both generic and trade formulations and include cefaclor (Ceclor, Raniclor: 2nd), cefadroxil (Duracef: 1st), cefdinir (Omnicef: 3rd), cefditoren (Spectracef: 3rd), cefixime (Suprax: 3rd), cefpodoxime (Vantin: 3rd), cefprozil (Cefzil: 2nd), ceftibuten (Cedax: 3rd), cephalexin (Keftab: Apo-Cephalex, Biocef, Keflex, NovoLexin, Nu-Cephalex: 1st), cefuroxime (Ceftin: 2nd), cephradine (Velosef: 1st), and loracarbef (Lorabid: 2nd). Cefuroxime and cephradine are also available in parenteral forms. The typical dose regimens for oral cephalosporins are 250 to 500 mg two to four times daily for 7 to 14 days. The oral cephalosporins are widely used in medicine for mild-to-moderate infections due to susceptible bacteria. The oral cephalosporins are generally well tolerated; adverse events can include diarrhea, nausea, abdominal pain, dyspepsia, headache, and rash. Rare but potentially severe adverse events include Clostridium difficile-associated diarrhea, hypersensitivity reactions, angioedema, anaphylaxis and Stevens Johnson syndrome/toxic epidermal necrolysis.
Hepatotoxicity
Oral cephalosporins can be associated with minor elevations in serum aminotransferase and alkaline phosphatase values, but these are generally mild, transient and not associated with symptoms or development of more severe liver injury. The frequency of these elevations is reported to be as high as 11%, but varies depending upon the frequency of monitoring, duration of therapy, and nature and severity of the underlying illness. Clinically apparent liver injury from oral cephalosporin use is rare, only isolated case reports having been published, and not all of the formulations have been linked to cases of liver injury. The clinical pattern of injury suggests that hepatotoxicity is largely a class effect from the cephalosporins, even though it is idiosyncratic and rare. The typical latency period has been 1 to 4 weeks with an abrupt onset of liver injury. The pattern of serum enzyme elevations is usually cholestatic, but mixed and hepatocellular instances have been reported. Liver injury is often accompanied by fever, rash and eosinophilia or other signs and symptoms of hypersensitivity. A history of penicillin allergy is not common.
Likelihood score: B (cephalosporins as a class are very likely but rare causes of clinically apparent liver injury, the association having been made largely with the most frequently used agents, such as cefazolin, cephalexin and ceftriaxone).
Mechanism of Injury
The mechanism of hepatic injury due to cephalosporins is unknown, but believed to be hypersensitivity and similar to that of other penicillins.
Outcome and Management
In most case reports, recovery has been rapid within 4 to 8 weeks without residual injury or persistent serum enzyme elevations. Among the few cases reported, none have been fatal. It is unclear whether there is cross sensitivity to the hepatic injury induced by cephalosporins. In many published cases, patients have been switched to an alternative cephalosporin without recurrence of injury, but it is more judicious to switch to another class of agents. Similarly, patients with a history of penicillin allergy have a higher rate of hypersensitivity reactions to cephalosporins, and they should be avoided or started with careful supervision and monitoring.
References to oral cephalosporin induced liver injury are provided in the introductory overview chapter on Cephalosporins.
Drug Class: Antiinfective Agents, Cephalosporins
CASE REPORT
Case 1. Cholestatic hepatitis related to cefuroxime.(1)
A 52 year old man was given a 10-day course of oral cefuroxime for recurrent folliculitis, but developed worsening skin rash and itching, and was found to be jaundiced 7 weeks after starting the antibiotic. Laboratory testing showed prominent elevations in serum AST and alkaline phosphatase with a total bilirubin of 8.4 mg/dL (Table). Tests for autoantibodies and for serologic evidence of hepatitis A, B and C were negative. Imaging tests of the liver including ultrasound, MRI and MRCP were unremarkable. The patient had a complex past medical history of diabetes, hypertension, coronary artery disease, chronic back pain and asthma for which he took several medications chronically, none of which had been changed recently. He abused alcohol for many years, but had abstained for 4 years. He had no known allergies. His cholestasis and liver disease were prolonged, and ultimately he underwent liver biopsy which showed changes of chronic inflammation and intrahepatic cholestasis compatible with drug induced liver disease. The diagnosis of cephalosporin induced liver injury had not been fully appreciated and he received two 10-day courses of cephalexin during this period of jaundice. These exposures were not associated with appreciable changes in serum enzyme levels, but were followed by an increase in serum bilirubin levels and skin rash (Table). Because of persistent pruritus, he was treated with ursodiol, cholestyramine and several antihistaminics. Ultimately, his rash and itching resolved; serum bilirubin fell to normal, but there were minor elevations in ALT and alkaline phosphatase that did not fall into the completely normal range for more than a year after the initial presentation.
Key Points
| Medication: | Cefuroxime, 500 mg daily for 10 days |
|---|---|
| Pattern: | Cholestatic (R=1.2) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 6 weeks |
| Recovery: | More than 6 months |
| Other medications: | Fluticasone and salmeterol inhalant and albuterol (for asthma), insulin (diabetes), gabapentin (neuropathy), lisinopril and hydrochlorothiazide (hypertension), cyclobenzaprine and the combination of hydrocodone and acetaminophen (low back pain) |
Laboratory Values
| Time after Starting | Time after Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comment |
|---|---|---|---|---|---|
| Ten day course of cefuroxime: weeks 1-2 | |||||
| 8 weeks | 6 weeks | 268 | 651 | 8.4 | Jaundice and pruritus |
| 9 weeks | 8 weeks | 264 | 595 | 7.1 | |
| Two courses of cephalexin: weeks 10-14 | |||||
| 15 weeks | 14 weeks | 284 | 720 | 11.8 | |
| Five day course of cefuroxime: week 19 | |||||
| 20 weeks | 19 weeks | 189 | 812 | 4.7 | Erythema |
| 21 weeks | 20 weeks | 191 | 859 | 3.6 | |
| 22 weeks | 21 weeks | 176 | 717 | 2.1 | Pruritus |
| 6 months | 6 months | 111 | 428 | 1.0 | |
| 7 months | 7 months | 69 | 174 | 0.7 | |
| 8 months | 8 months | 74 | 217 | 1.0 | Asymptomatic |
| 15 months | 15 months | 42 | 169 | 0.7 | |
| 18 months | 18 months | 32 | 131 | 0.4 | |
| 20 months | 20 months | 35 | 119 | 0.4 | |
| Normal Values | <35 | <130 | <1.2 | ||
Comment
The prolonged course of cholestasis with no obvious cause was most likely due to drug induced liver injury, and cefuroxime (a third generation, orally available cephalosporin) was the most likely cause. The long incubation period is unusual for cephalosporin related liver injury. Furthermore, rechallenge with cephalexin and then with cefuroxime appeared to worsen the course minimally, if at all, but may have been the reason for the delayed recovery. A year after the initial presentation, the patient was without symptoms, but serum enzymes were still mildly abnormal. Ultimately, however, even these mild biochemical abnormalities resolved.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cefuroxime – Generic, Ceftin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
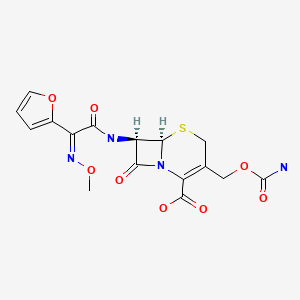
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cefuroxime | 55268-75-2 | C16-H16-N4-O8-S |
|
CITED REFERENCES
- 1.
- Alqahtani SA, Kleiner DE, Ghabril M, Gu J, Hoofnagle JH, Rockey DC., Drug-Induced Liver Injury Network (DILIN) Study Investigators. Identification and characterization of cefazolin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13:1328–36.e2. [PMC free article: PMC4472636] [PubMed: 25528012]
NOTE
References to both the oral and parenteral cephalosporins as well as review articles on the relative frequency of cephalosporin-related liver injury are given in the overview chapter on Cephalosporins.
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Cephalosporins, Parenteral.[LiverTox: Clinical and Researc...]Review Cephalosporins, Parenteral.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Different classes of antibiotics given to women routinely for preventing infection at caesarean section.[Cochrane Database Syst Rev. 2021]Different classes of antibiotics given to women routinely for preventing infection at caesarean section.Williams MJ, Carvalho Ribeiro do Valle C, Gyte GM. Cochrane Database Syst Rev. 2021 Mar 4; 3(3):CD008726. Epub 2021 Mar 4.
- Review [Review of oral cephalosporins. Basis for a rational choice].[Medicina (B Aires). 1994]Review [Review of oral cephalosporins. Basis for a rational choice].Forti IN. Medicina (B Aires). 1994; 54(5 Pt 1):439-58.
- Review Cephalosporins.[LiverTox: Clinical and Researc...]Review Cephalosporins.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Review of the spectrum and potency of orally administered cephalosporins and amoxicillin/clavulanate.[Diagn Microbiol Infect Dis. 2007]Review Review of the spectrum and potency of orally administered cephalosporins and amoxicillin/clavulanate.Sader HS, Jacobs MR, Fritsche TR. Diagn Microbiol Infect Dis. 2007 Mar; 57(3 Suppl):5S-12S. Epub 2007 Feb 9.
- Cephalosporins, Oral - LiverToxCephalosporins, Oral - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...