NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Reserpine is an oral antihypertensive medication that acts through inhibitor of alpha-adrenergic transmission and was one of the first antihypertensive agents introduced into clinical practice. Despite widescale use for many years, reserpine has not been shown to cause clinically apparent liver injury.
Background
Reserpine (re ser' peen) was one of the first antihypertensive agents developed for use in humans. It is an alkaloid extract of the Rauwolifia serpentine (thus its name) which is a climbing shrub found in India. Reserpine is thought to act by binding to adrenergic storage vesicles in neurons, inhibiting their capacity to concentrate and store norepinephrine and dopamine. The antihypertensive effect of reserpine correlates with the depletion of sympathetic amines in both the central nervous system and periphery. Reserpine is effective in lowering blood pressure and can be used alone or in combination with other antihypertensive medications. Reserpine was approved for use in the United States in 1955 but is currently rarely used, largely because of its central nervous system effects and the availability of many better tolerated and more potent antihypertensive medications. Reserpine continues to be available in generic forms as tablets of 0.1 and 0.25 mg. The typical maintenance dose in adults is 0.05 to 0.25 mg once daily. Side effects are common and include sedation, difficulty concentrating, fatigue, depression, dry mouth, headaches, dizziness, postural hypotension, male impotence and gastrointestinal upset.
Hepatotoxicity
Serum aminotransferase elevations during reserpine therapy are uncommon, but specific rates of such elevations in comparison to placebo treatment have not been reported. Despite many decades of use, reserpine has been implicated in few instances of clinically apparent acute liver injury, and none of them were particularly convincing. Published cases were marked by jaundice and abdominal pain arising a year after starting reserpine, but in combination with other known hepatotoxic agents (dihydrazine, phenobarbital, quinidine). The few cases that have been reported were self-limiting and resolved within a few months of stopping therapy. The last case of suspected reserpine associated liver injury was published more than 50 years ago.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Reserpine is metabolized by the cytochrome P450 (CYP) system to a major degree but neither induces or inhibits CYP activity, which may account in part for its relative lack of hepatotoxicity.
Drug Class: Antihypertensive Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Reserpine – Generic
DRUG CLASS
Antihypertensive Agent
Product labeling at DailyMed, National Library of Medicine, NIH
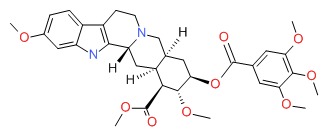
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Reserpine | 50-55-5 | C33-H40-N2-O9 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 21 May 2018
- Zimmerman HJ. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 639-71.(Expert review of hepatotoxicity published in 1999; reserpine rarely causes significant liver injury, only two case reports having been published despite its wide use).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs: antihypertensives. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of hypertensive agents; reserpine is not discussed).
- Michel T, Hoffman BB. Therapy of myocardial ischemia and hypertension. In, Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 745-88.(Textbook of pharmacology and therapeutics; “reserpine was the first drug that was found to interfere with the function of the sympathetic nervous system in human beings, and its use began the modern era of effective pharmacotherapy of hypertension”).
- Nagler A, Enat R. [Liver damage due to reserpine hypersensitivity]. Harefuah 1984; 107: 130-1. Hebrew. [PubMed: 6510817](52 year old woman developed jaundice 1 year after starting combination of dihydralazine and reserpine for hypertension [bilirubin 8 mg/dL, AST 480 U/L, Alk P 263 U/L], resolving within 1 month of stopping and recurring after 1 month of restarting both; may have been due to dihydralazine, but positive in vitro reactions occurred with reserpine alone).
- Moeckel W. [On the clinical picture of jaundice with intrahepatic cholestasis caused by drugs]. Med Klin 1965; 60: 294-8. German. [PubMed: 14258124](Five cases of drug induced cholestatic hepatitis; case 3 was 66 year old man who developed jaundice after being on reserpine, phenobarbital and quinidine for one year [bilirubin 17.4 mg/dL, Alk P 2 times ULN, eosinophils 12%]; while phenobarbital and quinidine can also cause liver injury, the case was attributed to reserpine on the basis of in vitro stimulation tests).
- Drugs for hypertension. Treat Guidel Med Lett 2009; 7: 1-10. [PubMed: 19107095](Brief overview of currently available drugs for hypertension with guidelines on their use and information on prices and toxicities: “Reserpine is an effective antihypertensive, but is seldom used now because in doses much higher than currently recommended, it can cause severe depression”).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases; no cases were attributed to reserpine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 39 [4%] were due to antihypertensive agents but none were believed to be due to reserpine).
- Drugs for hypertension. Med Lett Drugs Ther 2017; 59 (1516): 41-8. [PubMed: 28263286](Concise overview of the currently available drugs for hypertension with guidelines on their use and information on prices and toxicities; reserpine is not discussed).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- OBSERVATIONS ON THE ANTIHYPERTENSIVE AND SEDATIVE EFFECTS OF MEBUTAMATE, MEPROBAMATE AND RESERPINE.[Can Med Assoc J. 1963]OBSERVATIONS ON THE ANTIHYPERTENSIVE AND SEDATIVE EFFECTS OF MEBUTAMATE, MEPROBAMATE AND RESERPINE.MORIN Y, TURMEL L, GRANTHAM H, FORTIER J. Can Med Assoc J. 1963 Nov 9; 89(19):980-2.
- Modification of reserpine induced rigidity by dopaminergic and alpha-adrenergic drugs.[Acta Neurol Scand. 1985]Modification of reserpine induced rigidity by dopaminergic and alpha-adrenergic drugs.Anderson RJ. Acta Neurol Scand. 1985 Dec; 72(6):584-9.
- The thermogenic actions of alpha 2-adrenoceptor agonists in reserpinized mice are mediated via a central postsynaptic alpha 2-adrenoceptor mechanism.[Br J Pharmacol. 1989]The thermogenic actions of alpha 2-adrenoceptor agonists in reserpinized mice are mediated via a central postsynaptic alpha 2-adrenoceptor mechanism.Bill DJ, Hughes IE, Stephens RJ. Br J Pharmacol. 1989 Jan; 96(1):133-43.
- Review [ACUTE ADRENERGIC INSUFFICIENCY].[Laval Med. 1964]Review [ACUTE ADRENERGIC INSUFFICIENCY].DERY R. Laval Med. 1964 May; 35:501-4.
- Review ADRENERGIC MECHANISMS IN THE TREATMENT OF ESSENTIAL HYPERTENSION.[Am J Cardiol. 1963]Review ADRENERGIC MECHANISMS IN THE TREATMENT OF ESSENTIAL HYPERTENSION.ABRAMS WB, MOE RA, BATES H, WALLEN M, ODZE M, CREWS A, POCELINKO R. Am J Cardiol. 1963 Nov; 12:711-20.
- Reserpine - LiverToxReserpine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...