NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
OVERVIEW
Introduction
Zidovudine is a nucleoside analogue and reverse transcriptase inhibitor used in combination with other agents in the therapy and prophylaxis of the human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS). Zidovudine is a rare, but well established cause of clinically apparent acute and chronic liver injury.
Background
Zidovudine (zye doe' vue deen) is a synthetic analogue of thymidine (3’-azido-3’- deoxythymidine: AZT) that is phosphorylated intracellularly and then acts in competing with the natural substrate, thymidine triphosphate, for incorporation into growing HIV DNA chain causing inhibition of the viral reverse transcriptase and chain termination. Zidovudine was the first antiretroviral agent that was approved for use in treating HIV infection in the United States [1987] and was subsequently frequently used in antiretroviral regimens for many years. Recently, zidovudine has been replaced by better tolerated nucleoside analogues and it is no longer commonly used in developed countries. Zidovudine is currently indicated for the treatment of HIV infection in combination with other HIV medications such as lamivudine and abacavir. Zidovudine is available as a single agent in multiple generic forms and under the trade name Retrovir in 100 mg capsules, 300 mg tablets, and as an oral syrup; in combination with lamivudine generically or as Combivir; and in combination with abacavir and lamivudine generically or as Trizivir. The recommended dose of zidovudine in adults is 300 mg orally twice daily or 1 mg/kg every 4 hours. Common side effects include asthenia, constipation, headache, insomnia, loss of appetite, malaise, nausea, and vomiting. Less common but potentially severe adverse events include lactic acidosis, pancreatitis, peripheral neuropathy, myopathy, anemia and neutropenia, and hypersensitivity reactions including Stevens Johnson syndrome.
Hepatotoxicity
Chronic therapy with zidovudine is associated with modest serum enzyme elevations that are generally transient and asymptomatic and do not require dose modification. In prospective studies, 1 to 3% of recipients have developed ALT elevations above 5 times the upper limit of normal. The abnormalities, however, may be related to other underlying conditions such as hepatitis B or C and triggered by reconstitution syndrome.
Several forms of clinically apparent liver injury have been associated with zidovudine therapy: an acute cholestatic hepatitis, severe acute fatty liver and with lactic acidosis, and a chronic liver injury that presents with noncirrhotic portal hypertension, most likely due to nodular regenerative hyperplasia.
Rare instances of idiosyncratic cholestatic hepatitis have been reported with zidovudine therapy, typically arising within 1 to 4 weeks of starting treatment (Case 1). The liver injury is usually mild to moderate in severity and self-limited in course. Immunoallergic and autoimmune features are not common.
In addition, zidovudine has been associated with instances of severe hepatic steatosis and lactic acidosis. The liver injury typically arises after 2 to 6 months of therapy with prodromal symptoms of nausea, vomiting, anorexia and weakness, followed by dyspnea, jaundice and confusion. Lactic acid levels are elevated and metabolic acidosis may be severe and progressive. Serum enzymes are only modestly elevated and may initially be normal. Jaundice and signs of liver dysfunction develop late, but can eventually be associated with acute hepatic failure and death. Liver histology shows marked steatosis which is initially microvesicular and later macrovesicular and associated with cholestasis. In progressive cases, fibrosis and Mallory bodies are found. Similar cases occur with didanosine and stavudine which are more frequent causes of this syndrome. Risk factors for mitochondrial injury due to zidovudine include female sex, older age, preexisting liver disease, obesity, alcohol use and concurrent use of other di-deoxynucleosides.
Finally, long term therapy with zidovudine and other first generation nucleoside analogues has been associated with development of noncirrhotic portal hypertension. Symptoms are typically suggestive of cirrhosis, with fatigue, weight loss, ascites and/or variceal hemorrhage, but liver histology shows little or no fibrosis. Jaundice is uncommon. Laboratory testing demonstrates minor elevations in serum aminotransferase and alkaline phosphatase levels but normal levels of serum bilirubin, albumin and INR. Splenomegaly is common and thrombocytopenia is often the first manifestation of the hepatic abnormalities. Liver biopsies can be deceptively benign, with scant fibrosis and minor nonspecific changes. The disturbed hepatic architecture may not be obvious unless reticulum stains are done that show the typical nodularity and two-cell thick plates characteristic of nodular regeneration. The cause of nodular regenerative hyperplasia is not known but is suspected to be due to chronic vascular injury to the small veins and arterioles in portal areas.
Likelihood score: B (likely cause of clinically apparent acute and chronic liver disease).
Mechanism of Injury
The cause of the microvesicular fat and hepatic failure with lactic acidosis appears to be due to inhibition of the mitochondrial gamma polymerase by zidovudine leading to mitochondrial depletion and dysfunction. Similar mitochondrial injury may occur in the pancreas causing pancreatitis, in muscle causing myopathy and in peripheral nerves causing neuropathy. The cause of other forms of clinically apparent liver injury due to zidovudine is unknown, but hypersensitivity may play a role. A similar mechanism may underlie the chronic liver injury from zidovudine which results in noncirrhotic portal hypertension. Genetic studies of children with inherited forms of noncirrhotic portal hypertension have shown homozygous mutations in genes involved with purine synthesis that are important in mitochondrial replication and integrity.
Outcome and Management
The severity of liver injury caused by zidovudine ranges from mild and transient enzyme elevations to severe fatty liver, hepatic failure and lactic acidosis leading to death. In milder instances when the agent is withdrawn early, recovery occurs but is typically slow, requiring 1 to 3 months before return of liver enzymes and lactate levels to normal. Rechallenge may lead to recurrence and should be avoided. Patients should also avoid exposure to the other nucleoside analogues linked to this syndrome, particularly didanosine and stavudine. Various interventions have been used in attempts to treat the severe lactic acidosis and hepatic steatosis induced by nucleoside analogues. These interventions have included bicarbonate infusions, thiamine, l-carnitidine as well as renal dialysis and mechanical ventilation. Intravenous 20% glucose decreases lactic acid levels in some patients. Liver transplantation has reversed lactic acidosis in the rare patient that has undergone emergency transplantation, but this option is rarely practical.
Nodular regenerative hyperplasia is a long term complication of zidovudine therapy and generally improves once the agent is withdrawn and the patient switched to other antiretroviral agents. Therapy is generally directed at control of the complications of portal hypertension, including esophageal varices, ascites and hepatic encephalopathy. Patients with this complication should not receive first generation nucleoside analogues (didanosine, stavudine, zidovudine). Severe intercurrent medical conditions such as pneumonia or sepsis can cause worsening of the portal hypertension and signs and symptoms of hepatic decompensation (jaundice, worsening ascites, hepatic encephalopathy).
Drug Class: Antiviral Agents, Antiretroviral Agents
Other Drugs in the Subclass, Nucleoside Analogues: Abacavir, Adefovir, Didanosine, Emtricitabine, Entecavir, Lamivudine, Stavudine, Telbivudine, Tenofovir
CASE REPORTS
Case 1. Cholestatic hepatitis due to zidovudine.(1)
A 39 year old man with HIV and AIDS was started on zidovudine and developed fever and abdominal pain 5 days later. Serum enzymes were minimally elevated, but bilirubin was normal. Zidovudine was stopped, but he required hospitalization 6 days later because of worsening abdominal pain, jaundice and fever. At this point, serum bilirubin was 7.9 mg/dL and both ALT and alkaline phosphatase were several fold elevated (Table). Rash and eosinophilia were not mentioned. Ultrasound of the liver showed no evidence of biliary obstruction. Serum tests for hepatitis A and B were normal. He drank little alcohol and had no known exposures to hepatitis. He improved on no specific therapy and was discharged; liver tests abnormalities resolved within 4 weeks. He was then restarted on zidovudine at a reduced dose. Seven days later he redeveloped fever and abdominal pain and liver tests were again abnormal. Zidovudine was stopped and all abnormalities resolved within 4 weeks. He subsequently had no further evidence of liver disease, but died of AIDS-related complications approximately one-and-a-half years later.
Key Points
| Medication: | Zidovudine (100 mg five times daily) |
|---|---|
| Pattern: | Cholestatic (R=0.7) |
| Severity: | 3+ |
| Latency: | 5-6 days |
| Recovery: | 4 weeks |
| Other medications: | Trimethoprim-sulfamethoxazole, dicyclomine |
Laboratory Values
| Time After Starting | Time After Stopping | AST* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Pre | 49 | 184 | 0.3 | |
| Zidovudine (200 mg every 6 hours) given for 7 days | |||||
| 7 days | 0 | 31 | 126 | 0.4 | |
| 12 days | 6 days | 182 | 828 | 4.8 | |
| 14 days | 8 days | 256 | 1587 | 5.7 | |
| 16 days | 10 days | 238 | 1564 | 4.7 | |
| 18 days | 12 days | 168 | 1173 | 1.0 | |
| 24 days | 18 days | 70 | 782 | ||
| 4 weeks | 24 days | 38 | 368 | 0.7 | |
| 5 weeks | 4 weeks | 28 | 288 | 0.5 | |
| Zidovudine (100 mg every 6 hours) restarted for 7 days | |||||
| 6 weeks | 0 | 88 | 633 | ||
| 7 weeks | 1 week | 42 | 380 | 0.4 | |
| 12 weeks | 5 weeks | 24 | 126 | 0.3 | |
| Normal Values | <35 | <115 | <1.2 | ||
- *
Estimated from Figure and converted from times upper limit of normal to specific values based upon normal values shown.
Comment
Case of cholestatic hepatitis with short latency (<1 week) induced by zidovudine. The injury had a prodrome of fever and abdominal pain arising after 5 days of therapy that preceded the elevations in liver enzymes and serum bilirubin. Jaundice arose several days after zidovudine was stopped, serum bilirubin peaking 1 week later. The article did not mention skin rash, lymphadenopathy, lymphocytosis or eosinophilia, but the abrupt onset and presence of fever suggested an immunoallergic cause. The recurrence of cholestatic liver enzyme elevations on reexposure to zidovudine strengthened the association of the liver injury with this drug. The importance of zidovudine in therapy of HIV infection at the time of this clinical report justified the rechallenge effort to exonerate zidovudine as the cause of the liver injury.
Case 2. Lactic acidosis with severe hepatic steatosis due to zidovudine therapy.(2)
A 48 year old man with HIV and AIDS on long term zidovudine therapy developed nausea, abdominal pain and dyspnea. He had severe lactic acidosis with a bicarbonate level of 5 mg/dL, and serum lactate of 20.2 mmol/L (normal <3.0). Liver tests showed ALT 109 U/L and alkaline phosphatase of 207 U/L. His liver tests had previously been normal. He tested negative for serological evidence of hepatitis B or C. Other medications which he had been taking chronically included ranitidine and trimethoprim-sulfamethoxazole. After admission, he worsened rapidly with progressive obtundation, respiratory failure and intractable metabolic acidosis. An autopsy showed a massive liver (3.7 kg) with both macro- and microvesicular fatty change.
Key Points
| Medication: | Zidovudine |
|---|---|
| Pattern: | Mild elevations only |
| Severity: | 5+ (fatal lactic acidosis and hepatic failure) |
| Latency: | Several years |
| Recovery: | None |
| Other medications: | Trimethoprim-sulfamethoxazole, ranitidine |
Comment
Severe but typical course of lactic acidosis with microvesicular fatty liver that can occur with nucleoside analogue therapy. Despite the severity of the liver involvement, serum enzyme elevations may be normal or only modestly elevated. Initial signs of hepatic synthetic failure may be fall of albumin or rising prothrombin time and hepatic encephalopathy. Frequently, as in this case, lactic acidosis is the major clinical problem and cause of multiorgan failure. Pancreatitis can also complicate the course of nucleoside analogue induced mitochondrial injury and may be the immediate cause of death. The underlying pathogenesis of this form of hepatotoxicity appears to be mitochondrial injury or depletion caused by inhibition of the mitochondrial gamma polymerase which is responsible for replication of mitochondria DNA. Different nucleoside analogues have different degrees of likelihood of causing mitochondrial toxicity: fialuridine >> stavudine, zalcitabine and didanosine > zidovudine >> lamivudine, abacavir, emtricitabine, telbivudine, tenofovir, adefovir and entecavir (the last group may not cause this syndrome at all). In addition, different nucleoside analogues appear to cause different degrees of mitochondrial injury in different tissues. Thus, myopathy and neuropathy are most frequent with zidovudine, pancreatitis with didanosine and stavudine, and lactic acidosis with microvesicular fatty liver with fialuridine.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Zidovudine – Generic, Retrovir®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Zidovudine | 30516-87-1 | C10-H13-N5-O4 |
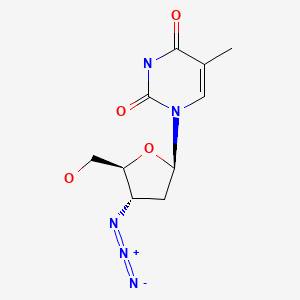
|
CITED REFERENCES
- 1.
- Dubin G, Braffman MN. Zidovudine-induced hepatotoxicity. Ann Intern Med. 1989;110:85. [PubMed: 2908831]
- 2.
- Fortgang IS, Belitsos PC, Chaisson RE, Moore RD. Hepatomegaly and steatosis in HIV-infected patients receiving nucleoside analog antiretroviral therapy. Am J Gastroenterol. 1995;90:1433–6. [PubMed: 7661164]
ANNOTATED BIBLIOGRAPHY
References updated: 25 June 2020
Abbreviations used: AIDS, acquired immune deficiency syndrome; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPA, hepatoportal sclerosis; HVPG, hepatic venous pressure gradient; mt, mitochondrial; NASH, nonalcoholic steatohepatitis; NCPH, noncirrhotic portal hypertension; NRH, nodular regenerative hyperplasia; TE, transient elastography.
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-18.(Review of hepatotoxicity of antiviral agents including zidovudine).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1137-58.(Textbook of pharmacology and therapeutics).
- National Institutes of Health. http://aidsinfo
.nih.gov/guidelines. (Clinical guidelines on the use of antiretroviral agents in HIV-1 infected adults, adolescents and children). - Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:192–7. [PubMed: 3299090](Controlled trial in 282 patients with AIDs reported that rates of serum enzyme elevations were similar in placebo as zidovudine-treated patients).
- Melamed AJ, Muller RJ, Gold JW, Campbell SW, Kleinberg MI, Armstrong D. Possible zidovudine-induced hepatotoxicity. JAMA. 1987;258:2063. [PubMed: 3477654](3 of 28 patients with HIV infection developed liver test abnormalities 2-3 weeks after starting zidovudine [AST of 161, 196 and 684 U/L, Alk P of 112 and 351 U/L], resolving rapidly with stopping and not recurring with reexposure: no mention of bilirubin or symptoms).
- Dubin G, Braffman MN. Zidovudine-induced hepatotoxicity. Ann Intern Med. 1989;110:85. [PubMed: 2908831](39 year old man with HIV and AIDS developed fever and abdominal pain 7 days after starting zidovudine [bilirubin 6.5 mg/dL, ALT 122 U/L, Alk P 598 U/L], resolving rapidly with stopping and recurring within 7 days upon restarting: Case 1).
- Jolliet P, Widman JJ. Reye’s syndrome in adult with AIDS. Lancet. 1990;335:1457. [PubMed: 1972225](26 year old woman with HIV infection on zidovudine for 1 year developed fever and cough and was given aspirin [3 g/day], developed lethargy and abnormal liver tests 12 days later with progressive liver failure and death; macrovesicular fat considered typical of Reyes syndrome found on liver biopsy).
- Hochster H, Dieterich D, Bozzette S, Reichman RC, Connor JD, Liebes L, Sonke RL, et al. Toxicity of combined ganciclovir and zidovudine for cytomegalovirus disease associated with AIDS. An AIDS Clinical Trials Group Study. Ann Intern Med. 1990;113:111–7. [PubMed: 2163228](40 patients with HIV and CMV infection were treated with zidovudine and intravenous ganciclovir; anemia and neutropenia were common; 1 patient developed hepatitis thought to be zidovudine related, resolving on stopping and recurring on restarting zidovudine [timing and details not given]).
- Kahn JO, Lagakos SW, Richman DD, Cross A, Pettinelli C, Liou S-H, Broom M, et al. A controlled trial comparing continued zidovudine with didanosine in human immunodeficiency virus infection. N Engl J Med. 1992;327:581–7. [PubMed: 1353607](913 patients with HIV infection on zidovudine were randomized to switch to didanosine [500 or 750 mg/day] or continue zidovudine; pancreatitis occurred in 7% and 13% of didanosine, but only 3% of zidovudine-treated patients; no mention of ALT levels of hepatotoxicity).
- Division of AIDS, National Institute of Allergy and Infectious Diseases. Table for grading severity of adult adverse experiences. Version 1.0, Clarification August 2009. Website accessed November 2011. http://www
.docstoc.com /docs/42821660/DAIDS-Toxicity-Table. (Tables for grading ALT, AST, Alk P and bilirubin elevations for the AIDS Clinical Trials Group, all expressed as upper limits of normal [ULN]; for ALT, AST, Alk P and GGT: grade 1 [mild]=1.25-2.5; grade 2 [moderate]=2.6-5.0; grade 3 [severe]=5.1-10.0; and grade 4 [potentially life-threatening]=>10.0 times ULN). - Chen SC, Barker SM, Mitchell DH, Stevens SM, O’Neill P, Cunningham AL. Concurrent zidovudine-induced myopathy and hepatotoxicity in patients treated for human immunodeficiency virus(HIV) infection. Pathology. 1992;24:109–11. [PubMed: 1641255](38 year old man with AIDS on zidovudine for 4 months developed muscle and abdominal pain [bilirubin 0.8 mg/dL, ALT, 115 U/L, Alk P 62 U/L, CPK 1926 U/L], liver biopsy showing macrovesicular steatosis and abnormalities, resolving within 3 months of stopping).
- Gradon JD, Chapnik EK, Sepkowitz DV. Zidovudine-induced hepatitis. J Intern Med. 1992;231:317–8. [PubMed: 1556529](38 year old man developed tender hepatomegaly 3 months after starting zidovudine and dapsone [bilirubin 2.0 mg/dL, ALT 120 U/L, Alk P 104 U/L], worsening in next 4 weeks despite stopping dapsone [bilirubin 9.7 mg/dL, ALT 873 U/L], but rapidly resolving within 10 days of stopping zidovudine).
- Shriner K, Goetz M. Severe hepatotoxicity in a patient receiving both acetaminophen and zidovudine. Am J Med. 1992;93:94–6. [PubMed: 1626578](31 year old man with AIDS, malnutrition and pancreatitis on long term zidovudine and TMP/SMS developed fever, rash, cellulitis and marked ALT elevations 3 days after starting iv cephalothin and receiving 3.3 g of acetaminophen [bilirubin 1.2 mg/dL], resolving rapidly upon stopping, but role of zidovudine unlikely).
- Chattha G, Arieff AI, Cumings G, Tierney LM Jr. Lactic acidosis complicating the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118:37–9. [PubMed: 8416156](Report of 7 patients with HIV infection who developed lactic acidosis of unknown cause who presented with nausea, anorexia and weight loss followed by dyspnea, stupor and death [in 4]; 4 on zidovudine, 1 ganciclovir and 1 clofazimine; initial arterial pH 7.09-7.27, lactate 10.4-17.4 mmol/L).
- Shintaku M, Nasu K, Shimizu T. Fulminant hepatic failure in an AIDS patient: possible zidovudine-induced hepatotoxicity. Am J Gastroenterol. 1993;88:464–6. [PubMed: 8438867](38 year old man with hemophilia and HIV-HBV coinfection developed jaundice within 3 weeks of starting zidovudine [bilirubin 6.0 mg/dL, ALT 2,267 U/L], progressing to hepatic failure and death, with autopsy showing massive necrosis and cirrhosis; cause unclear, but possibly due to immune reconstitution and acute reactivation of hepatitis B).
- Freiman JP, Helfert KE, Hammrell MR, Stein DS. Hepatomegaly with severe steatosis in HIV-seropositive patients. AIDS. 1993;7:379–85. [PubMed: 8471200](8 patients with HIV who developed severe hepatomegaly and marked steatosis which was associated with hepatic failure and lactic acidosis, 6 being fatal and 2 with recovery; all had received zidovudine, but for varying periods and often stopped well before onset of symptoms from hepatotoxicity).
- Le Bras P, D’Oiron R, Quertainmont Y, Halfon P, Caquet R. Metabolic, hepatic and muscular changes during zidovudine therapy: a drug-induced mitochondrial disease? AIDS. 1994;8:716–7. [PubMed: 8060560](32 year old man with hemophilia and HIV infection developed severe myopathy and fatty liver 8 months after starting zidovudine [CPK 1138 U/L and mild lactic acidosis], resolving with stopping drug).
- McKenzie R, Fried MW, Sallie R, Conjeevaram H, Di Bisceglie AM, Park Y, Savarese B, et al. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med. 1995;333:1099–105. [PubMed: 7565947](Description of syndrome of lactic acidosis, hepatic failure and pancreatitis arising after 8-11 weeks of fialuridine treatment in 15 patients with chronic hepatitis B; among 7 patients affected, 5 died of intractable lactic acidosis and 2 survived but required emergency liver transplantation).
- Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. 1995;1:417–22. [PubMed: 7585087](Review of mechanisms for mitochondrial injury by nucleoside analogues including inhibition of mitochondrial DNA polymerase gamma).
- Styrt B, Freiman JP. Hepatotoxicity of antiviral agents. Gastroenterol Clin North Am. 1995;24:839–52. [PubMed: 8749901](Early review of liver toxicity of antiviral agents; covering the first four nucleoside analogues used for HIV infection, zidovudine, didanosine, zalcitabine and stavudine).
- Wassef M, Keiser P. Hypersensitivity to zidovudine: report of a case of anaphylaxis and review of the literature. Clin Infect Dis. 1995;20:1387–9. [PubMed: 7620029](41 year old man with HIV infection, developed weakness and confusion 1 hour after a second dose of zidovudine with erythema, facial swelling and fever [ALT 157 U/L], resolving within 48 hours of stopping and recurring within 1 hour of single dose rechallenge with zidovudine; no mention of jaundice).
- Fortgang IS, Belitsos PC, Chaisson RE, Moore RD. Hepatomegaly and steatosis in HIV-infected patients receiving nucleoside analog antiretroviral therapy. Am J Gastroenterol. 1995;90:1433–6. [PubMed: 7661164](Retrospective review of 1836 patients treated for HIV infection found 322 [18%] with liver abnormalities and two [1.3 per 1000 person-years] with fatal lactic acidosis and fatty liver, both on zidovudine only).
- Olano JP, Borucki MJ, Wen JW, Haque AK. Massive hepatic steatosis and lactic acidosis in a patient with AIDS who was receiving zidovudine. Clin Infect Dis. 1995;21:973–6. [PubMed: 8645849](35 year old woman developed nausea, vomiting and weakness 5 months after starting zidovudine [bilirubin 0.9 mg/dL, ALT 85 U/L, Alk P 85 U/L], worsening over next week with severe lactic acidosis and pancreatitis despite stopping zidovudine promptly; autopsy showed massively enlarged liver [3.6 kg] with marked steatosis, but little inflammation or necrosis).
- Aggarwal A, al Talib K, Alabrash M. Type B lactic acidosis in an AIDS patient treated with zidovudine. Md Med J. 1996;45:929–31. [PubMed: 8942169](33 year old woman with HIV infection developed anorexia, weight loss, and tachypnea 9 months after starting zidovudine [lactate 16.3 mmol/L, pH 7.3 falling to 7.0, CT showing fatty liver, enzymes and bilirubin not given], dying of progressive lactic acidosis 3 days later).
- Henry K, Acosta EP, Jochimsen E. Hepatotoxicity and rash associated with zidovudine and zalcitabine chemoprophylaxis. Ann Intern Med. 1996;124:855. [PubMed: 8610958](Two nurses receiving zidovudine and zalcitabine for prevention of HIV infection after accidental needlestick for 3 weeks had fever, rash and nausea and liver test abnormalities, resolving rapidly with stopping drugs).
- Charton-Bain MC, Flamant M, Aubertin JM, Belair MF, Gilquin J, Kazatchkine M, Bruneval P. Gastroenterol Clin Biol. 1997;21:979–81. [Lactic acidosis and hepatic mitochondrial changes during a treatment with zidovudine] [PubMed: 9587562](33 year old man with HIV infection developed lactic acidosis 8 months after starting zidovudine [pH 7.25, lactate 8 mmol/L, ALT and Alk P normal], with biopsy showing marked steatosis and abnormal mitochondria).
- Sundar K, Suarez M, Banogon PE, Shapiro JM. Zidovudine-induced fatal lactic acidosis and hepatic failure in patients with acquired immunodeficiency syndrome: report of two patients and review of the literature. Crit Care Med. 1997;25:1425–30. [PubMed: 9267960](47 year old man and 57 year old woman with HIV infection presented with weakness and fatigue 6 and 9 months after starting zidovudine [bilirubin 2.7 and 0.8 mg/dL, ALT 85 and 109 U/L and Alk P 59 U/L], with progressive lactic acidosis and death; autopsy showed extensive hepatic steatosis).
- Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JAM, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as a common pathway. AIDS. 1998;12:1735–44. [PubMed: 9792373](Review of mitochondrial function and role of mitochondrial toxicity or depletion in the adverse side effects of nucleoside analogues).
- Blanche S, Rustin P, Slama A, Barret B, Firtion G, Ciraru-Vigneron N, Lacrois C, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–9. [PubMed: 10509500](Screening for mitochondrial symptoms among children born to HIV-positive mothers treated with zidovudine during pregnancy identified 6 with mitochondrial dysfunction, ages a few months to 4 years, hyperlactatemia in 5, liver enzyme elevations in 3, hyperamylasemia in 2).
- Acosta BS, Grimsley EW. Zidovudine-associated type B lactic acidosis and hepatic steatosis in an HIV-infected patient. South Med J. 1999;92:421–3. [PubMed: 10219365](34 year old woman with HIV infection developed nausea and weakness 1 year after starting zidovudine, with severe lactic acidosis but normal liver tests initially, progression to multiorgan failure and death, autopsy showed massive hepatomegaly and steatosis).
- Chariot P, Drogou I, de Lacroix-Szmania I, Eliezer-Vanerot M, Chazaud B, Lombès A, Schaeffer A, et al. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J Hepatol. 1999;30:156–60. [PubMed: 9927163](57 year old man with HIV infection developed nausea and fatigue with progressive lactic acidosis and death 6 months after starting a second course of zidovudine [bilirubin normal, ALT 8 times ULN, Alk P normal], autopsy showing massive micro- and macro-steatosis).
- Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. [PubMed: 10632283](Among 298 patients with HIV infection, ALT elevations above 5 times ULN occurred in 10.4% per year during antiretroviral treatment; factors associated with ALT elevations included ritonavir [27.3%] and coinfection with either HCV or HBV; ALT with bilirubin elevations occurred in 3 patients; 2 on indinavir and all 3 with coinfection).
- Velasco M, Guijarro C. Elevated liver enzymes following initiation of antiretroviral therapy. JAMA. 2000;283:2526–7. [PubMed: 10815112](Letter in response to Sulkowski et al. [JAMA 2000] pointing out that antiretroviral therapy can cause immune reconstitution and flares of hepatitis B or C, which may be misdiagnosed as hepatotoxicity).
- Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Elevated liver enzymes following initiation of antiretroviral therapy. JAMA. 2000;283:2526–7. [PubMed: 10815113](Reply to Velasco and Guijarro pointing at that the majority of the ALT elevations described could not be attributed to immune reconstitution).
- Pai VB, Koranyi K, Nahata MC. Acute hepatitis and bleeding possibly induced by zidovudine and ritonavir in an infant with HIV infection. Pharmacotherapy. 2000;20:1135–40. [PubMed: 10999509](9 year old boy with HIV infection and multiple complications developed fever, thrombocytopenia and hepatitis 2 months after starting zidovudine, lamivudine and ritonavir, with progressive liver failure and severe bleeding after liver biopsy; difficult to assign specific causality to a specific agent).
- Hill JB, Sheffield JS, Zeeman GG, Wendel GD Jr. Hepatotoxicity with antiretroviral treatment of pregnant women. Obstet Gynecol. 2001;98:909–11. [PubMed: 11704198](Two cases, 28 year old woman on zidovudine, lamivudine and efavirenz developed jaundice at 18 weeks gestation [bilirubin 20.3 mg/dL, ALT 421 U/L], remaining jaundiced until delivered at 27 weeks, resolving within 5 months after delivery; 22 year old woman started on lamivudine, zidovudine and nelfinavir at 14 weeks gestation and developed jaundice 10 weeks later [bilirubin 8.9 mg/dL, ALT 1598 U/L], progressing to acute liver failure and death).
- John M, Moore CB, James IR, Nolan D, Upton RP, McKinnon EJ, Mallal SA. Chronic hyperlactatemia in HIV-infected patients taking antiretroviral therapy. AIDS. 2001;15:717–23. [PubMed: 11371686](349 patients with HIV infection were screened for lactate levels on multiple occasions; 2 had lactic acidosis and both were symptomatic and on stavudine: estimated incidence of 3.9 per 1000 person years).
- Church JA, Mitchell WG, Gonzalez-Gomez I, Christensen J, Vu TH, Dimauro S, Boles RG. Mitochondrial DNA depletion, near-fatal metabolic acidosis, and liver failure in an HIV-infected child treated with combination antiretroviral therapy. J Pediatr. 2001;138:748–51. [PubMed: 11343055](2 year old boy treated for 18 months with didanosine, zidovudine and nelfinavir developed lactic acidosis and hepatic failure [bilirubin 30.6 mg/dL, AST 224 U/L], resolving slowly with stopping drugs).
- Coghlan ME, Sommadossi JP, Jhala NC, Many WJ, Saag MS, Johnson VA. Symptomatic lactic acidosis in hospitalized antiretroviral-treated patients with human immunodeficiency virus infection: a report of 12 cases. Clin Infect Dis. 2001;33:1914–21. [PubMed: 11692304](Experience with 12 cases of lactic acidosis in patients with HIV infection seen over 6 years, typically with anorexia, nausea and weight loss for several weeks, AST variably elevated, 11 on stavudine, 9 didanosine, 1 zidovudine alone; 6 with pancreatitis, 6 having liver biopsies all showed macro- and micro-steatosis; five died).
- Clark SJ, Creighton S, Portmann B, Taylor C, Wendon JA, Cramp ME. Acute liver failure associated with antiretroviral treatment for HIV: a report of six cases. J Hepatol. 2002;36:295–301. [PubMed: 11830344](6 patients with HIV infection who developed acute liver failure on stavudine [n=5], lamivudine [n=3], didanosine [n=2], saquinavir [n=2], efavirenz [n=2], nevirapine [n=2], or nelfinavir, delavirdine or zidovudine [n=1] for 1-3 months [peak bilirubin 2.7-32 mg/dL, AST 240-8650 U/L, Alk P 122-191 U/L]; 2 with signs of hypersensitivity; two with hepatitis B; 5 died, autopsies showing massive necrosis; one with massive steatosis; likely multiple causes).
- Hillaire S, Bonte E, Denninger MH, Casadevall N, Cadranel JF, Lebrec D, Valla D, et al. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002;51:275–80. [PMC free article: PMC1773310] [PubMed: 12117894](Among 28 cases of NCPH diagnosed between 1994 and 1998, none were diagnosed with HIV infection and no discussion of drug relatedness; 12 had a prothrombotic disorder).
- Falcó V, Rodríguez D, Ribera E, Martínez E, Miró JM, Domingo P, Diazaraque R, et al. Severe nucleoside-associated lactic acidosis in human immunodeficiency virus-infected patients: report of 12 cases and review of the literature. Clin Infect Dis. 2002;34:838–46. [PubMed: 11850865](Between 1997-2000, 12 cases of lactic acidosis in HIV-infected patients identified at 4 hospitals in Spain; ~1:1000 patient-years of treatment; all were receiving nucleoside analogues for 1-36 months; 1 case attributed to zidovudine, 11 to stavudine [1 also on didanosine], ALT 30-524 U/L, 33% fatality rate).
- Spengler U, Lichterfeld M, Rockstroh JK. Antiretroviral drug toxicity—a challenge for the hepatologist? J Hepatol. 2002;36:283–94. [PubMed: 11830343](Review of the diagnosis of drug induced liver disease in patients with HIV on antiretroviral agents, with discussion of mechanisms including mitochondrial toxicity and hypersensitivity reactions).
- Koch RO, Graziadei IW, Zangerle R, Romani N, Maier H, Vogel W. Acute hepatic failure and lactate acidosis associated with antiretroviral treatment for HIV. Wien Klin Wochenschr. 2003;115:135–40. [PubMed: 12674693](HIV-positive woman taking stavudine and didanosine for 18 months developed lactic acidosis with renal and hepatic failure, but recovered with stopping agents; liver biopsy showed microvesicular steatosis and giant mitochondria).
- Bonnet F, Bonarek M, Morlat P, Mercie P, Dupon M, Gemain MC, Malvy D, et al. Risk factors for lactic acidosis in HIV-1-infected patients treated with nucleoside reverse-transcriptase inhibitors: a case-control study. Clin Infect Dis. 2003;36:1324–8. [PubMed: 12746780](Case control study of 9 patients [5 women] with HIV infection and lactic acidosis, 6 with hepatomegaly, 5 with jaundice, 8 on stavudine, 7 on didanosine, 6 on zidovudine; 6 died; risk factors were renal insufficiency and low CD4 counts, but numbers of cases were few).
- Arenas-Pinto A, Grant AD, Edwards S, Weller IVD. Lactic acidosis in HIV-1 infected patients: a systematic review of published cases. Sex Transm Infect. 2003;79:340–3. [PMC free article: PMC1744718] [PubMed: 12902594](Review of 217 published cases of lactic acidosis; 53% female, all were taking at least one nucleoside for 1-36 months, 61% on stavudine, 33% didanosine, 31% zidovudine, 30% lamivudine; 92% had hepatic steatosis on biopsy or autopsy; 48% died).
- Lonergan JT, Barber RE, Mathews WC. Safety and efficacy of switching to alternative nucleoside analogues following symptomatic hyperlactatemia and lactic acidosis. AIDS. 2003;17:2495–9. [PubMed: 14600521](12 patients with HIV infection on antiretrovirals who developed hyperlactatemia >5 mmol/L [2 with acidosis; all on stavudine] stopped therapy and improved, restarting nucleosides without stavudine was tolerated in all but one case [on zidovudine], who later tolerated lamivudine and abacavir).
- Kontorinis N, Dieterich D. Hepatotoxicity of antiretroviral therapy. AIDS Rev. 2003;5:36–43. [PubMed: 12875106](Review of hepatotoxicity of antiretroviral drugs; definition of hepatotoxicity in antiretroviral studies; grade 1=1.25-2.5 times, grade 2=2.6-5 times, grade 3=5.1-10 times and grade 4=>10 times ULN or baseline ALT values; abacavir and lamivudine are least likely to cause hepatotoxicity).
- Ogedegbe AE, Thomas DL, Diehl AM. Hyperlactataemia syndromes associated with HIV therapy. Lancet Infect Dis. 2003;3:329–37. [PubMed: 12781504](Review of mechanisms of hyperlactatemia with antiretroviral therapy, occurs mostly with use of nucleoside analogues, stavudine, didanosine and zidovudine, attributed to mitochondrial depletion, but other mechanisms may be involved).
- Ofotokun I, Pomeroy C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med. 2003;11:55–9. [PubMed: 12717043](Review of sex differences in adverse events; higher frequency of mitochondrial toxicity and hypersensitivity in women than men).
- Ogedegbe AO, Sulkowski MS. Antiretroviral-associated liver injury. Clin Liver Dis. 2003;7:475–99. [PubMed: 12879995](Review of hepatotoxicity of antiretrovirals; ALT elevations above 5 times ULN reported in 7% with zidovudine, 16% didanosine, 9-13% stavudine, <1% lamivudine, tenofovir and abacavir, 3-10% protease inhibitors, 10% nevirapine and 8% efavirenz; recommend monitoring at 4 weeks and then every 12 weeks, stopping if ALT levels are >10 times ULN or if symptoms of liver injury are present, monitoring more closely if ALT levels are elevated).
- Abrescia N, D’Abbraccio M, Figoni M, Busto A, Maddaloni A, De Marco M. Hepatotoxicity of antiretroviral drugs. Curr Pharm Des. 2005;11:3697–710. [PubMed: 16305505](Review of hepatotoxicity of antiretrovirals; major syndrome with nucleoside analogues is mitochondrial injury with lactic acidosis and severe hepatomegaly and steatosis).
- Jain MK. Drug-induced liver injury associated with HIV medications. Clin Liver Dis. 2007;11:615–39. vii-viii. [PubMed: 17723923](Review of hepatotoxicity of antiretroviral medications; ALT elevations occur in 2-18% of patients, but often resolve spontaneously even without dose modification; classes of injury include hypersensitivity [nevirapine, efavirenz, abacavir], mitochondrial injury [stavudine, didanosine, zidovudine], flares of hepatitis B [lamivudine, emtricitabine, tenofovir], flares of hepatitis C [any potent regimen], idiosyncratic injury [ritonavir, nevirapine, efavirenz], cholestatic hepatitis [many agents]).
- Wester CW, Okezie OA, Thomas AM, Bussmann H, Moyo S, Muzenda T, Makhema J, et al. Higher-than-expected rates of lactic acidosis among highly active antiretroviral therapy-treated women in Botswana: preliminary results from a large randomized clinical trial. J Acquir Immune Defic Syndr. 2007;46:318–22. [PubMed: 18090299](Among 650 patients starting antiretroviral therapy, 2% developed hyperlactaemia [>4.4 mmol/L] and 1% lactic acidosis [all female, trend towards older age and higher body mass index] all on stavudine, didanosine, and/or zidovudine; 6 died, 4 of pancreatitis).
- Bolhaar MG, Karstaedt AS. A high incidence of lactic acidosis and symptomatic hyperlactatemia in women receiving highly active antiretroviral therapy in Soweto, South Africa. Clin Infect Dis. 2007;45:254–60. [PubMed: 17578788](Among 1735 adults with HIV infection started on antiretroviral therapy, 3% developed hyperlactatemia; 23 [1%] with lactic acidosis [22 women and 22 on stavudine, 1 on didanosine and zidovudine], 30% mortality; 44 had symptomatic hyperlactatemia [37 women, all 44 on stavudine, 3 also with didanosine], able to switch to zidovudine without recurrence, 2 of 3 relapsed on restarting stavudine).
- Lactic Acidosis International Study Group. Risk factors for lactic acidosis and severe hyperlactataemia in HIV-1-infected adults exposed to antiretroviral therapy. AIDS. 2007;21:2455–64. [PubMed: 18025882](Retrospective case control study of 110 cases with hyperlactataemia vs 220 controls, identified risk factors of older age, female gender, low CD4 counts, and use of stavudine, didanosine or both).
- Mallet V, Blanchard P, Verkarre V, Vallet-Pichard A, Fontaine H, Lascoux-Combe C, Pol S. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS. 2007;21:187–92. [PubMed: 17197809](Among 8 patients with HIV infection referred for evaluation of liver disease of unknown cause, all had nodular regenerative hyperplasia and had received didanosine [and many received stavudine or zidovudine] for 1 to 2 years [bilirubin 0.2-2.0 mg/dL, ALT 0.4-2.0 times ULN, Alk P 0.9-19.1 times ULN, platelets 71-149,000/μL], all had varices and 5 had ascites).
- Castelnuovo B, Nanyonjo A, Kamya M, Ocama P. Is it safe to switch from stavudine to zidovudine after developing symptomatic hyperlactatemia? Afr Health Sci. 2008;8:133–4. [PMC free article: PMC2584324] [PubMed: 19357764](60 year old woman developed symptomatic lactatemia 19 months after starting stavudine, lamivudine and nevirapine; switching to zidovudine was followed by fall of lactate levels to normal [5.2→1.6 mmol/L], but 10 months later levels rose again [3.7 mmol/L]).
- Stead D, Osler M, Boulle A, Rebe K, Meintjes G. Severe hyperlactataemia complicating stavudine first-line antiretroviral therapy in South Africa. Antivir Ther. 2008;13:937–43. [PubMed: 19043928](Retrospective analysis of 75 patients with HIV infection and symptomatic hyperlactatemia; 95% women, all on stavudine [1.7 per 100 patient-years], 71% with lactic acidosis, 12 died; 30 switched to zidovudine without recurrence).
- Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, et al. International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. [PubMed: 18677028](Most recent recommendations on use of antiviral therapy in adults with HIV infection including use of recently approved agents, raltegravir, maraviroc and etravirine).
- Inductivo-Yu I, Bonacini M. Highly active antiretroviral therapy-induced liver injury. Current Drug Safety. 2008;3:4–13. [PubMed: 18690975](Review of drug induced liver injury due to antiretroviral agents).
- Soriano V, Puoti M, Garcia-Gascó P, Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. [PubMed: 18090386](Review of hepatotoxicity of antiretroviral drugs with recommendations on management, stopping therapy if symptoms arise, with overt jaundice [direct bilirubin], evidence of mitochondrial toxicity, ALT >10 times ULN, ALT at lower levels if newly marketed agent; important to rule out other causes; problematic agents include didanosine, stavudine and zidovudine, nevirapine and efavirenz, full dose ritonavir and tipranavir).
- Bae WH, Wester C, Smeaton LM, Shapiro RL, Lockman S, Onyait K, Thior I, et al. Hematologic and hepatic toxicities associated with antenatal and postnatal exposure to maternal highly active antiretroviral therapy among infants. AIDS. 2008;22:1633–40. [PMC free article: PMC2664540] [PubMed: 18670224](Prospective monitoring found that only 1 of 69 infants born to antiretroviral treated mothers and none of 109 infants born to drug-therapy unexposed mothers with HIV infection developed ALT elevations >5 times ULN during the first 7 months of life).
- Kumarasamy N, Venkatesh KK, Cecelia AJ, Devaleenal B, Lai AR, Saghayam S, Balakrishnan P, Yepthomi T, Poongulali S, Flanigan TP, Solomon S, Mayer KH. Spectrum of adverse events after generic HAART in southern Indian HIV-infected patients. AIDS Patient Care STDS. 2008;22:337–44. [PubMed: 18422462](Among 3154 patients with HIV infection treated with antiretroviral agents over a 4 year period at a single center in Southern India, hepatitis occurred in 3.5% usually within the first 3 months; lactic acidosis was more common in women and was associated with stavudine use).
- Van Dyke RB, Wang L, Williams PL. Pediatric AIDS Clinical Trials Group 219C Team. Toxicities associated with dual nucleoside reverse-transcriptase inhibitor regimens in HIV-infected children. J Infect Dis. 2008;198:1599–608. [PMC free article: PMC2737265] [PubMed: 19000014](Among 2233 children receiving two nucleoside reverse-transcriptase inhibitors, 0.6-1.6% developed hepatitis, 8.3-13.5% acidosis; overall ALT elevations were rare [<1%]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 7 were attributed to antiretroviral agents, 2 nevirapine, 1 efavirenz and 4 miscellaneous combinations).
- Ingiliz P, Benhamou Y. Elevated liver enzymes in HIV monoinfected patients on HIV therapy: what are the implications? J HIV Ther. 2009;14:3–7. [PubMed: 19731558](Review of the causes of serum enzyme elevations during antiretroviral therapy; nucleoside reverse transcriptase inhibitors are capable of causing lactic acidosis, most commonly with zalcitabine, didanosine, stavudine and zidovudine; abacavir has been linked to hypersensitivity reactions and rare instances of hepatic failure).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, 3 antiretroviral agents were among the top 40 cases, including zidovudine [8th, 106 cases], lamivudine [26th, 45 cases] and nevirapine [36th, 37 cases]).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 4 to antiretroviral agents, including 3 to combinations with stavudine and 1 to abacavir).
- Schiano TD, Uriel A, Dieterich DT, Fiel MI. The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV. Virchows Arch. 2011;458:231–5. [PubMed: 21057809](45 year old man with HIV infection developed abnormal liver tests 4 years after starting didanosine [bilirubin normal, ALT 87 U/L, Alk P 286 U/L], biopsy showing nodular regeneration which progressed despite stopping and 5 years later he presented with varices and hepatomegaly [bilirubin 1.0 mg/dL, ALT 43 U/L, Alk P 166 U/L, platelets 104,000/μL]).
- Lopez-Delgado JC, Mendiluce RM, Pinol TS, Fernández XP, Sanchez L, Vicente RG. Urgent liver transplantation for nevirapine-induced acute liver failure: report of a case and review of the literature. Ann Transplant. 2012;17:122–7. [PubMed: 22466918](40 year old woman developed fever and jaundice 4 weeks after switching nevirapine for lopinavir/ritonavir in a antiretroviral regimen [bilirubin 5.0 mg/dL, ALT 5200 U/L, GGT 258 U/L, INR 4.5], with progressive hepatic failure and successful liver transplant 3 days later).
- Nielsen-Saines K, Watts DH, Veloso VG, Bryson YJ, Joao EC, Pilotto JH, Gray G, et al. NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368–79. [PMC free article: PMC3590113] [PubMed: 22716975](Controlled trial of 3 zidovudine based regimens for newborns of HIV infected mothers found ALT elevations above 3 times ULN in 14% of those receiving zidovudine alone, but none developed clinically apparent liver injury).
- Agu KA, Oparah AC. Adverse drug reactions to antiretroviral therapy: Results from spontaneous reporting system in Nigeria. Perspect Clin Res. 2013;4:117–24. [PMC free article: PMC3700325] [PubMed: 23833736](Among 1119 adverse drug reactions reported among HIV treated subjects in Nigeria, only 2 [0.1%] were due to hepatotoxicity).
- Pugi A, Bonaiuti R, Maggini V, Moschini M, Tuccori M, Leone R, Rossi M, et al. Safety profile of antiviral medications: a pharmacovigilance study using the Italian spontaneous-reporting database. Am J Health Syst Pharm. 2013;70:1039–46. [PubMed: 23719881](Among 863 spontaneous reports of a drug reaction to antiviral agents over a 22 year period in Italy, 11 were due to lactic acidosis, mostly due to stavudine; zidovudine was most commonly linked to anemia).
- Vispo E, Cevik M, Rockstroh JK, Barreiro P, Nelson M, Scourfield A, Boesecke C, et al. European Network of Clinical Trials (NEAT). Genetic determinants of idiopathic noncirrhotic portal hypertension in HIV-infected patients. Clin Infect Dis. 2013;56:1117–22. [PubMed: 23315321](Case control study of 22 HIV infected patients with noncirrhotic portal hypertension [NCPH] and 58 controls for single nucleotide polymorphisms in genes for enzymes involved in purine metabolism identified 2 single nucleotide polymorphisms in the 5-nucleotidase and 2 in the xanthine oxidase genes associated with presence of NCPH).
- Verheij J, Schouten JN, Komuta M, Nevens F, Hansen BE, Janssen HL, Roskams T. Histological features in western patients with idiopathic non-cirrhotic portal hypertension. Histopathology. 2013;62:1083–91. [PubMed: 23600724](Histological analysis of 70 patients with NCPH, 13 with HIV infection who were more likely to have NRH [93% vs 46%] and who had a similar risk for underlying thrombophilic condition [31% vs 40%]).
- Kupiec KE, Johnson JW, Barroso LF, Wrenn RH, Williamson JC. Zidovudine as modern day salvage therapy for HIV infection. AIDS Patient Care STDS. 2014;28:570–4. [PMC free article: PMC4216478] [PubMed: 25365419](In a retrospective analysis of safety and efficacy of salvage therapy including zidovudine in 69 patients with HIV infection, adverse events included nausea and diarrhea, but there were no instances of lactic acidosis or lipodystrophy).
- Cooper DA, Heera J, Ive P, Botes M, Dejesus E, Burnside R, Clumeck N, et al. Efficacy and safety of maraviroc vs. efavirenz in treatment-naive patients with HIV-1: 5-year findings. AIDS. 2014;28:717–25. [PMC free article: PMC3940293] [PubMed: 24983542](In a 5 year follow up of studies comparing maraviroc to efavirenz combined with zidovudine and lamivudine, ALT levels greater than 5 times ULN occurred in 8 [2.2%] on maraviroc and 5 [1.4%] on efavirenz, about half of whom also had bilirubin elevations, but none of whom died of liver failure).
- Sood A, Castrejón M, Saab S. Human immunodeficiency virus and nodular regenerative hyperplasia of liver: A systematic review. World J Hepatol. 2014;6:55–63. [PMC free article: PMC3953810] [PubMed: 24653794](A review of the literature on nodular regenerative hyperplasia in HIV infected patients).
- Parikh ND, Martel-Laferriere V, Kushner T, Childs K, Vachon ML, Dronamraju D, Taylor C, et al. Clinical factors that predict noncirrhotic portal hypertension in HIV-infected patients: a proposed diagnostic algorithm. J Infect Dis. 2014;209:734–8. [PubMed: 23911709](Comparison of 35 HIV infected persons with NCPH to 68 controls identified multiple clinical differences; authors propose a diagnostic algorithm that includes exposure to didanosine, splenomegaly, low platelet count or serum enzyme elevations as suggestive of diagnosis and warranting further evaluation).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 5 of which [3%] were attributed to antiretroviral agents including nevirapine, zidovudine and lamivudine).
- Yajima K, Uehira T, Otera H, Koizumi Y, Watanabe D, Kodama Y, Kuzushita N, et al. A case of non-cirrhotic portal hypertension associated with anti-retroviral therapy in a Japanese patient with human immunodeficiency virus infection. J Infect Chemother. 2014;20:582–5. [PubMed: 25034388](35 year old Japanese woman with HIV infection presented with ascites and gastrointestinal bleeding 4 years after stopping a 5 year course of didanosine [bilirubin 0.2 mg/dL, ALT 82 U/L, AST 83 U/L, Alk P 268 U/L, platelets 207,000 /µL, TE=9.3 kPa, biopsy showing no cirrhosis], with improvement and resolution of ascites and abnormal liver tests after control of bleeding and avoidance of nucleoside analogs).
- Brescini L, Orsetti E, Gesuita R, Piraccini F, Marchionni E, Staffolani S, Castelli P, et al. Evaluating liver fibrosis by transient elastometry in patients with HIV-HCV coinfection and monoinfection. Hepat Mon. 2014;14:e15426. [PMC free article: PMC4199183] [PubMed: 25337140](Among 354 adults undergoing transient elastography (TE), abnormal stiffness was found in 13% with HIV infection alone, 39% with HCV infection alone and 51% with both; treatment with stavudine and didanosine being associated with higher TE scores).
- Turon F, Silva-Junior G, Hernandez-Gea V, Garcia-Pagan JC. Hipertensión portal idiopática no cirrótica. Gastroenterol Hepatol. 2015;38:556–62. [Idiopathic non-cirrhotic portal hypertension] Spanish. [PubMed: 26321321](Review of noncirrhotic portal hypertension including epidemiology, pathogenesis, clinical presentation and management).
- Arora A, Sarin SK. Multimodality imaging of obliterative portal venopathy: what every radiologist should know. Br J Radiol. 2015;88:20140653. [PMC free article: PMC4614245] [PubMed: 25514699](Review of the radiologic findings of noncirrhotic portal hypertension that accompanies obliterative portal venopathy; typical findings being increased spleen size and stiffness with normal or shrunken liver, portal vein enlargement and varices).
- Ioannou GN, Bryson CL, Weiss NS, Boyko EJ. Associations between lipodystrophy or antiretroviral medications and cirrhosis in patients with HIV infection or HIV/HCV coinfection. Eur J Gastroenterol Hepatol. 2015;27:577–84. [PubMed: 25769096](In an analysis of the Veterans Administration Healthcare system Clinical Care Registry, long term use of didanosine but not other antiretroviral agents was found to be a risk factor for cirrhosis in patients with HIV monotherapy in contrast to patients with HIV-HCV coinfection, in whom all long term antiretroviral therapies were risk factors for cirrhosis).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 cases [1.3%] were attributed to antiretroviral agents, including one to zidovudine and one to didanosine but none to stavudine).
- Kovari H, Sabin CA, Ledergerber B, Ryom L, Reiss P, Law M, Pradier C, et al. Antiretroviral drugs and risk of chronic alanine aminotransferase elevation in human immunodeficiency virus (HIV)-monoinfected persons: the data collection on adverse events of anti-HIV drugs study. Open Forum Infect Dis. 2016;3:ofw009. [PMC free article: PMC4767274] [PubMed: 26925429](Among 21,495 persons observed for an average of 4-5 years in a prospective observation study of adverse events among HIV positive patients without HBV or HCV on antiretroviral therapy, 6368 [30%] developed chronically elevated liver tests [6 per 100 patient years] and risk factors included didanosine, stavudine and tenofovir therapy but not lamivudine, abacavir or most protease inhibitors).
- Vilarinho S, Sari S, Yilmaz G, Stiegler AL, Boggon TJ, Jain D, Akyol G, et al. Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension. Hepatology. 2016;63:1977–86. [PMC free article: PMC4874872] [PubMed: 26874653](Complete exome sequencing of DNA from 8 children with idiopathic noncirrhotic portal hypertension identified an extremely rare gene variant which was homozygous in all 8 children, the gene being deoxyguanosine kinase, an enzyme required for mitochondrial DNA replication that is also inhibited by didanosine perhaps explaining its hepatotoxicity).
- Ryom L, Lundgren JD, De Wit S, Kovari H, Reiss P, Law M, El-Sadr W, et al. D:A:D Study Group. Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. AIDS. 2016;30:1731–43. [PubMed: 26752282](Among 45,544 individuals followed for a median of 8.4 years in a prospective observation study of adverse events in patients with HIV infection on antiretroviral drugs, 209 developed end stage liver disease and 110 hepatocellular carcinoma, the combined rate being 1 per 1000 patient years; increase risk for these outcomes occurred in those with HCV and HBV infection but was also linked to long term therapy with stavudine, didanosine, and tenofovir).
- Lee M, Izzy M, Akki A, Tanaka K, Kalia H. Nodular regenerative hyperplasia: a case of rare prognosis. J Investig Med High Impact Case Rep. 2017;5:2324709617690742. [PMC free article: PMC5405903] [PubMed: 28491877](26 year old woman with perinatally acquired HIV infection developed splenomegaly and abnormal liver tests while on tenofovir, emtricitabine and atazanavir/ritonavir [bilirubin 0.7 mg/dL, ALT 83 U/L, AST 80 U/L, Alk P 260 U/L, platelets 72,000, INR 1.2, biopsy showing minimal fibrosis and nodular regeneration] who subsequently was found to have a hepatic mass on imaging suspected to be HCC; no mention of previous therapy with didanosine or stavudine).
- Ahmad AK, Atzori S, Taylor-Robinson SD, Maurice JB, Cooke GS, Garvey L. Spleen stiffness measurements using point shear wave elastography detects noncirrhotic portal hypertension in human immunodeficiency virus. Medicine (Baltimore). 2019;98:e17961. [PMC free article: PMC6882591] [PubMed: 31764798](Among 25 patients with HIV infection undergoing shearwave elastography, spleen stiffness was more reliable than liver stiffness in assessing noncirrhotic portal hypertension, being elevated in 6 of 11 cases of NCPH but in none of 14 controls).