NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
OVERVIEW
Introduction
Clofarabine is a purine analogue and antineoplastic agent used in the therapy of acute lymphoblastic leukemia (ALL) in children. Clofarabine is associated with frequent transient serum enzyme elevations during therapy, but they are usually asymptomatic and transient and it has only rarely been implicated in causing clinically apparent acute liver injury with jaundice.
Background
Clofarabine (kloe far' a been) is a purine analogue that is used in the treatment of acute lymphoblastic leukemia (ALL) in children and is used off-label in other forms of leukemia and myelodysplastic syndromes. Clofarabine is a fluorinated arabinosyladenine derivative that is converted intracellularly to the active triphosphate, which is believed to compete with adenine triphosphate for uptake and use by DNA polymerase leading to inhibition of DNA synthesis. Clofarabine was found to have activity against acute leukemia and was approved for use as an antineoplastic agent in the United States in 2004. Current indications are therapy of acute lymphoblastic leukemia in children after failure of prior therapies. Clofarabine is available as a powder for injection generically and under the trade name Clolar. The typical pediatric dose for ALL is 52 mg/m2 intravenously once daily for 5 days, with repeat courses every 2 to 6 weeks. Common side effects include bone marrow suppression, leucopenia, infections, fever, nausea, vomiting, anorexia, diarrhea, headache, fatigue, mucositis and skin rash. Clofarabine has been associated with rare instances of capillary leak syndrome marked by hypotension, tachypnea, edema, and progressive pulmonary and renal failure which is frequently fatal. Corticosteroids are typically given with clofarabine to help prevent this syndrome.
Hepatotoxicity
In clinical trials, serum enzymes elevations occurred in up to 75% of patients treated with clofarabine as monotherapy for refractory or relapsed acute leukemia. These elevations usually arose within 5 to 10 days of starting therapy and were generally transient and asymptomatic. The elevations rarely required dose adjustment or delay in therapy. Cases of clinically apparent liver injury due to clofarabine have been reported to occur, but few details were available and most patients were receiving other cancer chemotherapeutic agents. A single case report of toxic epidermal necrosis and fulminant hepatic failure in a child with ALL receiving clofarabine has been published. In high doses, clofarabine has been associated with very high rates of serum enzyme elevations and hyperbilirubinemia that are dose limiting. Instances of capillary leak syndrome and possibly sinusoidal obstruction syndrome have also been reported.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Hepatotoxicity from clofarabine is likely due to direct toxicity, as is typical for other purine analogues.
Outcome and Management
The severity of the liver injury linked to clofarabine therapy is generally self-limited and mild, and resolves with stopping therapy. However, instances of hepatic failure have been reported in patients receiving cancer chemotherapeutic regimens that include clofarabine. For these reasons, the product label recommends prospective monitoring of liver tests and immediate discontinuation of clofarabine for ALT elevations above 5 times ULN or the appearance of jaundice or symptoms of hepatitis. Liver injury is most frequent with higher doses. There is little evidence of cross sensitivity to liver injury among the various antineoplastic or antiviral purine analogues.
Drug Class: Antineoplastic Agents, Antimetabolites
Other Drugs in the Subclass, Purine Analogues: Azathioprine, Cladribine, Fludarabine, Mercaptopurine, Nelarabine, Pentostatin, Thioguanine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Clofarabine – Clolar®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
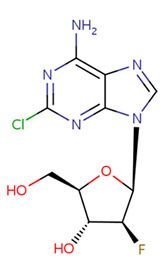
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Clofarabine | 123318-82-1 | C10-H11-Cl-F-N5-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 17 October 2017
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999 discusses the purine analogues pentostatin, cladribine and fludarabine, but not clofarabine or nelarabine).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of hepatotoxicity of anticancer agents does not discuss clofarabine).
- Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan DP. Purine analogs. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1701-5.(Textbook of pharmacology and therapeutics).
- Kantarjian H, Gandhi V, Cortes J, Verstovsek S, Du M, Garcia-Manero G, Giles F, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood 2003; 102: 2379-86. [PubMed: 12791647](Among 62 adults with various forms of refractory leukemia treated with clofarabine [52 mg/m2 daily for 5 days every 2-6 weeks], ALT elevations occurred in 74% and hyperbilirubinemia in 50%, but there were no liver related serious adverse events).
- Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, Rytting M, Brandt M, Keating M, Plunkett W, Kantarjian H. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood 2004; 103: 784-9. [PubMed: 14551141](Dose finding study of clofarabine in 25 patients with advanced leukemia; the dose limiting toxicities were skin rash and hepatotoxicity, but liver injury resolved in all patients; few details given).
- Pui CH, Jeha S, Kirkpatrick P. Clofarabine. Nat Rev Drug Discov 2005; 4: 369-70. [PubMed: 15902772](Brief review of structure, mechanism of action, efficacy and clinical indications for clofarabine shortly after its approval for use by the FDA).
- Curran MP, Perry CM. Clofarabine: in pediatric patients with acute lymphoblastic leukemia. Paediatr Drugs 2005; 7: 259-64. [PubMed: 16117562](Review of the structure, mechanism of action, pharmacokinetics, efficacy and safety of clofarabine in children with ALL; mentions that transient elevations in serum enzymes can occur and that four cases of capillary leak syndrome due to clofarabine have been reported).
- Faderl S, Gandhi V, O'Brien S, Bonate P, Cortes J, Estey E, Beran M, et al. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood 2005; 105: 940-7. [PubMed: 15486072](Among 32 adults given clofarabine and cytarabine for previously treated acute leukemia, hyperbilirubinemia occurred in 67-80% and ALT elevations in 42-65%, generally between day 7 and 28 of the first course, but resolving in all).
- Jeha S, Gaynon PS, Razzouk BI, Franklin J, Kadota R, Shen V, Luchtman-Jones L, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol 2006; 24: 1917-23. [PubMed: 16622268](Among 61 children with previously treated ALL who received several courses of clofarabine, ALT elevations above 5 times ULN occurred in 43%, usually peaking by day 7 of therapy and resolving by day 16).
- Gandhi V, Plunkett W. Clofarabine and nelarabine: two new purine nucleoside analogs. Curr Opin Oncol 2006; 18: 584-90. [PubMed: 16988579](Review of clofarabine and nelarabine, both of which were recently approved for use in ALL and T cell leukemia or lymphoma, dose limiting toxicities being hepatic for clofarabine, neurologic for nelarabine).
- Johnston DL, Mandel KM. Fatal skin and liver toxicity in a patient treated with clofarabine. Pediatr Blood Cancer 2008; 50: 1082. [PubMed: 17957757](9 year old girl with relapsed ALL developed liver enzyme elevations 2 days after starting clofarabine, followed by facial edema, rash, desquamation, jaundice and death from liver failure within 2 weeks of starting chemotherapy; few details given).
- Locatelli F, Testi AM, Bernardo ME, Rizzari C, Bertaina AP, Pession A, Giraldi E, et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol 2009; 147: 371-8. [PubMed: 19747360](Among 25 children with relapsed ALL treated with a single course of clofarabine, cyclophosphamde and etoposide, liver toxicity was frequent [64%], but invariably mild and asymptomatic, arising within 3-10 days of starting and resolving within 10 days).
- Jeha S, Razzouk B, Rytting M, Rheingold S, Albano E, Kadota R, Luchtman-Jones L, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Oncol 2009; 27: 4392-7. [PMC free article: PMC2744276] [PubMed: 19652076](Among 42 children with AML treated with clofarabine, 43% developed ALT elevations above 5 times ULN and 12% hyperbilirubinemia, but none developed clinically apparent liver injury or sinusoidal obstruction syndrome).
- Baytan B, Ozdemir O, Gunes AM, Döz O. Clofarabine-induced capillary leak syndrome in a child with refractory acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2010; 32: 144-6. [PubMed: 20057324](12 year old girl with refractory ALL developed hypotension on day 4 of a second course of clofarabine, etoposide and cyclophosphamide with subsequent edema, renal and pulmonary failure and progressive capillary leak syndrome resulting in death).
- Advani AS, Gundacker HM, Sala-Torra O, Radich JP, Lai R, Slovak ML, Lancet JE, et al. Southwest Oncology Group Study S0530: a phase 2 trial of clofarabine and cytarabine for relapsed or refractory acute lymphocytic leukaemia. Br J Haematol 2010; 151: 430-4. [PMC free article: PMC3058291] [PubMed: 21113977](Among 37 adults with refractory ALL treated with clofarabine and cytarabine, only 17% achieved a complete response and toxicities were common; no mention of hepatotoxicity or ALT elevations).
- Hijiya N, Thomson B, Isakoff MS, Silverman LB, Steinherz PG, Borowitz MJ, Kadota R, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood 2011; 118: 6043-9. [PMC free article: PMC3731655] [PubMed: 21967976](Pilot study of combination of clofarabine [40 mg/kg/day] with etoposide and cyclophosphamide in children with ALL who had failed previous therapy found unacceptable toxicity, 4 of first 8 patients with severe hepatotoxicity suggestive of sinusoidal obstruction syndrome, not seen with lower doses).
- Faderl S, Garcia-Manero G, Jabbour E, Ravandi F, Borthakur G, Estrov Z, Gandhi V, et al. A randomized study of 2 dose levels of intravenous clofarabine in the treatment of patients with higher-risk myelodysplastic syndrome. Cancer 2012; 118: 722-8. [PMC free article: PMC4180241] [PubMed: 21751197](Pilot study of two doses of clofarabine in 58 adults with myelodysplastic syndromes found higher rates of bilirubin and ALT elevations in patients treated with the higher dose).
- Advani AS, McDonough S, Coutre S, Wood B, Radich J, Mims M, O'Donnell M, Et al. SWOG S0910: a phase 2 trial of clofarabine/cytarabine/epratuzumab for elapsed/refractory acute lymphocytic leukaemia. Br J Haematol 2014; 165: 504-9. [PMC free article: PMC4209396] [PubMed: 24579885](Among 35 adults with refractory ALL treated with clofarabine, cytarabine and epratuzumab [anti-CD22], the response rate was 52%, but adverse events were common, including ALT elevations above 5 times ULN in 11 patients [31%], one of whom had "hepatic failure", but there were no liver related deaths ).
- Martínez-Cuadrón D, Montesinos P, Oriol A, Salamero O, Vidriales B, Bergua J, Herrera P, et al. Phase II trial to assess the safety and efficacy of clofarabine in combination with low-dose cytarabine in elderly patients with acute myeloid leukemia. Ann Hematol 2014; 93: 43-6. [PubMed: 24081577](Among 11 elderly adults with AML treated with clofarabine with low doses of cytarabine, the response rate was 27%, but the mortality rate rose to 73% at 8 weeks and one death was attributed to hepatic and renal failure).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 cases [5%] were attributed to antineoplastic agents, but none to clofarabine).