NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
OVERVIEW
Introduction
Griseofulvin is a fungistatic agent used to treat superficial fungal skin infections such as tinea capitis and pedis. Griseofulvin therapy can cause transient mild-to-moderate serum aminotransferase elevations and has very rarely been linked to clinically apparent acute drug induced liver injury.
Background
Griseofulvin (gris" ee oh ful' vin) is a metabolic product of Penicillium griseofulvum with potent activity against fungal agents. Its antifungal activity is believed to be due to disruption of the mitotic spindle of fungal cells, which interferes with cell division. Griseofulvin may also inhibit fungal DNA replication. Griseofulvin has been used for superficial dermatophyte infections (tinea corporis, capitis, barbae, cruris, pedis or unguium) for many years. Griseofulvin is available in generic forms as tablets of 500 mg, and an oral suspension in several generic forms and under the brand names of Grifulvin V and as film coated tablets of 125 and 250 mg as Gris-PEG. Because of the availability of other more potent antifungal agents with a wider sprectum of action, griseofulvin is now rarely used. The usual recommended dose is 500 to 1000 mg daily depending upon the type and severity of the infection. Common side effects include nausea, vomiting and headache.
Hepatotoxicity
Transient mild-to-moderate elevations in serum aminotransferase levels occur in up to 5% of patients treated with griseofulvin, but these abnormalities are usually asymptomatic and resolve even with continuation of the medication. Clinically apparent hepatotoxicity is rare and only isolated case reports have been published. The liver injury is typically cholestatic and usually arises within the first few months of therapy. Signs of hypersensitivity such as fever, rash and eosinophilia are rare but griseofulvin can include hypersensitivity reactions and at least one case of DRESS syndrome accompanied by serum aminotransferase elevations has been reported with its use. Published cases of griseofulvin induced liver injury have all been self-limited, recovery requiring 1 to 3 months. Griseofulvin can increase intrahepatic levels of protoporphyrin and induce acute attacks of porphyria in patients with acute intermittent porphyria in remission.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of clinically apparent hepatotoxicity from griseofulvin is unknown, but it is extensively metabolized by the liver which may lead to production of a toxic intermediate. In high doses, griseofulvin can interfere with the mitotic spindle in mammalian cells by binding to microtubular proteins. Griseofulvin also inhibits ferrocheletase activity the enzyme that inserts iron into the prophyrin ring and, therefore, interferes with porphyrin metabolism and can trigger attacks of acute porphyria.
Outcome and Management
The severity of the liver injury due to griseofulvin ranges from mild and transient enzyme elevations to marked cholestatic hepatitis. Typically, improvements are seen within a few weeks of discontinuation. Rechallenge may lead to recurrence and should be avoided. No cases of acute liver failure or chronic bile duct injury have been reported due to griseofulvin. There is no evidence for cross sensivity to hepatic injury between griseofulvin and other antifungal agents.
Drug Class: Antifungal Agents
CASE REPORT
Case 1. Griseofulvin induced hepatitis.
[Modified from: Chiprut RD, Viteri A, Jamroz C, Dyck WP. Intrahepatic cholestasis after griseofulvin administration. Gastroenterology 1976; 70: 1141-3. PubMed Citation]
A 63 year old man developed anorexia, fatigue and pruritus 3 weeks after restarting griseofulvin (500 mg twice daily), but didn’t stop treatment until ten days later when he was admitted for evaluation. He was jaundiced but without rash or fever. Laboratory results showed elevations in bilirubin, AST and alkaline phosphatase (Table). Liver tests had been normal in the past, including five months previously when he had a hypersensitivity reaction to griseofulvin (which he had been taking for 5 months and was then stopped for several weeks and then restarted). In addition, he underwent bilateral renal artery endartectomy for severe hypertension and required several blood transfusions approximately 2 months before presentation with jaundice. He did not drink alcohol and took no other medications. Testing showed absence of HBsAg and autoantibodies. A liver biopsy showed intrahepatic cholestasis that was interpreted as compatible with drug induced liver injury. After stopping griseofulvin, he rapidly became asymptomatic and all liver tests were normal three months later.
Key Points
| Medication: | Griseofulvin |
| Pattern: | Cholestatic (R=0.7) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 3 weeks (reexposure) to symptoms, 5 weeks to jaundice |
| Recovery: | Within 3 months |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | AST (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | 0 | 46 | 24 | 0.9 | Surgery |
| 5 weeks | 0 | 75 | 43 | 3.4 | Admission |
| 6 weeks | 1 week | 26 | 35 | 2.4 | Liver biopsy |
| 10 weeks | 5 weeks | 54 | 32 | 1.1 | |
| 4.5 months | 3 months | 30 | 17 | 0.7 | |
| ~1 year | ~1 year | 30 | 15 | 0.8 | |
| Normal Values | <40 | <13 | <1.2 | ||
Comment
This patient developed a mild cholestatic hepatitis 3 weeks after restarting griseofulvin, having a previous history compatible with a drug-hypersensitivity reaction (facial edema and pruritus). A liver biopsy was compatible with drug induced cholestasis as was the improvement that followed discontinuation. Because he received a blood transfusion approximately 2 months before developing symptoms of liver injury, the possibility of hepatitis C (which had yet to be described in 1976) also exists, but the pattern of enzyme elevation and liver biopsy were not typical of viral hepatitis. Despite griseofulvin’s known effects on the liver in mice and rats (causing Mallory body formulation), there have been very few case reports of liver injury due to griseofulvin published in the literature.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Griseofulvin – Generic, Grifulvin V®
DRUG CLASS
Antifungal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
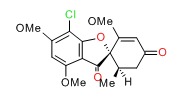
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Griseofulvin | 126-07-8 | C17-H17-Cl-O6 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 14 March 2018
- Zimmerman HJ. Antifungal agents. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 609-11.(Expert review of hepatotoxicity of antifungal agents published in 1999 mentions that griseofulvin is an interesting experimental hepatoxin [causing Mallory body formation in rats], but produces little hepatic injury in humans).
- Moseley RH. Antifungal agents. Hepatoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 470-3.(Review of hepatotoxicity of antifungal agents mentions that griseofulvin causes a variety of acute and chronic effects in rodents, but hepatotoxicity is rare in humans; individual case reports have been published of cholestatic jaundice and precipitation of acute intermittent porphyria).
- Bennett JE. Antifungal agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1571-92.(Textbook of pharmacology and therapeutics; griseofulvin is fungistatic against several species of dermatophytes, but has no effect on bacteria or other fungi).
- Osment LS. The many effects of griseofulvin. Ala J Med Sci 1969; 6: 392-8. [PubMed: 4903304](Review of griseofulvin structure, mechanism of action, efficacy and safety; griseofulvin is fungistatic, it induces hepatic microsomal enzymes and can trigger acute attacks of porphyria in susceptible patients, common side effects are gastrointestinal upset, headache and rash; it rarely causes liver injury).
- Redeker AG, Sterling RE, Bronow RS. Effect of griseofulvin in acute intermittent porphyria. JAMA 1964; 188: 466-8. [PubMed: 14125225](Two patients with acute intermittent porphyria were given griseofulvin [1 g/day]; urine pyrole excretion increased within 2-5 days and one developed severe abdominal and leg pain, tachycardia and hypertension with elevation of ALT from 38 to 129 U/L).
- Berman A, Franklin RL. Precipitation of acute intermittent porphyria by griseofulvin therapy. JAMA 1968; 192: 1005-7. [PubMed: 14290427](43 year old woman developed attack of acute porphyria [abdominal pain, nausea and restlessness] 7 days after starting griseofulvin [500 mg/day], with bilirubin 0.7 mg/dL, ALT 29 U/L, Alk P normal, and urine porphobilinogen positive).
- Brienstrup H, Sogaard-Andersen J. Intrahepatic cholestasis after griseofulvin treatment. Ugeskr Laeger 1966; 128: 145-6. Danish. [PubMed: 5914466](14 year old boy developed jaundice 4 months after starting griseofulvin [bilirubin 6.1 mg/dL, ALT 312 U/L, Alk P ~3 times ULN], with recovery over next few months).
- Chiprut RD, Viteri A, Jamroz C, Dyck WP. Intrahepatic cholestasis after griseofulvin administration. Gastroenterology 1976; 70: 1141-3. [PubMed: 131731](63 year old man developed fatigue and pruritus 3 weeks after starting griseofulvin [bilirubin 3.4 mg/dL, AST 75 U/L and Alk P ~3 times ULN], resolving within few months of stopping: Case 1).
- Gotz H, Reichenberger M. Ergebnisse einer Fragebogenaktion bei 1670 Dermatologen der Bundes-Republik Deutschland uber Nebenwirkungen dei der Griseofulvintherapie. [Results of questionnaires of 1670 dermatologists in West Germany concerning the side effects of griseofulvin therapy]. Hautarzt 1975; 11: 485-92. [PubMed: 4655974](1670 German dermatologists answered a questionnaire regarding griseofulvin; only 5% reported having seen liver injury during therapy, the majority of which was serum aminotransferase elevations without jaundice or symptoms).
- Smith AG, De Matteis F. Drugs and the hepatic porphyrias. Clin Haematol 1980; 9: 399-425. [PubMed: 7398153](Extensive review of the effects of drugs on heme metabolism and porphyria; griseofulvin appears to inhibit hepatic ferrochelatase, leading to accumulation of protoporphyrin).
- Simon N, Berko G, Polay A, Kocsis G. Der Einflus der Griseofulvintherapie auf die Leber function und den Porphyrin-Stofwechsel. [Influence of griseofulvin treatment on liver function and porphyrine metabolism]. Arch Dermatol Forsch 1981; 241: 148-55. [PubMed: 4255210](Griseofulvin therapy in humans leads to transient increase in serum AST and erythrocyte porphyrin levels, but this effect is highly variable and abnormalities usually return to normal within a few weeks).
- Hay RJ, Clayton YM, Griffiths WA, Dowd PM. A comparative double blind study of ketoconazole and griseofulvin in dermatophytosis. Br J Dermatol 1985; 112: 691-6. [PubMed: 3890924](Controlled trial of ketoconazole vs griseofulvin for up to one year in 74 patients with dermatophytoses; similar efficacy; side effects were mild and there was no evidence of liver toxicity in either group “as assessed clinically or by liver function tests.”).
- Lambert DR, Siegle RJ, Camisa C. Griseofulvin and ketoconazole in the treatment of dermatophyte infections. Int J Dermatol 1989; 28: 300-4. [PubMed: 2666321](Review of efficacy and safety of griseofulvin and ketoconazole for superficial mycoses; rate of hepatotoxicity of ketoconazole ranges from 1:10,000 to 1:15,000, and is more common among women and in persons over 40 years of age; recommends routine monitoring of liver enzymes; in contrast, griseofulvin is “remarkably safe” and no deaths have been attributed directly to the drug).
- Hay RJ. Risk/benefit ratio of modern antifungal therapy: focus on hepatic reactions. J Am Acad Dermatol 1993; 29: S50-4. [PubMed: 8315062](Review article on hepatotoxicity of antifungal agents, griseofulvin, ketoconazole, fluconazole, itraconazole and terbinafine; does not recommend routine monitoring, but stresses need to discontinue agent for hepatic injury with symptoms).
- Watanabe M, Akagi S, Kohge N, Uchida Y, Jguyen TX, Hirakawa K, Fukumoto S. Laparoscopy of griseofulvin-induced liver injury presenting a wide depression. Endoscopy 1994; 26: 514-5. [PubMed: 7956975](42 year old man developed abnormal liver tests 4 months after starting griseofulvin [bilirubin 1.2 mg/dL, ALT 170 rising to 369 U/L despite stopping alcoholic intake]; liver biopsy showed mild inflammation without fibrosis, abnormalities resolved with stopping griseofulvin).
- Polo CF, Buzalh AM, Vazquez ES, Afonso SG, Navone NM, del Carmen Battle AM. Griseofulvin-induced hepatopathy due to abnormalities in heme pathway. Gen Pharmac 1997; 29: 207-10. [PubMed: 9251900](In mice, griseofulvin inhibits ferrocheletase which is responsible for inserting iron into the protoporphyrin ring and can induce attacks of protoporphyria).
- Wingfield AB, Fernandez-Obregon AC, Wignall FS, Greer DL. Treatment of tinea imbricate: a randomized clinical trial using griseofulvin, terbinafine, itraconazole and fluconazole. Br J Dermatol 2004; 150: 119-26. [PubMed: 14746625](Randomized trial of four antifungals for 4 weeks for tinea imbricate in 86 patients in New Guinea; griseofulvin and terbinafine were effective; itraconazole and fluconazole were not; only one patient had ALT elevation [3-fold: terbinafine]).
- Song J, Deresinski S. Hepatotoxicity of antifungal agents. Curr Opin Investig Drugs 2005; 6: 170-7. [PubMed: 15751740](Extensive review of hepatotoxicity of antifungal agents; although griseofulvin is rarely associated with hepatotoxicity, it has been linked to rare cases of cholestatic hepatitis arising after 1-4 months of therapy).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 8 cases were attributed to antifungal agents, but no cases were attributed to griseofulvin).
- Elewski BE, Cáceres HW, DeLeon L, El Shimy S, Hunter JA, Korotkiy N, Rachesky IJ, et al. Terbinafine hydrochloride oral granules versus oral griseofulvin suspension in children with tinea capitis: results of two randomized, investigator-blinded, multicenter, international, controlled trials. J Am Acad Dermatol 2008; 59: 41-54. [PubMed: 18378354](Controlled trial of 6 weeks of terbinafine vs griseofulvin in 1549 children with tinea capitis; side effects were similar; low rate of serum biochemistry abnormalities, although 2 patients on griseofulvin stopped therapy because of serum aminotransferase elevations).
- Kao WY, Su CW, Huang YS, Chou YC, Chen YC, Chung WH, Hou MC, et al. Risk of oral anti-fungal agent-induced liver injury in Taiwanese. Br J Clin Pharmacol 2014; 77: 180-9. [PMC free article: PMC3895359] [PubMed: 23750489](Analysis of Taiwan national health Insurance database from 2002-2008 identified 52 patients with drug induced liver injury among 90,847 users of oral antifungal agents, rates were were 4.3 per 10,000 persons taking griseofulvin [8 cases], but none were fatal).
- Raschi E, Poluzzi E, Koci A, Caraceni P, Ponti FD. Assessing liver injury associated with antimycotics: Concise literature review and clues from data mining of the FAERS database. World J Hepatol 2014; 6: 601-12. [PMC free article: PMC4163743] [PubMed: 25232453](Analysis of spontaneous reports of drug induced liver injury to the FDA between 2004 and 2011 identified 1964 cases attributed to antifungal agents, only 3 of which were linked to griseofulvin, none of which were fatal).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 14 cases [1.6%] were attributed to antifungal agents including 7 due to terbinafine, 6 triazoles [4 fluconazole, 1 ketoconazole and 1 voriconazole], 1 micafungin, but none due to griseofulvin).
- Smith RJ, Boos MD, McMahon P. Probable griseofulvin-induced drug reaction with eosinophilia and systemic symptoms in a child. Pediatr Dermatol 2016; 33: e290-1. (9 year old boy developed fever, rash, adenopathy and liver test abnormalities 3 weeks after starting griseofulvin [bilirubin not provided, ALT 31 rising to 282 U/L, Alk P 170 rising to 285 U/L, eosinophils 310/µ. [PubMed: 27397873]L], rash, fever and laboratory abnormalities resolving within 2-4 weeks of starting prednisone therapy).
- Kyriakidis I, Tragiannidis A, Munchen S, Groll AH. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 2017; 16: 149-65. [PubMed: 27927037](Review of the hepatotoxicity of antifungal agents states that all antifungal agents may cause hepatic toxicity, mentions that griseofulvin has been linked to rare cases of transient liver injury, but not to acute liver failure).