Context and Policy Issues
Breast cancer has continued to be the most common type of cancer in Canada, and the second leading cause of death from cancer in Canadian women.1 In 2017, there was an estimated 103,200 new cases of cancer in Canadian women with breast cancer making up 26% of those cases (26,300 women), leading to 5,000 deaths.2 Breast cancer most frequently begins in the lobules or the milk ducts (carcinoma). Breast carcinoma can stay within the lobules or ducts (non-invasive, or carcinoma in situ), or can spread to the surrounding tissues of the breast or to lymph nodes and other parts of the body (invasive carcinoma).3 Breast cancer cells may migrate from a primary tumor to axillary lymph nodes, a condition that is most accurately diagnosed by histological examination of the lymph nodes by biopsy. Breast cancer cells migrate to one or a few lymph nodes, called sentinel lymph nodes, before involving others; the status of sentinel lymph nodes may predict the status of the remaining lymph nodes.4,5 Sentinel lymph node biopsy (SLNB) has been used to identify cancer cells in patients with invasive breast cancer and no evidence of lymph node involvement on ultrasound, avoiding a potentially morbid axillary lymph node dissection.6 SLNB involves the injection of a radioactive isotope prior to surgery which is then used to identify and biopsy the sentinel node. In cases where the patient does not have access to a hospital nuclear medicine department, they may be required to travel to receive surgery, or have radioactive materials transported to their local centre to receive surgery. Recommendations for these situations, including the transportation of radioactive material or patients between health facilities to perform SLNB are unclear.
This Rapid Response report aims to review the evidence-based guidelines associated with the use of SLNB for the management of breast cancer.
Research Question
What are the evidence-based guidelines regarding methods for sentinel lymph node biopsy for the management of breast cancer?
Key Findings
Five evidence-based guidelines were identified. SLNB is recommended for axillary staging for patients with early-stage or invasive breast cancer without clinically or pathologically positive lymph nodes. SLNB is recommended in patients with ductal carcinoma in situ (DCIS) when mastectomy was performed, and not for women with a preoperative diagnosis of DCIS who are having breast-conserving surgery, unless they are considered to be at high risk of invasive disease. SLNB is recommended for women who have operable breast cancer who have multicentric tumors, prior breast and/or axillary surgery, or preoperative or neoadjuvant systemic therapy. SLNB, rather than full axillary nodal clearance, is the standard of care in patients with primary breast cancer. The use of SLNB is recommended after neoadjuvant (preoperative) therapy for staging and management of patients presenting with a clinically negative axilla. There was no information found related to transportation of radioactive materials or patients between health facilities.
Methods
A limited literature search was conducted on key resources including PubMed, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. Methodological filters were applied to limit the retrieval to systematic reviews, health technology assessments, and meta-analyses, and guidelines. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2014 and March 25, 2019.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2014. Guidelines that were not evidence-based or had unclear methodology were also excluded.
Critical Appraisal of Individual Studies
The included guidelines were assessed using the AGREE II checklist.7 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
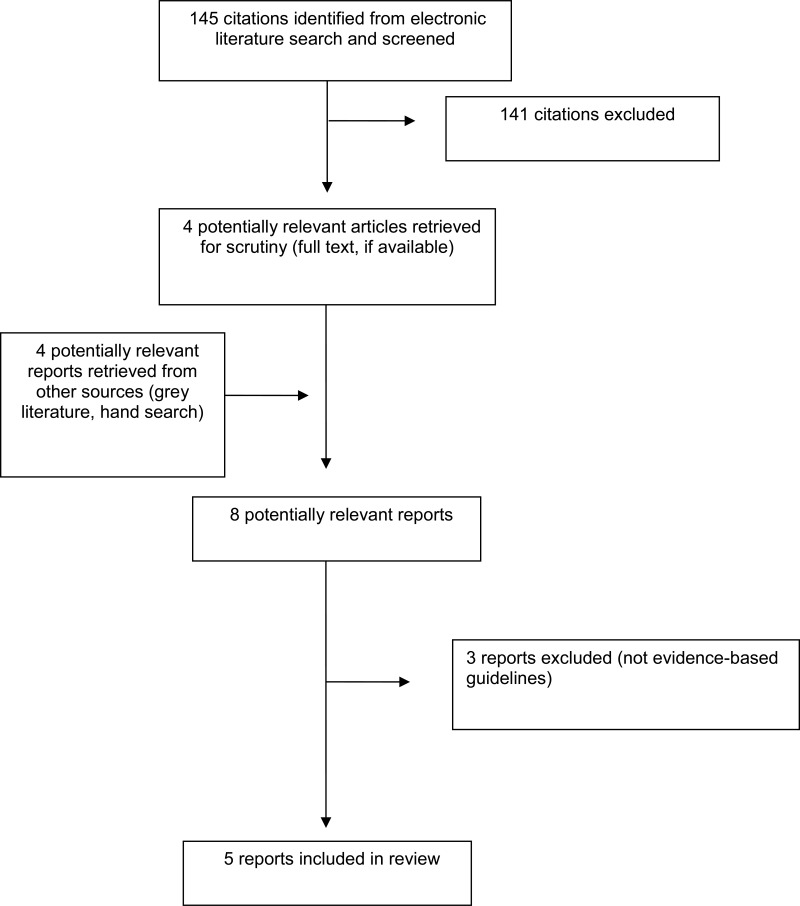
A total of 145 citations were identified in the literature search. Following screening of titles and abstracts, 141 citations were excluded and four potentially relevant reports from the electronic search were retrieved for full-text review. Four potentially relevant publications were retrieved from the grey literature search. Of these potentially relevant articles, three publications were excluded for various reasons, while five publications met the inclusion criteria and were included in this report. Appendix 1 describes the PRISMA flowchart of the study selection.
Summary of Study Characteristics
Five evidence-based guidelines were included. Breast cancer affects women and men; in the included guidelines, the term ‘women’ was used for statements and recommendations specific to women (such as breast-conserving surgery) and ‘people’ in all other cases. This Rapid Response report uses language consistent with that used in the source guideline documents.
One guideline was developed by the National Institute for Health and Care Excellence (NICE) for the diagnosis and management of early and locally advanced breast cancer;8 guideline content and recommendations were based on a structured review of the literature up to 2017, the evidence and recommendation ratings were adopted from the classification developed by the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) workgroup. While the methods indicate that the GRADE system was used to assign strength to each recommendation, the grading and strength of recommendations did not seem to be reported in the final document.
One guideline was developed by the American Society of Clinical Oncology (ASCO) for the diagnosis and management of early stage breast cancer;9 guideline content and recommendations were based on a structured review of the literature up to 2016, the evidence and recommendation ratings were adopted from the classification developed by the GRADE workgroup.
One guideline was developed by Cancer Care Ontario for SLNB for early breast cancer;10 guideline content and recommendations were based on a structured review of the literature up to 2015, the evidence and recommendation ratings were adopted from the classification developed by the GRADE workgroup. While the methods indicate that the GRADE system was used to assign strength to each recommendation, the grading and strength of recommendations did not seem to be reported in the final document.
One guideline was developed by the European Society of Medical Oncology (ESMO) for the diagnosis and management of primary breast cancer;11 details on literature review were not reported, the evidence and recommendation ratings were based on the Infectious Disease Society of America grading system. The guideline was published in 2015.
One guideline was developed by the Alberta Provincial Breast Tumour Team for patients with pre-operative breast cancer;12 guideline content and recommendations were based on a structured review of the literature up to 2014. The evidence grading system was unclear. The level of evidence and strength of recommendation were not reported.
Characteristics of the included guidelines are detailed in Appendix 2.
Summary of Critical Appraisal
The included guidelines8–12 had a clear scope and purpose, the recommendations were specific and unambiguous, the methods used for formulating the recommendations were clearly described in most guidelines, health benefits, side effects, and risks were stated in the recommendations, and the procedures for updating the guidelines provided and target users of the guideline are clearly defined. Other than the ESMO guideline, the methods for searching for and selecting the evidence were clear. This rigour of development and clarity of presentation would increase the users’ confidence in the accuracy and reliability of the recommendations. Cost factors were considered in two guidelines,8,12 and not in the rest. It was unclear whether the guidelines were piloted among target users, or whether patients’ views and preferences were sought. All specialties related to the assessment and management of breast cancer were included in the development of the guidelines.
Details of the critical appraisal of the included studies are presented in Appendix 3.
Summary of Findings
Evidence-based guidelines regarding the use of sentinel lymph node biopsy for the management of breast cancer
Five evidence-based guidelines issued recommendations regarding the use of sentinel lymph node biopsy for the management of breast cancer.8–12 The 2018 NICE guideline8 stated that SLNB should be used in patients with invasive breast cancer who did not have evidence of lymph node involvement on ultrasound or had a negative ultrasound-guided needle biopsy. SLNB should not be performed routinely for women with a preoperative diagnosis of ductal carcinoma in situ (DCIS) who are having breast-conserving surgery, unless they are considered to be at high risk of invasive disease. SLNB was recommended for all people who are having a mastectomy for DCIS. The strength of the recommendation was not reported. The ASCO guideline in 20179 recommended SLNB for women who have operable breast cancer who have multicentric tumors (moderately recommended), DCIS when mastectomy is performed (weakly recommended), prior breast and/or axillary surgery (strongly recommended), or preoperative or neoadjuvant systemic therapy (moderately recommended). The Cancer Care Ontario guideline in 201610 recommended SLNB as the preferred method of axillary staging for all patients with a clinical presentation of early-stage breast cancer without clinically or pathologically positive lymph nodes. The strength of the recommendation was not reported. The ESMO guideline in 201511 stated that SLNB, rather than full axillary nodal clearance, is the standard of care in patients with primary breast cancer (strongly recommended), and SLNB can be considered in patients who had neoadjuvant (preoperative) systemic therapy if the axillary lymph node is negative (generally recommended). The 2014 Alberta Health Services guideline12 stated that there is sufficient evidence to recommend the use of SLNB after neoadjuvant (preoperative) therapy for staging and management of patients presenting with a clinically negative axilla. The strength of recommendation was not reported.
Further detail regarding the included guidelines is presented in Appendix 4.
Limitations
The majority of recommendations on the use of SLNB for the management of breast cancer were based on evidence of limited quality; the recommendations need to be interpreted with caution.
Conclusions and Implications for Decision or Policy Making
The majority of recommendations on the use of SLNB for the management of breast cancer were based on evidence of limited quality; the recommendations need to be interpreted with caution. SLNB is recommended for staging and management of: 1. patients with early-stage or invasive breast cancer without clinically or pathologically positive lymph nodes; 2. patients with DCIS when mastectomy was performed; 3. for women who have operable breast cancer who have multicentric tumors, prior breast and/or axillary surgery, or preoperative or neoadjuvant systemic therapy; 4. patients with primary breast cancer; 5. Patients after neoadjuvant (preoperative) therapy presenting with a clinically negative axilla. SLNB was not recommended for women with a preoperative diagnosis of DCIS who were having breast-conserving surgery, unless they were considered to be at high risk of invasive disease. There were no recommendations found on the transportation of radioactive materials or patients between health facilities to perform SLNB.
There are uncertainties in the evidence leading to many recommendations on the use of SLNB in patients with breast cancer, except where SLNB was strongly recommended as the standard of care unless axillary node involvement was proven, and for women who have operable breast cancer who have prior breast and/or axillary surgery, Results from more high-quality trials are needed to elucidate the role of SLNB in the diagnosis and management of breast cancer.
References
- 1.
- 2.
- 3.
- 4.
Harlow
S. Sentinel lymph node biopsy in breast cancer: techniques. In: Post
TW, ed.
UpToDate. Waltham (MA): UpToDate; 2017.
www.uptodate.com. Accessed 2019 Apr 23
- 5.
Harlow
S. Overview of sentinel lymph node biopsy in breast cancer. In: Post
TW, ed.
UpToDate. Waltham (MA): UpToDate; 2018.
www.uptodate.com. Accessed 2019 Apr 23
- 6.
Harlow
S. Management of the regional lymph nodes in breast cancer. In: Post
TW, ed.
UpToDate. Waltham (MA): UpToDate; 2018.
www.uptodate.com. Accessed 2019 Apr 23
- 7.
- 8.
- 9.
Lyman
GH, Somerfield
MR, Bosserman
LD, Perkins
CL, Weaver
DL, Giuliano
AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update.
J Clin Oncol. 2017;35(5):561–564. [
PubMed: 27937089]
- 10.
The Expert Panel on SLNB in Breast Cancer. Sentinel lymph node biopsy in early-stage breast cancer. In: George
R, Kennedy
E, eds. Program in Evidence-Based Care, Evidence-Based Series. Toronto (ON): Cancer Care Ontario; 2016.
- 11.
Senkus
E, Kyriakides
S, Ohno
S, Penault-Llorca
F, Poortmans
P, Rutgers
E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.
Annals Oncol. 2015
Sep;26(Suppl 5):v8–v30. [
PubMed: 26314782]
- 12.
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Guidelines
View in own window
| Guideline Development Group, Year | Scope and Interventions | Target Population; Intended users | Evidence Collection, Selection, and Synthesis | Recommendations Development and Evaluation | Grading system |
|---|
| National Institute for Health and Care Excellence (NICE), 20188 | Guideline on diagnosing and managing early and locally advanced breast cancer | Patients with early and locally advanced breast cancer Clinicians involved in the assessment and treatment of patients with breast cancer | Systematic structured evidence review up to 2017 for Medline, Embase, Cochrane library database | Clinical recommendations were developed on the basis of the best available evidence | The evidence and recommendation rating were adopted from the classification developed by the GRADE workgroup. The GRADE system primarily involves consideration of the following factors: overall study quality (or overall risk of bias or study limitations), consistency of evidence, directness of evidence, and precision of evidence |
| American Society of Clinical Oncology (ASCO), 20179 | Guideline on the use of SLNB for patients with early-stage breast cancer | Patients with early breast cancer Clinicians involved in the assessment and treatment of patients with breast cancer | Systematic structured evidence review up to 2016 for PubMed, Cochrane library database | Clinical recommendations were developed on the basis of the best available evidence | The evidence and recommendation rating were adopted from the classification developed by the GRADE workgroup. The GRADE system primarily involves consideration of the following factors: overall study quality (or overall risk of bias or study limitations), consistency of evidence, directness of evidence, and precision of evidence. |
| Cancer Care Ontario, Breast Cancer Guideline Development Group, 201610 | Guideline on the use of SLNB in early-stage breast cancer | Patients with early breast cancer Clinicians involved in the assessment and treatment of patients with breast cancer | Systematic structured evidence review up to 2014 for Medline, Embase, Cochrane library database | Clinical recommendations were developed on the basis of the best available evidence | The evidence and recommendation rating were adopted from the classification developed by the GRADE workgroup. The GRADE system primarily involves consideration of the following factors: overall study quality (or overall risk of bias or study limitations), consistency of evidence, directness of evidence, and precision of evidence. |
| European Society of Medical Oncology (ESMO), 201511 | Guideline for primary breast cancer diagnosis and treatment | Patients with breast cancer Clinicians involved in the assessment and treatment of patients with breast cancer | Not reported | Clinical recommendations were developed on the basis of the best available evidence | Levels of evidence and grades of recommendation were adapted from the Infectious Diseases Society of America-United States Public Health Service Grading System |
| Alberta Health Services, 201412 | Guideline for neo-adjuvant (pre-operative) therapy for breast cancer | Patients with breast cancer Clinicians involved in the assessment and treatment of patients with breast cancer | Systematic structured evidence review up to 2014 for Medline, PubMed | Clinical recommendations were developed on the basis of the best available evidence | Unclear |
SLNB = sentinel lymph node biopsy
Appendix 3. Critical Appraisal of Included Publications
Table 3Summary of Critical Appraisal of Included Guideline using AGREE II7
View in own window
| First Author, Publication Year | Strengths | Limitations |
|---|
| NICE, 20188 |
scope and purpose of the guidelines are clear the recommendations are specific and unambiguous the method for searching for and selecting the evidence are clear methods used for formulating the recommendations are clearly described health benefits, side effects and risks were stated in the recommendations procedure for updating the guidelines provided target users of the guideline are clearly defined potential cost implications of applying the recommendation were included
|
|
| American Society of Clinical Oncology (ASCO), 20179 |
scope and purpose of the guidelines are clear the recommendations are specific and unambiguous the method for searching for and selecting the evidence are clear methods used for formulating the recommendations are clearly described health benefits, side effects and risks were stated in the recommendations procedure for updating the guidelines provided target users of the guideline are clearly defined
|
unclear whether the guideline was piloted among target users unclear whether patients’ views and preferences were sought potential cost implications of applying the recommendation not included
|
| Cancer Care Ontario, Breast Cancer Guideline Development Group, 201610 |
scope and purpose of the guidelines are clear the recommendations are specific and unambiguous the method for searching for and selecting the evidence are clear methods used for formulating the recommendations are clearly described health benefits, side effects and risks were stated in the recommendations procedure for updating the guidelines provided target users of the guideline are clearly defined
|
unclear whether the guideline was piloted among target users unclear whether patients’ views and preferences were sought potential cost implications of applying the recommendation not included
|
| European Society of Medical Oncology (ESMO), 201511 |
scope and purpose of the guidelines are clear the recommendations are specific and unambiguous the method for searching for and selecting the evidence are clear methods used for formulating the recommendations are clearly described health benefits, side effects and risks were stated in the recommendations procedure for updating the guidelines provided target users of the guideline are clearly defined
|
unclear whether the guideline was piloted among target users unclear whether patients’ views and preferences were sought potential cost implications of applying the recommendation not included
|
| Alberta Health Services, 201412 |
scope and purpose of the guidelines are clear the recommendations are specific and unambiguous the method for searching for and selecting the evidence are clear methods used for formulating the recommendations are clearly described health benefits, side effects and risks were stated in the recommendations procedure for updating the guidelines provided target users of the guideline are clearly defined potential cost implications of applying the recommendation were included
|
|
SLNB = sentinel lymph node biopsy
Appendix 4. Main Study Findings and Author’s Conclusions
Table 4Main Study Findings and Authors’ Conclusions
View in own window
| Recommendations | Strength of Evidence |
|---|
| NICE, 20188 |
|---|
Invasive breast cancer 1.4.1 Perform minimal surgery, rather than lymph node clearance, to stage the axilla for people with invasive breast cancer and no evidence of lymph node involvement on ultrasound or a negative ultrasound-guided needle biopsy. Sentinel lymph node biopsy (SLNB) is the preferred technique. [2009] 1.4.2 Perform SLNB using the dual technique with isotope and blue dye. [2009] Ductal carcinoma in situ 1.4.4 Do not perform SLNB routinely for women with a preoperative diagnosis of DCIS who are having breast-conserving surgery, unless they are considered to be at high risk[1] of invasive disease. People at high risk of invasive disease include those with a palpable mass or extensive microcalcifications. [2009] 1.4.5 Offer SLNB to all people who are having a mastectomy for DCIS. [2009] (p7,8) | Level of evidence and strength of recommendation not reported |
| ASCO, 20179 |
|---|
Recommendation 3. Clinicians may offer SNB for women who have operable breast cancer who have the following circumstances: 3.1. Multicentric tumors 3.2. Ductal carcinoma in situ when mastectomy is performed. 3.3. Prior breast and/or axillary surgery 3.4. Preoperative/neoadjuvant systemic therapy (p562) | 3.1 Evidence quality: intermediate (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate) Strength of recommendation: moderate 3.2 Evidence quality: insufficient (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate) Strength of recommendation: weak 3.3 Evidence quality: intermediate (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate) Strength of recommendation: strong 3.4 Evidence quality: intermediate (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate) Strength of recommendation: moderate |
| Cancer care Ontario, Breast Cancer Guideline Development Group, 201610 |
|---|
| “SLNB is recommended as the preferred method of axillary staging for all patients with a clinical presentation of early-stage breast cancer in the absence of clinically or pathologically positive lymph nodes” (p2) | Level of evidence and strength of recommendation not reported |
| ESMO, 201511 |
|---|
SLNB rather than full axillary nodal clearance, is now the standard of care, unless axillary node involvement is proven [II, A]. In patients undergoing preoperative systemic therapy, SLNB carried out after systemic therapy demonstrated lower detection rates and higher rates of falsenegatives. However, if the axilla is negative on ultrasound and/or PET/CT scanning, carried out before the start of systemic therapy, a post-systemic therapy SNLB can be considered [V, B]. (Table 7)
| Level of evidence: II: Small, randomised trials or large, randomised trials with a suspicion of bias (lower methodological quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity) V: Studies without the control group, case reports, experts opinions Strength of recommendation: A: Strong evidence for efficacy with a substantial clinical benefit, strongly recommended B: Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended |
| Alberta Health Services, 201412 |
|---|
| “There is sufficient evidence to recommend the use of sentinel lymph node dissection after NAT for staging and management of patients presenting with a clinically negative axilla” (p6) | Not reported |
NAT = neoadjuvant therapy; SLNB or SNB = sentinel lymph node biopsy;
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Sentinel lymph node biopsy for the management of breast cancer: a review of guidelines. Ottawa: CADTH; 2019 Apr. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governmentsor any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.