Introduction
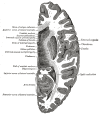
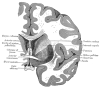
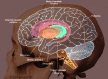
The putamen, combined with the globus pallidus, forms the lentiform nucleus; and with the caudate nucleus, it shapes the striatum, which is a subcortical structure that forms the basal ganglia. The putamen is involved in learning and motor control, including speech articulation, language functions, reward, cognitive functioning, and addiction.[1][2][3] Research has noted putaminal dysfunctions in various motor and cognitive dysfunctions, namely Parkinson disease, Huntington disease, Alzheimer disease, depression, obsessive-compulsive disorder, Wilson disease, and autism.
Structure and Function
The basal ganglia are a group of deep brain nuclei that divide into the putamen, caudate nucleus, nucleus accumbens, and globus pallidus. The putamen, caudate, and nucleus accumbens collectively form the striatum. Different parts of the striatum receive afferent input from different cortical regions and project their efferent output to the cortex through the thalamus.[4][5] The anterior putamen connects with the associative regions in the cortex, and the posterior portion connects with the primary motor cortex and the supplementary motor area.[4][6]
The striatum plays a significant role in various brain functions, including motor control and learning, language, reward, cognitive functioning, and addiction through the functional cortico-striato-thalamocortical neural pathways.[3][7] Traditionally, the basal ganglia structures are known for their motor functions. However, it is well studied now that the basal ganglia are not only involved in purely motor functions but also are associated with more complex goal-directed behaviors, including emotion, motivation, and cognition components to express a particular movement.[1] Therefore a pathologic state (e.g., neurodegeneration, hemorrhage, etc.) in the striatum can lead to a broad range of clinical manifestations from motor dysfunction such as Parkinson disease to various psychiatric disorders.[3][8]
The putamen is also involved in modulating the sensory as well as motor aspects of pain.[9]
Embryology
From cephalic to caudal, the primary three primitive brain vesicles differentiate to the prosencephalon (forebrain), mesencephalon (midbrain), and rhombencephalon (hindbrain). By the end of the fifth week of gestation, the prosencephalon differentiates to the telencephalon (cerebral hemispheres) and the diencephalon.[10] The telencephalon gives rise to most of the components of the basal ganglia which includes the caudate and the putamen.[7] The globus pallidus originates from neuroblasts in the wall of the 3rd ventricle of the diencephalon.
Blood Supply and Lymphatics
The putamen receives its vascular supply from the perforating branches of the anterior cerebral artery (ACA) and middle cerebral artery (MCA), also known as the lenticulostriate arteries, with variations of the predominance of either ACA or MCA supply.[11] Although the brain tissue with the highest metabolic rate lacks a conventional lymphatic system responsible for cleansing waste, the brain parenchyma owns its specific lymphatic drainage pathways,[12][13] which are composed of[14][15][16][17][18][19][20]:
- Perivascular drainage pathways
- Glymphatic system
- Meningeal lymphatic vessels, olfactory/cervical lymphatic drainage route and their association with CSF circulation
- The connection among different components of the brain lymphatic drainage system
The putamen, as a portion of basal ganglia, has a similar drainage system as the whole brain with structural differences in perivascular spaces compared to the cerebral cortex.[21]
Nerves
The putamen, situated in the striatal/dorsal portion of the basal ganglia, functions in harmony with the cortex through a complex cortico-basal ganglia network to perform and produce complex behaviors. It appears to be the coordination between separate functional channels and integration across a function that directs a coordinated behavior to exhibit and also be modified based on external and internal stimuli.[1] The three primary afferent input sources to the striatum are the cerebral cortex, thalamus, and primarily dopaminergic cells of the brain stem. Accordingly, outputs from the striatum travel to the pallidum complex and the substantia nigra, pars reticulata, and pars compacta.[1]
Physiologic Variants
Volumetric changes of the putamen have been linked to different neurologic and psychiatric disorders. Researchers have conducted large imaging meta-analyses to establish the physiologic variation of the putamen to make it comparable to its pathologic condition and determine its physiological state. It has been proven in almost all studies that the volume of putamen declines in size in both genders by aging.[22][3] However, the effect of gender and hemispherical asymmetry is still controversial.[22][23][3]
Surgical Considerations
The putamen, as a common structure affected by a hypertensive cerebral hemorrhage, elicits a large range of presentations based on the magnitude of the initial blood extravasation.[24] Despite the controversy, surgical evacuation of the intracerebral hemorrhage is a mainstay therapeutic approach to decompress the mass effect and also eliminate the cytotoxic edema resulting from the ischemia and the degraded blood products.[25] Current recommendations to remove putaminal bleed include large size hematoma with life-threatening herniation, especially in young aged patients.[26] The other indication of removal of hematoma includes the presence of hemiparesis owing to the compression of the internal capsule from the hematoma that can be confirmed by the application of the MR tractography study.[26]
There are different surgical approaches chosen based on the hematoma size and position, the patient's hemodynamical stability, underlying etiology, resource availability, and the surgeon's preference.[27][25][28] For example, the putaminal hemorrhages associated with arteriovenous malformations make it a contraindication for endoscopic surgery.[29] Therefore, it necessitates changing the operating method from the endoscopic to the microscopic approach.[27] Traditionally, a craniotomy was the first-line surgical treatment; but due to its high mortality and morbidity rate, endoscopy, and most recently, navigation guided or stereotactic aspiration has been the favored technique.[26] Although less invasive, the efficiency of hematoma evacuation is still low in endoscopy surgery owing to obscure visualization and limited or incomplete hemostasis.[28][25] Therefore, a stainless-steel tube helps to guide the endoscope during the evacuation procedure.[28] Moreover, a standard technique of endoscopy, precise targeting of the lesions, non-eloquent assessment with minimal brain retraction, and maintaining optimal hemostasis are critical factors to eliminate postoperative complications.[25]
Despite all the disagreements over the timing of surgery and appropriate surgical approach (craniotomy, stereotactic or endoscopic) for hematoma evacuation, the most important factor to keep in mind is fast decompression to control the intracranial pressure, not the complete evacuation.[29]
Clinical Significance
The putamen is a common site for hypertensive bleed as well as infarction. The corkscrew pattern of lenticulostriate vessels (increases intraluminal pressure), as well as the formation of Charcot-Bouchard aneurysm secondary to fibrinoid necrosis, predisposes to its rupture.[24][30] On the contrary, lipohyalinosis and micro-atheroma formation account for resulting in infarction.[24]
Bilateral putaminal hemorrhages, though rare, can occur in cases of bleeding disorders, methanol intoxication, metastatic lesions, and amyloid angiopathies.
There can be a multispectral presentation in cases with small putaminal strokes, and these can categorize as follows[24]:
- Mixed motor and sensory
- Pure motor
- Pure sensory
- Ataxic hemiparesis
- Dysarthria with clumsy hands
- Hemiballism and hemichorea
There can be aphasia due to putaminal bleed in the dominant hemisphere, whereas spatial and hemineglect occur in right putaminal bleeds.[24] The presence of a conjugate eye deviation (CED) has been regarded as a poor prognostic marker in these bleeds.[31]
The putamen correlates with a broad spectrum of movement disorders and psychiatric diseases. The most well-known movement disorder related to putamen is Parkinson disease, which is the result of dopamine depletion in the posterior putamen and presents with rigidity, tremor, ataxia, and impairment of balance. Changes in putamen volume link to a large number of diseases.[22] While some disorders increase the volume of the putamen, including bipolar disorder,[32] Tourette syndrome,[33] attention-deficit-hyperactivity disorder,[34] researchers have seen a decline in volume in major depressive disorder,[35] Williams syndrome,[36][37] autism, schizophrenia,[38] and suicide attempters.[39] Interestingly, obsessive-compulsive disorder (OCD) prevents putaminal volume loss associated with normal aging.[40] It also has been documented that repetitive behaviors of OCD develop in the putaminal lesions.[41] Other pathologic conditions linked to dysfunction of putamen include Huntington disease, Lewy body disorders, Alzheimer disease, Wilson disease,[42] motor, and cognitive impairment following putaminal hemorrhage, gait dysregulation following a stroke, and bilateral putaminal necrosis as a sequela of methanol toxicity.[43][44]
Other Issues
Interesting findings regarding the involvement of the putamen in various physiological states[8][45][46][45]:
- Studies have demonstrated that the total volume of bilateral putamen in bilinguals is greater compared to monolingual individuals.
- A recent neuropsychological study evaluated the volumes of subcortical structures regarding the ability to recognize facial expressions of emotions. The results elicited the putamen volume inversely correlated with the recognition of the fearful face.
- The putamen, along with some other brain regions, mainly orbitofrontal cortices in the left and right hemispheres, is involved in the recognition of a mother’s own infant versus other infants, demonstrating the significant role of striatal structures in maternal behavior and love.
Figure
Putamen & Caudate nucleus Image courtesy Dr Chaigasame
References
- 1.
- Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016 Mar;18(1):7-21. [PMC free article: PMC4826773] [PubMed: 27069376]
- 2.
- Viñas-Guasch N, Wu YJ. The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct Funct. 2017 Dec;222(9):3991-4004. [PubMed: 28585051]
- 3.
- Koikkalainen J, Hirvonen J, Nyman M, Lötjönen J, Hietala J, Ruotsalainen U. Shape variability of the human striatum--Effects of age and gender. Neuroimage. 2007 Jan 01;34(1):85-93. [PubMed: 17056276]
- 4.
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995 Jan;20(1):91-127. [PubMed: 7711769]
- 5.
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003 Dec;26(4):317-30. [PubMed: 14729134]
- 6.
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357-81. [PubMed: 3085570]
- 7.
- Fazl A, Fleisher J. Anatomy, Physiology, and Clinical Syndromes of the Basal Ganglia: A Brief Review. Semin Pediatr Neurol. 2018 Apr;25:2-9. [PMC free article: PMC6039104] [PubMed: 29735113]
- 8.
- Uono S, Sato W, Kochiyama T, Kubota Y, Sawada R, Yoshimura S, Toichi M. Putamen Volume is Negatively Correlated with the Ability to Recognize Fearful Facial Expressions. Brain Topogr. 2017 Nov;30(6):774-784. [PubMed: 28748407]
- 9.
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, McHaffie JG, Coghill RC. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011 Jul;134(Pt 7):1987-2004. [PMC free article: PMC3122370] [PubMed: 21616963]
- 10.
- Mortazavi MM, Adeeb N, Griessenauer CJ, Sheikh H, Shahidi S, Tubbs RI, Tubbs RS. The ventricular system of the brain: a comprehensive review of its history, anatomy, histology, embryology, and surgical considerations. Childs Nerv Syst. 2014 Jan;30(1):19-35. [PubMed: 24240520]
- 11.
- Djulejić V, Marinković S, Georgievski B, Stijak L, Aksić M, Puškaš L, Milić I. Clinical significance of blood supply to the internal capsule and basal ganglia. J Clin Neurosci. 2016 Mar;25:19-26. [PubMed: 26596401]
- 12.
- Sun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF, Yuan H, Colvin RA, Yang XY. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog Neurobiol. 2018 Apr-May;163-164:118-143. [PubMed: 28903061]
- 13.
- Laman JD, Weller RO. Editorial: route by which monocytes leave the brain is revealed. J Leukoc Biol. 2012 Jul;92(1):6-9. [PubMed: 22745459]
- 14.
- Engelhardt B, Carare RO, Bechmann I, Flügel A, Laman JD, Weller RO. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016 Sep;132(3):317-38. [PMC free article: PMC4992028] [PubMed: 27522506]
- 15.
- Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G. Cerebrovascular Smooth Muscle Cells as the Drivers of Intramural Periarterial Drainage of the Brain. Front Aging Neurosci. 2019;11:1. [PMC free article: PMC6357927] [PubMed: 30740048]
- 16.
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012 Aug 15;4(147):147ra111. [PMC free article: PMC3551275] [PubMed: 22896675]
- 17.
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015 Jun 29;212(7):991-9. [PMC free article: PMC4493418] [PubMed: 26077718]
- 18.
- Bradley WG. CSF Flow in the Brain in the Context of Normal Pressure Hydrocephalus. AJNR Am J Neuroradiol. 2015 May;36(5):831-8. [PMC free article: PMC7990574] [PubMed: 25355813]
- 19.
- Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008 May 14;5:10. [PMC free article: PMC2412840] [PubMed: 18479516]
- 20.
- Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11(1):26. [PMC free article: PMC4326185] [PubMed: 25678956]
- 21.
- Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997 Oct;191 ( Pt 3)(Pt 3):337-46. [PMC free article: PMC1467691] [PubMed: 9418990]
- 22.
- Halkur Shankar S, Ballal S, Shubha R. Study of normal volumetric variation in the putamen with age and sex using magnetic resonance imaging. Clin Anat. 2017 May;30(4):461-466. [PubMed: 28281277]
- 23.
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Free S, Lemieux L, Myers R, Duncan JS, Brooks DJ, Koepp MJ, Hammers A. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. Neuroimage. 2007 Nov 01;38(2):261-70. [PubMed: 17851093]
- 24.
- Ghetti G. Putaminal hemorrhages. Front Neurol Neurosci. 2012;30:141-4. [PubMed: 22377882]
- 25.
- Hsieh PC, Cho DY, Lee WY, Chen JT. Endoscopic evacuation of putaminal hemorrhage: how to improve the efficiency of hematoma evacuation. Surg Neurol. 2005 Aug;64(2):147-53; discussion 153. [PubMed: 16051009]
- 26.
- Munakomi S, Agrawal A. Advancements in Managing Intracerebral Hemorrhage: Transition from Nihilism to Optimism. Adv Exp Med Biol. 2019;1153:1-9. [PubMed: 30888664]
- 27.
- Suyama D, Kumar B, Watanabe S, Tanaka R, Yamada Y, Kawase T, Kato Y. Endoscopic Approach to Putaminal Bleed. Asian J Neurosurg. 2019 Jan-Mar;14(1):63-66. [PMC free article: PMC6417331] [PubMed: 30937010]
- 28.
- Chen CC, Cho DY, Chang CS, Chen JT, Lee WY, Lee HC. A stainless steel sheath for endoscopic surgery and its application in surgical evacuation of putaminal haemorrhage. J Clin Neurosci. 2005 Nov;12(8):937-40. [PubMed: 16275100]
- 29.
- Suyama D, Kumar B, Watanabe S, Tanaka R, Yamada Y, Kawase T, Kato Y. Endoscopic Approach to Cerebellar and Large Putaminal Bleed. Asian J Neurosurg. 2019 Jan-Mar;14(1):72-76. [PMC free article: PMC6417327] [PubMed: 30937012]
- 30.
- Gupta K, M Das J. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 17, 2023. Charcot-Bouchard Aneurysm. [PubMed: 31971704]
- 31.
- Sato S, Koga M, Yamagami H, Okuda S, Okada Y, Kimura K, Shiokawa Y, Nakagawara J, Furui E, Hasegawa Y, Kario K, Arihiro S, Nagatsuka K, Minematsu K, Toyoda K. Conjugate eye deviation in acute intracerebral hemorrhage: stroke acute management with urgent risk-factor assessment and improvement--ICH (SAMURAI-ICH) study. Stroke. 2012 Nov;43(11):2898-903. [PubMed: 22984006]
- 32.
- DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004 Feb;6(1):43-52. [PubMed: 14996140]
- 33.
- Roessner V, Overlack S, Schmidt-Samoa C, Baudewig J, Dechent P, Rothenberger A, Helms G. Increased putamen and callosal motor subregion in treatment-naïve boys with Tourette syndrome indicates changes in the bihemispheric motor network. J Child Psychol Psychiatry. 2011 Mar;52(3):306-14. [PubMed: 20883521]
- 34.
- Xu B, Jia T, Macare C, Banaschewski T, Bokde ALW, Bromberg U, Büchel C, Cattrell A, Conrod PJ, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot JL, Paillère Martinot ML, Nees F, Orfanos DP, Paus T, Poustka L, Smolka MN, Walter H, Whelan R, Schumann G, Desrivières S., IMAGEN Consortium. Impact of a Common Genetic Variation Associated With Putamen Volume on Neural Mechanisms of Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2017 May;56(5):436-444.e4. [PubMed: 28433093]
- 35.
- Sachs-Ericsson NJ, Hajcak G, Sheffler JL, Stanley IH, Selby EA, Potter GG, Steffens DC. Putamen Volume Differences Among Older Adults: Depression Status, Melancholia, and Age. J Geriatr Psychiatry Neurol. 2018 Jan;31(1):39-49. [PubMed: 29251178]
- 36.
- Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Karmiloff-Smith A, Murphy DG, Murphy KC. Brain structural differences associated with the behavioural phenotype in children with Williams syndrome. Brain Res. 2009 Mar 03;1258:96-107. [PubMed: 19118537]
- 37.
- Zou L, Song Y, Zhou X, Chu J, Tang X. Regional morphometric abnormalities and clinical relevance in Wilson's disease. Mov Disord. 2019 Apr;34(4):545-554. [PubMed: 30817852]
- 38.
- Cheung C, Yu K, Fung G, Leung M, Wong C, Li Q, Sham P, Chua S, McAlonan G. Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PLoS One. 2010 Aug 18;5(8):e12233. [PMC free article: PMC2923607] [PubMed: 20805880]
- 39.
- Jollant F, Wagner G, Richard-Devantoy S, Köhler S, Bär KJ, Turecki G, Pereira F. Neuroimaging-informed phenotypes of suicidal behavior: a family history of suicide and the use of a violent suicidal means. Transl Psychiatry. 2018 Jun 19;8(1):120. [PMC free article: PMC6008434] [PubMed: 29921964]
- 40.
- de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, Stein DJ, Fouche JP, Soriano-Mas C, Sato JR, Hoexter MQ, Denys D, Nakamae T, Nishida S, Kwon JS, Jang JH, Busatto GF, Cardoner N, Cath DC, Fukui K, Jung WH, Kim SN, Miguel EC, Narumoto J, Phillips ML, Pujol J, Remijnse PL, Sakai Y, Shin NY, Yamada K, Veltman DJ, van den Heuvel OA. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014 Mar;171(3):340-9. [PubMed: 24220667]
- 41.
- Figee M, Wielaard I, Mazaheri A, Denys D. Neurosurgical targets for compulsivity: what can we learn from acquired brain lesions? Neurosci Biobehav Rev. 2013 Mar;37(3):328-39. [PubMed: 23313647]
- 42.
- Poujois A, Mikol J, Woimant F. Wilson disease: brain pathology. Handb Clin Neurol. 2017;142:77-89. [PubMed: 28433113]
- 43.
- Yang JH, Lee HD, Kwak SY, Byun KH, Park SH, Yang D. Mechanism of cognitive impairment in chronic patients with putaminal hemorrhage: A diffusion tensor tractography. Medicine (Baltimore). 2018 Jul;97(29):e11035. [PMC free article: PMC6086472] [PubMed: 30024496]
- 44.
- Bhatia R, Kumar M, Garg A, Nanda A. Putaminal necrosis due to methanol toxicity. Pract Neurol. 2008 Dec;8(6):386-7. [PubMed: 19015300]
- 45.
- Burgaleta M, Sanjuán A, Ventura-Campos N, Sebastian-Galles N, Ávila C. Bilingualism at the core of the brain. Structural differences between bilinguals and monolinguals revealed by subcortical shape analysis. Neuroimage. 2016 Jan 15;125:437-445. [PubMed: 26505300]
- 46.
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biol Psychiatry. 2008 Feb 15;63(4):415-23. [PubMed: 17686467]
Disclosure: Mehrnoosh Ghandili declares no relevant financial relationships with ineligible companies.
Disclosure: Sunil Munakomi declares no relevant financial relationships with ineligible companies.
Publication Details
Author Information and Affiliations
Authors
Mehrnoosh Ghandili1; Sunil Munakomi2.Affiliations
Publication History
Last Update: January 30, 2023.
Copyright
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Publisher
StatPearls Publishing, Treasure Island (FL)
NLM Citation
Ghandili M, Munakomi S. Neuroanatomy, Putamen. [Updated 2023 Jan 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.