15.1. INTRODUCTION
Dietary fat has frequently been blamed for the increase in prevalence of obesity (Bray et al., 2004). Epidemiological studies have demonstrated a positive relationship between high-fat diets and excess energy intake due to their high energy density and palatability (Prentice and Poppitt, 1996). However, this association is confounded by differences in physical activity, smoking, and food availability and variety (Willett, 1998; Bray et al., 2004). Furthermore, epidemiological studies investigating the association between high fat intake and obesity have been inconsistent (Seidell, 1998; Willett, 1998).
Preload studies have shown that fat exerts the weakest effect on satiety compared to carbohydrate and protein, suggesting that fat may lead to “passive overconsumption” (Blundell et al., 1993). But when preloads were matched for energy density and palatability, differences in satiety were not obvious (Geliebter, 1979; Stubbs and Harbron, 1996; McCrory et al., 2000), pointing to energy density as the key driver of satiety under experimental conditions. Furthermore, lipids suppress later food intake when present in the small intestine of both humans and animals (Welch et al., 1988; Greenberg et al., 1990; Drewe et al., 1992; Woltman and Reidelberger, 1995; Castiglione et al., 1998; Van Wymwlbeke et al., 1998).
Relatively few studies have investigated the responses of specific fats and fatty acids on food intake. Furthermore, studies have used different fats and fatty acids making it almost impossible to draw conclusions. However, it is clear that not all fats are equal in their effect on appetite and associated biological processes.
15.2. DIETARY FATS AND SATIETY: FAT STRUCTURE
The effect of fats on satiety has been investigated in four areas associated with fat structure: chain length, degree of saturation, degree of esterification, and functionality of specific fat molecules, particularly conjugated linoleic acid (CLA) and Olibra® (Lipid Technologies Provider AB, Karishamn, Sweden).
15.2.1. Chain Length
Studies on the effect of fatty acid chain length on satiety have shown that medium-chain triacylglycerols (MCT, 8–12 C) are more satiating than long-chain triacylglycerols (LCT) in animals (Friedman et al., 1983) and humans (Stubbs and Harbron, 1996; Rolls et al., 1988; Van Wymelbeke et al., 1998, 2001; St-Onge et al., 2003). MCT consumed as a preload resulted in lower energy intake 30 min later compared to LCT in healthy individuals (Rolls et al., 1988) (Figure 15.1). A breakfast high in MCT (30%) resulted in lower energy intake (220 kcal) at lunch 4 h later compared to a high oleic acid breakfast (30%) in healthy individuals (St-Onge et al., 2003). A similar study also found that food intake at lunch was lower after a high MCT breakfast (43 g) compared to high oleic or high saturated fat breakfast in men (Van Wymelbeke et al., 1998). The same authors found lower intake at dinner when a high MCT lunch was consumed (Van Wymelbeke et al., 2001).
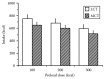
FIGURE 15.1
Mean energy intake (kcal) from an ad libitum lunch in 12 individuals. Lunch was offered 30 min after a preload of 100 (10 g), 200 (20 g), and 300 (30 g) kcal of MCT or LCT. There was a significant reduction in calories (14%–15% fewer calories) (more...)
Studies on weight loss have shown that adding MCT to a very low calorie diet improved satiety and resulted in a higher rate of weight loss without affecting fat-free mass (FFM) compared to LCT in the first 2 weeks of the diet in obese women (Krotkiewski, 2001). As well, consumption of 18–24 g/day of MCT with a weight reduction diet resulted in lower endpoint body weight and a trend toward greater fat mass loss after 16 weeks compared to LCT in overweight subjects (St-Onge and Bosarge, 2008).
Mode of intake (oral vs. gastrointestinal infusions) plays an important role on the effect of chain length on appetite. MCT is more satiating compared to LCT when taken orally in humans (Stubbs and Harbron, 1996; Rolls et al., 1988; Van Wymelbeke et al., 1998, 2001; St-Onge et al., 2003). However, when infused in the stomach, fatty acids with different chain lengths did not show different effects on satiety in rats (Maggio and Koopmans, 1982). On the other hand, intraduodenal infusion of long-chain fatty acids (sodium oleate) inhibited food intake, whereas infusion of medium-chain fatty acids (sodium caprylate) had no effect on food intake in humans (Matzinger et al., 2000).
The time between fat ingestion and subsequent meal has been shown to be an important variable on the effect of chain length on appetite. In diabetic rats, 1.5 mL of MCT suppressed subsequent food intake in the first 2 h after the preload, whereas the reduction in intake with 1.5 mL LCT occurred after 2–4 h compared to a no preload control (Friedman et al., 1983). The difference is proposed to be due to a differential rate of delivery of the ingested lipid to the liver (Friedman et al., 1983). MCT have an advantage over triacylglycerols in getting hydrolyzed and absorbed. Furthermore, MCT are absorbed into the portal system and are rapidly taken up and oxidized by the liver, whereas, LCT are packed into chylomicrons that bypass the liver via the lymphatic system, favoring uptake of LCT into the adipose tissue and muscle. In the mitochondria, MCT do not require acylcarnitine transferase to cross the inner mitochondrial membrane, and therefore, it is not a rate-limiting step in MCT oxidation as it is for LCT (Bremer, 1983). As a result, plasma ketone bodies are increased, which is an indication of enhanced hepatic acid oxidation (Krotkiewski, 2001; Van Wymelbeke et al., 2001). Satiety has been associated with increased fatty acid oxidation in the liver (Langhans, 1996).
Fatty acid chain length seems to be a determinant of gut hormone secretion. Only fatty acids with a chain length greater than C12 are able to stimulate the secretion of Cholecystokinin (CCK), Gastric Inhibitory Peptide (GIP), neurotensin, and pancreatic polypeptide (PP) (McLaughlin et al., 1999; Barbera et al., 2000; Drewe et al., 2008). MCT (<C12) ingested or given intraduodenally did not stimulate CCK or neurotensin release in humans (Matzinger et al., 2000; Drewe et al., 2008). Peptide YY (PYY) secretion is stimulated by both MCT and LCT, but the magnitude and concentration was greater after LCT compared to MCT (Maas et al., 1998).
Overall, when compared to LCT, MCT suppress energy intake when consumed in high amounts. However, intake of high quantities of MCT has been linked to adverse events such as nausea, vomiting, gastrointestinal discomfort, and abdominal discomfort, which limits the amount of MCT that can be incorporated in the diet.
15.2.2. Saturation
Within the same chain length, a greater degree of unsaturation is associated with enhanced satiety, but studies have been inconsistent. In the C-18 fatty acids, linoleic acid resulted in lower appetite and short-term food intake compared to oleic and stearic acids when administered intraduodenally in human subjects (French et al., 2000) (Figure 15.2) or incorporated in foods (Lawton et al., 2000). In weanling Zucker rats, a daily gavage of 100 μL of γ-linolenic acid suppressed food intake and weight gain compared to soy oil (Phinney et al., 1993; Thurmond et al., 1993). γ-Linolenic acid (890 mg/day) has been shown to reduce weight regain in formerly obese humans compared to oleic acid after 1 year of supplementation (Schirmer and Phinney, 2007). On the other hand, a 2-week study of ingestion of oils with different degrees of saturation has reported no difference in satiety scores, energy intake, or weight in overweight subjects consuming a diet high in oleic or γ-linoleic or α-linolenic acid (45 mL oil) (Kamphuis et al., 2001). In overweight subjects, polyunsaturated fatty acid (PUFA) and monounsaturated fatty acid (MUFA) in a preload (63–87 g) suppressed appetite and short-term food intake to the same extent (Flint et al., 2003). Similarly, muffins rich in MUFA (40 g) decreased food intake and appetite in a similar manner to muffins rich in saturated fatty acids (40 g) in lean subjects (Alfenas and Mattes, 2003). However, a recent study found that in rats, MUFA (600 mg/kg/12 h) were able to suppress food intake and decrease body weight to a larger extent compared to PUFA (Vogler et al., 2008).
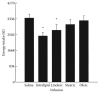
FIGURE 15.2
The effect of upper intestine infusion of C-18 fatty acid-enriched oils on food intake. Infusions were given for 100 min at a rate of 1 mL (8.3 kJ)/min (200 mL/L emulsions). At 90 min after the start of the infusion, subjects were given a liquid meal (more...)
Several mechanisms have been suggested for the association between the degree of unsaturation of fatty acids and satiety. These mechanisms include both peripheral and central pathways for appetite regulation. In the periphery, plasma CCK in humans (Beardshall et al., 1989) and apolipoprotein A-IV (ApoA-IV) in rats (Kalogeris et al., 1996) have been shown to be more potently released following ingestion of linoleic acid-containing oils compared to other less unsaturated fatty acids. Diunsaturated oil resulted in the highest release of CCK followed by monounsaturated oil with saturated fat showing no effect on plasma CCK release (Beardshall et al., 1989). However, other studies have failed to show a relationship between plasma CCK concentrations and degree of unsaturation (French et al., 2000). Another gut hormone, GLP-1, is increased with α-linolenic acid, an unsaturated long-chain free fatty acid (FFA), in a dose-dependent manner in vitro, whereas medium-chain and saturated long-chain fatty acids did not have any effect (Hirasawa et al., 2005). In the same study but in rats, α-linolenic acid resulted in the highest plasma GLP-1 secretion compared to other unsaturated and saturated fatty acids of same chain length (Hirasawa et al., 2005). Centrally, serotonin (5HT), a neurotransmitter, has also been implicated in the association between appetite and unsaturation (Friedman et al., 1986; Mullen and Martin, 1992). In diet-induced obese mice, Neuropeptide Y (NPY) mRNA in the arcuate nucleus was decreased and pro-opiomelanocortin mRNA was increased with the administration of n – 3 PUFA (Huang et al., 2004). Furthermore, it was suggested that n – 3 PUFA (α-linolenic, eicosapentaenoic acid [EPA], docosahexaenoic acid [DHA]) can exert anorexigenic effects in the peripheral endocannabinoid system by acting as antagonists to n – 6 PUFA (Oda, 2007). n – 3 PUFA competes with the n – 6 PUFA derivatives, anandamide and 2-arachidonoyl glycerol, which are major agonists for the endocannabinoid system (CB1) (Oda, 2007). In mice, the concentration of n – 3 PUFA in the brain of mice is inversely related to 2-arachidonoyl glycerol concentrations (Watanabe et al., 2003).
In conclusion, a greater number of double bonds seems to be associated with enhanced satiety when given in high amounts, but large amounts of fat cannot be recommended for human nutrition. Whether moderate amounts of PUFA can affect food intake remains to be established.
15.2.3. Esterification
The degree of esterification of dietary fats seems to play a role in satiety, but only a limited number of studies address it. One-monoglycerides infused into the duodenum of pigs suppressed subsequent intake in excess of its energy content compared to oleic acid. CCK was shown to be the mediator of this effect by administering a CCK antagonist and abolishing the reported suppression of intake by monoglycerides (Gregory and Rayner, 1987; Gregory et al., 1989). However, in humans, one-monoglycerides, given at 25% of daily energy intake, behaved in a similar manner to triglycerides with regard to appetite and energy intake in the short term and on the subsequent day (Johnstone et al., 1998a,b).
Diacylglycerols (DG), more specifically the 1,3-diacylglycerol isomer, have been reported to lower hunger, appetite, and desire-to-eat over 12 h compared to triacylglycerols when incorporated in foods (~26 g) (Kamphuis et al., 2003b). In terms of weight control, DG consumption is associated with decreased total and visceral body fat accumulation in both rats (Murase et al., 2001) and humans (Nagao et al., 2000; Maki et al., 2002). Suggested mechanisms include enhanced β-oxidation and greater energy expenditure due to the availability of free fatty acids in the portal circulation (Murata et al., 1997). DG have a similar energy value to triacylglycerols (9 kcal/g) (Taguchi et al., 2001). Similar to MCT, DG are incorporated into the portal vein and transported to the liver where they are mostly oxidized (Breckenridge and Kuksis, 1975). However, the potential effect of DG on satiety still requires further investigation.
15.2.4. Functional Fats
15.2.4.1. Conjugated Linoleic Acid
CLAs are a group of geometric and positional isomers of linoleic acid that occur naturally in food (e.g., dairy products, beef). CLA intake from dietary sources is generally <600 mg/day (Kovacs and Mela, 2006). CLA has been implicated with a number of potential health benefits including weight control. Most of the physiologic effects of CLA rest with the trans-10, cis-12 and cis-9, trans-11 isomers, linked to weight control effects; and trans-10, cis-12, associated with body composition changes (Pariza et al., 2001).
The few studies assessing the effect of long-term CLA supplementation on appetite have found inconsistent results in human subjects. Two studies found no effect (Blankson et al., 2000; Medina et al., 2000), while another study found that 13 weeks of mixed isomer CLA intake (1.8 and 3.6 g) increased fullness and satiety compared to baseline (Kamphuis et al., 2003a).
Studies on CLA supplementation and weight control in human subjects have shown that daily intake of 3.4 g of CLA (equal parts of both isomers) reduced total body fat (Blankson et al., 2000) and abdominal fat (Riserus et al., 2001). Furthermore, two other studies have also shown significant reductions in plasma leptin concentrations with daily intake of 3 g of CLA (isomer mix) for 64 days (Medina et al., 2000) and an inverse association with plasma leptin concentrations after 8 weeks of CLA intake (isomer mix) (Belury et al., 2003). Increased β-oxidation has been suggested as a mechanism. For example, infusion of 1% CLA mixture into perfused livers of rats for 2 weeks produced more ketone bodies than 1% linoleic acid-fed rats (Sakano et al., 1999), suggesting that fatty acid oxidation was increased.
While further explorations are required into the effect of CLA on appetite regulation, it is worthwhile noting that CLA trans-10, cis-12 has been shown to induce inflammation and hyperinsulinemia in animal models (Poirier et al., 2006) and impair insulin sensitivity in a few clinical trials (Riserus et al., 2002, 2004; Moloney et al., 2004), which raises concerns regarding supplementation with CLA trans-10, cis-12. Further research is needed in order to asses the health benefits or risks of CLA on the long term.
15.2.4.2. Olibra
Olibra is a novel fat emulsion consisting of a mixture of fractionated palm oil (40%) and fractionated oat oil (2.5%) in water. Olibra has been associated with increased satiety and decreased energy intake (~1 MJ difference) at a meal 4 h later when added to yogurt (5 g) in nonobese, overweight, and obese subjects. The decrease in intake was maintained for the rest of the day (Burns et al., 2000, 2001, 2002). However, a recent study has failed to find an effect on food intake and appetite (Logan et al., 2006). Speculated mechanisms include the physiochemical stability of the emulsion resulting in delayed digestion and stimulation of distal small intestine receptors and gut hormone secretion, but there is no supporting evidence in the literature. One study reported an increase in plasma GLP-1 response but only at 180 min after 25 weeks consumption of 10 g of Olibra (Diepvens et al., 2007). However, the mechanism of action requires further clarification.
15.3. FAT IN RELATION TO OTHER MACRONUTRIENTS AND SATIETY
15.3.1. Fiber
Combining fat with fiber has been shown to increase the satiating potential of fat (French and Read, 1994; Burton-Freeman, 2000; Burton-Freeman et al., 2002). Fiber intake is associated with enhanced satiety and reduced food intake (Howarth et al., 2001; Samra and Anderson, 2007). The properties of some fibers that prolong the contact of dietary fats with the intestinal mucosa and retard fat digestion may contribute to enhanced satiety. Partially hydrolyzed guar gum (PHGG) (6 g) added to yogurt has been shown to decrease bioaccessibility of fat in a dynamic model of the gastrointestinal tract (Minekus et al., 2005). Accordingly, PHGG was able to suppress postprandial serum lipid concentrations in healthy volunteers with moderate hypertriglyceridemia after a high-fat high-cholesterol meal (Kondo et al., 2004). Viscous fiber (12 g guar gum) added to high-fat treatment prolonged satiety and slowed gastric emptying compared to a high-fat treatment without fiber (French and Read, 1994). This effect may be partly mediated through enhanced release of cholecystokinin. Adding fiber (20 g) to a low-fat treatment (16–23 g) increased plasma CCK secretion and subjective satiety to a similar extent as a high-fat treatment (31–40 g) without fiber in healthy individuals (Burton-Freeman et al., 2002).
Combining fat with fiber is an interesting venue to enhance the satiating potential of fat-containing products and therefore merits further exploration.
15.3.2. Carbohydrate
Short-term studies investigating satiety after meals varying in fat-to-CHO ratios have been inconsistent (Fryer et al., 1955; Driver, 1988; van Amelsvoort et al., 1989; Foltin et al., 1990). Meals with a high-fat–carbohydrate ratio either show a weaker suppression of hunger (van Amelsvoort et al., 1989; Cotton et al., 2007) or have the same effect (van Amelsvoort et al., 1989; Foltin et al., 1990; de et al., 1992; Stubbs et al., 1995) compared to meals with a low-fat–carbohydrate ratio. Such inconsistencies could result from diverse experimental parameters and not upon actual differences in the metabolic properties of the macronutrients themselves. For example, variations in the type of fat and carbohydrate used, palatability and energy density of the test meal, population studied (gender, age, dietary restraint, body mass index, etc.), and the mode of test food delivery could result in different outcomes between studies.
15.4. FACTORS BEHIND THE IMPACT OF FAT ON SATIETY
15.4.1. Physical Properties
The chemical composition of fatty acids affects the physical properties of the triacylglycerol molecule. For example, the melting point of the fatty acid is inversely related to its degree of unsaturation (Table 15.1). Therefore, the degree of unsaturation is likely to affect the ease of emulsification of the triacylglycerol in the digestive tract, which is predicted to markedly affect the ease of digestion and absorption of fatty acids. The result is modulation of the rate of interaction between fatty acids and satiety signals on the intestinal wall (Small, 1991).
TABLE 15.1
Effect of Increasing Unsaturation of Component Fatty Acids on the Melting Point of Pure Long-Chain Triacylglycerols
15.4.2. Cephalic Modulation
Cephalic stimulation with fat is associated with modulation of digestive processes and appetite. Gilbertson et al. have shown that different fatty acids can be discriminated by taste receptors on the tongue of rats (Gilbertson et al., 1997). K+ currents on the tongue were shown to be markedly inhibited by linoleic and linolenic acids but not by stearic or oleic acid. These findings seem to parallel the human food intake data (Figure 15.1). K+ currents are identified in other tissues including the duodenum (Gilbertson, 1998), suggesting that similar signaling from the small intestine in response to fatty acids may occur. In general, presence of food in the mouth is associated with modulation of digestive processes (Helman, 1988; Teff and Engelman, 1996). Cephalic stimulation with fats, particularly long-chain unsaturated fatty acids, elicit several digestive processes including gastric lipase (Wojdemann et al., 1997) and pancreatic digestive enzymes secretion (Hiraoka et al., 2003), CCK release (Wisen et al., 1992), and pancreatic polypeptide secretion (Crystal and Teff, 2006). Stimulation of the human tongue with full-fat soft cheese increases the serum lipid response to an intragastric load of triacylglycerol when compared with nonfat soft cheese or no stimulation (Mattes, 1996). Subjects were blinded on the nature of the cheese and were not able to distinguish between the cheese samples in sensory tests suggesting that a specific chemosensory or tactile mechanism from the mouth mediated this change.
Few studies have investigated the association between oral stimulation of fat and satiety. A study has shown that oral stimulation with different fats by modified-sham-feeding (MSF) resulted in increased feelings of satiety compared with water in human subjects, with linoleic acid showing the strongest response (Smeets and Westerterp-Plantenga, 2006). On the other hand, sham feeding a high-fat cake increased food intake at the next meal compared to nonfat cake in restrained eaters (Crystal and Teff, 2006).
15.4.3. Oxidative Qualities
Fatty acid oxidation in the liver seems to be associated with appetite and food intake (Friedman and Tordoff, 1986; Friedman et al., 1986; Langhans and Scharrer, 1987; Stubbs et al., 1995). Studies on the effect of fat oxidation on food intake suggest that fat that is oxidized generates a satiety signal and, in contrast, fat that is stored is less satiating (Friedman, 1998). In rats, feeding is stimulated when the oxidation of long-chain fatty acids is inhibited by methyl palmoxirate, which blocks carnitine palmitoyl-transferase-1 and decreases transport of long-chain fatty acids to the mitochondria (Friedman and Tordoff, 1986; Friedman et al., 1990). Perhaps because MCT do not require carnitine palmitoyltransferase-1 for their transport into the mitochondria, they are more readily oxidized in the mitochondria compared to LCT, which need carnitine palmitoyltransferase-1 (Williams et al., 1968). The fast oxidation rate may partly explain the reported reduced feeding response after MCT intake compared to LCT (Friedman et al., 1990).
15.4.4. Gut Hormones
Regulation of appetite upon fat consumption has been shown to be mediated by a number of gut hormones. Administration of the CCK-A receptor antagonist loxiglumide suppressed the reduction of food intake resulting from intraduodenal fat administration; suggesting that CCK is a candidate mediator of this interaction (Matzinger et al., 2000). Duodenal fat administration in animals has been shown to result in elevated concentrations of plasma CCK (Schwartz et al., 1999). Another candidate peptide is enterostatin, which is produced from pancreatic colipase in equimolar amounts to colipase in the intestine (Erlanson-Albertsson and York, 1997). Furthermore, long-chain triglycerides have been shown to suppress plasma ghrelin (Heath et al., 2004) and stimulate secretion of CCK, GIP, neurotensin, PP, and PYY in humans (Spiller et al., 1984; Maas et al., 1998; Barbera et al., 2000) and ApoA-IV (Liu et al., 2003) in rats. PUFA stimulated the release of plasma GLP-1 in mice (Hirasawa et al., 2005).
15.4.5. Delayed Fat Digestion
Dietary fat is usually digested and absorbed in the duodenum, but if digestion and absorption of fat occurs in the distal sections of the small intestine, it stimulates a strong feedback signal associated with slowing of gastrointestinal transit and release of various satiety hormones (Read et al., 1984; Spiller et al., 1984; Welch et al., 1985; Van Citters and Lin, 1999). Infusion of corn oil into the jejunum induced early satiety and reduced energy intake in healthy volunteers compared to infusion in the duodenum (Welch et al., 1985, 1988). Furthermore, administration of a compound that retards fat digestion, by inhibiting the lipase–colipase-mediated fat hydrolysis, was associated with reduced food intake and elevated concentrations of plasma CCK and enterostatin in rats (Mei et al., 2006). Therefore, delaying fat digestion might be a promising method to enhance the satiating effect of fats and deserves further research.
15.4.6. Inhibited Fat Digestion
Products of fat digestion seem to be essential for a fat-induced satiety response. Inhibition of fat digestion by administration of a lipase inhibitor (tetrahydrolipstatin) has been shown to decrease proximal gastric relaxation (Feinle et al., 2001) and antropyloroduodenal motility (Feinle et al., 2003) resulting from intraduodenal fat administration. Furthermore, the suppression of fat breakdown modulates gut hormone release by decreasing the secretion of CCK, GLP-1, PP, and PYY and the suppression of ghrelin (Feinle et al., 2001, 2003; Feinle-Bisset et al., 2005) resulting from intraduodenal infusion of triacylglycerol. When Orlistat, a lipase inhibitor, was administered with a high-fat preload (70% fat), suppression of food intake at the next meal was prevented (Feinle et al., 2003) and the fat-induced increase in circulating neurotensin and CCK was blocked in healthy subjects (Drewe et al., 2008). These observations suggest that digestion of fats with the consequent release of free fatty acids into the small intestine is important for the effects of fat on gastrointestinal function and energy intake (Little et al., 2007).
15.4.7. Length of Small Intestine Exposed to Fat
Gastric emptying rate and intestinal transit time seem to be dependent on the length and region of small intestine exposed to dietary fats. Studies on dogs implanted with intestinal fistulae have demonstrated that regardless of the region exposed, gastric emptying was inhibited only when more than 15 cm of the small intestine was exposed to fat (sodium oleate) with the highest degree of inhibition achieved after exposure of more than 150 cm (Lin et al., 1989, 1990). However, exposure of the proximal small intestine to fat produced a stronger inhibition of gastric emptying compared to the distal small intestine (Lin et al., 1990).
Intestinal transit is inhibited by fat in both the proximal (jejunal brake) (Lin et al., 1996a) and distal gut (ileal brake) (Read et al., 1984; Spiller et al., 1984). However, contrary to gastric emptying, intestinal transit was more potently inhibited by fat in the distal than in the proximal small intestine in dogs implanted with intestinal fistulae (Lin et al., 1997). PYY has been suggested as the primary mediator of fat-induced ileal brake (Lin et al., 1996b). PYY releasing cells are located in the ileum and colon (Adrian et al., 1985; Taylor, 1985); therefore, fat in the proximal gut can release PYY indirectly via CCK (McFadden et al., 1992) or by direct stimulation of these endocrine cells in the distal small intestine (Aponte et al., 1988).
Suppression of energy intake has also been reported to be dependent on the length of small intestine exposed to fat. In rats, energy intake was decreased only when the entire small intestine was exposed to fat. Exposing only 35 cm of the jejunum to fat did not affect energy intake (Meyer et al., 1998).
15.5. CONCLUSION
In the present chapter, we tried to answer the question: Is dietary fat satiating? Within a controlled environment, yes, fats do have an effect on satiety and appear to regulate appetite through several mechanisms including the release of appetite hormones and inhibition of gastric emptying and intestinal transit. Certain types of fats are more satiating than others. However, in free-living conditions, the situation is complicated. Several genetic, psychological, and behavioral factors interact with physiological and metabolic systems on their effect on food behavior in free-living individuals. For instance, it has been found that certain individuals are genetically “immune” to the effects of a high-fat diet, suggesting that the consumption of a high-fat diet does not universally lead to weight gain. These individuals habitually consume a high-fat diet and remain lean and are therefore labeled as having “high-fat phenotypes” while others gain weight and are labeled as having “low-fat phenotypes” (Cooling and Blundell, 2001). Furthermore, in a free-living environment, the presence of highly palatable foods, a characteristic of high-fat foods, could chronically activate the hedonic system which would promote higher appetite and more energy intake (Lowe and Levine, 2005). The hedonic system is regarded as pleasure associated with eating palatable foods. In an environment with unlimited availability of highly palatable foods, there is a concern of how much the homeostatic appetite regulatory mechanisms can override the hedonic components and hyperresponsiveness to palatable foods (Blundell et al., 2005).
REFERENCES
- Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. [PubMed: 3840109]
- Alfenas RC, Mattes RD. Effect of fat sources on satiety. Obes Res. 2003;11:183–187. [PubMed: 12582212]
- van Amelsvoort JM, van SP, Kraal JH, Lussenburg RN, Houtsmuller UM. Effects of varying the carbohydrate:fat ratio in a hot lunch on postprandial variables in male volunteers. Br J Nutr. 1989;61:267–283. [PubMed: 2650734]
- Aponte GW, Taylor IL, Soll AH. Primary culture of PYY cells from canine colon. Am J Physiol. 1988;254:G829–G836. [PubMed: 3377082]
- Barbera R, Peracchi M, Brighenti F, Cesana B, Bianchi PA, Basilisco G. Sensations induced by medium and long chain triglycerides: Role of gastric tone and hormones. Gut. 2000;46:32–36. [PMC free article: PMC1727792] [PubMed: 10601051]
- Beardshall K, Frost G, Morarji Y, Domin J, Bloom SR, Calam J. Saturation of fat and cholecystokinin release: Implications for pancreatic carcinogenesis. Lancet. 1989;2:1008–1010. [PubMed: 2572741]
- Belury MA, Mahon A, Banni S. The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J Nutr. 2003;133:257S–260S. [PubMed: 12514304]
- Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr. 2000;130:2943–2948. [PubMed: 11110851]
- Blundell JE, Burley VJ, Cotton JR, Lawton CL. Dietary fat and the control of energy intake: Evaluating the effects of fat on meal size and postmeal satiety. Am J Clin Nutr. 1993;57:772S–777S. [PubMed: 8475895]
- Blundell JE, Stubbs RJ, Golding C, Croden F, Alam R, Whybrow S, Le NJ, Lawton CL. Resistance and susceptibility to weight gain: Individual variability in response to a high-fat diet. Physiol Behav. 2005;86:614–622. [PubMed: 16225895]
- Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: A review of animal, clinical and epidemiological studies. Physiol Behav. 2004;83:549–555. [PubMed: 15621059]
- Breckenridge WC, Kuksis A. Diaglycerol biosynthesis in everted sacs of rat intestinal mucosa. Can J Biochem. 1975;53:1170–1183. [PubMed: 1192259]
- Bremer J. Carnitine—Metabolism and functions. Physiol Rev. 1983;63:1420–1480. [PubMed: 6361812]
- Burns AA, Livingstone MB, Welch RW, Dunne A, Robson PJ, Lindmark L, Reid CA, Mullaney U, Rowland IR. Short-term effects of yoghurt containing a novel fat emulsion on energy and macronutrient intakes in non-obese subjects. Int J Obes Relat Metab Disord. 2000;24:1419–1425. [PubMed: 11126337]
- Burns AA, Livingstone MB, Welch RW, Dunne A, Reid CA, Rowland IR. The effects of yoghurt containing a novel fat emulsion on energy and macronutrient intakes in non-overweight, overweight and obese subjects. Int J Obes Relat Metab Disord. 2001;25:1487–1496. [PubMed: 11673771]
- Burns AA, Livingstone MB, Welch RW, Dunne A, Rowland IR. Dose–response effects of a novel fat emulsion (Olibra) on energy and macronutrient intakes up to 36 h post-consumption. Eur J Clin Nutr. 2002;56:368–377. [PubMed: 11965514]
- Burton-Freeman B. Dietary fiber and energy regulation. J Nutr. 2000;130:272S–275S. [PubMed: 10721886]
- Burton-Freeman B, Davis PA, Schneeman BO. Plasma cholecystokinin is associated with subjective measures of satiety in women. Am J Clin Nutr. 2002;76:659–667. [PubMed: 12198015]
- Castiglione KE, Read NW, French SJ. Food intake responses to upper gastrointestinal lipid infusions in humans. Physiol Behav. 1998;64:141–145. [PubMed: 9662077]
- Cooling J, Blundell JE. High-fat and low-fat phenotypes: Habitual eating of high- and low-fat foods not related to taste preference for fat. Eur J Clin Nutr. 2001;55:1016–1021. [PubMed: 11641752]
- Cotton JR, Burley VJ, Weststrate JA, Blundell JE. Dietary fat and appetite: Similarities and differences in the satiating effect of meals supplemented with either fat or carbohydrate. J Hum Nutr Diet. 2007;20:186–199. [PubMed: 17539869]
- Crystal SR, Teff KL. Tasting fat: Cephalic phase hormonal responses and food intake in restrained and unrestrained eaters. Physiol Behav. 2006;89:231–220. [PubMed: 16846622]
- de GC, Hulshof T, Weststrate JA, Jas P. Short-term effects of different amounts of protein, fats, and carbohydrates on satiety. Am J Clin Nutr. 1992;55:33–38. [PubMed: 1728818]
- Diepvens K, Soenen S, Steijns J, Arnold M, Westerterp-Plantenga M. Long-term effects of consumption of a novel fat emulsion in relation to body-weight management. Int J Obes (Lond). 2007;32:510–8. [PubMed: 17299383]
- Drewe J, Gadient A, Rovati LC, Beglinger C. Role of circulating cholecystokinin in control of fat-induced inhibition of food intake in humans. Gastroenterology. 1992;102:1654–1659. [PubMed: 1568575]
- Drewe J, Mihailovic S, D’Amato M, Beglinger C. Regulation of fat-stimulated neurotensin secretion in healthy subjects. J Clin Endocrinol Metab. 2008;93:1964–1970. [PubMed: 18303078]
- Driver CJ. The effect of meal composition on the degree of satiation following a test meal and possible mechanisms involved. Br J Nutr. 1988;60:441–449. [PubMed: 3219315]
- Erlanson-Albertsson C, York D. Enterostatin—A peptide regulating fat intake. Obes Res. 1997;5:360–372. [PubMed: 9285845]
- Feinle C, Rades T, Otto B, Fried M. Fat digestion modulates gastrointestinal sensations induced by gastric distention and duodenal lipid in humans. Gastroenterology. 2001;120:1100–1107. [PubMed: 11266374]
- Feinle C, O’Donovan D, Doran S, Andrews JM, Wishart J, Chapman I, Horowitz M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G798–G807. [PubMed: 12684211]
- Feinle-Bisset C, Patterson M, Ghatei MA, Bloom SR, Horowitz M. Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metab. 2005;289:E948–E953. [PubMed: 15998659]
- Flint A, Helt B, Raben A, Toubro S, Astrup A. Effects of different dietary fat types on postprandial appetite and energy expenditure. Obes Res. 2003;11:1449–1455. [PubMed: 14694208]
- Foltin RW, Fischman MW, Moran TH, Rolls BJ, Kelly TH. Caloric compensation for lunches varying in fat and carbohydrate content by humans in a residential laboratory. Am J Clin Nutr. 1990;52:969–980. [PubMed: 2239795]
- French SJ, Read NW. Effect of guar gum on hunger and satiety after meals of differing fat content: Relationship with gastric emptying. Am J Clin Nutr. 1994;59:87–91. [PubMed: 8279409]
- French SJ, Conlon CA, Mutuma ST, Arnold M, Read NW, Meijer G, Francis J. The effects of intestinal infusion of long-chain fatty acids on food intake in humans. Gastroenterology. 2000;119:943–948. [PubMed: 11040181]
- Friedman MI. Fuel partitioning and food intake. Am J Clin Nutr. 1998;67:513S–518S. [PubMed: 9497162]
- Friedman MI, Tordoff MG. Fatty acid oxidation and glucose utilization interact to control food intake in rats. Am J Physiol. 1986;251:R840–R845. [PubMed: 3777211]
- Friedman MI, Edens NK, Ramirez I. Differential effects of medium- and long-chain triglycerides on food intake of normal and diabetic rats. Physiol Behav. 1983;31:851–855. [PubMed: 6686684]
- Friedman MI, Tordoff MG, Ramirez I. Integrated metabolic control of food intake. Brain Res Bull. 1986;17:855–859. [PubMed: 3801940]
- Friedman MI, Ramirez I, Bowden CR, Tordoff MG. Fuel partitioning and food intake: Role for mitochondrial fatty acid transport. Am J Physiol. 1990;258:R216–R221. [PubMed: 2301636]
- Fryer JH, Moore NS, Williams HH, Young CM. A study of the interrelationship of the energy-yielding nutrients, blood glucose levels, and subjective appetite in man. J Lab Clin Med. 1955;45:684–696. [PubMed: 14368035]
- Geliebter AA. Effects of equicaloric loads of protein, fat, and carbohydrate on food intake in the rat and man. Physiol Behav. 1979;22:267–273. [PubMed: 441167]
- Gilbertson TA. Role of the taste system in ingestive behavior. Studies in NaCl and fatty acid transduction. Ann N Y Acad Sci. 1998;855:860–867. [PubMed: 9929702]
- Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: Gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–C1210. [PubMed: 9142845]
- Greenberg D, Smith GP, Gibbs J. Intraduodenal infusions of fats elicit satiety in sham-feeding rats. Am J Physiol. 1990;259:R110–R118. [PubMed: 2375420]
- Gregory PC, Rayner DV. The influence of gastrointestinal infusion of fats on regulation of food intake in pigs. J Physiol. 1987;385:471–481. [PMC free article: PMC1192355] [PubMed: 3656166]
- Gregory PC, McFadyen M, Rayner DV. Duodenal infusion of fat, cholecystokinin secretion and satiety in the pig. Physiol Behav. 1989;45:1021–1024. [PubMed: 2506588]
- Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol. 2004;180:273–281. [PubMed: 14765979]
- Helman CA. Chewing gum is as effective as food in stimulating cephalic phase gastric secretion. Am J Gastroenterol. 1988;83:640–642. [PubMed: 3376919]
- Hiraoka T, Fukuwatari T, Imaizumi M, Fushiki T. Effects of oral stimulation with fats on the cephalic phase of pancreatic enzyme secretion in esophagostomized rats. Physiol Behav. 2003;79:713–717. [PubMed: 12954413]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. [PubMed: 15619630]
- Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59:129–139. [PubMed: 11396693]
- Huang XF, Xin X, McLennan P, Storlien L. Role of fat amount and type in ameliorating diet-induced obesity: Insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro-opiomelanocortin mRNA expression. Diabetes Obes Metab. 2004;6:35–44. [PubMed: 14686961]
- Johnstone AM, Ryan LM, Reid CA, Stubbs RJ. Breakfasts high in monoglyceride or triglyceride: No differential effect on appetite or energy intake. Eur J Clin Nutr. 1998a;52:603–609. [PubMed: 9725662]
- Johnstone AM, Ryan LM, Reid CA, Stubbs RJ. Overfeeding fat as monoglyceride or triglyceride: Effect on appetite, nutrient balance and the subsequent day’s energy intake. Eur J Clin Nutr. 1998b;52:610–618. [PubMed: 9725663]
- Kalogeris TJ, Monroe F, Demichele SJ, Tso P. Intestinal synthesis and lymphatic secretion of apolipoprotein A-IV vary with chain length of intestinally infused fatty acids in rats. J Nutr. 1996;126:2720–2729. [PubMed: 8914941]
- Kamphuis MM, Westerterp-Plantenga MS, Saris WH. Fat-specific satiety in humans for fat high in linoleic acid vs fat high in oleic acid. Eur J Clin Nutr. 2001;55:499–508. [PubMed: 11423927]
- Kamphuis MM, Lejeune MP, Saris WH, Westerterp-Plantenga MS. Effect of conjugated linoleic acid supplementation after weight loss on appetite and food intake in overweight subjects. Eur J Clin Nutr. 2003a;57:1268–1274. [PubMed: 14506488]
- Kamphuis MM, Mela DJ, Westerterp-Plantenga MS. Diacylglycerols affect substrate oxidation and appetite in humans. Am J Clin Nutr. 2003b;77:1133–1139. [PubMed: 12716663]
- Kondo S, Xiao JZ, Takahashi N, Miyaji K, Iwatsuki K, Kokubo S. Suppressive effects of dietary fiber in yogurt on the postprandial serum lipid levels in healthy adult male volunteers. Biosci Biotechnol Biochem. 2004;68:1135–1138. [PubMed: 15170121]
- Kovacs EM, Mela DJ. Metabolically active functional food ingredients for weight control. Obes Rev. 2006;7:59–78. [PubMed: 16436103]
- Krotkiewski M. Value of VLCD supplementation with medium chain triglycerides. Int J Obes Relat Metab Disord. 2001;25:1393–1400. [PubMed: 11571605]
- Langhans W. Metabolic and glucostatic control of feeding. Proc Nutr Soc. 1996;55:497–515. [PubMed: 8832815]
- Langhans W, Scharrer E. Evidence for a vagally mediated satiety signal derived from hepatic fatty acid oxidation. J Auton Nerv Syst. 1987;18:13–18. [PubMed: 3819311]
- Lawton CL, Delargy HJ, Brockman J, Smith FC, Blundell JE. The degree of saturation of fatty acids influences post-ingestive satiety. Br J Nutr. 2000;83:473–482. [PubMed: 10953671]
- Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrient. Am J Physiol. 1989;256:G404–G411. [PubMed: 2919683]
- Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed to nutrient. Am J Physiol. 1990;259:G1031–G1036. [PubMed: 2260658]
- Lin HC, Zhao XT, Wang L. Jejunal brake: Inhibition of intestinal transit by fat in the proximal small intestine. Dig Dis Sci. 1996a;41:326–329. [PubMed: 8601377]
- Lin HC, Zhao XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology. 1996b;110:1491–1495. [PubMed: 8613054]
- Lin HC, Zhao XT, Wang L. Intestinal transit is more potently inhibited by fat in the distal (ileal brake) than in the proximal (jejunal brake) gut. Dig Dis Sci. 1997;42:19–25. [PubMed: 9009111]
- Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: Implications for the pathophysiology of obesity. Am J Clin Nutr. 2007;86:531–541. [PubMed: 17823414]
- Liu M, Shen L, Doi T, Woods SC, Seeley RJ, Tso P. Neuropeptide Y and lipid increase apolipoprotein AIV gene expression in rat hypothalamus. Brain Res. 2003;971:232–238. [PubMed: 12706239]
- Logan CM, McCaffrey TA, Wallace JM, Robson PJ, Welch RW, Dunne A, Livingstone MB. Investigation of the medium-term effects of Olibra trade mark fat emulsion on food intake in non-obese subjects. Eur J Clin Nutr. 2006;60:1081–1091. [PubMed: 16538239]
- Lowe MR, Levine AS. Eating motives and the controversy over dieting: Eating less than needed versus less than wanted. Obes Res. 2005;13:797–806. [PubMed: 15919830]
- Maas MI, Hopman WP, Katan MB, Jansen JB. Release of peptide YY and inhibition of gastric acid secretion by long-chain and medium-chain triglycerides but not by sucrose polyester in men. Eur J Clin Invest. 1998;28:123–130. [PubMed: 9541126]
- Maggio CA, Koopmans HS. Food intake after intragastric meals of short-, medium, or long-chain triglyceride. Physiol Behav. 1982;28:921–926. [PubMed: 7100293]
- Maki KC, Davidson MH, Tsushima R, et al. Consumption of diacylglycerol oil as part of a reduced-energy diet enhances loss of body weight and fat in comparison with consumption of a triacylglycerol control oil. Am J Clin Nutr. 2002;76:1230–1236. [PubMed: 12450887]
- Mattes RD. Oral fat exposure alters postprandial lipid metabolism in humans. Am J Clin Nutr. 1996;63:911–917. [PubMed: 8644686]
- Matzinger D, Degen L, Drewe J, Meuli J, Duebendorfer R, Ruckstuhl N, D’Amato M, Rovati L, Beglinger C. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut. 2000;46:688–693. [PMC free article: PMC1727908] [PubMed: 10764713]
- McCrory MA, Fuss PJ, Saltzman E, Roberts SB. Dietary determinants of energy intake and weight regulation in healthy adults. J Nutr. 2000;130:276S–279S. [PubMed: 10721887]
- McFadden DW, Rudnicki M, Kuvshinoff B, Fischer JE. Postprandial peptide YY release is mediated by cholecystokinin. Surg Gynecol Obstet. 1992;175:145–150. [PubMed: 1636140]
- McLaughlin J, Grazia LM, Jones MN, D’Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116:46–53. [PubMed: 9869601]
- Medina EA, Horn WF, Keim NL, Havel PJ, Benito P, Kelley DS, Nelson GJ, Erickson KL. Conjugated linoleic acid supplementation in humans: Effects on circulating leptin concentrations and appetite. Lipids. 2000;35:783–788. [PubMed: 10941880]
- Mei J, Lindqvist A, Krabisch L, Rehfeld JF, Erlanson-Albertsson C. Appetite suppression through delayed fat digestion. Physiol Behav. 2006;89:563–568. [PubMed: 16952381]
- Meyer JH, Tabrizi Y, DiMaso N, Hlinka M, Raybould HE. Length of intestinal contact on nutrient-driven satiety. Am J Physiol. 1998;275:R1308–R1319. [PubMed: 9756564]
- Minekus M, Jelier M, Xiao JZ, Kondo S, Iwatsuki K, Kokubo S, Bos M, Dunnewind B, Havenaar R. Effect of partially hydrolyzed guar gum (PHGG) on the bioaccessibility of fat and cholesterol. Biosci Biotechnol Biochem. 2005;69:932–938. [PubMed: 15914912]
- Moloney F, Yeow TP, Mullen A, Nolan JJ, Roche HM. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2004;80:887–895. [PubMed: 15447895]
- Mullen BJ, Martin RJ. The effect of dietary fat on diet selection may involve central serotonin. Am J Physiol. 1992;263:R559–R563. [PubMed: 1384352]
- Murase T, Mizuno T, Omachi T, Onizawa K, Komine Y, Kondo H, Hase T, Tokimitsu I. Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice. J Lipid Res. 2001;42:372–378. [PubMed: 11254749]
- Murata M, Ide T, Hara K. Reciprocal responses to dietary diacylglycerol of hepatic enzymes of fatty acid synthesis and oxidation in the rat. Br J Nutr. 1997;77:107–121. [PubMed: 9059234]
- Nagao T, Watanabe H, Goto N, Onizawa K, Taguchi H, Matsuo N, Yasukawa T, Tsushima R, Shimasaki H, Itakura H. Dietary diacylglycerol suppresses accumulation of body fat compared to triacylglycerol in men in a double-blind controlled trial. J Nutr. 2000;130:792–797. [PubMed: 10736331]
- Oda E. n-3 Fatty acids and the endocannabinoid system. Am J Clin Nutr. 2007;85:919. [PubMed: 17344516]
- Pariza MW, Park Y, Cook ME. The biologically active isomers of conjugated linoleic acid. Prog Lipid Res. 2001;40:283–298. [PubMed: 11412893]
- Phinney SD, Tang AB, Thurmond DC, Nakamura MT, Stern JS. Abnormal polyun-saturated lipid metabolism in the obese Zucker rat, with partial metabolic correction by gamma-linolenic acid administration. Metabolism. 1993;42:1127–1140. [PubMed: 8412765]
- Poirier H, Shapiro JS, Kim RJ, Lazar MA. Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes. 2006;55:1634–1641. [PubMed: 16731825]
- Prentice AM, Poppitt SD. Importance of energy density and macronutrients in the regulation of energy intake. Int J Obes Relat Metab Disord. 1996;20(Suppl 2):S18–23. [PubMed: 8646267]
- Read NW, McFarlane A, Kinsman RI, et al. Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology. 1984;86:274–280. [PubMed: 6690354]
- Riserus U, Berglund L, Vessby B. Conjugated linoleic acid (CLA) reduced abdominal adipose tissue in obese middle-aged men with signs of the metabolic syndrome: A randomised controlled trial. Int J Obes Relat Metab Disord. 2001;25:1129–1135. [PubMed: 11477497]
- Riserus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabet Care. 2002;25:1516–1521. [PubMed: 12196420]
- Riserus U, Smedman A, Basu S, Vessby B. Metabolic effects of conjugated linoleic acid in humans: The Swedish experience. Am J Clin Nutr. 2004;79:1146S–1148S. [PubMed: 15159248]
- Rolls BJ, Gnizak N, Summerfelt A, Laster LJ. Food intake in dieters and nondieters after a liquid meal containing medium-chain triglycerides. Am J Clin Nutr. 1988;48:66–71. [PubMed: 3389331]
- Sakano M, Miyanaga F, Kawahara S, et al. Dietary conjugated linoleic acid reciprocally modifies ketogenesis and lipid secretion by the rat liver. Lipids. 1999;34:997–1000. [PubMed: 10574665]
- Samra RA, Anderson GH. Insoluble cereal fiber reduces appetite and short-term food intake and glycemic response to food consumed 75 min later by healthy men. Am J Clin Nutr. 2007;86:972–979. [PubMed: 17921373]
- Schirmer MA, Phinney SD. Gamma-linolenate reduces weight regain in formerly obese humans. J Nutr. 2007;137:1430–1435. [PubMed: 17513402]
- Schwartz GJ, Whitney A, Skoglund C, Castonguay TW, Moran TH. Decreased responsiveness to dietary fat in Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors. Am J Physiol. 1999;277:R1144–R1151. [PubMed: 10516256]
- Seidell JC. Dietary fat and obesity: An epidemiologic perspective. Am J Clin Nutr. 1998;67:546S–550S. [PubMed: 9497168]
- Small DM. The effects of glyceride structure on absorption and metabolism. Annu Rev Nutr. 1991;11:413–434. [PubMed: 1892708]
- Smeets AJ, Westerterp-Plantenga MS. Satiety and substrate mobilization after oral fat stimulation. Br J Nutr. 2006;95:795–801. [PubMed: 16571160]
- Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake—Inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. [PMC free article: PMC1432347] [PubMed: 6706215]
- St-Onge MP, Bosarge A. Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am J Clin Nutr. 2008;87:621–626. [PMC free article: PMC2874190] [PubMed: 18326600]
- St-Onge MP, Ross R, Parsons WD, Jones PJ. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res. 2003;11:395–402. [PubMed: 12634436]
- Stubbs RJ, Harbron CG. Covert manipulation of the ratio of medium- to long-chain triglycerides in isoenergetically dense diets: Effect on food intake in ad libitum feeding men. Int J Obes Relat Metab Disord. 1996;20:435–444. [PubMed: 8696422]
- Stubbs RJ, Ritz P, Coward WA, Prentice AM. Covert manipulation of the ratio of dietary fat to carbohydrate and energy density: Effect on food intake and energy balance in free-living men eating ad libitum. Am J Clin Nutr. 1995;62:330–337. [PubMed: 7625339]
- Taguchi H, Nagao T, Watanabe H, Onizawa K, Matsuo N, Tokimitsu I, Itakura H. Energy value and digestibility of dietary oil containing mainly 1,3-diacylglycerol are similar to those of triacylglycerol. Lipids. 2001;36:379–382. [PubMed: 11383689]
- Taylor IL. Distribution and release of peptide YY in dog measured by specific radioimmunoassay. Gastroenterology. 1985;88:731–737. [PubMed: 3838162]
- Teff KL, Engelman K. Oral sensory stimulation improves glucose tolerance in humans: Effects on insulin, C-peptide, and glucagon. Am J Physiol. 1996;270:R1371–R1379. [PubMed: 8764306]
- Thurmond DC, Tang AB, Nakamura MT, Stern JS, Phinney SD. Time-dependent effects of progressive gamma-linolenate feeding on hyperphagia, weight gain, and erythrocyte fatty acid composition during growth of Zucker obese rats. Obes Res. 1993;1:118–125. [PubMed: 16353349]
- Van Citters GW, Lin HC. The ileal brake: A fifteen-year progress report. Curr Gastroenterol Rep. 1999;1:404–409. [PubMed: 10980979]
- Van Wymelbeke VV, Himaya A, Louis-Sylvestre J, Fantino M. Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr. 1998;68:226–234. [PubMed: 9701177]
- Van Wymelbeke VV, Louis-Sylvestre J, Fantino M. Substrate oxidation and control of food intake in men after a fat-substitute meal compared with meals supplemented with an isoenergetic load of carbohydrate, long-chain triacylglycerols, or medium-chain triacylglycerols. Am J Clin Nutr. 2001;74:620–630. [PubMed: 11684530]
- Vogler O, Lopez-Bellan A, Alemany R, Tofe S, Gonzalez M, Quevedo J, Pereg V, Barcelo F, Escriba PV. Structure-effect relation of C18 long-chain fatty acids in the reduction of body weight in rats. Int J Obes (Lond). 2008;32:464–473. [PubMed: 18059405]
- Watanabe S, Doshi M, Hamazaki T. n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostag Leukot Essent Fatty Acids. 2003;69:51–59. [PubMed: 12878451]
- Welch I, Saunders K, Read NW. Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroenterology. 1985;89:1293–1297. [PubMed: 4054521]
- Welch IM, Sepple CP, Read NW. Comparisons of the effects on satiety and eating behaviour of infusion of lipid into the different regions of the small intestine. Gut. 1988;29:306–311. [PMC free article: PMC1433617] [PubMed: 3356362]
- Willett WC. Is dietary fat a major determinant of body fat? Am J Clin Nutr. 1998;67:556S–562S. [PubMed: 9497170]
- Williams JR, Browning ET, Scholz R, Kreisber RA, Fritz IB. Inhibition of fatty acid stimulation of gluconeogenesis by (+)-decanoylcarnitine in perfused rat liver. Diabetes. 1968;17:194–208. [PubMed: 4296238]
- Wisen O, Bjorvell H, Cantor O, Johansson C, Theodorsson E. Plasma concentrations of regulatory peptides in obesity following modified sham feeding (MSF) and a liquid meal test. Regul Pept. 1992;39:43–54. [PubMed: 1349761]
- Wojdemann M, Olsen O, Norregaard P, Sternby B, Rehfeld JF. Gastric lipase secretion after sham feeding and cholinergic blockade. Dig Dis Sci. 1997;42:1070–1075. [PubMed: 9149064]
- Woltman T, Reidelberger R. Effects of duodenal and distal ileal infusions of glucose and oleic acid on meal patterns in rats. Am J Physiol. 1995;269:R7–R14. [PubMed: 7631905]
Publication Details
Author Information and Affiliations
Authors
Rania Abou Samra.Copyright
Publisher
CRC Press/Taylor & Francis, Boca Raton (FL)
NLM Citation
Samra RA. Fats and Satiety. In: Montmayeur JP, le Coutre J, editors. Fat Detection: Taste, Texture, and Post Ingestive Effects. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15.

