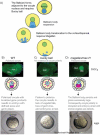
The divisions of the germline stem cell are asymmetric in that they generate a renewing cell and a cell with the capacity to differentiate as an oocyte. Thereafter, the asymmetric meiotic divisions of oocytes produce one large cell, the egg, and two small polar bodies. In addition to asymmetric divisions, asymmetries are present in the form of localized cellular structures, organelles (mitochondria, endoplasmic reticulum, the oocyte nucleus), and also in the distribution of proteins and RNAs within the oocyte. All or a subset of these asymmetries are present in nearly all animals (Figure 3). For example, in most animals, the nucleus is in a central position within the early oocyte, but as oogenesis progresses, the oocyte nucleus moves to the oocyte periphery, or cortex (Figure 3). The side of the cell where the oocyte nucleus is localized in late-stage vertebrate oocytes is generally defined as the animal pole (Figure 3). Earlier asymmetries in oocytes are also shared among animals.
The Balbiani body was first noted more than 100 years ago and since has been identified in primary oocytes of all animals that have been examined including mammals although the constituents vary among species (Figure 4). The Balbiani body is a transient collection of organelles, inclusions, and molecules that assembles adjacent to the nucleus of primary oocytes (Figures 3 and 4) (Guraya, 1979). In frog, fly, fish, mouse, and other mammals, including humans, the Balbiani body is asymmetrically positioned and is composed of endoplasmic reticulum, mitochondria, Golgi, and proteins (Pepling et al., 2007; Billett and Adam, 1976; Bukovsky et al., 2004; Kloc et al., 2008; de Smedt et al., 2000; Kloc et al., 2004). In addition to these organelles, RNAs, in particular, those encoding germ plasm and patterning molecules are sequestered in the Balbiani body of zebrafish and frogs (Kloc et al., 2004a; Wilk et al., 2005). In zebrafish and in frogs, the Balbiani body functions as an integral component of a vegetal transport pathway thought to entrap mRNAs and other gene products necessary for germ cell formation and patterning of the embryo. First, these molecules colocalize with the endoplasmic reticulum, and later, they are positioned at the vegetal oocyte cortex. Thus, the Balbiani body functions to segregate these germ plasm and vegetal patterning molecules within the oocyte (reviewed by Kloc et al., 2004a).
Balbiani bodies share many features with P-bodies and stress bodies of other cell types. P bodies and stress bodies are distinct cellular granules containing mRNAs and enzymes that mediate mRNA turnover or storage. Hence, it is reasonable to speculate that the role of the Balbiani body might be to protect messages from being degraded and/or to prevent expression or activation of patterning molecules before they are needed. This also provides a mechanism to spatially restrict their activity. Consistent with these models, mediators of translational activation or repression are among the molecules sequestered in the Balbiani body of early oocytes (Collier et al., 2005; Marnef et al., 2009). Notably, the maternal mRNAs required at later stages to establish the germline of the embryo are localized to the Balbiani body. In some cases, however, the corresponding proteins are not localized to the Balbiani body: for example, Vasa protein is present in the cytoplasm rather than localized in the Balbiani body in zebrafish (Knaut et al., 2000). Thus, at least in the case of vasa, the mRNA present in the Balbiani body seems to be translationally repressed or the protein is actively excluded or not retained. In fetal ovaries of humans and mice, where Balbiani bodies are also present, Dazl protein and Vasa protein are detected throughout the cytoplasm of primary oocytes and are not limited to the Balbiani body (Anderson et al., 2007).
Recently, the highly conserved Rap55 protein, also known as Trailer hitch in Drosophila, was reported to localize to the Balbiani body of primary oocytes in the neonatal mouse ovary (Pepling et al., 2007). In Drosophila ovaries, Trailer hitch localizes to the fusome-associated endoplasmic reticulum and to other organelle clusters in the oocyte, including the anterior-positioned Balbiani body of newly formed follicles, where Trailer hitch mediates mRNA trafficking (Wilhelm et al., 2005; Pepling et al., 2007; Roper, 2007). The conserved localization of Trailer hitch supports an ancient function for the Balbiani body in the transport of organelles and possibly RNAs, although Balbiani body-localized RNAs have not been reported in mammals. Although the Balbiani body in the mouse has been proposed to function to select the healthiest mitochondria in oocytes, its developmental role remains to be demonstrated. In humans, like mouse, the Balbiani body is present as a perinuclear aggregate that includes mitochondria and other organelles and is transiently present in a spherical conformation (Figure 4) (Gondos, 1987). The developmental requirement for the Balbiani body in humans and whether it is also an indicator of the prospective animal–vegetal axis of the late-stage oocyte and egg remains to be determined.
Although the germ cell-specific mRNAs, such as, vasa, nanos, gasz, and dazl, are conserved and localize to the Balbiani body of fish and frog oocytes, Balbiani body or polarized localization of these mRNAs in the early oocytes of mammals has not been observed so far (Houston et al., 1998; Maegawa et al., 1999; Nishi et al., 1999; Houston and King, 2000; McNeilly et al., 2000; Mita and Yamashita, 2000; Yan et al., 2002; Yan et al., 2004; Braat et al., 1999; Knaut et al., 2000; Kloc et al., 2001; Kloc et al., 2002; Kosaka et al., 2007). One explanation for the absence of localized germ plasm mRNAs in mammalian oocytes can be attributed to the different modes or developmental timing of primordial germ cell specification in mammals compared with fish and frogs (reviewed by Wylie, 2000; McLaren, 2003). Specifically, in fish and frogs, the primordial germ cells are specified from maternally inherited germ plasm, whereas in the mouse, the primordial germ cells are induced during gastrulation. In addition to the germ plasm mRNAs, several molecules necessary for the development of the embryonic axes of zebrafish and Xenopus are localized asymmetrically along the animal–vegetal axis in oocytes. These molecules will be discussed later in the discussion of dorsal–ventral axis formation.
Molecular Control of Balbiani Body Development
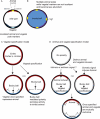
Despite awareness of the Balbiani body for more than 100 years and its conserved presence in the primary oocytes of all animals examined, how Balbiani body formation is regulated at the cellular and molecular level and its essential developmental function are not understood. The conserved germline cyst architecture discussed earlier, namely, the intercellular bridges and fusome have been postulated to function as or to position a polarity cue or Balbiani body precursor materials (Figure 5) (Kloc et al., 2004; Kloc et al., 2004a; Kloc et al., 2008). However, so far, mutants are not available to functionally test whether oocyte polarity or Balbiani body “specification” is inherited from asymmetries or cues positioned within the vertebrate germline cyst (Figure 5). From studies conducted in invertebrate and vertebrate genetic systems combined, only three genes regulating Balbiani body development have been described to date and are described below.
Milton: Kinesin Adaptors and Mitochondrial Allocation
Milton, a kinesin-associated adaptor protein, was identified in Drosophila for its function in allocation of mitochondria from the nurse cells to the Balbiani body of the oocyte (Figure 5) (Cox and Spradling, 2006). The milton alleles described in Drosophila do not impede RNA localization or disrupt axis specification (Cox and Spradling, 2003; Cox and Spradling, 2006). This finding indicates that Milton has a dedicated function in regulating mitochondria allotment to the Balbiani body, whereas transient recruitment of RNAs, such as oskar, to the Balbiani body is regulated independent of Milton and, apparently, also independent of mitochondria acquisition. Only a subset of the mitochondria present in vertebrate oocytes localizes within the Balbiani body, and nurse cells have not been identified in vertebrates. (Marinos and Billett, 1981; Pepling et al., 2007; Kloc et al., 2004a; Kosaka et al., 2007; Marlow and Mullins, 2008; Kloc et al., 2008; Zhang et al., 2008). Thus, mitochondrial acquisition must occur via a mechanism that does not involve allocation from the nurse cells in vertebrates.
In one study, mitochondria of Xenopus oocytes were labeled using a combination of activity-dependent and -independent mitochondrial markers or with dual emission mitochondrial probes that mark mitochondria and also provide an indication of membrane potential (mitochondrial activity) (Wilding et al., 2001). (Wilding et al. (2001) observed that the most highly active mitochondria were adjacent to the nucleus or within the Balbiani body. These findings suggest one model whereby the most active mitochondria could be selectively localized to the Balbiani body. It is possible that Milton acts in an analogous manner in vertebrates to allocate, or rather to select specific (e.g., the most active) mitochondria for recruitment to the Balbiani body and to regulate the number of mitochondria associated with the Balbiani body. Whether Milton or another kinesin-associated adaptor protein plays a conserved role in regulating mitochondria distribution in vertebrate oocytes remains to be determined.
Bucky Ball: A Novel Regulator of Oocyte Asymmetry
Bucky ball was identified based on its maternal-effect egg polarity phenotype in zebrafish (Dosch et al., 2004). In the primary oocytes of zebrafish, bucky ball mutants mitochondria are not localized in an aggregate and no Balbiani body forms (Figure 6) (Marlow and Mullins, 2008; Bontems et al., 2009). In fact, Bucky ball protein is required to establish this earliest known oocyte asymmetry and for all examined asymmetries thereafter, including specification of the first embryonic axis to form in vertebrates, the animal–vegetal axis (Marlow and Mullins, 2008; Bontems et al., 2009).
The oocytes of frogs and fish are highly polarized as can be seen in the localization of mRNAs and proteins along the oocyte axis. The Balbiani body is the earliest known indicator of the asymmetry, and the mRNAs that localize to this structure are the first known to localize. Thus, the Balbiani body-mediated pathway is referred to as the early vegetal localization pathway (reviewed by Kloc and Etkin, 2005; King et al., 2005; Minakhina and Steward, 2005). Many of the mRNAs that localize to the Balbiani body are germ plasm components; however, a few mRNAs encoding regulators of embryonic patterning (e.g., Wnt11 in frogs, Syntabulin in zebrafish) also utilize the early Balbiani body-mediated vegetal pathway. The majority of localized mRNAs encoding molecules involved in specification or patterning of the later developing dorsal–ventral axis localize via later localization pathways. The late pathway for localization to the vegetal pole operates at stages after the Balbiani body is no longer present, but it relies on an intact cytoskeletal network thought to be organized by the earlier Balbiani body vegetal pathway (e.g., by molecules localized during or after the Balbiani body-mediated phase; reviewed by Kloc and Etkin, 2005; King et al., 2005; Minakhina and Steward, 2005). Other RNAs localize to the opposite side of the cell, at the animal pole, and only become asymmetrically distributed at later stages of oocyte development when the Balbiani body is no longer present.
Notably, in bucky ball mutants, even the late localizing vegetal pole RNAs, which do not transit through the Balbiani body, require bucky ball function. It remains to be determined whether this is due to the absence of the Balbiani body or represents an additional function of Bucky ball. Conversely, animal pole-localized mRNAs are no longer limited to the animal pole; instead, these RNAs are found around the circumference of the oocyte cortex (Marlow and Mullins, 2008; Bontems et al., 2009) (Figure 7). The expanded animal character in bucky ball mutants supports a model, whereby establishing vegetal pole identity breaks symmetry and regulates or alternatively indirectly influences the extent of the animal territory; however, the mechanism linking patterning of the two poles is not known. It is possible that the animal and vegetal poles are defined by a mechanism involving mutual exclusion of protein complexes at the cell cortex (e.g., similar to the mutual exclusion of Par protein complexes that polarize diverse cell types and pattern the anterior–posterior axes in C. elegans and Drosophila (Figure 7C); reviewed by Wodarz, 2002; Nance, 2005; Goldstein and Macara, 2007). Alternatively, independent signals (from within the oocyte or from somatic cells) are required to specify the vegetal and animal regions and activate mutually antagonistic programs to pattern the animal–vegetal axis (Figure 7D). So far, a “vegetalized” (i.e., expanded vegetal at the expense of animal pole) maternal-effect mutant to support active induction of animal identity has not been discovered. Thus, it is possible that the animal pole is the default state in the absence of vegetal pole specification, which, once induced, would act to inhibit or override the default animal program. Even if the animal is at a default state, evidence for active patterning of the animal pole (probably mediated in part by signals from the surrounding somatic cells) can be seen in the localization of markers such as Vg1 in bucky ball mutants (Figure 7B). Vg1 is normally only present in the medial animal region, but is found in multiple discrete domains at the oocyte cortex in bucky ball mutants, which is consistent with the presence of multiple animal poles rather than a simple expansion of animal character into vegetal regions (Figure 7B) (Marlow and Mullins, 2008).
Bucky ball is required both to assemble the Balbiani body, a marker of vegetal in zebrafish, and to regulate development of the animal–vegetal axis. A key question that remains to be addressed is whether the animal–vegetal polarity and Balbiani body defects are interdependent (i.e., defective polarity causes the Balbiani body assembly defect or vice versa). Alternatively, it is possible that Balbiani body assembly and axis formation are separable processes that independently require Bucky ball function. To distinguish whether defective oocyte polarity in late–stage oocytes reflects a constant requirement for Buc protein to maintain polarity or if these defects are a secondary consequence of the earlier polarity defect will require conditional rescue of bucky ball mutant phenotypes or conditional inactivation of the Buc protein. Genetic mutants, like the Drosophila milton mutants, that uncouple aspects of Balbiani body development from development of the first axis (the animal–vegetal axis in vertebrates) have yet to be discovered in vertebrates.
The molecular identity of the bucky ball gene does not provide insight into the mechanism by which Buc mediates oocyte polarity and Balbiani body formation. The buc gene encodes a novel protein of unknown function first identified in Xenopus as a vegetally localized transcript, Xvelo (Bontems et al., 2009; Claussen and Pieler, 2004). Mammals also have bucky ball-like genes; however, the human buc-like genes lack predicted open reading frames (Bontems et al., 2009). Whether the human buc-like genes have retained function perhaps as noncoding mRNAs and can compensate for any aspect of bucky ball function in zebrafish mutants remains to be investigated. The absence of identifiable functional domains in the Bucky ball protein makes it difficult to assign Bucky ball to a known signaling pathway. Currently, no other gene required to promote Balbiani body formation is known in vertebrates or invertebrate model systems. Thus, biochemical studies, additional genetic screens, and analysis of conditional knockouts are needed to identify the molecular regulators of Balbiani body formation and oocyte polarity, including novel components of the Bucky ball pathway, to fully understand how polarity is established and maintained in vertebrate oocytes.
MACF1/Magellan: A Role for Microtubule: Actin Cross-linkers in Balbiani Body Dynamics
Like bucky ball, magellan was identified through zebrafish maternal-effect screens based on the animal–vegetal polarity phenotypes of eggs (Dosch et al., 2004). In contrast to bucky ball mutant oocytes, which fail to form Balbiani bodies, primary oocytes of magellan mutants are able to recruit germ plasm mRNAs and mitochondria to establish a Balbiani body that initially resembles the Balbiani body of wild-type oocytes (Gupta et al., 2010). Similar to wild type, the Balbiani body of magellan mutant oocytes undergoes expansion, but then it grows abnormally large and persists in later-stage oocytes because of the failure of the Balbiani body to transit to the oocyte cortex (Figure 6) (Gupta et al., 2010). Consequently, germ plasm mRNAs are not delivered to the cortex and are effectively trapped in the persisting Balbiani body. In wild-type oocytes, acetylated (i.e., stable) microtubules are observed in the cytoplasm between the nucleus and the cortex around the circumference of primary oocytes; acetylated microtubules do not reach the cortex in magellan mutant oocytes, but instead surround the nucleus (Gupta et al., 2010). Thus, anchoring microtubules to the cortex may be a key step in Balbiani body-mediated cargo delivery and turnover. Magellan also maintains a centrally positioned nucleus (Gupta et al., 2010). The extent to which the large Balbiani body and displacement of the nucleus phenotypes are linked is not known. The excess growth of the Balbiani body of magellan mutants could preclude the nucleus from occupying a central position, or these defects may represent distinct functions of Magellan. Defective nuclear position phenotypes are not observed in bucky ball mutants.
Both Bucky ball and Magellan regulate animal–vegetal polarity and are essential for delivery of cargo to the vegetal cortex (Figure 6). Bucky ball promotes Balbiani body assembly, whereas Magellan is required for Balbiani body translocation and dispersal. The opposite phenotypes of zebrafish bucky ball and magellan mutants with regard to Balbiani body development raise the interesting possibility that the two genes function in the same pathway. Examination of whether bucky ball or magellan gene product abundance or localization is dependent on one another, and epistasis studies to determine the compound mutant phenotype will reveal whether these genes regulate Balbiani body development as components of a single pathway or independent pathways converging on Balbiani body dynamics.
The magellan mutant disrupts microtubule actin crosslinking factor 1, a cytoskeletal cross-linking protein previously identified as a fusome component known as short stop (shot) in Drosophila (Roper and Brown, 2004). Together, the molecular identity of the magellan gene, the defective attachment of microtubules to the cortex in magellan mutant oocytes, and prior known functions of Macf-1 as an organizer of the microtubule cytoskeleton indicate a mechanism whereby Magellan maintains nuclear position and facilitates translocation of the Balbiani body to the vegetal cortex via regulation of the microtubule cytoskeleton (Figure 6). Drosophila, a shot/macf1 mutant, also has oocyte polarity and microtubule defects. Specifically, shot is necessary for polarizing the microtubules within the germline cyst, for oocyte determination, and for asymmetric accumulation of factors in Drosophila oocytes (Roper and Brown, 2004). The earlier polarity phenotypes in Drosophila oocytes compared with zebrafish magellan mutants may reflect the nature of the mutant alleles (e.g., it is possible that one of the multiple splice forms of macf1 persists in zebrafish magellan mutants and carries out the earlier function).
In the mouse, MacF1 is essential for proper patterning of the anterior–posterior axis. Knockout of MacF1 is zygotic lethal; homozygous mutants arrest at gastrulation due to failure to specify axial mesoderm, the absence of which ultimately leads to defective posterior body formation (Chen et al., 2006). Defective posterior body formation in the MacF1 knockout is associated with reduced activity of the Wnt pathway, which is known to promote posterior body formation (discussed in more detail in the chapter on dorsal–ventral axis patterning) (Chen et al., 2006). The studies of Chen and colleagues support a model whereby MacF1 mediates translocation of Axin to the cell membrane where Axin is then degraded. Thus, MacF1 regulates posterior axis formation as a component of a complex that promotes Wnt activity. Whether MacF1 also has an essential maternal function in the mouse has not been examined. Notably, Drosophila, shot/macf1 mutants, do not have phenotypes reminiscent of loss of wnt/wingless mutant phenotypes. The lack of wnt phenotypes in Drosophila, the large size of the macf1 gene, and the existence of multiple splice forms of macf1 present in vertebrates may reflect a novel function for macf1 in vertebrates or represent a less severe phenotype if some function remains intact. However, these possibilities remain to be investigated.
In oocytes of mammals, the distribution of mitochondria and ER is also dynamic during oogenesis, and mitochondria quality correlates with outcome in fertility clinics (reviewed by Jansen, 2000). It is not clear whether there is an analogous link between the position of the Balbiani body in early oocytes of mammals and the position of the animal–vegetal axis of late-stage oocytes and eggs.
Publication Details
Copyright
Publisher
Morgan & Claypool Life Sciences, San Rafael (CA)
NLM Citation
Marlow FL. Maternal Control of Development in Vertebrates: My Mother Made Me Do It! San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Oocyte Polarity and the Embryonic Axes: The Balbiani Body, an Ancient Oocyte Asymmetry.