This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Show detailsContinuing Education Activity
Pulsus paradoxus refers to a systolic pressure drop greater than 10mmHg during inspiration. Pulsus paradoxus results from alterations in the mechanical forces imposed on the chambers of the heart and pulmonary vasculature and is often due to pericardial disease, particularly cardiac tamponade and to a lesser degree constrictive pericarditis. However, it is important to recognize that pulsus paradoxus may be seen in non-pericardial cardiac diseases such as right ventricular myocardial infarction and restrictive cardiomyopathy. Additionally, non-cardiac conditions, including pulmonary diseases like severe chronic obstructive pulmonary disease, asthma, tension pneumothorax, large bilateral pleural effusion, and pulmonary embolism can lead to pulsus paradoxus. Other causes include any condition resulting in cardiac compression, such as iatrogenic compression during surgery, marked obesity, and pectus excavatum. Finally, pulsus paradoxus may also manifest secondary to severe hypovolemic shock. This activity describes the pathophysiology, evaluation, and management of pulsus paradoxus and highlights the role of the interprofessional team in the care of patients with pulsus paradoxus.
Objectives:
- Identify the etiology of pulsus paradoxus.
- Describe the presentation of a patient with pulsus paradoxus.
- Outline the management options for pulsus paradoxus.
- Explain the importance of improving coordination amongst the interprofessional team to enhance care for patients affected by pulsus paradoxus.
Introduction
Pulsus paradoxus refers to an exaggerated fall in a patient’s blood pressure during inspiration by greater than 10 mm Hg. Pulsus paradoxus results from alterations in the mechanical forces imposed on the chambers of the heart and pulmonary vasculature often due to pericardial disease, particularly cardiac tamponade and to a lesser degree constrictive pericarditis. However, it is important to understand that pulsus paradoxus may be seen in non-pericardial cardiac diseases such as right ventricular myocardial infarction and restrictive cardiomyopathy. Additionally, non-cardiac disease states can occasionally lead to pulsus paradoxus including pulmonary disease (severe chronic obstructive pulmonary disease [COPD], asthma, tension pneumothorax, large bilateral pleural effusions, pulmonary embolism), as well as any cause of cardiac compression (iatrogenic during surgery, marked obesity, pectus excavatum). Finally, pulsus paradoxus may also manifest secondary to severe hypovolemic shock.[1][2][3][4]
Etiology
As discussed above, pulsus paradoxus typically manifests in patients with pericardial disease, notably, cardiac tamponade and constrictive pericarditis. Other cardiac causes include right ventricular infarction and restrictive cardiomyopathy. Additionally, non-cardiac disease states can also lead to pulsus paradoxus including pulmonary disease (severe COPD, asthma, tension pneumothorax, large bilateral pleural effusions, pulmonary embolism), as well as any cause of cardiac compression (iatrogenic during surgery, marked obesity, pectus excavatum).[5][6][7]
Epidemiology
The epidemiology of pulsus paradoxus is difficult to define due to the heterogeneity of the diseases that lead to its manifestation. The incidence of cardiac tamponade leading to pulsus paradoxus has been poorly documented. A single-center retrospective study of 136 patients admitted with the diagnosis of cardiac tamponade, the main causes were malignancy (32%), infection (24%), idiopathic (16%), iatrogenic (15%), postmyocardial infarction (7%), uremic (4%), and other causes (2%). However, the number who developed pulsus paradoxus was not noted. Although the statistics regarding pulsus paradoxus in cardiac tamponade are limited, it is believed to occur in a majority of cases. In one prospective study of 15 patients with cardiac tamponade, pulsus paradoxus was present in 10 of the 15 patients (66.6%).
Pathophysiology
To truly understand the pathophysiology of pulsus paradoxus, we must explore the normal respirophasic effects of chest mechanics on blood pressure. In healthy individuals, there are normal phasic changes in cardiac output that occur with respiration. During inspiration, there is a small decrease in systemic arterial pressure of less than 10 mm Hg. As we inhale, intrapleural pressure drops; there is a decrease in intrathoracic pressure that promotes venous inflow into the chest increasing right heart filling. However, this does not equate to an increased filling of the left heart during inspiration. This is because, as one inhales, the lungs expand and pull radial traction on the pulmonary vasculature increasing its capacitance, momentarily sequestering blood in the chest, and dropping blood flow to the left heart, decreasing pre-load and consequently cardiac output. The opposite occurs during expiration, thus systolic pressure normally decreases during inspiration and increases during expiration. So why does the term pulsus paradoxus imply the drop in blood pressure during inspiration is paradoxical? The term pulsus paradoxus was coined by historic German physician Adolph Kussmaul who was referring to the palpated pulse of affected patients being of variable strength despite regular precordial activity.[8][9][10]
The pathophysiology of pulsus paradoxus is complex and varies depending on the etiology; there are several mechanisms involved. In cardiac tamponade resulting in pulsus paradoxus, the physiologic drop in cardiac output is exaggerated for several reasons; however, the most significant is enhanced ventricular chamber interaction, often referred to as ventricular interdependence. The increased pericardial pressure limits the ability of the right ventricular free wall to expand and accommodate the inflow of blood during inspiration. The result is increased bowing of the ventricular septum into the left ventricle as blood fills the right heart, resulting in a lower left ventricular end diastolic volume, a lower stroke volume and resulting lower systolic pressure. In more simple terms, in a non-compliant pericardial space, for the right heart to fill more in inspiration, the left heart must fill less. As pericardial pressure increases, the compliance of the ventricles decreases until, under extreme pressure, the effective compliance of all chambers meets that of the pericardial space. In advanced tamponade, intrapericardial pressure will be the key factor determining diastolic cardiac pressures. This is the reason that a clinician will see an equalization of chamber pressures during diastole in cardiac tamponade.
In constrictive pericarditis, a separate mechanism may be involved. During normal inspiration, both intrapleural and intrapericardial pressure fall in tandem to roughly the same degree, and this pressure drop is transmitted to the cardiac chambers. In constrictive pericarditis, the thickened pericardium will prevent the normal decrease in pressure from reaching the ventricles; thus, the normal drop in filling pressures will be blunted by the thick pericardium. This is important because in the pulmonary veins (which are extrapericardial), the pressure drop will be greater than left ventricular diastolic pressure drop (intrapericardial), and the gradient for left ventricular filling will be decreased, resulting in decreased left ventricular filling. The opposite occurs during expiration.
Another mechanism to consider is the effect of inspiration on left ventricular transmural pressure. The left ventricular transmural pressure is the sum of the intracavitary pressure minus the negative intrathoracic pressure generated during inspiration. As the left ventricle contracts during inspiration, it must do so against negative intrathoracic pressure. This increases left ventricular wall stress and afterload and effectively decreases systolic aortic pressure. This mechanism is exaggerated in severe pulmonary disease that requires significant negative intrathoracic pressures during inspiration such as COPD, asthma, and obstructive sleep apnea.
History and Physical
Pulsus paradoxus is a clinical exam finding that may be seen in a variety of disease processes. It is a key exam finding in pericardial effusion as it may herald cardiac tamponade. In pericardial effusion, the presence of pulsus paradoxus has a greater than 80% sensitivity for tamponade, and strongly increases the likelihood that tamponade is present. However, the absence of pulsus paradoxus does not exclude a diagnosis of cardiac tamponade. If present, pulsus paradoxes may be an indication that hemodynamic collapse is imminent; thus, all patients with suspected tamponade and pulsus paradoxus should be evaluated for urgent or emergent pericardial drainage.
Disease states in which cardiac tamponade may occur WITHOUT pulsus paradoxus include the following:
- Intracardiac shunts or moderate to severe valvular insufficiency
- Co-existing disease that significantly increases left or right ventricular diastolic pressure such severe systemic hypertension, aortic stenosis, or cor pulmonale
- Aortic dissection resulting in pericardial effusion/tamponade
- Cardiac tamponade in hypovolemia
Pulsus paradoxus may be seen in constrictive pericarditis, though with a frequency less than that seen in tamponade. A notable exception to this is constrictive pericarditis with pericardial effusion (effusive-constrictive pericarditis) in which a severe pulsus paradoxus is likely to be seen.
As noted above, there are many disease states aside from pericardial pathology that may lead to pulsus paradoxus. The most common of these is severe asthma or COPD. In these disease states, the negative intrathoracic pressure generated is greatly amplified compared to healthy individuals, sometimes close to 40 mm Hg below atmospheric pressure. These wide pressure swings will increase left ventricular transmural pressure and effectively increase LV afterload. Additionally, the pressure will be transmitted to the intrathoracic aorta and downstream vessels. This may manifest as pulsus paradoxus.
An important thing to keep in mind when considering pulsus paradoxus while discerning between pericardial and non-pericardial disease is the following: pulsus paradoxus in non-pericardial disease will usually manifest with a drop in systolic and diastolic pressures. This is in contrast to pericardial disease in which the drop is mainly in systolic pressure, diastolic pressure is usually minimally affected, and thus pulse pressure will be narrower.
Evaluation
Measuring Pulsus Paradoxus
For patients without indwelling arterial access, pulsus paradoxus is best measured with a manual sphygmomanometer and stethoscope. Automatic blood pressure cuffs cannot accurately measure for pulsus paradoxus. Assessment is made by inflating the cuff until all Korotkoff are absent, then very slowly releasing pressure from the cuff. The first sounds auscultated will be heard only during expiration, and this pressure should be noted. Next, as cuff pressure is dropped further, the pressure should be noted when Korotkoff sounds are heard during both inspiration and expiration. The variation between these 2 systolic pressures is what quantifies pulsus paradoxus. Severe pulsus paradoxus may be able to be appreciated as a weakening or disappearance of the palpated pulse during inspiration. Under certain circumstances, pulsus paradoxus may be able to be seen with respirophasic variations in the patient’s pulse oximetry waveform as well.
An important tip when assessing for pulsus paradoxus is to ensure normal tidal volume breathing in the patient. Do not instruct them to change their breathing pattern as the depth of respiration influences the magnitude of pulsus paradoxus and will be amplified in patients with pulmonary disease.
For patients with indwelling arterial access, measuring pulsus paradoxus is as simple as watching the waveform and noting the difference in systolic pressure during the respiratory cycle. Because the drop in blood pressure is secondary to a drop in left ventricular stroke volume, the change in pressure noted during pulsus paradoxus will primarily reflect a decrease in both systolic and pulse pressure; diastolic pressure is usually minimally affected.
Further evaluation of patients with pulsus paradoxus will depend on the etiology. Importantly, if there is known or suspected pericardial effusion, then a diagnosis of cardiac tamponade should be considered, and an ECG, chest radiography, and trans-thoracic echocardiography should be obtained in the absence of hemodynamic compromise. If hemodynamic instability is present, emergent drainage of pericardial fluid should be considered.
Treatment / Management
Pulsus paradoxus is not a disease state. It is a physiologic manifestation of an underlying disease process and treatment should address the underlying pathology. If cardiac tamponade is suspected and pulsus paradoxus is present, consideration should be made for urgent or emergent pericardiocentesis. An exception to this would be pericardial effusion secondary to aortic dissection or myocardial rupture; these patients require emergency surgical intervention.[11][12]
Differential Diagnosis
- Cardiogenic shock
- Constrictive pericarditis
- Effusive-Constrictive pericarditis
- Pneumothorax
- Pulmonary embolism
Enhancing Healthcare Team Outcomes
Pulsus paradoxus is best managed by an interprofessional team including nurses. Pulsus paradoxus is not a disease state. It is a physiologic manifestation of an underlying disease process and treatment should address the underlying pathology. If cardiac tamponade is suspected and pulsus paradoxus is present, consideration should be made for urgent or emergent pericardiocentesis.
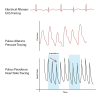
Figure
Diagram of electrical alterans, pulsus alterans, and pulsus paradoxus. Contributed by Rian Kabir, MD
References
- 1.
- Arnold DH, Vukovic AA, Arnold CG, Penrod C, Pologe JA. Prediction of acute asthma exacerbation severity and interrater reliability of manual pulsus paradoxus measurement. Ann Allergy Asthma Immunol. 2019 Jun;122(6):653-654. [PMC free article: PMC6555652] [PubMed: 30928416]
- 2.
- Malahfji M, Arain S. Reversed Pulsus Paradoxus in Right Ventricular Failure. Methodist Debakey Cardiovasc J. 2018 Oct-Dec;14(4):298-300. [PMC free article: PMC6369612] [PubMed: 30788016]
- 3.
- Doukky R, Improvola G, Shih MJ, Costello BT, Munoz-Pena JM, Golzar Y, Margeta B, Bai CJ. Usefulness of Oximetry Paradoxus to Diagnose Cardiac Tamponade. Am J Cardiol. 2019 Feb 01;123(3):498-506. [PubMed: 30477799]
- 4.
- Alerhand S, Carter JM. What echocardiographic findings suggest a pericardial effusion is causing tamponade? Am J Emerg Med. 2019 Feb;37(2):321-326. [PubMed: 30471929]
- 5.
- Pologe JA, Wolley KL, Arnold DH. Respiratory waveform variation can prevent pulsus paradoxus measurement by sphygmomanometry. J Asthma. 2019 Jul;56(7):687-692. [PMC free article: PMC6395523] [PubMed: 29972658]
- 6.
- Uong A, Brandwein A, Crilly C, York T, Avarello J, Gangadharan S. Pleth Variability Index to Assess Course of Illness in Children with Asthma. J Emerg Med. 2018 Aug;55(2):179-184. [PubMed: 30056835]
- 7.
- Al Shakaki M, Dell'Aquila AM, Rukosujew A. Symptoms of massive cardiac tamponade during support of biventricular assist device. Int J Artif Organs. 2018 May;41(5):245-246. [PubMed: 29558840]
- 8.
- Shyy W, Knight RS, Kornblith A, Teismann NA. Point-of-Care Diagnosis of Cardiac Tamponade Identified by the Flow Velocity Paradoxus. J Ultrasound Med. 2017 Nov;36(11):2197-2201. [PubMed: 28503752]
- 9.
- Perel A. The value of dynamic preload variables during spontaneous ventilation. Curr Opin Crit Care. 2017 Aug;23(4):310-317. [PubMed: 28614095]
- 10.
- Hess DR. Pulse Oximetry: Beyond SpO2. Respir Care. 2016 Dec;61(12):1671-1680. [PubMed: 27899542]
- 11.
- Repessé X, Vieillard-Baron A, Geri G. Value of measuring esophageal pressure to evaluate heart-lung interactions-applications for invasive hemodynamic monitoring. Ann Transl Med. 2018 Sep;6(18):351. [PMC free article: PMC6186557] [PubMed: 30370278]
- 12.
- Hamzaoui O, Monnet X, Teboul JL. Pulsus paradoxus. Eur Respir J. 2013 Dec;42(6):1696-705. [PubMed: 23222878]
Disclosure: Matthew Van Dam declares no relevant financial relationships with ineligible companies.
Disclosure: Brian Fitzgerald declares no relevant financial relationships with ineligible companies.
- Review Pulsus paradoxus.[Clin Respir J. 2018]Review Pulsus paradoxus.Sarkar M, Bhardwaj R, Madabhavi I, Gowda S, Dogra K. Clin Respir J. 2018 Aug; 12(8):2321-2331.
- Review Pulsus paradoxus.[Eur Respir J. 2013]Review Pulsus paradoxus.Hamzaoui O, Monnet X, Teboul JL. Eur Respir J. 2013 Dec; 42(6):1696-705. Epub 2012 Dec 6.
- Using a human cardiovascular-respiratory model to characterize cardiac tamponade and pulsus paradoxus.[Theor Biol Med Model. 2009]Using a human cardiovascular-respiratory model to characterize cardiac tamponade and pulsus paradoxus.Ramachandran D, Luo C, Ma TS, Clark JW Jr. Theor Biol Med Model. 2009 Aug 6; 6:15. Epub 2009 Aug 6.
- The paradoxical pulse in tamponade: mechanisms and echocardiographic correlates.[Echocardiography. 1994]The paradoxical pulse in tamponade: mechanisms and echocardiographic correlates.Hoit BD, Shaw D. Echocardiography. 1994 Sep; 11(5):477-87.
- Pulsus paradoxus; historical and clinical perspectives.[Int J Cardiol. 2010]Pulsus paradoxus; historical and clinical perspectives.Abu-Hilal MA, Mookadam F. Int J Cardiol. 2010 Feb 4; 138(3):229-32. Epub 2009 May 22.
- Pulsus Paradoxus - StatPearlsPulsus Paradoxus - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...