NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Diabetes mellitus is a common and debilitating disease that affects a variety of organs including the skin. Between thirty and seventy percent of patients with diabetes mellitus, both type 1 and type 2, will present with a cutaneous complication of diabetes mellitus at some point during their lifetime. A variety of dermatologic manifestations have been linked with diabetes mellitus; these conditions vary in severity and can be benign, deforming, and even life-threatening. Such skin changes can offer insight into patients’ glycemic control and may be the first sign of metabolic derangement in undiagnosed patients with diabetes. Recognition and management of these conditions is important in maximizing the quality of life and in avoiding serious adverse effects in patients with diabetes mellitus. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
The changes associated with diabetes mellitus can affect multiple organ systems. Between thirty and seventy percent of patients with diabetes mellitus, both type 1 and type 2, will present with a cutaneous complication of diabetes mellitus at some point during their lifetime (1). Dermatologic manifestations of diabetes mellitus have various health implications ranging from those that are aesthetically concerning to those that may be life-threatening. Awareness of cutaneous manifestations of diabetes mellitus can provide insight into the present or prior metabolic status of patients. The recognition of such findings may aid in the diagnosis of diabetes, or may be followed as a marker of glycemic control. The text that follows describes the relationship between diabetes mellitus and the skin, more specifically: (1) skin manifestations strongly associated with diabetes, (2) non-specific dermatologic signs and symptoms associated with diabetes, (3) dermatologic diseases associated with diabetes, (4) common skin infections in diabetes, and (5) cutaneous changes associated with diabetes medications.
SKIN MANIFESTATIONS STRONGLY ASSOCIATED WITH DIABETES MELLITUS
Acanthosis Nigricans
EPIDEMIOLOGY
Acanthosis nigricans (AN) is a classic dermatologic manifestation of diabetes mellitus that affects men and women of all ages. AN is more common in type 2 diabetes mellitus (2) and is more prevalent in those with darker-skin color. AN is disproportionately represented in African Americans, Hispanics, and Native Americans (3). AN is observed in a variety of endocrinopathies associated with resistance to insulin such as acromegaly, Cushing syndrome, obesity, polycystic ovarian syndrome, and thyroid dysfunction. Unrelated to insulin resistance, AN can also be associated with malignancies such as gastric adenocarcinomas and other carcinomas (4).
PRESENTATION
AN presents chronically as multiple poorly demarcated plaques with grey to dark-brown hyperpigmentation and a thickened velvety to verrucous texture. Classically, AN has a symmetrical distribution and is located in intertriginous or flexural surfaces such as the back of the neck, axilla, elbows, palmer hands (also known as “tripe palms”), inframammary creases, umbilicus, or groin. Affected areas are asymptomatic; however, extensive involvement may cause discomfort or fetor. Microscopy shows hyperkeratosis and epidermal papillomatosis with acanthosis. The changes in skin pigmentation are primarily a consequence of hyperkeratosis, not changes in melanin. AN can present prior to the clinical diagnosis of diabetes; the presence of AN should prompt evaluation for diabetes mellitus and for other signs of insulin resistance.
PATHOGENESIS
The pathogenesis of AN is not completely understood. The predominant theory is that a hyperinsulin state activates insulin growth factor receptors (IGF), specifically IGF-1, on keratinocytes and fibroblasts, provoking cell proliferation, resulting in the aforementioned cutaneous manifestations of AN (5,6).
TREATMENT
Treatment of AN may improve current lesions and prevent future cutaneous manifestations. AN is best managed with lifestyle changes such as dietary modifications, increased physical activity, and weight reduction. In patients with diabetes, pharmacologic adjuvants, such as metformin, that improve glycemic control and reduce insulin resistance are also beneficial (7). Primary dermatologic therapies are usually ineffective especially in patients with generalized involvement. However, in those with thickened or macerated areas of skin, oral retinoids or topical keratolytics such as ammonium lactate, retinoic acid, or salicylic acid can be used to alleviate symptoms (8-10).
Diabetic Dermopathy
EPIDEMIOLOGY
Dermopathy (DD), also known as pigmented pretibial patches or diabetic shin spots, is the most common dermatologic manifestations of diabetes, presenting in as many as one-half of those with diabetes (11). Although disputed, some consider the presence of DD to be pathognomonic for diabetes. DD has a strong predilection for men and those older than 50 years of age (12). Although DD may antecede the onset of diabetes, it occurs more frequently as a late complication of diabetes and in those with microvascular disease. Nephropathy, neuropathy, and retinopathy are regularly present in patients with DD. An association with cardiovascular disease has also been identified, with one study showing 53% of non-insulin-dependent diabetes mellitus with DD had coexisting coronary artery disease (13).
PRESENTATION
DD initially presents with rounded, dull, red papules that progressively evolve over one-to-two weeks into well-circumscribed, atrophic, brown macules with a fine scale (figure 1). Normally after about eighteen to twenty-four months, lesions dissipate and leave behind an area of concavity and hyperpigmentation. At any time, different lesions can present at different stages of evolution. The lesions are normally distributed bilaterally and localized over bony prominences. The pretibial area is most commonly involved, although other bony prominences such as the forearms, lateral malleoli or thighs may also be involved. Aside from the aforementioned changes, patients are otherwise asymptomatic. DD is a clinical diagnosis that should not require a skin biopsy. Histologically, DD is rather nonspecific; it is characterized by lymphocytic infiltrates surrounding vasculature, engorged blood vessels in the papillary dermis, and dispersed hemosiderin deposits. Moreover, the histology varies based on the stage of the lesion. Immature lesions present with epidermal edema as opposed to epidermal atrophy which is representative of older lesions (14).

Figure 1.
Diabetic Dermopathy
PATHOGENESIS
The origin of DD remains unclear, however, mild trauma to affected areas (15), hemosiderin and melanin deposition (16), microangiopathic changes (17), and destruction of subcutaneous nerves (18) have all been suggested.
TREATMENT
Treatment is typically avoided given the asymptomatic and self-resolving nature of DD as well as the ineffectiveness of available treatments. However, DD often occurs in the context of microvascular complications and neuropathies (12); hence, patients need to be examined and followed more rigorously for these complications. Although it is important to manage diabetes and its complications accordingly, there is no evidence that improved glycemic control alters the development of DD.
Diabetic Foot Syndrome
EPIDEMIOLOGY
Diabetic Foot Syndrome (DFS) encompasses the neuropathic and vasculopathic complications that develop in the feet of patients with diabetes. Although preventable, DFS is a significant cause of morbidity, mortality, hospitalization, and reduction in quality of life of patients with diabetes. The incidence and prevalence of DFS in patients with diabetes is 1% to 4% and 4% to 10%, respectively (19). Furthermore, DFS is slightly more prevalent in type 1 diabetes compared with type 2 diabetes (20). A more comprehensive review of diabetic foot syndrome can be found in The Diabetic Foot chapter of Endotext.
PRESENTATION
DFS presents initially with callosities and dry skin related to diabetic neuropathy. In later stages, chronic ulcers and a variety of other malformations of the feet develop. Between 15% and 25% of patients with diabetes will develop ulcers (21). Ulcers may be neuropathic, ischemic, or mixed. The most common type of ulcers are neuropathic ulcers, a painless ulceration resulting from peripheral neuropathy. Ulcers associated with peripheral vascular ischemia are painful but less common. Ulcers tend to occur in areas prone to trauma, classically presenting at the site of calluses or over bony prominences. It is common for ulcers to occur on the toes, forefoot, and ankles. Untreated ulcers usually heal within one year, however, fifty percent of patients with diabetes will have recurrence of the ulcer within three years (22). The skin of affected patients, especially in those with type 2 diabetes, is more prone to fungal infection and the toe webs are a common port of entry for fungi which can then infect and complicate ulcers (23). Secondary infection of ulcers is a serious complication that can result in gangrenous necrosis, osteomyelitis, and may even require lower extremity amputation. Another complication, diabetic neuro-osteoarthropathy (also known as Charcot foot), is an irreversible debilitating and deforming condition involving progressive destruction of weight-bearing bones and joints. Diabetic neuro-osteoarthropathy occurs most frequently in the feet and can result in collapse of the midfoot, referred to as “rocker-bottom foot.” Moreover, a reduction of the intrinsic muscle volume and thickening of the plantar aponeurosis can cause a muscular imbalance that produces a clawing deformation of the toes. An additional complication of diabetes and neuropathy involving the feet is erythromelalgia. Erythromelalgia presents with redness, warmth, and a burning pain involving the lower extremities, most often the feet. Symptoms may worsen in patients with erythromelalgia with exercise or heat exposure and may improve with cooling (24).
PATHOGENESIS
The pathogenesis of DFS involves a combination of inciting factors that coexist together: neuropathy (25), atherosclerosis (25), and impaired wound healing (26). In the setting of long-standing hyperglycemia, there is an increase in advanced glycosylation end products, proinflammatory factors, and oxidative stress which results in the demyelination of nerves and subsequent neuropathy (27,28). Single-cell RNA sequencing revealed that there is a unique subset of fibroblasts that overexpress factors associated with healing within the wound bed as opposed to the wound edge (28A). Additionally, wound healers demonstrate an increase in M1 macrophages as opposed to non-wound healers which have an increase in M2 macrophages. The effect on sensory and motor nerves, can blunt the perception of adverse stimuli and produce an altered gait, increasing the likelihood of developing foot ulcers and malformations. Also damage to autonomic nerve fibers causes a reduction in sweating which may leave skin in the lower extremity dehydrated and prone to fissures and secondary infection (29). In addition to neuropathy, accelerated arterial atherosclerosis can lead to peripheral ischemia and ulceration (30). It has been reported that diabetic patients with Charcot neuroarthropathy are associated with greater impairment of cutaneous microvascular reactivity when compared to non-complicated diabetic groups (30A). Finally, hyperglycemia impairs macrophage functionality as well as increases and prolongs the inflammatory response, slowing the healing of ulcers (31).
TREATMENT
Treatment should involve an interdisciplinary team-based approach with a focus on prevention and management of current ulcers. Prevention entails daily surveillance, appropriate foot hygiene, and proper footwear, walkers, or other devices to minimize and distribute pressure. An appropriate wound care program should be used to care for ongoing ulcers. Different classes of wound dressing should be considered based on the wound type. Hydrogels, hyperbaric oxygen therapy, topical growth factors, and biofabricated skin grafts are also available (19). The clinical presentation should indicate whether antibiotic therapy or wound debridement is necessary (19). In patients with chronic treatment resistant ulcers, underlying ischemia should be considered; these patients may require surgical revascularization or bypass.
Diabetic Thick Skin
Skin thickening is frequently observed in patients with diabetes. Affected areas of skin can appear thickened, waxy, or edematous. These patients are often asymptomatic but can have a reduction in sensation and pain. Although different parts of the body can be involved, the hands and feet are most frequently involved. Ultrasound evaluation of the skin can be diagnostic and exhibit thickened skin. Subclinical generalized skin thickening is the most common type of skin thickening. Diabetic thick skin may represent another manifestation of scleroderma-like skin changes or limited joint mobility, which are each described in more detail below.
Scleroderma-Like Skin Changes
EPIDEMIOLOGY
Scleroderma-like skin changes are a distinct and easily overlooked group of findings that are commonly observed in patients with diabetes. Ten to fifty percent of patients with diabetes present with the associated skin findings (32). Scleroderma-like skin changes occurs more commonly in those with type 1 diabetes and in those with longstanding disease (33). There is no known variation in prevalence between males and females, or between racial groups.
PRESENTATION
Scleroderma-like skin changes develop slowly and present with painless, indurated, occasionally waxy appearing, thickened skin. These changes occur symmetrically and bilaterally in acral areas. In patients with scleroderma-like skin changes the acral areas are involved, specifically the dorsum of the fingers (sclerodactyly), proximal interphalangeal, and metacarpophalangeal joints. Severe disease may extend centrally from the hands to the arms or back. A small number of patients with diabetes may develop more extensive disease, which presents earlier and with truncal involvement. The risk of developing nephropathy and retinopathy is increased in those with scleroderma-like skin changes who also have type 1 diabetes (33,34). The aforementioned symptoms are also associated with diabetic hand syndrome which may present with limited joint mobility, palmar fibromatosis (Dupuytren's contracture), and stenosing tenosynovitis (“trigger finger”) (35). The physical exam finding known as the “prayer sign” (inability to flushly press palmar surfaces on each hand together) may be present in patients with diabetic hand syndrome and scleroderma-like skin changes (36). On histology, scleroderma-like skin changes reveal thickening of the dermis, minimal-to-absent mucin, and increased interlinking of collagen. Although on physical exam scleroderma may be difficult to distinguish from these skin changes, scleroderma-like skin changes are not associated with atrophy of the dermis, Raynaud’s syndrome, pain, or telangiectasias.
PATHOGENESIS
Although not fully understood, the pathogenesis is believed to involve the strengthening of collagen as a result of reactions associated with advanced glycosylation end products or a buildup of sugar alcohols in the upper dermis (37,38).
TREATMENT
Scleroderma-like skin changes is a chronic condition that is also associated with joint and microvascular complication. Therapeutic options are extremely limited. One observational report has suggested that very tight blood sugar control may result in the narrowing of thickened skin (39). In addition, aldose reductase inhibitors, which limit increases in sugar alcohols, may be efficacious (38). In patients with restricted ranges of motions, physical therapy can help to maintain and improve joint mobility.
Limited Joint Mobility
Limited Joint Mobility (LJM), also known as diabetic cheiroarthropathy, is a relatively common complication of long-standing diabetes mellitus. The majority of patients with LJM also present with scleroderma-like skin changes (38,40). The prevalence of LJM is 4% to 26% in patients without diabetes and 8% to 58% in patients with diabetes (41). LJM presents with progressive flexed contractures and hindered joint extension, most commonly involving the metacarpophalangeal and interphalangeal joints of the hand. The earliest changes often begin in the joints of the fifth finger before then spreading to involve the other joints of the hand (38). Patients may present with an inability to flushly press the palmar surfaces of each of their hands together (“prayer sign”) (figure 2) or against the surface of a table when their forearms are perpendicular to the surface of the table (“tabletop sign”) (42). These changes occur as a result of periarticular enlargement of connective tissue. The pathogenesis likely involves hyperglycemia induced formation of advanced glycation end-products, which accumulate to promote inflammation and the formation of stiffening cross-links between collagen (43). LJM is strongly associated with microvascular and macrovascular changes and diagnosis of LJM should prompt a workup for related sequela (44). Patients with LJM may also be at increased risk for falls (45). There are no curative treatments. Symptomatic patients may benefit from non-steroidal anti-inflammatory drugs or targeted injection of corticosteroids (43). LJM is best managed with improved glycemic control (46), as well as, regular stretching to maintain and minimize further limitations in joint mobility.
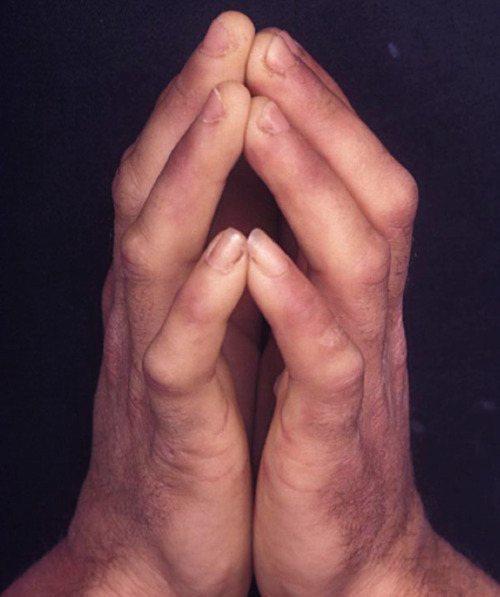
Figure 2.
Limited Joint Mobility
Scleredema Diabetocorum
EPIDEMIOLOGY
Scleredema diabeticorum is a chronic and slowly progressive sclerotic skin disorder that is often seen in the context of diabetes. Whereas 2.5% to 14% of all patients with diabetes have scleredema, over 50% of those with scleredema present with concomitant diabetes (47). Scleredema has a proclivity for those with a long history of diabetes. It remains unclear whether there is a predilection for scleredema in those with type 1 diabetes (48) compared to those with type 2 diabetes (48). Women are affected more often than men (49). Although all ages are affected, scleredema occurs more frequently in those over the age of twenty (48,49).
PRESENTATION
Scleredema presents with gradually worsening indurated and thickened skin. These skin changes occur symmetrically and diffusely. The most commonly involved areas are the upper back, shoulders, and back of the neck. The face, chest, abdomen, buttocks, and thighs may also be involved; however, the distal extremities are classically spared. The affected areas are normally asymptomatic but there can be reduced sensation. Patients with severe longstanding disease may develop a reduced range of motion, most often affecting the trunk. In extreme cases, this can lead to restrictive respiratory problems. A full thickness skin biopsy may be useful in supporting a clinical presentation. The histology of scleredema displays increased collagen and a thickened reticular dermis, with a surrounding mucinous infiltrate, without edema or sclerosis.
PATHOGENESIS
Although many theories center on abnormalities in collagen, there is no a consensus regarding the pathogenesis of scleredema. The pathogenesis of scleredema may involve an interplay between non-enzymatic glycosylation of collagen, increased fibroblast production of collagen, or decreases in collagen breakdown (50,51).
TREATMENT
Scleredema diabeticorum is normally unresolving and slowly progressive over years. Improved glycemic control may be an important means of prevention but evidence has not shown clinical improvements in those already affected by scleredema diabeticorum. A variety of therapeutic options have been proposed with variable efficacy. Some of these therapies include immunosuppressants, corticosteroids, intravenous immunoglobulin, and electron-beam therapy (52). Phototherapy with UVA1 or PUVA may be effective in those that are severely affected (52). Independent of other treatments, physical therapy is an important therapeutic modality for patients with scleredema and reduced mobility (53).
Necrobiosis Lipoidica
EPIDEMIOLOGY
Necrobiosis lipoidica (NL) is a rare chronic granulomatous dermatologic disease that is seen most frequently in patients with diabetes. Although nearly one in four patients presenting with NL will also have diabetes, less than 1% of patients with diabetes will develop NL (54). For unknown reasons, NL expresses a strong predilection for women compared to men (55). NL generally occurs in type 1 diabetes during the third decade of life, as opposed to type 2 diabetes in which it commonly presents in the fourth or fifth decades of life (54). The majority of cases of NL presents years after a diagnosis of diabetes mellitus; however, 14% to 24% of cases of NL may occur prior to or at the time of diagnosis (56). One study evaluating comorbidities and diabetic complications in patients with NL found high rates of smoking, hypertension, hyperlipidemia, obesity, coronary artery disease, myocardial infarction, thyroid disease, poor kidney function, and poor glucose control (56A). The highest comorbidity rates in patients with NL were patients with type 2 diabetes.
PRESENTATION
NL begins as a single or group of firm well-demarcated rounded erythematous papules (figure 3). The papules then expand and aggregate into plaques characterized by circumferential red-brown borders and a firm yellow-brown waxen atrophic center containing telangiectasias. NL occurs bilaterally and exhibits Koebnerization. Lesions are almost always found on the pretibial areas of the lower extremities. Additional involvement of the forearm, scalp, distal upper extremities, face, or abdomen may be present on occasion, and the heel of the foot or glans penis even more infrequently. If left untreated, only about 15% of lesions will resolve within twelve years. Despite the pronounced appearance of the lesions, NL is often asymptomatic. However, there may be pruritus and hypoesthesia of affected areas, and pain may be present in the context of ulceration. Ulceration occurs in about one-third of lesions, and has been associated with secondary infections and squamous cell carcinoma. The histology of NL primarily involves the dermis and is marked by palisading granulomatous inflammation, necrobiotic collagen, a mixed inflammatory infiltrate, blood vessel wall thickening, and reduced mucin.
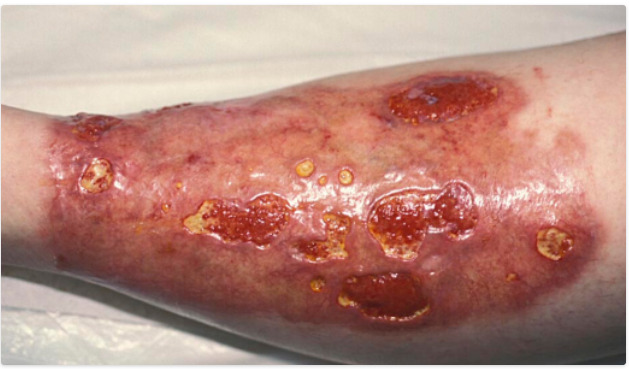
Figure 3.
Necrobiosis Lipoidica
PATHOGENESIS
The pathogenesis of NL is not well understood. The relationship between diabetes and NL has led some to theorize that diabetes-related microangiopathy is related to the development of NL (54). Other theories focus on irregularities in collagen, autoimmune disease, neutrophil chemotaxis, or blood vessels (57).
TREATMENT
NL is a chronic, disfiguring condition that can be debilitating for patients and difficult for clinicians to manage. Differing degrees of success have been reported with a variety of treatments; however, the majority of such reports are limited by inconsistent treatment responses in patients and a lack of large controlled studies. Corticosteroids are often used in the management of NL and may be administered topically, intralesionally, or orally. Corticosteroids can be used to manage active lesions, but is best not used in areas that are atrophic. Success has also been reported with calcineurin inhibitors (e.g., cyclosporine), anti-tumor necrosis factor inhibitors (e.g., infliximab), pentoxifylline, antimalarials (e.g., hydroxychloroquine), PUVA, granulocyte colony stimulating factor, dipyridamole, and low-dose aspirin (54). Appropriate wound care is important for ulcerated lesions; this often includes topical antibiotics, protecting areas vulnerable to injury, emollients, and compression bandaging. Surgical excision of ulcers typically has poor results. Some ulcerated lesion may improve with split-skin grafting. Although still recommended, improved control of diabetes has not been found to lead to an improvement in skin lesions. Patients with newly diagnosed NL should be screened for hypertension, hyperlipidemia, and thyroid disease (56A).
Bullosis Diabeticorum
EPIDEMIOLOGY
Bullosis diabeticorum (BD) is an uncommon eruptive blistering condition that presents in those with diabetes mellitus. Although BD can occasionally present in early-diabetes (58), it often occurs in long-standing diabetes along with other complications such as neuropathy, nephropathy, and retinopathy. In the United States, the prevalence of BD is around 0.5% amongst patients with diabetes and is believed to be higher in those with type 1 diabetes (13). BD is significantly more common in male patients than in female patients (59). The average age of onset is between 50 and 70 years of age (59).
PRESENTATION
BD presents at sites of previously healthy-appearing skin with the abrupt onset of one or more non-erythematous, firm, sterile bullae. Shortly after forming, bullae increase in size and become more flaccid, ranging in size from about 0.5 cm to 5 cm. Bullae frequently present bilaterally involving the acral areas of the lower extremities. However, involvement of the upper extremities and even more rarely the trunk can be seen. The bullae and the adjacent areas are nontender. BD often presents acutely, classically overnight, with no history of trauma to the affected area. Generally, the bullae heal within two to six weeks, but then commonly reoccur. Histological findings are often non-specific but are useful in distinguishing BD from other bullous diseases. Histology, typically shows an intraepidermal or subepidermal blister, spongiosis, no acantholysis, minimal inflammatory infiltrate, and normal immunofluorescence.
PATHOGENESIS
There is an incomplete understanding of the underlying pathogenesis of BD and no consensus regarding a leading theory. Various mechanisms have been proposed, some of which focus on autoimmune processes, exposure to ultraviolet light, variations in blood glucose, neuropathy, or changes in microvasculature (60).
TREATMENT
BD resolve without treatment and are therefore managed by avoiding secondary infection and the corresponding sequelae (e.g., necrosis, osteomyelitis). This involves protection of the affected skin, leaving blisters intact (except for large blisters, which may be aspirated to prevent rupture), and monitoring for infection. Topical antibiotics are not necessary unless specifically indicated, such as with secondary infection or positive culture.
NONSPECIFIC DERMATOLOGIC SIGNS AND SYMPTOMS
Ichthyosiform Changes of the Shins
Ichthyosiform changes of the shins presents with large bilateral areas of dryness and scaling (sometimes described as “fish scale” skin) (figure 4). Although cutaneous changes may occur on the hands or feet, the anterior shin is most classically involved. These cutaneous changes are related to rapid skin aging and adhesion defects in the stratum corneum (61). The prevalence of ichthyosiform changes of the shins in those with type 1 diabetes has been reported to be between 22% to 48% (33,62). These changes present relatively early in the disease course of diabetes. There is no known difference in prevalence between males and females (33). The development of ichthyosiform changes of the shins is related to production of advanced glycosylation end products and microangiopathic changes. Treatment is limited but topical emollients or keratolytic agents may be beneficial (61).
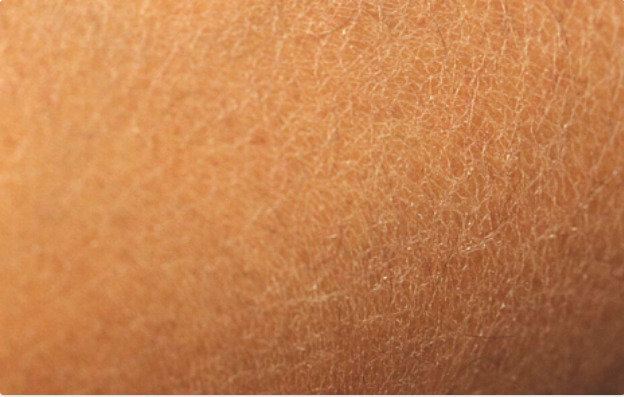
Figure 4.
Acquired ichthyosiform changes
Xerosis
Xerosis is one of the most common skin presentations in patients with diabetes and has been reported to be present in as many as 40% of patients with diabetes (63). Xerosis refers to skin that is abnormally dry. Affected skin may present with scaling, cracks, or a rough texture. These skin changes are most frequently located on the feet of patients with diabetes. It has been reported that diabetic patients that are obese will experience more severe hypohidrosis of the feet (63A). In patients with diabetes, xerosis occurs often in the context of microvascular complications (40). To avoid complications such as fissures and secondary infections, xerosis can be managed with emollients like ammonium lactate (64).
Acquired Perforating Dermatosis
EPIDEMIOLOGY
Perforating dermatoses refers to a broad group of chronic skin disorders characterized by a loss of dermal connective tissue. A subset of perforating dermatoses, known as acquired perforating dermatoses (APD), encompasses those perforating dermatoses that are associated with systemic diseases. Although APD may be seen with any systemic diseases, it is classically observed in patients with chronic renal failure or long-standing diabetes (65). APD occurs most often in adulthood in patients between the ages of 30 and 90 years of age (65,66). The prevalence of APD is unknown. It is estimated that of those diagnosed with APD about 15% also have diabetes mellitus (67). In a review, 4.5% to 10% of patients with chronic renal failure presented with concurrent APD (68,69).
PRESENTATION
APD presents as groups of hyperkeratotic umbilicated-nodules and papules with centralized keratin plugs. The lesions undergo Koebnerization and hence the extensor surfaces of the arms and more commonly the legs are often involved; eruptions also occur frequently on the trunk. However, lesions can develop anywhere on the body. Lesions are extremely pruritic and are aggravated by excoriation. Eruptions may improve after a few months but an area of hyperpigmentation typically remains. Histologically, perforating dermatoses are characterized by a lymphocytic infiltrate, an absence or degeneration of dermal connective tissue components (e.g., collagen, elastic fibers), and transepidermal extrusion of keratotic material.
PATHOGENESIS
The underlying pathogenesis is disputed and not fully understood. It has been suggested that repetitive superficial trauma from chronic scratching may induce epidermal or dermal derangements (70). The glycosylation of microvasculature or dermal components has been suggested as well. Other hypotheses have implicated additional metabolic disturbances, or the accumulation of unknown immunogenic substances that are not eliminated by dialysis (65). APD is also considered a form of prurigo nodularis (70A).
TREATMENT
APD can be challenging to treat and many of the interventions have variable efficacy. Minimizing scratching and other traumas to involved areas can allow lesions to resolve over a period of months. This is best achieved with symptomatic relief of pruritus. Individual lesions can be managed with topical agents such as keratolytics (e.g., 5% to 7% salicylic acid), retinoids (e.g., 0.01% to 0.1% tretinoin), or high-potency steroids (71). Refractory lesions may respond to intralesional steroid injections or cryotherapy (71). A common initial approach is a topical steroid in combination with emollients and an oral antihistamine. Generalized symptoms may improve with systemic therapy with oral retinoids, psoralen plus UVA light (PUVA), allopurinol (100 mg daily for 2 to 4 months), or oral antibiotics (doxycycline or clindamycin) (72). Additionally, as APD is a form of prurigo nodularis, the use of immunomodulating agents such as dupilumab may be effective in treating the condition. There is evidence of dupilumab monotherapy effectively treating certain forms of APD (72A). Nevertheless, effective management of the underlying systemic disease is fundamental to the treatment of APD. In those with diabetes, APD is unlikely to improve without improved blood sugar control. Moreover, dialysis does not reduce symptoms; however, renal transplantation can result in the improvement and resolution of cutaneous lesions.
Eruptive Xanthomas
EPIDEMIOLOGY
Eruptive xanthomas (EX) is a clinical presentation of hypertriglyceridemia, generally associated with serum triglycerides above 2,000 mg/dL (73). However, in patients with diabetes, lower levels of triglycerides may be associated with EX. The prevalence of EX is around one percent in type 1 diabetes and two percent in type 2 diabetes (74,75). Serum lipid abnormalities are present in about seventy-five percent of patients with diabetes (76).
PRESENTATION
EX has been reported as the first presenting sign of diabetes mellitus, granting it can present at any time in the disease course. EX presents as eruptions of clusters of glossy pink-to-yellow papules, ranging in diameter from 1 mm to 4 mm, overlying an erythematous area (figure 5). The lesions can be found on extensor surfaces of the extremities, the buttocks, and in areas susceptible in Koebnerization. EX is usually asymptomatic but may be pruritic or tender. The histology reveals a mixed inflammatory infiltrate of the dermis which includes triglyceride containing macrophages, also referred to as foam cells.

Figure 5.
Eruptive Xanthomas
PATHOGENESIS
Lipoprotein lipase, a key enzyme in the metabolism of triglyceride rich lipoproteins, is stimulated by insulin. In an insulin deficient state, such as poorly controlled diabetes, there is decreased lipoprotein lipase activity resulting in the accumulation of chylomicrons and other triglyceride rich lipoproteins (77). Increased levels of these substances are scavenged by macrophages (78). These lipid-laden macrophages then collect in the dermis of the skin where they can lead to eruptive xanthomas.
TREATMENT
EX can resolve with improved glycemic control and a reduction in serum triglyceride levels (79). This may be achieved with fibrates or omega-3-fatty acids in addition to an appropriate insulin regimen (80). A more comprehensive review of the treatment of hypertriglyceridemia can be found in the Triglyceride Lowering Drugs section of Endotext.
Acrochordons
Acrochordons (also known as soft benign fibromas, fibroepithlial polyps, or skin tags) are benign, soft, pedunculated growths that vary in size and can occur singularly or in groups (figure 6). The neck, axilla, and periorbital area, are most frequently involved, although other intertriginous areas can also be affected. Skin tags are common in the general population, but are more prevalent in those with increased weight or age, and in women. It has been reported that as many as three out of four patients presenting with acrochordons also have diabetes mellitus (81). Patients with acanthosis nigricans may have acrochordons overlying the affected areas of skin. Although disputed, some studies have suggested that the amount of skin tags on an individual may correspond with an individual's risk of diabetes or insulin resistance (82). Excision or cryotherapy is not medically indicated but may be considered in those with symptomatic or cosmetically displeasing lesions.
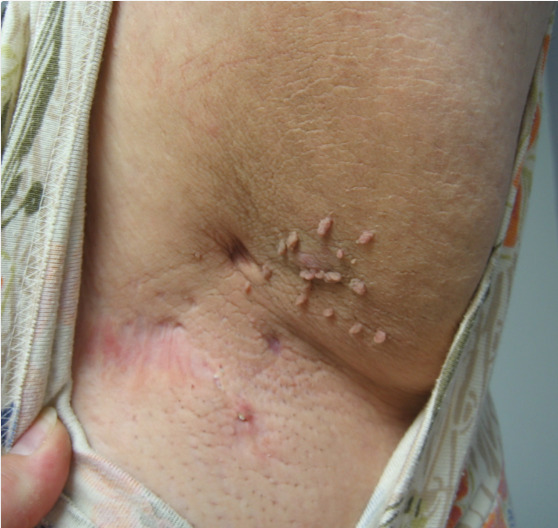
Figure 6.
Acrochordons
Diabetes-Associated Pruritus
Diabetes can be associated with pruritus, more often localized than generalized. Affected areas can include the scalp, ankles, feet, trunk, or genitalia (83,84). Pruritus is more likely in patients with diabetes who have dry skin or diabetic neuropathy. Specifically, for type 2 diabetes, risk factors for pruritus were identified to be age, duration of disease, diabetic peripheral neuropathy, diabetic retinopathy, diabetic chronic kidney disease, and fasting plasma glucose levels (84A). Involvement of the genitalia or intertriginous areas may occur in the setting of infection (e.g., candidiasis). Treatments include topical capsaicin, topical ketamine-amitriptyline-lidocaine, oral anticonvulsants (e.g., gabapentin or pregabalin), and, in the case of candida infection, antifungals.
Huntley’s Papules (Finger Pebbles)
Huntley’s papules, also known as finger pebbles, are a benign cutaneous finding affecting the hands. Patients present with clusters of non-erythematous, asymptomatic, small papules on the dorsal surface of the hand, specifically affecting the metacarpophalangeal joints and periungual areas. The clusters of small papules can develop into coalescent plaques. Other associated cutaneous findings include hypopigmentation and induration of the skin. Huntley’s papules are strongly associated with type 2 diabetes and may be an early sign of diabetic thick skin (85,86). Topical therapies are usually ineffective; however, patients suffering from excessive dryness of the skin may benefit from 12% ammonium lactate cream (87).
Keratosis Pilaris
Keratosis pilaris is a very common benign keratotic disorder. Patients with keratosis pilaris classically present with areas of keratotic perifollicular papules with surrounding erythema or hyperpigmentation (figure 7). The posterior surfaces of the upper arms are often affected but involvement of the thighs, face, and buttocks can also be seen. Compared to the general population, keratosis pilaris occurs more frequently and with more extensive involvement of the skin in those with diabetes (33,62). Keratosis pilaris can be treated with various topical therapies, including salicylic acid, moisturizers, and emollients.
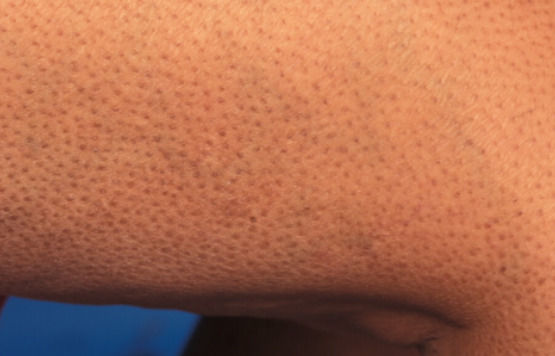
Figure 7.
Keratosis Pilaris
Pigmented Purpuric Dermatoses
Pigmented purpuric dermatoses (also known as pigmented purpura) are associated with diabetes, more often in the elderly, and frequently coexists with diabetic dermopathy (88,89). Pigmented purpura presents with non-blanching copper-colored patches involving the pretibial areas of the legs or the dorsum of the feet. The lesions are usually asymptomatic but may be pruritic. Pigmented purpuric dermatoses occur more often in late-stage diabetes in patients with nephropathy and retinopathy as a result of microangiopathic damage to capillaries and sequential erythrocyte deposition (90).
Palmar Erythema
Palmar erythema is a benign finding that presents with symmetric redness and warmth involving the palms. The erythema is asymptomatic and often most heavily affects the hypothenar and thenar eminences of the palms. The microvascular complications of diabetes are thought to be involved in the pathogenesis of palmar erythema (91). Although diabetes associated palmar erythema is distinct from physiologic mottled skin, it is similar to other types of palmar erythema such as those related to pregnancy and rheumatoid arthritis.
Periungual Telangiectasias
As many as one in every two patients with diabetes are affected by periungual telangiectasias (92). Periungual telangiectasias presents asymptomatically with erythema and telangiectasias surrounding the proximal nail folds (71). Such findings may occur in association with “ragged” cuticles and fingertip tenderness. The cutaneous findings are due to venous capillary dilatation that occurs secondary to diabetic microangiopathy. Capillary abnormalities, such as venous capillary tortuosity, may differ and can represent an early manifestation of diabetes-related microangiopathy (93).
Rubeosis Faciei
Rubeosis faciei is a benign finding present in about 7% of patients with diabetes; however, in hospitalized patients, the prevalence may exceed 50% (94). Rubeosis faciei presents with chronic erythema of the face or neck. Telangiectasias may also be visible. The flushed appearance is often more prominent in those with lighter colored skin. The flushed appearance is thought to occur secondary to small vessel dilation and microangiopathic changes. Complications of diabetes mellitus, such as retinopathy, neuropathy, and nephropathy are also associated with rubeosis faciei (90). Facial erythema may improve with better glycemic control and reduction of caffeine or alcohol intake.
Yellow Skin and Nails
It is common for patients with diabetes, particularly elderly patients with type 2 diabetes, to present with asymptomatic yellow discolorations of their skin or fingernails. These benign changes commonly involve the palms, soles, face, or the distal nail of the first toe. The accumulation of various substances (e.g., carotene, glycosylated proteins) in patients with diabetes may be responsible for the changes in complexion; however, the pathogenesis remains controversial (95).
Onychocryptosis
Onychocryptosis, or ingrown toenails, have been reported in patients with diabetes, specifically type 2 diabetes (95A). The great toes are most affected. It is hypothesized that this nail change can occur in diabetic patients because onychocryptosis is correlated with increased body mass index, trauma, weak vascular supply, nail plate dysfunction, and subungual hyperkeratosis.
DERMATOLOGIC DISEASES ASSOCIATED WITH DIABETES
Generalized Granuloma Annulare
EPIDEMIOLOGY
Although various forms of granuloma annulare exist, only generalized granuloma annulare (GGA) is thought to be associated with diabetes. It is estimated that between ten and fifteen percent of cases of GGA occur in patients with diabetes (96). Meanwhile, less than one percent of patients with diabetes present with GGA. GGA occurs around the average age of 50 years. It occurs more frequently in women than in men, and in those with type 1 diabetes (97).
PRESENTATION
GGA initially presents with groups of skin-colored or reddish, firm papules which slowly grow and centrally involute to then form hypo- or hyper-pigmented annular rings with elevated circumferential borders. The lesions can range in size from 0.5 cm to 5.0 cm. The trunk and extremities are classically involved in a bilateral distribution. GGA is normally asymptomatic but can present with pruritus. The histology shows dermal granulomatous inflammation surrounding foci of necrotic collagen and mucin. Necrobiosis lipoidica can present similarly to GGA; GGA is distinguished from necrobiosis lipoidica by its red color, the absence of an atrophic epidermis, and on histopathology: the presence of mucin and lack of plasma cells.
PATHOGENESIS
The pathogenesis of GGA is incompletely understood. It is believed to involve an unknown stimulus that leads to the activation of lymphocytes through a delayed-type hypersensitivity reaction, ultimately initiating a proinflammatory cascade and granuloma formation (98).
TREATMENT
GGA has a prolonged often unresolving disease course and multiple treatments have been suggested to better manage GGA. However, much of the information stems from small studies and case reports. Antimalarials, retinoids, corticosteroids, dapsone, cyclosporine, PUVA, and calcineurin inhibitors have been suggested as therapies (98).
Psoriasis
Psoriasis is a chronic immune-mediated inflammatory disorder that may present with a variety of symptoms, including erythematous, indurated, and scaly areas of skin. Psoriasis has been found to be associated with a variety of risk factors, such as hypertension and metabolic syndrome, that increase the likelihood of cardiovascular disease. The development of diabetes mellitus, an additional cardiovascular risk factor, has been strongly associated with psoriasis (99). In particular, younger patients and those with severe psoriasis may be more likely to develop diabetes in the future (99)
Lichen Planus
Lichen planus is a mucocutaneous inflammatory disorder characterized by firm, erythematous, polygonal, pruritic, papules. These papules classically involve the wrists or ankles, although the trunk, back, and thighs can also be affected. A number of studies have cited an association between lichen planus and abnormalities in glucose tolerance testing. Approximately one in four patients with lichen planus have diabetes mellitus (100). Although the association is contested, it has been reported that patients with diabetes may also be more likely to develop oral lichen planus (101).
Vitiligo
Vitiligo is an acquired autoimmune disorder involving melanocyte destruction. Patients with vitiligo present with scattered well-demarcated areas of depigmentation that can occur anywhere on the body, but frequently involves the acral surfaces and the face. Whereas about 1% of the general population is affected by vitiligo, vitiligo is much more prevalent in those with diabetes mellitus. Vitiligo occurs more frequently in women and is also more common in type 1 than in type 2 diabetes mellitus (96,98). Coinciding vitiligo and type 1 diabetes mellitus may be associated with endocrine autoimmune abnormalities of the gastric parietal cells, adrenal, or thyroid (102). A more comprehensive review of polyglandular autoimmune disorders can be found in the Autoimmune Polyglandular Syndromes section of Endotext.
Hidradenitis Supparativa
Hidradenitis supparativa (HS) is a chronic inflammatory condition characterized by inflamed nodules and abscesses located in intreginious areas such as the axilla or groin. These lesions are often painful and malodorous. HS is frequently complicated by sinus formation and the development of disfiguring scars. HS occurs more often in women than men and usually presents in patients beginning in their twenties (103). Compared to the general population, diabetes mellitus is three-times more common in patients with HS (104). It is recommended that patients with HS be screened for diabetes mellitus. There is no standardized approach to the treatment of HS, although some benefits have been reported with the use of antibiotics, retinoids, antiandrogens, or immunomodulators such as tumor necrosis factor (TNF) inhibitors (105).
Glucagonoma
Glucagonoma is a rare neuroendocrine tumor that most frequently affects patients in their sixth decade of life (106). Patients with glucagonoma may present with a variety of non-specific symptoms. However, necrolytic migratory erythema (NME) is classically associated with glucagonoma and presents in 70% to 83% of patients (106) (107). NME is characterized by erythematous erosive crusted or vesicular eruptions of papules or plaques with irregular borders. The lesions may become bullous or blistered, and may be painful or pruritic. The abdomen, groin, genitals, or buttocks are frequently involved, although cheilitis or glossitis may also be present. Biopsy at the edge of the lesion may demonstrate epidermal pallor, necrolytic edema, and a perivascular inflammatory infiltrate (108). Patients with glucagonoma may also present with diabetes mellitus. In patients with glucagonoma, diabetes mellitus frequently presents prior to NME (107). Approximately 20% to 40% of patients will present with diabetes mellitus before the diagnosis of glucagonoma (107,109). Of those patients diagnosed with glucagonoma but not diabetes mellitus, 76% to 94% will eventually develop diabetes mellitus (110). A more comprehensive review of glucagonoma can be found in the Glucagonoma section of Endotext.
Skin Infections
The prevalence of cutaneous infections in patients with diabetes is about one in every five patients (111). Compared with the general population, patients with diabetes mellitus are more susceptible to infections and more prone to repeated infections. A variety of factors are believed to be involved in the vulnerability to infection in patients with uncontrolled diabetes, some of these factors include angiopathy, neuropathy, hindrance of the anti-oxidant system, abnormalities in leukocyte adherence, chemotaxis, and phagocytosis, as well as, a glucose-rich environment facilitates the growth of pathogens.
BACTERIAL
Erysipelas and cellulitis are cutaneous infections that occur frequently in patients with diabetes. Erysipelas presents with pain and well-demarcated superficial erythema. Cellulitis is a deeper cutaneous infection that presents with pain and poorly-demarcated erythema. Folliculitis is common among patients with diabetes, and is characterized by inflamed, perifollicular, papules and pustules. Treatment for the aforementioned conditions depends on the severity of the infection. Uncomplicated cellulitis and erysipelas are typically treated empirically with oral antibiotics, whereas uncomplicated folliculitis may be managed with topical antibiotics. Colonization with methicillin-resistant Staphylococcus aureus (MRSA) is not uncommon among patients with diabetes (112); however, it is debated as to whether or not colonized patients are predisposed to increased complications (113) such as bullous erysipelas, carbuncles, or perifollicular abscesses. Regardless, it is important that appropriate precautions are taken in these patients and that antibiotics are selected that account for antimicrobial resistance.
Infection of the foot is the most common type of soft tissue infection in patients with diabetes. If not managed properly, diabetic foot infections can become severe, possibly leading to sepsis, amputation, or even death. Although less severe, the areas between the toes and the toenails are also frequently infected in patients with diabetes. Infections can stem from monomicrobial or polymicrobial etiologies. Staphylococcal infections are the most common (114), although complications with infection by Pseudomonas aeruginosa are also common (115). Pseudomonal infection of the toenail may present with a green discoloration, which may become more pronounced with the use of a Wood’s light. Treatment frequently requires coordination of care from multiple medical providers. Topical or oral antibiotics and surgical debridement may be indicated depending on the severity of the infection.
Necrotizing fasciitis is an acute life-threatening infection of the skin and the underlying tissue. Those with poorly controlled diabetes are at an increased risk for necrotizing fasciitis. Necrotizing fasciitis presents early with erythema, induration, and tenderness which may then progress within days to hemorrhagic bullous. Patients will classically present with severe pain out of proportion to their presentation on physical exam. Palpation of the affected area often illicit crepitus. Involvement can occur on any part of the body but normally occurs in a single area, most commonly affecting the lower extremities. Fournier’s gangrene refers to necrotizing fasciitis of the perineum or genitals, often involving the scrotum and spreading rapidly to adjacent tissues. The infection in patients with diabetes is most often polymicrobial. Complications of necrotizing fasciitis include thrombosis, gangrenous necrosis, sepsis, and organ failure. Necrotizing fasciitis has a mortality rate of around twenty percent (116). In addition, those patients with diabetes and necrotizing fasciitis are more likely to require amputation during their treatment (117). Treatment is emergent and includes extensive surgical debridement and broad-spectrum antibiotics.
Erythrasma is a chronic asymptomatic cutaneous infection, most often attributed to Corynebacterium minutissiumum. Diabetes mellitus, as well as obesity and older age are associated with erythrasma. Erythrasma presents with non-pruritic non-tender clearly demarcated red-brown finely scaled patches or plaques. These lesions are commonly located in intriginuous areas such as the axilla or groin. Given the appearance and location, erythrasma can be easily mistaken for tinea or Candidia infection; in such cases, the presence of coral-red fluorescence under a Wood’s light can confirm the diagnosis of erythrasma. Treatment options include topical erythromycin or clindamycin, Whitfield’s ointment, and sodium fusidate ointment. More generalized erythrasma may respond better to oral erythromycin.
Malignant otitis externa is a rare but serious infection of the external auditory canal that occurs most often in those with a suppressed immune system, diabetes mellitus, or of older age. Malignant otitis externa develops as a complication of otitis externa and is associated with infection by Pseudomonas aeruginosa. Patients with malignant otitis externa present with severe otalgia and purulent otorrhea. The infection can spread to nearby structures and cause complications such as chondritis, osteomyelitis, meningitis, or cerebritis. If untreated, malignant otitis externa has a mortality rate of about 50%; however, with aggressive treatment the mortality rate can been reduced to 10% to 20% (118). Treatment involves long-term systemic antibiotics with appropriate pseudomonal coverage, hyperbaric oxygen, and possibly surgical debridement.
FUNGAL
Candidiasis is a frequent presentation in patients with diabetes. Moreover, asymptomatic patients presenting with recurrent candidiasis should be evaluated for diabetes mellitus. Elevated salivary glucose concentrations (119) and elevated skin surface pH in the intertriginous regions of patients with diabetes (120) may promote an environment in which candida can thrive. Candida infection can involve the mucosa (e.g., thrush, vulvovaginitis), intertriginous areas (e.g., intertrigo, erosion interdigital, balanitis), or nails (e.g., paronychia). Mucosal involvement presents with pruritus, erythema, and white plaques which can be removed when scraped. Intertriginous Candida infections may be pruritic or painful and present with red macerated, fissured plaques with satellite vesciulopustules. Involvement of the nails may present with periungual inflammation or superficial white spots. Onchyomycosis may be due to dermatophytes (discussed below) or Candidal infection. Onchomycosis, characterized by subungual hyperkeratosis and oncholysis, is present in nearly one in two patients with type 2 diabetes mellitus. Candidiasis is treated with topical or oral antifungal agents. Patients also benefit from improved glycemic control and by keeping the affected areas dry.
Although it remains controversial, dermatophyte infections appear to be more prevalent among patients with diabetes (121-123). Various regions of the body may be affected but tinea pedis (foot) is the most common dermatophyte infection effecting patients; it presents with pruritus or pain and erythematous keratotic or bullous lesions. Relatively benign dermatophyte infections like tinea pedis can lead to serious sequela, such as secondary bacterial infection, fungemia, or sepsis, in patients with diabetes if not treated hastily. Patients with diabetic neuropathy may be especially vulnerable (124). Treatment may include topical or systemic antifungal medications depending on the severity.
Mucormycosis is a serious infection that is associated with type 1 diabetes mellitus, particularly common in those who develop diabetic ketoacidosis. A variety of factors including hyperglycemia and a lower pH, create an environment in which Rhizopus oryzae, a common pathogen responsible for mucormycosis, can prosper. Mucormycosis may present in different ways. Rhino-orbital-cerebral mucormycosis is the most common presentation; it develops quickly and presents with acute sinusitis, headache, facial edema, and tissue necrosis. The infection may worsen to cause extensive necrosis and thrombosis of nearby structures such as the eye. Mucormycosis should be treated urgently with surgical debridement and intravenous amphotericin B. When it is not suitable to administer amphotericin B in patients, the alternative use of new triazoles, posaconazole and isavuconazole, may be beneficial treatments (124A).
Lastly, abnormal toe web findings (e.g., maceration, scale, or erythema) may be an early marker of irregularities in glucose metabolism and of undiagnosed diabetes mellitus (125). Additionally, such findings may be a sign of epidermal barrier disruption, a precursor of infection (125).
CUTANEOUS CHANGES ASSOCIATED WITH DIABETES MEDICATIONS
Insulin
A number of localized changes are associated with the subcutaneous injection of insulin. The most common local adverse effect is lipohypertrophy, which affects less than thirty percent of patients with diabetes that use insulin (126,127). Lipohypertrophy is characterized by localized adipocyte hypertrophy and presents with soft dermal nodules at injection sites. Continued injection of insulin at sites of lipohypertrophy can result in delayed systemic insulin absorption and capricious glycemic control. With avoidance of subcutaneous insulin at affected sites, lipohypertrophy normally improves over the course of a few months.
Furthermore, lipoatrophy is an uncommon cutaneous finding which occurred more frequently prior to the introduction of modern purified forms of insulin. Lipoatrophy presents at insulin injection sites over a period of months with round concave areas of adipose tissue atrophy. Allergic reactions to the injection of insulin may be immediate (within one hour) or delayed (within one day) and can present with localized or systemic symptoms. These reactions may be due to a type one hypersensitivity reaction to insulin or certain additives. However, allergic reactions to subcutaneous insulin are rare, with systemic allergic reactions occurring in only 0.01% of patients (126). Other cutaneous changes at areas of injection include the development of pruritus, induration, erythema, nodular amyloidosis, or calcification.
Oral Medications
Oral hypoglycemic agents may cause a number of different cutaneous adverse effects such as erythema multiforme or urticaria. DPP-IV inhibitors, such as vildagliptin, can be associated with inflamed blistering skin lesions, including bullous pemphigoid and Stevens-Johnson syndrome, as well as, angioedema (128,129). Allergic skin and photosensitivity reactions may occur with sulfonylureas (130). The sulfonylureas, chlorpropamide and tolbutamide, are associated with the development of a maculopapular rash during the initial two months of treatment; the rash quickly improves with stoppage of the medication (131,132). In certain patients with genetic predispositions, chlorpropamide may also cause acute facial flushing following alcohol consumption (133). SGLT-2 inhibitors have been associated with an increased risk of genital fungal infections and Fournier’s gangrene (134) (for details see Endotext chapter Oral and Injectable (Non-Insulin) Pharmacological Agents for the Treatment of Type 2 Diabetes) (135).
CONCLUSION
Diabetes mellitus is associated with a broad array of dermatologic conditions (Table 1). Many of the sources describing dermatologic changes associated with diabetes mellitus are antiquated; larger research studies utilizing modern analytic tools are needed to better understand the underlying pathophysiology and treatment efficacy. Although each condition may respond to a variety of specific treatments, many will improve with improved glycemic control. Hence, patient education and lifestyle changes are key in improving the health and quality of life of patients with diabetes mellitus.
Table 1.
Frequent Skin Manifestations of Diabetes Mellitus
| DISEASE | APPEARANCE | COMMON LOCATIONS | SYMPTOMS | TREATMENT |
|---|---|---|---|---|
| Acanthosis Nigricans | Multiple poorly demarcated plaques with grey to dark-brown hyperpigmentation, and a thickened velvety to verrucous texture | Back of the neck, axilla, elbows, palmer hands, inframammary creases, umbilicus, groin | Typically, asymptomatic | Improved glycemic control, oral retinoids, ammonium lactate, retinoic acid, salicylic acid |
| Diabetic Dermopathy | Rounded, dull, red papules that progressively evolve over one-to-two weeks into well-circumscribed, atrophic, brown macules with a fine scale; lesions present in different stages of evolution at the same time | Pretibial area, lateral meoli, thighs | Typically, asymptomatic | Self-resolving |
| Diabetic Foot Syndrome | Chronic ulcers, secondary infection, diabetic neuro-osteoarthropathy, clawing deformity | Feet | Typically, asymptomatic but may have abnormal gait | Interdisciplinary team-based approach involving daily surveillance, appropriate foot hygiene, proper footwear/walker, wound care, antibiotics, wound debridement, surgery |
| Scleroderma-like Skin Changes | Slowly developing painless, indurated, occasionally waxy appearing, thickened skin | Acral areas: dorsum of the fingers, proximal interphalangeal areas, metacarpophalangeal joints | Typically, asymptomatic but may have reduced range of motion | Improved glycemic control, aldose reductase inhibitors, physical therapy |
| Ichthyosiform Skin Changes | Large bilateral areas of dryness and scaling (may be described as “fish scale” skin) | Anterior shins, hands, feet | Typically, asymptomatic | Emollients, Keratolytics |
| Xerosis | Abnormally dry skin that may also present with scaling or fissures | Most common on the feet | Typically, asymptomatic | Emollients |
| Pruritus | Normal or excoriated skin | Often localized to the scalp, ankles, feet, trunk, or genitalia; however, it may be generalized | Pruritus | Topical capsaicin, topical ketamine-amitriptyline-lidocaine, oral anticonvulsants, antifungals |
REFERENCES
- 1.
- Meurer M, Stumvoll M, Szeimies R-M. Hautveränderungen bei Diabetes mellitus. Der Hautarzt. 2004;55(5):428–35.
- 2.
- Stuart CA, Gilkison CR, Smith MM, Bosma AM, Keenan BS, Nagamani M. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clinical Pediatrics. 1998;37(2):73–79. [PubMed: 9492114]
- 3.
- Stuart CA, Driscoll MS, Lundquist KF, Gilkison CR, Shaheb S, Smith MM. Acanthosis nigricans. Journal of basic and clinical physiology and pharmacology. 1998;9(2-4):407–18. [PubMed: 10212845]
- 4.
- Yeh JS, Munn SE, Plunkett TA, Harper PG, Hopster DJ, du Vivier AW. Coexistence of acanthosis nigricans and the sign of Leser-Trelat in a patient with gastric adenocarcinoma: a case report and literature review. Journal of the American Academy of Dermatology. 2000;42(2):357–62. [PubMed: 10640933]
- 5.
- Bhagyanathan M, Dhayanithy D, Parambath VA, Bijayraj R. Acanthosis nigricans: A screening test for insulin resistance–An important risk factor for diabetes mellitus type-2. Journal of family medicine and primary care. 2017;6(1):43. [PMC free article: PMC5629898] [PubMed: 29026747]
- 6.
- Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance. American journal of clinical dermatology. 2004;5(3):199–203. [PubMed: 15186199]
- 7.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatology online journal. 2008;14(9) [PubMed: 19061584]
- 8.
- Blobstein SH. Topical therapy with tretinoin and ammonium lactate for acanthosis nigricans associated with obesity. Cutis. 2003;71(1):33–34. [PubMed: 12553628]
- 9.
- Ehsani A, Noormohammadpour P, Goodarzi A, et al. Comparison of long-pulsed alexandrite laser and topical tretinoin-ammonium lactate in axillary acanthosis nigricans: A case series of patients in a before-after trial. Caspian journal of internal medicine. 2016;7(4):290. [PMC free article: PMC5153522] [PubMed: 27999648]
- 10.
- Sinha S, Schwartz RA. Juvenile acanthosis nigricans. Journal of the American Academy of Dermatology. 2007;57(3):502–08. [PubMed: 17592743]
- 11.
- Bustan RS, Wasim D, Yderstræde KB, Bygum A. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state-a systematic review. Danish medical journal. 2017;64(1) [PubMed: 28007053]
- 12.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: A subtle sign with grave implications. Journal of the American Academy of Dermatology. 2008;58(3):447–51. [PubMed: 18155320]
- 13.
- Romano G, Moretti G, Di Benedetto A, et al. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes research and clinical practice. 1998;39(2):101–06. [PubMed: 9597379]
- 14.
- Huntley AC. The cutaneous manifestations of diabetes mellitus. Journal of the American Academy of Dermatology. 1982;7(4):427–55. [PubMed: 6216269]
- 15.
- Houck GM, Morgan MB. A reappraisal of the histologic findings of pigmented pretibial patches of diabetes mellitus. Journal of cutaneous pathology. 2004;31(2):141–44. [PubMed: 14690458]
- 16.
- McCASH S. Emanuel PO. Defining diabetic dermopathy. The Journal of dermatology. 2011;38(10):988–92. [PubMed: 21762390]
- 17.
- Kaňková K, Záhejský J, Márová I, et al. Polymorphisms in the RAGE gene influence susceptibility to diabetes-associated microvascular dermatoses in NIDDM. Journal of Diabetes and its Complications. 2001;15(4):185–92. [PubMed: 11457670]
- 18.
- Kiziltan M, Benbir G. Clinical and nerve conduction studies in female patients with diabetic dermopathy. Acta diabetologica. 2008;45(2):97–105. [PubMed: 18357406]
- 19.
- Amin N, Doupis J. Diabetic foot disease: from the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World journal of diabetes. 2016;7(7):153. [PMC free article: PMC4824686] [PubMed: 27076876]
- 20.
- Sämann A, Tajiyeva O, Müller N, et al. Prevalence of the diabetic foot syndrome at the primary care level in Germany: a cross‐sectional study. Diabetic Medicine. 2008;25(5):557–63. [PubMed: 18346154]
- 21.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. Jama. 2005;293(2):217–28. [PubMed: 15644549]
- 22.
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. The Lancet. 2005;366(9498):1719–24. [PubMed: 16291066]
- 23.
- Mlinarić-Missoni E, Kalenić S, Važić-Babić V. Species distribution and frequency of isolation of yeasts and dermatophytes from toe webs of diabetic patients. Acta dermatovenerologica Croatica 2005;13(2):0-0.
- 24.
- Davis MD, O'fallon WM, Rogers RS III, Rooke TW. Natural history of erythromelalgia: presentation and outcome in 168 patients. Archives of Dermatology. 2000;136(3):330–36. [PubMed: 10724194]
- 25.
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. The lancet. 2003;361(9368):1545–51. [PubMed: 12737879]
- 26.
- Lobmann R, Schultz G, Lehnert H. Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes care. 2005;28(2):461–71. [PubMed: 15677818]
- 27.
- Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. International journal of molecular sciences. 2016;17(6):917. [PMC free article: PMC4926450] [PubMed: 27294922]
- 28.
- Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. International journal of endocrinology 2014;2014.
- 28A.
- Theocharidis G, Thomas BE, Sarkar D, Mumme HL, Pilcher WJR, Dwivedi B, Sandoval-Schaefer T, Sîrbulescu RF, Kafanas A, Mezghani I, Wang P, Lobao A, Vlachos IS, Dash B, Hsia HC, Horsley V, Bhasin SS, Veves A, Bhasin M. Single cell transcriptomic landscape of diabetic foot ulcers. Nat Commun. 2022 Jan 10;13(1):181. doiPMCID: PMC8748704. [PMC free article: PMC8748704] [PubMed: 35013299] [CrossRef]
- 29.
- Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med 2001;68(11):928-30, 32, 34-44.
- 30.
- Peripheral Arterial Disease in People With Diabetes. Diabetes Care. 2003;26(12):3333–41. [PubMed: 14633825]
- 30A.
- Lanting SM, Chan TL, Casey SL, Peterson BJ, Chuter VH. Cutaneous microvascular reactivity in Charcot neuroarthropathy: a systematic review and meta-analysis. J Foot Ankle Res. 2022 Mar 1;15(1):17. doiPMCID: PMC8886937. [PMC free article: PMC8886937] [PubMed: 35232466] [CrossRef]
- 31.
- Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PloS one. 2010;5(3):e9539. [PMC free article: PMC2832020] [PubMed: 20209061]
- 32.
- Ahn C, Yosipovitch G, Huang W. Diabetes and the Skin. In: Callen J, Jorizzo J, Zone J, Piette W, Rosenbach M, Vleugels RA, eds. Dermatological Signs of Systemic Disease. Edinburgh: Elsevier, 2017:205-14.
- 33.
- Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes care. 1998;21(4):506–09. [PubMed: 9571332]
- 34.
- Brik R, Berant M, Vardi P. The scleroderma‐like syndrome of insulin‐dependent diabetes mellitus. Diabetes/Metabolism Research and Reviews. 1991;7(2):121–28. [PubMed: 1794257]
- 35.
- Yosipovitch G, Yosipovitch Z, Karp M, Mukamel M. Trigger finger in young patients with insulin dependent diabetes. The Journal of rheumatology. 1990;17(7):951–52. [PubMed: 2213763]
- 36.
- Kim RP, Edelman SV, Kim DD. Musculoskeletal complications of diabetes mellitus. Clinical Diabetes. 2001;19(3):132–35.
- 37.
- Haustein UF. Scleroderma‐like lesions in insulin‐dependent diabetes mellitus. Journal of the European Academy of Dermatology and Venereology. 1999;13(1):50–53. [PubMed: 10565631]
- 38.
- Yosipovitch G, Loh KC, Hock OB. Medical pearl: Scleroderma-like skin changes in patients with diabetes mellitus. Journal of the American Academy of Dermatology. 2003;49(1):109–11. [PubMed: 12833019]
- 39.
- Rho YW, Suhr KB, Lee JH, Park JK. A clinical observation of scleredema adultorum and its relationship to diabetes. The Journal of dermatology. 1998;25(2):103–07. [PubMed: 9563277]
- 40.
- Sawatkar G, Kanwar A, Dogra S, Bhadada S, Dayal D. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 south Asian patients. British Journal of Dermatology. 2014;171(6):1402–06. [PubMed: 24773124]
- 41.
- Gerrits EG, Landman GW, Nijenhuis-Rosien L, Bilo HJ. Limited joint mobility syndrome in diabetes mellitus: A minireview. World journal of diabetes. 2015;6(9):1108. [PMC free article: PMC4530324] [PubMed: 26265997]
- 42.
- Fitzgibbons PG, Weiss A-PC. Hand manifestations of diabetes mellitus. The Journal of hand surgery. 2008;33(5):771–75. [PubMed: 18590861]
- 43.
- Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility in diabetes and ageing: recent advances in pathogenesis and therapy: SAGE Publications Sage UK: London, England, 2010.
- 44.
- Frost D, Beischer W. Limited joint mobility in type 1 diabetic patients. Diabetes care. 2001;24(1):95–99. [PubMed: 11194249]
- 45.
- Lopez-Martin I, Benito Ortiz L, Rodriguez-Borlado B, Cano Langreo M, Garcia-Martinez FJ, Martin Rodriguez MF. (Association between limited joint mobility syndrome and risk of accidental falls in diabetic patients). Semergen. 2015;41(2):70–5. [PubMed: 24906788]
- 46.
- Lindsay JR, Kennedy L, Atkinson AB, et al. Reduced prevalence of limited joint mobility in type 1 diabetes in a UK clinic population over a 20-year period. Diabetes Care. 2005;28(3):658–61. [PubMed: 15735204]
- 47.
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. A multicentre study of characteristics, comorbidities, course and therapy in 44 patients. Journal of the European Academy of Dermatology and Venereology. 2015;29(12):2399–404. [PubMed: 26304054]
- 48.
- Cole GW, Headley J, Skowsky R. Scleredema Diabeticorum: A Common and Distinct Cutaneous Manifestation of Diabetes Mellitus. Diabetes Care. 1983;6(2):189–92. [PubMed: 6851809]
- 49.
- Carrington PR, Sanusi I, Winder PR, Turk LL, Jones C, Millikan LE. Scleredema adultorum. International journal of dermatology. 1984;23(8):514–22. [PubMed: 6209231]
- 50.
- Martin C, Requena L, Manrique K, Manzarbeitia F, Rovira A. Scleredema diabeticorum in a patient with type 2 diabetes mellitus. Case reports in endocrinology 2011;2011.
- 51.
- Varga J, Gotta S, Li L, Sollberg S, Leonardo M. Scleredema adultorum: case report and demonstration of abnormal expression of extracellular matrix genes in skin fibroblasts in vivo and in vitro. British Journal of Dermatology. 1995;132(6):992–99. [PubMed: 7662581]
- 52.
- Ferreli C, Gasparini G, Parodi A, Cozzani E, Rongioletti F, Atzori L. Cutaneous Manifestations of Scleroderma and Scleroderma-Like Disorders: a Comprehensive Review. Clinical Reviews in Allergy & Immunology. 2017:1–31. [PubMed: 28712039]
- 53.
- Bray SM, Varghese S, English JC. Ultrasonic massage and physical therapy for scleredema: improving activities of daily living. Archives of dermatology. 2010;146(4):453–54. [PubMed: 20404247]
- 54.
- Reid SD, Ladizinski B, Lee K, Baibergenova A, Alavi A. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. Journal of the American Academy of Dermatology. 2013;69(5):783–91. [PubMed: 23969033]
- 55.
- O’toole E, Kennedy U, Nolan J, Young M, Rogers S, Barnes L. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140(2):283–6. [PubMed: 10233223]
- 56.
- Muller SA, Winkelmann R. Necrobiosis lipoidica diabeticorum: a clinical and pathological investigation of 171 cases. Archives of dermatology. 1966;93(3):272–81. [PubMed: 5910868]
- 56A.
- Severson KJ, Patel MH, Brumfiel CM, Breen I, Butterfield RJ, Nelson SA, Sekulic A, Pittelkow MR, Mangold AR. Comorbidities and diabetic complications in patients with necrobiosis lipoidica. J Am Acad Dermatol. 2022 Apr;86(4):891–894. Epub 2021 Mar 17. [PubMed: 33722550] [CrossRef]
- 57.
- Kota SK, Jammula S, Kota SK, Meher LK, Modi KD. Necrobiosis lipoidica diabeticorum: A case-based review of literature. Indian journal of endocrinology and metabolism. 2012;16(4):614. [PMC free article: PMC3401767] [PubMed: 22837927]
- 58.
- Lopez PR, Leicht S, Sigmon JR, Stigall L. Bullosis diabeticorum associated with a prediabetic state. Southern medical journal. 2009;102(6):643–44. [PubMed: 19434030]
- 59.
- Larsen K, Jensen T, Karlsmark T, Holstein PE. Incidence of bullosis diabeticorum–a controversial cause of chronic foot ulceration. International wound journal. 2008;5(4):591–96. [PMC free article: PMC7951251] [PubMed: 19006577]
- 60.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. International journal of dermatology. 2000;39(3):196–200. [PubMed: 10759959]
- 61.
- Patel N, Spencer LA, English JC, Zirwas MJ. Acquired ichthyosis. Journal of the American Academy of Dermatology. 2006;55(4):647–56. [PubMed: 17010746]
- 62.
- Pavlović MD, Milenković T, Dinić M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes care. 2007;30(8):1964–67. [PubMed: 17519431]
- 63.
- Goyal A, Raina S, Kaushal SS, Mahajan V, Sharma NL. Pattern of cutaneous manifestations in diabetes mellitus. Indian journal of dermatology. 2010;55(1):39. [PMC free article: PMC2856371] [PubMed: 20418975]
- 63A.
- Iacopi E, Riitano N, Dini V, Berta R, Pieruzzi L, Janowska A, Anselmino M, Piaggesi A, Romanelli M. Using Skin Bioengineering to Highlight How Weight and Diabetes Mellitus Modify the Skin in the Lower Limbs of Super-Obese Patients. Diabetes Metab Syndr Obes. 2020 Mar 16;13:729–738. doiPMCID: PMC7083633. [PMC free article: PMC7083633] [PubMed: 32214836] [CrossRef]
- 64.
- Pavicic T, Korting HC. Xerosis and callus formation as a key to the diabetic foot syndrome: dermatologic view of the problem and its management. JDDG. Journal der Deutschen Dermatologischen Gesellschaft. 2006;4(11):935–41. [PubMed: 17081268]
- 65.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty‐two cases. Journal of the European Academy of Dermatology and Venereology. 2006;20(6):679–88. [PubMed: 16836495]
- 66.
- Karpouzis A, Giatromanolaki A, Sivridis E, Kouskoukis C. Acquired reactive perforating collagenosis: current status. The Journal of dermatology. 2010;37(7):585–92. [PubMed: 20629824]
- 67.
- Poliak SC, Lebwohl MG, Parris A, Prioleau PG. Reactive perforating collagenosis associated with diabetes mellitus. New England Journal of Medicine. 1982;306(2):81–84. [PubMed: 7053490]
- 68.
- Abdelbaqi‐Salhab M, Shalhub S, Morgan MB. A current review of the cutaneous manifestations of renal disease. Journal of cutaneous pathology. 2003;30(9):527–38. [PubMed: 14507400]
- 69.
- Robles-Mendez J, Vazquez-Martinez O, Ocampo-Candiani J. Skin manifestations of chronic kidney disease. Actas Dermo-Sifiliográficas. 2015;106(8):609–22. [PubMed: 26093993]
- 70.
- Morton C, Henderson I, Jones M, Lowe J. Acquired perforating dermatosis in a British dialysis population. British Journal of Dermatology. 1996;135(5):671–77. [PubMed: 8977664]
- 70A.
- Forouzandeh M, Stratman S, Yosipovitch G. The treatment of Kyrle's disease: a systematic review. J Eur Acad Dermatol Venereol. 2020 Jul;34(7):1457–1463. Epub 2020 Feb 5. [PubMed: 31919924] [CrossRef]
- 71.
- Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med. 2008;75(11):772–74. [PubMed: 19068958]
- 72.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis. JDDG. Journal der Deutschen Dermatologischen Gesellschaft. 2013;11(8):723–29. doi(published Online First: Epub Date)|. [PubMed: 23718268] [CrossRef]
- 72A.
- Ying Y, Shuang C, Zhen-Ying Z. Dupilumab may be an alternative option in the treatment of acquired reactive perforating collagenosis combined with AD. Immun Inflamm Dis. 2022 Mar;10(3):e574. Epub 2021 Dec 24PMCID: PMC8926492. [PMC free article: PMC8926492] [PubMed: 34953055] [CrossRef]
- 73.
- Sugandhan S, Khandpur S, Sharma VK. Familial chylomicronemia syndrome. Pediatric dermatology. 2007;24(3):323–25. [PubMed: 17542893]
- 74.
- Mahajan S, Koranne R, Sharma S. Cutaneous manifestation of diabetes melitus. Indian Journal of Dermatology, Venereology, and Leprology. 2003;69(2):105. [PubMed: 17642848]
- 75.
- Ahmed K, Muhammad Z, Qayum I. Prevalence of cutaneous manifestations of diabetes mellitus. J Ayub Med Coll Abbottabad. 2009;21(2):76–9. [PubMed: 20524475]
- 76.
- Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–99. [PMC free article: PMC4392164] [PubMed: 25725623]
- 77.
- Naik NS. Eruptive xanthomas. Dermatology online journal. 2001;7(2) [PubMed: 12165227]
- 78.
- Zaremba J, Zaczkiewicz A, Placek W. Eruptive xanthomas. Advances in Dermatology and Allergology/Postȩpy Dermatologii i Alergologii 2013;30(6):399.
- 79.
- Parker F. Xanthomas and hyperlipidemias. Journal of the American Academy of Dermatology. 1985;13(1):1–30. [PubMed: 4031142]
- 80.
- Ales Z, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomedical Papers. 2014;158(2):181–88. [PubMed: 24781043]
- 81.
- Margolis J. Letter: Skin tags in diabetes mellitus. N Engl J Med. 1976;295:172–3. [PubMed: 1272340]
- 82.
- Levy L, Zeichner JA. Dermatologic manifestation of diabetes. Journal of diabetes. 2012;4(1):68–76. [PubMed: 21848812]
- 83.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes care. 2010;33(1):150–55. [PMC free article: PMC2797961] [PubMed: 20040674]
- 84.
- Valdes-Rodriguez R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta dermato-venereologica. 2015;95(4):417–21. [PubMed: 25203328]
- 84A.
- Yang QP, Chen YY, Li Z, Xu M. Major Risk Factors Analysis of Pruritus Complicated by Type 2 Diabetes Mellitus and the Effect of Comprehensive Nursing Intervention. Front Surg. 2022 Feb 15;9:842884. doiPMCID: PMC8887712. [PMC free article: PMC8887712] [PubMed: 35242808] [CrossRef]
- 85.
- Guarneri C, Guarneri F, Borgia F, Vaccaro M. Finger pebbles in a diabetic patient: Huntley's papules. International journal of dermatology. 2005;44(9):755–56. [PubMed: 16135146]
- 86.
- Tabor CA, Parlette EC. Cutaneous manifestations of diabetes: signs of poor glycemic control or new-onset disease. Postgraduate medicine. 2006;119(3):38–44. [PubMed: 17128644]
- 87.
- Murphy-Chutorian B, Han G, Cohen SR. Dermatologic manifestations of diabetes mellitus. Endocrinology and Metabolism Clinics. 2013;42(4):869–98. [PubMed: 24286954]
- 88.
- Liau M, Long V, Yang S, Tan K, Aw D. Pigmented purpuric dermatosis in Singapore: a clinic-epidemiological characterisation. Hong Kong Journal of Dermatology & Venereology. 2016;24(2):65–69.
- 89.
- Lithner F. Purpura, pigmentation and yellow nails of the lower extremities in diabetics. Journal of Internal Medicine. 1976;199(1‐6):203–08. [PubMed: 1258701]
- 90.
- Demirseren DD, Emre S, Akoglu G, et al. Relationship between skin diseases and extracutaneous complications of diabetes mellitus: clinical analysis of 750 patients. American journal of clinical dermatology. 2014;15(1):65–70. [PubMed: 24135944]
- 91.
- Serrao R, Zirwas M, English JC. Palmar erythema. American journal of clinical dermatology. 2007;8(6):347–56. [PubMed: 18039017]
- 92.
- Landau J, Davis E. The small blood-vessels of the conjunctiva and nailbed in diabetes mellitus. The Lancet. 1960;276(7153):731–34. [PubMed: 13758645]
- 93.
- Oumeish OY. Skin disorders in patients with diabetes. Clinics in dermatology. 2008;26(3):235–42. [PubMed: 18640520]
- 94.
- Duff M, Demidova O, Blackburn S, Shubrook J. Cutaneous manifestations of diabetes mellitus. Clinical Diabetes. 2015;33(1):40–48. [PMC free article: PMC4299750] [PubMed: 25653473]
- 95.
- Bristow I. Non‐ulcerative skin pathologies of the diabetic foot. Diabetes/metabolism research and reviews. 2008;24(S1) [PubMed: 18357585]
- 95A.
- Yesudian PD, Nwabudike LC, de Berker D. Nail changes in diabetes. Clin Exp Dermatol. 2022 Jan;47(1):9–15. Epub 2021 Sep 21. [PubMed: 34293827] [CrossRef]
- 96.
- Mendes AL, Miot HA, Haddad V Junior. Diabetes mellitus and the skin. Anais brasileiros de dermatologia. 2017;92(1):8–20. [PMC free article: PMC5312172] [PubMed: 28225950]
- 97.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Annals of dermatology. 2009;21(2):113–19. [PMC free article: PMC2861218] [PubMed: 20523767]
- 98.
- Cyr PR. Diagnosis and management of granuloma annulare. American family physician. 2006;74(10) [PubMed: 17137003]
- 99.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: A systematic review and meta-analysis. JAMA Dermatology. 2013;149(1):84–91. [PubMed: 23407990]
- 100.
- Seyhan M, Özcan H, Sahin I, Bayram N, Karincaoğlu Y. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes research and clinical practice. 2007;77(2):198–202. [PubMed: 17275122]
- 101.
- Guggenheimer J, Moore PA, Rossie K, et al. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: I. Prevalence and characteristics of non-candidal lesions. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2000;89(5):563–69. [PubMed: 10807712]
- 102.
- Van den Driessche A, Eenkhoorn V, Van Gaal L, De Block C. Type 1 diabetes and autoimmune polyglandular syndrome: a clinical review. Neth J Med. 2009;67(11):376–87. [PubMed: 20009114]
- 103.
- Von der Werth J, Williams H. The natural history of hidradenitis suppurativa. Journal of the European Academy of Dermatology and Venereology. 2000;14(5):389–92. [PubMed: 11305381]
- 104.
- Bui T-L, Silva-Hirschberg C, Torres J, Armstrong AW. Hidradenitis suppurativa and diabetes mellitus: A systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2017 [PubMed: 29056237]
- 105.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. Journal of the American Academy of Dermatology. 2009;60(4):539–61. [PubMed: 19293006]
- 106.
- Eldor R, Glaser B, Fraenkel M, Doviner V, Salmon A, Gross DJ. Glucagonoma and the glucagonoma syndrome–cumulative experience with an elusive endocrine tumour. Clinical endocrinology. 2011;74(5):593–98. [PubMed: 21470282]
- 107.
- Wermers RA, Fatourechi V, Wynne AG, Kvols LK, Lloyd RV. The Glucagonoma Syndrome Clinical and Pathologic Features in 21 Patients. Medicine. 1996;75(2):53–63. [PubMed: 8606627]
- 108.
- Kovács RK, Korom I, Dobozy A, Farkas G, Ormos J, Kemény L. Necrolytic migratory erythema. Journal of cutaneous pathology. 2006;33(3):242–45. [PubMed: 16466513]
- 109.
- Kindmark H, Sundin A, Granberg D, et al. Endocrine pancreatic tumors with glucagon hypersecretion: a retrospective study of 23 cases during 20 years. Medical Oncology. 2007;24(3):330–37. [PubMed: 17873310]
- 110.
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. Journal of general internal medicine. 2013;28(11):1525–29. [PMC free article: PMC3797362] [PubMed: 23681843]
- 111.
- Macedo GMC, Nunes S, Barreto T. Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetology & metabolic syndrome. 2016;8(1):63. [PMC free article: PMC5006568] [PubMed: 27583022]
- 112.
- Kutlu SS, Cevahir N, Akalin S, et al. Prevalence and risk factors for methicillin-resistant Staphylococcus aureus colonization in a diabetic outpatient population: a prospective cohort study. American journal of infection control. 2012;40(4):365–68. [PubMed: 21864943]
- 113.
- Hartemann‐Heurtier A, Robert J, Jacqueminet S, et al. Diabetic foot ulcer and multidrug‐resistant organisms: risk factors and impact. Diabetic Medicine. 2004;21(7):710–15. [PubMed: 15209763]
- 114.
- Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian journal of endocrinology and metabolism. 2012;16 Suppl1:S27. [PMC free article: PMC3354930] [PubMed: 22701840]
- 115.
- Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67(3):351–68. [PubMed: 17335295]
- 116.
- Bonne SL, Kadri SS. Evaluation and management of necrotizing soft tissue infections. Infectious Disease Clinics. 2017;31(3):497–511. [PMC free article: PMC5656282] [PubMed: 28779832]
- 117.
- Dworkin M, Westercamp M, Park L, McIntyre A. The epidemiology of necrotizing fasciitis including factors associated with death and amputation. Epidemiology & Infection. 2009;137(11):1609–14. [PubMed: 19351432]
- 118.
- Bhandary S, Karki P, Sinha B. Malignant otitis externa: a review. Pacific health dialog. 2002;9(1):64–67. [PubMed: 12737420]
- 119.
- Sashikumar R, Kannan R. Salivary glucose levels and oral candidal carriage in type II diabetics. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2010;109(5):706–11. [PubMed: 20416536]
- 120.
- Yosipovitch G, Tur E, Cohen O, Rusecki Y. Skin surface pH in intertriginous areas in NIDDM patients: possible correlation to candidal intertrigo. Diabetes Care. 1993;16(4):560–63. [PubMed: 8462378]
- 121.
- Saunte DML, Holgersen JB, Hædersdal M, et al. Prevalence of toe nail onychomycosis in diabetic patients. Acta dermato-venereologica. 2006;86(5):425–28. [PubMed: 16955188]
- 122.
- Alteras I, Saryt E. Prevalence of pathogenic fungi in the toe-webs and toe-nails of diabetic patients. Mycopathologia. 1979;67(3):157–59. [PubMed: 384255]
- 123.
- Romano C, Massai L, Asta F, Signorini A. Prevalence of dermatophytic skin and nail infections in diabetic patients. Mycoses. 2001;44(3‐4):83–86. [PubMed: 11413928]
- 124.
- Rich P. Onychomycosis and tinea pedis in patients with diabetes. Journal of the American Academy of Dermatology. 2000;43(5):S130–S34. [PubMed: 11044289]
- 124A.
- Sharma A, Goel A. Mucormycosis: risk factors, diagnosis, treatments, and challenges during COVID-19 pandemic. Folia Microbiol (Praha). 2022 Feb 26;:1–25. doiEpub ahead of printPMCID: PMC8881997. [PMC free article: PMC8881997] [PubMed: 35220559] [CrossRef]
- 125.
- Sinikumpu S-P, Auvinen J, Jokelainen J, et al. Abnormal skin in toe webs is a marker for abnormal glucose metabolism. A cross-sectional survey among 1,849 adults in Finland. Scientific reports. 2017;7:9125. [PMC free article: PMC5567349] [PubMed: 28831117]
- 126.
- Richardson T, Kerr D. Skin-related complications of insulin therapy. American journal of clinical dermatology. 2003;4(10):661–67. [PubMed: 14507228]
- 127.
- Lima AL, Illing T, Schliemann S, Elsner P. Cutaneous Manifestations of Diabetes Mellitus: A Review. American Journal of Clinical Dermatology. 2017:1–13. [PMC free article: PMC8977104] [PubMed: 27988837]
- 128.
- Attaway A, Mersfelder TL, Vaishnav S, Baker JK. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors. A case report and review of literature. Journal of dermatological case reports. 2014;8(1):24. [PMC free article: PMC3989094] [PubMed: 24748908]
- 129.
- Byrd JS, Minor DS, Elsayed R, Marshall GD. DPP-4 inhibitors and angioedema: a cause for concern? Annals of Allergy, Asthma & Immunology. 2011;106(5):436–38. [PubMed: 21530877]
- 130.
- Hitselberger JF, Fosnaugh RP. Photosensitivity due to chlorpropamide. JAMA. 1962;180(1):62–63. [PubMed: 13907785]
- 131.
- Walker G, Kinsell LW. Clinical experience with chlorpropamide. California medicine. 1961;94(6):344. [PMC free article: PMC1574396] [PubMed: 13782713]
- 132.
- Walker G, Slater J, Westlake E, Nabarro J. Clinical experience with tolbutamide. British medical journal. 1957;2(5040):323. [PMC free article: PMC1961998] [PubMed: 13446468]
- 133.
- Leslie R, Pyke D. Chlorpropamide-alcohol flushing: a dominantly inherited trait associated with diabetes. Br med J. 1978;2(6151):1519–21. [PMC free article: PMC1608771] [PubMed: 728707]
- 134.
- Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Current medical research and opinion. 2014;30(6):1109–19. [PubMed: 24517339]
- 135.
- Feingold KR. Oral and Injectable (Non-Insulin) Pharmacological Agents for the Treatment of Type 2 Diabetes. 2021 Aug 28. In: Feingold KR, Anawalt B, Boyce A, et al editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–.
- Review Diabetes Mellitus in People with Cancer.[Endotext. 2000]Review Diabetes Mellitus in People with Cancer.Joharatnam-Hogan N, Carter TJ, Reynolds N, Ho JH, Adam S, Board R. Endotext. 2000
- Review Hypoglycemia During Therapy of Diabetes.[Endotext. 2000]Review Hypoglycemia During Therapy of Diabetes.Davis HA, Spanakis EK, Cryer PE, Davis SN. Endotext. 2000
- Review Diabetes Mellitus After Solid Organ Transplantation.[Endotext. 2000]Review Diabetes Mellitus After Solid Organ Transplantation.Pham PT, Sarkar M, Pham PM, Pham PC. Endotext. 2000
- Review Pregestational Diabetes Mellitus.[Endotext. 2000]Review Pregestational Diabetes Mellitus.Cleary EM, Thung SF, Buschur EO. Endotext. 2000
- Review Classification of Diabetes Mellitus.[Endotext. 2000]Review Classification of Diabetes Mellitus.Solis-Herrera C, Triplitt C, Reasner C, DeFronzo RA, Cersosimo E. Endotext. 2000
- Skin Manifestations of Diabetes Mellitus - EndotextSkin Manifestations of Diabetes Mellitus - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
