Abstract
Multiple sclerosis (MS) is a long-lasting inflammatory neurodegenerative disease of the central nervous system caused by an inappropriate attack of the body’s immune system on its own cells. To date, its etiology remains highly enigmatic, with insufficient evidence on the exact cause triggering the disease. Many studies have highlighted the role of different environmental and genetic factors in its etiopathogenesis, each adding a new wedge to MS conundrum and therefore making it a multifactorial and polygenic disease. One of the entrants in the risk factor category for MS is vitamin D, and there is sufficient evidence to suggest its role in increasing the risk of MS development. MS patients have lower levels of vitamin D, and in conjunction with other factors like low sunlight intensity and genetic variations in vitamin D metabolic pathway genes, vitamin D has been adjudged as a potent risk factor for MS. The biological effects of vitamin D in the body are mediated by the vitamin D receptor that acts as a transcription factor after activation by vitamin D and subsequent heterodimerization with the retinoid-X receptor. This allows regulation of protein expression of target genes involved in diverse cellular processes including immune response and vitamin D metabolism. It clearly suggests use of vitamin D supplementation as an unconventional option for MS treatment; however, much work needs to be done to precisely determine the level and/or dosage of vitamin D required for achieving optimum therapeutic response in patients without causing adverse effects.
Key words:
Deficiency, Exposure, Multiple sclerosis, Sunlight, Vitamin DIntroduction
Multiple sclerosis (MS) is a chronic multifactorial and polygenic autoimmune disease of the central nervous system (CNS), affecting predominantly young to middle-aged adults, especially females (1). It was Jean-Martin Charcot who described MS for the first time in 1868 (2). Escalating evidence has shown that it is the outcome of inappropriate immune response, characterized by auto-inflammation, making it a highly unpredictable disease (3). It is accompanied by a wide continuum of signs and symptoms which vary from person to person depending on the area of CNS damage (1, 3). Its epidemiology is variable across the globe, which indicates that MS etiology is governed by numerous geographic and environmental factors (4, 5). Presently, it is estimated that there are over 2.3 million people in the world living with MS, clearly indicating an increase in the number when compared to the 2008 estimate (6). A large body of epidemiological evidence supports the consensus view that it is a heterogeneous disease which results from complex interactions between susceptibility genes and one or more environmental factors during the course of growth and development of a person (1, 7–11). However, no single gene or environmental factor has been unambiguously identified as the causative agent, and it is likely that the cumulative effects of several genes and environmental factors lead to disease onset (12). To date, the exact cause of this debilitating neurological disease remains convoluted; however, significant attempts have been made to discover environmental agents associated with it (8).
Epidemiological and experimental data suggest low vitamin D levels to be associated with disease predisposition in cancer, schizophrenia, cardiovascular ailments, rheumatoid arthritis, and autoimmune diseases such as systemic lupus erythematosus, type 1 diabetes, and MS (13–17). The association between vitamin D and MS has become a burning issue across the globe and in the recent years there has been a tremendous increase in studies on the same (18, 19). The aim of this chapter is to explore the association between vitamin D deficiency and MS risk, and to present the latest knowledge and developments on the role of vitamin D as a risk factor for MS
Vitamin D and Its Biological Role
Vitamin D is a pro-hormone belonging to the category of fat-soluble group of vitamins. It is a secosteroid and is primarily responsible for maintaining calcium homeostasis by facilitating absorption and utilization of minerals; as a result, it acts as a major contributor toward bone formation and homeostasis (15, 20–24). The naturally occurring form of vitamin D is biologically inactive and requires hydroxylation in the liver and kidney for activation (25). It exists in two main forms in humans: D2–ergocalciferol (plant derived) and D3–cholecalciferol (animal derived) (25). Small quantities of vitamin D can be obtained from food; however, its primary source is generated by exposure to sunlight (15, 25–27). Vitamin D in skin is present in the form of pro-vitamin D3 or 7-dehydrocholesterol and is converted to pre-vitamin D3 photochemically by ultraviolet B (UV-B) rays from the sun and later on converted to vitamin D3 by isomerization (23). This vitamin D3 from skin, food, or supplements is transported to liver by vitamin D–binding proteins (GC group-specific component), where it is converted to 25-hydroxyvitamin D3 (25(OH)D3) or calcidiol through the process of hydroxylation by one or more cytochrome P450 vitamin D 25-hydroxylases like vitamin D-25-hydroxylase (CYP2R1 cytochrome P450, family 2, subfamily R, member 1) (28, 29). In kidneys, 25(OH)D3 is further hydroxylated to 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) or calcitriol by 25-hydroxyvitamin D-1-alpha-hydroxylase (CYP27B1 cytochrome P450, family 27, subfamily B, member 1) (15, 29). The schematic pathway for vitamin D synthesis is given in Figure 1. The breakdown product of vitamin D is calcitroic acid which is generated through hydroxylation of 1,25(OH)2D3 by 1,25-dihydroxyvitamin D 24-hydroxylase (CYP24A1 cytochrome P450, family 24, subfamily A, member 1) (15, 30).
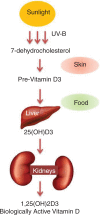
Figure 1
Biosynthetic pathway of vitamin D in humans. Vitamin D is synthesized in a series of events involving sunlight exposure and hydroxylation by liver and kidney enzymes.
In humans, the most biologically active form of vitamin D is 1,25(OH)2D3; however, the vitamin D levels in the body are represented by 25(OH)D3 concentrations due to its longer half-life than 1,25(OH)2D3 (31). The optimal concentration of vitamin D in the body remains a perplexing issue and as a result there exist several definitions for defining vitamin D status of a person. Generally, vitamin D deficiency and insufficiency has been defined as a serum level of 25(OH) D3 <50 nmol/L or 52.5–72.5 nmol/L, respectively (32, 33). Vitamin D deficiency is highly prevalent across the globe, affecting almost every population irrespective of age and gender (15, 17).
Vitamin D plays an essential role in innate and acquired immunity by acting as an immunomodulator regulating the production of type 1 and type 2 helper T-cell cytokines (Th1, Th2) (34), suggesting its key role in governing immune and inflammatory responses within the body (35). It plays a key role in several other processes like cellular growth, proliferation, differentiation, and apoptosis; DNA repair and oxidative stress; and membrane transport and adhesion (15, 22–24). Recent studies have proposed that its supportive role in immune response reflects its involvement in the prevention of various diseases including brain disorders and cancer (15, 33, 36, 37). The graphical representation of diverse roles played by vitamin D at the cellular level is shown in Figure 2.
Figure 2
Pictorial representation of diverse roles played by vitamin D.
The various biological responses of vitamin D are mediated through the vitamin D receptor (VDR) signaling due to its ubiquitous expression in immune cells as well as within CNS (38, 39). The binding of vitamin D (1, 25 (OH)2D3, calcitriol) to VDR and its subsequent activation leads to its heterodimerization with the retinoid-X receptor (RXR), resulting in modulation of vitamin D responsive gene expression by translocation of heterodimer complex (1, 25 (OH)2D3-VDR/RXR) to nucleus, and its recruitment on vitamin D response elements (VDRE) of target genes (24, 40). The schematic pathway for vitamin D–based signaling is given in Figure 3. Depending on the site of recruitment of VDR complex, it may result in induction of transcription at the promoter site or regulate expression at enhancer sites (41, 42). This allows for the regulation of protein expression of target vitamin D–sensitive genes involved in diverse cellular processes including immune response and vitamin D metabolism and therefore the outcome of this mechanism could be changed from pro-inflammatory to anti-inflammatory, thereby modulating the disease risk (38, 43). The direct manifestations of immunomodulatory effects of vitamin D are inhibition of Th1 cytokine production and Th17 cell differentiation, and stimulation of Th2 cytokines and T-regulatory cells, resulting in a shift in immune response (34).
Status of Vitamin D in MS
MS RISK AND VITAMIN D
The geographical distribution of MS is highly variable (4) and the causal factors known to play a role in its development are fusion of genetic and environmental components, thereby reflecting the role of epigenetics in its development (7, 44, 45). The pattern of its distribution across the globe is believed to be irregular with several exceptions; however, it shows higher prevalence in regions away from the equator (higher altitudes) where there is lower sunlight exposure (46, 47). A recent study has provided substantial evidence in support of latitude gradient shown by MS prevalence (48). Globally, vitamin D is low in general population and also in certain diseases including MS (17). The first report to suggest connection between MS and sunlight was the one by Goldberg et al. (49). Several studies have suggested that reduced levels of vitamin D are associated with a higher risk of MS as serum levels of vitamin D have been found to be lower in patients than controls (50–56). Many studies have observed a correlation between season of birth and MS risk as is evident from the fact that there is lower sunlight intensity in winter when compared to summer, reflecting the possibility of an association between mother’s exposure to sunlight during pregnancy, vitamin D levels or its dietary intake, and MS susceptibility (57).
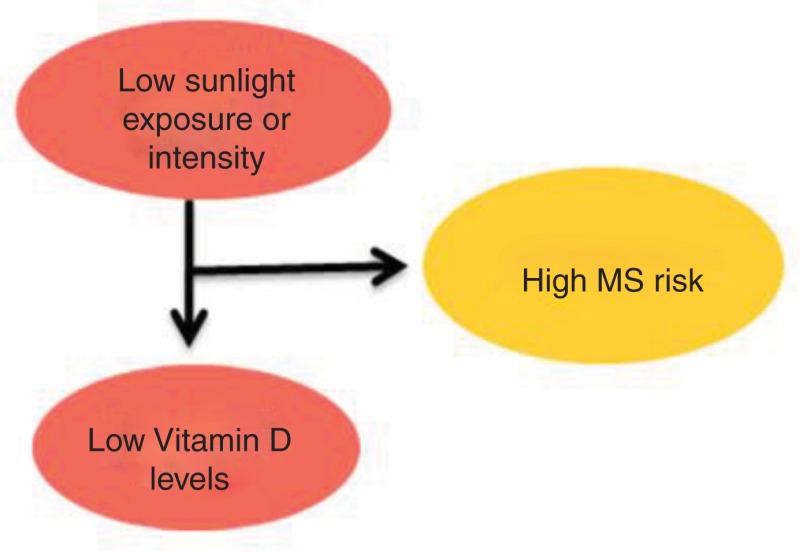
Since the major source of vitamin D is sunlight-induced synthesis, it is evident that decreased sunlight exposure leads to reduced levels of vitamin D and thus higher MS risk (53–55). The decreased MS susceptibility has been linked to early sunlight exposure in life, especially during childhood and adolescence (50–52). The graphical representation of the link between sunlight, vitamin D, and MS risk is shown in Figure 4. Interestingly, migration studies have shown that MS risk changes with migration from one place to another; however, age at migration plays a key role in determining the disease risk of the migrant (58, 59). Recent studies have suggested an association between vitamin D levels and MS relapse rate as well as the degree of disability, and it was seen that patients with higher serum levels of vitamin D showed a lower relapse rate while lower levels of vitamin D appeared to be associated with higher levels of disability in patients measured in terms of expanded disability status scale (EDSS) score (60–63).

Figure 4
Pictorial representation of association between sun exposure, vitamin D, and MS risk.
Although there has been a lot of research on vitamin D status and MS risk in adult-onset cases, there is lack of data on association with pediatric-onset MS (64–66). A recent meta-analysis based on Mendelian randomization has used instrumental variable analysis to provide evidence for causal and independent association between low vitamin D levels and increased body mass index (BMI) with the risk of developing pediatric MS (64). In addition, there is evidence suggesting vitamin D–based regulation of klotho and nuclear factor-erythroid-2-related factor 2 (Nrf2) signaling pathways to be responsible for MS development as they are believed to maintain calcium and redox homeostasis within the body (67) and as a result klotho and Nrf2 in conjunction with vitamin D (vitamin D-klotho-Nrf2) act as keepers of several cell signaling pathways including myelin synthesis pathway (68). Even though there is evidence suggesting the role of vitamin D as a potent environmental risk factor for MS, further studies are required to evaluate precisely whether vitamin D status governs MS susceptibility independently or in combination with sun exposure. Furthermore, research to elucidate the duration and time of exposure and the role of other related epidemiological factors on MS susceptibility are warranted.
GENETICS OF VITAMIN D AND MS
Genetic link of vitamin D status in MS has long been hypothesized and several small-scale studies have been carried out to explore the association of polymorphisms in vitamin D–related genes with MS risk. The most consistent genetic regions found to be associated with the status of vitamin D in MS are vitamin D metabolism genes—CYP24A1, CYP27B1, and DBP/GC (encoding vitamin D–binding protein) (69, 70). It is anticipated that these genes may increase MS risk by modulating vitamin D metabolic pathway, thereby affecting vitamin D levels (70). The other crucial gene has been the vitamin D–based signaling gene VDR, particularly FokI, ApaI, TaqI, and BsmI variants, although a recent study has reported conflicting results (71, 72). A meta-analysis by Huang et al. provided evidence against their association (73). A recent investigation provided strong evidence for the role of VDR in the regulation of gene expression in immune cells of myeloid lineage which clearly indicates the importance of these genes in maintaining cellular tolerance (74). At the same time, it was observed that MS susceptibility loci including CYP27B1 and CYP24A1 showed high expression in myeloid cells, clearly reflecting the role of this interconnected regulatory pathway in therapeutic intervention of MS (74). In addition, it has been demonstrated that the main MS susceptibility governing genetic variant-major histocompatibility complex, class II region, DR beta 1 (HLA-DRB1) contains VDRE in its promoter region, which strongly suggests that their expression is governed by vitamin D (75). In fact, strong correlation has been observed between the increase in expression level of HLA-DRB1 and vitamin D, providing solid evidence for functional implication of vitamin D in MS (75). Several other genes implicated in predicting serum concentrations of vitamin D and subsequent risk of developing MS include NADSYN1 (nicotinamide adenine dinucleotide synthetase) and DHCR7 (7-dehydrocholesterol) (76). Moreover, several genes involved in MS predisposition are also regulated by vitamin D as predicted by in silico analysis, clearly signifying the role of vitamin D as a modulator of MS risk (77) (Figure 5).

Figure 5
In silico analysis depicting interactions between several vitamin D responsive MS candidate genes, confirming the effect of vitamin D on MS (http://www.stringdb.org).
Furthermore, a recent cross-sectional study by Laursen et al. showed the association between age at onset of MS and vitamin D–related genetic and environmental factors including GC, CYP2R1, CYP27B1, CYP24A1, and HLA-DRB1*1501 (78). Significant association was observed between younger age of MS onset and low sunlight exposure, higher BMI at the age of 20, and HLA-DRB1*1501, reflecting their independent effect on age at disease onset. Also, no association was found between age at onset and rest of the vitamin D–related genetic and environmental factors (78). Accordingly, vitamin D appears to be a potent environmental risk factor in MS, exerting its effect at the genetic level by interacting with genetic elements associated with MS. The concordance observed within genetic and epidemiological data clearly signifies the application of vitamin D supplementation as a promising treatment option for MS.
VITAMIN D AS A FUTURE TREATMENT OPTION FOR MS
There is compelling evidence to suggest that reduced risk of MS is associated with higher sunlight exposure and increased levels of vitamin D, thus suggesting a protective effect of vitamin D supplementation on MS (79). The current research based on large datasets is being targeted on using vitamin D supplementation as an alternative approach for MS treatment; however, there is still lack of convincing evidence for its effect on disease progression (80). The exact mechanism governing vitamin D–mediated regulation of immune response has to be completely elucidated for exploiting it as a future treatment option for MS. The experimental studies hitherto have suggested that the immune effects of vitamin D are not exerted at physiologic concentrations which results in hypercalcemia, reflecting an increase in calcium levels within the body (34). The previous studies based on low sample numbers have not been able to reveal convincing clinical effects of vitamin D in mitigating MS symptoms (81). Hence, there is lack of concrete evidence providing substantial support in using vitamin D intervention for MS management. At the same time, the outcome of numerous genetic studies reiterate the fact that the studies based on vitamin D and MS should be conducted by considering the independent effect of different vitamin D–linked genetic and environmental factors on vitamin D levels within the body (82).
Keeping in mind the role of vitamin D as an immunomodulator and a risk factor for MS, its supplementation could be the most promising cost-effective treatment for MS in comparison with conventional disease-modifying therapy; thus, it could eventually prove beneficial for lowering MS burden across the globe. However, the major concerns that remain undetermined regarding its application are precise dosage, timing, response, and efficacy. Since MS is highly prevalent in women than men, it will be interesting to study the effect of gender on immunomodulatory response of vitamin D intervention. Also, keeping in view the role of genetic background of a person in determining treatment response, it becomes mandatory to conduct vitamin D–based randomized controlled trials to study the ultimate effects in different individuals with a particular genotype.
Conclusion
MS remains a mysterious disease posing several challenges for investigation; however, considerable progress has been made in unscrambling its etiology. Although there has been a remarkable progress in the research focusing on the role of vitamin D as a risk factor for MS, studies are warranted to explore the exact mechanism behind the impact of vitamin D levels on disease course, severity, and relapse. The precise effect of vitamin D on MS progression is yet to be determined. There is an urgent requirement for understanding the molecular mechanisms behind this association and exploring vitamin D supplementation as a future therapeutic option for MS. At the same time, increased attention should be given to establish the optimum levels of vitamin D that can be used for achieving desired clinical and immunomodulatory effects in MS patients with lesser adverse reactions of hypercalcemia.
Since vitamin D exerts its immunomodulatory effects through binding of VDR, cellular expression of VDR can be a crucial determinant for MS pathogenesis.Vitamin D, being the ligand of VDR, is highly dependent on environmental influences; thus VDR analysis provides an excellent possibility to investigate gene–environment interaction. Understanding how polymorphisms in vitamin D metabolic pathway genes can affect expression at mRNA as well as at protein level may help in delineating the role of vitamin D–based pathway behind MS risk, enabling therapies targeting vitamin D–based signaling pathway. Furthermore, it will help in defining the critical targets involved in vitamin D metabolism and its regulation. This will aid in revealing the clinical immunomodulatory application of vitamin D for MS patients, and provide the basis for using vitamin D supplementation as a future therapeutic alternative for MS management. In addition, this approach can also provide evidence as to whether vitamin D can serve as a reliable clinical marker for MS progression, degree of disability or severity, and for predicting the outcome of disease for better management.
Acknowledgments: The research work on MS was supported by the grants provided to the Women-Scientist, Dr. Insha Zahoor, by the Department of Science and Technology (DST), Govt. of India, New Delhi, under the Women Scientists Scheme-A (WOS-A) vide Order No.: SR/WOS-A/LS-72/2013(G)
Conflict of interest: The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this book chapter.
Copyright and permission statement: To the best of our knowledge, the materials included in this chapter do not violate copyright laws. All original sources have been appropriately acknowledged and/or referenced. Where relevant, appropriate permissions have been obtained from the original copyright holder(s).
References
- 1.
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008 Oct;372(9648):1502–1517. [PubMed: 18970977] [CrossRef]
- 2.
- Clanet M. Jean-Martin Charcot. 1825 to 1893. Int MS J. 2008 Jun;15(2):59–61. [PubMed: 18782501]
- 3.
- Weinshenker BG. Epidemiology of multiple sclerosis. Neurol Clin. 1996 May;14(2):291–308. [PubMed: 8827172] [CrossRef]
- 4.
- Rosati G. The prevalence of multiple sclerosis in the world: An update. Neurol Sci. 2001 Apr;22(2):117–139. [PubMed: 11603614] [CrossRef]
- 5.
- Aguirre-Cruz L, Flores-Rivera J, De La Cruz-Aguilera DL, Rangel-Lopez E, Corona T. Multiple sclerosis in Caucasians and Latino Americans. Autoimmunity. 2011 Nov;44(7):571–575. [PubMed: 21875378] [CrossRef]
- 6.
- Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014 Sep;83(11):1022–1024. [PMC free article: PMC4162299] [PubMed: 25200713] [CrossRef]
- 7.
- Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008 Mar;7(3):268–277. [PubMed: 18275928] [CrossRef]
- 8.
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol. 2007 Apr;61(4):288–299. [PubMed: 17444504] [CrossRef]
- 9.
- Handunnetthi L, Ramagopalan SV, Ebers GC. Multiple sclerosis, vitamin D, and HLA-DRB1*15. Neurology. 2010 Jun;74(23):1905–1910. [PMC free article: PMC2882222] [PubMed: 20530326] [CrossRef]
- 10.
- Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: Genes, syndromes, and therapies. Lancet Neurol. 2009 Nov;8(11):1056–1072. [PubMed: 19833297] [CrossRef]
- 11.
- Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012 Nov;8(11):602–612. [PMC free article: PMC4467212] [PubMed: 23045241] [CrossRef]
- 12.
- Milo R, Kahana E. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev. 2010 Mar;9(5):A387–394. [PubMed: 19932200] [CrossRef]
- 13.
- Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol. 2013 Oct;45(2):256–266. [PubMed: 23238772] [CrossRef]
- 14.
- Burton JM, Costello FE. Vitamin D in multiple sclerosis and central nervous system demyelinating disease—A review. J Neuroophthalmol. 2015 Jun;35(2):194–200. [PubMed: 25985434] [CrossRef]
- 15.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul;357(3):266–281. [PubMed: 17634462] [CrossRef]
- 16.
- Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010 Jun;9(6):599–612. [PubMed: 20494325] [CrossRef]
- 17.
- Hossein-Nezhad A, Holick MF. Vitamin D for health: A global perspective. Mayo Clin Proc. 2013 Jul;88(7):720–755. [PMC free article: PMC3761874] [PubMed: 23790560] [CrossRef]
- 18.
- Holick MF, Cook S, Suarez G, Rametta M. Vitamin D deficiency and possible role in multiple sclerosis. Eur Neurol Rev. 2015;10(2):131–138. [CrossRef]
- 19.
- Mormile R. Vitamin D intake and its protective role in multiple sclerosis: The Checkmate to Survivin? Iran J Pharm Res. 2016 Spring;15(2):383–384. [PMC free article: PMC5018265] [PubMed: 27642308]
- 20.
- Bouillon R, Van Cromphaut S, Carmeliet G. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J Cell Biochem. 2003 Feb;88(2):332–339. [PubMed: 12520535] [CrossRef]
- 21.
- Bell TD, Demay MB, Burnett-Bowie SA. The biology and pathology of vitamin D control in bone. J Cell Biochem. 2010 Sep;111(1):7–13. [PMC free article: PMC4020510] [PubMed: 20506379] [CrossRef]
- 22.
- Wolf G. The discovery of vitamin D: The contribution of Adolf Windaus. J Nutr. 2004 Jun;134(6):1299–1302. [PubMed: 15173387]
- 23.
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014 Mar;21(3):319–329. [PMC free article: PMC3968073] [PubMed: 24529992] [CrossRef]
- 24.
- Trochoutsou A, Kloukina V, Samitas K, Xanthou G. Vitamin-D in the immune system: Genomic and non-genomic actions. Mini Rev Med Chem. 2015;15(11):953–963. [PubMed: 25985946] [CrossRef]
- 25.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006 Mar;81(3):353–373. [PubMed: 16529140] [CrossRef]
- 26.
- Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: A global perspective of current status. J Nutr. 2005 Feb;135(2):310–316. [PubMed: 15671233]
- 27.
- Norman AW. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008 Aug;88(2):491S–499S. [PubMed: 18689389]
- 28.
- Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: Metabolism. Endocrinol Metab Clin North Am. 2010 Jun;39(2):243–253. [PMC free article: PMC2879391] [PubMed: 20511049] [CrossRef]
- 29.
- Hsu F, Kent WJ, Clawson H, Kuhn RM, Diekhans M, Haussler D. The UCSC known genes. Bioinformatics. 2006 May;22(9):1036–1046. [PubMed: 16500937] [CrossRef]
- 30.
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004 Dec;80 6 Suppl:1689S–1696S. [PubMed: 15585789]
- 31.
- Hollis BW. Assessment of vitamin D nutritional and hormonal status: What to measure and how to do it. Calcif Tissue Int. 1996 Jan;58(1):4–5. [PubMed: 8825231] [CrossRef]
- 32.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US); 2011. [PubMed: 21796828]
- 33.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011 July;96(7):1911–1930. [PubMed: 21646368] [CrossRef]
- 34.
- van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J Steroid Biochem Mol Biol. 2005 Oct;97(1–2):93–101. [PubMed: 16046118] [CrossRef]
- 35.
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008 Aug;4(8):404–412. [PubMed: 18594491] [CrossRef]
- 36.
- Nowson CA, McGrath JJ, Ebeling PR, Haikerwal A, Daly RM, Sanders KM, et al. Vitamin D and health in adults in Australia and New Zealand: A position statement. Med J Aust. 2012 Jun;196(11):686–687. [PubMed: 22708765] [CrossRef]
- 37.
- Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: An updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011 Dec;155(12):827–838. [PubMed: 22184690] [CrossRef]
- 38.
- Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol. 2008 Feb;194(1–2):7–17. [PubMed: 18177949] [CrossRef]
- 39.
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005 Jan;29(1):21–30. [PubMed: 15589699] [CrossRef]
- 40.
- Smolders J, Peelen E, Thewissen M, Menheere P, Tervaert JW, Hupperts R, et al. The relevance of vitamin D receptor gene polymorphisms for vitamin D research in multiple sclerosis. Autoimmun Rev. 2009 Jun;8(7):621–626. [PubMed: 19393206] [CrossRef]
- 41.
- Pike JW, Meyer MB. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Endocrinol Metab Clin North Am. 2010 Jun;39(2):255–269. [PMC free article: PMC2879406] [PubMed: 20511050] [CrossRef]
- 42.
- Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010 Oct;20(10):1352–1360. [PMC free article: PMC2945184] [PubMed: 20736230] [CrossRef]
- 43.
- Hanwell HE, Banwell B. Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim Biophys Acta. 2011 Feb;1812(2):202–212. [PubMed: 20674744] [CrossRef]
- 44.
- Goodin DS. The genetic basis of multiple sclerosis: A model for MS susceptibility. BMC Neurol. 2010 Oct;10:101. [PMC free article: PMC2994805] [PubMed: 21029420] [CrossRef]
- 45.
- Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: Risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010 Jul;9(7):727–739. [PubMed: 20610348] [CrossRef]
- 46.
- Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol. 2007 Apr;254(4):471–477. [PubMed: 17377831] [CrossRef]
- 47.
- Kampman MT, Brustad M. Vitamin D: A candidate for the environmental effect in multiple sclerosis–observations from Norway. Neuroepidemiology. 2008;30(3):140–146. [PubMed: 18382112] [CrossRef]
- 48.
- Simpson S Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J Neurol Neurosurg Psychiatry. 2011 Oct;82(10):1132–1141. [PubMed: 21478203] [CrossRef]
- 49.
- Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: Decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med. Hypotheses. 1986 Oct;21(2):193–200. [PubMed: 3537648] [CrossRef]
- 50.
- Mansouri B, Asadollahi S, Heidari K, Fakhri M, Assarzadegan F, Nazari M, et al. Risk factors for increased multiple sclerosis susceptibility in the Iranian population. J Clin Neurosci. 2014 July;21(12):2207–2211. http://doi
.org/10.1016/j .jocn.2014.04.020. [PubMed: 25082407] - 51.
- BjØrnevik K, Riise T, Casetta I, Drulovic J, Granieri E, HolmØy T, et al. Sun exposure and multiple sclerosis risk in Norway and Italy: The EnvIMS study. Mult Scler. 2014 July;20(8):1042–1049. [PubMed: 24414538] [CrossRef]
- 52.
- Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology. 2007 July;69(4):381–388. [PubMed: 17646631] [CrossRef]
- 53.
- Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. 2011 Feb;76(6):540–548. [PubMed: 21300969] [CrossRef]
- 54.
- van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor BV, et al. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: Case-control study. BMJ. 2003 Aug;327(7410):316. [PMC free article: PMC169645] [PubMed: 12907484] [CrossRef]
- 55.
- Baarnhielm M, Hedstrom AK, Kockum I, Sundqvist E, Gustafsson SA, Hillert J, et al. Sunlight is associated with decreased multiple sclerosis risk: No interaction with human leukocyte antigen-DRB1*15. Eur J Neurol. 2012 Jul;19(7):955–962. [PubMed: 22289117] [CrossRef]
- 56.
- Pandit L, Ramagopalan SV, Malli C, D’Cunha A, Kunder R, Shetty R. Association of vitamin D and multiple sclerosis in India. Mult Scler. 2013 Oct;19(12):1592–1596. [PubMed: 23519972] [CrossRef]
- 57.
- Dobson R, Giovannoni G, Ramagopalan S. The month of birth effect in multiple sclerosis: Systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry. 2013 Apr;84(4):427–432. [PubMed: 23152637] [CrossRef]
- 58.
- Oren Y, Shapira Y, Agmon-Levin N, Kivity S, Zafrir Y, Altman A, et al. Vitamin D insufficiency in a sunny environment: A demographic and seasonal analysis. Isr Med Assoc J. 2010 Dec;12(12):751–756. [PubMed: 21348404]
- 59.
- Kurtzke JF, Dean G, Botha DP. A method for estimating the age at immigration of white immigrants to South Africa, with an example of its importance. S Afr Med J. 1970 Jun;44(23):663–669. [PubMed: 5427147]
- 60.
- Runia TF, Hop WC, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012 July;79(3):261–266. [PubMed: 22700811] [CrossRef]
- 61.
- Simpson S Jr, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010 Aug;68(2):193–203. [PubMed: 20695012]
- 62.
- Shahbeigi S, Pakdaman H, Fereshtehnejad SM, Nikravesh E, Mirabi N, Jalilzadeh G. Vitamin D3 concentration correlates with the severity of multiple sclerosis. Int J Prev Med. 2013 May;4(5):585–591. [PMC free article: PMC3733190] [PubMed: 23930170]
- 63.
- Thouvenot E, Orsini M, Daures JP, Camu W. Vitamin D is associated with degree of disability in patients with fully ambulatory relapsing-remitting multiple sclerosis. Eur J Neurol. 2015 Mar;22(3):564–569. [PubMed: 25530281] [CrossRef]
- 64.
- Gianfrancesco MA, Stridh P, Rhead B, Shao X, Xu E, Graves JS, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset, MS. Neurology. 2017 Apr;88(17):1623–1629. [PMC free article: PMC5405763] [PubMed: 28356466] [CrossRef]
- 65.
- Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Goltzman D, et al. Vitamin D and risk of multiple sclerosis: A Mendelian randomization study. PLoS Med. 2015 Aug;12(8):e1001866. [PMC free article: PMC4549308] [PubMed: 26305103] [CrossRef]
- 66.
- Rhead B, Bäärnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016 Oct;2(5):e97. [PMC free article: PMC5022843] [PubMed: 27652346] [CrossRef]
- 67.
- Berridge MJ. Vitamin D: A custodian of cell signalling stability in health and disease. Biochem Soc Trans. 2015 Jun;43(3):349–358. [PubMed: 26009175] [CrossRef]
- 68.
- Chen CD, Sloane JA, Li H, Aytan N, Giannaris EL, Zeldich E, et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci. 2013 Jan;33(5):1927–1939. [PMC free article: PMC3711388] [PubMed: 23365232] [CrossRef]
- 69.
- Orton SM, Ramagopalan SV, Para AE, Lincoln MR, Handunnetthi L, Chao MJ, et al. Vitamin D metabolic pathway genes and risk of multiple sclerosis in Canadians. J Neurol Sci. 2011 Jun;305(1–2):116–120. [PubMed: 21440908] [CrossRef]
- 70.
- Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011 Aug;476(7359):214–219. [PMC free article: PMC3182531] [PubMed: 21833088] [CrossRef]
- 71.
- Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004 Sep;338(2):143–156. [PubMed: 15315818] [CrossRef]
- 72.
- Huang J, Xie ZF. Polymorphisms in the vitamin D receptor gene and multiple sclerosis risk: A meta-analysis of case-control studies. J Neurol Sci. 2012 Feb;313(1–2):79–85. [PubMed: 22029942] [CrossRef]
- 73.
- Cox MB, Ban M, Bowden NA, Baker A, Scott RJ, Lechner-Scott J. Potential association of vitamin D receptor polymorphism Taq1 with multiple sclerosis. Mult Scler. 2012 Jan;18(1):16–22. [PubMed: 21816760] [CrossRef]
- 74.
- Booth DR, Ding N, Parnell GP, Shahijanian F, Coulter S, Schibeci SD, et al. Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun. 2016 Jun;17(4):213–219. [PMC free article: PMC4895389] [PubMed: 26986782] [CrossRef]
- 75.
- Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton SM, Dyment DA, et al. Expression of the multiple sclerosis-associated MHC class II Allele HLADRB1*1501 is regulated by vitamin D. PLoS Genet. 2009 Feb;5(2):e1000369. [PMC free article: PMC2627899] [PubMed: 19197344] [CrossRef]
- 76.
- Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010 Jul;19(13):2739–2745. [PMC free article: PMC2883344] [PubMed: 20418485] [CrossRef]
- 77.
- Cree BA. Multiple sclerosis genetics. Handb Clin Neurol. 2014;122:193–209. [PubMed: 24507519] [CrossRef]
- 78.
- Laursen JH, Søndergaard HB, Sørensen PS, Sellebjerg F, Oturai AB. Association between age at onset of multiple sclerosis and vitamin D level-related factors. Neurology. 2016 Jan;86(1):88–93. [PubMed: 26446064] [CrossRef]
- 79.
- Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocinol. 2013 Jan;5(1):51–108. [PMC free article: PMC3897598] [PubMed: 24494042] [CrossRef]
- 80.
- Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: Exploratory outcomes from a double-blind randomised controlled trial. Mult Scler. 2012 Aug;18(8):1144–1151. [PubMed: 22354743] [CrossRef]
- 81.
- James E, Dobson R, Kuhle J, Baker D, Giovannoni G, Ramagopalan SV. The effect of vitamin D-related interventions on multiple sclerosis relapses: A meta-analysis. Mult Scler. 2013 Oct;19(12):1571–1579. [PubMed: 23698130] [CrossRef]
- 82.
- Niino M, Miyazaki Y. Genetic polymorphisms related to vitamin D and the therapeutic potential of vitamin D in multiple sclerosis. Can J Physiol Pharmacol. 2015 Jan;93(5):319–325. [PubMed: 25798693] [CrossRef]
Publication Details
Author Information and Affiliations
Authors
Insha Zahoor 1 and Ehtishamul Haq1.
1 and Ehtishamul Haq1.Affiliations
 Author for correspondence: Insha Zahoor, Bioinformatics Centre, Ground Floor, Science Block, University of Kashmir, Hazratbal, Srinagar, Jammu and Kashmir, 190006, India. E-mail: moc.liamg@11roohazahsni
Author for correspondence: Insha Zahoor, Bioinformatics Centre, Ground Floor, Science Block, University of Kashmir, Hazratbal, Srinagar, Jammu and Kashmir, 190006, India. E-mail: moc.liamg@11roohazahsniCopyright
Licence: This open access article is licenced under Creative Commons Attribution 4.0 International (CC BY-NC 4.0). https://creativecommons.org/licenses/by-nc/4.0/
Publisher
Codon Publications, Brisbane (AU)
NLM Citation
Zahoor I, Haq E. Vitamin D and Multiple Sclerosis: An Update. In: Zagon IS, McLaughlin PJ, editors. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis [Internet]. Brisbane (AU): Codon Publications; 2017 Nov 27. Chapter 5. doi: 10.15586/codon.multiplesclerosis.2017.ch5