NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | 186Re-Labeled [N-[2[[3-(3,3-diphosphonopropylcarbamoyl)propyl]-2-thioethylamino]acetyl]-2-aminoethylenethiolate] oxorhenium (V) |
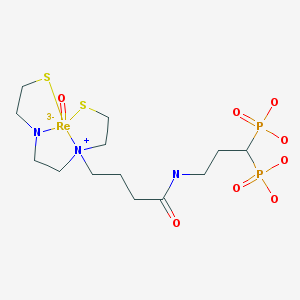
|
| Abbreviated name: | [186Re]MAMA-BP | |
| Synonym: | ||
| Agent Category: | Compound | |
| Target: | Hydroxyapatite: Bone imaging Molecular target: Farnesyl disphosphate (pyrophosphate) synthase | |
| Target Category: | Enzyme | |
| Method of detection: | Single-photon emission computed tomography (SPECT); gamma planar imaging | |
| Source of signal / contrast: | 186Re | |
| Activation: | No | |
| Studies: |
| Click on the above structure of [186Re]MAMA-BP for additional information in PubChem. |
Background
[PubMed]
Bisphosphonates (BPs) or nitrogen-containing bisphosphonates (NBPs) are often used for the management of pain palliation and disorders related to skeletal tissue, including those arising from cancer metastases, because these compounds have a very high affinity for hydroxyapatite (HA), a component of the bone matrix. These phosphonates or their derivatives tend to accumulate in osteoclasts located at areas of increased bone metabolism by inhibiting the enzyme farnesyl diphosphate (or pyrophosphate) synthase, an important regulatory enzyme of the cellular mevalonate pathway, which is involved in protein prenylation (1). The molecular mechanism of action of BPs and the NBPs has been described by Drake et. al. (2). Several BPs and NBPs are commercially available for clinical use to treat different bone disorders, and there are ongoing clinical trials approved by the United States Food and Drug Administration to evaluate these compounds for the treatment of various bone ailments. In addition, BPs are often labeled with 99mTc or 186/188Re and used for the imaging and treatment of pain as a result of bone metastases from cancer such as that of the breast or the prostate (3). However, these compounds have limited efficacy primarily because they exist either as a mixture of anionic compounds with varying properties (e.g., 99mTc- labeled methyl diphosphonate (MDP)) or are unstable (e.g., 86Re-labeled 1-hydroxyethylidene-1,1-diphosphonate) under in vivo conditions, resulting in a reduced uptake at targeted bone areas and an increased accumulation in non-target soft tissue such as the gastric lining of the stomach (4). The limited clinical utility of radiolabeled BPs was suggested to be caused by the dual activities exhibited by the compounds: one phosphonate group acts as a radionuclide chelator, and the other phosphonate group binds to the target(s).Therefore, due to the close proximity of the two groups, one activity may be interfering with the other (4).
In an effort to solve the stability problems observed with the 186Re-labeled NBPs, Ogawa et al. developed two new NBPs, 186Re-[N-[2-[[3-(3,3-diphosphonopropylcarbamoyl)propyl]-2-thioethylamino]acetyl]-2-aminoethylenethiolate] oxorhenium (V) ([186Re]MAMA-BP) and its hydroxylated derivative, 186Re-[N-[2-[[4-[(4-hydroxy-4,4-diphosphonobutyl)amino]-4-oxobutyl]-2-thioethylamino]acetyl]-2-aminoethanethiolate] oxorhenium (V) ([186Re]MAMA-HBP) (3). The investigators then compared the two compounds for their affinity to HA under in vitro conditions and studied the biodistribution of the radiochemicals in normal mice. This chapter presents the results obtained with [186Re]MAMA-BP. Results obtained with [186Re]MAMA-HBP are presented in a separate chapter of MICAD (5).
Synthesis
[PubMed]
A 186Re-glucoheptonate ligand exchange reaction was used for the synthesis of [186Re]MAMA-BP and 186Re-1-hydroxyethylidene-1,1-diphosphonate ([186Re]-MAMA-HEDP; used as a control in the various studies) and is detailed elsewhere (6). The radiochemical yield of [186Re]MAMA-BP was reported to be between 21.3 ± 4.5 and 32.0 ± 4.1% with a purity of 96.4 ± 1.4%. Specific activity of the radiolabeled compounds was not reported.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The in vitro stability of [186Re]MAMA-BP after a 24-h incubation in 0.1 M phosphate-buffered saline (pH, 7.0) saturated with 95% O2 and 5% CO2 at 37°C was reported to be 81.8 ± 1.7% as determined with reversed-phase high-performance liquid chromatography or thin-layer chromatography. Under the same conditions, the stability of [186Re]MAMA-HBP was reported to be 75.55 ± 1.57%.
The HA binding of [186Re]MAMA-BP was determined by using different amounts (1–25 mg/mL) of commercially available HA beads suspended in Tris/HCl-buffered saline (pH, 7.4) as described by Ogawa et al. (3). The percent of HA binding was determined with an equation given elsewhere (3). Approximately 40% of [186Re]MAMA-BP bound to the HA beads at the lowest concentration (compared with ~70% for [186Re]MAMA-HBP) and increased to ~95.0% at the highest concentration (compared with ~97.5% for [186Re]MAMA-HBP). Results from this study indicated that [186Re]MAMA-HBP had a higher affinity for HA than [186Re]MAMA-BP.
Animal Studies
Rodents
[PubMed]
The biodistribution of [186Re]MAMA-BP and [186Re]MAMA-HBP was investigated in normal, male ddY mice after intravenous administration of the respective labeled compounds (3). For this study, another NBP, [186Re]HEDP, was used as a control. The animals (n = 5–6 mice per time point) were euthanized at designated time points ranging from 10 min to 24 h postinjection (p.i.). Organs of interest (liver, kidney, blood, intestine, stomach, and the complete left femur) were removed from the cadavers and weighed, and the amount of radioactivity accumulated in the tissues was measured, and the data were presented as percent injected dose per gram tissue (% ID/g).
The tissue distribution pattern of radioactivity from [186Re]MAMA-BP was reported to be similar to that observed with [186Re]MAMA-HBP and [186Re]MAMA-HEDP (3). Maximum label was deposited on the femur at all time points with the radiochemicals (21.38 ± 3.83% ID/g with [186Re]MAMA-BP, 24.80 ± 2.41% ID/g with [186Re]MAMA-HBP, and 13.09 ± 2.90% ID/g with [186Re]MAMA-HEDP) at 24 h p.i., followed by the kidneys (varying from 2.43 ± 0.53% ID/g with [186Re]MAMA-BP to 0.42 ± 0.10% ID/g with [186Re]MAMA-HEDP) at the same time point. The femur/blood ratios of radioactivity were ~1,200 with [186Re]MAMA-HBP compared with ~800 and ~400 with [186Re]MAMA-BP and [186Re]MAMA-HEDP, respectively. In addition, the blood clearance of radioactivity from [186Re]MAMA-HEDP was slower than that of the other two 186Re-labeled MAMA-bisphosphonates, and it had a lower accumulation in the liver.
Ogawa et al. calculated the radiation dose estimates for the three bisphosphonates for adult patients by monoexponential extrapolation of the biodistribution data (3). Although the red-marrow/bone-surface and total-body/bone-surface ratios for all the radiolabeled compounds were the same, the effective-dose/bone-surface ratio of [186Re]MAMA-HEDP was higher than that of either [186Re]MAMA-BP or [186Re]MAMA-HBP.
References
- 1.
- Kimmel D.B. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86(11):1022–33. [PubMed: 17959891]
- 2.
- Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–45. [PMC free article: PMC2667901] [PubMed: 18775204]
- 3.
- Ogawa K., Mukai T., Arano Y., Otaka A., Ueda M., Uehara T., Magata Y., Hashimoto K., Saji H. Rhemium-186-monoaminemonoamidedithiol-conjugated bisphosphonate derivatives for bone pain palliation. Nucl Med Biol. 2006;33(4):513–20. [PubMed: 16720243]
- 4.
- Torres Martin de Rosales R., Finucane C., Mather S.J., Blower P.J. Bifunctional bisphosphonate complexes for the diagnosis and therapy of bone metastases. Chem Commun (Camb). 2009;(32):4847–9. [PMC free article: PMC7116767] [PubMed: 19652801]
- 5.
- Chopra, A., 186Re-Labeled [N-2[[4-[(4-hydroxy-4,4-diphosphonobutyl)amino]-4-oxobutyl]-2-thioethylamino]acetyl]-2-aminoethylenethiolate] conjugated to hydroxyl-bisphosphonate. Molecular Imaging and Contrast agent Database (MICAD) [database online]. National Library of Medicine, NCBI, Bethesda, MD, USA. Available from www
.micad.nih.gov, 2004 -to current. - 6.
- Ogawa K., Mukai T., Arano Y., Hanaoka H., Hashimoto K., Nishimura H., Saji H. Design of a radiopharmaceutical for the pallation of painful bone metastases: rhenium-186-labeled bisphosphonate derivative. J. Label. Compd. Radiopharm. 2004;47:753–761.
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review (186)Re-Labeled [N-[2-[[4-[(4-hydroxy-4,4-diphosphonobutyl)amino]-4-oxobutyl]-2-thioethylamino]acetyl]-2-aminoethanethiolate] oxorhenium (V).[Molecular Imaging and Contrast...]Review (186)Re-Labeled [N-[2-[[4-[(4-hydroxy-4,4-diphosphonobutyl)amino]-4-oxobutyl]-2-thioethylamino]acetyl]-2-aminoethanethiolate] oxorhenium (V).Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (99m)Tc-Labeled mercaptoacetylglycylglycylglycine conjugated to hydroxy-bisphosphonate.[Molecular Imaging and Contrast...]Review (99m)Tc-Labeled mercaptoacetylglycylglycylglycine conjugated to hydroxy-bisphosphonate.Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (99m)Tc-Labeled 6-hydrazinopyridine-3-carboxylic acid conjugated to hydroxy-bisphosphonate.[Molecular Imaging and Contrast...]Review (99m)Tc-Labeled 6-hydrazinopyridine-3-carboxylic acid conjugated to hydroxy-bisphosphonate.Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (99m)Tc/(188)Re-Labeled dipicolylamine-alendronate.[Molecular Imaging and Contrast...]Review (99m)Tc/(188)Re-Labeled dipicolylamine-alendronate.Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Rhemium-186-monoaminemonoamidedithiol-conjugated bisphosphonate derivatives for bone pain palliation.[Nucl Med Biol. 2006]Rhemium-186-monoaminemonoamidedithiol-conjugated bisphosphonate derivatives for bone pain palliation.Ogawa K, Mukai T, Arano Y, Otaka A, Ueda M, Uehara T, Magata Y, Hashimoto K, Saji H. Nucl Med Biol. 2006 May; 33(4):513-20.
- 186Re-Labeled [N-[2[[3-(3,3-diphosphonopropylcarbamoyl)propyl]-2-thioethylamino]...186Re-Labeled [N-[2[[3-(3,3-diphosphonopropylcarbamoyl)propyl]-2-thioethylamino]acetyl]-2-aminoethylenethiolate] oxorhenium (V) - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro