NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Balk E, Raman G, Chung M, et al. Comparative Effectiveness of Management Strategies for Renal Artery Stenosis [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Oct. (Comparative Effectiveness Reviews, No. 5.)
This publication is provided for historical reference only and the information may be out of date.

Comparative Effectiveness of Management Strategies for Renal Artery Stenosis [Internet].
Show detailsBackground
Renal artery stenosis (RAS) is defined as the narrowing of the lumen of the renal artery. Atherosclerosis accounts for 90 percent of cases of RAS and usually involves the ostium and proximal third of the main renal artery and the perirenal aorta.1 RAS is becoming increasingly common because of atherosclerosis in an aging population; in addition, there is an increased prevalence of atherosclerotic RAS (ARAS) among elderly with diabetes, hyperlipidemia, aortoiliac occlusive disease, coronary artery disease, and hypertension. ARAS is a progressive disease that may occur alone or in combination with hypertension and ischemic kidney disease.1 The prevalence of ARAS in the general population remains poorly defined, although the prevalence may vary from 30 percent among patients with coronary artery disease (CAD) identified by angiography2 to 50 percent among elderly or those with diffuse atherosclerotic vascular diseases.3 In the United States 12 to 14 percent of new patients entering dialysis programs have been found to have ARAS.4
Optimal strategies for evaluating patients suspected of having RAS remain unclear. Patients with moderate to high risk atherovascular diseases who present with uncontrolled hypertension or unexplained abnormal kidney function tests are generally evaluated for RAS.1,5,6 A reduction in estimated glomerular filtration rate (GFR) of at least 30 percent from baseline following angiotensin converting enzyme (ACE) inhibitor or angiotensin-receptor blockers (ARB) therapy is a clinical clue suggestive of RAS.7 A variety of physiological studies to assess the renin-angiotensin system and perfusion studies to assess renal blood flow are available. However, the clinical clues can be nonspecific and physiologic studies have limited usefulness in ARAS, especially, among the elderly. The initial evaluation relies on imaging techniques such as duplex ultrasonography, magnetic resonance angiography (MRA), computed tomographic angiography (CTA), and radionuclide renal scanning. The success rates of these noninvasive imaging techniques depend on operator’s experience, body habitus, the presence of bowel gas, and may be less reliable visualizing distal segments of renal arteries. Currently, catheter angiography remains the reference standard for the evaluation of the degree of stenosis in RAS.
Most authorities consider the goals of therapy to be improvement in uncontrolled hypertension, preservation or salvage of kidney function, and improvement in symptoms and quality of life. Treatment alternatives include medications alone or revascularization of the stenosed renal artery or arteries. Combination therapy with multiple antihypertensive agents, usually including ACE inhibitors or ARBs, calcium channel blockers, and or beta blockers, are frequently prescribed with a goal of normalizing blood pressure. Some clinicians also recommend statins to lower low density lipoprotein (LDL) cholesterol and antiplatelet agents, such as aspirin or clopidogrel, to reduce thrombosis. The current standard for revascularization in most patients is percutaneous transluminal angioplasty with stent placement across the stenosis. Angioplasty without stent placement is less commonly employed. Revascularization by surgical reconstruction is generally used for only patients with complicated renal artery anatomy or for patients who require pararenal aortic reconstructions for aortic aneurysms or severe aortoiliac occlusive disease.
The American College of Cardiology and the American Heart Association recently published guidelines for the management of patients with peripheral arterial disease, including RAS.8,9 These guidelines provide recommendations about which patients should be considered for revascularization; however, there remains considerable uncertainty on which intervention provides the best clinical outcomes. Among patients treated with medical therapy alone, there is the risk for deterioration of kidney function with worsening morbidity and mortality. Renal artery revascularization may provide immediate improvement in kidney function and blood pressure; however, as with all invasive interventions, it may result in substantial morbidity and mortality in some patients.
ACE inhibitors and ARBs are effective in controlling renovascular hypertension in 86 to 92 percent of these patients, but the loss of kidney function due to reduction in transcapillary filtration pressure can result in acute or chronic kidney disease.1 Indications and timing of revascularization for ARAS are topics of considerable debate. The American Heart Association lists three clinical criteria for revascularization: 1) hypertension (accelerated, refractory, or malignant), 2) renal salvage, and 3) cardiac disturbance syndromes (recurrent “flash” pulmonary edema or unstable angina with significant RAS).10 This must be weighed against the morbidity and mortality risks of revascularization.
Placement of renal artery stents can resolve dissections, minimize stenosis recoil and restenosis, and correct translesional pressure gradients. The evidence for durability of benefit is unclear; the majority of the published studies on stent placement in ARAS had followup duration of less than two years. Comparison among studies on the effect of revascularization on hypertension and kidney function is limited because of differences in medical therapy, target blood pressure, and criteria for improvement.1 The American College of Cardiology and the American Heart Association recently published guidelines for the management of patients with peripheral arterial disease, including renal artery stenosis.8,9 Nevertheless, considerable controversy remains regarding optimal strategies for evaluation and management of patients with ARAS; the evidence supporting a benefit of aggressive treatment remains unclear.
Meanwhile, a Medicare claims analysis found that the rate of percutaneous renal artery revascularization has rapidly increased between 1996 and 2000 with the number of renal artery interventions increasing from 7,660 to 18,520. However, there is marked disparity in use across geographical regions.11 Therefore, the Agency for Healthcare Research and Quality (AHRQ) is commissioning an expedited review of the evidence on the effectiveness of renal artery angioplasty with stent placement versus aggressive medical therapy. This review was commissioned under Section 1013 of the Medicare Modernization Act, that instructs to conduct comparative-effectiveness reviews on medications and devices. AHRQ has requested the Tufts-New England Medical Center Evidence-based Practice Center (Tufts-NEMC EPC) to conduct a review of the literature on the Comparative Effectiveness of Management Strategies for Renal Artery Stenosis.
Scope and Key Questions
This report summarizes the evidence evaluating the effect and safety of angioplasty (with or without stents, or surgical revascularization) and medical treatments in the treatment of ARAS, particularly after long-term followup. Key questions addressed in this report are:
- For patients with atherosclerotic renal artery stenosis in the modern management era (i.e., since JNC-5 in 1993*), what is the evidence on the effects of aggressive medical therapy (i.e., antihypertensive, antiplatelet, and antilipid treatment) compared to renal artery angioplasty with stent placement on long-term clinical outcomes (at least 6 months) including blood pressure control, preservation of kidney function, flash pulmonary edema, other cardiovascular events, and survival?
- 1a. What are the patient characteristics, including etiology, predominant clinical presentation, and severity of stenosis, in the studies?
- 1b. What adverse events and complications have been associated with aggressive medical therapy or renal artery angioplasty with stent placement?
- What clinical, imaging, laboratory and anatomic characteristics are associated with improved or worse outcomes when treating with either aggressive medical therapy alone or renal artery angioplasty with stent placement?
- What treatment variables are associated with improved or worse outcomes of renal artery angioplasty with stent placement, including periprocedural medications, type of stent, use of distal protection devices, or other adjunct techniques?
Analytic Framework
We applied the analytic framework depicted in Figure 1 to answer the key questions in the evaluation of the treatment modalities for ARAS. This framework addressed relevant clinical outcomes. It also examined clinical predictors that affected treatment outcomes. While evidence from high quality randomized controlled trials was preferred, these data were rare, so nonrandomized and uncontrolled studies were used to augment the evidence.
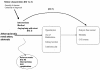
Figure 1
Analytic framework for evaluating the effectiveness and safety of treatments for renal artery stenosis. Arrows depict studies sought to address key questions formulated in this report
Types of Participants
The population of interest for this report is adults with ARAS that is of sufficient severity to warrant aggressive management, either due to resistant hypertension, evidence of kidney damage, or the high likelihood of poor outcomes. Because of the variety of techniques used to diagnose and define RAS, the definitions used by study authors were accepted. Where possible this review is limited to studies of patients with a high proportion of ARAS (as opposed to fibromuscular dysplasia and other diseases). In addition, only studies of revascularization where the large majority of patients had only procedures to correct ARAS (as opposed to aortic disease or renal artery aneurysm) were included.
Types of Interventions
The primary interventions of interest are angioplasty with stent placement and aggressive medical treatment, as defined in the key questions. However, given the state of the evidence, this review also covers angioplasty without stent placement, surgical revascularization, any medical treatment, and so-called “natural history” studies where a variety of generally undefined strategies are employed.
Types of Outcome Measures
The primary outcomes of interest include long-term (6 months or more) mortality, kidney function, hypertension, cardiovascular disease, and related outcomes, in addition to adverse events and complications (including 30-day mortality).
Types of Studies
The ideal study to answer the key questions would be a randomized controlled trial directly comparing the primary interventions of interest. However, given the paucity of randomized trials and of nonrandomized comparative studies, this review evaluates studies of cohorts of patients who received one treatment (or one set of treatments) without a control group. In addition, because of continued changes in management of hypertension and of RAS over the past 20 years or more, older noncomparative studies of patients enrolled prior to the publication of JNC-5 (as described above and in the Methods section) in 1993 were not reviewed.
CORAL Trial
A randomized, multicenter clinical trial sponsored by National Institutes of Health (NIH), the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial contrasts the effect of renal artery stent placement with optimum medical therapy (including antihypertensive drugs, a statin, and aspirin) and clopidogrel (an antiplatelet agent) to optimum medical therapy alone in patients with hemodynamically significant ARAS and systolic hypertension.12
The first line antihypertensive treatment will be either an ARB (candesartan) alone or with hydrochlorothiazide. Study eligibility criteria continue to evolve. The latest agreed upon criteria (Rundback JH. Personal communication, Jun 4, 2006) include adults with ARAS ≥60 percent and systolic hypertension on two or more antihypertensive medications. Those with high stage kidney disease (serum creatinine ≥3.0 mg/dL) at enrollment, very small kidneys (<7 cm), as well as certain patients with cardiovascular disease are being excluded. Other eligibility criteria apply.
The trial started in April 2004 and plans to follow approximately 2,200 North American patients at up to 100 clinical sites for the occurrence of a composite cardiovascular and kidney endpoint, including cardiovascular or kidney-related death, myocardial infarction, hospitalization for congestive heart failure, stroke, doubling of serum creatinine level, and need for renal replacement therapy. The study is expected to last about 3 to 5.5 years. This study is to be completed in 2010 and no results are available at this time.
Footnotes
- *
5th Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (1993). These guidelines marked a substantial change from previous guidelines in treatment recommendations for hypertension, including more aggressive blood pressure targets. This time point also marks when ACE inhibitors began to be used more routinely for patients with severe hypertension.
- Introduction - Comparative Effectiveness of Management Strategies for Renal Arte...Introduction - Comparative Effectiveness of Management Strategies for Renal Artery Stenosis
Your browsing activity is empty.
Activity recording is turned off.
See more...
