NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
McTigue K, Harris R, Hemphill MB, et al. Screening and Interventions for Overweight and Obesity in Adults [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2003 Dec. (Systematic Evidence Reviews, No. 21.)
This publication is provided for historical reference only and the information may be out of date.
We present in this chapter the findings from our review according to each key question of the analytic framework (Table 2 and Figure 5). Tables and figures called out for the first time in this chapter can be found at the end of the chapter; evidence tables appear in Appendix B.
Key Question No. 1: Does Screening for Overweight and Obesity Affect Health Outcomes?
We found no randomized controlled trials (RCTs) evaluating the efficacy of obesity screening programs (the overarching key question). Lacking direct evidence linking screening and mortality, morbidity, mental health outcomes, or functioning, we turned to indirect evidence on the components that a screening and intervention initiative would involve. These are the internal linkages depicted in the analytic framework.
Key Question No. 2: What is the Prevalence of Overweight and Obesity?
Data from the National Center for Health Statistics show that, over the past 40 years, obesity prevalence, estimated from measured height and weight, has increased from 13% to 27% of the U.S. adult population.7, 8 The prevalence of overweight, a less severe problem, rose from 31% to 34%. Thus, an estimated 61% of the U.S. population has a problem of excess weight or body fat. Age, sex, and ethnic prevalence differentials exist, as discussed in Chapter 1.
Key Question No. 3: Is There a Reliable and Valid Screening Test?
The most commonly used screening test for obesity, and the one upon which the clinical definition is based, is the body mass index (BMI, calculated as weight in kilograms divided by height in meters squared). BMI is thus a measure of weight adjusted for height.
In 1996, the U.S. Preventive Services Task Force (USPSTF) reviewed literature showing that BMI is easy to measure, highly reliable, and closely correlated (0.7–0.8) with body fat content in adults and children.2 BMI is correlated with percentage of body fat (R2 = 0.68–0.7453 and R2 = 0.47–0.92, depending on age)54 and with body fat mass (R2 = 0.95, in men; R2 = 0.98, in women).53 Validity may vary by characteristics of the population. For example, the degree of body fat and BMI differ somewhat by ethnicity.55–57 The elderly generally show a higher proportion of internal fat, and BMI correlates least strongly with body fat percentage in elderly adults;54 however, estimates of body fat percentage from BMI for the elderly have shown an error comparable to that for young adults (approximately 4%).58 The clinical relevance of BMI measurement is clear from an established prospective link between BMI and multiple adverse health outcomes.18, 21–24, 27, 48, 49
Some limitations of BMI do exist. For example, the correlation between body fat and BMI is age dependent and does not incorporate body fat distribution, which is an independent risk factor for health outcomes.59, 60 In addition, BMI does not take into account “fitness” (the weight of muscle vs the weight of fat in a heavily muscled individual), which is also associated with mortality independent of BMI.61
Other measures of adiposity (eg, waist-to-hip ratio, waist circumference) have been proposed to capture the increased cardiovascular risk seen with central adiposity. Central, or abdominal, adiposity has been most closely linked with cardiovascular risk in several prospective studies.
In the Atherosclerosis Risk in Communities (ARIC) study, either BMI ≥30 (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.4–2.0) or a waist-hip ratio >0.98 (OR,1.5; 95% CI, 1.3–1.8) was linked with increased risk of the multiple metabolic syndrome, adjusted for age, sex, ethnicity, and center.62 Ten-year death rates from the Health Professional Follow-up Study suggest that the relationship between central adiposity and mortality may be age dependent in men. In this study, overall and cardiovascular mortality in men increased linearly with baseline BMI in younger men (initially <65 years of age) and had no relationship with BMI in older men (initially at least age 65); by contrast, waist circumference predicted risk of overall and cardiovascular mortality in younger men and cardiovascular death among the older men.63 In a cohort of Iowa women, the waist-hip ratio was a better predictor of total or coronary heart disease mortality than BMI; hypertension incidence was more strongly linked with general obesity than with abdominal obesity; and all measures of obesity were strongly linked with diabetes incidence.25 Of particular note, even women in the lowest BMI quintile had marked increased risk of diabetes if they also had a high waist-hip ratio.25 In another prospective study of women, waist-hip ratio and waist circumference were independently associated with increased risk of coronary heart disease, even among those with BMI < 25.64
As BMI has been linked with a wider range of health outcomes, entry criteria for most studies are based on BMI. Obesity treatment trials typically reported either change in weight (directly proportional to BMI) or BMI; they did not reliably report measures of fat distribution. Because of these factors, we focused our analysis of screening tools on BMI.
Key Question No. 4a: Do Any Interventions Lead to Sustained Weight Reduction?
We identified 3 major forms of treatment for obesity that can be offered through various health care settings: counseling and behavioral interventions aimed at lifestyle intervention, pharmacotherapy, and surgery. We present results on these types of interventions below; details of the main studies cited appear in the evidence tables in Appendix B. As reflected in the analytic framework, our interest is both in intermediate outcomes such as sustained weight loss and maintenance of weight loss or those related to glucose tolerance, blood pressure, and lipid status, and in various health outcomes (discussed below).
Counseling and Behavioral Interventions
Counseling and behavioral interventions include a variety of approaches, all aimed at promoting a change in diet or exercise. These treatments can be delivered with or without behavioral intervention. The latter comprises strategies to help patients acquire the skills, motivations, and supports to change their diet and exercise patterns.
Prior Systematic Reviews
The National Institutes of Health (NIH) panel reviewed 29 counseling-based trials, with follow-up of at least 12 months, in which net weight loss (weight loss in intervention group minus weight loss in control group) could be calculated from evidence table data (Table 4). They found that average weight change in the intervention groups varied from a gain of 8 kg to a loss of 21.6 kg; corrected for change in control groups, weight change was +1.9 kg to -8.8 kg. In considering trials of ≥ 1 month duration, they found 38 in which counseling for low-calorie diets (1,000–1,200 kilo-calorie [kcal] per day) could reduce body weight by an average of 8% over 3 to 12 months and decrease abdominal fat.3 While very-low-calorie diets produced greater initial weight loss than low-calorie diets, effects were similar over follow-up beyond 1 year. Counseling for physical activity (in 24 RCTs) led to modest weight loss (2% to 3% of weight) and reduction in abdominal fat independent of the effect of caloric reduction. The combination of counseling for a reduced calorie diet and increased physical activity produced greater weight loss and reduction in abdominal fat than either approach alone. Review of 36 studies indicated that behavior therapy was a useful adjunct to other weight loss approaches over 1 year and that longer-term efficacy depended on continuing the intervention.
Upon review of 13 diet or behavioral therapy trials, the Canadian Task Force on Preventive Health Care found that weight reduction was most effective during supervised dietary treatment, with subsequent gradual weight regain. In 6 trials, with 24 to 60 months of follow-up, net weight change was 0 kg to -4.5 kg.46
The UK National Health Service (NHS) likewise found that behavioral interventions, combined with diet or exercise, appear to be effective and may be more so if of longer duration.47 In 24 studies, net weight change was similar to that found in other reviews: +1.4 to -10.6 kg over 12 to 60 months. The authors concluded that long-term follow-up and maintenance strategies should be an integral part of a weight loss program.47
Studies of Efficacy of Counseling and Behavioral Interventions
We identified 17 randomized trials of fair or good quality in which interventions were based on counseling (including diet, exercise, or some combination of the 2) and delivered with or without behavioral therapy.52, 65–80 In 1 additional trial, the intervention or control activity was delivered by county of residence in a nonrandom fashion.81 Evidence Table 1, Appendix B, records details of these 18 studies. Sustained weight loss was defined as a change in body weight (in pounds, kilograms, or kilograms per meter squared of height [ie, BMI]) for at least 1 year.
We recorded the mode of intervention in Evidence Table 1 as well as the degree of intensity of the intervention. We considered interventions to be of moderate intensity if they involved monthly person-to-person contact with the participant, during the first 3 months of the intervention. Those with more frequent contact were considered high intensity and those with less frequent contact low intensity; cut-offs were driven by the distribution of program intensity we found represented in the literature. The following discussion takes up trials of high, medium, and low intensity in that order. We classified trials according to the type of intervention - ie, type of behavior counseled (diet or exercise [D, E respectively in Evidence Table 1]) and presence of a behavioral therapy component in the delivery of the intervention (B in Evidence Table 1). More detail on counseling interventions is presented in Appendix C.
Trials often entailed both weight loss and maintenance phases. These were analyzed separately from trials that involved either only a loss phase or only a maintenance phase (following successful weight loss). We break out the 2 maintenance-only trials in the discussion below.67, 72
Comparison of effects is summarized in Figure 6. For this, we plotted the difference in mean weight change between intervention and control groups, at end-points as close as possible to 1 year, for all trials for which we could calculate the difference or the investigators could report the difference. If variance data were available in the articles or we could calculate them, we also depicted the 95% confidence intervals. Data are arrayed for high-, then moderate-, and then low-intensity studies; those with good internal validity are listed ahead of those with fair internal validity.
High-Intensity Interventions for Weight Loss. In all, 11 RCTs employed high-intensity interventions at the group or individual level.52, 65, 66, 68, 69, 71, 73, 74, 76, 79, 80 Of these, 6 RCTs compared high-intensity counseling intervention with a true control (no-intervention) group. Four achieved significant reductions in weight in the treatment groups (average loss: 2.7–5.5 kg improvement over controls at 12 months to more than 2 years of follow-up).65, 66, 69, 79
Of these, a Finnish trial is of particular note: individual-based diet and exercise advice with a behavioral component (7 sessions over the first year) led the intervention group to lose 3.4 kg more than controls over the first year.66 The investigators noted a high frequency of response (where response was defined as 5% weight loss): 43% of the intervention group versus 5% of the controls.66 Similar results were subsequently found for the participants in the Diabetes Prevention Program in the United States: high-intensity counseling for diet and exercise, with behavioral therapy, led to an average 5.5 kg loss in the intervention group versus controls over an average of 2.8 years.79
Two trials with a true control were less successful. One, with a group-based approach, showed borderline-significant reduction in weight in the treatment groups.74 Another combined an intense individual and group approach; the treatment group had improved weight at 6 months, but did not differ significantly from controls at 12 to 30 months of follow-up.68
Because prior studies have shown that combinations of diet, exercise, and behavioral therapy tend to be more effective than a single treatment, we evaluated 5 additional trials in which 1 combination of treatments was found to be superior to another, even in the setting of no true control (ie, no treatment) arm.52, 71, 73, 76, 80 One study evaluating choice of behavioral interventions found that a diet and exercise approach combined with behavioral choice treatment led to, on average, 5.8 kg of additional weight loss compared with traditional behavioral therapy.73 Another trial examined different exercise regimens in the setting of diet and behavioral therapy: people who were supplied with treadmill machines for use at home, and instructed to do short bouts of exercise, lost more weight (4.3 kg more) over 18 months than those with similar instructions but no home equipment.71 A trial comparing various 1-year, 26-session counseling programs found that combining meal replacements with a dietician-led group intervention was more effective than such counseling alone; short counseling sessions (10 to 15 minutes) with a health care provider combined with meal replacements led to similar degrees of weight loss as 1-hour dietician-led group sessions.80
A single trial examined the combination of counseling and behavioral therapy with pharmacotherapy for obesity.52 Compared with sibutramine alone, the addition of a lifestyle intervention (behavioral therapy) led to a 7.3 kg weight reduction, and the further addition of a portion-controlled low-calorie diet led to weight loss of 12.8 kg over 1 year. Finally, Wing and Anglin studied weight change by race, rather than weight loss per se. The data in Figure 6 thus reflect differences between black and white populations estimated from their graph-based results.76 Graphical data (significance not noted) suggest that weight loss was greater with behavioral therapy and an intermittent very-low-calorie diet than with behavioral therapy with a continuous low-calorie diet for white and black participants. However, over the 1-year follow-up, weight loss was lower in blacks than whites, primarily from faster regain.
Three studies (1 with 2 different physical activity interventions) reported weight change results first at 12 to 18 months, and then with prolonged follow-up.66, 69, 74 As seen in prior reviews, subjects showed a tendency towards weight regain. However, in 3 of the 4 interventions, a modest statistically significant weight loss of at least 2 kg was maintained 24 to 36 months after initiation.65, 66, 74 Two programs specifically included long-term maintenance interventions.65, 74
Moderate-Intensity Interventions for Weight Loss. We identified 2 moderate-intensity, counseling-based weight loss interventions, both with a behavioral component.70, 77 A 1-year, group-focused intervention showed effectiveness similar to that of the nonpharmacological high-intensity interventions (3.5 kg).70 A second intervention for multiple issues related to cardiac risk showed no significant improvement in weight at 18 months.77
Low-Intensity Interventions for Weight Loss. The low-intensity weight loss interventions that we reviewed were not effective. In 1 RCT, Jeffrey and French looked at the difference in weight change between intervention and control groups; this study was designed for weight gain prevention and showed no difference in men or in women of 2 socioeconomic strata at the end of 1 year.75
Two other studies (not shown in Figure 6) also investigated low-intensity approaches.78, 81 An RCT that did not measure baseline weight for controls found that, after 3 years, skilled manual laborers who underwent either a single or an annual nurse-run preventive medicine examination weighed 0.4 kg less than coworkers who received no health checks.78 Finally, a group-based study, delivered according to nonrandomized intervention or control counties, was likewise ineffective.81
Interventions for Weight Loss in the Overweight. Limited data address the efficacy of counseling-based interventions in the overweight. A well-done high-intensity intervention on participants with baseline mean BMI levels of 25 promoted weight loss in a range similar to that found in trials including only obese participants.65 However, 1 low- and 1 moderate-intensity intervention in which participants' baseline BMI was in the upper range of overweight were ineffective.75, 77
Interventions for Maintenance of Weight Loss. Two trials, neither with a true control group, evaluated counseling and behavioral plans for weight maintenance only.67, 72 In the high-intensity trial, members of a weight-focused treatment arm regained 2.1 kg less over 18 months than those receiving exercise-focused treatment.72 Weight-focused participants maintained, on average, 90% of their weight loss over that time (compared with 54% in the exercise-focused arm). A low-intensity weight maintenance intervention also found a significant difference between treatments:67 among self-selected women who had already lost more than 20 pounds, the prescription of pre-measured low-calorie liquid meal replacements led to an average of 5 kg lower weight over 1 year.
Summary of the Effectiveness of Counseling and Behavioral Treatment
Data on effectiveness of counseling and behavioral interventions must be understood in light of several caveats. Although several trials were of “good” quality (internal validity), most were judged only “fair,” with limitations such as small sample size, potential selection bias (trials often enrolled volunteers), and high drop-out rates. Studies tended to report mean group weight change (eg, an average weight change of -5 kg in a group of 50 participants) and not frequency of response to the interventions (eg, 15 [30%] of 50 participants achieved loss of 10% of their initial body weight). We were not able to assess differences by sex or ethnic background in response to counseling and behavioral interventions; most trials included only women and either did not specify ethnicity or involved primarily white samples. One study did examine weight loss in black and white participants; initial weight loss in the black groups was followed by rapid weight regain, so that at the end of the study, sustained weight loss was considerably greater in white participants.76 Finally, the vast majority of these trials enrolled either only obese participants or samples in which average baseline BMI was in the high-overweight range. We were not able to assess the effectiveness of interventions specifically for those who are overweight but not obese.
Our findings agreed with those of the prior systematic reviews: generally, the counseling and behavioral interventions showed small to moderate degrees of weight loss sustained over at least 1 year. In the updated searches, higher-intensity trials and combinations of interventions appeared to promote better outcomes. However, treating patients with individual- versus group-based approaches did not appear to have a strong influence on success. As previous reviews have noted, trials with follow-up beyond 1 year tended to show a loss of effect; several studies, however, have shown modest weight loss maintained at 24 to 36 months. In addition, the success of weight maintenance trials is encouraging. Weight loss methods may need to be paired with longer-term maintenance interventions for sustained improvement.
Pharmacotherapy Interventions
Prior Systematic Reviews
Pharmacological treatment options for obesity are intended either to help promote or to sustain weight loss, in coordination with lifestyle change. Drug options have changed markedly since the major prior systematic reviews for obesity. The evidence concerning sibutramine (a dopamine, norepinephrine, and serotonin re-uptake inhibitor) and orlistat (a gastrointestinal lipase inhibitor) has increased. Both are approved by the U.S. Food and Drug Administration (FDA) for weight loss and weight maintenance after prior weight loss, in persons with BMI > 30 or persons with BMI > 27 with other risk factors such as hypertension, diabetes, or dyslipidemia; they should be used in conjunction with a reduced-calorie diet. FDA approval was based upon studies of up to 2 years' duration. Because of safety concerns, dexfenfluramine, fenfluramine, phenylpropanolamine, and the combination of fenfluramine/phentermine—previously central players in the pharmacotherapy of obesity—are no longer available in the United States.
Because available medications have changed so substantially since the publication of the 3 earlier large comprehensive obesity reviews, we do not present their findings here, although we did review relevant content. For example, we reviewed the 1996 National Task Force on the Prevention and Treatment of Obesity systematic review of clinical trials up to 6 months of drugs available at that time. Arterburn and Noel had also reviewed these same articles in 2001,82 so we focused on the more recent review. In our data synthesis step, we included the findings of the systematic reviews along with the additional studies that we reviewed in detail.
Arterburn and Noel concluded that limited evidence shows that sibutramine is more effective than placebo in promoting modest weight loss (2.8–4.2 kg in 7 RCTs over 0.5–24 months) in healthy adults, and in those with controlled hypertension, but that subjects regain weight after stopping treatment.51 Orlistat was found to have a modest effect on body weight (an average of 3.5 kg loss in 10 RCTs). Both medications lacked long-term evidence of safety. Phentermine (7.4 kg average loss in 1 RCT) and mazindol (3.8 kg average loss in 1 RCT) caused modest weight loss in adults who were more than 15% overweight; again, these drugs lacked long-term evidence on safety. Small RCTs found limited and inconsistent evidence for the efficacy of diethylpropion (2 RCTs) and fluoxetine (2 RCTs) for weight loss compared with placebo.
Pharmacotherapy Intervention Studies Reviewed
We identified 13 RCTs of the efficacy of pharmacotherapy for weight loss that had been published since the 1996 USPSTF guidelines and that fit our inclusion criteria. Of these, 6 trials evaluated sibutramine,83–88 6 covered orlistat,89–94 and 1 involved metformin.95 We did not re-review any trials that Arterburn and Noel had covered.51 We added 1 medication, metformin, to the list of drugs reviewed previously, as we had located several trials addressing its use specifically for weight loss. Evidence Tables 2, 3, and 4 in Appendix B present details on trials of sibutramine, orlistat, and metformin, respectively.
Inclusion criteria for our review included (a) fair or good quality, placebo-controlled RCTs on humans; (b) body weight or BMI as a primary trial outcome; (c) trial duration of at least 6 months; (d) trial population generalizable to a typical U.S. primary care population; (e) English language; and (f) no review by Arterburn and Noel. We categorized studies as either “weight loss” (minimum of 6-month follow-up) or “weight maintenance” (minimum of 1-year follow-up after successful weight loss). Eight weight-loss trials and 3 weight-maintenance trials were judged to be of fair or good quality (1 study had both a loss and a maintenance arm).
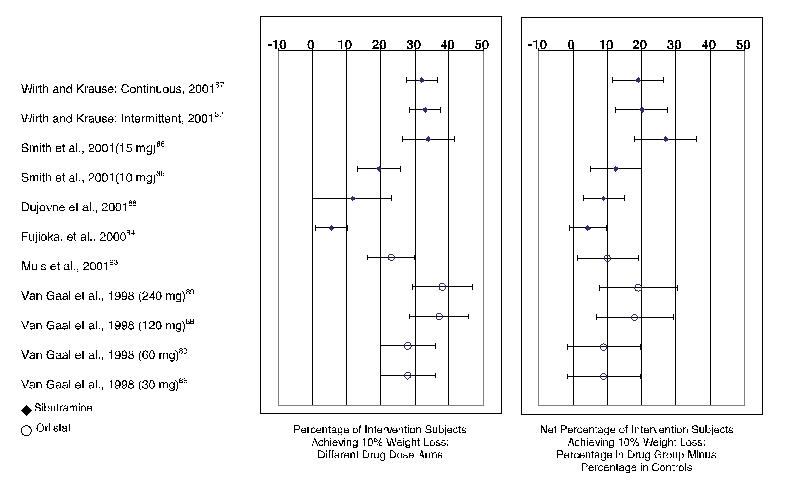
To depict the various findings from the trials focused on weight loss, we record in Figures 7 and 8 the mean weight changes and the percentage frequency of response (10% weight loss), respectively.
Pharmacotherapy Interventions for Weight Loss
Sibutramine. Of the 6 sibutramine trials (Evidence Table 2),83–88 5 concerned weight loss and lasted 6 to 12 months.84–88 Sibutramine-treated participants achieved an average of 2.8 kg to 4.8 kg more weight loss than placebo (Figure 7). Four trials recorded frequency of response; for example, 27% to 65% of sibutramine-treated patients achieved 5% weight loss and 6% to 38% of patients lost 10% of their initial body weight.84, 86–88 Sibutramine-treated participants achieved 5% of total body weight loss 19% to 37% more often than controls (depending on drug dose). As shown in Figure 8, patients on sibutramine achieved a 10% weight loss 5% to 27% more often than controls (Figure 8, right hand panel). Differences by dose of drug or mode of use (Figure 8, left-hand panel) were also of interest; however, by and large dosage did not materially affect outcomes. For instance, in 1 trial, weight loss obtained with sibutramine therapy did not differ between people treated with continuous therapy and those treated with intermittent therapy.87
Orlistat. Of the 6 orlistat trials (see Evidence Table 3),89–93, 96 we reviewed 5 assessing weight loss over 6 to 12 months duration; orlistat-treated participants treated in the normal dosing range (120 mg 3 times a day [Figure 7]) lost significantly more weight than controls: on average, 2.8 kg to 4.5 kg (or 1.2–1.5 kg/m2) more than control participants.89–93 In 1 small study (n = 55), the average weight of orlistat treated patients dropped 5.8 kg more than that of controls, but the difference was not statistically significant.94 Only 2 of the orlistat trials we reviewed reported frequency of response.89, 93 In these trials, a 10% weight loss response occurred in 23% to 38% of orlistat-treated participants depending on dose (Figure 8, left-hand panel). Between 9% and 19% more of orlistat group members were able to achieve 10% loss of their body weight than members of the control group (Figure 8, right-hand panel).
Metformin. Only 1 metformin trial met quality criteria (Evidence Table 4).95 It included patients with diabetes who had inadequate blood glucose control despite oral therapy for at least 1 year. Metformin-treated patients did no better than controls in terms of weight loss.
Pharmacotherapy Interventions for Maintenance of Weight Loss
Sibutramine. James et al reported weight change from baseline through 6 months of successful treatment with sibutramine, followed by an 18-month period of sibutramine maintenance therapy.83 Over the 2 years, sibutramine-treated participants lost, on average, 4 kg more than their placebo-treated counterparts. They were also more likely (41% of sibutramine vs 14% of placebo participants) to maintain 80% of their initial weight loss.
Orlistat. In the trial conducted by Karhunen et al, participants received 2 years of therapy: orlistat for 2 years, placebo for 2 years, or 1 year of each.91 The investigators did not record orlistat versus placebo results. Patients who were treated with orlistat for 1 or 2 years obtained “significantly more” weight loss over 2 years than participants treated with placebo. During the second year of treatment, orlistat was no more effective than placebo, on average, and discontinuing orlistat appeared to lead to excess weight regain. That is, those treated with orlistat for the first year and then changed to placebo had a mean 6.3 kg weight gain during the second year, whereas those treated with placebo throughout gained, on average, only 3 kg over the second year of the study.
In another trial, initiated after 6 months of successful dieting, participants treated with an orlistat dose of at least 60 mg 3- times a day lost more weight over 1 year and were less likely to experience marked weight regain than placebo-treated patients.90 Those treated with 120 mg 3- times daily also achieved a larger percentage of weight change and were more likely to maintain 75% of their initial weight loss than placebo-treated patients.
Summary of the Efficacy of Pharmacotherapy
Again, findings must be interpreted in light of several considerations. One is internal validity: most trials were of fair quality, although a few were judged to be of good quality. A second is the degree to which participants were overweight in the trials. These trials did not assess drug effectiveness by degree of overweight. Some included participants with baseline BMI in the high 20s, but average baseline weight was consistently in the obese range (ie, BMI ≥ 30), and pharmacotherapy for weight loss is approved only for the obese. As with pharmacotherapy trials, participants were primarily women of European origin. We evaluated several pharmacotherapy trials with relatively short-term follow-up (less than 1 year); although their findings were generally within the range seen for the longer studies, sustained weight loss was not established in so short a time.
Finally, although most trials did employ a form of intention-to-treat analysis, typically the investigators analyzed their data according to a “last observation carried forward” protocol—the final weight outcome available was used as the final weight for those participants who dropped out of the study. As weight loss tends to be maximal within the first 6 months of therapy, failure to measure body weight at the endpoint of longer trials risks overestimating the tendency towards sustained weight loss. We would prefer to review such results alongside analysis of trial “completers,” but such an approach was infrequent.
Overall, fairly long-term data for sibutramine and orlistat suggested that these drugs have modest but potentially prolonged effects. Although average weight loss was consistently modest, the percentage of patients achieving clinically significant loss (5%–10% of body weight) was frequently substantial. Weight maintenance trials suggested that prolonged drug therapy confers some benefit but that discontinuation of pharmacotherapy may lead to rapid weight regain.
Surgical Approaches
Bariatric surgical procedures are restrictive or malabsorptive in nature. The 3 techniques most commonly used in randomized trials are primarily restrictive. Gastric bypass involves complete gastric partitioning with anastomosis of the proximal gastric segment to a jejunal loop. Adjustable gastric banding involves placing an inflatable band around the stomach that can be adjusted to different diameters.97 Vertical banded gastroplasty entails partial gastric partitioning at the proximal gastric segment with placement of a gastric outlet stoma of fixed diameter.46 Although this literature still reports on this technique, clinical practice patterns appear to be shifting away from it. Gastric bypass, adjustable gastric banding, and vertical banded gastroplasty can all be performed either laparoscopically or through an open technique.
The duodenal switch procedure is a relatively new malabsorptive technique; although fairly common in clinical practice, we did not find any RCTs evaluating its effectiveness. Another malabsorptive method, jejunoileal bypass, is no longer recommended because of excessive malabsorption.47
Prior Systematic Reviews
The 3 previous obesity reviews all evaluated the impact of surgery on people with obesity. Because of practical and ethical constraints to a true randomized, blinded, placebo-controlled surgical obesity trial, high-quality evidence for obesity surgery is limited. Reviews have relied primarily on randomized unblinded trials in which neither arm was a true control (eg, comparisons between surgical techniques). Here, we summarize their findings for current procedures with at least 1 year of follow-up. The Canadian Task Force on Preventive Health Care analyzed 4 randomized trials and 1 prospective cohort study.46 In these studies, mean weight loss following surgery was 17 kg to 46 kg after 2 to 5 years. Postoperative mortality and morbidity rates were low; 1 surgery-related death occurred (0.002% of subjects), and post-operative morbidity was less than 5%. The Centre for Reviews and Dissemination from the University of York's review of 6 trials showed weight loss of 9.7 kg to 57.9 kg.47 In the NIH National Heart, Lung and Blood Institute review, 5 randomized surgical trials led to weight loss of 10 kg to 159 kg over 12 to 48 months.3
Studies of the Effectiveness of Surgical Interventions for Weight Loss
Randomized Trials. Our review of randomized trials with comparison arms included trials of fair to good quality with follow-up of at least 1 year and weight loss or BMI change as an outcome. We identified 2 trials that had not been reported by the previous systematic reviews.97, 98 Details are in Evidence Table 5. One compares laparoscopic versus open banding techniques,97 and the other, 2 laparoscopic banding techniques.98
In the de Wit et al study, 50 obese (BMI >40) patients were randomized to either open or laparoscopic adjustable silicone gastric banding.97 Both groups showed large average weight declines over 1 year: 35 kg in the laparoscopic and 34 kg in the open surgical group. Accordingly, BMI dropped from 51.3 to 39.7 in the laparoscopic group and from 49.7 to 39.1 in the open group. Complications included incisional hernias (14.3%), umbilical hernia (2%), and infection (2%); no subject died.
Weiner et al randomized 101 consecutive patients to 1 of 2 groups of laparoscopic gastric banding—retrogastric placement (RGP) or esophagogastric placement (EGP).98 Weight loss was substantial in both groups: more than 40 kg in both groups at 18 months. Post-operative complications included 3 band slippages (3%, which were subsequently corrected with additional laparoscopic surgery), 1 pouch dilation (1%), vomiting in 6 patients (6%), and dysphagia in 6 patients (6%); no patient died. Of note, results from a quality-of-life questionnaire revealed that more than 96% of participants reported excellent well-being at the 18-month follow-up.
Cohort Studies. We used well-designed cohort studies as a second form of evidence for obesity surgery. RCTs are the preferable form of evidence, but because the constraints against a large RCT for obesity surgery are extensive, this may be a situation in which carefully evaluated cohort data are appropriate. We searched for controlled cohort studies with weight loss as a primary outcome, in which the patient population was well described, and identified a single large on-going study - the Swedish Obese Subjects (SOS) study.99, 100 In addition, we examined extensive uncontrolled cohort (case series) evidence to assess the safety of obesity surgical techniques and their applicability to primary care populations.
The SOS study provides the best cohort evidence: a large ongoing multi-center trial with nonrandomized matched controls involving volunteers at 750 primary health care centers and 26 county and university hospitals in Sweden. The surgical arm is divided equally among 3 surgical techniques: gastric banding, vertical banded gastroplasty, and gastric bypass. Controls are treated with “the best non-surgical options available” (p. S3).100
Two-year data revealed weight loss of 28 kg (23% of total weight) among the surgical patients and 0.5 kg among controls. The percentages of weight reduction after gastric banding, vertical banded gastroplasty, and gastric bypass were 21%, 23%, and 33%, respectively. Eight-year follow-up data showed a 20-kg weight loss (16.5% of total weight) for 251 patients in the surgical group; over this same period, the 232 control patients gained 0.7 kg.100 The reported post-operative mortality rate was 0.2%. As in the randomized trials, morbidity was low; among 1,164 patients, complications included bleeding (0.9%), wound complications (1.8%), abdominal infection (2.1%), thromboembolic events (0.8%), pulmonary symptoms (6.2%), and miscellaneous events (4.8%).100
Summary of Effectiveness of Surgical Treatment
Overall, considering RCTs, controlled cohort studies, and uncontrolled cohort studies, the degree of weight reduction obtained with surgical interventions is consistently dramatic. Cohort evidence suggests that this weight loss may be prolonged and that it can be achieved in patient populations with multiple comorbidities.
We must, however, note some limitations to this literature. Surgical data are limited by the lack of placebo-controlled RCT evidence, and the internal validity of the trials we reviewed was of only fair quality. Again, the investigators tended to report average treatment effect, rather than frequency of response.
Finally, obesity surgery has been performed for only a select group of patients; the NIH obesity panel recommends it only for those people with a BMI >40 or a BMI of 35 to 40 with at least 1 obesity-related comorbidity.3 National data indicate that 5% to 6% of the general population has a BMI in this range,12 so a substantial number of people may meet these criteria.
Key Question No. 4b: Do Interventions Improve Other Intermediate Health Outcomes?
Prior reviews have established that counseling-based weight loss can improve various intermediate health outcomes (in the analytic framework in Chapter 2, Figure 5, these are maintenance of weight loss, blood pressure, gylcemic control, and serum lipids). In this review, we did not attempt to re-establish either this link or that between our intermediate health outcomes and final health outcomes. The NIH review found that weight loss by lifestyle modifications reduced blood pressure in overweight nonhypertensive patients.3 Likewise, multiple trials of weight loss via lifestyle intervention in hypertensive patients - generally achieving 5 kg to 10 kg weight loss - led to reductions in systolic and diastolic blood pressure (3–7 mm Hg, and 3 mm Hg, respectively), less incident hypertension, and few antihypertensive medications. Only 1 RCT producing weight loss in hypertensive patients reported no significant change in blood pressure.
Trials reviewing weight loss in a similar range reduced serum triglycerides, increased high-density lipoprotein (HDL) cholesterol, and reduced low-density lipoprotein (LDL) cholesterol. In addition, weight loss from lifestyle modification lowered blood glucose levels in overweight and obese persons without diabetes and reduced blood glucose and hemoglobin A1c (HbA1c) in some subjects with diabetes.
Because pharmacotherapy can potentially alter body chemistry and thus affect physiology differently from other means of weight loss, we did assess new data looking specifically at the ability of drug-induced weight loss to affect intermediate health outcomes. Health outcomes for pharmacotherapy are limited to data from trials lasting no more than 2 years. Three trials assessed the effect of sibutramine treatment on HbA1c.83–85 Two, lasting 24 to 104 weeks, found no significant drug versus placebo effect;83, 84 the third found that sibutramine-treated participants, over 24 weeks, had a 2.2% greater decline in HbA1c than placebo.85 Three trials examined fasting or nonfasting serum glucose in relation to sibutramine therapy;83, 84, 86 none found a significant difference compared with placebo.
Orlistat therapy was linked to slightly lower total cholesterol after 24 to 52 weeks: 8% lower in 2 trials,90, 93 and 2.7mg/dL to 8.5 mg/dL (0.07–0.22 mmol/L) lower in another.92 Sibutramine showed less consistent total cholesterol findings. Three trials found no significant drug versus placebo effect;83, 84, 86 another found a 20.4 mg/dL improvement over 24 weeks.85
Sibutramine and orlistat showed inconsistent effects on triglycerides83, 84, 86, 88, 92 and on HDL.85, 88 One trial found a significant decrease in HDL cholesterol with orlistat therapy (1.5–1.9 mg/dL with >90 mg daily dose),90 and another a 3.49% increase in HDL cholesterol.92 Two trials found a significant increase in HDL cholesterol (5 mg/dL in one and 2.1 mg/dL in the other) with sibutramine,83, 88 and 2 others reported no significant effect.84, 85 Orlistat was associated with reductions in LDL cholesterol (3.1–10 mg/dL reduction vs placebo) in 1 trial90 and a 10% reduction in another.93 Sibutramine, in 4 different trials, had no effect on LDL cholesterol.83–85, 88 Overall, we found mixed evidence of improvements of secondary health outcomes among the relatively short-term pharmacotherapy trials reviewed here; this inconsistency may be attributable to altered metabolism by medications or to inadequate length of follow-up.
In surgical cohort data, substantial weight loss was linked with dramatic improvements of intermediate health outcomes. In one cohort of 330 patients with baseline glucose abnormalities (half with impaired glucose tolerance and half with type 2 diabetes), 90% complete postsurgical follow-up revealed normal fasting blood glucose and glycosylated hemoglobin in 91% of subjects.101 Long-term follow-up (>5 years) of a group of 109 surgical patients found the rate of development of diabetes to be 0.15 cases per 100 person years; in 27 controls (patients who qualified for surgery but subsequently declined the procedure), the rate was 2.72 cases per 100 person years.102 Marked improvements in lipid profiles have also been noted.103 In the SOS,104 participants were initially matched on age, sex, height, weight, waist, blood pressure, cholesterol and triglyceride levels, and diabetes. In the 2-year follow-up, the investigators reported lower odds of hypertension (odds ratio [OR], 0.38; CI, 0.22–0.65), diabetes (OR, 0.02; CI, 0.00–0.16), hypertriglyceridemia (OR, 0.10; CI, 0.04–0.25), and low HDL cholesterol (OR, 0.28; CI, 0.16–0.49) in the surgical patients compared with the controls. However, blood pressure slowly returned to levels similar to the control group. Thus, they did not report a long-term improvement in blood pressure.105
Key Question No. 5: Do Interventions Improve Final Health Outcomes
To examine the question of effects of sustained weight loss, we divided our analysis of health effects into intermediate outcomes (discussed above) and final health outcomes. Even moderate intentional weight loss (5%–10% of initial body weight) has been shown to reduce the severity of comorbidities associated with obesity. Limited observational data suggest that intentional weight loss in the obese can lead to reduced mortality.106, 107
We looked for new evidence that weight loss can affect mortality, morbidity, mental health, and daily functioning. Two recent trials provide strong evidence that behaviorally mediated weight loss can prevent diabetes.66, 79 We found very limited evidence evaluating other outcomes in the setting of modest controlled weight loss: 1 trial evaluating 2 types of behavioral therapy showed borderline improved self-esteem in both treatment groups.73 The National Task Force on the Prevention and Treatment of Obesity found that dieting did not lead to problems in psychological function or eating disorders.60 The health benefits of weight loss in overweight patients are less clear.
Key Question No. 6: What are the Harms of Screening and Treatment?
Screening or Counseling and Behavioral Interventions
We did not find studies evaluating the harms of screening or of counseling and behavioral interventions. Nonetheless, a potential risk does exist, particularly as the stigma of obesity is well established.
Medications
In their review of sibutramine harms, Arterburn and Noel found that common adverse effects occurred somewhat more frequently in drug than control arms (10%–30% vs 8%–19%), but no serious adverse events were reported.51 They noted reports of mean increases in systolic and diastolic blood pressure (1–3 mm Hg) and heart rate (4–5 beats per minute); in people with controlled hypertension, the incidence of clinically significant increases in blood pressure was similar in intervention and control participants. They also found a 3.9% dropout for hypertension in the sibutramine trials they reviewed.
Among the sibutramine trials that we reviewed, several showed high dropout rates; however, attrition was generally lower for sibutramine patients than for those on placebo. Attrition attributable to an adverse event was almost identical in drug and placebo arms in 5 sibutramine trials;83, 84, 86–88 it was not noted in the sixth.85
Even though reports of adverse events were common, when rates were specified they tended not to differ between drug and placebo participants. The most frequent side effects included insomnia, nausea, hypertension, dry mouth, dizziness, and confusion; side effects were usually not severe. The most concerning side effect is hypertension; the average increase in blood pressure was small: 0 mm Hg to 3.5 mm Hg higher in drug-treated patients in 3 studies,86–88 2.1 mm Hg higher in a fourth,84 and 5% higher in a fifth.83 Two studies reported dropout because of hypertension, but it did not differ between drug and placebo arms.84, 88 One study noted that an average increase of 6.7 beats per minute in pulse rate was associated with sibutramine.88
Orlistat adverse effects are primarily gastrointestinal. Arterburn and Noel found that 22% to 27% of orlistat-treated participants reported oily spotting, flatulence, and fecal urgency (vs 1%–7% of control participants).51 Of the RCTs they reviewed, 4 showed an increased incidence of vitamin deficiency with orlistat treatment; 1 suggested reduced intestinal absorption of contraceptive pills with this medication. In addition, in 1 RCT of participants with coronary heart disease risk factors, more serious adverse events (10% vs 2.6%; unidentified in nature) occurred with orlistat than placebo.
In the orlistat trials we reviewed, attrition rates were generally similar (within 5%) between drug and placebo arms. Dropout because of an adverse event generally was slightly more common (0%–7%) in orlistat-treated participants, although in 1 group it was an additional 12%.90
In the 3 trials that reported overall adverse event rates, 10% to 18% more of the drug-treated participants reported problems than did those in the placebo groups.89, 92, 93 The most common side effects were gastrointestinal (flatus, abdominal pain, fecal urgency): 14% to 37% more orlistat patients than controls reported the problems in the 3 studies, but they were usually mild. One trial reported the frequency of severe gastrointestinal events to be 1% to 9% more in drug-treated participants versus placebo.89 Two studies noted that orlistat led to gastrointestinal disturbance leading to attrition in 1% to 10% of participants.
Adverse effects of other obesity medications may be of concern. Arterburn and Noel's review of harms of pharmacotherapy found no evidence of serious adverse reactions for phentermine, but unclear incidence rates of potentially serious side effects for mazindol and diethylpropion (pulmonary hypertension [mazindol and diethylpropion] and psychosis [mazindol]).51 They found that fluoxetine has been linked with gastrointestinal symptoms, sleep disturbance, sweating, tremor, amnesia, and thirst, and systematic review evidence of 10% to 15% incidence of anxiety, diarrhea, dry mouth, headache, and nausea.51 The single metformin trial we reviewed did not provide enough information to evaluate its profile of side effects.95
Surgical Approaches
We evaluated multiple surgical cohort studies, with follow-up of at least 1 year, to assess adverse effects. Adverse outcome data included 2 series in which patients had substantial comorbidity,108, 109 and multiple studies in which a modest degree of comorbidity was present. Generally, mortality rates were very low. In 12 cohorts treated with vertical banded gastroplasty, mortality ranged from 0% to 1.5%; if these rates were pooled, this represents a total of 3 deaths in 1,165 patients.108–119 Two of those studies included patients with substantial comorbid conditions,108, 109 as did 2 of the gastric bypass cohorts.109, 120 Among 9 cohorts of gastric bypass patients, mortality was 0% to 1.5% (10 deaths in 1,397 patients).101, 113, 120–126 Adjustable gastric banding mortality was similarly low: 0% to 1.6% in 16 cohorts.119, 127–141
Vertical banded gastroplasty was associated with several types of complications. Re-operation rate was reported to be 20% to 25% over 3 to 5 years.112, 115 Wound infection was noted in 8% to 32% of patients in 3 studies.109, 112, 113 Less frequent adverse events (<6%) included gastric leaks, stomal stenosis, and pouch dilatations. In gastric bypass patients, wound infection was reported in 8% to 20% of patients.113, 124, 125 Staple failure occurred in 15% of patients in 1 study with 14 years of follow-up;101 the same cohort developed vitamin B12 deficiency in 40% of participants. Other notable morbidity included diarrhea in 13% of 1 group125 and gastrointestinal hemorrhage in 3% of another.113 In patients undergoing adjustable gastric banding, morbidity was often from re-operation (1%–20%),127, 130, 133–136, 140, 142 band dislocation, leakage, or slippage (0.4%–8%).128–130, 132, 133, 135–137, 142
In addition to these procedure-specific adverse consequences, surgical patients require long-term follow-up and multivitamin supplementation. We found no evidence of psychological harms, but there is a suggestion of psychological benefit to weight reduction in surgery studies.
Figures
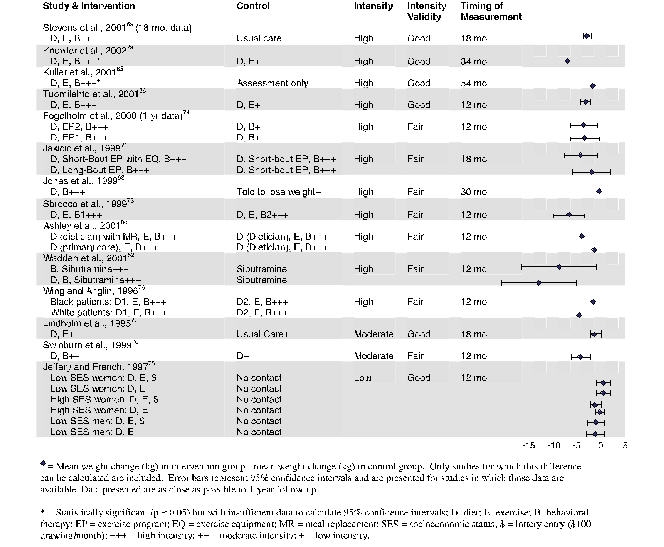
Figure 6. Differences in Mean Weight Between Intervention and Control Groups for Counseling and Behavioral Interventions

Figure 7. Differences in Mean Weight Between Intervention and Control Groups for Pharmacotherapy Interventions