A new version of this publication is available.
This publication is provided for historical reference only and the information may be out of date.
3.1. Surgical site infection risk factors: epidemiology and burden worldwide
Background
SSIs are potential complications associated with any type of surgical procedure. Although SSIs are among the most preventable HAIs (1, 2), they still represent a significant burden in terms of patient morbidity and mortality and additional costs to health systems and service payers worldwide (3–11). SSI is both the most frequently studied and the leading HAI reported hospital-wide in LMICs (3, 4). For these reasons, the prevention of SSI has received considerable attention from surgeons and infection control professionals, health care authorities, the media and the public. In particular, there is a perception among the public that SSIs may reflect a poor quality of care (12). The aim of this review is to provide an update of the global data on SSI with a special focus on LMICs, notably to assess infection rates, associated risk factors and the economic burden.
Summary of the available evidence
1. Burden of SSI
a. Evidence from high-income countries
i. USA
In 2010, an estimated 16 million surgical procedures were performed in acute care hospitals in the USA (13). In a recent report on the rates of national and state HAIs based on data from 2014, 3654 hospitals reported 20 916 SSI among 2 417 933 surgical procedures performed in that year (5).
Of note, between 2008 and 2014 there was an overall 17% decrease in SSI in the 10 main surgical procedures. As an example, there was a decrease of 17% in abdominal hysterectomy and 2% in colon surgery (5).
By contrast, a multi-state HAI prevalence survey conducted in 2011 estimated that there were 157 000 SSIs related to any inpatient surgery and SSI was ranked as the second most frequently reported HAI between 2006 and 2008 (14). Another study reported data from the National Healthcare Safety Network (NHSN) between 2006 and 2008 that included 16 147 SSIs following 849 659 surgical procedures across all groups, representing an overall SSI rate of 1.9% (15).
AMR patterns of HAI in the USA have been described (16) and compared to a previous report (17). Among the 1029 facilities that reported one or more SSI, Staphylococcus aureus was the most commonly reported pathogen overall (30.4%), followed by coagulase-negative staphylococci (11.7%), Escherichia coli (9.4%) and Enterococcus faecalis (5.9%). Table 3.1.1 summarizes the distribution of the top seven reported pathogens (16).
Table 3.1.1
Distribution and percentage of pathogenic isolates associated with SSI and resistant to selected antimicrobial agents, NHSN, 2009–2010.
To investigate the costs of SSI, a study used the 2005 hospital stay data from the US Nationwide Inpatient Sample, which represents 1054 hospitals from 37 states. Extra hospital stay attributable to SSI was 9.7 days with increased costs of US$ 20 842 per admission. From a national perspective, SSI cases were associated with 406 730 extra hospital days and hospital costs exceeding US$ 900 million. An additional 91 613 readmissions for the treatment of SSI accounted for a further 521 933 days of care at a cost of nearly US$ 700 million (18).
Applying two different consumer price index adjustments to account for the rate of inflation in hospital resource prices, the Centers for Disease Control and Prevention estimated that the aggregate attributable patient hospital costs for SSI infection ranged between US$ 1087 and US$ 29 443 per infection (adjusted for the 2007 US$ level). Using the consumer price index for urban consumers and inpatient hospital services, SSI is considered as the HAI with the largest range of annual costs (US$ 3.2–8.6 billion and US$ 3.5–10 billion, respectively) (19).
ii. European countries
The European point prevalence survey of HAIs and antimicrobial use conducted in 2011–2012 showed that SSIs are the second most frequent HAI in hospitals (20). A recent report from the ECDC on SSI surveillance of SSI provided data for 2010 and 2011 (6) from 20 networks in 15 European Union countries and one European Economic Area country using a standardized protocol (21). Hip prosthesis was the most frequently reported surgical procedure and represented 33% of all operations. The cumulative incidence of patients with SSI was the highest in colon surgery with 9.5% (episodes per 100 operations), followed by 3.5% for coronary artery bypass graft, 2.9% for caesarean section, 1.4% for cholecystectomy, 1.0% for hip prosthesis, 0.8% for laminectomy and 0.75% for knee prosthesis (6). The results showed also decreasing trends in SSI incidence in several types of procedure (caesarean section, hip prosthesis and laminectomy) (Figure 3.1.1), thus suggesting that prevention efforts, including surveillance, were successful in participating hospitals (6, 22).
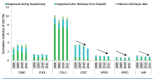
Figure 3.1.1
Cumulative incidence for SSI by year and type of procedure: European Union/European Economic Area countries, 2008–2011. Data source: ECDC, HAI-Net SSI patient-based data 2008–2011 (http://ecdc.europa.eu/en/activities/surveillance/Pages/data-access.aspx#sthash.hHYRJ9ok.dpuf, (more...)
A study published in 2004 reviewed data from 84 studies and estimated the economic costs of SSIs in Europe to range between € 1.47–19.1 billion. It predicted also that the average patient stay would increase by approximately 6.5 days and cost 3 times as much to treat an infected patient. The analysis suggested that the SSI-attributable economic burden at that time was likely to be underestimated (10).
In France, it was estimated that 3% of surgical procedures resulted in infection for a total annual cost of nearly € 58 million. Moreover, patients who experienced SSI had a significantly increased mortality risk (from 4- to 15-fold) and a 3-fold increased length of hospital stay (23).
The prevalence of SSI in Switzerland was reported to be 5.4% in a study conducted in 50 acute care hospitals participating in the Swiss Nosocomial Infection Prevalence surveillance programme (24). Another study described a 13-year multicentre SSI surveillance scheme performed from 1998 to 2010. Reported SSI rates were: 18.2% after 7411 colectomies; 6.4% after 6383 appendicectomies; 2.3% after 7411 cholecystectomies; 1.7% after 9933 herniorrhaphies; 1.6% after 6341 hip arthroplasties; and 1.3% after 3667 knee arthroplasties (25).
In Italy, the SSI rate reported by the “Sistema Nazionale di Sorveglianza delle Infezioni del Sito Chirurgico” (national SSI surveillance system) from 355 Italian surgical wards between 2009 and 2011 was 2.6% episodes per 100 procedures (1628 cases/60 460 procedures); 60% of SSIs were diagnosed through 30-day post-discharge surveillance. SSI rates were higher in colon (9.0%) and rectal surgery (7.0%), laparotomy (3.1%) and appendectomy (2.1%) (26).
In England, the most recent summary of data collected by National Health Service hospitals reported cumulative SSI rates from January 2008 to March 2013. The highest rate was reported among large bowel surgery (8.3%; 95% CI: 7.9–8.7 per 1000 inpatient days), followed by small bowel surgery (4.9%; 95% CI: 4.3–5.7), bile duct, liver and pancreatic surgery (4.9%; 95% CI: 4.1–5.9) and cholecystectomy (4.6%; 95% CI: 3.1–6.6). The lowest rate was reported for knee prosthesis (0.4%; 95% CI: 0.3–0.4) (8).
Data collected from April 2010 to March 2012 estimated that the median additional length of stay attributable to SSI was 10 days (7–13 days), with a total of 4694 bed-days lost over the 2-year period (27).
iii. Australia
A study evaluated the time trends in SSI rates and pathogens in 81 Australian health care facilities participating in the Victorian Healthcare Associated Infection Surveillance System. A total of 183 625 procedures were monitored and 5123 SSIs were reported. S. aureus was the most frequently identified pathogen, and a statistically significant increase in infections due to ceftriaxone-resistant E. coli was observed (relative risk: 1.37; 95% CI: 1.10–1.70) (9).
iv. Japan
Data from the Japan nosocomial infection surveillance system showed that 470 hospitals voluntarily participated in SSI surveillance in 2013 (28, 29). A retrospective study evaluated also the influence of SSI on the postoperative duration of hospitalization and costs between 2006 and 2008 after abdominal or cardiac surgery. Overall, the mean postoperative hospitalization was 20.7 days longer and the mean health care expenditure was US$ 8791 higher in SSI patients. SSI in abdominal surgery extended the average hospitalization by 17.6 days and increased the average health care expenditure by US$ 6624. Among cardiac surgical patients, SSI extended the postoperative hospitalization by an average of 48.9 days and increased health care expenditure by an average of US$ 28 534 (30). A recent study assessed SSI rates and risk factors after colorectal surgery using the Japan nosocomial infection surveillance system national database. The cumulative incidence of SSI for colon and rectal surgery was 15.0% (6691/44 751 procedures) and 17.8% (3230/18 187 procedures), respectively (31).
v. Republic of Korea
A prospective multicentre surveillance study published in 2000 concluded that SSI constituted 17.2% of all HAIs reported from 15 acute care hospitals (32, 33). The 2009 national SSI surveillance system report described the incidence and risk factors for SSI in 7 types of procedures. The SSI rate per 100 operations was 3.68% (22/1169) after craniotomies, 5.96% (14/235) for ventricular shunt operations, 4.25% (75/1763) for gastric operations, 3.37% (22/653) for colon surgery, 5.83% (27/463) for rectal surgery, 1.93% (23/1190) for hip joint replacement and 2.63% (30/1139) for knee joint replacement (34).
A web-based surveillance of SSIs was performed between 2010 and 2011 to determine the incidence of SSIs after 15 surgical procedures in 43 hospitals. The overall SSI rate represented 2.10% of the total of 18 644 operations and differed after various types of surgery (35). In addition, a systematic review of the literature published between 1995 and 2010 on the epidemiological and economic burden of SSI in the Republic of Korea reported an overall incidence of SSI ranging from 2.0% to 9.7% (36). SSIs were associated with increased hospitalization costs and each episode of SSI was estimated to cost around an additional 2 000 000 Korean Republic won (approximately US$ 1730). Postoperative stays for patients with SSIs were 5 to 20 days longer (36).
In a recent study conducted between 2008 and 2012, the SSI rate following gastrectomy was 3.12% (522/16 918), 2.05% (157/7656) for total hip arthroplasty and 1.90% (152/7648) for total knee arthroplasty. There was a significant trend of decreased crude SSI rates over 5 years (37).
vi. Gulf Council Countries
We were not able to retrieve published national data on SSI rates from any of the Gulf Council Countries (Bahrain, Kingdom of Saudi Arabia, Kuwait, Oman, Qatar and the United Arab Emirates). However, in Saudi Arabia, a 5-year analysis of SSI in orthopaedic surgery in one hospital estimated a rate of 2.55% (38). Another study from the King Abdulaziz Medical City (Saudi Arabia) compared SSI rates for herniorraphy and cholecystectomy in 2007 to 1999–2000. In 2007, SSI rates per 100 operations in 2007 were 0.88% for herniorrhaphy and 0.48% for cholecystectomy, while in 2007, rates were reduced by 80% for herniorrhaphy (P=0.049) and 74% for cholecystectomy (P=0.270) (39).
vii. Singapore
In a systematic literature review (2000 to 2012) (40) of the burden of HAI in South-East Asia, the pooled incidence of SSI was 7.8% (95% CI: 6.3–9.3). A study conducted between January and March 2008 in a tertiary care hospital in Singapore reported an SSI incidence of 8.3% for general, neurologic and orthopaedic surgical procedures (41).
viii. Uruguay
The national incidence data on SSI for 2012–2013 reported that the incidence rate for appendectomy was 3.2%, 2.5% for cardiac surgery, 6.2% for cholecystectomy and 15.4% for colon surgery (42).
ix. Chile
The 2013 national report on HAI surveillance showed a SSI rate of 3.09% for coronary bypass surgery and 1.89% for hip joint replacement. Infection rates in cholecystectomy performed via laparotomy were 4.12% (95% CI: 2.8–6.11) times higher than laparoscopic cholecystectomy (P<0.0001) (43).
b. WHO systematic reviews on SSI in LMICs
The WHO report on the global burden of endemic HAI provided SSI data from LMICs. The pooled SSI incidence was 11.8 per 100 surgical patients undergoing surgical procedures (95% CI: 8.6–16.0) and 5.6 per 100 surgical procedures (95% CI: 2.9–10.5). SSI was the most frequent HAI reported hospital-wide in LMICs and the level of risk was significantly higher than in developed countries (3, 4).
Recently, WHO conducted an update of the systematic literature review of from 1995 to 2015 with a special focus on SSI in LMICs (WHO unpublished data). A total of 231 articles in English, French, German, Spanish and Portuguese were included. The pooled SSI rate was 11.2 per 100 surgical patients (95% CI: 9.7–12.8) for incidence/prospective studies. There was no statistical difference in SSI rates when stratified by study quality, patient age groups, geographic regions, country income, SSI definition criteria, type of setting or year of publication. However, there were statistical differences between studies according to the type of surgical population procedures (P=0.0001) and the number of patients per study (P=0.0004).
In incidence studies, the SSI rate was higher for procedures in oncology (17.2%; 95% CI: 15.4–19.1), orthopaedic (15.1%; 95% CI: 10.2–20.6), general surgery (14.1%; 95% CI: 11.6–16.8) and paediatric surgery (12.7%; 95% CI: 6.7–20.3). The SSI rate expressed as the number of SSI infections per 100 surgical operations was reported in 57 (24.7%) studies. The pooled SSI rate using this measure was 6.1% (95% CI: 5.0–7.2) for incidence/prospective studies (Figure 3.1.2).
Table 3.1.2
Summary of SSI rates in different countries.
Some studies (44–50) investigated SSI rates after caesarean section surgery and showed a substantial variability in the definition of SSI and in reported rates. High rates of SSI following caesarean section were reported in several LMICs: 16.2% in a study from Nigeria (44), 19% from Kenya (45), 10.9% from Tanzania (46) and 9.7% by Viet Nam (47). In 2 studies from Brazil, one reported a rate of 9.6% (48) and the other a higher rate of 23.5% (49). In comparison, a much lower average SSI rate of 2.9% is reported in Europe (6, 21).
2. Factors increasing the risk of SSI
Many factors influence surgical wound healing and determine the potential for infection (51). These include patient-related (endogenous) and process/procedural-related (exogenous) variables that affect a patient’s risk of developing an SSI. Some variables are obviously not modifiable, such as age and gender. However, other potential factors can be improved to increase the likelihood of a positive surgical outcome, such as nutritional status, tobacco use, correct use of antibiotics and the intraoperative technique.
The usefulness of risk assessment and the definition of risk is debatable as there are very few studies that have an altered patient outcome based on information gained by risk assessment (52, 53). One study analysed a 2-year data report of the NHSN for all surgical procedures and used stepwise logistic regression to develop specific risk models by procedure category. The study concluded that a set of new models using existing data elements collected through the NHSN improved predictive performance, compared to the traditional NHSN risk index stratification (15).
A systematic review of 57 studies from both high-income countries and LMICs identified the following factors associated with an increased risk of SSI in adjusted analysis: a high body mass index; a severe score according to the US National Nosocomial Infections Surveillance (NNIS) risk index; severe wound class; diabetes; and a prolongation of surgery duration (54). A meta-analysis of prospective cohort studies suggested that diabetes mellitus is significantly associated with an increased risk of SSI (55). The national nosocomial surveillance system protocol in Italy identified a longer duration of surgery, an American Society of Anesthesiologists score of at least 3 and a pre-surgery hospital stay of at least 2 days as factors associated with an increased risk of SSI, while videoscopic procedures reduced SSI rates (26). In the Republic of Korea, a systematic review of the epidemiological and economic burden identified diabetes, the absence or >1 hour administration of antibiotic prophylaxis and the type of wound classification (contaminated or dirty) as risk factors significantly associated with SSI by multivariate analyses (36). In addition, the NNIS risk index identified trauma, re-operation and age (60–69 years) as risk factors for SSI after total hip arthroplasty (37).
In a recent unpublished systematic review conducted by WHO, a total of 14 observational studies (no RCTs) (56–69) describing the relationship between surgical volume and the risk of SSI were identified. There was a substantial heterogeneity in the definitions of volume, surgical procedures studied and SSI measurement. Thus, separate meta-analyses were performed to evaluate SSI rates between high vs. low and medium vs. low hospital volume, and high vs. low and medium vs. low surgeon volume. A moderate quality of evidence showed that surgical procedures performed in high-/medium-volume hospitals have lower SSI rates compared to low-volume hospitals (OR: 0.69; 95% CI: 0.55–0.87 and OR: 0.80; 95% CI: 0.69–0.94, respectively). In addition, there was a moderate quality of evidence that surgical procedures performed by high- or medium-volume surgeons have lower SSI rates (OR: 0.67; 95% CI: 0.55–0.81 and OR: 0.73; 95% CI: 0.63–0.85, respectively) compared to low-volume hospitals. However, there was controversial evidence when high- and medium-volume hospitals were compared and it remains unclear whether there is a linear relationship between procedure/surgeon volume and the SSI rate.
Conclusions
Despite robust data on the burden of SSI in some countries or regions, accurate estimates of the global burden in terms of SSI rates and the economic aspects still remain a goal for the future. As an example, SSI and overall HAI data are not yet included in the list of diseases for which the global burden is regularly estimated by WHO or other international organizations gathering data on global health. Although SSI rates vary between countries and geographical regions, they represent an important problem, with a significantly higher burden in developing countries. If SSI rates are to serve as a quality indicator and comparison benchmark for health care facilities, countries and the public, they must be determined in a reliable way that produces robust infection rates to ensure valid comparisons. There is a global need to address changes to SSI definitions, strengthen and validate SSI data quality, and to conduct robust SSI economic and burden studies.
References
- 1.
- Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121(2):182–205. [PubMed: 4014115]
- 2.
- Harbarth S, Sax H, Gastmeier P. The preventable proportion of nosocomial infections: an overview of published reports. J Hosp Infect. 2003;54(4):258–66. quiz 321. [PubMed: 12919755]
- 3.
- Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–41. [PubMed: 21146207]
- 4.
- Report on the burden of endemic health care-associated infection worldwide A systematic review of the literature. Geneva: World Health Organization; 2011. [accessed 10 August 2016]. http://apps
.who.int/iris /bitstream/10665 /80135/1/9789241501507_eng.pdf. - 5.
- National and state healthcare-associated infections progress report. Atlanta (GA): National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention; 2016. [accessed 10 August 2016]. http://www
.cdc.gov/HAI /pdfs/progress-report /hai-progress-report.pdf. - 6.
- Surveillance of surgical site infections in Europe 2010–2011. Stockholm: European Centre for Disease Prevention and Control; 2013. [accessed 10 August 2016]. http://ecdc
.europa.eu /en/publications/Publications /SSI-in-europe-2010-2011.pdf. - 7.
- English national point prevalence survey on healthcare associated infections and antimicrobial use, 2011. Preliminary data. London: Health Protection Agency; 2012.
- 8.
- Surveillance of surgical site infections in NHS hospitals in England (2012/13). London: Public Health England; 2013.
- 9.
- Worth LJ, Bull AL, Spelman T, Brett J, Richards MJ. Diminishing surgical site infections in Australia: time trends in infection rates, pathogens and antimicrobial resistance using a comprehensive Victorian surveillance program, 2002–2013. Infect Control Hosp Epidemiol. 2015;36(4):409–16. [PubMed: 25782895]
- 10.
- Leaper DJ, van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, et al. Surgical site infection - a European perspective of incidence and economic burden. Int Wound J. 2004;1(4):247–73. [PMC free article: PMC7951634] [PubMed: 16722874]
- 11.
- Humphreys H. Preventing surgical site infection. Where now? J Hosp Infect. 2009;73(4):316–22. [PubMed: 19700219]
- 12.
- Birgand G, Lepelletier D, Baron G, Barrett S, Breier A-C, Buke C, et al. Agreement among healthcare professionals in ten European countries in diagnosing case-vignettes of surgical site infections. PLoS One. 2013;8(7):e68618. [PMC free article: PMC3706413] [PubMed: 23874690]
- 13.
- Surgical site infection (SSI) event. Atlanta (GA): Centers for Disease Control and Prevention; 2016. [accessed 10 August 2016]. www
.cdc.gov/nhsn/PDFs /pscManual/9pscSSIcurrent.pdf. - 14.
- Magill SSEJ, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med. 2014;370(13):1198–208. [PMC free article: PMC4648343] [PubMed: 24670166]
- 15.
- Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol. 2011;32(10):970–86. [PubMed: 21931247]
- 16.
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. [PubMed: 23221186]
- 17.
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. [PubMed: 18947320]
- 18.
- de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–97. [PubMed: 19398246]
- 19.
- Scott RD. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. Atlanta (GA): Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, Centers for Disease Control and Prevention; 2009. [accessed 10 August 2016]. http://www
.cdc.gov/HAI /pdfs/hai/Scott_CostPaper.pdf. - 20.
- European Centre for Disease Prevention and Control. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals. Stockholm: European Centre for Disease Prevention and Control; 2013. [accessed 10 August 2016]. http://www
.ecdc.europa .eu/en/publications /Publications/Forms/ECDC_DispForm .aspx?ID=1155. - 21.
- Surveillance of surgical site infections in European hospitals – HAISSI protocol. Stockholm: European Centre for Disease Prevention and Control; 2012. [accessed 10 August 2016]. http://ecdc
.europa.eu /en/publications/Publications /120215_TED_SSI_protocol.pdf. - 22.
- Cosgrove SE, Ristaino P, Caston-Gaa A, Fellerman DP, Nowakowski EF, Carroll KC, et al. Caveat emptor: the role of suboptimal bronchoscope repair practices by a third-party vendor in a pseudo-outbreak of pseudomonas in bronchoalveolar lavage specimens. Infect Control Hosp Epidemiol. 2012;33(3):224–9. [PubMed: 22314057]
- 23.
- Lamarsalle L, Hunt B, Schauf M, Szwarcensztein K, Valentine WJ. Evaluating the clinical and economic burden of healthcare-associated infections during hospitalization for surgery in France. Epidemiol Infect. 2013;141(12):2473–82. [PMC free article: PMC3821401] [PubMed: 23445665]
- 24.
- Sax H, Uçkay I, Balmelli C, Bernasconi E, Boubaker K, Mühlemann K, et al. Overall burden of healthcare-associated infections among surgical patients: results of a national study. Ann Surg. 2011;253(2):365–70. [PubMed: 21217517]
- 25.
- Staszewicz W, Eisenring MC, Bettschart V, Harbarth S, Troillet N. Thirteen years of surgical site infection surveillance in Swiss hospitals. J Hosp Infect. 2014;88(1):40–7. [PubMed: 25063012]
- 26.
- Marchi M, Pan A, Gagliotti C, Morsillo F, Parenti M, Resi D, et al. The Italian national surgical site infection surveillance programme and its positive impact, 2009 to 2011. Euro Surveill. 2014;19(21) 20815. [PubMed: 24906378]
- 27.
- Jenks PJ, Laurent M, McQuarry S, Watkins R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J Hosp Infect. 2014;86(1):24–33. [PubMed: 24268456]
- 28.
- Ministry of Health, Labour and Welfare. Japan nosocomial infections surveillance. 2016. [accessed 10 August 2016]. https://www
.nih-janis .jp/english/about/index.html. - 29.
- Morikane K, Konishi T, Harihara Y, Nishioka M, Kobayashi H. Implementation and establishment of nationwide surgical site infections surveillance in Japan. Am J Infect Control. 2005;33(5):e175–e6.
- 30.
- Kusachi S, Kashimura N, Konishi T, Shimizu J, Kusunoki M, Oka M, et al. Length of stay and cost for surgical site infection after abdominal and cardiac surgery in Japanese hospitals: multi-center surveillance. Surg Infect (Larchmt). 2012;13(4):257–65. [PubMed: 22871224]
- 31.
- Morikane K, Honda H, Yamagishi T, Suzuki S, Aminaka M. Factors associated with surgical site infection in colorectal surgery: the Japan nosocomial infections surveillance. Infect Control Hosp Epidemiol. 2014;35(6):660–6. [PubMed: 24799642]
- 32.
- Kim JM, Park ES, Jeong JS, Kim KM, Kim JM, Oh HS, et al. Multicenter surveillance study for nosocomial infections in major hospitals in Korea. Nosocomial Infection Surveillance Committee of the Korean Society for Nosocomial Infection Control. Am J Infect Control. 2000;28(6):454–8. [PubMed: 11114615]
- 33.
- Park SJ, Lee KY, Park JW, Lee JG, Choi HJ, Chun HK, et al. A preliminary study for the development of indices and the current state of surgical site infections (SSIs) in Korea: the Korean Surgical Site Infection Surveillance (KOSSIS) program. Ann Surg Treat Res. 2015;88(3):119–25. [PMC free article: PMC4347042] [PubMed: 25741490]
- 34.
- Kim YK, Kim HY, Kim ES, Kim HB, Uh Y, Jung SY, et al. Korean surgical site infection surveillance system report, 2009. Korean J Nosocomial Infect Control. 2010;15(1):1–13.
- 35.
- Kim YK, Kim HY, Kim ES, Kim HB, Jin HY, Lee JY, et al. Korean surgical site infection surveillance system report: data summary from July 2010 through June 2011. Korean J Nosocomial Infect Control. 2012;17(1):1–12.
- 36.
- Lee KY, Coleman K, Paech D, Norris S, Tan JT. The epidemiology and cost of surgical site infections in Korea: a systematic review. J Korean Surg Soc. 2011;81(5):295–307. [PMC free article: PMC3228997] [PubMed: 22148121]
- 37.
- Choi HJ, Adiyani L, Sung J, Choi JY, Kim HB, Kim YK, et al. Five-year decreased incidence of surgical site infections following gastrectomy and prosthetic joint replacement surgery through active surveillance by the Korean Nosocomial Infection Surveillance System. J Hosp Infect. 2016;93(4):339–46. [PubMed: 26944901]
- 38.
- Al-Mulhim FABM, Sadat-Ali M, Alomran AS, Azam MQ. Prevalence of surgical site infection in orthopedic surgery: a 5-year analysis. Int Surg. 2014;99(3):264–8. [PMC free article: PMC4027911] [PubMed: 24833150]
- 39.
- El Beltagy KE, E-SA, Sallah M, Memish ZA. Surgical site infection rates for herniorrhaphy and cholecystectomy in a tertiary care hospital in Saudi Arabia. J Chemother. 2010;22(1):44–7. [PubMed: 20227992]
- 40.
- Ling MLAA, Madriaga G. The burden of healthcare-associated infections in Southeast Asia: a systematic literature review and meta-analysis. Clin Infect Dis. 2015;60(11):1690–9. [PubMed: 25676799]
- 41.
- Young BNT, Teng C, Ang B, Tai HY, Lye DC. Nonconcordance with surgical site infection prevention guidelines and rates of surgical site infections for general surgical, neurological, and orthopedic procedures. Antimicrob Agents Chemother. 2011;55(10):4659–63. [PMC free article: PMC3186972] [PubMed: 21825293]
- 42.
- Sistema Nacional de vigilancia de las infecciones hospitalarias. Montevideo (Uruguay): Ministerio de Salud Publica; 2014. [accessed 10 August 2016]. http://www
.msp.gub.uy /publicaci%C3%B3n/sistema-nacional-de-vigilancia-de-infecciones-hospitalarias. - 43.
- Informe de vigilancia de infecciones asociadas a la atencifin en salud. Santiago (Chile): Departamento De Calidad y Formacifin, Programa Control DE IAAS, Ministerio de Salud; 2013.
- 44.
- Morhason-Bello IO, Oladokun A, Adedokun BO, Obisesan KA, Ojengbede OA, Okuyemi OO. Determinants of post-caesarean wound infection at the University College Hospital, Ibadan, Nigeria. Niger J Clin Pract. 2009;12(1):1–5. [PubMed: 19562911]
- 45.
- Koigi-Kamau R, Kabare LW, Wanyoike-Gichuhi J. Incidence of wound infection after caesarean delivery in a district hospital in central Kenya. East Afr Med J. 2005;82(7):357–61. [PubMed: 16167709]
- 46.
- Mpogoro FJ, Mshana SE, Mirambo MM, Kidenya BR, Gumodoka B, Imirzalioglu C. Incidence and predictors of surgical site infections following caesarean sections at Bugando Medical Centre, Mwanza, Tanzania. Antimicrob Resist Infect Control. 2014;3:25. [PMC free article: PMC4131772] [PubMed: 25126415]
- 47.
- Tran TS, Jamulitrat S, Chongsuvivatvong V, Geater A. Postoperative hospital-acquired infection in Hungvuong obstetric and gynaecological hospital, Vietnam. J Hosp Infect. 1998;40(2):141–7. [PubMed: 9819693]
- 48.
- Couto RC, Pedrosa TM, Nogueira JM, Gomes DL, Neto MF, Rezende NA. Post-discharge surveillance and infection rates in obstetric patients. Int J Gynaecol Obstet. 1998;61(3):227–31. [PubMed: 9688482]
- 49.
- Cardoso Del Monte MC, Pinto Neto AM. Postdischarge surveillance following cesarean section: the incidence of surgical site infection and associated factors. Am J Infect Control. 2010;38(6):467–72. [PubMed: 20226571]
- 50.
- Rizvi M, Rizvi MW, Shaheen, Sultan A, Khan F, Shukla I, et al. Emergence of coryneform bacteria as pathogens in nosocomial surgical site infections in a tertiary care hospital of North India. J Infect Public Health. 2013;6(4):283–8. [PubMed: 23806703]
- 51.
- Buggy D. Can anaesthetic management influence surgical wound healing? Lancet. 2000;356(9227):355–7. [PubMed: 10972364]
- 52.
- Boyd O, Jackson N. How is risk defined in high-risk surgical patient management? Crit Care. 2005;9(4):390–6. [PMC free article: PMC1269426] [PubMed: 16137389]
- 53.
- Gibbons C, Bruce J, Carpenter J, Wilson AP, Wilson J, Pearson A, et al. Identification of risk factors by systematic review and development of risk-adjusted models for surgical site infection. Health Technol Assess. 2011;15(30):1–156. iii–iv. [PubMed: 21884656]
- 54.
- Korol E, Johnston K, Waser N, Sifakis F, Jafri HS, Lo M, et al. A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS One. 2013;8(12):e83743. [PMC free article: PMC3867498] [PubMed: 24367612]
- 55.
- Zhang Y, Zheng QJ, Wang S, Zeng SX, Zhang YP, Bai XJ, et al. Diabetes mellitus is associated with increased risk of surgical site infections: A meta-analysis of prospective cohort studies. Am J Infect Control. 2015;43(8):810–5. [PubMed: 26234220]
- 56.
- Anderson DJ, Hartwig MG, Pappas T, Sexton DJ, Kanafani ZA, Auten G, et al. Surgical volume and the risk of surgical site infection in community hospitals: size matters. Ann Surg. 2008;247(2):343–9. [PubMed: 18216543]
- 57.
- Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-nationwide inpatient sample. J Bone Joint Surg (Am). 2003;85-a(9):1775–83. [PubMed: 12954837]
- 58.
- Jalisi S, Bearelly S, Abdillahi A, Truong MT. Outcomes in head and neck oncologic surgery at academic medical centers in the United States. Laryngoscope. 2013;123(3):689–98. [PubMed: 23444189]
- 59.
- Meyer E, Weitzel-Kage D, Sohr D, Gastmeier P. Impact of department volume on surgical site infections following arthroscopy, knee replacement or hip replacement. BMJ Qual Saf. 2011;20(12):1069–74. [PubMed: 21768211]
- 60.
- Muilwijk J, van den Hof S, Wille JC. Associations between surgical site infection risk and hospital operation volume and surgeon operation volume among hospitals in the Dutch nosocomial infection surveillance network. Infect Control Hosp Epidemiol. 2007;28(5):557–63. [PubMed: 17464915]
- 61.
- Namba RS, Inacio MC, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg (Br). 2012;94(10):1330–8. [PubMed: 23015556]
- 62.
- Patel HJ, Herbert MA, Drake DH, Hanson EC, Theurer PF, Bell GF, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg. 2013;96(5):1560–5. discussion 5–6. [PubMed: 23998408]
- 63.
- Wu SC, Chen CC, Ng YY, Chu HF. The relationship between surgical site infection and volume of coronary artery bypass graft surgeries: Taiwan experience. Infect Control Hosp Epidemiol. 2006;27(3):308–11. [PubMed: 16532422]
- 64.
- Geubbels EL, Wille JC, Nagelkerke NJ, Vandenbroucke-Grauls CM, Grobbee DE, de Boer AS. Hospital-related determinants for surgical-site infection following hip arthroplasty. Infect Control Hosp Epidemiol. 2005;26(5):435–41. [PubMed: 15954480]
- 65.
- Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg (Am). 2001;83-a(11):1622–9. [PubMed: 11701783]
- 66.
- Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg (Am). 2004;86-a(9):1909–16. [PubMed: 15342752]
- 67.
- Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg (Am). 1997;79(4):485–94. [PubMed: 9111392]
- 68.
- Nguyen NT, Paya M, Stevens CM, Mavandadi S, Zainabadi K, Wilson SE. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Ann Surg. 2004;240(4):586–93. discussion 93–4. [PMC free article: PMC1356460] [PubMed: 15383786]
- 69.
- Shah SN, Wainess RM, Karunakar MA. Hemiarthroplasty for femoral neck fracture in the elderly surgeon and hospital volume-related outcomes. J Arthroplasty. 2005;20(4):503–8. [PubMed: 16124968]
3.2. Surgical site infection surveillance: definitions, methods and impact
The surveillance of HAI is one of the core components of an effective IPC programme (1, 2). However, defining, detecting, reporting and interpreting HAI, including SSI, is challenging and requires expertise, time and resource dedication.
Definitions of surveillance and SSI
Surveillance is defined as “the ongoing, systematic collection, analysis, interpretation and evaluation of health data closely integrated with the timely dissemination of these data to those who need it” (3).
There are many definitions of SSI and a systematic review identified as many as 41 different definitions. However, only five were described as being standardized definitions created by multidisciplinary groups (Table 3.2.1) (4). More than one third of included studies used the US CDC definitions (either 1988 or 1992). While the authors of this review suggest that a single definition allows longitudinal analysis and benchmarking, they conclude by stating that “there is no single, objective gold standard test for surgical wound infection” (4). In addition, many countries use the HAI SSI protocol developed by the ECDC (http://ecdc.europa.eu/en/healthtopics/Healthcare-associated_infections/surgical-site-infections/Pages/SSI.aspx, accessed 20 May 2016).
Table 3.2.1
Definitions of SSI.
Aims of surveillance
The primary aim of surveillance is the collection of data on SSI rates in order to obtain a measure of the magnitude of the problem. These data must then be analysed to identify and investigate trends, including a careful interpretation of results. Finally, surveillance data should guide the identification of improvement actions and evaluate the effectiveness of these interventions. In this context, the feedback of SSI rates to relevant stakeholders is important.
Should surveillance be conducted?
The positive impact of HAI surveillance was first described in the landmark study on the efficacy of a nosocomial infection control programme conducted in the USA in the 1970s. In this trial, it was shown that an IPC programme with both surveillance and control components could lower SSI rates significantly (5). Importantly, surveillance of SSI is part of the WHO safe surgery guidelines (6). Many countries have introduced mandatory surveillance of HAI, including SSI, such as the UK and certain states in the USA, whereas other countries have voluntary-based surveillance, such as France, Germany and Switzerland. However, there are considerable differences related to the types of surveillance, as well as in the length and type of surveillance (7, 8). Increasingly, national networks and “networks of networks” are being created, such as the CDC NHSN, the ECDC HAI Surveillance Network (HAI-Net) and the International Nosocomial Infection Control Consortium.
By using standardized definitions of HAI and specifically SSI, these networks allow inter-hospital comparisons and benchmarking. An essential component of these surveillance networks is feedback to individual hospitals, as discussed below.
It has been postulated that a “surveillance effect” might occur, similar to the Hawthorne effect in clinical trials, that is, the simple fact of being conscious that one is being observed may independently lead to improved practices or improved adherence to guidelines (9).
Another way in which a successful surveillance programme may decrease SSI rates is that the feedback given to the institution may prompt investigation of why its rates are higher than the benchmark. Certain process indicators (if not already collected) may then identify the reason for “underperformance” and prompt local initiatives to improve performance on these indicators. There is conflicting evidence that conducting surveillance as part of a network has a positive impact on SSI rates (Table 3.2.2). Some studies report a successful reduction of SSI rates after participation in a surveillance network (10–12), while others report no effect (13). However, there is an important methodological issue that could “dilute” the reduction in the time trend of SSI rates, which is the fact of adding smaller hospitals in a network without taking into account their year of participation in the network. This obstacle was overcome in an analysis of German data where hospitals were stratified by year of participation (9) and in an analysis of Dutch (14) and Swiss (13) data where SSI rates were stratified by surveillance time to operation in consecutive one-year periods using the first year of surveillance as a reference. The Dutch and German studies reported decreasing time trends of SSI rates after surveillance, whereas the Swiss study did not.
Table 3.2.2
Temporal trends of SSI rates after surveillance in selected networks.
Conversely, as shown in clinical trials, intensive surveillance may lead to the detection of higher SSI rates than under standard surveillance conditions. As an example, in a recent clinical trial comparing skin antiseptic agents for caesarean section, the SSI rate was 4.0% in one arm and 7.3% in the other (15). These rates seem higher than the most recently available data from the ECDC, which show an SSI rate of 2.9% (inter-country range: 0.4%–6.8%) (16).
Establishing a surveillance system
According to the US Association for Professionals in Infection Control and Epidemiology (20), there is “no single or “right” method of surveillance design or implementation” (21). However, the following minimal requirements for ensuring quality of surveillance have been identified by the Association (21).
- A written plan that states goals, objects and elements of surveillance process
- Constant rigour of intensity of surveillance
- Consistent elements of surveillance (for example, definitions, calculation methods)
- Adequate human resources (professionals trained in epidemiology)
- Informatic services, computer support
- Evaluation methods.
For a surveillance programme to be successful, there should be a method of data validation to ensure that data are accurate and reliable (22), particularly for benchmarking purposes, as discussed further (23).
Methods for conducting surveillance
In the field of SSI, most surveillance systems target colorectal surgery and total hip and knee arthroplasty. The most common outcome indicator is the cumulative SSI incidence (or SSI rate). Detecting SSI using prevalence methods is less reliable given the high proportion of SSIs that manifest after discharge.
For any given period, denominator data represent the total number of procedures within each category. The number of patients can be used also as the denominator, but it is less precise because more than one infection can occur in the same patient. Numerator data will be the number of SSIs in that same period. Demographic data (age, sex, timing and choice of antimicrobial prophylaxis, American Society of Anesthesiologists score, duration of the operation and wound contamination class) are recorded for all patients, including the site of infection and type of SSI (superficial, deep, organ/space) for those with SSI. Linkage with microbiological data may also be useful.
The gold standard is prospective direct surveillance, although it is time- and labour-intensive and costly (24). The CDC recommendations describe indirect methods of surveillance (sensitivity of 84–89%; specificity 99.8%) as a combination of:
- Review of microbiology reports and patient medical records.
- Surgeon and/or patient surveys.
- Screening for readmission and/or return to the OR.
- Other information, such as coded diagnoses, coded procedures, operative reports or antimicrobials ordered. (24)
The importance of post-discharge surveillance
It is estimated that a significant proportion of SSIs are detected following patient discharge. This proportion varies across settings and according to different definitions, but it has been estimated to be between 13% to 71% (25). The fact that hospital length of stay has been steadily decreasing over the past decades has probably contributed to shifting the burden from inpatient to outpatient infections. Moreover, implant-associated infections may not become apparent until one year after the procedure. For this reason, many surveillance networks recommend the practice of post-discharge surveillance. There is no known gold standard procedure for post-discharge surveillance and a systematic review identified only 7 reports of studies comparing different surveillance methods (26). Due to variations in data collection and classification, as well as missing information regarding diagnostic criteria, no synthesis of post-discharge surveillance data was possible. The authors concluded that more research is required regarding the measurement of SSI after hospital discharge.
There has been recent controversy regarding the CDC decision to shorten post-discharge surveillance to 90 days instead of one year after certain procedures (27). This change was aimed at simplifying post-discharge surveillance and reducing delayed feedback, but it has not been universally adopted as yet (28). A report compared historical prospective SSI surveillance data from a USA network to the retrospective application of the new CDC definitions (29). The authors found that 9.6% of SSIs detected by the former definition went undetected with the new definitions; 28.8% of these undetected SSIs concerned hip and knee prostheses. The proportion of missed SSIs varied by procedure, but they were high for hip (8.8%) and knee prostheses (25.1%). Another report from the Dutch SSI surveillance network analysed the influence of the duration and method of post-discharge surveillance on SSI rates in selected procedures (30). The proportion of missed SSIs was variable, but they were 6% and 14% for hip and knee prostheses, respectively. More importantly, the study showed that the new CDC method of performing post-discharge surveillance was associated with a higher risk of not detecting a SSI when compared with the former method.
How to report surveillance data
Although most surveillance systems report SSI rates, there has been debate in the literature regarding the best choice of outcome indicator. Some authors argue that the incidence density of in-hospital SSI is a more suitable choice of outcome indicator by taking into account different lengths of hospital stay and different post-discharge surveillance methods (31). This indicator requires recording the date of patient discharge.
In order to adjust for variations in case-mix, it is recommended to present risk-adjusted SSI rates in addition to crude rates (32). The most commonly used method of risk adjustment is the NNIS risk index whose aim is to predict the occurrence of an SSI in a given patient (33). This risk index has been updated and includes procedure-specific factors that improve its predictive power, but it is not widely used (28, 34). Of note, collecting data for the NNIS risk index may be difficult in settings with limited resources where very limited information is reported in patient records. As an example, in a recent systematic review conducted by WHO, only 14 of 231 SSI surveillance studies from developing countries reported using the NNIS risk index (WHO unpublished data).
Some surveillance systems report standardized infection ratios, which is the ratio between the observed and the expected infection rates (35, 36). A ratio higher than 1.0 indicates that more SSIs occurred than were expected, whereas a ratio lower than 1.0 indicates the opposite (36). The simplest manner to calculate the expected number of SSIs is by multiplying the number of operations in each procedure category by the SSI rate and dividing by 100. This accounts for the case-mix and is therefore a risk-adjusted summary measure (36).
Other surveillance systems (UK, Switzerland) use a funnel plot to improve the precision of the estimates of SSI rates, which are dependent on the number of operations performed. SSI rates are plotted against the number of procedures for each hospital and 95% CIs are drawn. In this manner, outliers (hospitals with unusually high rates) can be easily identified (37).
Difficulties associated with surveillance
Active surveillance is a resource- and time-consuming activity. Constraints can be both in financial terms and/or in the availability of trained and dedicated staff. Surveillance data need validation and interpretation by supervising IPC professionals and/or epidemiologists. A major and very common constraint to HAI surveillance in developing countries is the lack of reliable microbiology support. However, this may have a less significant impact on SSI surveillance as a clinical diagnosis can often be made without microbiological confirmation. Thus, the correct collection of clinical data (preferably electronically) is essential for a successful surveillance system. Another difficulty in low-income countries is the high loss of patient follow-up for post-discharge surveillance due to long distances between surgical care services and the patient’s place of residence and/or the patient’s financial constraints. Based on some interesting publications (38), WHO has developed an adapted approach to SSI post-discharge surveillance by issuing pre-discharge instructions to the patient to allow him/her to recognize signs of infection and maintain follow-up through telephone calls. Finally, in the absence of effective infection control programmes and societies (local and national), it is difficult to introduce a sustainable surveillance system.
Use of surveillance for benchmarking
The use of HAI surveillance data, including SSIs, has been advocated for benchmarking purposes (23). Benchmarking can be used for several purposes, including for the publication of “league tables” as in the UK and USA (39). In addition, it is also used in the USA as the basis for modifying hospital payments to facilities paid by Medicare (24). There are advantages and disadvantages of benchmarking as there are important pitfalls that should be actively avoided. There is a possibility that surveillance systems with more intensive and sensitive surveillance methods that result in higher SSI rates may be unfairly penalised.
Even in the presence of uniform standardized definitions, several studies have shown that inter-rater agreement for SSI is rather low (40–42). One study evaluated inter-rater agreement by submitting 12 case-vignettes of suspected SSI to IPC physicians and surgeons from 10 European countries (41). It was found that there was poor agreement regarding SSI diagnosis and the type of SSI, with variations between and within countries. An analysis of data submitted from 11 countries to the ECDC HELICS (Hospitals in Europe for Infection Control through Surveillance) network showed that there was a substantial variation not only in terms of case-mix (as measured by the NNIS risk index score), but also in the reporting of SSI (highly variable inter-country proportions of superficial SSI ranging from 20–80%) and the length and intensity of postoperative follow-up (31).
An audit of SSI surveillance methods in England showed that differences in data collection methods and data quality were associated with large differences in SSI rates (43). What is striking is that even in the presence of mandatory surveillance with a clearly defined national protocol, a substantial proportion of responders (15%) used alternative definitions (43).
Conclusions
Ideally, surveillance of SSI should be an integral part of IPC programmes of health care organizations and priorities for public health agencies worldwide. However, caution must be exerted when interpreting SSI data, especially when making comparisons, due to a possible heterogeneity of definitions used, surveillance methods, risk stratification and reporting.
Further studies are needed to determine the most sensitive methods of diagnosing SSI, both for in-patients and as part of PDS, and the most efficient methods of collecting data. It is of the utmost importance to develop and test reliable adapted definitions and surveillance methods for settings with limited resources. The role of automated computerized algorithms needs to be also further evaluated. Similarly, the role of SSI surveillance data for benchmarking purposes needs to be clarified, especially when public reporting is involved.
References
- 1.
- Core components for infection prevention and control programmes. Geneva: World Health Organization; 2009.
- 2.
- Zingg W, Holmes A, Dettenkofer M, Goetting T, Secci F, Clack L, et al. Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet Infect Dis. 2015;15(2):212–24. [PubMed: 25467650]
- 3.
- Centers for Disease Control and Prevention. Guidelines for evaluating surveillance systems. Morb Mortal Wkly Rep (MMWR). 1988;37(5):1–18.
- 4.
- Bruce J, Russell EM, Mollison J, Krukowski ZH. The quality of measurement of surgical wound infection as the basis for monitoring: a systematic review. J Hosp Infect. 2001;49(2):99–108. [PubMed: 11567554]
- 5.
- Haley RW, Quade D, Freeman HE, Bennett JV. The SENIC project. Study on the efficacy of nosocomial infection control (SENIC project). Summary of study design. Am J Epidemiol. 1980;111(5):472–85. [PubMed: 6246798]
- 6.
- Guidelines for safe surgery: safe surgery saves lives. Geneva: World Health Organization; 2009. [PubMed: 23762968]
- 7.
- Wilson AP, Kiernan M. Recommendations for surveillance priorities for healthcare-associated infections and criteria for their conduct. J Antimicrob Chemother. 2012;67(Suppl. 1):i23–8. [PubMed: 22855875]
- 8.
- National Institute for Health and Care Excellence. NICE guideline. Healthcare-associated infections: prevention and control. 2011. [accessed 19 July 2016]. https://www
.nice.org.uk/guidance/ph36. - 9.
- Gastmeier P, Schwab F, Sohr D, Behnke M, Geffers C. Reproducibility of the surveillance effect to decrease nosocomial infection rates. Infect Control Hosp Epidemiol. 2009;30(10):993–9. [PubMed: 19719414]
- 10.
- Brandt C, Sohr D, Behnke M, Daschner F, Ruden H, Gastmeier P. Reduction of surgical site infection rates associated with active surveillance. Infect Control Hosp Epidemiol. 2006;27(12):1347–51. [PubMed: 17152033]
- 11.
- Astagneau P, L’Heriteau F, Daniel F, Parneix P, Venier AG, Malavaud S, et al. Reducing surgical site infection incidence through a network: results from the French ISO-RAISIN surveillance system. J Hosp Infect. 2009;72(2):127–34. [PubMed: 19380181]
- 12.
- Mannien J, van den Hof S, Muilwijk J, van den Broek PJ, van Benthem B, Wille JC. Trends in the incidence of surgical site infection in the Netherlands. Infect Control Hosp Epidemiol. 2008;29(12):1132–8. [PubMed: 18991504]
- 13.
- Staszewicz W, Eisenring MC, Bettschart V, Harbarth S, Troillet N. Thirteen years of surgical site infection surveillance in Swiss hospitals. J Hosp Infect. 2014;88(1):40–7. [PubMed: 25063012]
- 14.
- Geubbels EL, Nagelkerke NJ, Mintjes-De Groot AJ, Vandenbroucke-Grauls CM, Grobbee DE, De Boer AS. Reduced risk of surgical site infections through surveillance in a network. Int J Qual Health Care. 2006;18(2):127–33. [PubMed: 16484315]
- 15.
- Tuuli MG, Liu J, Stout MJ, Martin S, Cahill AG, Odibo AO, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–55. [PMC free article: PMC4777327] [PubMed: 26844840]
- 16.
- Surveillance of surgical site infections in Europe 2010–2011. Stockholm: European Centre for Disease Prevention and Control; 2013.
- 17.
- Fifth report of the mandatory surveillance of surgical site infection in orthopaedic surgery. London: Health Protection Agency; Dec, 2009. [accessed 19 July 2016]. April 2004 to March 2009. http://webarchive
.nationalarchives .gov.uk/20140714084352/ http://www .hpa.org.uk /webc/HPAwebFile/HPAweb_C/1259151994683. - 18.
- Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121(2):182–205. [PubMed: 4014115]
- 19.
- Astagneau P, L’Heriteau F. Surveillance of surgical-site infections: impact on quality of care and reporting dilemmas. Curr Opin Infect Dis. 2010;23(4):306–10. [PubMed: 20485163]
- 20.
- Association for Professionals in Infection Control. Vision and mission. [accessed 19 July 2016]. http://www
.apic.org/About-APIC /Vision-and-Mission. - 21.
- Lee TB, Montgomery OG, Marx J, Olmsted RN, Scheckler WE. Association for Professionals in Infection Control. Recommended practices for surveillance: Association for Professionals in Infection Control and Epidemiology (APIC), Inc. Am J Infect Control. 2007;35(7):427–40. [PubMed: 17765554]
- 22.
- Mannien J, van der Zeeuw AE, Wille JC, van den Hof S. Validation of surgical site infection surveillance in the Netherlands. Infect Control Hosp Epidemiol. 2007;28(1):36–41. [PubMed: 17230385]
- 23.
- Haustein T, Gastmeier P, Holmes A, Lucet JC, Shannon RP, Pittet D, et al. Use of benchmarking and public reporting for infection control in four high-income countries. Lancet Infect Dis. 2011;11(6):471–81. [PubMed: 21616457]
- 24.
- Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control. 2014;35(06):605–27. [PMC free article: PMC4267723] [PubMed: 24799638]
- 25.
- Holtz TH, Wenzel RP. Postdischarge surveillance for nosocomial wound infection: a brief review and commentary. Am J Infect control. 1992;20(4):206–13. [PubMed: 1524269]
- 26.
- Petherick ES, Dalton JE, Moore PJ, Cullum N. Methods for identifying surgical wound infection after discharge from hospital: a systematic review. BMC Infect Dis. 2006;6:170. [PMC free article: PMC1697816] [PubMed: 17129368]
- 27.
- Surgical site infection (SSI) event. Atlanta (GA): Centers for Disease Control and Prevention; 2013. [accessed 19 July 2016]. http://www
.cdc.gov/nhsn /pdfs/pscmanual/9pscssicurrent.pdf. - 28.
- Surveillance of surgical site infections in European hospitals – HAISSI protocol. Version 1.02. Stockholm: European Centre for Disease Prevention and Control; 2012. [accessed 19 July 2016]. http://ecdc
.europa.eu /en/publications/Publications /120215_TED_SSI_protocol.pdf. - 29.
- Dicks KV, Lewis SS, Durkin MJ, Baker AW, Moehring RW, Chen LF, et al. Surveying the surveillance: surgical site infections excluded by the January 2013 updated surveillance definitions. Infect Control Hosp Epidemiol. 2014;35(5):570–3. [PMC free article: PMC4219409] [PubMed: 24709727]
- 30.
- Koek MB, Wille JC, Isken MR, Voss A, van Benthem BH. Post-discharge surveillance (PDS) for surgical site infections: a good method is more important than a long duration. Euro Surveill. 2015;20(8) 21042. [PubMed: 25742435]
- 31.
- Wilson J, Ramboer I, Suetens C. HELICS-SSI working group. Hospitals in Europe Link for Infection Control through Surveillance (HELICS). Inter-country comparison of rates of surgical site infection--opportunities and limitations. J Hosp Infect. 2007;65(Suppl. 2):165–70. [PubMed: 17540264]
- 32.
- O’Neill E, Humphreys H. Use of surveillance data for prevention of healthcare-associated infection: risk adjustment and reporting dilemmas. Curr Opin Infect Dis. 2009;22(4):359–63. [PubMed: 19474727]
- 33.
- Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91(3B):152S–7S. [PubMed: 1656747]
- 34.
- Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol. 2011;32(10):970–86. [PubMed: 21931247]
- 35.
- Rioux C, Grandbastien B, Astagneau P. The standardized incidence ratio as a reliable tool for surgical site infection surveillance. Infect Control Hosp Epidemiol. 2006;27(8):817–24. [PubMed: 16874641]
- 36.
- Gaynes RP, Culver DH, Horan TC, Edwards JR, Richards C, Tolson JS. Surgical site infection (SSI) rates in the United States, 1992–1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clin Infect Dis. 2001;33(Suppl. 2):S69–77. [PubMed: 11486302]
- 37.
- Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med. 2005;24(8):1185–202. [PubMed: 15568194]
- 38.
- Aiken AM, Wanyoro AK, Mwangi J, Mulingwa P, Wanjohi J, Njoroge J, et al. Evaluation of surveillance for surgical site infections in Thika Hospital, Kenya. J Hosp Infect. 2013;83(2):140–5. [PMC free article: PMC3580288] [PubMed: 23332563]
- 39.
- Jarvis WR. Benchmarking for prevention: the Centers for Disease Control and Prevention’s National Nosocomial Infections Surveillance (NNIS) system experience. Infection. 2003;31(Suppl. 2):44–8. [PubMed: 15018472]
- 40.
- Hedrick TL, Harrigan AM, Sawyer RG, Turrentine FE, Stukenborg GJ, Umapathi BA, et al. Defining surgical site infection in colorectal surgery: an objective analysis using serial photographic documentation. Dis Colon Rectum. 2015;58(11):1070–7. [PubMed: 26445180]
- 41.
- Birgand G, Lepelletier D, Baron G, Barrett S, Breier AC, Buke C, et al. Agreement among healthcare professionals in ten European countries in diagnosing case-vignettes of surgical-site infections. PLoS One. 2013;8(7):e68618. [PMC free article: PMC3706413] [PubMed: 23874690]
- 42.
- Wilson AP, Gibbons C, Reeves BC, Hodgson B, Liu M, Plummer D, et al. Surgical wound infection as a performance indicator: agreement of common definitions of wound infection in 4773 patients. BMJ. 2004;329(7468):720. [PMC free article: PMC518898] [PubMed: 15367425]
- 43.
- Tanner J, Padley W, Kiernan M, Leaper D, Norrie P, Baggott R. A benchmark too far: findings from a national survey of surgical site infection surveillance. J Hosp Infect. 2013;83(2):87–91. [PubMed: 23332352]
3.3. Importance of a clean environment in the operating room and decontamination of medical devices and surgical instruments
3.3.1. Environment
For many years, environmental contamination was considered to be less important than many other factors in contributing to HAI. However, recent evidence shows that a contaminated health care environment plays a significant role in the transmission of microorganisms (1,2). It is essential that the operating room (OR) is thoroughly cleaned on a daily basis. Proper mechanical ventilation is also necessary to prevent surgical wound contamination from unfiltered air drawn into the OR and to dilute and remove microorganisms shed in skin scales (3). Specific guidance on the most appropriate ventilation systems in the OR and an evidence-based recommendation on laminar flow are included in chapter 4.23 of these guidelines.
Environmental cleaning and waste management in the OR
Cleaning consists of the removal of dust, soil and contaminants on environmental surfaces and ensures a hygienic and healthy environment both for patients and staff. The environment should be thoroughly cleaned and general principles of good practice should be taken into consideration (Box 3.3.1). Cleaning requirements for various surfaces are detailed in Table 3.3.1.
Box 3.3.1
General principles for environmental cleaning.
Table 3.3.1
Cleaning requirements for various surface types in ORs.
At the beginning of each day, all flat surfaces should be wiped with a clean, lint-free moist cloth to remove dust and lint. Between cases, hand-touch surfaces (Figure 3.3.1) and surfaces that may have come in contact with patients’ blood or body fluids, should be wiped clean first by using a detergent solution and then disinfected according to hospital policy and allowed to dry.
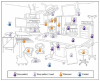
Figure 3.3.1
Example of cleaning frequencies in preoperative and postoperative care areas. Reproduced with permission from reference .
All spills must be carefully cleaned up and the surface cleaned and disinfected according to hospital policy. Domestic heavy duty gloves should be always worn to undertake this task. Use a single-use plastic apron if contamination of the body is likely. Use of a gown and mask is not necessary. If there is a risk of spills with chemicals, the use of a face shield or goggles should be considered, depending on the type of chemical products used for disinfection. All waste from the OR should be collected and removed in closed leak-proof containers; soiled linen should be placed in plastic bags for collection. All reusable medical devices should be sent for reprocessing to the sterile services department or the decontamination unit. The operating table should be cleaned and wiped with a detergent solution, including the mattress and the surface. All surfaces that have come in contact with a patient or a patient’s body fluids must be cleaned and disinfected using an appropriate disinfectant solution according to local protocols.
At the end of every day, it is necessary to perform a total cleaning procedure. All areas of the surgical suite, scrub sinks, scrub or utility areas, hallways and equipment should be thoroughly cleaned, regardless of whether they were used or not during the last 24 hours. Soiled linen should be removed in closed leak-proof containers. All contaminated waste containers should be removed and replaced with clean containers. Sharps’ containers should be closed and removed when they are three quarters full. All surfaces should be cleaned from top to bottom using a detergent, followed by a disinfectant if necessary, and then allowed to dry. To reduce the microbial contamination of environmental surfaces, such as walls, ceilings and floors, they should be thoroughly cleaned from top to bottom with a detergent and allowed to dry. The routine use of a disinfectant or fumigation of the OR is not necessary even after contaminated surgery.
3.3.2. Decontamination of medical devices and surgical instruments
Decontamination is a complex and highly specialized subject. This section provides a brief summary on the decontamination and reprocessing of reusable medical devices and patient care equipment.
In countries with established programmes, decontamination is a speciality in its own right and is an independent, quality-assured and accountable service delivered to health care institutions. The entire process of decontamination is highly regulated and governed by clearly defined guidelines and standards, which are established at both national and international (International Organization for Standardization) levels. This ensures validation of the processes and patient safety (7–10).
In LMICs, decontamination science is in its infancy and few structured decontamination programmes exist, as was evident during the recent Ebola outbreak. In these countries, where the lack of sterile instruments and/or the availability of a properly designed OR and sterile services department have a considerable impact, SSI can be described as surgery-associated infection (11,12). In response to this need, the WHO/Pan American Health Organization (PAHO) have produced a decontamination and reprocessing manual for health care facilities (13) to support and guide operational activities to improve standards of care.
In the USA, the term decontamination does not include cleaning and refers to all reprocessing following on thereafter. In the UK and Europe, decontamination relates to the entire process, including cleaning, and this term is used in this chapter (see Table 3.3.2).
Table 3.3.2
Glossary of terms.
Essentials of decontamination
All medical devices that are reprocessed, such as surgical instruments, must undergo rigorous cleaning prior to decontamination and sterilization procedures. Soaking contaminated medical devices prior to cleaning in disinfectants of any kind is not sufficient or recommended (14). Regardless of the type of operative procedure, the decontamination steps in reprocessing surgical instruments and other medical devices are the same. The life cycle of decontamination illustrates (Figure 3.3.2) the salient features of decontamination, with each step being as important as the next.
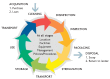
Figure 3.3.2
The cycle of decontamination of a reusable surgical instrument. Reproduced with permission from reference .
Risk assessment of contaminated instruments
The risk of transferring microorganisms from instruments and equipment is dependent on the following factors:
- the presence of microorganisms, their number, and their virulence;
- the type of procedure that is going to be performed (invasive or non-invasive);
- the body site where the instrument or equipment will be used.
Risk assessment for the reprocessing of medical devices was best described by Spaulding (15) and has since been modified. After thorough cleaning, the decision to disinfect or sterilize is based on whether the device is stable to heat or not. In addition, the body site where the instrument or equipment will be used/have contact with will determine whether cleaning or high level disinfection or sterilization is required. According to the Spaulding classification, medical devices are categorized as critical, semi-critical or non-critical according to the degree of risk of infection transmission (Table 3.3.3).
Table 3.3.3
Spaulding classification of equipment decontamination (15).
Decontamination facility
The work space
All reprocessing of medical devices must take place in the sterile services department, which should be a separate demarcated department or in a designated decontamination area. Many countries have centralized decontamination areas (central sterile services department) and provide services to the OR, wards and clinical areas. Centralized decontamination processes make the decontamination process cheaper, increase the process safety and enhance its quality. A structured transportation system for clean and used equipment must also be in place. Of note, when the decontamination area space is very limited (usually just one room) and reprocessing is expected to take place in the smallest and least appropriate space with old equipment and overcrowded surfaces, the risk of contamination of clean trays is highly likely. Decontamination of medical devices in clinical areas is not recommended.
Standard operating procedures for decontamination and sterilization
All decontamination units must have written policies and procedures for each stage of the decontamination process and should include:
- formal staff qualification, education/training and competency assessment;
- cleaning;
- high-level disinfection (all processes available);
- preparation and packaging of medical devices;
- sterilizer operating procedures;
- monitoring and documenting of chemical or cycle parameters;
- workplace health and safety protocols specific to the chemical sterilant;
- handling, storage and disposal of the sterilant according to the manufacturer’s instructions for use and local regulations;
- use of physical, chemical and/or biological indicators;
- quality systems;
- validation of cleaning, disinfection and sterilization.
Provisions for hand hygiene and personal protective equipment
Equipped hand hygiene stations should be available at the entrance and exit of the sterile services department or decontamination areas. Appropriate personal protective equipment must be provided at each entry point into the sterile services department or decontamination area. Personal protective equipment is designed to be disposable, but it is reused in some low-resource settings. This is acceptable provided that the personal protective equipment, for example, an apron, is cleaned by wiping with a damp cloth and allowed to dry. The apron should then be wiped with 70% alcohol and allowed to dry. A discard bucket for used personal protective equipment must be provided at the exit point, preferably near the wash hand basin.
The workflow
There should be clearly demarcated areas during the reprocessing of medical devices, such as the dirty area where the items are received and cleaned, the inspection-assembly-packaging and the sterilization or high-level disinfection areas, and finally those dedicated to the storage of sterile packs and their transportation. It is recommended that these areas be physically demarcated to avoid cross-contamination from dirty to clean. When this is not possible because of lack of space, obstacles should be placed in order to only permit a unidirectional movement of staff and equipment from dirty to clean without any possibility of overlap.
Transportation of used medical devices
Once devices have been used in the clinical area such as the OR, they should be prepared for transportation to the sterile services department by counting and collecting the devices, rinsing them under cold running water, allowing excess water to drain away, and placing them in a closed container or tray, which will keep them moist until they are removed. These trays (and the accompanying checklist) should be transported in a robust trolley, preferably with closed sides, to the decontamination area. Soaking of medical devices in disinfectant prior to cleaning or during transportation is not recommended as there is a danger of spilling contaminated fluids (13) (Box 3.3.2). Used devices should be received, checked and sorted for cleaning in the “dirty” area. Cleaning is normally done either manually or by automated methods.
Box 3.3.2
Recommendations related to the soaking of instruments in disinfectant prior to cleaning.
Manual cleaning
Cleaning by hand will require well-trained operators to wear appropriate personal protective equipment (waterproof aprons, domestic heavy duty gloves, face cover to protect mucous membranes and head cover [optional]), dilute the detergent accurately according to the manufacturer’s guidelines, open up all the hinges on the devices and clean these by holding the item below the surface of the water (water temperature no more than 50°C) while using a soft nylon brush to remove debris. Visual inspection of the hinges, teeth and serrated edges should be carried out to ensure cleanliness. There is no controlled validation of manual cleaning apart from protein detection, which is expensive. Water or air pressure guns are used to blow through and clear lumen devices.
Automated cleaning
Reprocessing medical devices through a washer disinfector is safer and usually more efficient. Devices are cleaned using water jets, then washed with detergent and warm water, followed by a thermal disinfection cycle (some machines have a drying cycle). The load is substantial, although some washer disinfectors are capable of reprocessing up to 60 trays per hour. Most importantly, each cycle is validated with physical and biological parameters (13).
Inspection, assembly and packaging
Using a magnifying glass and good lighting, clean devices are carefully checked to confirm cleanliness and being fit for purpose and then reassembled. If the medical device is found not to be clean, it is returned for re-cleaning; damaged devices are replaced and the completed tray is wrapped ready for sterilization. Packaging is usually done by double wrapping for surgical trays or sterilization pouches for single items. Packaging material should be robust, permeable to steam, but maintain a fluid barrier, and should protect the sterility of the package prior to use.
Methods of decontamination
Steam sterilization
Most surgical devices are heat-resistant and therefore steam is the preferred sterilizing agent globally. It is inexpensive, efficient, easily maintained and widely available, compared with chemical sterilizers. There are several types of autoclaves/sterilizers. All of them work on the same principle of converting water to steam and holding the steam just below boiling point (saturated) so that there is maximum (latent) heat held in a semi-gaseous state. The steam makes contact with the load in the chamber and releases the heat, thus resulting in sterilization. The time that the steam is in contact with the devices is crucial and is known as the “holding time”.
Types of autoclaves
- The pre-vacuum steam sterilizer is the most widely-used sterilizer and is suitable for the sterilization of wrapped clean instruments, gowns, drapes, towelling and other dry materials required for surgery. Air removal is part of the cycle and thus it is suitable for medical devices with lumens and porous loads.
- Downward (gravity) displacement sterilizers are designed for sterilizing bio-hazardous waste, solutions and instruments. They are now obsolete and have many drawbacks as sterility cannot be assured and they are less reliable than pre-vacuum sterilizers. They are not the best option for wrapped packs or porous materials. Air removal is by gravity displacement and they are also not suitable for medical devices with lumens.
- Non-vacuum steam sterilizers: self-contained (benchtop) sterilizers are sometimes used, but they are only suitable for relatively few or simple items. Table top sterilizers may be used in outpatient departments, dental surgeries and some family planning clinics, but they should not be considered for use in ORs and they are also not suitable for medical devices with lumens.
Sterilization by chemical (low temperature) automatic methods
Chemical gas (low temperature) sterilization is used to sterilize heat- and moisture-sensitive devices. It should be noted that these methods are expensive to install and to run. The mechanics are complex and well-trained staff should be employed if this method is used. Manual chemical sterilization is not recommended because the process cannot be controlled and may lead to occupational health issues.
Sterilization with gaseous chemical methods should be carried out in chambers with automated cycles that provide safety for the user and guarantee the process. Medical device compatibility will vary with each low temperature sterilization method. Low temperature (gas) sterilization can be achieved using a number of different chemicals for example, ethylene oxide, hydrogen peroxide gas/plasma, ozone, low temperature and steam formaldehyde.
Immediate use sterilization system or “flash” sterilization
An Immediate use sterilization sysstem or “flash” sterilization is a common term that describes the fast sterilization of non-porous and/or non-cannulated surgical instruments in an unwrapped condition in downward displacement steam instrument sterilizers located close to the point where the instruments will be used immediately. In the past, “flash” sterilization was the main means of providing sterile instruments for surgery. Special high-speed sterilizers are usually located in the OR in order to process unwrapped instruments and instruments for urgent use. For example, the only available hand piece is dropped on the floor in the middle of the procedure and this single instrument needs to be sterilized in a rush. These sterilizers operate at 134° C for 3–10 minutes. “Flash” sterilization delivers the instruments wet and very hot into the OR environment. Of note, “flash” sterilization should never replace the lack of material or instruments for a programmed surgical procedure.
If an immediate use sterilization system must be used, it should be used only after all of the following conditions have been met:
- Work practices should ensure proper cleaning, inspection and arrangement of instruments before sterilization.
- The physical layout of the area ensures direct delivery of sterilized items to the point of use.
- Procedures are developed, followed and audited to ensure aseptic handling and staff safety during transfer of the sterilized items from the sterilizer to the point of use.
Validation
In sterile services, it is the process and not the procedure, which is usually tested and validated to ensure high quality assurance and the reliability of the process. There are both simple and complex methods to check that the surgical package has been through the correct decontamination process. Validation of the sterilization process has to take place at every step and can be quite confusing for the sterile services department staff. For details, please refer to the WHO/PAHO decontamination and reprocessing manual for health care facilities (13).
Loan instruments
It is common practice for expensive medical devices used for operations, such as instruments for orthopaedics, neurology or implants and transplants, to be rented (“loaned”) from supply companies and brought to the OR. Often the companies deliver the sets directly to the OR and recuperate them directly, thus bypassing the sterile services department. These medical devices are used between several hospitals and the greatest concern is that often there is no control of correct reprocessing of these devices. In LMICs, many companies supplying loan instruments do not have facilities to reprocess medical devices and they are often moved from one health care facility to another without adequate reprocessing. In these circumstances, there is often very little documentation about where or how the medical devices have been used. In a very comprehensive document published by the UK Institute of Decontamination Sciences, which outlines the relationship between the OR, the supply company and the sterile services department, it is clear that the ultimate responsibility for patient safety and quality of sterilization lies with the sterile services department in the health care facility and not the supply company (14). Therefore, it is vital that all medical devices destined for the OR must transit via the sterile services department of that health care facility and are validated as safe to use.
Storage of sterile packs
After sterilization, the packs are removed and allowed to cool. If there is an adequate supply of surgical trays and equipment, appropriate storage in the sterile services department has to be provided before the packs are dispatched to the OR. The proper storage of sterile instruments and equipment is essential to ensure that the product maintains its level of sterilization or disinfection. The storage area for sterile packs has specific requirements.
- Store in a clean, dry environment (that is, far from moisture sources) that is protected from any damage. It is recommended that the storage containers should not be made of absorbent material, such as wood.
- The area must be bright, light and airy with good air circulation. The temperature must be moderate without wide fluctuations during the day.
- The storage area should have an adequate level of lighting and the walls should be smooth and easy to clean.
- Access to the area should be restricted.
- The packs should be placed on open racks rather than closed shelves in a single layer to prevent moisture from accumulating between the packs.
- The labels must be visible and clear.
- The pack inspection register should be clearly visible. The racks must be at least 10 cm off the ground and from the ceiling.
- Before use, packages should be inspected in order to verify that they meet the requirements of a sterile product.
User sterility check
Use of sterile instruments in the operating room
1. Role of the nurse who lays out the sterile surgical instruments on the operating trolley in the operating room
The nurse who prepares the operating trolley should check that:
- the preparation area is quiet, clean and undisturbed;
- the packs are not wet (no moisture);
- the packaging of the pack is intact, not torn or opened;
- there are no water marks from condensation indicating non-sterility;
- the chemical indicator strip is present and has a uniform change of colour;
- the internal indicator shows sterilization;
- the devices are clean;
- the surfaces of the devices are intact;
- the devices are fit for use.
2. Role of the scrub nurse
The scrub nurse should check to ensure that:
- the devices are ready and fit for use;
- the devices are not dirty or broken;
- there are an adequate number of devices for the procedure (to avoid opening several packs or resorting to an immediate use sterilization system);
- the pack indicators are placed in the patient notes;
- the surgeon is aware of any shortage of equipment or devices.
3. Role of the surgeon and surgical team
The surgeon should ensure that before making an incision:
- the operating field is sterile and clearly defined;
- the devices are visibly clean;
- the devices are fit for purpose;
- all the necessary equipment is available;
- there is no unnecessary delay on the operating table because of a lack of instruments;
- the pack indicators are in the patient notes and are satisfactory.
On completion of the surgical procedure, OR staff should:
- check that all instruments are present before returning to the sterile services department;
- rinse the instruments as per the standard operating protocol;
- ensure that items are securely contained in a leak-proof container before transportation to the sterile services department;
- inform the sterile services department of any issues with the surgical instruments, for example, a broken device.
Decontamination of endoscopes
An increasing number of diagnostic and therapeutic procedures are now being carried out using rigid or flexible endoscopes (16). Effective decontamination will protect the patient from infection, ensure the quality of diagnostic procedures and samples and prolong the life of the equipment (17) (Table 3.3.4). The source of infection may be due to:
Table 3.3.4
Types of endoscopic procedures.
- the previous patient or inadequate decontamination of the endoscope before reuse;
- endogenous skin, bowel or mucosal flora;
- contaminated lubricants, dyes, irrigation fluid or rinse water;
- inadequate decontamination of the reprocessing equipment.
Staff should be aware of the complexities of the endoscopes they are processing to ensure that the construction of the endoscope is fully understood. Failures in decontamination, particularly for flexible endoscopes, have been reported due to failure to access all channels of the endoscope. Irrespective of the method of disinfection or sterilization, cleaning is an essential stage in the decontamination procedure and the manufacturers’ instructions should be followed at all times. An endorsement of compatibility of the endoscope with the decontamination process is essential.
Rigid endoscopes are relatively easy to clean, disinfect and sterilize as they do not have the sophistication of functionality, construction and channel configuration and compatibility issues that exist with flexible endoscopes. Where possible, all reprocessing of autoclavable endoscopes and their accessories should take place in a sterile services department or dedicated decontamination unit as the process controls and validation are already in place. It should never take place in the clinical area (17).
Flexible endoscopes are heat-sensitive and require chemical disinfection (or low temperature disinfection) (18). Decontamination of flexible endoscopes should take place in a dedicated well-ventilated room (up to 12 air changes per hour) away from the procedure room. There should be adequate ventilation to remove potentially harmful disinfectant vapour. The room should be equipped with a sink with sufficient capacity to accommodate the largest endoscopes and a dedicated wash hand basin equipped with soap and disposable paper towels.
There should be a workflow direction within the room from dirty to clean to avoid the possibility of recontamination of decontaminated endoscopes from those just used on a patient. Systems should be in place to indicate which endoscopes are ready for patient use and recorded either manually or by an automated endoscope reprocessor. Modern units will have a 2-room system with pass-through washer disinfectors to separate the clean and dirty areas. Storage of endoscopes should be organized to avoid any recontamination of processed endoscopes. There should be sufficient storage for the consumables used during the decontamination procedure, for example, personal protective equipment, chemicals, cleaning brushes and sufficient capacity for waste disposal.
References
- 1.
- Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27(4):665–90. [PMC free article: PMC4187643] [PubMed: 25278571]
- 2.
- Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Morbid Mortal Wkly Rep (MMWR). 2003. [accessed 16 February 2016]. pp. 1–48. http://www
.cdc.gov/hicpac /pdf/guidelines/eic_in_HCF_03.pdf. [PubMed: 12836624] - 3.
- Health technical memorandum (HTM) 03-01: heating and ventilation of health sector buildings. Leeds: Department of Health; 2007. [accessed 16 February 2016]. https://www
.gov.uk/government /publications /guidance-on-specialised-ventilation-for-healthcare-premises-parts-a-and-b. - 4.
- Best practices for environmental cleaning for prevention and control of infections in all health care settings. 2nd edition. Ontario: Provincial Infectious Diseases Advisory Committee; 2012. [Accessed 16 February 2016]. https://www
.publichealthontario .ca/en/eRepository /Best_Practices _Environmental_Cleaning_2012.pdf. - 5.
- Woodhead K, Taylor EW, Bannister G, Chesworth T, Hoffman P, Humphreys H. Behaviours and rituals in the operating theatre. J Hosp Infect. 2002;51:241–55. [accessed 16 February 2016]; http://www
.his.org.uk /files/2313/7338/2940/Theatre_rituals .pdf. [PubMed: 12183138] - 6.
- Spruce L, Wood A. Back to basics: environmental cleaning. AORN J. 2014;100:55–61. [PubMed: 24973185]
- 7.
- Perioperative standards and recommended practices. Denver (CO): AORN, Inc; 2014. Recommended practices for environmental cleaning; pp. 255–76.
- 8.
- ISO 17665-1:2006. Sterilization of health care products - moist heat - Part 1: requirements for the development, validation and routine control of a sterilization process for medical devices. Geneva: International Organization for Standardization; 2006.
- 9.
- ISO/TS 17665-2:2009. Sterilization of health care products - moist heat - Part 2: guidance on the application of ISO 17665-1. Geneva: International Organization for Standardization; 2009.
- 10.
- ISO/TS 17665-3:2013. Sterilization of health care products - moist heat - Part 3: guidance on the designation of a medical device to a product family and processing category for steam sterilization. Geneva: International Organization for Standardization; 2013.
- 11.
- ISO 15883-2:2006. Washer-disinfectors - Part 2: requirements and tests for washer-disinfectors employing thermal disinfection for surgical instruments, anaesthetic equipment, bowls, dishes, receivers, utensils, glassware, etc. Geneva: International Organization for Standardization; 2006.
- 12.
- Hirsch T, Hubert H, Fischer S, Lahmer A, Lehnhardt M, Steinau HU, et al. Bacterial burden in the operating room: impact of airflow systems. Am J Infect Control. 2012;40:e228–32. [PubMed: 22542026]
- 13.
- Khan MA, Manan F, Khan A, Ahmad I, Alam Q. Pattern of isolation and sensitivity in surgical patients presented to Khyber teaching hospital. Khyber J Med Surg. 2013;6:222–25.
- 14.
- Health building note 13 (HBN13): sterile services department. Norwich: Her Majesty’s Stationery Office; 2004. http://www
.gov.uk/government /uploads/system /uploads/attachment_data /file/148489/HBN_13.pdf. - 15.
- Health technical memorandum 01-01: management and decontamination of surgical instruments (medical devices) used in acute care. London: Department of Health; 2013. [accessed 16 February 2016]. https://www
.gov.uk/government /publications /management-and-decontamination-of-surgical-instruments-used-in-acute-care. [PubMed: 23413631] - 16.
- Spaulding EH. Chemical disinfection of medical and surgical materials. In: Lawrence CA, Block SS, editors. Disinfection, sterilization and preservation. Philadelphia (PA): Lea & Febiger; 1968. pp. 517–31g.
- 17.
- Kovaleva J, Peters FT, van der Mei HC, Degener JE. Transmission of infection by flexible Gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013;26(2):231–54. [PMC free article: PMC3623380] [PubMed: 23554415]
- 18.
- Health technical memorandum 01-06: decontamination of flexible endoscopes. London: Department of Health; 2013. [accessed 16 February 2016]. https://www
.gov.uk/government /publications /management-and-decontamination-of-flexible-endoscopes.
Publication Details
Copyright
All rights reserved. Publications of the World Health Organization are available on the WHO website (http://www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; email: tni.ohw@sredrokoob).
Requests for permission to reproduce or translate WHO publications–whether for sale or for non-commercial distribution–should be addressed to WHO Press through the WHO website (http://www.who.int/about/licensing/copyright_form).
Publisher
World Health Organization, Geneva
NLM Citation
Global Guidelines for the Prevention of Surgical Site Infection. Geneva: World Health Organization; 2016. 3, IMPORTANT ISSUES IN THE APPROACH TO SURGICAL SITE INFECTION PREVENTION.


