NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Rostom A, Dube C, Lewin G. Use of Aspirin and NSAIDs to Prevent Colorectal Cancer [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007 Mar. (Evidence Syntheses, No. 45.)
This publication is provided for historical reference only and the information may be out of date.
Our comprehensive literature search yielded 1,788 citations. Screening yielded 362 potentially relevant articles that were obtained in full for further review. Of these, 66 studies met our eligibility criteria and were included in the evidence report. More than half of these articles (n=39) were companion or duplicate articles., and nineteen of these were excluded on that basis, 15 – 33 (Table 2, Appendix 8) as well as two 34, 35 of four 34 – 37 studies from different authors with overlapping patient populations. Although excluded, the duplicate and companion articles were used to fill in any missing data not reported in the articles that we used. One study was also excluded because the patient population encompassed a significant proportion of subjects with a personal history of CRC. 38 The final study sample included 39 unique studies of effectiveness and five economic evaluations (see Figure 2 [Appendix 8], QUOROM flowchart [Appendix 7], and Table 3 [Appendix 8]). Five 39 – 43 of the included 44 articles and an additional one 44 addressed the question of the effect of chemoprevention on FOBT.
Table 2
CRC/NSAIDS review: list of duplicates and related studies grouped by name*.
Table 3
CRC/NSAIDS review: list of included studies*.
We identified a great deal of variability in the conduct of the observational studies. These differences centered predominately on the methods of: ascertainment of cases and controls; NSAID exposure measurement and its ascertainment; and, ascertainment of the outcomes of interest. As described in the methods, the presented framework (Figure 1, Appendix 8) was used to divide the studies into appropriate subgroups, based on both clinical and statistical factors.
Key Question 1A
Does aspirin/NSAID use in healthy adults (>18 years of age) decrease CRC mortality and/or all-cause mortality?
Two cohort studies assessed the effect of ASA and non-ASA NSAIDs on CRC-related mortality. Thun et al., 45 in a large study of 662,424 patients, found a statistically significant reduction in CRC mortality for men (relative risk [RR]=0.58; 95% CI: 0.36–0.93) and women (RR=0.61; 95% CI: 0.38–0.97) without a history of colon cancer at enrolment (i.e., average risk) and use of ASA for greater than 15 years. The results for ASA use for 1 to 15 years was statistically significant for men (RR=0.72; 95% CI:0.52–0.99), but not women (RR=0.72; 95% CI: 0.51–1.02). Lipworth et al., 46 in a study of 113,538 participants who filled at least one ibuprofen prescription over a 6-year period, reported increases in all-cause mortality (SMR=1.21; 95% CI: 1.19–1.24) and no reduction in mortality from bowel (SMR=1.05; 95% CI: 0.9–1.2) or rectal cancer (SMR=1.26; 95% CI: 1.0–1.5).
Key Question 1B
Does aspirin/NSAID use in healthy adults (>18 years of age) decrease CRC incidence?
RCT data
Only one RCT, the Physicians Health Study (PHS), assessed the effect of ASA on CRC incidence. 47 This study failed to demonstrate a benefit of ASA use on invasive CRC prevention (at 5 yrs: RR=1.15; 95% CI: 0.80–1.65; at 12 yrs: RR=1.03; 95% CI: 0.83–1.28). 47 This study also failed to demonstrate a statistically significant difference in stage of CRC at diagnosis, nor differences in rectal bleeding between the ASA and placebo groups.
Observational study data
The effectiveness of ASA/NSAID chemoprevention on CRC incidence was assessed in eight cohort 42, 43, 48 – 53 and 12 case-control studies. 36, 54 – 64 A detailed description of the included studies, including their study population and outcome measures, are presented in Evidence Tables 1.2, 1.3, 2.2, and 2.3 (Appendix 8).
Cohort study data: CRC incidence
Regular use of ASA in average-risk individuals was assessed in five studies. 42, 48 – 50, 52 One study demonstrated a non-significant increased risk of CRC with ASA use in men and women separately, and due to its method of reporting, we could not use it in the statistical analysis. 53 Together, the remaining four studies demonstrated a 22% reduction in CRC incidence (RR = 0.78; 95% CI: 0.63–0.97) (Figure 2).

Figure
Figure 2. Cohort studies—average risk population and CRC incidence.
Four studies assessed the effect of duration of ASA use on CRC incidence. 42, 48, 49, 52 Two studies showed no statistically significant reduction of CRC regardless of duration. 48, 52 One study demonstrated no benefit of ASA use for less than 5 to 9 years, but a statistically significant reduction in CRC risk with greater than 20 years of use (RR=0.56; 95% CI: 0.36–0.9). 49 Another report demonstrated a statistically significant reduction in the risk of CRC with ASA use of more than twice per week for more than 2 years (RR= 0.54; 95% CI: 0.34–0.83) and for more than 4 years (RR=0.35; 95% CI: 0.16–0.75). 48
The effect of non ASA-NSAIDs on CRC incidence was assessed in one study which also provided data on duration of use and dose response. 43 Overall, patients with greater than 12 months of non-ASA NSAID use, including use in the preceding year, had a statistically significant reduction in CRC incidence (RR= 0.61; 95% CI: 0.48–0.77). In the dose analyses, only the larger sample “medium” dose endpoint reached statistical significance (RR=0.59; 95% CI: 0.45–0.77).
Use of non-ASA NSAIDs for 1 to 3 years was associated with statistically significant reductions in CRC incidence (RR=0.65; 95% CI: 0.48–0.87), but just failed to reach statistical significance at 4 to 6 years (RR=0.49; 95% CI: 0.24–1.0) (Figure 2).
Case-control data: CRC frequency
Significant heterogeneity precluded us from combining studies reporting the effect of regular use of ASA on CRC frequency (Figure 3). Four studies reported widely varying statistically significant reductions in the RR of CRC with regular ASA use (RR=0.3 to 0.98), 33, 56, 57, 59 while the other three studies reported non-significant trends in favour of ASA use (RR=0.8–0.9). 36, 54, 62 These studies differed considerably in the methods for assessment of ASA exposure and outcome assessment.

Figure
Figure 3. Case-control studies—CRC incidence.
The effect of duration of ASA use on CRC frequency was assessed in five studies. 23, 33, 36, 54, 56 The RRs of CRC with ASA use of 1 to 3 years, and 4 to 6 years were 0.85 (95% CI: 0.72–1.0) and 0.68 (95% CI: 0.54–0.87), respectively. A single study assessed longer durations of use from 7 to 9 years to greater than 14 years, with the individual estimates not reaching statistical significance. 23 Four studies assessed the effect of the recency of use of ASA in this setting. 23, 33, 36, 54 ASA use greater than 1 year from study onset did not reduce the RR of CRC (RR= 0.99; 95%CI: 0.84–1.17), 33, 36, 54 but its use within 1 year of study onset resulted in a statistically significant reduction in the RR of CRC in two 23, 33 of the four studies. 23, 33, 36, 54 (Heterogeneity precluded pooling.)
Dose response was assessed in two studies. 23, 36 Rosenberg assessed dosages from 162.5 mg/day to greater than 650 mg/day in a small sample of patients, and found that only the 325 mg/day was associated with reductions in CRC frequency (RR=0.60; 95% CI: 0.5–0.9). 23 Rodriguez assessed dosages of 75 mg to 300 mg/day, and found that only the 300 mg/day dose resulted in a statistically significant reduction in the frequency of CRC (RR=0.60; 95% CI: 0.4–0.9). 36
Based on four studies, regular use of non-ASA NSAIDs was associated with reductions in CRC frequency.(RR=0.70; 95%: 0.63–0.78). 33, 36, 57, 62 An additional study found a statistically significant reduction in CRC frequency (OR=0.30; 95% CI: 0.08–0.98), but could not be pooled with the others because of the method it used to quantify regular NSAID use. 59 The same analysis for “any NSAID” showed significant statistical heterogeneity. However, five of six studies demonstrated a statistically significant reduction in CRC frequency. 23, 55, 58, 63, 64
Duration of use was assessed in one study for non-ASA NSAIDs, 36 and in four studies for “any NSAID.” 23, 55, 62, 63 Some of the subgroups demonstrated significant heterogeneity. The pooled estimates reached statistical significance for “any NSAID” use for the durations of 4 to 6 years (RR=0.38; 95% CI: 0.23–0.620), and for 10 years or greater (RR=0.56; 95% CI: 0.39–0.82).
Dose response was assessed by Peleg using the “calculated cumulative dose” (CCD; defined based on NSAID dosage equivalence and cumulative use). “Any NSAID” use at the moderate (320–700 mg) and high CCDs (>700 mg) was associated with a statistically significant reductions in CRC frequency (RR=0.19; 95% CI: 0.09–0.52, and RR=0.22; 95% CI: 0.09–0.56, respectively). No significant reductions in frequency were observed with use of “any NSAID” at the lowest CCD (RR=0.58; 95% CI: 0.26–1.32).
In this analysis group, regular use of ASA and non-ASA NSAIDs appear to reduce the incidence of CRC. Recent use, and increased dose and duration of use, appear to result in a greater reduction of CRC incidence.
Key Question 2
What is the magnitude of decreased CRA incidence due to aspirin/NSAID chemoprevention in healthy adults?
RCT data
Four RCTs assessed the effectiveness of chemoprevention on CRA incidence (Figure 4; Appendix 8, Evidence Tables 1.1 and 2.1). 47, 65 – 67 Two of these studies assessed the effect of ASA in patients with a prior history of CRAs, 65, 66 and one assessed the effect in average-risk individuals. 47 Overall, the use of ASA in doses of 81–325 mg/day resulted in modest reductions in CRAs. These reductions reached statistical significance in two studies. 65, 66 The PHS 47 failed to show a statistically significant reduction in CRAs with ASA (RR=0.86; 95% CI: 0.68–1.1). Baron et al. 65 found a statistically significant benefit when participants took 81 mg of ASA but not 325 mg, for prevention of recurrence of any (RR=0.83; 95% CI: 0.70–0.98 - for 81 mg) or advanced CRAs (RR=0.58; 95% CI: 0.37–0.90 - for 81 mg), respectively. Neither dose was effective at reducing the recurrence of small CRAs (<1.0 cm). In the last study, Benamouzig et al. 66 reported statistically significant reductions in the mean number of CRAs (0.45 vs 0.86 for ASA and placebo, respectively, p=0.01), the recurrence of greater than three CRAs (RR=0.3; 95% CI: 0.1–0.89) or one or more CRAs greater than 1.0 cm (RR=0.44; 95% CI: 0.24–0.82). There were no statistically significant reductions for CRAs greater than 1.0 cm, or for the various categories of advanced adenomas. These authors also reported no statistically significant difference in CRA recurrence between the 160 mg of lysine ASA and 300 mg groups (35% vs 25%, respectively; p=0.23). When these last two studies were combined, a statistically significant reduction in CRA incidence was observed (RR= 0.82; 95% CI: 0.7–0.95) for low-dose ASA.

Figure
Figure 4. RCT studies—incidence of adenomas.
One RCT assessed the effect of sulindac (non-ASA NSAID) on the regression of CRAs (<1.0 cm) found at flexible sigmoidoscopy. 67 In this short 4-month study, 300 mg of sulindac failed to show a statistically significant regression of the identified CRAs.
The results of this analysis group were mixed. Two RCTs showed a reduction in CRAs with ASA. 68, 69 On the other hand, the large PHS did not show a benefit of ASA on CRA incidence. However, participants in this study were relatively young males (mean age 53.2 years) who used a relatively small amount of ASA (325mg of ASA every second day). Participants were also not necessarily free of CRAs at study onset, and outcomes were collected through mailed questionnaires. In contrast, the two positive RCTs were colonoscopy-based studies in populations with a prior history of CRAs, and required an absence of polyps at the start of the study.
Observational study data
The effectiveness of ASA/NSAID chemoprevention on CRA incidence was assessed in four cohort studies 39, 42, 70, 71 and 12 case-control studies. 26, 37, 41, 56, 63, 72 – 78 A detailed description of the included studies, including their study population, and outcome measures are presented in Evidence Tables 1.2, and 1.3 (Appendix 8). A summary of the breakdown of the study types, numbers, and outcome measures is presented in Table 1 (Appendix 8). The age and gender distributions of the included studies are presented in Table 6 (Appendix 8).
Table 1
Hierarchical framework for data analysis.
Table 6
Summary of study populations.
Cohort study data: CRA incidence
Regular use of ASA in average-risk individuals was associated with a statistically significant reduction in CRA occurrence (RR=0.72; 95% CI: 0.61–0.85). 39, 42 The effect was similar for small and large polyps, and for polyps with advanced histology. 39 A single study of average-risk women assessed the effect of duration of ASA use, and demonstrated a non-significant trend towards a reduction in CRA incidence with increased duration of use from 1 to 3 years to greater than 20 years. 39 This same study also assessed dose response. Taking less than five ASA tablets per week did not significantly reduce the risk of CRA, but taking 6 to 14 or greater than 14 tablets were each associated with a statistically significant reduction in CRA incidence (RR= 0.68; 95% CI: 0.55–0.84; and RR=0.57; 95% CI: 0.42–0.77, respectively) (Figure 5).

Figure
Figure 5. Cohort studies—incidence of adenomas (CRA).
In two studies, regular use of ASA by higher-risk patients appeared to reduce the risk of CRAs, 70, 71 although this effect reached statistical significance in only one study (RR=0.52; 95% CI: 0.31–0.89). 70 In Tangrea et al., 71 doses of ASA greater than 325 mg/day were associated with a statistically significant reduction in the risk of CRAs (RR=0.54; 95% CI: 0.3–0.96). The same study also found that regular use of any NSAID in higher-risk patients reduced the incidence of CRAs (RR= 0.64; 95% CI: 0.48–0.85) (Figure 5).
Case-control data: CRA frequency
Regular use of ASA in average-risk individuals significantly reduced CRA frequency in a pooled analysis of five studies (RR=0.87; 95% CI: 0.77–0.98) (Figure 6). 37, 56, 72, 77, 79 The duration and dose response for average-risk individuals was assessed in a single study that reported a non-significant trend in favour of higher ASA dose and longer duration of use. 37
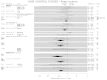
Figure
Figure 6. Case-control studies—incidence of adenomas.
The regular use of non-ASA NSAIDs 37, 72, 73, 79 or “any NSAID” 41, 63, 72, 74, 75 in average-risk individuals was associated with significant reductions in CRA frequency (RR=0.54 [95% CI: 0.4–0.74] and RR= 0.57 [95% CI: 0.46–0.71], respectively). Also with “any NSAID” use, there were statistically significant reductions in CRAs when these agents were used for less than 5 years (RR=0.55 [95% CI: 0.39–0.77]; and even greater reductions with use longer than 5 years (RR= 0.43 [95% CI:0.26–0.70]. 41, 63, 72, 74 Dose response was assessed in a single study, 63 which reported a statistically significant reduction in CRA frequency (RR=0.31; 95% CI: 0.11–0.84) for the highest CCD, but not for the lower CCDs (Figure 6).
A single study assessed ASA and non-ASA NSAID use in higher risk individuals, using both population and hospital-based controls. 76 When using the population-based controls, the reduction in CRAs did not reach statistical significance for any endpoint except for the “any NSAID use” for greater than 5 years (RR=0.21; 95% CI: 0.04–0.99). Likewise, using hospital-based controls, only the reduction in CRAs with ASA for greater than 5 years was associated with significant reductions in CRA frequency (RR=0.09; 95% CI: 0.01–0.82) (Figure 6).
Overall, the data suggests that regular use of ASA and non-ASA NSAIDs reduces the risk of CRA. Higher doses, and longer duration of use (trend), appear to be more effective at reducing CRA incidence than low-dose, short-term use. In this analysis group, dose response, duration, and higher-risk patient data was limited.
Key Question 3
What is the magnitude of decreased CRA incidence on CRC in healthy adults?
Given the wide acceptance of the polyp to cancer pathway (and association), it was felt that there was no need to re-establish this association for the current report.
Key Question 4
What is the magnitude of harms of aspirin/NSAID use in healthy adults (i.e., increased major GI bleeding, hemorrhagic stroke or nephropathy)?
Twenty-eight systematic reviews were identified for this question. The term review will be equivalent to systematic review in all the descriptions made below. The review characteristics and results are described in the set of Evidence Tables 3 (Appendix 8).
4a. Harms due to aspirin use
There were 11 reviews addressing the magnitude of harms due to ASA use in an adult population (Evidence Table 3.1, Appendix 8), 2, 80 – 89 none of which addressed its nephrotoxocity.
General. Five systematic reviews addressed general ASA harms in the adult population. 2, 80 – 83 The outcomes measured were: all-cause mortality, mortality due to harms, and withdrawal due to harms with the use of ASA.
All-cause mortality. All-cause mortality was measured in all the reviews. 2, 80 – 83 However, mortality and withdrawals due to harms with aspirin use were not reported across the reviews.
For primary prevention, a non-significant reduction in mortality rate was observed for ASA compared with placebo: OR=0.93 (95% CI: 0.84–1.02) 2 and RR=0.94 (95% CI: 0.87–1.01). 81 For secondary prevention, a significantly lower all-cause mortality rate was detected with ASA compared with placebo: RR: 0.82 [95% CI: 0.70–0.99] 83 and RR: 0.85 [95% CI: 0.8–0.9] 82 The all-cause mortality did not differ between ASA alone, oral anticoagulant drugs (OAD) or a combination of OAD and ASA 80 , yet one trial (ASPECT-2) observed a significantly lower mortality rate in the OAD group compared with ASA alone (1.2% vs. 4.5%, p<0.05). 90
CV harms: There were seven reviews addressing the magnitude of CV harms associated with ASA use in an adult population. 2, 80 – 85 The CV events reported were: acute MI, stroke (all, hemorrhagic or ischemic), and death due to CV events.
Three reviews reported the mortality due to CV events. 2, 81, 82 For primary prevention, mortality due to CV events was not significantly different between ASA and placebo [OR: 0.87 (95% CI: 0.70–1.09) 2 and RR: 0.93 [95% CI: 0.83–1.03]. 81 For secondary prevention, there was a significant reduction in the mortality due to CV events with ASA (RR: 0.84 [95% CI: 0.79–0.90]). 82
Six reviews reported the risk of acute MI with ASA use. 2, 80 – 83, 85 For primary prevention, a significantly lower risk of MI was reported for ASA compared with placebo in two reviews [OR: 0.72 (95% CI: 0.60– 0.87) 2 and 0.74 (95% CI: 0.68–0.82)] 81 . In a third review, although the data was not pooled, a significant absolute risk reduction in MIs was reported in one trial comparing the use of ASA with placebo in patients with arterial hypertension (ARR=0.5%, NNT=200) 85 For secondary prevention, two reviews reported a significant reduction in MI risk with ASA use compared with placebo (RR: 0.68 [95% CI: 0.62–0.74] 82 and RR: 0.7 [95% CI: 0.7–0.9] 83 ). In one review of ASA use compared with OAD use, one RCT showed a significantly lower incidence of MI (WARIS-II: ASA= 9.7% vs. OAD= 7.4%, p<0.001), while the other trials showed no difference. 80
Six reviews reported on the risk of acute stroke (including hemorrhagic and ischemic stroke) with ASA use. 2, 81 – 85 In primary prevention trials, the risk of stroke was no different between ASA and placebo: OR=1.02 [95% CI: 0.85–1.23] 2 and RR=1.20 (0.96–1.49) 81 in healthy males; RR=1.02 (95% CI: 0.86–1.21) in patients with vascular risk factors 81 ; and, OR=0.94 [95% CI: 0.76–1.17] in patients with hypertension. 85 One review also reported a non-significant OR of 1.4 (95% CI: 0.9–2.0) for hemorrhagic stroke. 2 For secondary prevention, the overall risk of stroke was not statistically different between ASA and placebo (RR 0.88 [95% CI: 0.76–1.02] 82 and RR of 0.8 [95% CI: 0.7–1.0], 83 respectively). However, the risk of hemorrhagic stroke was increased by 84% with ASA use (RR: 1.84 [1.24–2.74]). 82 In secondary prevention trials, Serebruany et al. found higher rates of hemorrhagic stroke with higher doses of ASA used (ASA <100 mg/day: 0.3% [95% CI: 0.2–0.4]; ASA 100–325 mg/day: 0.3% [95% CI: 0.2–0.3]; ASA >325 mg/day: 1.1% [95% CI: 0.7–1.5]), while the risk of ischemic stroke was decreased by 18% (RR: 0.82 [0.73–0.92]). 82
GI harms. GI harms of aspirin (ASA) were considered in seven reviews. 2, 83, 84, 86 – 89 The included reviews summarized data from RCTs, 2, 83, 84, 86, 88, 89, 91 cohort, 2, 87, 88 and case-control studies, 87, 88 and some considered both low and high doses of ASA. 84, 92
ASA was consistently associated with a statistically significant elevated risk of GI bleeding (Evidence Table 3.1, Appendix 8). In the systematic reviews of RCTs, this increase in RR ranged from 1.6 to 2.5 times that seen with non-ASA users; in the systematic review of cohorts it was 2.2 times, and in the systematic review of case controls it was 3.1 times. The use of ASA was also associated with an increased risk of adverse GI symptoms such as nausea and dyspepsia (OR: 1.7 [95% CI:1.5–1.8]). 89
A dose effect has been suggested for ASA-induced GI toxicity. One systematic review pooled GI bleeding incidence among large CV studies and found that 2.5% (95% CI: 2.2–2.6) of patients taking >100 mg of ASA/day suffered a GI bleed compared with 1.1% (95% CI: 0.9–1.3) taking less than 100 mg/day. 84 Tramer et al. found that ulcer bleeds or perforation occurred in 0.34% and 0.86% of patients taking low (325 mg q 2 days) and high dose ASA (2.5–5.2 g per day), respectively (difference statistically significant). 88 Similarly, Roderick et al. found a greater risk of GI bleeding with high-dose ASA (1600 mg) (OR: 2.8 [95% CI: 1.3–5.7]), than with a lower dose of 300 mg/day (OR: 1.6 [95% CI: 0.7–4.0). 89 Another systematic review of RCTs demonstrated an increased risk of GI bleeding with low-dose ASA (50–162.5 mg) (RR: 1.59 [95% CI: 1.40–181]), but the rate of GI bleeding with the higher dose (>162 mg) was not statistically different (RR: 1.68 [95% CI: 1.51–1.88]). 86
Hayden et al. 2 estimated that 3/1000 middle-aged men would suffer a GI bleed over a 5-year period of continuous ASA use, and the rate would be as high as 2/1000 patients per year if older, higher-risk patients were considered. Roderick et al. also suggested that the GI bleeding rate with ASA (300 mg) is 60% higher than with placebo, and represents an attributable rate of 2.5 events/1000 patient-years. 89 The risk of hospitalization because of GI bleeding is also increased (OR: 1.9 [95% CI: 1.1–3.1]), though death from GI bleeding per se is rare. 89 Of the reviews that reported on this latter outcome, 83, 88, 89 only one death was recorded with ASA use. 88
4b. Harms due to NSAID use (other than aspirin or COX-2 inhibitors - non-ASA NSAIDs)
CV harms. Only one of the included reviews reported on the CV harms of non-ASA NSAIDs. 93 This cumulative meta-analysis of RCTs of the CV harms of rofecoxib (described below) also reported a systematic review of 11 observational studies of the risk of MI with NSAIDs. Ten of those studies used data from large administrative or clinical databases. The pooled analysis (but not a cumulative one) found a small statistically significant protective effect with naproxen (OR: 0.86; 95% CI: 0.75–0.99). Similar results were obtained when analyses were based on comparisons with non-naproxen NSAIDs (OR: 0.86; 95% CI: 0.75–0.99). These results, particularly the first one, need to be interpreted with caution, especially since there was substantial inter-study heterogeneity in the analyses (I2 = 68%). Unfortunately, this meta-analysis did not report on the risk of CV harms with non-naproxen NSAIDs.
GI harms. The included systematic reviews of the GI harms of NSAIDs summarized data from RCTs, 88, 94 – 97 cohort, 88, 94, 98 and case-control studies. 88, 94, 98 Two of the systematic reviews of RCTs focused mainly on prevention of NSAID-induced upper GI toxicity through the use of prophylactic agents or the use of COX-2 inhibitors. 96, 97 One of these reported on the rate of GI complications in patients taking NSAIDs. 96
The doses of NSAIDs described in the included systematic reviews were those generally indicated for that particular NSAID. Dose effects were defined differentially by doses of a given NSAID within a study and/or by comparison to other NSAIDs using dose equivalency tables.
All the included studies reported an increased risk of peptic ulceration and GI hemorrhage with NSAIDs use. Among those taking these medications for greater than 4 weeks, 19% have gastric ulcers, 96 6% duodenal ulcers, 96 and approximately 20% to 24% have gastroduodenal ulcers greater than 3 mm in size. 88, 96 If any ulcers are considered, estimates as high as 40% have been reported, though it is felt that over 80% of these are not clinically significant. 96 The best RCT evidence of the risk with NSAIDs of complicated peptic ulcers, that is, ulcers with perforation, obstruction or bleeding (POB), was derived from the original MUCOSA study, 11, 96, 99 and collaborated by data from the NSAID arms of the COX-2 inhibitors trials. 96, 100, 101 These studies demonstrated a POB rate of approximately 1.5% to 2% per year in average-risk individuals taking standard non-ASA NSAIDs. The risk of POBs is considerably higher and can reach 10% in higher-risk individuals, such as those with previous peptic ulcer, older age, and comorbid conditions, such as CV disease. 11, 96, 100, 102
In a systematic review by Ofman et al., 94 the risk of perforation or bleeding in NSAID users compared with non-users was elevated in the pooled analyses for RCTs (OR= 5.36; 95% CI: 1.79–16.1), cohort (RR=2.7; 95% CI: 2.1–3.5), and case-control studies (OR=3.0; 95% CI: 2.5–3.7). The same authors assessed the risk of adverse GI symptoms with NSAIDs in a separate publication. 95 Overall, 4.8% of patients reported dyspeptic symptoms. Dyspepsia was greater with higher NSAID doses (RR=2.6; 95% CI: 1.5–4.5) and high dyspepsia NSAIDs (indomethacin, meclofenamate, and piroxicam) (RR=2.2;1.5–3.2), than with low-dose NSAIDs overall (RR=1.3; 95%CI: 0.9–1.8), although these confidence intervals overlapped.
In a study that predominately looked at the risk of NSAID complications in relation to H. pylori status, Huang et al. 98 found that NSAIDs alone were associated with a statistically significant increased risk of endoscopically-detected ulcers (OR: 5.14; 95% CI: 1.35–19.6) and peptic ulcer bleeds (OR:4.79; 95% CI: 3.78–6.06). Tramer et al. reported that peptic ulcer bleeds occurred in 4.8% of patients taking NSAIDs. 88 The results of this systematic review were reported as the absolute risk difference (ARD) for those taking NSAIDs compared with non-NSAID users. The authors found that, among the included RCTs, the ARD for NSAID-induced perforation or bleeding was 0.48%, while it was 0.22% in the included cohort studies. The ARD for death for users of ASA and non-ASA NSAIDs was 0.008% in RCTs and cohort studies. The authors estimated that the RR of death from NSAIDs was 3.4, and suggested that it may be an underestimate (95% CI: 1.3–8.7). 88 Using a biological progression model based on the proportion of patients with NSAID ulcers that subsequently bleed, Tramer et al. estimated that the increased risk of death among NSAID users was closer to 0.08% (ARD). 88
The risk of non-ASA-NSAID-induced upper GI toxicity can be reduced through the use of a concomitant prophylactic agent. Only one study of the use of these agents, MUCOSA, 99 considered the reduction of clinically important NSAID ulcer complications such as perforation or bleeding. Here, misoprostol was associated with a 40% RRR (OR: 0.598; 95% CI: 0.364–0.982) in combined clinical ulcer complications. 11, 96, 99 The remaining agents (H2-receptor antagonists and proton pump inhibitors) have only been evaluated in endoscopic ulcer studies. 11, 96 In the systematic review by Rostom et al., 11, 96 double-dose H2-receptor antagonists (equivalent to ranitidine 300 mg twice daily) were associated with statistically significant reductions in the risk of duodenal (RR: 0.26; 95% CI: 0.11–0.65) and gastric ulcers (RR: 0.44; 95% CI: 0.26–0.74). Standard dose H2-receptor antagonists were not effective at reducing the risk of NSAID induced gastric ulcers. Proton pump inhibitors significantly reduced the risk of both endoscopic duodenal (RR: 0.19; 95% CI: 0.09–0.37) and gastric ulcers (RR: 0.40; 95% CI: 0.32–0.51). 11, 96
4c. Harms due to COX-2 inhibitor use (including COX-2 selective)
Fourteen systematic reviews investigated the harms due to COX-2 inh use (Evidence Table 3.3, Appendix 8, and Appendix 4 for full text).
COX-2 inhibitors appear to be better tolerated, and have fewer adverse effects causing withdrawal than standard NSAIDs. These agents cause significantly fewer GI symptoms, endoscopically detected ulcers, and clinically important ulcer complications (perforation, hemorrhage or bleeding) than standard NSAIDs. The coadministration of ASA with a COX-2 inhibitor appears to abolish the GI safety advantage of these agents. COX-2 inhibitors have been suggested to have a greater risk of CV events than placebo. The data on the CV harms are rapidly changing and are detailed further in the discussion and Appendix 4.
Other Considerations
What is the cost and cost effectiveness of aspirin/NSAID use in preventing CRC?
Five economic evaluations of the use of NSAIDs in adenoma and/or CRC prevention were identified (Evidence Table 4). 40, 103 – 106 All the studies used decision analysis and a Markov model within a U.S. economical and clinical practice context.
A. NSAID chemoprophylaxis in average-risk populations
Suleiman et al. 103 simulated a population of 100,000 50-year-old average-risk subjects followed until death. The model included four mutually exclusive interventions: 1) no screening, no chemoprevention; 2) colonoscopy every 10 years (every 3 years if adenomas were identified); 3) ASA 325 mg per day; and, 4) colonoscopy every 10 years along with ASA 325 mg per day.
Key probabilities and assumptions are presented in Evidence Table 4 (Appendix 8). The efficacy of colonoscopy at preventing CRC was 75%; that of ASA was 50%, and that of ASA combined with colonoscopy was 87.5%. The authors also assumed that ASA use affected polyp growth in the same magnitude as it affected CRC growth. Subject compliance with the intervention was assumed to be 100%, and the effect of ASA on CV outcomes was not incorporated into the model.
Costs were in U.S. dollars, discounted at 3% from a third-party payer perspective. The cost of colonoscopy was $696, that of ASA, including both drug cost and that of complications, was $172 per patient per year.
Colonoscopy every 10 years saved 7,951 LYs (life years), at a total cost of $223,780,829; chemoprevention saved 5,301 LYs at a total cost of $386,920,810. Compared with no intervention, the incremental cost-effectiveness ratios (ICERs) were $10,983, $47,249 and $41,929 per LY saved for colonoscopy, chemoprevention and colonoscopy/chemoprevention, respectively. When added to colonoscopy every 10 years, the ICER of ASA 325 mg daily is $227,607/LY saved. Sensitivity analysis showed that the costs of chemoprevention (which includes drug cost and cost of complications) need to fall below $70 per patient per year to become more cost-effective than colonoscopy.
The authors concluded that daily ASA use as chemoprevention for CRC was at present not cost-effective because of the relatively large costs associated with its adverse effects, as well as its relative inefficacy compared with colonoscopy. 103
In a very similar model, Ladabaum et al. 40 evaluated the cost effectiveness of ASA at 325 mg per day in average-risk U.S. subjects, compared with two different screening strategies: (1) colonoscopy every 10 years (every 5 years if polyps were discovered) (“COLON”); and (2) flexible sigmoidoscopy every 5 years combined with yearly fecal occult blood testing (“FS/FOBT”). In the later strategy, any positive test would be followed by colonoscopy.
The model simulated an infinitely large population of 50-year-old average-risk U.S. subjects followed until the age of 80.
Key probabilities and assumptions are presented in Evidence Table 4 (Appendix 8). It was assumed that the efficacy of ASA at preventing CRC was 30% (lower than in Suleiman et al.'s study 103 ), and that the compliance with screening was only 25%. The effect of ASA on CV outcomes was modelled in sensitivity analysis.
Costs were also in U.S. dollars, discounted at 3% and from a third-party payer perspective. The cost of colonoscopy was comparable to Suleiman et al.'s study, that of ASA therapy $4 per patient per year, with a probability of ASA-related complications of 2–16 per 10,000 patient-years, each event costing $15,000.
Results show that, compared with no screening, FS/FOBT or COLON markedly reduced CRC mortality. When ASA was added to no screening or to screening, the additional decrease in cancer deaths was offset by ASA-related deaths. The ICERs for screening were $16,844 for each life-year ($16,844/LY) saved and $20,172/LY saved for FS/FOBT and COLON, respectively. As an adjunct to FS/FOBT, ASA increased costs and decreased LYs because of related complications. Sensitivity analysis showed that the cost effectiveness of daily ASA use as chemoprevention for CRC was highly dependent on its efficacy, on its complication rate and on the degree of compliance to screening in the population.
The authors also examined the effect of ASA as an adjunct to screening if it decreased CV death as well as CRC incidence. If ASA decreased CV mortality by 0.1% and CRC incidence by 30%, its use as an adjunct to screening would cost $10,039 and $8,976 per LY saved for FS/FOBT and COLON, respectively.
It was concluded that ASA therapy could not be considered a substitute for screening. Although ASA chemoprevention seems cost-effective in an unscreened population, it is much less effective than screening, which is highly cost effective. The use of ASA as an adjunct to screening is cost-effective if it can reduce CRC risk by 40% to 80% and has low complication rates. If, in addition, ASA was modelled to decrease CV mortality by at least 0.1%, its use as an adjunct to screening would remain cost-effective as long as its chemopreventive efficacy is 30% or greater.
Ladabaum et al. 106 also modelled the use of COX-2 inhibitors (celecoxib or rofecoxib) in the same average-risk U.S. population, using similar probabilities and costs for the natural history of the disease as well as the screening tests. The authors restricted the comparison to that of colonoscopy every 10 years. The efficacy of COX-2 inhibitors was assumed to be 30% (similar to that of ASA in their prior report), it was assumed that their effect is similar on polyp and CRC growth, the rate of excess major complications and death with COX-2 inhibitors were assumed to be 0, and the cost of COX-2 inh therapy was estimated at $325 per year. Sensitivity analysis was used to explore the effects of: varying COX-2 inh's chemopreventive efficacy from 0 to 100%; assuming a differential effect on polyp and CRC growth; using a COX-2 inh exclusively in individuals younger than 65; increasing the rate of excess major GI complications to 0.1% per year and of death from COX-2 to 2% to 8% per complication; varying the doses of COX-2 inhibitors, with yearly costs of COX-2 chemoprevention ranging from $81.25 to $1300; and evaluating whether COX-2 inh chemoprevention would allow for less frequent screening. The effect of COX-2 on CV mortality was not modelled.
Compared with no intervention, colonoscopy every 10 years saved 0.065LY/person and its ICER was $20,200/LY saved, whereas COX-2 inh saved 0.027LY/person with an astronomical ICER of $233,300/LY saved. In comparison to screening alone, the addition of a COX-2 inh saved an extra 0.008LY/person and its ICER was of $823,800/LY saved. If, in combination with COX-2 inh chemoprevention, colonoscopy was decreased to every 20 years, the ICER became $3,313,000/LY saved. In the strategies incorporating chemoprevention, COX-2 use accounted for 66% to 92% of total costs.
In summary, use of a COX-2 inh was both less effective and more costly than screening alone. These results were highly sensitive to the chemopreventive efficacy and the cost of COX-2 inhibitors. COX-2 inh use alone would need to reduce CRC risk by 60% at a cost of $0.25/day to approach the cost effectiveness of colonoscopy every 10 years. Their use as an adjunct to colonoscopy screening incurred ICERs lower than $100,000/LY saved only if their chemopreventive efficacy was greater or equal to 60% and their cost was $0.25/day.
Hur et al. compared the cost effectiveness of ASA 325mg daily with that of celecoxib 400 mg bid in a population of 50-year-old average-risk U.S. men followed for 10 years. 104 It was assumed that both ASA and celecoxib (Celebrex) had the same efficacy for RR of CRC. It was also assumed that ASA reduced the risk of CV events, while celecoxib had no impact on CV morbidity and mortality. The model, not accounting for the impact of screening or that of chemoprevention on cancer incidence, essentially compared the costs, cardioprotective effects, and toxicities of ASA versus celecoxib. The results of the base-case analysis showed comparable efficacies (7.60 and 7.57 QALYs for ASA and celecoxib, respectively), but at an enormous cost difference ($181 and $23,403 for ASA and celecoxib, respectively). Sensitivity analysis showed that coxibs became more effective than ASA if the relative ulcer rate on coxibs (COX-2 inhibitors) was 93% lesser than in the base-case, if the combined relative MI/ulcer rate for coxibs was decreased by 60% and if the relative bleeding rate on ASA was increased by 550%.
B. NSAID chemoprophylaxis in higher-risk populations
Ladabaum et al, 106 using the same Markov model as described in their average-risk analyses, compared the daily use of COX-2 inhibitors (celecoxib or rofecoxib) with either “do nothing,” colonoscopy every 5 years, or with the combination of colonoscopy every 5 years with daily COX-2, in an infinitely large U.S. population of 50-year-old subjects with one or two affected first-degree relatives. It was assumed that the risk of CRC was 2.6 and 3.6 times that of the average-risk population (derived from SEER data 1973-1994) for subjects with one and two affected first-degree relatives, respectively. Other assumptions and costs were similar to those described for their COX-2 chemoprevention in the analysis of average-risk subjects; the same sensitivity analyses were also performed, but in addition, it was used to determine if screening and/or chemoprevention should be instituted by the age of 40 instead of 50. The effect of COX-2 inhibitors on CV mortality was not modelled.
The ICERs for the interventions were similar whether screening began at age 40 or at age 50 for these subjects. Screening colonoscopy, either every 5 or 10 years, costs under $6,500/LY saved as compared with no intervention. The ICER of colonoscopy screening every 5 years compared with every 10 years was $19,800 and $10,900/LY saved, for persons with one and two affected first-degree relatives, respectively. As per average-risk subjects, use of a COX-2 inhibitor alone in higher-risk groups was both less effective and more costly than screening alone. Moreover, chemoprevention combined with colonoscopy every 10 years lost LYs and was more costly than colonoscopy every 5 years. Two-way sensitivity analysis showed that, in persons with two affected first-degree relatives, COX-2 inhibitors alone would need to reduce cancer risk by 70% at $0.50/day to approach the effectiveness and cost effectiveness of colonoscopy every 5 years ($4,800 vs $2,600/LY saved compared with no intervention, respectively). In that same higher-risk group, the ICER of COX-2 inhibitor use as an adjunct to colonoscopy every 5 years, compared with colonoscopy every 5 years alone, would be less than $100,000/LY saved only if they reduced cancer risk by 60% or more at $0.50/day, and would be less than $50,000/LY saved if they reduced cancer risk by 50% or more at $0.25/day.
In summary, colonoscopy every 5 years in subjects with one or two affected first-degree relatives was cost effective, whereas COX-2 inh use alone is both less effective and more expensive than screening. COX-2 inh use as an adjunct to colonoscopy every 5 years could be considered relatively cost effective if COX-2 inhibitors could reduce cancer risk by at least 50% and if their daily cost was of $0.50 or less.
Arguedas et al. compared 1) surveillance colonoscopy 3 years after the initial polypectomy and every 5 years once no polyps were recovered, 2) chemoprevention with celecoxib 200 mg/day, to 3) no surveillance, no chemoprevention 105 in subjects with a prior history of colonic adenoma followed for 10 years.
Key probabilities and assumptions are presented in Evidence Table 4 (Appendix 8). The cancer RR on COX-2 inhibitors was 50% (range 0%–100%); the rate of peptic ulcer disease on celecoxib was 0.02/y (range 0.01–0.15) and the rate of withdrawal from COX-2 inhibitors due to side effects was 0.01/y (range 0–0.02/y). Subjects who withdrew from celecoxib would undergo the surveillance colonoscopy protocol and compliance with any intervention was assumed to be 100%. The cost of celecoxib therapy was $1,766/y (range $25–$1,766). The effect of COX-2 inhibitors on CV mortality was not modelled.
Colonoscopic surveillance was more effective and considerably less costly than chemoprevention, saving 0.01995LY at a cost of $558 compared with 0.00579LY for $9,931 with celecoxib. Compared with no intervention, the ICERs for colonoscopy and chemoprevention were $27,970 and $407,498/LY saved, respectively. Sensitivity analysis showed that the results were not sensitive to the rate of polyp formation (i.e., increasing or decreasing the magnitude of patient risk) but that, as per Suleiman's study, varying the cost and/or the chemopreventive effect of celecoxib would affect the ICERs. For example, celecoxib use could be economically advantageous (ICER less than $50,000/LY saved) if it reduced polyp recurrence by 50% and cost $0.10/day, or reduced recurrence by 75% and cost $0.35/day.
In summary, the use COX-2 inhibitors does not appear to be cost effective either compared with screening or as an adjunct to screening. In higher-risk patients, COX-2 inhibitors would have to reduce polyp recurrence by 50% or more and cost less than $0.50/day.
C. The impact of NSAID chemoprevention on FOBT testing
This section was added at the request of the USPSTF and is located in Appendix 6.
- Results - Use of Aspirin and NSAIDs to Prevent Colorectal CancerResults - Use of Aspirin and NSAIDs to Prevent Colorectal Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...

