NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological Agents. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100B.)
Human T-cell lymphotropic virus Type 1 was considered by a previous IARC Working Group in 1996 (IARC, 1996). Since that time, new data have become available, these have been incorporated in the Monograph, and taken into consideration in the present evaluation.
1. Exposure Data
1.1. Taxonomy, structure, and biology
1.1.1. Taxonomy
Retroviruses can be classified according to the morphology of their virion core or according to sequence homologies that become evident after phylogenetic analyses. Human T-lymphotropic virus type 1 (HTLV-1) is a member of the delta-type retrovirus group, other members of which include HTLV types 2, 3 and 4, bovine leukaemia virus (BLV), and simian T-cell leukaemia virus (STLV) types 1, 2 and 3 (Matsuoka & Jeang, 2007). STLV-1 is found in Old World monkeys and great apes. HTLV-1 and STLV-1 are thought to originate from common ancestors (Vandamme et al., 1998). Together with the STLVs, HTLVs form the primate T–cell lymphotropic viruses (PTLV) group. The PTLVs belong to the complex retrovirus family since, in addition to the structural gag, pol and env genes, their genome also contains regulatory and accessory genes. Among these retroviruses, HTLV-1 and STLV-1 induce T-cell neoplasms (Tsujimoto et al., 1987; Gallo, 2002), BLV causes a B-cell neoplastic disease in cattle and in sheep, and HTLV-3 and HTLV-4 have not been clearly associated with any haematological disease (Mahieux & Gessain, 2009). A recent report demonstrated that HTLV-2 infection is linked with higher lymphocyte and platelet counts, although it has not been yet associated with oncogenesis (Bartman et al., 2008).
1.1.2. Structure of the virion
The structure of retroviruses is reviewed in the Monograph on HIV-1 in this volume. HTLVs are enveloped viruses with a diameter of approximately 80–100 nm. The HTLV virions contain two covalently bound genomic RNA strands, which are complexed with the viral enzymes reverse transcriptase (RT; with associated RNAse H activity), integrase and protease, and the capsid proteins. The outer part of the virions consists of a membrane-associated matrix protein and a lipid layer intersected by the envelope proteins (IARC, 1996).
1.1.3. Structure of the viral genome
As stated above, HTLV-1 is a complex retrovirus that contains regulatory genes (tax and rex) and accessory genes (p12, p13, p30 and HBZ), in addition to structural genes (gag, pol and env) (see Fig. 1.1). The Gag precursor protein (53 kD) is translated from unspliced genomic RNA. This protein is cleaved into p19 (matrix), p24 (capsid), and p15 (nucleocapsid) by the viral protease. The protease–polymerase products are generated by two frameshifts, which produce protease, reverse transcriptase, and integrase. The Env precursor protein is translated from a single-spliced mRNA, and is cleaved by a cellular protease into the extracellular protein, gp46, and the transmembrane protein, gp21 (Seiki et al., 1983; Sakalian & Hunter, 1998; Matsuoka & Jeang, 2007; Verdonck et al., 2007).

Fig. 1.1
Scheme of the HTLV-1 genome: alternatively spliced mRNAs and putative proteins encoded by each mRNA are shown
1.1.4. Host range
HTLV-1 naturally infects humans. However, several publications have clearly demonstrated that HTLV-1 can experimentally be inoculated to different animals, including rabbits, rats, mice, and New World monkeys (Lairmore et al., 2005).
1.1.5. Target cells
HTLV-1 can infect different cell types (T cells, B cells, dendritic cells, fibroblasts, etc.) in tissue culture. However, it can transform only T cells both in vitro and in vivo. HTLV-1 induces the clonal proliferation of T lymphocytes, mainly CD4-positive T cells, and to a lesser extent, CD8-positive T cells (Etoh et al., 1997; Cavrois et al., 1998; Yasunaga et al., 2001). Proliferation is thought to be mediated by one or several viral genes, such as tax, rex, p12, p13, p30, or HBZ.
HTLV-1 infection of dendritic cells has been recently shown to play a major role in HTLV-1 cell-to-cell transmission (Jones et al., 2008). In experimentally infected squirrel monkeys (S. Sciureus), HTLV-1 was mainly detected in the lymphoid organs, which were therefore suggested to be a major reservoir of the virus (Kazanji et al., 2000).
1.1.6. Life cycle, replication, and regulation of gene expression
The glucose transporter 1 (GLUT1), neuropilin 1, and heparan sulfate proteoglycan form the HTLV-1 receptor complex (Manel et al., 2003; Ghez et al., 2006). These proteins are ubiquitously expressed in cultured cells, therefore allowing HTLV-1 to infect a variety of cell types in vitro.
The life cycle of HTLV-1 is similar to that of other retroviruses. A characteristic of HTLV-1 is that it is mainly spread through cell-to-cell contact, although the exact mechanism is still a matter of debate (Igakura et al., 2003). After reverse transcription and integration into the genome, HTLV-1 propagates through clonal expansion of infected cells (Etoh et al., 1997; Cavrois et al., 1998). The limited use of the viral reverse transcriptase explains the remarkable genetic stability of HTLV-1. This is why the administration of reverse transcriptase inhibitors in vivo does not influence provirus load (Miyazato et al., 2006; Taylor et al., 2006). Consequently, the HTLV-1 provirus sequence variability is very low (Gessain et al., 1992; Van Dooren et al., 2004). This striking genetic stability is used as a molecular tool to follow the migration of infected populations in the recent or distant past to gain new insights into the origin, evolution, and modes of transmission of such retroviruses and their hosts. The few nucleotide substitutions observed among virus strains are indeed specific to the geographic origin of the patients rather than being linked to the pathology.
Three modes of transmission are known for HTLV-1, and for each of these routes, cell-to-cell contact is required. The transmission is discussed in Section 1.2.
(a) Regulation of gene expression
Viral gene expression initiates from the 5′Long Terminal Repeat (LTR), and is highly dependent on the Tax protein. The details on the regulation of the viral LTR are described in Section 4. However, Tax is a major target of cytotoxic T cells in vivo (Koenig et al., 1993), and Tax-expressing cells are, therefore, rapidly eliminated by cytotoxic T cells (Hanon et al., 2000; Asquith et al., 2007; Asquith & Bangham, 2008). Despite a strong cytotoxic T-cell response, proliferation of HTLV-1-infected cells in vivo is likely to depend on viral gene expression (Asquith et al., 2007). Epigenetic changes to the 5′LTR may control viral gene expression in vivo, enabling escape from cytotoxic T cells. A recent report demonstrated that the administration of valproate, a histone deacetylase inhibitor, to tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) patients decreased the provirus load in vivo without, however, improving the clinical symptoms (Lezin et al., 2007). The mechanism by which the infected cells are eliminated is unknown, but these results indicate that increasing viral expression may represent a potential approach to decreasing the proviral load.
1.2. Epidemiology of infection
In 1977, Takatsuki et al. described regions in Japan with high frequencies of T-cell-associated lymphoproliferative disorders and proposed that these diseases shared a viral etiology (Takatsuki et al., 1977). This led to the discovery of HTLV-1 just a few years later, and it became the first human retrovirus to be implicated as a causative agent for human malignancy (Poiesz et al., 1980; Hinuma et al., 1981). Its discovery paved the way to a greater understanding of retroviruses, notably HIV, and their effects on humans. It is now apparent that HTLVs have been infecting humans for thousands of years. An estimated 15–20 million persons worldwide are infected with HTLV-1 (Gessain & de Thé, 1996) [The Working Group noted that the accuracy of this estimate is unknown], and a vaccine is not yet available.
All HTLV types have simian counterparts, and the viral strains found are predominantly related to geography rather than pathology (Slattery et al., 1999). These are all believed to have originated from Africa, the only continent where all PTLVs have been found. From there, PTLV migrated to Asia, where it evolved into STLV-1. This Asian STLV-1 virus type diffused through India, Japan, and Indonesia before returning to Africa, where phylogenetic analyses and anthropological studies place PTLV-1 spread among non-human primates at approximately 27300 years ago (95% confidence interval [CI]: 19100–35500) (Van Dooren et al., 2001).
Interspersed patterns of STLV-1 and HTLV-1 strains suggest frequent interspecies transmissions between humans and primates in Africa. Evidence of these frequent crossings are distinguished by the four major geographic subtypes: cosmopolitan HTLV-1 subtype A, Central African subtype B, Melanesian subtype C, and subtype D, also found in Central Africa (Cassar et al., 2007). The slave trade and an increase in human immigration and mobility facilitated the expansion of HTLV-1 into the New World, Japan, the Middle East, and North Africa (Verdonck et al., 2007). The majority of infected individuals from these regions are infected with cosmopolitan subtype A (HTLV-1A) (Proietti et al., 2005).
1.2.1 Prevalence, geographic distribution
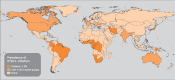
Even though the global geographic distribution of HTLV-1 has been well defined in the literature, fine-scale variations in HTLV-1 prevalence are less well understood. HTLV-1 is often found in micro-epidemic regions surrounded by regions with low prevalence (Figure 1.2). For example, regions of Kyushu and Okinawa, Japan, have rates as high as 20%; whereas, neighbouring the People’s Republic of China and the Republic of Korea have rates of less than 0.1% (Proietti et al., 2005). In general, regions of high endemicity include south-western Japan, parts of subSaharan Africa, the Caribbean Islands, and South America (IARC, 1996). Infection has also been detected in Melanesia, the Solomon Islands, and among Australian Aborigines; there is only low prevalence in Europe and North America.
There is a characteristic age- and gender-dependence of HTLV-1 seroprevalence in many populations. HTLV-1 prevalence increases with age and is higher in women (Beilke & Murphy, 2006). Published studies from several countries including Jamaica, Japan, Brazil, and the United States of America (Beilke & Murphy, 2006) have demonstrated similar trends, in addition to a significant increase of seropositivity in low socioeconomic strata, and in those with a history of blood transfusions. HTLV-1 is most prevalent in populations that have a low geographic mobility, and correspondingly higher rates of vertical and sexual transmission.
1.2.2 Transmission, and risk factors for infection
There are three main modes of transmission in HTLV-1 infection: vertical transmission, sexual transmission, and parenteral transmission. Each has its respective risk factors: prolonged breastfeeding; unprotected sex with an infected partner, multiple lifetime sexual partners, infection with sexually transmitted diseases (STDs); blood transfusion before institution of HTLV-antibody screening of donors, and injection drug use.
(a) Vertical transmission
The highest rates of HTLV-1 transmission are due to breastfeeding, and in southern Japan, the overall infection rate of breastfed children by HTLV-1-carrier mothers is estimated at 10–30% (Tajima & Hinuma, 1992). There may be a higher rate of transmission of mother-to-female compared to mother-to-male infants for HTLV in French Guyana (Ureta-Vidal et al., 1999). In Japan, there does not appear to be gender differences in HTLV-1 before the age of 20 years (Hino, 1990). In a longitudinal study of Japanese children born to carrier mothers, those who did not seroconvert by the age of 3 years remained seronegative until the age of 18 years (Kusuhara et al., 1987). In-utero infectivity is much lower, probably because of limited trafficking of HTLV-1-infected lymphocytes across the placenta. The risk of infection in children has been shown to correspond to the proviral load in the mother’s breast milk, and to the duration of breastfeeding (Wiktor et al., 1997). In Japan, the avoidance of breastfeeding by an HTLV-1-infected mother reduced the transmission from 20% to 3%, and efforts to eliminate breastfeeding or to at least reduce its duration to less than 12 months by seropositive mothers, significantly reduced HTLV-1 transmission to children. In a subset of children with HTLV-1 positive cord blood, none had seroconverted (Hino et al., 1996).
(b) Sexual transmission
The second main type of transmission is sexual. One prospective study of heterosexual discordant couples (one partner seropositive, one partner seronegative) suggested higher male- to- female transmission (Stuver et al., 1993), but another did not (Roucoux et al., 2005). In Latin America, gender differences in sexual practices and the seroprevalence of STDs between populations might partially explain these discrepancies (Plancoulaine et al., 1998; Sanchez-Palacios et al., 2003). HTLV-1 carriers infect their spouses at low rates (1–2 per 100 person–years); however, within long-term sexual relationships, HTLV-1 proviral load and lower rates of condom use were shown to increase transmission efficiency (Kaplan et al., 1996; Iga et al., 2002; Roucoux et al., 2005).
(c) Parenteral transmission
HTLV-1 transmission is also known to occur by the transfusion of cellular blood components, requiring testing of blood products by blood banks in high prevalence regions. The “residual risk” of transfusion-transmitted HTLV-1 infection after serological testing has been estimated as 1 in 641000 blood units transfused in the USA (95%CI: 256000–2000000) (Schreiber et al., 1996). Transmission through this route has been reduced by improvements in the sensitivity of serological assays for HTLV-1 and leukoreduction of blood products, so the current residual risk is probably less than 1 per million blood units transfused. Although nucleic-acid testing of blood products has been introduced for HIV, hepatitis C and hepatitis B viruses (HCV, HBV), it has not yet been developed for HTLV-1, because HTLV-1 would require a cell-based rather than a plasma-based assay (Murphy et al., 1999). HTLV-1 is also transmitted via needle-sharing associated with injection drug use. However, compared to HTLV-2, the prevalence of HTLV-1 is relatively low among injection drug users and their sexual partners in the USA, Italy, Spain, Brazil, and Argentina (Gotuzzo, 2000), perhaps because injection drug use is less common among HTLV-1- as opposed to HTLV-2-risk groups (Roucoux & Murphy, 2004). However, increasing human mobility and cultural interaction create the opportunity for increased HTLV-1 transmission by this route.
1.2.3 Persistence, latency and natural history of infection
The lifetime risk of developing adult T-cell leukaemia/lymphoma (ATLL) has been estimated at 2–4% among HTLV-1 carriers, and the latency period from primary infection until ATLL onset is about 60 years in Japan, and 40 years in Jamaica (Tajima, 1988; Murphy et al., 1989; Takatsuki et al., 1994; Hanchard, 1996; Yasunaga & Matsuoka, 2007). The incubation period for HTLV-associated myelopathy is thought to be shorter: 10–20 years after sexual transmission but as little as 6 months after transfusion-transmitted HTLV-1 infection (Gout et al., 1990). Several viral and immunological markers have been proposed as markers for predicting which infected subjects will progress from latency to disease (see Section 2.1), but prospective validation of these markers is lacking. HTLV-1 may also be associated with increased overall mortality (Arisawa et al., 2003; Orland et al., 2004).
Compared to HIV, the HTLV-1’s genome is very stable, with proviral integration predominating over production of viral RNA particles (Mortreux et al., 2003). Occasionally, abnormal, multilobulated lymphocytes “flower cells” can be observed in the peripheral blood (Hisada et al., 1998; Sacher et al., 1999). Aside from detection in the peripheral blood, infected cells have also been detected in the cerebrospinal fluid, an indication of HTLV-1 ability to cross the blood–brain barrier (Mortreux et al., 2003). In an assessment of the patterns of HTLV-1 proviral DNA and antibody titre levels among transfusion recipients, in early infection, proviral loads are initially elevated with corresponding low antibody titres, and as proviral load begins to decrease, antibody titres increase, and later remain stable within each of the cases (Manns et al., 1999). Proviral load may also be related to the route of infection, with transfusion-transmitted HTLV associated with a higher proviral load (Murphy et al., 2004).
Platelet and lymphocyte counts may be chronically elevated in HTLV-1 (Glynn et al., 2000). Both higher platelet counts and lower eosinophils counts were found to be significantly associated with HTLV-1 status among blood donors from the USA (Bartman et al., 2008). HTLV-1 participants also had a small increase in absolute lymphocyte count compared with controls, which was not statistically significant.
HTLV-1 preferentially targets CD4-positive T cells, and infection is transmitted through direct cell-to-cell contact. Recent reports also support a role for dendritic cells in HTLV-1 transmission (Jones et al., 2008). Once inside the cell, the HTLV-1 provirus integrates itself into the host genome. A study. demonstrated a significant rate of viral integration within genes (as opposed to non-coding regions of the genome), which suggests an HTLV-1 preference to insert in growth-related genes over random integration (Hanai et al., 2004). Because of clonal expansion, T cells with identical integration sites are considered to have originated from the same infected cell. More sensitive assays with the ability to amplify regions of the host genome adjacent to the integration site, such as inverse polymerase chain reaction (PCR), may be used to identify clones of infected T lymphocytes in asymptomatic carriers (Okayama et al., 2004; Tanaka et al., 2005).
In contrast to HIV, which produces a large amount of cell-free virions in plasma, HTLV-1 increases its copy number through the proliferation of infected cells, and infection is maintained through this expansion. Early expression of viral tax protein and HTLV-1 accessory proteins induce and maintain initial replication (Manns et al., 1999). An increased proviral load derives from this persistent clonal expansion of virus-infected cells (Wattel et al., 1995). After initial infection, individuals are asymptomatic with proviral loads ranging from 102–105 per million peripheral blood mononuclear cells (Wattel et al., 1992; Etoh et al., 1999; Mortreux et al., 2003). The proviral load remains relatively stable within a single infected individual over several years (Mortreux et al., 2003; Kwaan et al., 2006).
2. Cancer in Humans
2.1. T-cell malignancies
2.1.1. HTLV-1 infection and ATLL
As described in the previous IARC Monograph (IARC, 1996), ATLL occurs almost exclusively in areas where HTLV-1 infection is endemic, such as Japan, the Caribbean, and West Africa. In other areas, cases are usually found among immigrants from endemic populations. Evidence of HTLV-1 infection was initially found in at least 90% of ATLL cases and subsequently, HTLV-1 infection became part of the diagnostic criteria for ATLL. In ATLL, the virus is monoclonally integrated into the tumour cells. The previous IARC Working Group concluded that HTLV-1 infection was a necessary cause of ATLL (IARC, 1996). The current Monograph will focus primarily on recent evidence on predictors or risk of ATLL in HTLV-1 carriers. To date, no other malignancies have been convincingly liked to HTLV-1.
Since 1996, several case series have been published on ATLL occurring in diverse HTLV-1-endemic and –non-endemic populations. These include reports from Japan (Tsukasaki et al., 1999); Brazil (Farias de Carvalho et al., 1997; Barbosa et al., 1999; Pombo De Oliveira et al., 1999); Argentina (Marin et al., 2002); Chile (Cabrera et al., 2003); the Commonwealth of Dominica (Adedayo & Shehu, 2004); and Hong Kong Special Administrative Region (Chan & Liang, 1996; Au & Lo, 2005; see Table 2.1 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.1.pdf). One report from the Hong Kong Special Administrative Region estimated an HTLV-1 prevalence of only 4×10−5 but identified six cases of apparent ATLL by systematically screening all non-Hodgkin lymphoma cases for HTLV-1 antibody (Au & Lo, 2005).
To date, there are a total of six cohort analyses based in Japan that document the incidence of ATLL among HTLV-1 carriers, and confirm that male carriers have about a 3–5 fold higher risk of developing ATLL than female carriers (Tokudome et al. 1991; Iwata et al., 1994; Arisawa et al., 2000, 2003, 2006; Hisada et al., 2001). It is worth noting that because HTLV-1-seropositivity is part of the diagnosis of ATLL, relative risks for ATLL can not be calculated (see Table 2.2 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.2.pdf). These cohort studies confirm the causal relationship between HTLV-1 infection and the incidence of ATLL, and also underline the higher risk among infected men. However, the reason for the apparently higher disease penetrance in HTLV-1-infected men than HTLV-1-infected women seen in Japan, and whether it exists elsewhere, is unknown. Modelling data from the Carribean have not identified such male excesses (Murphy et al., 1989; see Table 2.3 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.3.pdf).
There are four case–control analyses nested within prospective HTLV-1 cohorts in Japan that have compared incident ATLL cases with matched HTLV-1 carriers. These have focused on viral or serological predictors of either ATLL or the prevalence of circulating abnormal lymphocytes that resemble ATLL cells (Hisada et al., 1998a, b; Arisawa et al., 2002; Okayama et al., 2004; see Table 2.4 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.4.pdf). Pre-diagnosis predictors of ATLL included a higher proviral load, higher antibody titres, and a higher prevalence of soluble interleukin-2 receptor-α (sIL2-R). The predictors for higher levels of abnormal lymphocytes were a higher proviral load and male gender. However, these studies are based on a small number of incident cases, and have not been repeated in other HTLV-1-infected populations.
2.1.2. Host susceptibility
As noted above, there is consistent evidence from cohorts in Japan that male carriers have a higher risk compared to female carriers for this malignancy.
It has long been proposed that the risk of ATLL is strongly related to very early HTLV-1 infection. Testing this hypothesis would be difficult, requiring prospective follow-up of large birth cohorts. However, in a recent update of an ongoing study in the Caribbean of mothers of ATLL- and of HTLV-1-associated TSP/HAM patients, 35/36 mothers (97.2%) of ATLL cases were seropositive versus 5/15 mothers (33%) of TSP/HAM patients (P<0.001; Bartholomew et al., 1998). The cells of the sole seronegative mother of an ATLL case were also negative on PCR. However, the father and one older sister of this ATLL case were both HTLV-1 seropositive. All patients in the study were breastfed, and none of the patients or their mothers had a history of a blood transfusion. These findings support the proposal that early and/or mother-to-child infection with HTLV-1 plays an important role in the genesis of ATLL.
Finally, much of the notion of the epidemiology of ATLL is based on epidemiological studies conducted in Japan. It is important to note that geographically defined social environments may alter the natural history of this infection. Specifically, the peak incidence of ATLL occurs earlier and at a reduced frequency among HTLV-1 carriers in the Caribbean in comparison to Japan. Several studies compared viral and immune markers between HTLV-1 carriers and uninfected controls, and between Jamaican and Japanese HTLV-1 carriers as an approach to determine why the penetrance of ATLL may differ between populations.
Hisada et al. (2004) analysed viral markers between a matched set of Jamaican (n = 51) and Japanese carriers (n = 51) (Hisada et al., 2004). They found that the anti-HTLV-1 titres were higher among the Jamaicans (P=0.03), as was the prevalence of antibodies against the tax protein (anti-tax) (P=0.002). There was no significant difference in proviral load between the Jamaican and Japanese carriers.
Birmann et al. (2009) further added a matched HTLV-1 seronegative subject to each carrier within each population reported in Hisada et al. (2004) to evaluate a set of serum immune markers (Birmann et al., 2009). HTLV-1 infection was associated with activated T-cell immunity among the Jamaican subjects as indicated by a higher prevalence of a low EBV nuclear antigens (EBNA-1:EBNA-2) ratio, higher serum levels of sIL2R, and of soluble CD30. The results among the Japanese subjects indicated diminished T-cell immunity among the carriers, as indicated by lower C-reactive protein levels. [The Working Group noted that the observed population differences in non-carriers between the two populations and the impact of HTLV-1 infection within the population in immune profiles may begin to explain the divergent natural history of HTLV-1 infection in the two sentinel populations, and highlight the importance of social environments. Factors that may contribute to these findings include differences in age-specific infection rates, co-existing infections and nutritional status.] See Table 2.5 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.5.pdf.
2.2. Other malignancies
2.2.1. Cutaneous T-cell lymphoma
One study reported that 60 patients with mycosis fungoides had detectable tax-related proteins in peripheral blood mononuclear cell samples; of these, they reported that 83% also had antibodies to HTLV-1 tax proteins (Pancake et al., 1996). However, several large studies on patients with cutaneous T-cell malignancies, including many cases of mycosis fungoides in the USA, Republic of Korea, Japan, Spain, Europe, Mali, and Taiwan (China) could not replicate these results, and did not confirm an association with HTLV-1 (Wood et al., 1996, 1997; Bazarbachi et al., 1997; Kikuchi et al., 1997a, b; Chang et al., 1998; Fouchard et al., 1998; Kim et al., 1998; see Table 2.6 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.6.pdf). Overall, there is no consistent evidence that HTLV-1 is associated with cutaneous T-cell malignancies, with the exception of ATLL presenting in the skin.
2.2.2. B- and T-cell lymphomas
Since the previous IARC Monograph (IARC, 1996), several case series have examined HTLV-1 infection in B- and T-cell lymphomas (Farias de Carvalho et al., 1997; Marin et al., 2002; Cabrera et al., 2003; Suefuji et al., 2003; Adedayo & Shehu, 2004). With the exception of some T-cell lymphomas which may, in retrospect, have been cases of ATLL (Marin et al., 2002), there was no evidence that HTLV-1 infection played a role in these lymphomas (see Table 2.7 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.7.pdf).
The majority of primary gastric lymphomas are of B-cell origin, and only rarely of T-cell phenotype (Isaacson et al., 2008). However, a study reported that 18/58 lymphoma-type ATLL patients had gastric involvement of ATLL cells; and of these, three had primary gastric lymphoma (Sakata et al., 2001). Another report described 67 cases of surgically resected primary gastric lymphoma, of which five were found to have T-cell lymphoma: two were HTLV-1 positive and three were HTLV-1 negative (Shimada-Hiratsuka et al., 1997). A final report described 14 T-cell lymphomas in 233 cases of primary gastric lymphoma (Nakamura & Tsuneyoshi, 1998). [The Working Group remarked that although further research may be useful, it is not clear if gastric lymphoma represents a separate entity, or simply ATLL involving the stomach or adjacent lymphoid tissue.] See Table 2.8 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.8.pdf
Except for a few cases of ATLL, two studies were unable to find HTLV-1 in post-transplant lymphoproliferative disorders. Gentile et al. (1998) reported three patients who developed T-cell lymphoproliferative disorders after renal transplantation, but none had evidence of HTLV-1 infection. The second study reported on 24 cases of post-transplant lymphoid proliferation in Japan: 12 B-cell, ten T-cell, and two natural killer phenotype. A total of 5/10 T-cell tumours were classified as ATLL (Hoshida et al., 2001). See Table 2.9 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.9.pdf.
In one study, 12 cases of leukaemia of large granular lymphocytes with and tested for IgG antibodies to proteins related to HTLV-1 were reported (Starkebaum et al., 1987). Sera from 6/12 cases reacted with the HTLV-1 proteins, while none of the ‘control’ serum (from healthy persons and patients with other disorders) reacted. By analogy, an initial report of HTLV-2 and large granular lymphocytes (Loughran et al., 1992) was not confirmed in a controlled study (Loughran et al., 1994). A set of 27 specimens from cases of splenic lymphoma on Kyushu Island, Japan – an HTLV-1-endemic area – were tested for anti-HTLV-1 antibody, and all were negative (Kumagawa et al., 2001).
2.2.3. Non-lymphomatous tumours
DNA from HTLV-1 and human foamy virus has been detected in recent studies of thymoma, and in 2002, a study reported that 11/12 samples from thymoma patients (91.6%) from Italy tested positive for the tax gene of HTLV-1/2 and 9/12 samples (75%) positive for the tax and pol genes of HTLV-1 (Manca et al., 2002). However, a later study did not find any evidence for HTLV-1 or human foamy virus in 21 thymoma patients from the USA (Li et al., 2004).
Whether or not HTLV-1 is linked to an increased risk of solid malignancies has been studied with generally negative results. However, there is intriguing evidence for a possible protective effect of HTLV-1 on gastric carcinoma. In a prospective cohort study, 4136 adults living in four towns in the Nagasaki Prefecture in south-western Japan were followed for 6 years (Arisawa et al., 2003). A total of 1063 were seropositive for anti-HTLV-1 antibodies at baseline, including 439 (22.9%) men and 624 (26.2%) women. There were a total of 290 deaths in the cohort, with increased all-cause mortality for HTLV-1 both when ATLL was counted among the deaths (RR, 1.5; 95%CI: 1.2–1.9), and when ATLL deaths were excluded (RR, 1.3; 95%CI: 1.0–1.7). HTLV-1 was not associated with an increased risk of all-site cancer mortality after excluding cases of ATLL (RR, 1.1; 95%CI: 0.77–1.7). Other interesting findings from the study included a decreased incidence of gastric cancer (RR, 0.42; 95%CI: 0.17–0.99). These negative results for HTLV-1 and solid tumours are in contrast to previous cross-sectional studies, which reported a higher HTLV-1 seroprevalence among 394 non-transfused patients with non-ATLL malignancy than in age- and sex-matched healthy controls (Asou et al., 1986), and a higher prevalence of all malignant neoplasms among siblings of ATLL patients compared to siblings of HTLV-1 seronegative non-Hodgkin lymphoma cases (Kozuru et al., 1996).
In a recent retrospective cohort study in the Nagasaki Prefecture, Japan, 497 HTLV-1-positive and 497 HTLV-1-negative persons who did not have gastric cancer at baseline were followed with serial endoscopy of the stomach (Matsumoto et al., 2008). Helicobacter pylori antibodies were found in 61.7% of HTLV-1-positive cases compared to 71.6% of the HTLV-1-negative cases. There were 14 cases (2.8%) of gastric cancer in the HTLV-1-positive subjects compared to 35 cases (7%) in the age- and sex-matched HTLV-1-seronegatives (OR, 0.38; 95%CI: 0.21–0.70). [The Working Group noted that these data suggest that HTLV-1 infection may reduce the inflammation usually associated with H. pylori infection, and thereby reduce the risk of gastric carcinoma.]
[The Working Group noted that further investigation is warranted on the subject of persistent H. pylori infection among HTLV-1 seropositives to determine whether HTLV-1 infection may reduce the inflammatory response to H. pylori and reduce the risk of gastric carcinoma, as mentioned above (Arisawa et al., 2003; Matsumoto et al., 2008).]
Finally, one study of 85 cases of oesophageal squamous cell carcinoma in the Islamic Republic of Iran found no increase in HTLV-1 antibody prevalence compared to non-cancer controls (Mirsadraee et al., 2007).
See Table 2.10 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.10.pdf.
2.3. Cofactors
In addition to the roles of being of masculine gender and infected at a younger age (described above), undernutrition and repeated exposure to filariasis in childhood were proposed as potential risk factors for ATLL (Tajima & Hinuma, 1984).
Since then, there is some evidence that co-infection with Strongyloides stercoralis is detrimental to HTLV-1 carriers. Among 38 ATLL cases, those who were positive for S. stercoralis were younger at diagnosis then those uninfected (Plumelle et al., 1997).
Gabet et al. (2000) reported that among HTLV-1 carriers from West Guyana and Martinique, those who were co-infected with S. stercoralis had a substantially higher HTLV-1 proviral load, and substantially more oligoclonal expansion of HTLV-1-infected lymphocytes than carriers who were negative for S. stercoralis. These observations were substantiated in a Japanese population of HTLV-1 carriers (Satoh et al., 2002). [The Working Group noted that, together, these papers suggest that co-infection with S. stercoralis may increase the risk of ATLL in HTLV-1 carriers, and presents a potential for risk reduction with parasite treatment and control.]
One case series reported three cases of ATLL among eight HTLV-1 carriers within 2 years after immunosuppression with tacrolimus for liver transplantation (Kawano et al., 2006). In another study, five cases of ATLL were reported following immunosuppression for kidney transplantation (Hoshida et al., 2001).
See Table 2.11 available at http://monographs.iarc.fr/ENG/Monographs/vol100B/100B-07-Table2.11.pdf.
3. Cancer in Experimental Animals
In this Volume, the Working Group decided not to include a separate section on “Cancer in Experimental Animals” in the Monographs on viruses but rather to include description of such studies under Section 4 (below). The reasoning for this decision is explained in the General Remarks.
4. Other Relevant Data
4.1. Mechanism of HTLV-1-linked carcinogenesis
4.1.1. Transforming capacity of HTLV-1
In vivo, HTLV-1 infection has been reported not only in T lymphocytes, but also in B lymphocytes, myeloid cells, monocytes, and dendritic cells (Koyanagi et al., 1993; Manel et al., 2005; Jones et al., 2008). However, HTLV-1 immortalizes only T lymphocytes in vitro through the action of the Tax viral protein.
4.1.2. Biochemical and biological properties of HTLV-1 proteins
HTLV-1 belongs to the complex retroviruses family. The pX region, which is localized between the env gene and the 3′LTR, encodes regulatory genes (tax and rex), and accessory genes (p12, p13, p30 and HBZ) (Matsuoka & Jeang, 2007). These proteins not only control viral gene transcription, but also modulate the proliferation of infected cells. Indeed, the fact that HTLV-1 induces the proliferation of infected cells facilitates its transmission through cell-to-cell contact rather than through the release of viral particles (see also Section 1 and Fig.1.1).
(a) Rex
The Rex protein binds to Rex-responsive elements (RxRE), a highly stable stem-loop structure in the R/U3 region of the 3′LTR (Hanly et al., 1989). Rex regulates viral gene expression at the post-transcriptional level, by increasing the level of unspliced RNA in the nucleus, and by enhancing the nuclear export and the expression of the unspliced gag/pol and single-spliced env transcripts (Inoue et al., 1991).
(b) p12
The open reading frame I of the pX region of HTLV-1 encodes the protein p12, which is located in the endoplasmic reticulum and the Golgi. In quiescent primary lymphocytes and in vivo, p12 is important for establishing HTLV-1 infection and optimal viral infectivity. The p12 protein therefore facilitates host-cell activation, and the establishment of persistent infection (Collins et al., 1998; Albrecht et al., 2000; Nicot et al., 2005).
(c) p13
The protein p13 contains a mitochondrial-targeting signal, and exists in the nucleus and mitochondria. Mutation of the p13 gene impairs viral proliferation in vivo, indicating that p13 is critical for viral replication (Hiraragi et al., 2006). In addition, p13 expression is associated with a suppressed cell proliferation in vitro (Silic-Benussi et al., 2004).
(d) p30
The protein p30 is a nuclear and nucleolar protein (Koralnik et al., 1993) that binds to and retains the tax/rex mRNA within the nucleus. Therefore, p30 is a post-transcriptional negative regulator of viral replication and viral gene expression (Nicot et al., 2004).
(e) Tax
Tax, a 40-kD phosphoprotein, encoded from a spliced mRNA, is found mainly in the nucleus but also in the cytoplasm (Meertens et al., 2004). Tax interacts with several host factors (Boxus et al., 2008), which results in trans-activation of some genes, trans-repression of others, modulation of the cell cycle, and dysregulation of apoptosis (Matsuoka & Jeang, 2007).
The transduction of a pX-containing sequence into primary human T Lymphocytes by use of a defective simian herpesvirus is sufficient to immortalize these cells (Grassmann et al., 1989). However, since this vector could express not only tax but also the genes p12, p13, p30 and HBZ, it was difficult to conclude whether Tax was the only responsible viral protein for cell transformation. Subsequently, immortalization (IL-2-dependent growth) of human CD4-positive T cells was demonstrated in vitro by the use of a retroviral vector expressing only the tax gene (Akagi et al., 1995). In addition, the transforming ability of Tax was demonstrated in the Rat-1 fibroblast cell line in vitro in a soft-agar assay, and in vivo in nude mice (Tanaka et al., 1990). These findings clearly showed that Tax is oncogenic. In addition, several studies with animals transgenic for Tax have clearly demonstrated that Tax expression leads to the induction of tumours, confirming that Tax is oncogenic in vivo (see Section 4.1.6).
Transcription pathways activated by Tax include those of NF-κB, CREB, SRF and AP-1 (Azran et al., 2004). To turn on the NF-κB pathway, Tax binds to IKKγ, and activates the IKK complex, leading to phosphorylation of IκB (Jin et al., 1999). For survival, the Akt/PI3K pathway is also implicated in addition to the NF-κB pathway. Tax also activates this pathway by binding to the p85α subunit of PI3K, leading to activation of AP-1 (Peloponese & Jeang, 2006).
The transcription factor p53 is a crucial element in the cellular defence against tumour development. Mutations in the TP53 gene are frequently found in human cancers (IARC p53 database available online at http://www.p53.iarc.fr). Mutations in TP53 occur in less than 30% of adult T-cell leukaemia (ATLL) cells, depending on the clinical stage, which indicates that other mechanisms are involved. The precise mechanism that leads Tax to inhibit the function of p53 is still a matter of debate: some authors suggested that competition with the transcription co-activators CBP/p300 plays a major role (Mulloy et al., 1998; Suzuki et al., 1999), whereas others reported that activation of the NF-κB pathway was needed (Pise-Masison & Brady, 2005).
Tax activates the transcription of viral genes through three imperfect 21 base-pair repeat elements, the Tax-responsive element (TRE) (Fujisawa et al., 1986). The neighbouring GC-rich sequences of TRE are required for the binding of Tax (Paca-Uccaralertkun et al., 1994). The TRE contains sequences that are similar to that of the cyclic adenosine monophosphate (cAMP)-responsive element (CRE). CRE-binding protein/activating transcription factor (CREB/ATF) family members bind to TRE in a Tax-dependent manner (Franklin et al., 1993). Tax can interact with transcriptional co-activators, CREB-binding protein (CBP) and p300, that acetylate histones in the promoter region. In addition, CREB co-activators – termed transducers of regulated CREB activity (TORCs) – activate Tax-mediated viral gene transcription through the LTR. Tax interacts with TORCs (Siu et al., 2006). Thus, co-activators, TORCs, and CBP/p300 are necessary for the Tax-mediated activation of viral gene transcription.
Tax also inhibits transforming growth factor β (TGF-β)-mediated signals. It is likely that the inhibition of TGF-β-signalling enables HTLV-1-infected cells to escape TGF-β-mediated growth inhibition (Mori et al., 2001; Lee et al., 2002).
The Tax sequence contains several important domains that are involved in CREB and NF-κB activation. Recently, the C-terminal sequence of Tax was shown to contain a PDZ-binding motif. This PDZ-binding motif, which is absent from HTLV-2 Tax, seems critical for the ability of HTLV-1 Tax to transform cells in vitro (Rousset et al., 1998; Endo et al., 2002). It should be noted that Tax is not expressed in 60% of ATLL cases, due to deletions, epigenetic changes of the 5′LTR, and genetic changes in the Tax sequence (Matsuoka, 2005, 2010; Giam & Jeang, 2007).
(f) HBZ
The HTLV-1 bZIP factor (HBZ) is transcribed from the complementary strand of the proviral genome (Larocca et al., 1989; Gaudray et al., 2002). Viral transcription from the 5′LTR is highly dependent upon Tax expression and this is due to the presence of three TREs, as indicated above. Conversely, the transcription from the 3′LTR is dependent on Sp1 (Yoshida et al., 2008). Therefore, HBZ gene transcription is relatively constant, and is correlated with proviral load (Usui et al., 2008). Interestingly, and in contrast to the finding that the tax gene has not been frequently detected in ATLL cells, the HBZ mRNA could be detected in all ATLL cases. Even if defective proviruses are commonly detected in ATLL cells, the HBZ gene always remains intact (Miyazaki et al., 2007). Importantly, HBZ expression is associated with the proliferation of ATLL cells since the knock-down of HBZ in ATLL cells decreases the growth of the leukaemic cells (Satou et al., 2006), which further indicates that HBZ is critical and essential for the growth of these cells. Several transcription factors bind HBZ, including c-Jun, JunD, JunB, RelA/p65, p300, and CREB (Basbous et al., 2003; Hivin et al., 2007; Clerc et al., 2008).
4.1.3. Biological properties of HTLV-1 proteins relevant to carcinogenesis
(a) Immortalization
HTLV-1 can transform CD4-positive T lymphocytes in vitro. Among all the viral proteins, only Tax has the ability to immortalize CD4-positive T cells in vitro (see above).
(b) Genetic instability
Cytogenetic abnormalities that are specific to ATLL have not been found, but trisomies, deletions, and structural rearrangements are frequently reported in two of the four ATLL subtypes (acute leukaemia and lymphoma ATLL) (Kamada et al., 1992). This is therefore indicative of chromosomal instability in ATLL cells where the altered functions of several centrosome-associated proteins seem also to be involved in the Tax-driven aneuploidy (Afonso et al., 2007). As an example, the functions of HsMAD1 (also known as TXBP181) functions are impaired in Tax-expressing cells. HsMAD1 acts at the G2/M-checkpoint and has been found on the centrosome during metaphase. It is tempting to speculate that the loss of HsMAD1 function could be linked to the loss or modification of the centrosomal activity (Jin et al., 1998).
Tax has also been reported to interact with the anaphase-promoting complex/cyclosome (APC/C). This interaction leads to a premature mitotic exit, and may contribute to aneuploidy (Liu et al., 2005).
Recently, another partner of Tax, the centrosome-associated TAX1BP2 protein (also known as TXBP121) was also implicated in the Tax-dependent initiation of aneuploidy (Ching et al., 2006). By the use of in-situ fluorescence microscopy the authors demonstrated that Tax binds to and co-localizes with endogenous TAX1BP2, forming peri-nuclear dots. In the absence of Tax, overexpression of TAX1BP2 leads to a reduction in the number of cells that contain supernumerary centrosomes. In contrast, depletion of endogenous TAX1BP2 induces centrosome amplification. Therefore, Tax and TAX1BP2 have opposite effects. Besides, a Tax mutant that does not interact with TAX1BP2 can no longer induce centrosome duplication. This suggests that Tax targets TAX1BP2 to cause aneuploidy. In addition, during mitosis, Tax binds to Ran and RanBP1, which fragments spindle poles, and induces multipolar segregation (Peloponese et al., 2005).
(c) DNA-damage responses
Tax has been reported to suppress the expression of DNA polymerase β (Jeang et al., 1990), which is implicated in DNA repair. This suppression is associated with impaired DNA repair in HTLV-1-infected cells. In addition, it has been reported that Tax inhibits ATM-mediated DNA-damage response, resulting in premature DNA replication in the presence of genomic lesions (Chandhasin et al., 2008).
(d) Cell proliferation and differentiation
As mentioned above, the HTLV-1 provirus can be detected not only in CD4-positive T cells, but also in CD8-positive T cells as well as in dentric cells in vivo. Among CD4-positive T cells, the HTLV-1 provirus was detected in memory/effector T cells. After infection followed by a couple of cycles during which HTLV-1 uses its reverse transcriptase, the virus is amplified via clonal proliferation of the infected cells (Takemoto et al., 1994; Wattel et al., 1995). The same infected clones survive in vivo, indicating that clonal proliferation is persistent (Etoh et al., 1997; Cavrois et al., 1998). A prospective study has shown that this clonal proliferation is associated with the onset of ATLL in some cases (Okayama et al., 2004), although oligoclonal proliferation without ATLL occurs in most asymptomatic HTLV-1 carriers. Of note, the proviral load ranges from less than 0.1% up to 30% (of total peripheral blood mononuclear cells) in asymptomatic carriers. It is likely that a high proviral load is associated with a higher risk of developing ATLL (Tachibana et al., 1992).
4.1.4. Role of HTLV-1 in malignant conversion
(a) Requirement of HTLV-1 expression for cell growth
Viral gene expression differs between in-vitro-transformed cell lines and primary ATLL cells in a manner that is similar to the relation between EBV-transformed cells and Burkitt lymphoma cells. Tax expression is usually high in transformed cells in vitro but TAX gene transcription is detected in only about 40% of ATLL cells cultured ex vivo (Matsuoka & Jeang, 2007). Analyses of the HTLV-1 provirus identified three mechanisms that inactivate Tax expression: 1) genetic changes in the TAX gene sequence that lead to a premature stop codon or to insertions/deletions; 2) DNA methylation of the provirus; and, 3) deletion of the proviral 5′LTR (Matsuoka & Jeang, 2007). Deletion or DNA methylation of the 5′LTR silenced transcription of the viral genes, including TAX, REX, P12, P13, and P30. The 3′LTR, on the other hand, was intact and unmethylated in all ATLL cases examined, and HBZ was shown to be expressed in all ATLL cases tested, and to induce lymphocyte proliferation (Satou et al., 2006). Interestingly, there is a correlation between the proviral load and HBZ mRNA levels (Li et al., 2009).
(b) Persistence of the HTLV-1 genome
HTLV-1 induces ATLL in a subset of carriers after a long latency period. As an example, the cumulative lifetime risk of developing ATLL was estimated to be 6.6% for men and 2.1% for women among Japanese HTLV-1 carriers (Arisawa et al., 2000). ATLL cells retain the HTLV-1 provirus in the genome, but as stated above, defective proviruses are frequently detected, which are classified into two types. A type-1 defective provirus was found in 43% of all defective viruses; it lacks internal sequences such as gag, pol and env but retains both LTRs. A type-2 defective provirus lacks the 5′LTR and internal sequences. It is frequently observed in acute and lymphoma-type ATLLs whereas it is quite rare in chronic ATLL, indicating that this defective provirus is likely to be associated with disease progression (Tamiya et al., 1996). Detailed analyses show that the type-2 defective provirus can be generated before and after integration. A defective provirus formed after integration suggests that the deletion of the 5′LTR may block Tax expression, enabling ATLL cells to escape the host immune system. The frequency of type-2 defective proviruses is low in carriers, indicating that these defective proviruses were selected during leukaemogenesis. Another possibility is that infected cells with the type-2 defective provirus tend to transform into ATLL cells. ATLL cells with the type-2 defective provirus frequently cannot produce Tax as a result of the deletion of the promoter or the deletion of the second exon. However, all cases with the type-2 defective provirus maintain an intact HBZ gene sequence (Miyazaki et al., 2007).
As a mechanism of retroviral oncogenesis, the integrated LTR activates the transcription of cellular oncogenes, flanking integration sites. However, there are no common integration sites of HTLV-1 provirus in ATLL cells (Doi et al., 2005).
(c) Alterations of oncogenes and tumour-suppressor genes
Several ways by which tumour-suppressor genes can be inactivated have been demonstrated in cancer cells. Mutations of the TP53 gene occur in up to 40% of all ATLL patients (Sakashita et al., 1992; Yasunaga & Matsuoka, 2003). Deletion or mutation of the p16INK4A gene has also been reported. Such genetic changes in the p53 and p16INK4A gene sequence and/or function are detected in the more aggressive disease states, indicating that somatic DNA changes in these two genes are associated with the progression of ATLL (Yasunaga & Matsuoka, 2003).
Cytogenetic analysis of ATLL cells showed a common breakpoint cluster region in chromosome 10p11.2. Further analyses have shown that the transcription factor 8 (TCF8) is frequently disrupted by several mechanisms, including epigenetic silencing. Suppressed expression of TCF8 is associated with resistance to TGF-β. Mice carrying a mutation in TCF8 frequently developed thymic T-cell lymphoma, indicating that TCF8 is a tumour-suppressor gene (Hidaka et al., 2008).
There have been a few reports of cellular oncogenes in ATLL cells. By screening cDNA expression-libraries derived from leukaemic cells of ATLL patients for the potential to transform NIH3T3 mouse fibroblasts, a novel transforming gene, Tgat, was identified. Expression of Tgat in NIH3T3 cells resulted in cell transformation, indicated by anchorage-independent growth in semisolid medium, and tumorigenicity in nude mice (Yoshizuka et al., 2004).
4.1.5 Interaction between HTLV-1 and environmental agents
The frequency of opportunistic infections is fairly high among ATLL patients, indicating that T-cell-mediated immunity is severely impaired in such patients. The presence of the parasite S. stercoralis is commonly seen in immunosuppressed patients. In a study in the Japanese districts of Kyushu and Okinawa, where strongyloidiasis is endemic, 36 patients were identified as seropositive for HTLV-1. Fourteen of these patients (39%) had HTLV-1 DNA monoclonally integrated in their blood lymphocytes. It has been suggested that the parasitic infestation with S. stercoralis may act as a cofactor for HTLV-1-induced leukaemogenesis (Nakada et al., 1987).
4.1.6 Animal models for HTLV-1-associated cancers
Animals, including rabbits, rats, and monkeys can be experimentally infected with HTLV-1 (Lairmore et al., 2005). In rabbits and rats, HTLV-1 infection is persistent, and induces host immune response. However, HTLV-1 does not lead to definite diseases in these two species. A large number of Old World monkeys are naturally infected with STLV-1. This virus is almost identical at the nucleotide level with HTLV-1, and several cases of ATLL have been described in monkeys (Tsujimoto et al., 1987; Akari et al., 1998). Experimental infection with HTLV-1 of squirrel monkeys (S. Sciureus) led to a substantial decrease in the proliferation rate of the CD4-positive T-cell population in those infected animals that were affected by a pathology similar to ATLL in humans (Debacq et al., 2005). Co-infection of rhesus macaques (Macaca mulatta) with HTLV-1 and simian immunodeficiency virus 1 (SIV-1) increased the number of multilobulated lymphocytes in the circulation. The study showed that SIV-1 may have the potential to upregulate HTLV-1 and disease expression (Traina-Dorge et al., 2007). So far, non-human primates represent the only suitable animal model to study human ATLL.
Several groups have shown that HTLV-1 can infect immunocompetent mice, although in most of these studies, no viral mRNA production or HTLV-1 antibody response were detected. In addition, these mice did not show progression to ATLL (Lairmore et al., 2005). Several transgenic animal models have been established to study HTLV-1; the Tax protein has been shown to be oncogenic in several of these models. The type of tumour depends on the promoter used in each study: transgenic mice expressing Tax using the granzyme B promoter developed tumours of natural killer cells (Grossman et al., 1995), and transgenic mice with Tax expression via the lck promoter developed a disease that resembles ATLL (Hasegawa et al., 2006). The major difference between most of these animal tumours and ATLL is the fact that, as stated above, a subset of human ATLL cells do not express Tax. HBZ-transgenic mice have also been shown to display increased T-cell proliferation (Satou et al., 2006).
4.2. HTLV-1, host immune system, and genetic susceptibility
The host immune system influences the condition of viral infection, and the diseases induced by it. Large interindividual variations in proviral load are commonly observed between HTLV-1 carriers, but the amount of provirus is relatively constant in HTLV-1-infected individuals over time (Kwaan et al., 2006), suggesting that host factors, including the immune system, determine the provirus load. In spite of eliciting a strong immune response, HTLV-1 infection persists in vivo, mainly in CD4-positive T cells, and part of the CD8-positive T cells are infected by HTLV-1 (Yasunaga et al., 2001). An in vivo study of HTLV-1-infected cells used diuterium-labelled glucose to investigate lymphocyte kinetics, and showed that CD4+CD45RO+ and CD8+CD45RO+ T-lymphocyte proliferation was elevated in HTLV-1-infected subjects (Asquith et al., 2007). This was associated with viral gene expression, and indicates that active proliferation induced by viral infection induces the host immune response, and that the proviral load is determined by a balance between Cytotoxic T Lymphocytes activity and viral gene expression. The host immune system probably prevents the development of ATLL in vivo as suggested by a study where 3/8 HTLV-1-positive carriers, who were immunosuppressed during the course of a liver transplantation, developed ATLL (Kawano et al., 2006).
4.3. Synthesis
There is strong mechanistic evidence supporting the role of HTLV-1 in human carcinogenesis. The viral protein Tax has the ability to immortalize and to transform human T cells. At the leukaemic stage, the expression of Tax is often not maintained, but the viral protein HBZ continues to be expressed, and supports the sustained growth of the leukaemic cells (see Figure 4.1).
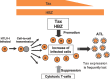
Fig. 4.1
Natural history of HTLV-1 infection leading to onset of ATL
5. Evaluation
There is sufficient evidence in humans for the carcinogenicity of HTLV-1. HTLV-1 causes adult T-cell leukaemia/lymphoma.
HTLV-1 is carcinogenic to humans (Group 1).
References
- Adedayo OA, Shehu SM. Human T-cell lymphotropic virus type 1 (HTLV-1) and lymphoid malignancies in Dominica: a seroprevalence study. Am J Hematol. 2004;77:336–339. [PubMed: 15551358] [CrossRef]
- Afonso PV, Zamborlini A, Saïb A, Mahieux R. Centrosome and retroviruses: the dangerous liaisons. Retrovirology. 2007;4:27. [PMC free article: PMC1855351] [PubMed: 17433108] [CrossRef]
- Akagi T, Ono H, Shimotohno K. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood. 1995;86:4243–4249. [PubMed: 7492783]
- Akari H, Ono F, Sakakibara I, et al. Simian T cell leukemia virus type I-induced malignant adult T cell leukemia-like disease in a naturally infected African green monkey: implication of CD8+ T cell leukemia. AIDS Res Hum Retroviruses. 1998;14:367–371. [PubMed: 9519898] [CrossRef]
- Albrecht B, Collins ND, Burniston MT, et al. Human T-lymphotropic virus type 1 open reading frame I p12(I) is required for efficient viral infectivity in primary lymphocytes. J Virol. 2000;74:9828–9835. [PMC free article: PMC102019] [PubMed: 11024109] [CrossRef]
- Arisawa K, Katamine S, Kamihira S, et al. A nested case-control study of risk factors for adult T-cell leukemia/lymphoma among human T-cell lymphotropic virus type-I carriers in Japan. Cancer Causes Control. 2002;13:657–663. [PubMed: 12296513] [CrossRef]
- Arisawa K, Sobue T, Yoshimi I, et al. Human T-lymphotropic virus type-I infection, survival and cancer risk in southwestern Japan: a prospective cohort study. Cancer Causes Control. 2003;14:889–896. [PubMed: 14682446] [CrossRef]
- Arisawa K, Soda M, Akahoshi M, et al. Human T-cell lymphotropic virus type-1 infection and risk of cancer: 15.4 year longitudinal study among atomic bomb survivors in Nagasaki, Japan. Cancer Sci. 2006;97:535–539. [PubMed: 16734733] [CrossRef]
- Arisawa K, Soda M, Endo S, et al. Evaluation of adult T-cell leukemia/lymphoma incidence and its impact on non-Hodgkin lymphoma incidence in southwestern Japan. Int J Cancer. 2000;85:319–324. [PubMed: 10652420] [CrossRef]
- Asou N, Kumagai T, Uekihara S, et al. HTLV-I seroprevalence in patients with malignancy. Cancer. 1986;58:903–907. [PubMed: 3013397] [CrossRef]
- Asquith B, Bangham CR. How does HTLV-I persist despite a strong cell-mediated immune response? Trends Immunol. 2008;29:4–11. [PMC free article: PMC6066088] [PubMed: 18042431] [CrossRef]
- Asquith B, Zhang Y, Mosley AJ, et al. In vivo T lymphocyte dynamics in humans and the impact of human T-lymphotropic virus 1 infection. Proc Natl Acad Sci U S A. 2007;104:8035–8040. [PMC free article: PMC1861853] [PubMed: 17483473] [CrossRef]
- Au WY, Lo JY. HTLV-1-related lymphoma in Hong Kong Chinese. Am J Hematol. 2005;78:80–81. [PubMed: 15609285] [CrossRef]
- Azran I, Schavinsky-Khrapunsky Y, Aboud M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology. 2004;1:20. [PMC free article: PMC514576] [PubMed: 15310405] [CrossRef]
- Barbosa HS, Bittencourt AL, Barreto de Araújo I, et al. Adult T-cell leukemia/lymphoma in northeastern Brazil: a clinical, histopathologic, and molecular study. J Acquir Immune Defic Syndr. 1999;21:65–71. [PubMed: 10235516] [CrossRef]
- Bartholomew C, Jack N, Edwards J, et al. HTLV-I serostatus of mothers of patients with adult T-cell leukemia and HTLV-I-associated myelopathy/tropical spastic paraparesis. J Hum Virol. 1998;1:302–305. [PubMed: 10195256]
- Bartman MT, Kaidarova Z, Hirschkorn D, et al. HTLV Outcomes Study (HOST) Investigators. Long-term increases in lymphocytes and platelets in human T-lymphotropic virus type II infection. Blood. 2008;112:3995–4002. [PMC free article: PMC2581993] [PubMed: 18755983] [CrossRef]
- Basbous J, Arpin C, Gaudray G, et al. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-Jun and modulates their transcriptional activity. J Biol Chem. 2003;278:43620–43627. [PubMed: 12937177] [CrossRef]
- Bazarbachi A, Soriano V, Pawson R, et al. Mycosis fungoides and Sezary syndrome are not associated with HTLV-I infection: an international study. Br J Haematol. 1997;98:927–933. [PubMed: 9326191] [CrossRef]
- Beilke M, Murphy E (2006). The human T-cell leukemia virus types 1 and 2 (HTLV-1 and 2). In: Viral and Immunological Malignancies. Volberding P, Palefsky J, editors. Hamilton, Ontario: BC Decker Inc, pp. 326-358.
- Birmann BM, Breen EC, Stuver S, et al. Population differences in immune marker profiles associated with human T-lymphotropic virus type I infection in Japan and Jamaica. Int J Cancer. 2009;124:614–621. [PMC free article: PMC2701897] [PubMed: 18989900] [CrossRef]
- Boxus M, Twizere JC, Legros S, et al. The HTLV-1 Tax interactome. Retrovirology. 2008;5:76. [PMC free article: PMC2533353] [PubMed: 18702816] [CrossRef]
- Cabrera ME, Marinov N, Guerra C, et al. [Chronic lymphoproliferative syndromes in Chile. A prospective study in 132 patients] Rev Med Chil. 2003;131:291–298. [PubMed: 12790078]
- Cassar O, Capuano C, Bassot S, et al. Human T lymphotropic virus type 1 subtype C melanesian genetic variants of the Vanuatu Archipelago and Solomon Islands share a common ancestor. J Infect Dis. 2007;196:510–521. [PubMed: 17624835] [CrossRef]
- Cavrois M, Leclercq I, Gout O, et al. Persistent oligoclonal expansion of human T-cell leukemia virus type 1-infected circulating cells in patients with Tropical spastic paraparesis/HTLV-1 associated myelopathy. Oncogene. 1998;17:77–82. [PubMed: 9671316] [CrossRef]
- Chan WP, Liang R. Sequence analysis of HTLV-1 provirus associated with adult T-cell leukemia/lymphoma in Hong Kong. Am J Hematol. 1996;51:94–95. [PubMed: 8571947] [CrossRef]
- Chandhasin C, Ducu RI, Berkovich E, et al. Human T-cell leukemia virus type 1 tax attenuates the ATM-mediated cellular DNA damage response. J Virol. 2008;82:6952–6961. [PMC free article: PMC2446947] [PubMed: 18434398] [CrossRef]
- Chang YT, Liu HN, Chen CL, Chow K-C. Detection of Epstein-Barr virus and HTLV-I in T-cell lymphomas of skin in Taiwan. Am J Dermatopathol. 1998;20:250–254. [PubMed: 9650697] [CrossRef]
- Ching YP, Chan SF, Jeang KT, Jin D-Y. The retroviral oncoprotein Tax targets the coiled-coil centrosomal protein TAX1BP2 to induce centrosome overduplication. Nat Cell Biol. 2006;8:717–724. [PubMed: 16767081] [CrossRef]
- Clerc I, Polakowski N, André-Arpin C, et al. An interaction between the human T cell leukemia virus type 1 basic leucine zipper factor (HBZ) and the KIX domain of p300/CBP contributes to the down-regulation of tax-dependent viral transcription by HBZ. J Biol Chem. 2008;283:23903–23913. [PMC free article: PMC3259792] [PubMed: 18599479] [CrossRef]
- Collins ND, Newbound GC, Albrecht B, et al. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed: 9616168]
- Debacq C, Héraud JM, Asquith B, et al. Reduced cell turnover in lymphocytic monkeys infected by human T-lymphotropic virus type 1. Oncogene. 2005;24:7514–7523. [PubMed: 16091751] [CrossRef]
- Doi K, Wu X, Taniguchi Y, et al. Preferential selection of human T-cell leukemia virus type I provirus integration sites in leukemic versus carrier states. Blood. 2005;106:1048–1053. [PubMed: 15840694] [CrossRef]
- Endo K, Hirata A, Iwai K, et al. Human T-cell leukemia virus type 2 (HTLV-2) Tax protein transforms a rat fibroblast cell line but less efficiently than HTLV-1 Tax. J Virol. 2002;76:2648–2653. [PMC free article: PMC135979] [PubMed: 11861831] [CrossRef]
- Etoh K, Tamiya S, Yamaguchi K, et al. Persistent clonal proliferation of human T-lymphotropic virus type I-infected cells in vivo. Cancer Res. 1997;57:4862–4867. [PubMed: 9354450]
- Etoh K, Yamaguchi K, Tokudome S, et al. Rapid quantification of HTLV-I provirus load: detection of monoclonal proliferation of HTLV-I-infected cells among blood donors. Int J Cancer. 1999;81:859–864. [PubMed: 10362130] [CrossRef]
- Farias de Carvalho SM, Pombo de Oliveira MS, Thuler LC, et al. HTLV-I and HTLV-II infections in hematologic disorder patients, cancer patients, and healthy individuals from Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:238–242. [PubMed: 9257659] [CrossRef]
- Fouchard N, Mahe A, Huerre M, et al. Cutaneous T cell lymphomas: mycosis fungoides, Sezary syndrome and HTLV-I-associated adult T cell leukemia (ATL) in Mali, West Africa: a clinical, pathological and immunovirological study of 14 cases and a review of the African ATL cases. Leukemia. 1998;12:578–585. [PubMed: 9557617] [CrossRef]
- Franklin AA, Kubik MF, Uittenbogaard MN, et al. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) ATF-2 response and cAMP element-binding protein (CREB). J Biol Chem. 1993;268:21225–21231. [PubMed: 8407959]
- Fujisawa J, Seiki M, Sato M, Yoshida M. A transcriptional enhancer sequence of HTLV-I is responsible for trans-activation mediated by p40 chi HTLV-I. EMBO J. 1986;5:713–718. [PMC free article: PMC1166849] [PubMed: 3011423]
- Gabet AS, Mortreux F, Talarmin A, et al. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene. 2000;19:4954–4960. [PubMed: 11042682] [CrossRef]
- Gallo RC. Human retroviruses after 20 years: a perspective from the past and prospects for their future control. Immunol Rev. 2002;185:236–265. [PubMed: 12190935] [CrossRef]
- Gaudray G, Gachon F, Basbous J, et al. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol. 2002;76:12813–12822. [PMC free article: PMC136662] [PubMed: 12438606] [CrossRef]
- Gentile TC, Hadlock KG, Uner AH, et al. Large granular lymphocyte leukaemia occurring after renal transplantation. Br J Haematol. 1998;101:507–512. [PubMed: 9633895] [CrossRef]
- Gessain A, de Thé G. Geographic and molecular epidemiology of primate T lymphotropic retroviruses: HTLV-I, HTLV-II, STLV-I, STLV-PP, and PTLV-L. Adv Virus Res. 1996;47:377–426. [PubMed: 8895837] [CrossRef]
- Gessain A, Gallo RC, Franchini G. Low degree of human T-cell leukemia/lymphoma virus type I genetic drift in vivo as a means of monitoring viral transmission and movement of ancient human populations. J Virol. 1992;66:2288–2295. [PMC free article: PMC289023] [PubMed: 1548762]
- Ghez D, Lepelletier Y, Lambert S, et al. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J Virol. 2006;80:6844–6854. [PMC free article: PMC1489069] [PubMed: 16809290] [CrossRef]
- Giam CZ, Jeang KT. HTLV-1 Tax and adult T-cell leukemia. Front Biosci. 2007;12:1496–1507. [PubMed: 17127397] [CrossRef]
- Glynn SA, Murphy EL, Wright DJ, et al. Laboratory abnormalities in former blood donors seropositive for human T-lymphotropic virus types 1 and 2: a prospective analysis. Arch Pathol Lab Med. 2000;124:550–555. [PubMed: 10747312]
- Gotuzzo E. Risk of transfusion-transmitted human T-cell lymphotropic virus-type I in Latin America. Int J Infect Dis. 2000;4:59–61. [PubMed: 10737839] [CrossRef]
- Gout O, Baulac M, Gessain A, et al. Rapid development of myelopathy after HTLV-I infection acquired by transfusion during cardiac transplantation. N Engl J Med. 1990;322:383–388. [PubMed: 2300089] [CrossRef]
- Grassmann R, Dengler C, Müller-Fleckenstein I, et al. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. [PMC free article: PMC287130] [PubMed: 2541443] [CrossRef]
- Grossman WJ, Kimata JT, Wong FH, et al. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. [PMC free article: PMC42636] [PubMed: 7862633] [CrossRef]
- Hanai S, Nitta T, Shoda M, et al. Integration of human T-cell leukemia virus type 1 in genes of leukemia cells of patients with adult T-cell leukemia. Cancer Sci. 2004;95:306–310. [PubMed: 15072587] [CrossRef]
- Hanchard B. Adult T-cell leukemia/lymphoma in Jamaica: 1986–1995. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13 Suppl 1:S20–S25. [PubMed: 8797699] [CrossRef]
- Hanly SM, Rimsky LT, Malim MH, et al. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 1989;3:1534–1544. [PubMed: 2482226] [CrossRef]
- Hanon E, Hall S, Taylor GP, et al. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood. 2000;95:1386–1392. [PubMed: 10666215]
- Hasegawa H, Sawa H, Lewis MJ, et al. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat Med. 2006;12:466–472. [PubMed: 16550188] [CrossRef]
- Hidaka T, Nakahata S, Hatakeyama K, et al. Down-regulation of TCF8 is involved in the leukemogenesis of adult T-cell leukemia/lymphoma. Blood. 2008;112:383–393. [PubMed: 18467597] [CrossRef]
- Hino S. Milk-borne transmission of HTLV-I from carrier mothers and its prevention. Hematology Reviews. 1990;3:223–233.
- Hino S, Katamine S, Miyata H, et al. Primary prevention of HTLV-I in Japan. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13 Suppl 1:S199–S203. [PubMed: 8797724] [CrossRef]
- Hinuma Y, Nagata K, Hanaoka M, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981;78:6476–6480. [PMC free article: PMC349062] [PubMed: 7031654] [CrossRef]
- Hiraragi H, Kim SJ, Phipps AJ, et al. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13(II) is required for viral infectivity in vivo. J Virol. 2006;80:3469–3476. [PMC free article: PMC1440407] [PubMed: 16537614] [CrossRef]
- Hisada M, Okayama A, Shioiri S, et al. Risk factors for adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Blood. 1998;92:3557–3561. [PubMed: 9808547]
- Hisada M, Okayama A, Shioiri S, et al. Risk factors for adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Blood. 1998;92:3557–3561. b. [PubMed: 9808547]
- Hisada M, Okayama A, Spiegelman D, et al. Sex-specific mortality from adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Int J Cancer. 2001;91:497–499. [PubMed: 11251972] [CrossRef]
- Hisada M, Okayama A, Tachibana N, et al. Predictors of level of circulating abnormal lymphocytes among human T-lymphotropic virus type I carriers in Japan. Int J Cancer. 1998;77:188–192. a. [PubMed: 9650550] [CrossRef]
- Hisada M, Stuver SO, Okayama A, et al. Persistent paradox of natural history of human T lymphotropic virus type I: parallel analyses of Japanese and Jamaican carriers. J Infect Dis. 2004;190:1605–1609. [PubMed: 15478065] [CrossRef]
- Hivin P, Basbous J, Raymond F, et al. The HBZ-SP1 isoform of human T-cell leukemia virus type I represses JunB activity by sequestration into nuclear bodies. Retrovirology. 2007;4:14. [PMC free article: PMC1805765] [PubMed: 17306025] [CrossRef]
- Hoshida Y, Li T, Dong Z, et al. Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer. 2001;91:869–875. [PubMed: 11275994] [CrossRef]
- IARC. Human immunodeficiency viruses and human T-cell lymphotropic viruses. IARC Monogr Eval Carcinog Risks Hum. 1996;67:1–424. [PMC free article: PMC5366879] [PubMed: 9190379]
- Iga M, Okayama A, Stuver S, et al. Genetic evidence of transmission of human T cell lymphotropic virus type 1 between spouses. J Infect Dis. 2002;185:691–695. [PubMed: 11865428] [CrossRef]
- Igakura T, Stinchcombe JC, Goon PK, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. [PubMed: 12589003] [CrossRef]
- Inoue J, Itoh M, Akizawa T, et al. HTLV-1 Rex protein accumulates unspliced RNA in the nucleus as well as in cytoplasm. Oncogene. 1991;6:1753–1757. [PubMed: 1923501]
- Isaacson P, Chott A, Nakamura S et al. (2008). Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Malt lymphoma). In: World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Swerdlow SH, Campo E, Harris NL et al., editors. Lyon: IARC Press, pp. 214-217.
- Iwata K, Ito S, Saito H, et al. Mortality among inhabitants of an HTLV-I endemic area in Japan. Jpn J Cancer Res. 1994;85:231–237. [PMC free article: PMC5919449] [PubMed: 8188520]
- Jeang KT, Widen SG, Semmes OJ 4th, Wilson SH. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–1084. [PubMed: 2309119] [CrossRef]
- Jin DY, Giordano V, Kibler KV, et al. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J Biol Chem. 1999;274:17402–17405. [PubMed: 10364167] [CrossRef]
- Jin DY, Spencer F, Jeang KT. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. [PubMed: 9546394] [CrossRef]
- Jones KS, Petrow-Sadowski C, Huang YK, et al. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14:429–436. [PubMed: 18376405] [CrossRef]
- Kamada N, Sakurai M, Miyamoto K, et al. Chromosome abnormalities in adult T-cell leukemia/lymphoma: a karyotype review committee report. Cancer Res. 1992;52:1481–1493. [PubMed: 1540956]
- Kaplan JE, Khabbaz RF, Murphy EL, et al. The Retrovirus Epidemiology Donor Study Group. Male-to-female transmission of human T-cell lymphotropic virus types I and II: association with viral load. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:193–201. [PubMed: 8680892] [CrossRef]
- Kawano N, Shimoda K, Ishikawa F, et al. Adult T-cell leukemia development from a human T-cell leukemia virus type I carrier after a living-donor liver transplantation. Transplantation. 2006;82:840–843. [PubMed: 17006333] [CrossRef]
- Kazanji M, Ureta-Vidal A, Ozden S, et al. Lymphoid organs as a major reservoir for human T-cell leukemia virus type 1 in experimentally infected squirrel monkeys (Saimiri sciureus): provirus expression, persistence, and humoral and cellular immune responses. J Virol. 2000;74:4860–4867. [PMC free article: PMC112009] [PubMed: 10775625] [CrossRef]
- Kikuchi A, Nishikawa T, Ikeda Y, Yamaguchi K. Absence of human T-lymphotropic virus type I in Japanese patients with cutaneous T-cell lymphoma. Blood. 1997;89:1529–1532. a. [PubMed: 9057633]
- Kikuchi A, Ohata Y, Matsumoto H, et al. Anti-HTLV-1 antibody positive cutaneous T-cell lymphoma. Cancer. 1997;79:269–274. b. [PubMed: 9010100] [CrossRef]
- Kim JE, Huh J, Cho K, Kim CW. Pathologic characteristics of primary cutaneous T-cell lymphoma in Korea. J Korean Med Sci. 1998;13:31–38. [PMC free article: PMC3054332] [PubMed: 9539316]
- Koenig S, Woods RM, Brewah YA, et al. Characterization of MHC class I restricted cytotoxic T cell responses to tax in HTLV-1 infected patients with neurologic disease. J Immunol. 1993;151:3874–3883. [PubMed: 7690819]
- Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. [PMC free article: PMC240398] [PubMed: 8445734]
- Koyanagi Y, Itoyama Y, Nakamura N, et al. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology. 1993;196:25–33. [PubMed: 8356797] [CrossRef]
- Kozuru M, Uike N, Muta K, et al. High occurrence of primary malignant neoplasms in patients with adult T-cell leukemia/lymphoma, their siblings, and their mothers. Cancer. 1996;78:1119–1124. [PubMed: 8780552] [CrossRef]
- Kumagawa M, Suzumiya J, Ohshima K, et al. Splenic lymphoproliferative disorders in human T lymphotropic virus type-I endemic area of japan: clinicopathological, immunohistochemical and genetic analysis of 27 cases. Leuk Lymphoma. 2001;41:593–605. [PubMed: 11378577] [CrossRef]
- Kusuhara K, Sonoda S, Takahashi K, et al. Mother-to-child transmission of human T-cell leukemia virus type I (HTLV-I): a fifteen-year follow-up study in Okinawa, Japan. Int J Cancer. 1987;40:755–757. [PubMed: 2891625] [CrossRef]
- Kwaan N, Lee TH, Chafets DM, et al. HTLV Outcomes Study (HOST) Investigators. Long-term variations in human T lymphotropic virus (HTLV)-I and HTLV-II proviral loads and association with clinical data. J Infect Dis. 2006;194:1557–1564. [PubMed: 17083040] [CrossRef]
- Lairmore MD, Silverman L, Ratner L. Animal models for human T-lymphotropic virus type 1 (HTLV-1) infection and transformation. Oncogene. 2005;24:6005–6015. [PMC free article: PMC2652704] [PubMed: 16155607] [CrossRef]
- Larocca D, Chao LA, Seto MH, Brunck TK. Human T-cell leukemia virus minus strand transcription in infected T-cells. Biochem Biophys Res Commun. 1989;163:1006–1013. [PubMed: 2476979] [CrossRef]
- Lee DK, Kim BC, Brady JN, et al. Human T-cell lymphotropic virus type 1 tax inhibits transforming growth factor-beta signaling by blocking the association of Smad proteins with Smad-binding element. J Biol Chem. 2002;277:33766–33775. [PubMed: 12097320] [CrossRef]
- Lezin A, Gillet N, Olindo S, et al. Histone deacetylase mediated transcriptional activation reduces proviral loads in HTLV-1 associated myelopathy/tropical spastic paraparesis patients. Blood. 2007;110:3722–3728. [PubMed: 17717136] [CrossRef]
- Li H, Loehrer PJ Sr, Hisada M, et al. Absence of human T-cell lymphotropic virus type I and human foamy virus in thymoma. Br J Cancer. 2004;90:2181–2185. [PMC free article: PMC2409482] [PubMed: 15150553]
- Li M, Kesic M, Yin H, et al. Kinetic analysis of human T-cell leukemia virus type 1 gene expression in cell culture and infected animals. J Virol. 2009;83:3788–3797. [PMC free article: PMC2663277] [PubMed: 19193802] [CrossRef]
- Liu B, Hong S, Tang Z, et al. HTLV-I Tax directly binds the Cdc20-associated anaphase-promoting complex and activates it ahead of schedule. Proc Natl Acad Sci USA. 2005;102:63–68. [PMC free article: PMC544051] [PubMed: 15623561] [CrossRef]
- Loughran TP Jr, Coyle T, Sherman MP, et al. Detection of human T-cell leukemia/lymphoma virus, type II, in a patient with large granular lymphocyte leukemia. Blood. 1992;80:1116–1119. [PubMed: 1355373]
- Loughran TPJ Jr, Sherman MP, Ruscetti FW, et al. Prototypical HTLV-I/II infection is rare in LGL leukemia. Leuk Res. 1994;18:423–429. [PubMed: 8207960] [CrossRef]
- Mahieux R, Gessain A. The human HTLV-3 and HTLV-4 retroviruses: new members of the HTLV family. Pathol Biol (Paris). 2009;57:161–166. [PubMed: 18456423]
- Manca N, Perandin F, De Simone N, et al. Detection of HTLV-I tax-rex and pol gene sequences of thymus gland in a large group of patients with myasthenia gravis. J Acquir Immune Defic Syndr. 2002;29:300–306. [PubMed: 11873081]
- Manel N, Battini JL, Taylor N, Sitbon M. HTLV-1 tropism and envelope receptor. Oncogene. 2005;24:6016–6025. [PubMed: 16155608] [CrossRef]
- Manel N, Kim FJ, Kinet S, et al. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115:449–459. [PubMed: 14622599] [CrossRef]
- Manns A, Miley WJ, Wilks RJ, et al. Quantitative proviral DNA and antibody levels in the natural history of HTLV-I infection. J Infect Dis. 1999;180:1487–1493. [PubMed: 10515807] [CrossRef]
- Marin O, Hasui K, Remondegui C, et al. Adult T-cell leukemia/lymphoma in Jujuy, north-west Argentina. Pathol Int. 2002;52:348–357. [PubMed: 12100517] [CrossRef]
- Matsumoto S, Yamasaki K, Tsuji K, Shirahama S. Human T lymphotropic virus type 1 infection and gastric cancer development in Japan. J Infect Dis. 2008;198:10–15. [PubMed: 18544011] [CrossRef]
- Matsuoka M. Human T-cell leukemia virus type I (HTLV-I) infection and the onset of adult T-cell leukemia (ATL). Retrovirology. 2005;2:27. [PMC free article: PMC1131926] [PubMed: 15854229] [CrossRef]
- Matsuoka M. Mechanism of oncogenesis by human T-cell leukemia virus type 1. Gan To Kagaku Ryoho. 2010;37:10–13. [PubMed: 20087027]
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–280. [PubMed: 17384582] [CrossRef]
- Meertens L, Chevalier S, Weil R, et al. A 10-amino acid domain within human T-cell leukemia virus type 1 and type 2 tax protein sequences is responsible for their divergent subcellular distribution. J Biol Chem. 2004;279:43307–43320. [PubMed: 15269214] [CrossRef]
- Mirsadraee M, Kalantari MR, Saffari A, Mahmoudi M. Association of HTLV1 infection and esophageal squamous cell carcinoma. J Gastrointest Cancer. 2007;38:15–18. [PubMed: 19065717] [CrossRef]
- Miyazaki M, Yasunaga J, Taniguchi Y, et al. Preferential selection of human T-cell leukemia virus type 1 provirus lacking the 5′ long terminal repeat during oncogenesis. J Virol. 2007;81:5714–5723. [PMC free article: PMC1900290] [PubMed: 17344291] [CrossRef]
- Miyazato P, Yasunaga J, Taniguchi Y, et al. De novo human T-cell leukemia virus type 1 infection of human lymphocytes in NOD-SCID, common gamma-chain knockout mice. J Virol. 2006;80:10683–10691. [PMC free article: PMC1641804] [PubMed: 16943297] [CrossRef]
- Mori N, Morishita M, Tsukazaki T, et al. Human T-cell leukemia virus type I oncoprotein Tax represses Smad-dependent transforming growth factor beta signaling through interaction with CREB-binding protein/p300. Blood. 2001;97:2137–2144. [PubMed: 11264182] [CrossRef]
- Mortreux F, Gabet AS, Wattel E. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. [PubMed: 12529656] [CrossRef]
- Mulloy JC, Kislyakova T, Cereseto A, et al. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol. 1998;72:8852–8860. [PMC free article: PMC110302] [PubMed: 9765430]
- Murphy EL, Hanchard B, Figueroa JP, et al. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer. 1989;43:250–253. [PubMed: 2917802] [CrossRef]
- Murphy EL, Lee TH, Chafets D, et al. HTLV Outcomes Study Investigators. Higher human T lymphotropic virus (HTLV) provirus load is associated with HTLV-I versus HTLV-II, with HTLV-II subtype A versus B, and with male sex and a history of blood transfusion. J Infect Dis. 2004;190:504–510. [PubMed: 15243924] [CrossRef]
- Murphy EL, Watanabe K, Nass CC, et al. Evidence among blood donors for a 30-year-old epidemic of human T lymphotropic virus type II infection in the United States. J Infect Dis. 1999;180:1777–1783. [PubMed: 10558931] [CrossRef]
- Nakada K, Yamaguchi K, Furugen S, et al. Monoclonal integration of HTLV-I proviral DNA in patients with strongyloidiasis. Int J Cancer. 1987;40:145–148. [PubMed: 2886441] [CrossRef]
- Nakamura S, Tsuneyoshi M. Primary gastric T-cell lymphoma with and without human T-lymphotropic virus type 1. Cancer. 1998;82:998–999. [PubMed: 9486597] [CrossRef]
- Nicot C, Dundr M, Johnson JM, et al. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat Med. 2004;10:197–201. [PubMed: 14730358] [CrossRef]
- Nicot C, Harrod RL, Ciminale V, Franchini G. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene. 2005;24:6026–6034. [PubMed: 16155609] [CrossRef]
- Okayama A, Stuver S, Matsuoka M, et al. Role of HTLV-1 proviral DNA load and clonality in the development of adult T-cell leukemia/lymphoma in asymptomatic carriers. Int J Cancer. 2004;110:621–625. [PubMed: 15122598] [CrossRef]
- Orland JR, Wang B, Wright DJ, et al. HOST Investigators. Increased mortality associated with HTLV-II infection in blood donors: a prospective cohort study. Retrovirology. 2004;1:4. [PMC free article: PMC419722] [PubMed: 15169553] [CrossRef]
- Paca-Uccaralertkun S, Zhao LJ, Adya N, et al. In vitro selection of DNA elements highly responsive to the human T-cell lymphotropic virus type I transcriptional activator, Tax. Mol Cell Biol. 1994;14:456–462. [PMC free article: PMC358395] [PubMed: 8264613]
- Pancake BA, Wassef EH, Zucker-Franklin D. Demonstration of antibodies to human T-cell lymphotropic virus-I tax in patients with the cutaneous T-cell lymphoma, mycosis fungoides, who are seronegative for antibodies to the structural proteins of the virus. Blood. 1996;88:3004–3009. [PubMed: 8874198]
- Peloponese JMJ Jr, Haller K, Miyazato A, Jeang KT. Abnormal centrosome amplification in cells through the targeting of Ran-binding protein-1 by the human T cell leukemia virus type-1 Tax oncoprotein. Proc Natl Acad Sci USA. 2005;102:18974–18979. [PMC free article: PMC1323167] [PubMed: 16365316] [CrossRef]
- Peloponese JMJ Jr, Jeang KT. Role for Akt/protein kinase B and activator protein-1 in cellular proliferation induced by the human T-cell leukemia virus type 1 tax oncoprotein. J Biol Chem. 2006;281:8927–8938. [PubMed: 16436385] [CrossRef]
- Pise-Masison CA, Brady JN. Setting the stage for transformation: HTLV-1 Tax inhibition of p53 function. Front Biosci. 2005;10:919–930. [PubMed: 15569630] [CrossRef]
- Plancoulaine S, Buigues RP, Murphy EL, et al. Demographic and familial characteristics of HTLV-1 infection among an isolated, highly endemic population of African origin in French Guiana. Int J Cancer. 1998;76:331–336. [PubMed: 9579568] [CrossRef]
- Plumelle Y, Gonin C, Edouard A, et al. Effect of Strongyloides stercoralis infection and eosinophilia on age at onset and prognosis of adult T-cell leukemia. Am J Clin Pathol. 1997;107:81–87. [PubMed: 8980372]
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–7419. [PMC free article: PMC350514] [PubMed: 6261256] [CrossRef]
- Pombo De Oliveira MS, Loureiro P, Bittencourt A, et al. The Brazilian ATLL Study Group. Geographic diversity of adult t-cell leukemia/lymphoma in Brazil. Int J Cancer. 1999;83:291–298. [PubMed: 10495418] [CrossRef]
- Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. [PubMed: 16155612] [CrossRef]
- Roucoux DF, Murphy EL. The epidemiology and disease outcomes of human T-lymphotropic virus type II. AIDS Rev. 2004;6:144–154. [PubMed: 15595431]
- Roucoux DF, Wang B, Smith D, et al. HTLV Outcomes Study Investigators. A prospective study of sexual transmission of human T lymphotropic virus (HTLV)-I and HTLV-II. J Infect Dis. 2005;191:1490–1497. [PubMed: 15809908] [CrossRef]
- Rousset R, Fabre S, Desbois C, et al. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene. 1998;16:643–654. [PubMed: 9482110] [CrossRef]
- Sacher RA, Luban NL, Ameti DI, et al. Low prevalence of flower cells in U.S.A. blood donors infected with human T-lymphotrophic virus types I and II. Br J Haematol. 1999;105:758–763. [PubMed: 10354142] [CrossRef]
- Sakalian M, Hunter E. Molecular events in the assembly of retrovirus particles. Adv Exp Med Biol. 1998;440:329–339. [PubMed: 9782300]
- Sakashita A, Hattori T, Miller CW, et al. Mutations of the p53 gene in adult T-cell leukemia. Blood. 1992;79:477–480. [PubMed: 1730092]
- Sakata H, Iwakiri R, Koyama T, et al. Human T-cell lymphotropic virus-associated primary gastric lymphoma. Dig Dis Sci. 2001;46:1381–1386. [PubMed: 11478487] [CrossRef]
- Sanchez-Palacios C, Gotuzzo E, Vandamme AM, Maldonado Y. Seroprevalence and risk factors for human T-cell lymphotropic virus (HTLV-I) infection among ethnically and geographically diverse Peruvian women. Int J Infect Dis. 2003;7:132–137. [PubMed: 12839715] [CrossRef]
- Satoh M, Toma H, Sugahara K, et al. Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4(+)25(+) HTLV-1-infected T-cells in human carriers of both HTLV-1 and S. stercoralis. Oncogene. 2002;21:2466–2475. [PubMed: 11971181] [CrossRef]
- Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA. 2006;103:720–725. [PMC free article: PMC1334651] [PubMed: 16407133] [CrossRef]
- Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–1690. [PubMed: 8637512] [CrossRef]
- Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983;80:3618–3622. [PMC free article: PMC394101] [PubMed: 6304725] [CrossRef]
- Shimada-Hiratsuka M, Fukayama M, Hayashi Y, et al. Primary gastric T-cell lymphoma with and without human T-lymphotropic virus type 1. Cancer. 1997;80:292–303. [PubMed: 9217043] [CrossRef]
- Silic-Benussi M, Cavallari I, Zorzan T, et al. Suppression of tumor growth and cell proliferation by p13II, a mitochondrial protein of human T cell leukemia virus type 1. Proc Natl Acad Sci U S A. 2004;101:6629–6634. [PMC free article: PMC404096] [PubMed: 15100416] [CrossRef]
- Siu YT, Chin KT, Siu KL, et al. TORC1 and TORC2 coactivators are required for tax activation of the human T-cell leukemia virus type 1 long terminal repeats. J Virol. 2006;80:7052–7059. [PMC free article: PMC1489057] [PubMed: 16809310] [CrossRef]
- Slattery JP, Franchini G, Gessain A. Genomic evolution, patterns of global dissemination, and interspecies transmission of human and simian T-cell leukemia/lymphotropic viruses. Genome Res. 1999;9:525–540. [PubMed: 10400920]
- Starkebaum G, Loughran TPJ Jr, Kalyanaraman VS, et al. Serum reactivity to human T-cell leukaemia/lymphoma virus type I proteins in patients with large granular lymphocytic leukaemia. Lancet. 1987;1:596–599. [PubMed: 2881134] [CrossRef]
- Stuver SO, Tachibana N, Okayama A, et al. Heterosexual transmission of human T cell leukemia/lymphoma virus type I among married couples in southwestern Japan: an initial report from the Miyazaki Cohort Study. J Infect Dis. 1993;167:57–65. [PubMed: 8418183] [CrossRef]
- Suefuji H, Ohshima K, Hayabuchi N, et al. HTLV-1 carriers with B-cell lymphoma of localized stage head and neck: prognosis, clinical and immunopathological features. Br J Haematol. 2003;123:606–612. [PubMed: 14616963] [CrossRef]
- Suzuki T, Uchida-Toita M, Yoshida M. Tax protein of HTLV-1 inhibits CBP/p300-mediated transcription by interfering with recruitment of CBP/p300 onto DNA element of E-box or p53 binding site. Oncogene. 1999;18:4137–4143. [PubMed: 10435595] [CrossRef]
- Tachibana N, Okayama A, Ishihara S, et al. High HTLV-I proviral DNA level associated with abnormal lymphocytes in peripheral blood from asymptomatic carriers. Int J Cancer. 1992;51:593–595. [PubMed: 1601522] [CrossRef]
- Tajima K. Malignant lymphomas in Japan: epidemiological analysis of adult T-cell leukemia/lymphoma (ATL). Cancer Metastasis Rev. 1988;7:223–241. [PubMed: 3067901] [CrossRef]
- Takatsuki K et al. (1977). Adult T-cell leukemia in Japan. In: Topics in Hematology. Seno S, Takaku S Irino S, editors. Amsterdam: Excerpta Medica, pp. 73–77.
- Takatsuki K, Yamaguchi K, Matsuoka M (1994). ATL and HTLV-I-related diseases. In: Adult T-cell Leukemia. Takatsuki K, editor. Oxford: Oxford University Press, pp. 1–27.
- Takemoto S, Matsuoka M, Yamaguchi K, Takatsuki K. A novel diagnostic method of adult T-cell leukemia: monoclonal integration of human T-cell lymphotropic virus type I provirus DNA detected by inverse polymerase chain reaction. Blood. 1994;84:3080–3085. [PubMed: 7949180]
- Tamiya S, Matsuoka M, Etoh K, et al. Two types of defective human T-lymphotropic virus type I provirus in adult T-cell leukemia. Blood. 1996;88:3065–3073. [PubMed: 8874205]
- Tanaka A, Takahashi C, Yamaoka S, et al. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. [PMC free article: PMC53412] [PubMed: 2300570] [CrossRef]
- Tanaka G, Okayama A, Watanabe T, et al. The clonal expansion of human T lymphotropic virus type 1-infected T cells: a comparison between seroconverters and long-term carriers. J Infect Dis. 2005;191:1140–1147. [PubMed: 15747250] [CrossRef]
- Taylor GP, Goon P, Furukawa Y, et al. Zidovudine plus lamivudine in Human T-Lymphotropic Virus type-I-associated myelopathy: a randomised trial. Retrovirology. 2006;3:63. [PMC free article: PMC1590049] [PubMed: 16984654] [CrossRef]
- Tajima K, Hinuma Y (1984). Epidemiological features of adult T-cell leukemia. In: Pathophysiological Aspects of Cancer Epidemiology. Mathe G, Rizenstein P, editors. Oxford: Pergamon Press, pp. 75–87.
- Tajima K, Hinuma Y. Epidemiology of HTLV-1/II in Japan and the world. Gann Monogr Cancer Res. 1992;39:129–149.
- Tokudome S, Maeda Y, Fukada K, et al. Follow-up of asymptomatic HTLV-I carriers among blood donors in Kyushu, Japan. Cancer Causes Control. 1991;2:75–78. [PubMed: 1873439] [CrossRef]
- Traina-Dorge VL, Martin LN, Lorino R, et al. Human T cell leukemia virus type 1 up-regulation after simian immunodeficiency virus-1 coinfection in the nonhuman primate. J Infect Dis. 2007;195:562–571. [PubMed: 17230416] [CrossRef]
- Tsujimoto H, Noda Y, Ishikawa K, et al. Development of adult T-cell leukemia-like disease in African green monkey associated with clonal integration of simian T-cell leukemia virus type I. Cancer Res. 1987;47:269–274. [PubMed: 2878717]
- Tsukasaki K, Imaizumi Y, Tawara M, et al. Diversity of leukaemic cell morphology in ATL correlates with prognostic factors, aberrant immunophenotype and defective HTLV-1 genotype. Br J Haematol. 1999;105:369–375. [PubMed: 10233406] [CrossRef]
- Ureta-Vidal A, Angelin-Duclos C, Tortevoye P, et al. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: implication of high antiviral antibody titer and high proviral load in carrier mothers. Int J Cancer. 1999;82:832–836. [PubMed: 10446450] [CrossRef]
- Usui T, Yanagihara K, Tsukasaki K, et al. Characteristic expression of HTLV-1 basic zipper factor (HBZ) transcripts in HTLV-1 provirus-positive cells. Retrovirology. 2008;5:34. [PMC free article: PMC2386809] [PubMed: 18426605] [CrossRef]
- Van Dooren S, Pybus OG, Salemi M, et al. The low evolutionary rate of human T-cell lymphotropic virus type-1 confirmed by analysis of vertical transmission chains. Mol Biol Evol. 2004;21:603–611. [PubMed: 14739252] [CrossRef]
- Van Dooren S, Salemi M, Vandamme AM. Dating the origin of the African human T-cell lymphotropic virus type-i (HTLV-I) subtypes. Mol Biol Evol. 2001;18:661–671. [PubMed: 11264418]
- Vandamme AM, Salemi M, Desmyter J. The simian origins of the pathogenic human T-cell lymphotropic virus type I. Trends Microbiol. 1998;6:477–483. [PubMed: 10036726] [CrossRef]
- Verdonck K, González E, Van Dooren S, et al. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–281. [PubMed: 17376384] [CrossRef]
- Wattel E, Mariotti M, Agis F, et al. Quantification of HTLV-1 proviral copy number in peripheral blood of symptomless carriers from the French West Indies. J Acquir Immune Defic Syndr. 1992;5:943–946. [PubMed: 1512694]
- Wattel E, Vartanian JP, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–2868. [PMC free article: PMC188982] [PubMed: 7707509]
- Wiktor SZ, Pate EJ, Rosenberg PS, et al. Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breast-feeding. J Hum Virol. 1997;1:37–44. [PubMed: 10195229]
- Wood GS, Salvekar A, Schaffer J, et al. Evidence against a role for human T-cell lymphotrophic virus type I (HTLV-I) in the pathogenesis of American cutaneous T-cell lymphoma. J Invest Dermatol. 1996;107:301–307. [PubMed: 8751960] [CrossRef]
- Wood GS, Schaffer JM, Boni R, et al. No evidence of HTLV-I proviral integration in lymphoproliferative disorders associated with cutaneous T-cell lymphoma. Am J Pathol. 1997;150:667–673. [PMC free article: PMC1858284] [PubMed: 9033279]
- Yasunaga J, Matsuoka M. Leukemogenesis of adult T-cell leukemia. Int J Hematol. 2003;78:312–320. [PubMed: 14686488] [CrossRef]
- Yasunaga J, Matsuoka M. Human T-cell leukemia virus type I induces adult T-cell leukemia: from clinical aspects to molecular mechanisms. Cancer Control. 2007;14:133–140. [PubMed: 17387298]
- Yasunaga J, Sakai T, Nosaka K, et al. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood. 2001;97:3177–3183. [PubMed: 11342446] [CrossRef]
- Yoshida M, Satou Y, Yasunaga J, et al. Transcriptional control of spliced and unspliced human T-cell leukemia virus type 1 bZIP factor (HBZ) gene. J Virol. 2008;82:9359–9368. [PMC free article: PMC2546946] [PubMed: 18653454] [CrossRef]
- Yoshizuka N, Moriuchi R, Mori T, et al. An alternative transcript derived from the trio locus encodes a guanosine nucleotide exchange factor with mouse cell-transforming potential. J Biol Chem. 2004;279:43998–44004. [PubMed: 15308664] [CrossRef]
- HUMAN T-CELL LYMPHOTROPIC VIRUS TYPE 1 - Biological AgentsHUMAN T-CELL LYMPHOTROPIC VIRUS TYPE 1 - Biological Agents
Your browsing activity is empty.
Activity recording is turned off.
See more...