NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Thyroid disease in pregnancy is a common clinical problem. During the past 2 years significant clinical and scientific advances have occurred in the field. This chapter reviews the physiology of thyroid and pregnancy focusing on iodine requirements and advances in placental function. There follows discussion on thyroid function tests in pregnancy and their interpretation noting ethnic variation in pregnancy range. Sections on iodine nutrition, thyroid autoantibodies and pregnancy complications, thyroid considerations in infertile women, hypothyroidism in pregnancy, thyrotoxicosis in pregnancy, thyroid nodules and cancer in pregnant women, fetal and neonatal considerations, thyroid disease and lactation, screening for thyroid dysfunction in pregnancy will inform the reader of the current information on these areas. Postpartum thyroid disease is also discussed. Current topical fields of importance include the role of isolated hypothyroxinemia on obstetric outcomes and neurodevelopment, the influence of thyroid autoantibodies on the same parameters and the effect of recent data on malformations associated with antithyroid drug therapy on management guidelines for thyrotoxicosis in pregnancy. It also seems as if pregnancy may have a deleterious effect on the progression differentiated thyroid cancer in pregnancy; this requires more confirmation. The intense debate on whether to screen for thyroid function in all pregnant women continues. Although the few randomised trials which have been performed are negative several areas of the world and some clinics in USA recommend screening. In general recent guidelines from USA and Europe find no evidence to support routine screening.
INTRODUCTION
During the past 3-4 decades there has been a major expansion of our knowledge regarding thyroid disorders associated with pregnancy... Thyroid disorders are common. The prevalence of hyperthyroidism is around 5 per 1000 and hypothyroidism about 3- 10 per 1000 in women. As the conditions are generally much more common in the female it is to be expected that they will appear during pregnancy. Developments in our understanding of thyroid physiology (1,2) and immunology (3) in pregnancy as well as improvements in thyroid function testing (4) have highlighted the importance of recognizing and providing appropriate therapy to women with gestational thyroid disorders (5). There has been much discussion and many publications on the optimal management of pregnant women who are hyper or hypothyroid(6,7). In addition. the impact of iodine deficiency on the mother and developing fetus(8), the adverse effects of maternal hypothyroidism on mental development in their offspring(9), the clinical importance of postpartum thyroiditis(10) have all been reviewed .. The field has advanced rapidly so that the evidence based guidelines on thyroid and pregnancy published in 2007 (11) are now being replaced with 4 further updated documents ; two from The American Thyroid Association (12,13) one from The American Endocrine society (14)and one from The European Thyroid Association(15) all with continuing international representation.
Pregnancy may affect the course of thyroid disorders and, conversely, thyroid diseases may affect the course of pregnancy. Moreover, thyroid disorders (and their management) may affect both the pregnant woman and the developing fetus
MATERNAL THYROID PHYSIOLOGY
Numerous hormonal changes and metabolic demands occur during pregnancy, resulting in profound and complex effects on thyroid function Table 14-1 summarizes the main physiologic changes that occur during a normal pregnancy, and which relate to thyroid function or thyroid function testing. These changes are discussed below.
Table 14-1Factors affecting Thyroid Physiology during normal Pregnancy
| Physiologic Change | Thyroid-related consequences |
| Increased renal I- clearance | Increased 24-hr RAIU |
| Decreased plasma I- and placental I- transport to the fetus | In I- deficient women, decreased T4, increased TSH, and goiter formation |
| Increased O2 consumption by fetoplacental unit, gravid uterus and mother | Increased BMR |
| First-trimester increase in hCG | Increased free T4 and T3 Decreased basal TSH (partial blunting of the pituitary-thyroid axis) |
| Increased serum TBG | Increased total T4 and T3 |
| Increased plasma volume | Increased T4 and T3 pool size |
| Inner-ring deiodination of T4 and T3 by placenta | Accelerated rates of T4 and T3 degradation and production |
Iodine and Pregnancy
Physiologic adaptation of the thyroidal economy associated with normal pregnancy is replaced by pathologic changes when pregnancy takes place in conditions with iodine deficiency or even only mild iodine restriction. Globally, the changes in maternal thyroid function that occur during gestation can be viewed as a mathematical fraction, with hormone requirements in the numerator and the availability of iodine in the denominator. When availability of iodine becomes deficient during gestation, at a time when thyroid hormone requirements are increased, this situation presents an additional challenge to the maternal thyroid 1,2. Figure 14-1 illustrates the steps through which pregnancy induces a specific challenge for the thyroid gland and the profound difference between glandular adaptation in conditions with iodine sufficiency or deficiency.
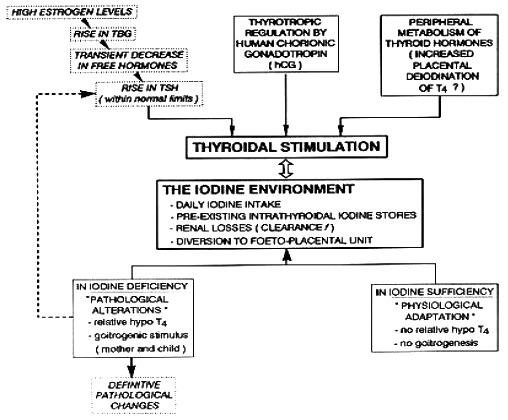
Figure 14- 1
From physiological adaptation to pathological alterations of the thyroidal economy during pregnancy. The scheme illustrates the sequence of events occurring for the maternal thyroid gland, emphasizing the role of iodine deficiency to stimulate the thyroidal machinery (from Glinoer, Ref 1).
Early in pregnancy there is an increase in renal blood flow and glomerular filtration which lead to an increase in iodide clearance from plasma (1,16). This results in a fall in plasma iodine concentrations and an increase in iodide requirements from the diet . In women with iodine sufficiency there is little thyroid impact of the obligatory increase in renal iodine losses, because the intrathyroidal iodine stores are plentiful at the time of conception and they remain unaltered throughout gestation. Pregnancy does not have a major influence on circulating iodine concentrations in iodine-sufficient regions. It should be noted, however, that the iodine excretion levels were unusually high in this study, ranging between 459-786 µg/day (17).
In regions where the iodine supply is borderline or low, the situation is clearly different and significant changes occur during pregnancy (1). Historic studies of radioiodine uptake have shown an increase (18). In addition, there is a further increment in iodine requirements, due to transplacental iodide transport necessary for iodothyronine synthesis by the fetal thyroid gland (19), which becomes progressively functional after the first trimester. When pregnancy takes place in conditions with borderline iodine availability, significant increments in both maternal and fetal thyroid volume occur, if no supplemental iodine is given during early pregnancy (20).
Thus during pregnancy, the physiologic changes that take place in maternal thyroid economy lead to an increase in thyroid hormone production of ~50% above preconception baseline hormone production. In order to achieve the necessary increment in hormone production, the iodine intake needs to be increased during early pregnancy.
Iodine deficiency present at critical stages during pregnancy and early childhood results in impaired development of the brain and consequently in impaired mental function (8,21). Iodine deficiency worldwide is a major cause of neurointellectual impairment and is discussed in detail in chapter 20.Although a variety of methods exists for the correction of iodine deficiency, the most commonly accepted and applied method is universal salt iodization (USI), i.e., the addition of suitable amounts of potassium iodide (or iodate) to all salt for human and livestock consumption. A WHO committee recommended appropriate iodine intakes for pregnant and lactating women as well as for children (Table 14-2) (22)
Table14- 2. Recommended iodine intake during pregnancy and lactation and categorization of iodine nutrition adequacy based on urinary iodine excretion
| Population Group | Median Urinary Iodine conc. | Category of Iodine intake |
| Pregnant women | 250 μg/d | |
| Lactating women | 250 μg/d | |
| Pregnant women | < 150 μg/L | Insufficient |
| 150 – 249 μg/L | Adequate | |
| 250 – 499 μg/L | More than adequate | |
| > 500 μg/L | Excessive | |
| Lactating women | < 100 μg/L | Insufficient |
| > 100 μg/L | Adequate (but see below) |
Patients with known or underlying autoimmune thyroid disorders or autonomous thyroid tissue may have side effects from excessive iodine intake, There is no clear evidence to define “how much more iodine may become too much iodine.” A recommendation was adopted to indicate that there is no proven further benefit in providing pregnant women with more than twice the daily RNI (recommended nutritional intake).
During breast-feeding, thyroid hormone production and urinary iodine excretion return to normal, but iodine is efficiently concentrated by the mammary gland. Since breast milk provides approximately 100 µg/d of iodine to the infant, it is recommended that the breast-feeding mother should continue to take 250 µg per day of iodine (see Table 14-2).
Although substantial progress has been made in the worldwide correction of iodine deficiency mainly by increasing the universal salt iodisation Nevertheless there have been many studies and reports from different world regions demonstrating the resurgence of iodine deficiency in pregnant women despite previous successful public health strategies to correct population deficiencies of the element. Therefore iodine deficiency requires constant monitoring, even after the implementation of iodine supplementation in pregnant women. Recently, iodine deficiency has re emerged in Australia and the UK and even in USA there are groups of the population with suboptimal iodine levels (24-26). The importance of iodine deficiency in pregnancy on childhood IQ has been emphasized (27). In addition there is increasing evidence of the benficial effect of iodine supplementation before and during pregnancy in ameliorating this problem (27)
Metabolism Of Iodine During Normal Pregnancy
After reduction to iodide, dietary iodine is rapidly absorbed from the gut. Then, iodide of dietary origin mixes rapidly with iodide resulting from the peripheral catabolism of thyroid hormones and iodothyronines by deiodination, and together they constitute the extra-thyroidal pool of inorganic iodide (PII). This pool is in a dynamic equilibrium with two main organs, the thyroid gland and the kidneys. Figure 14-2 schematically compares the kinetics of iodide in non-pregnant healthy adults with two different intake levels [a) adequate = 150 µg/day; and b) restricted = 70 µg/day] to the pregnancy situation with a comparable iodine intake of 70 µg/day. A normal adult utilizes ~80 µg of iodide to produce thyroid hormones (TH) and the system is balanced to fulfill these daily needs. When the iodine intake is adequate (150 µg/day, the average situation in the U.S., for instance) in non-pregnant conditions, a kinetic balance is achieved with a 35 % uptake of the available iodine by the thyroid (Figure 14-2; panel A). From the 80 µg of hormonal iodide produced each day by TH catabolism, 15 µg of iodide is lost in the feces, leaving 65 µg to be redistributed between the thyroid compartment (hence, providing 25 µg for daily TH production) and irreversible urinary losses. In such conditions, the metabolic balance is in equilibrium, with 150 µg of iodide ‘in’ & the same amount ‘out’, and 80 µg available for daily hormone production. Thus, with an iodine intake level of 150 µg/day (or above) in non-pregnant healthy adults, the system is able to maintain plentiful intra-thyroidal stores, in the order of 15-20 mg of iodine. In contrast, when the iodine intake is restricted to only 70 µg/day (a situation still seen in parts of Western Europe), the system must up-regulate the glandular iodide trapping mechanisms and increase the relative iodine intake to 50 (Figure 14-2; panel B). The higher uptake allows to recover 35 µg of iodine from dietary intake and 33 µg from TH catabolism but, in these conditions in a non-pregnant healthy adult, this is no longer strictly sufficient to sustain requirements for the production of TH, since 80 µg of iodide is still required daily. To compensate for the missing amount (i.e. ~10-12 µg), the system must use the iodine that is stored in the gland, which therefore becomes progressively depleted to lower levels (~2-5 mg of stable iodine). Over time, if the nutritional situation remains unchanged and despite some adaptation of urinary iodine losses, the metabolic balance becomes negative. The thyroid gland tries to adapt by an increased uptake, glandular hypertrophy, and a higher setting of the pituitary thyrostat.
During pregnancy, two fundamental changes take place. There is a significant increase in the renal iodide clearance (by ~1.3- to ~1.5-fold) and, concomitantly, a sustained increment in TH production requirements (by ~1.5-fold), corresponding to increased iodine requirements, from 80 to 120 µg iodide/day. Since the renal iodide clearance already increases in the first weeks of gestation and persists thereafter, this constitutes a non-avoidable urinary iodine loss, which tends to lower circulating PII levels and, in turn, induce a compensatory increase in the thyroidal clearance of iodide. These mechanisms underline the increased physiologic thyroidal activity during pregnancy. Panel C in Figure 9 indicates that when the daily iodine intake is only 70 µg during pregnancy, despite an increase in glandular uptake to 60 %, the equilibrium becomes more or less rapidly unbalanced, since the iodide entry resulting from both uptake and recycling is insufficient to fulfill the increased requirements for TH production.
Calculations show that, in such conditions, ~20 µg of iodine are missing daily and, in order to sustain TH production, the glandular machinery must draw from already depleted intra-thyroidal iodine stores. Thus in about one trimester after conception, the already low intra-thyroidal iodine stores become even more depleted and, when iodine deprivation prevails during the first half, it tends to become more severe with the progression of gestation to its final stages. A second mechanism of iodine deprivation for the mother occurs later in gestation, from the passage of a part of the available iodine from maternal circulation to the fetal-placental unit. The extent of iodine passage has not yet been precisely established. At mid-gestation, the fetal thyroid gland has already started to produce TH, indispensable for the adequate development of the fetus. In summary, augmentation of iodide trapping is the fundamental mechanism by which the thyroid adapts to changes in the iodine supply, and such mechanism is the key to understanding thyroidal adaptation to iodine deficiency. During pregnancy, increased hormone requirements and iodine losses alter the preconception steady-state. When the iodine supply is restricted (or more severely deficient), pregnancy triggers a vicious circle that leads to excessive glandular stimulation (27).
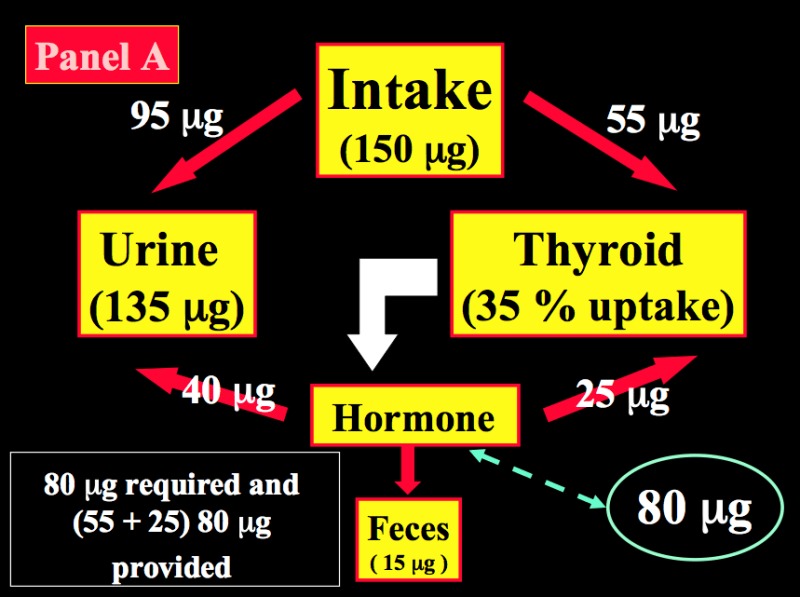
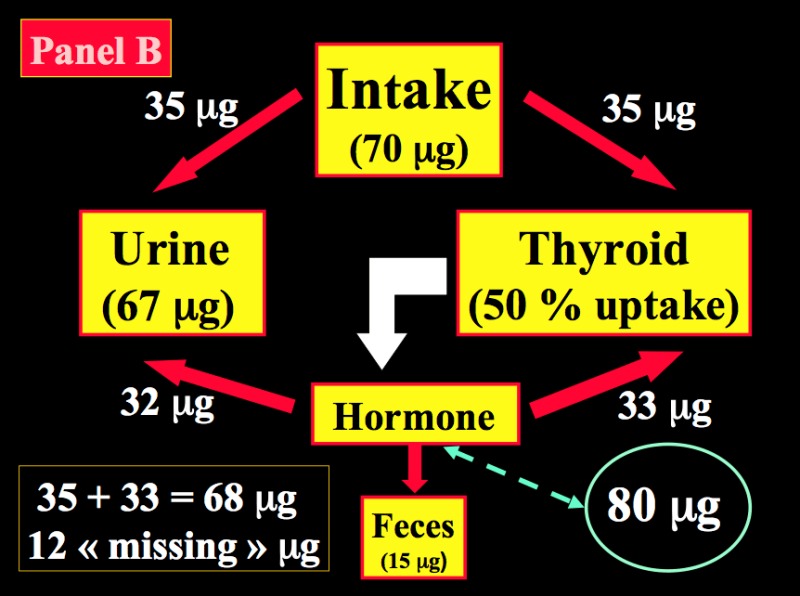
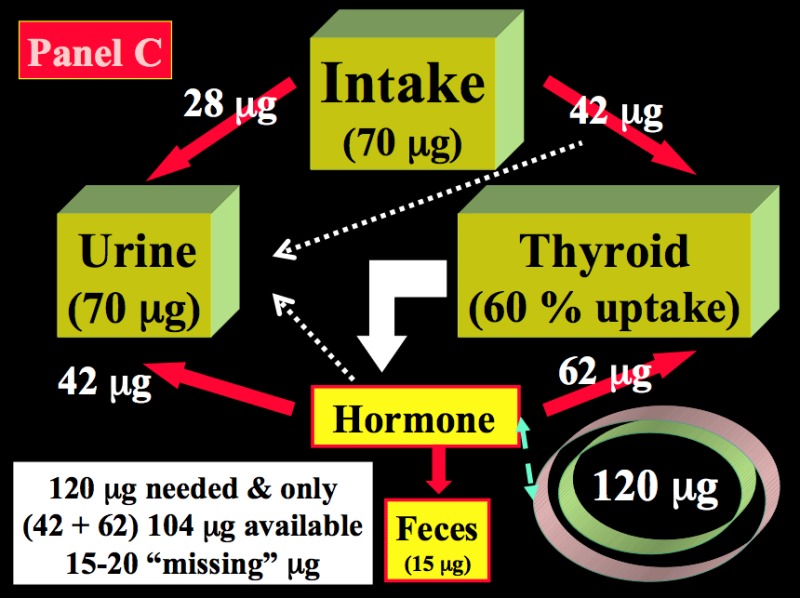
Figure 14-2
Schematic representation of the kinetics of iodide in healthy non-pregnant and pregnant adults. Panel A: non-pregnant adult with an adequate iodine intake of 150 µg/day. Panel B: non-pregnant adult with a restricted iodine intake, corresponding to 70 µg/day. Panel C: the latter condition is compared with an identically restricted level of iodine intake (i.e. 70 µg/day) in a pregnant woman. Daily TH production was set at 80 µg of iodine/day (in non-pregnant) and increased by 1.5-fold to 120 µg/day during pregnancy (from Glinoer, Ref 27).
Goiter Formation In Mother And Progeny
Iodine deficiency during pregnancy, even when considered to be only mild, results in prolonged enhanced thyroidal stimulation and leads to goitrogenesis in both mother and fetus (1). Pregnancy may therefore be considered as an ‘environmental’ factor to induce thyroid pathology in areas with a marginally reduced iodine intake. While goiter formation is not observed in pregnant women who reside in iodine-sufficient regions such as in the USA, several studies from Europe have shown that the thyroid volume (TV) increases significantly during pregnancy (1). In European regions with a sufficient iodine intake, changes in TV remain minimal (10-15% on the average), consistent mainly with vascular thyroid swelling during pregnancy (28). In other European regions with a lower iodine intake, observed changes were much larger, with TV increments ranging between 20-35% on the average, and many women exhibiting a doubling in thyroid size between 1st trimester and term (29,30). In Brussels for instance before iodine supplementation was systematically prescribed, almost 10% of women developed a goiter during pregnancy, which was only partially reversible after parturition (31). In fact there is a high prevalence of thyroid disorders in this region associated with mild iodine deficiency (32). Precise measurements of TV in newborns of these mothers indicated that TVs were 40% larger in newborns from non supplemented mothers (compared with newborns from iodine-supplemented mothers), and thyroid hyperplasia already present in 10% of these infants soon after birth (compared with none in newborns from the iodine-receiving mothers) (33).
Goitrogenesis associated with pregnancy may be one of the environmental factors explaining the preponderance of goiters in the female population. There is an association between parity and thyroid volume in an iodine deficient area (34) and this may be accentuated by active smoking(35) Rarely, a pre-existing goiter may increase in size abruptly during gestation, leading to tracheal compression and respiratory symptoms due partly to intrathyroidal hemorrhage (36,37).
The biochemical markers of enhanced thyroidal stimulation during an otherwise normal pregnancy, when iodine deficiency is present. are firstly relative hypothyroxinemia ( serum free T4 concentrations near (or below) the lower limit of the gestational reference range); Preferential T3 secretion (reflected by an elevated total T3/T4 molar ratio) and a progressive rise in TSH to reach levels that are twice (or even higher) the preconception serum TSH levels (38). In mild to moderate iodine deficiency conditions, serum thyroglobulin (Tg) increases progressively during gestation, so that at delivery, two thirds of women may have supra-normal Tg concentrations. Tg increments correlate well with gestational goitrogenesis, and hence constitute a useful prognostic marker of goiter formation, and its prevention by iodine supplementation (33). The best single parameter to evaluate the adequacy of iodine nutrition in a population is provided by measurements of the urinary iodine excretion (UIE) levels in a representative sample of the population. Although UIE is highly useful for public health estimations of iodine intake in populations, UIE alone is not a valid diagnostic criterion in individuals. Therefore, in the individual at risk of iodine deficiency the markers of thyroid stimulation already described are the best indicators of thyroid stress.
Treatment and Prevention of Maternal Goiter in Pregnancy
In countries with a longstanding and well-established USI program, pregnant women are not at risk of having iodine deficiency. Therefore, no systematic dietary fortification needs to be organized in the population. It should, however, be recommended individually to women to use vitamin/mineral tablets specifically prepared for pregnancy requirements and containing iodine supplements. In countries without an efficient USI program, or with an established USI program where the coverage is known to be only recent or partial, complementary approaches are required to reach the RNI for iodine. Such approaches include the use of iodine supplements in the form of potassium iodide (100-200 µg/day) or the inclusion of KI (125-150 µg/day) in vitamin/mineral preparations manufactured for pregnancy requirements. Finally in those areas with severe iodine deficiency and, in general, no accessible USI program and difficult socioeconomic conditions, it is recommended to administer iodized oil orally as early during gestation as possible. he importance of continuing monitoring of iodine status in the population cannot be overemphasized and has been discussed above.
To prevent gestational goitrogenesis, women should ideally be provided with an adequate level of iodine intake (~150 µg/day) already long before conception. Only then can a long term steady-state be achieved with sufficient intra-thyroidal iodine stores (10-20 mg), thus avoiding triggering of the thyroid machinery that occurs once gestation begins. To achieve such goal, public health authorities ought to implement dietary iodine supplementation national programs in the population. Correcting this public health problem has been the aim of a massive global campaign that was undertaken 15-20 years ago worldwide, based on universal salt iodization (USI), and that has shown remarkable progress so far (33). However, data demonstrate that silent iodine prophylaxis is not sufficient to restore an adequate iodine balance, and that more stringent prophylactic measures need to be taken by public health authorities.
How much supplemental iodine should be given to prevent goiter formation remains a matter of local appreciation and depends primarily on the extent of pre-existing iodine deprivation Since the ultimate goal is to restore and maintain a balanced iodine status in expecting mothers, this can be achieved in most instances with supplements of 100-200 µg of iodine per day given during pregnancy [Fig 14-3] In practice, this requires the administration of multivitamin pills designed specifically for pregnancy purposes and containing iodine supplements. It should be remembered that, because of the longstanding restriction in dietary iodine before the onset of a pregnancy, a lag period of approximately one trimester is inevitable before the benefits of iodine supplementation to improve thyroid function can be observed (33). Even then, despite iodine supplementation, iodine sufficiency may not be attained by all pregnant women (40). Because of the advocacy for salt restriction to reduce cardiovascular mortality a debate has ensued whereby use of iodinated salt seemed to be at odds with this strategy. However, simply increasing the iodine concentration in the salt can accommodate both the reduction in salt intake and the requirement to provide iodine in this way. This strategy has recently been endorsed by WHO (41).
Importantly, it has also emerged that insufficient iodine status is associated with poorer neurocognitive outcome in the offspring. While this has been accepted for many decades in relation to severe iodine (42) deficiency it is now seen to be the case in areas of mild iodine deficiency (43-45). The is accruing evidence that iodine supplementation in pregnancy even in women with mild iodine deficiency is beneficial in improving neurocognitive outcome in the child (46).
Finally, caution is needed to avoid iodine excess to the fetal (47) as well as the maternal (48) thyroid. The fetal thyroid gland is exquisitely sensitive to the inhibitory effects of high iodine concentrations, and a recent study showed that inhibitory effects of high iodine loads could lead to opposite variations in maternal and neonatal thyroid function, i.e. with facilitation of thyroid function in the mother but aggravation in the neonate (49).
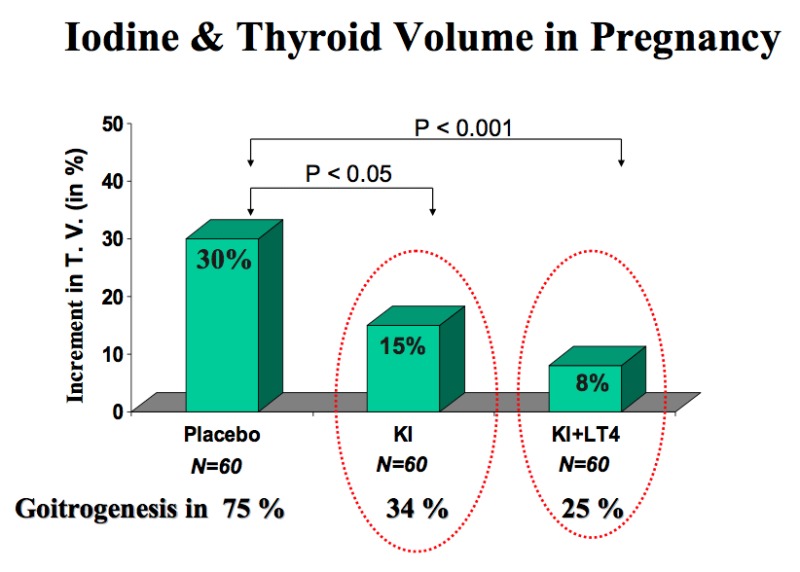
Figure 14-3
Randomized clinical trial with placebo versus KI (100 µg iodine/day) or KI + l-T4 (100 µg iodine/day and 100 µg T4/day) given during pregnancy in women with moderate iodine deficiency and laboratory features of thyroidal stimulation. In the placebo-treated group, TV increased by a mean 30% and goiter formation occurred in 75% of the women. In both actively-treated groups, the increments in TV were significantly reduced (to only 15% and 8%), as was goiter formation (from Glinoer, Ref 33).
In areas with severe iodine deficiency, iodine supplements have been administered to pregnant women using iodized salt, potassium iodide drops and iodized oil (given intramuscularly or orally), as emergency prophylactic and therapeutic approaches to avoid endemic cretinism. Several such programs have conclusively demonstrated their remarkable efficiency to prevent and treat endemic goiter, as well as to eradicate endemic cretinism (8). The results of such studies have indicated that pregnant women who reside in severely iodine-deficient regions can adequately be managed with iodine supplementation. However, except for emergency situations, there is presumably no need to use supra-physiologic amounts of iodine to normalize thyroid function parameters. Although it has not been possible, thus far, in the setting of difficult field studies to evaluate quantitatively the reduction in goiter size or goiter prevalence associated with the clear improvement in thyroid function, goiter reduction is undoubtedly a side benefit of the overall improvement in the iodine nutritional status (50, 51).
In summary, pregnancy is a strong goitrogenic stimulus for both the mother and fetus, even in areas with only a moderate iodine restriction or deficiency. Maternal goiter formation can be directly correlated with the degree of prolonged glandular stimulation that takes place during gestation. Goiters formed during gestation only partially regress after parturition, and pregnancy therefore constitutes one of the environmental factors that may help explain the higher prevalence of goiter and thyroid disorders in women, compared with men. Most importantly, goiter formation also takes place in the progeny, emphasizing the exquisite sensitivity of the fetal thyroid to the consequences of maternal iodine deprivation, and also indicating that the process of goiter formation already starts during the earliest stages of the development of the fetal thyroid gland. Iodine prophylaxis is best achieved using iodised salt. An equal if not more important benefit of using salt supplementation in gestation is the demonstrable positive effect on neurocognition in the child in areas of iodine deficiency or any degree. Monitoring of the population with urinary iodine measurements is essential.
Effects Of human Chorionic Gonadotrophin On Thyroid Function
Human chorionic Gonadotrophin (hCG) is a member of the glycoprotein hormone family that is composed of a common α-subunit and a non-covalently associated, hormone-specific β-subunit. The α-subunit of hCG consists of a polypeptide chain of 92 amino acid residues containing two N-linked oligosaccharide side-chains. The β-subunit of hCG consists of 145 residues with two N-linked and four O-linked oligosaccharide side-chains. The β-subunit of TSH is composed of 112 residues and one N-linked oligosaccharide. The β-subunits of both molecules possess 12 half-cysteine residues at highly conserved positions. Three disulfide bonds form a cystine knot structure, which is identical in both TSH and hCG and is essential for binding to their receptor (LH and hCG bind to the same receptor, the LHCG receptor). A single gene on chromosome 6 encodes for the common αsubunit, while the genes that encode for the β-subunits are clustered on chromosome 19, with seven genes (but only three actively transcribed) coding for β-hCG (52).
The structural homology between hCG and TSH provides already an indication that hCG may act as a thyrotropic agonist, by overlap of their natural functions. Human CG possesses an intrinsic (albeit weak) thyroid-stimulating activity and perhaps even a direct thyroid-growth-promoting activity (52). During normal pregnancy, the direct stimulatory effect of hCG on thyrocytes induces a small and transient increase in free thyroxine levels near the end of the 1st trimester (peak circulating hCG) and, in turn, a partial TSH suppression (1,52.) When tested in bioassays, hCG is only about 1/104 as potent as TSH during normal pregnancy. This weak thyrotropic activity explains why, in normal conditions, the effects of hCG remain largely unnoticed and thyroid function tests mostly unaltered.
The thyrotropic role of hCG in normal pregnancy is illustrated in fig 14- 4. The figure shows the inverse relationship between serum hCG and TSH concentrations, with a mirror image between the nadir of serum TSH and peak hCG levels at the end of the first trimester. The inset in the figure shows that the rise in serum free T4 is proportional to peak hCG values. At this period during gestation, 1/5th of otherwise euthyroid pregnant women have a transiently lowered serum TSH, even below the lower limit of the normal non pregnant reference range (53)
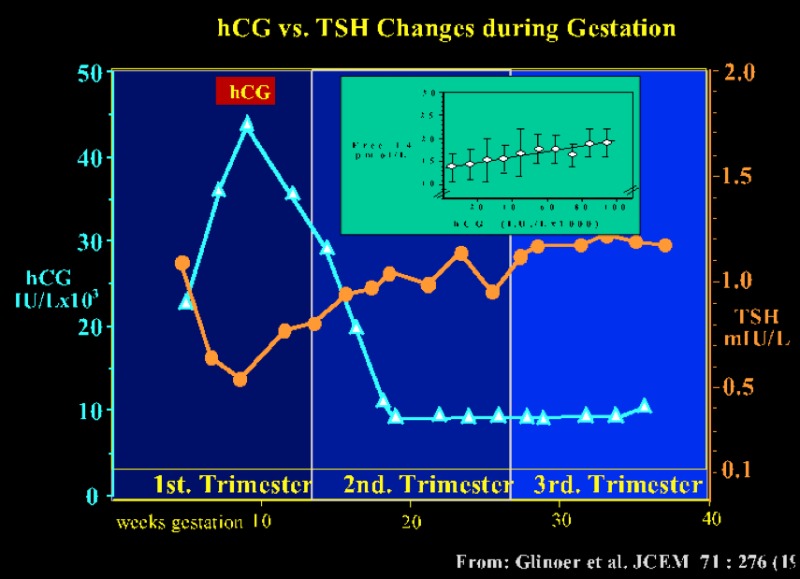
Figure 14-4
The pattern of serum TSH and hCG changes are shown as a function of gestation age in 606 healthy pregnant women. Between 8 and 14 weeks gestation, changes in serum hCG and TSH are mirror images of each other, and there is a significant negative correlation between the individual TSH (nadir) and peak hCG levels (P<0.001) (hCG: ▲-----▲ ; TSH: ●-----●). The inset shows a scattergram of serum free T4 levels in the same women plotted in relation to circulating hCG concentrations (by 10.000 IU/L increment) during the first half of gestation. The figure shows the direct relationship between free T4 and hCG, with progressively increasing free T4 levels (from Glinoer, Ref 53).
Experimental studies with desialylated and deglycosylated hCG, using T3 secretion as the response parameter (in a serum-free culture system with human thyroid follicles), have shown that removal of sialic acid or carbohydrate residues from native hCG transformed such hCG variants into thyroid stimulating super-agonists 54. Further evidence to support the patho-physiological role of hCG to stimulate excessively the human thyroid gland is can be found in studies of patients with hydatidiform mole and choriocarcinoma (see Chapter 13). In these conditions, clinical and biochemical manifestations of hyperthyroidism often occur and, as expected, the abnormal stimulation of the thyroid is rapidly relieved after appropriate surgical treatment (55).
Changes In Circulating Thyroid Hormone Binding Proteins
The increase in total serum T4 and T3 that occurs during pregnancy is due to an increase in serum thyroxine binding globulin (TBG) concentrations. Changes in TBG happen early and, by 16-20 weeks of gestation, TBG concentrations have doubled (1). The cause of the marked increase in serum TBG is probably multifactorial. TBG biosynthesis was increased, after estradiol priming, in primary cultures of hepatocytes from Rhesus monkeys (56) and changes in the glycosylation patterns of TBG, induced by estrogen, have indicated
that the increase in circulating levels of TBG was due in large part to a reduction of its plasma clearance (57). However, the lack of increase in other binding proteins (CBG & SHBG) by estrogen in HEP-G2 cells raised the possibility that other factors might be operative in the pregnant state. (57). Sera of pregnant or estrogen-treated individuals show a marked increase in the more heavily sialylated fractions of TBG. This increase in the sialic acid content of TBG inhibits the uptake of the protein by specific asialylo-glycoprotein receptors on hepatocytes, and the more heavily sialylated proteins from pregnant sera have therefore a longer plasma half-life (58). Such alterations in sialylation are not found in TBG isolated from patients with congenital TBG elevation, the latter being due to a true over-production of the protein (59) Thus, in addition to the stimulatory estrogen effects of estrogen on TBG synthesis, a major contribution to the increased TBG concentration during pregnancy is the reduced clearance of the protein. Delivery leads to a rapid reversal of this process and serum TBG concentrations return to normal within 4-6 weeks. Serum T4 and T3 also return to pregestational serum levels. In addition to the 2 to 3-fold increase in serum TBG, modest decreases in both serum transthyretin (TTR) and albumin are commonly found in pregnancy, but the physiological impact of these changes, if any, is unknown.
In a 42-year-old woman who had both established hypothyroidism and inherited TBG deficiency, the baseline TBG level was 70% below the average baseline level of non-TBG-deficient women (60).. During her pregnancies, serum TBG levels rose, although remaining at only one half the usual increment in TBG associated with normal pregnancy. Despite the patient’s low baseline TBG level and blunted pregnancy-associated TBG rise, she required an increase in her thyroxine replacement doses that mirrored those observed in hypothyroid, but non-TBG-deficient pregnant women. It was suggested therefore that an increase in TBG concentration was not the key determinant for the increase in thyroxine requirement in pregnancy. However, an alternative explanation was proposed (61). In the normal situation before pregnancy, the homeostasis of thyroid function is ensured by the equilibrium between a serum total T4 of ~100 nmol/L and a TBG concentration of ~260 nmol/L. This equilibrium implies, in turn, that ~75 % of the circulating T4 is bound to TBG and that ~35-40 % of circulating TBG is saturated by T4. During a normal pregnancy, the extracellular TBG pool expands from ~3,000 to ~7,000 nmol/L. Thus, for the homeostasis of free thyroid hormones to be maintained, the extra-thyroidal total thyroxine pool must parallel this expansion, and this can only be achieved by the thyroid gland filling up the progressively the increased hormonal pool during the first half of pregnancy (see Figure 14-5). In the exceptional case of Zigman, when this partially TBG-deficient patient was not pregnant, her serum total T4 was ~70 nmol/L and TBG ~80 nmol/L, indicating that her circulating TBG was almost completely saturated by T4, because of her severe restriction in the TBG binding capacity. However in the non pregnant condition, only a relatively small fraction of the patient’s circulating T4 could be bound to TBG: ~50%. When the patient became pregnant, her TBG deficiency was still partially responsive to estrogen induction and TBG increased 3-fold to ~240 nmol/L and total T4 to ~90 nmol/L. In other words, her total T4 concentrations had to be raised by ~30% (via an increase in thyroxine replacement), hence allowing to restore a TBG binding saturation level by T4 of ~35%, equivalent to what is observed at the onset of pregnancy in non-TBG-deficient women. Thus, the increment required in l-T4 dosage was precisely of the same proportion than that anticipated from the partial rise in serum TBG during pregnancy.

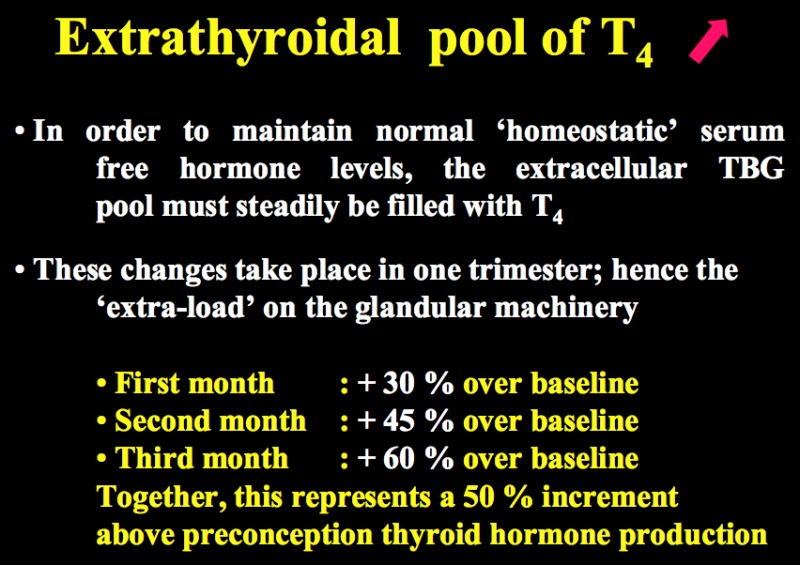
Figure 14-5
The upper panel illustrates the rapid changes that occur in serum total binding capacity of TBG during the first half of gestation under the influence of elevated estrogen levels. The lower panel shows that, in order to maintain unaltered free T4 levels, the markedly increased TBG extra-cellular pool must steadily be filled with increasing amounts of T4, until a new equilibrium is reached. This is achieved during pregnancy via an overall ~50% increase in thyroid hormone production.
Increased Plasma Volume
The increased plasma concentration of TBG, together with the increased plasma volume, results in a corresponding increase in the total T4 pool during pregnancy. While the changes in TBG are most dramatic during the first trimester, the increase in plasma volume continues until delivery. Thus, for free T4 concentration to remain unaltered, the T4 production rate must increase (or its degradation rate decrease) to allow for additional T4 to accumulate. One would predict that in a situation where the T4 input was constant, there would be an iterative increment in T4 as TBG increases, due to reduced T4 availability to degradation enzymes. The evidence that thyroxine requirements are markedly enhanced during pregnancy in hypothyroid treated women (see section on maternal hypothyroidism) strongly suggests that not only T4 degradation is decreased in early pregnancy but also that an increased T4 production occurs throughout gestation to maintain the homeostasis of free T4 concentrations (1)
Thyroxine Production Rate
The only direct measurements of T4 turnover rates in pregnancy were obtained nearly 40 years ago (62). In eight pregnant subjects (4 in 1st half & 4 in 2nd half of gestation), T4 turnover rates were estimated not to be significantly different from those of non-pregnant subjects. However, based on several considerations discussed above from more recent work, it can now be concluded that the T4 production rate is enhanced during pregnancy. Globally, it is accepted that there is a ~50 % increase in the production of T4 during gestation (1)
The Placenta
During the first trimester the human conceptus is surrounded by the placenta, which acts as an exchange unit for nutrients and waste products. The primary barrier to exchange between mother and fetus is the syncytiotrophoblast layer of the placental chorionic villi which has effective tight junctions and prevents the free diffusion of thyroid hormones across it.
The human placenta in addition to this cellular barrier also regulates the amounts of thyroid hormones passing from the mother to the fetus through its expression of placental thyroid hormone transporters, thyroid hormone binding proteins, iodothyronine deiodinases, sulfotransferases and sulfatases (63,64). The transport of iodine through the placenta is also important as the organ has shown to actively concentrate the anion (65). Oxytocin and hCG may also promote placental iodide uptake helping to protect against fetal iodine deficiency(66) Placental NIS protein levels are significantly correlated with gestational age during early pregnancy and increase with increased placental vascularization. This would lead to increased iodide supply to meet increased fetal requirements for thyroid hormone synthesis as the pregnancy progresses (67) The precise details of placental iodide concentration are unclear. It is interesting that in mothers who smoke placental iodide transport seemed unaffected despite high thiocyanate levels, suggesting that thiocyanate-insensitive iodide transporters alternative to NIS are active or that NIS in the placenta is autoregulated to keep iodide transport unaltered.(68).
Fetal circulating concentrations of total T3 are at least 10 fold lower than total T4. Unlike adults, the proportion of free unbound T4 is also higher than bound T4 in early gestation. Free T4 levels are determined by the fetal concentrations of the thyroid hormone binding proteins in the circulation and coelomic cavity and the amount of maternal T4 crossing the placenta. The concentration of free T4 in the coelomic fluid in the first trimester is approximately 50% of that found in the maternal circulation and could therefore exert biological effects in fetal tissues.
The human placenta expresses iodothyronine deiodinases type II (D2) (which activates T4 to T3) and type III (D3) (which inactivates T4 and T3). The principle subtype in the placenta is D3, having 200 times the activity of D2. D3 effectively metabolises most of the maternal T4 presented to the placenta.: still a physiologically relevant amount of T4 is transferred to the fetus. Both D2 and D3 activity per gram of placenta decrease with advancing gestation.( 64). Decidualization, which is a characteristic of the endometrium of the pregnant uterus and a response of maternal cells to the hormone progesterone, is also dependent on the strong expression and tight control of the type 3 deiodinase (69) to regulate the local T3 concentration.
A range of thyroid hormone transporters including monocarboxylate transporters (MCT) 8 and 10, system-L amnio acid transporters (LAT1 and LAT2) and organic anion transporting polypeptides (OATP) 1A2, 4A1 and Oatp1c1 have been located at the apical and basolateral membranes of the syncytiotrophoblasts (70,71). These transporters may facilitate thyroid hormone transfer across the cell barrier from the mother to the fetus ( Figure 14-6). In fact, it seems that the syncytiotrophoblast may control the quantity and forms of thyroid hormones taken up by the human placenta so that this this could be critical in regulating transplacental thyroid hormone supply from the mother to the fetus (72). Studies in the mouse with human placental tissue indicate that MCT8 makes a significant contribution to T3 uptake into human trophoblast cells and has a role in modulating human trophoblast cell invasion and viability (73). Transthyretin (TTR), a serum thyroid hormone binding protein, appears to play an important role in the delivery of maternal thyroid hormone to the developing fetus. (74).The human placenta secretes TTR into maternal and fetal circulations and the placental TTR secreted into the maternal placental circulation can be taken up by trophoblasts and translocated to the fetal circulation, forming a TTR shuttle system. This may have important implications for materno-fetal transfer of thyroid hormones (75).
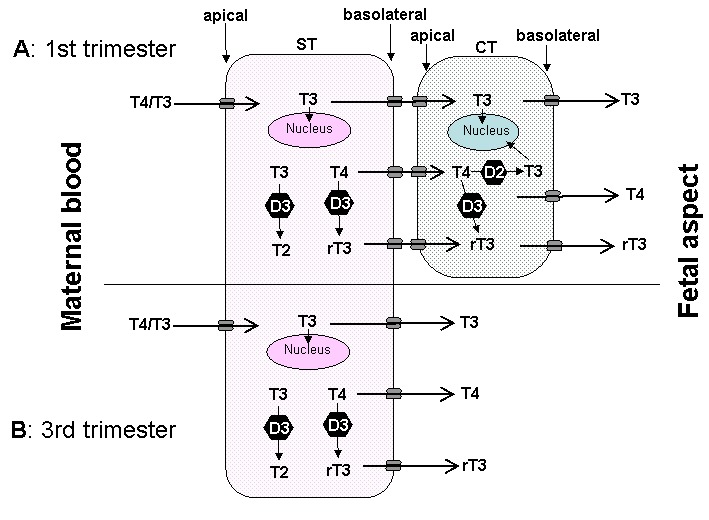
Fig 14-6
The passage of T4 and T3 from the maternal to fetal circulation requires negotiation through the apical membrane (maternal-facing) and the basolateral membrane (fetal-facing) of syncytiotrophoblasts (ST), and in the first half of pregnancy (A) through the plasma membranes of cytotrophoblasts (CT) as well. The localisation and function of the six different TH transporters ( ) present in the placenta may differ. These include monocarboxylate transporters (MCT) 8 and 10, system-L amnio acid transporters (LAT1 and LAT2) and organic anion transporting polypeptides (OATP) 1A2 and 4A1.There may also be other yet to be identified TH transporters. In addition, T4 and T3 are subject to metabolism by deioidnase type 2 (D2) and type 3 (D3) as they pass through the trophoblasts. (From 63)
In addition to the regulation of transplacental thyroid hormone transfer for fetal development, human placental development is itself is responsive to thyroid hormone from early in gestation with evidence of trophoblastic expression of thyroid hormone receptors. T3 has been shown to promote proliferation, invasion and production of epidermal growth factor by 1st trimester primary trophoblast cultures. In humans T3 has been shown to suppress apoptosis and down regulate Fas and Fas-ligand expression (76). It has been postulated that abnormal thyroid hormone levels could give rise to malplacentation which underlie the association between maternal thyroid dysfunction and adverse obstetric outcome.
The inner-ring deiodination of T4 catalyzed by the type 3 deiodinase enzyme is the source of high concentrations of reverse T3 present in amniotic fluid, and the reverse T3 levels parallel maternal serum T4 concentrations (77). This enzyme may function to reduce the concentrations of T3 and T4 in the fetal circulation (the latter being still contributed by 20-30 % from thyroid hormones of maternal origin at the time of parturition), although fetal tissue T3 levels can reach adult levels due to the local activity of the Type 2 deiodinase (see Chapter 15). Type 3 deiodinase may also indirectly provide a source of iodide to the fetus via iodothyronine deiodination. Despite the presence of placental Type 3 deiodinase, in circumstances in which fetal T4 production is reduced or maternal free T4 markedly increased, transplacental passage occurs and fetal serum T4 levels are about one third of normal.(78). Thyroxine can be detected in amniotic fluid prior to the onset of fetal thyroid function, indicating its maternal origin by transplacental transfer (79).. Figure 14-7 depicts the steep maternal to fetal gradient of total T4 concentrations in early pregnancy stages. Between 6-12 weeks gestation, if maternal total T4 concentration is set to represent 100%, the total T4 concentration in the coelomic fluid would represent 0.07% and T4 in the amniotic cavity as little as 0.0003-0.0013% of maternal total T4 concentrations. (Because of low levels of binding proteins in the amniotic cavity, the ratio of amniotic/serum FT4 is much higher.) Thus, the placental barrier to maternal iodothyronines is not impermeable to the transplacental passage of thyroid hormones of maternal origin, even in the 3rd trimester (63). Even though very small quantitatively, such concentrations may qualitatively represent an extremely important source of thyroid hormones to ensure the adequate development of the feto-maternal unit.
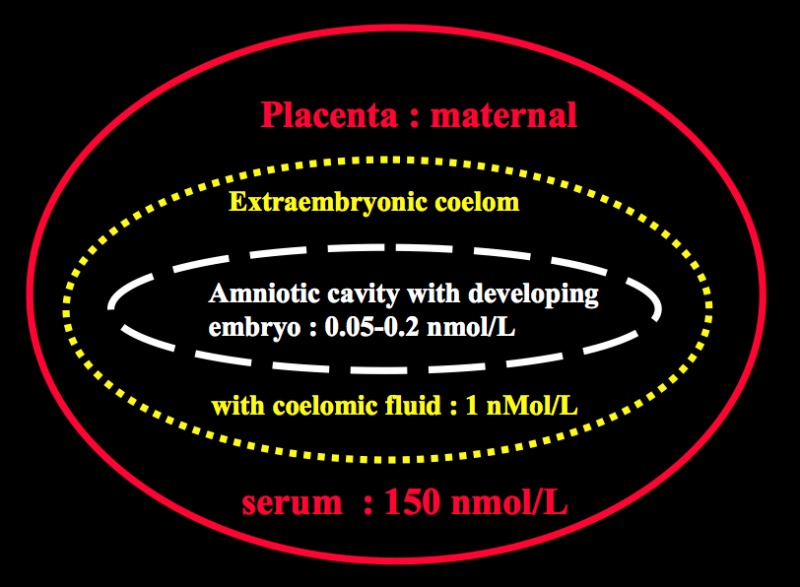
Figure14- 7Steep gradient between maternal concentrations of thyroid hormones (Total T4) and those measured in the coelomic fluid and amniotic cavity with the developing embryo, during early stages of gestation.
A review concluded that a local action of thyroid hormones on female reproductive organs and embryo seemed to be crucial for a successful pregnancy and alterations of the highly regulated local activity of thyroid hormones may play an important, previously underestimated role in early pregnancy and pregnancy loss (80). Furthermore, studies in rats suggest that transcriptomic profiling of the utero-placental compartments, in addition to analysis of mRNA expression of key thyroid hormone placental signaling genes, may predict offspring obesity (81) It is important to note that there is increasing evidence that placental and angiogenic factors are affected by thyroid hormones (82). In isolated human decidual cells T3 regulates angiogenic growth factor and cytokine secretion in a cell-type specific and gestational age specific manner(83). In a large number of women from the Generation R study it was found that high levels of pro- and anti-angiogenic factors may be a risk factor for adverse pregnancy outcomes through their effects on maternal thyroid function (84). Overall thyroid hormones modulate inflammatory processes and are implicated in placental development and disease (85).
Immunological and hormonal aspects of normal pregnancy (Table 14-3)
Pregnancy has a significant effect on the immune system, in order to maintain the fetal-maternal allograft, which is not rejected despite displaying paternal histocompatability antigens. While there is no overall immunosuppression during pregnancy, clinical improvement usually occurs in patients with immunological disorders such as rheumatoid arthritis (RA) when they become pregnant. Clinical improvement occurs as well in psoriatic arthritis and Graves' disease. On the other hand, systemic lupus erythematosus (SLE) may flare during pregnancy.
Table 14-3 Immunological and Hormonal Features of Pregnancy
Clinical: Improvement in Graves’ hyperthyroidism
Rheumatoid arthritis
Psoriatic arthritis and other autoimmune diseases
Trophoblast: HLA G expression
Fas ligand expression
Lymphocytes: Th2 response
Th2 cytokines produced by the fetal/placental unit
Critical role of Treg cells (CD4+CD25+) in maternal tolerance
Possible role of Th17 lymphocyte subset
Hormones: Progesterone increase – reduction in B cell activity
Oestrogen increase – Fall in autoantibody levels
Cortisol,1,25 vitamin D and norepinephrine all affect the immune response
Other Galectin-1
The trophoblast does not express the classical major histocompatibility complex (MHC) class Ia or II which are needed to present antigenic peptides to cytotoxic cells and T helper cells respectively. Instead HLA-G, a non-classical MHC Ib molecule is expressed which may be a ligand for the natural killer (NK) cell receptor so protecting the fetus from NK cell damage ; it may also activate CD8+ T-cells that may have a suppressor function. Human trophoblasts also express the Fas ligand abundantly, thereby contributing to the immune privilege in this unique environment possibly by mediating apoptosis of activated Fas expressing lymphocytes of maternal origin.
T-cell subset studies in pregnancy are discrepant, as peripheral blood CD4+ and CD8+ cell levels have been variously reported to decline, remain unchanged and increase during pregnancy. Although, the distinction between Th1 (T cell helper 1) and Th2 (T cell helper 2) immune responses in humans remains less clear than in the mouse the general agreement is that in pregnancy there is a bias towards a Th2 response (3) This seems to be achieved by the fetal/ placental unit producing Th2 cytokines, which inhibit Th1. Th1 cytokines are potentially harmful to the fetus as, for example, interferon alpha (IFNα) is a known abortifacient. The characterisation of regulatory T cells (CD4+CD25+), a T cell subset that can prevent experimental autoimmune disease, has improved the understanding of the immunological maintenance of pregnancy. It is now thought that these cells are one of the main groups of T cell subsets which allow tolerance of the fetal semi allograft. They may be found in the decidua as well as in the maternal circulation and regulate autoimmune responses.(3)
Assessment of Thyroid Function in Pregnancy
As there is significant overlap between the symptoms experienced by normal euthyroid pregnant women and those with thyroid dysfunction clinical diagnosis is not always straightforward. Because thyroid physiology is altered in pregnancy it has become clear during the past decade that normative gestational reference ranges for thyroid hormone analytes are necessary. Most clinical laboratory reports only provide non-pregnant reference intervals for the interpretation of laboratory results...
The range of normal serum total T4 is modified during pregnancy under the influence of a rapid increase in serum TBG levels. The TBG plateau is reached at mid-gestation (see Figure 14-8, upper left panel) . If one uses total T4 to estimate thyroid function, the non pregnant reference range (5-12 µg/dl; 50-150 nmol/L) can be multiplied t by 1.5 during pregnancy. However, it should be noted that since total T4 values only reach a plateau around mid-gestation, such adaptation is only fully valid during the 2nd half of gestation (see Figure 14-8, upper right panel) . Thus, the use of total T4 does not provide an accurate estimate of thyroid function during early gestation.. However, the free thyroxine index (“adjusted T4“) appears to be a reliable assay during pregnancy (87).
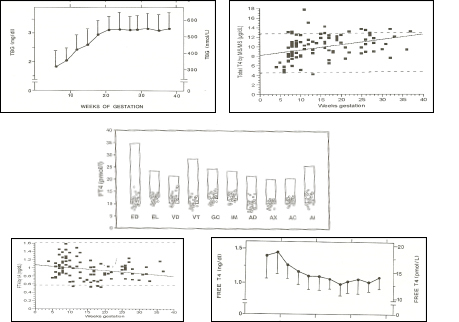
Figure 14-8
Upper left panel: pattern of changes in serum TBG concentrations (mean + sd) in 606 normal pregnant women ( Ref 1). Upper right panel: pattern of changes in serum total T4 concentrations (individual results) in 98 normal pregnancies ( Ref 86). Middle panel: free T4 measurements in 29 women in the 9th month of gestation, using equilibrium dialysis (ED), and 9 different immunoassays (EL: Elecsys; VD: Vidas; VT: Vitros ECi; GC: Gamma-Coat; IM: Immunotech; AD: Advantage; AX: AxSYM; AC: ACS: 180; AI: AIA Pack). The boxes show the non-pregnant upper and lower reference intervals. The percentages given in the upper part of the figure show the mean decrement (in percent) of serum free T4 values compared with the mean free T4 reference value for non-pregnant subjects, provided by the manufacturer. It can be seen that free T4 values were decreased by 40% when measured by ED, and by 17-34% depending on the immunoassay employed . Lower left panel: pattern of changes in serum free T4 concentrations (individual results) in 98 normal pregnancies in the USA, with an adequate iodine intake (Ref 86). Lower right panel: pattern of changes in serum free T4
Although gestation specific reference intervals for thyroid function tests are not currently in routine use in most laboratories there has been intense activity world wide in the development of such ranges (4). . Irrespective of the techniques used to measure free T4 during pregnancy, there is a characteristic pattern of serum free T4 changes during normal pregnancy. This pattern includes a slight and temporary rise in free T4 during the first trimester (due to the thyrotropic effect of hCG) and a tendency for serum free T4 values to decrease progressively during later gestational stages (88). In iodine-sufficient conditions, the physiologic free T4 decrement that is observed during the second and third trimester remains minimal (~10%), while it is enhanced (~20-25%) in iodine-deficient nutritional conditions (see Figure 14-4, lower left and right panels, respectively).
Unfortunately, few if any FT4 immunoassay manufacturers provide appropriate normal pregnancy-related reference intervals that are method-specific (specific for the method used for hormone analysis). It is therefore imperative that method- and gestation-specific reference intervals for FT4 are derived in the appropriate reference populations to prevent misinterpretation of thyroid status in pregnant women. While ‘gold standard methodology’ (e.g. tandem mass spectrometry) is useful for accurate standardisation of values (89), in practice the use of kit assays for free thyroid hormones as well as routine estimation of total bound hormones are used. These are based on analogue methods that rely on the concentrations of binding-proteins, are method-dependent and may give misleading FT4 and FT3 values in pregnancy. For example a commercially available FT4 assay has shown that it correlates more closely to total T4 assays than to FT4 measured following physical separation from binding proteins (90). A comparison of 5 different commercial assays for FT4 and FT3 showed significant interassay variation underlining the necessity for individual laboratory based reference ranges (91). Reference values for FT4 were different when measured by 7 different kits(92).Even in the same region, the use of gestational age specific reference ranges from different laboratories led to misclassification (93). FT4 assays are considered to be flawed and unreliable during pregnancy (87) but there are data showing that, despite susceptibility towards binding protein alterations, these assays may indeed reflect the gold standard assays (94). A mathematical analysis of measurement of total T4 or Free T4 in pregnancy concluded that free hormone measurement is indeed as good as the total assay (95). Gestational reference ranges for theses hormones as well as TSH should be available in every hospital dealing with pregnancy. (4). Table 14-4 shows selected reference ranges for FT4, FT3 and TSH published up to 2008. Since 2008 further country data has been documented (103-107). with emphasis being placed on obtaining first trimester ranges . Concern has been noted with regard to previous suggestions that the upper limit for TSH should be 2.5mIU/L in the first trimester(108) because of ethnic variation(109). A more realistic figure may be 3.0-4.0 mIU/L(110).
Table 14-4
Selected Trimester-Specific, Method-Specific FT4, FT3 and TSH Medians (±SD) or Means* (±SE) and Reference Intervals
| Country (ref) | Gestation
(n=) | FT4 Median
(Reference Interval) or Mean (±SEM)* | TSH Median (Reference Interval) or Mean (±SEM) | FT3 Median (Reference Interval) or Mean (±SEM) | FT4/FT3
Instrument |
| pmol/L | mIU/L | pmol/L | |||
| Australia 2008 (96)
Means* | T11 (1,817) | 13.5 (10.4-17.8) | 0.74 (0.02-2.15) | 4.35 (3.3-5.7) | Abbott Architect i |
| Non-pregnant (100) | (9.0-19.0) | (0.40-4.00) | (3.0-5.5) | ||
| Canada2 2008 (97) | T1 (224) | 15.0 (11.0-19.0) | Roche Cobas e601/E-170 | ||
| T2 (240) | 13.5 (9.7-17.5) | ||||
| T3 (211) | 11.7 (8.1-15.3) | ||||
| India 2008
(98)
ID2 | T1 (107) | 14.46 (12.00-19.45) 4 | 2.1 (0.60-5.00) 4 | 4.4 (1.92-5.86)4 | Roche Cobas e411/Elecsys |
| T2 (137) | 13.4 (9.48-19.58) 4 | 2.4 (0.40-5.78) 4 | 4.3 (3.20-5.70)4 | ||
| T3 (87) | 13.28 (11.30-17.71) 4 | 2.1 (0.74-5.70) 4 | 4.1 (3.30-5.18)4 | ||
| Switzerland 2007
(99)
ID2 | T1 (783) | 13.79 (10.53-18.28) TT45 110.64 (72.27-171.18) nmol/L | 1.04 (0.88-2.83) | 4.67 (3.52-6.22) TT3 1.78 (1.25-2.72) | Abbott Architect i2000SR |
| T2 (528) | 12.17 (9.53-15.68) TT43 134.84 (94.77- 182.51) nmol/L | 1.02 (0.20-2.79) | 4.47 (3.41-5.78) TT3 2.15 (1.43-3.16) | ||
| T3 (598) | 11.08 (8.63-13.61) TT43 136.65 (94.88- 193.35) nmol/L | 1.13 (0.31-2.90) | 4.27 (3.33-5.59) TT3 2.19 (1.40-3.16) | ||
| USA 2008 (100) | T1 (585) | 9.9 (6.8-13.0) FT4 pmol/L | 1.1 (0.04-3.60) | Siemens Immulite 2000 | |
| USA 2008
(101) | T1 (9,562) | 1.13 (1.00-1.20) FT4 ng/dL | 1.05 (0.63-1.66) | Siemens Immulite 2000 | |
| T2 (9,562) | 1.013 (0.92-1.11) ng/dL | 1.23 (0.82-1.78) | |||
| USA 2007
[NHANES III] (88) Means | T1 (71) | TT43 141.35 (3.07) nmol/L (123.64-158.29) | 0.91 (0.17) 0.28-1.06 | Roche | |
| T2 (83) | TT43 152.95 (2.17) nmol/L (146.36-165.13) | 1.03 (0.20) 0.57-1.28 | |||
| T3 (62) | TT43 142.64 (3.73) nmol/L (126.46-160.69) | 1.32 (0.27) 0.69-2.87 | |||
| USA
(86) | T1 (59) | FT4 1.13 (0.23) ng/dL TT43 114.29 (34.36) nmol/L | 1.13 (0.69) | LC/MS/MS API 4000 | |
| T2 (35) | FT4 0.92 (0.30) ng/dL TT43 137.32 (24.97) nmol/L | 1.13 (0.54) | |||
| T3 (26) | FT4 0.86 (0.21) ng/dL TT43 138.48 (25.74) nmol/L | 1.04 (0.61) | |||
| Non-pregnant (26) | FT4 0.93 (0.25) ng/dL TT43 91.63 (10.17) nmol/L | 1.73 (1.13) | |||
|
USA 2007 (102) | T2 (2,551) | FT4 12.0 (9.3-15.2) TT4 128 (89.0-176.0) | 1.14 (0.15-3.11) | FT3 4.85 (3.82-5.96) TT3 2.62 (1.82-3.68) | Abbot Architect i2000SR |
| * Means as marked. All are geometric means (±Standard error of the mean, SEM).
1 T 1=First trimester, Gestation weeks (GW) 1-14; T2=Second trimester, GW 15-28 ; T3=Third trimester, GW 29-40 2 Iodine nutrition status was not assessed in this study; iodine deficiency has not been ruled out. 3 To convert to SI units use www 4Reference interval is 90% 5 IQR=interquartile range Adapted from ref 4 | |||||
In general, serum TSH concentrations provide the first clinical indicator for thyroid dysfunction. Due to the log-linear relationship between TSH and FT4, very small changes in T4 concentrations will provoke very large changes in serum TSH. However, in pregnancy, thyroid and pituitary functions are less stable. During early gestation, TSH is suppressed by 20-50% by week 10 due to the steep increase in hCG concentrations. Therefore, maternal serum TSH does not provide a good indicator for the control of treatment of thyroid dysfunction in the first trimester unless trimester specific ranges are available. False readings can lead to maternal under-replacement with LT4, or overtreatment with anti-thyroid drugs both of which can result in both maternal hypothyroidism and an increased risk for adverse fetal brain development. TSH is however the best measure of thyroid function during the 2nd and 3rd trimesters
Reliable trimester-specific (or gestation-specific) reference intervals for TSH are also now available, being based on an adequate sample size comprised singleton pregnancies in an iodine sufficient, antibody-free population ( see fig 14-9) The importance of the reference range is shown by the fact that 28% of singleton pregnancies with a serum TSH greater than 2 standard deviations above the mean would not have been identified when using the nonpregnant serum TSH range. The individual genetic set-points of a population may result in an intra-individual variability of the thyroid hormone levels, reflected by the reference intervals (112). Also, Afro Americans in USA have lower TSH values in gestation (113), and data from The Netherlands has also documented ethnic differences in thyroid function tests in pregnancy (114). These should be recognized when deriving normative reference ranges.
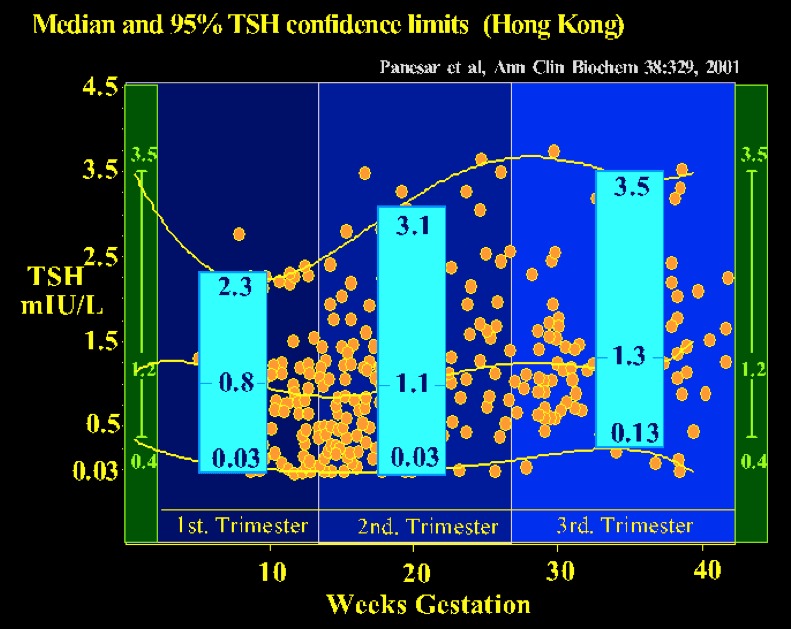
Figure 14-9
Gestation-related reference intervals for serum TSH in a Chinese population (343 healthy pregnant women & 63 non-pregnant controls). The median, 2.5th and 97.5th percentiles for serum TSH values are shown in the blue boxes for each trimester. Gestation-specific reference intervals for TSH should alleviate the potential risk of misinterpretation of thyroid function tests in pregnancy (from Panesar, Ref 111).
In summary, TSH levels may be misleading in the first trimester and T4 values either total or free will give a more accurate estimate of clinical status. Later in gestation TSH levels are reliable whereas T4 may fall especially in the 3rd trimester but this does not indicate hypothyroidism. In some cases, serum anti-TPO antibodies, anti-Tg and/or TSH receptor antibody levels can provide other information; TPO antibodies can predict the risk of hypothyroidism. Ethnic differences in trimester specific reference ranges should be noted. For example the upper limit of TSH in the first trimester was much higher than 2.5 mIU/L in Chinese pregnant women(108). Large differences in thyroid function reference intervals between different populations of pregnant women are seen due to assay variation as well as ethnicity and body mass index (115,116) In pregnant women with low TSH hyperthyroidism, TSH receptor antibodies are observed in 60–70% of the cases.
Hyperemesis Gravidarum
Vomiting occurs in normal pregnancy during the 1st trimester and ceases usually by the 15th week. Prolonged nausea and severe vomiting in early pregnancy that causes greater than a 5% weight loss, dehydration and ketonuria is defined as Hyperemesis Gravidarum (HG) and occurs in 0.5-10 cases per 1,000 pregnancies (117). Hyperemesis is associated with high hCG levels occurring at this time, but the exact cause remains uncertain. For unknown reasons, HG is more prevalent in Asian than Caucasian women. Norwegian data from 1967 to 2005 showed a prevalence of 0.9% but it affected 2.2% of Pakistani women; 1.9% of Turkish women and 0.5% of Norwegian women (118); a familial aggregation suggesting strong evidence for a genetic component of HG. has been suggested (119) When the charge-isoforms profiles of circulating hCG were compared in HG women with different ethnic backgrounds (Samoan vs. European). an increase in total serum hCG concentrations as well as an increase in the proportion of acidic hCG variants in the women suffering from HG, compared with matched control subjects was noted (120). The same study also confirmed the known association between hCG concentrations in early pregnancy and elevations in thyroid hormone levels .While there was no major association between HG and ethnic background, the authors observed a high prevalence of recurrent HG and a familial predisposition for this condition, suggesting that either long-term environmental factors or genetic factors may play a crucial role in the pathogenesis of HG and gestational transient non autoimmune thyrotoxicosis (121)
Thirty to sixty percent of patients with HG have elevations of serum free thyroid hormone concentrations with a suppressed TSH . Women with hyperemesis and elevated thyroid hormone levels most commonly do not have other clinical evidence of primary thyroid disease, such as Graves’ disease. A minor proportion of these patients may have clinical hyperthyroidism, termed ‘gestational hyperthyroidism’ or ‘gestational transient thyrotoxicosis’ (GTT). Graves’ disease can also occur coincident with hyperemesis . Many common signs and symptoms of hyperthyroidism may be mimicked by a normal pregnancy. The clinical challenge is therefore to differentiate between these two disorders
The etiology of excessive thyroid stimulation is considered to be hCG itself (or derivatives of hCG) via a direct stimulation of the thyroid cells through binding of hCG to the TSH receptor (52).. A case of severe HG was reported where the gestational thyrotoxicosis associated with HG was due to a mutation of the TSH receptor, providing hypersensitivity to hCG (122 ). Only one other similar case has since been reported world wide (123). In virtually all patients with gestational hyperthyroidism, appropriate fluid replacement will lead to resolution of the clinical symptoms. As gestation proceeds and hCG levels progressively fall, normal thyroid function is resumed. In severe (but rare) cases, antithyroid drug treatment may be required (described in more detail below). Several investigators have observed that there may even be more subtle form of hyperthyroidism associated with morning sickness (124) Severity of emesis was correlated with serum free T4 and hCG levels and inversely with the degree of TSH suppression (124), suggesting strongly that HG may reflect the extreme end of the spectrum of physiological changes that occur at this time in normal pregnancy (Fig 14-10). It is possible that high hCG levels cause both an increased estrogen secretion as well as thyroid hyperfunction, and in turn explain the coexistence of nausea and vomiting with hyperthyroidism.
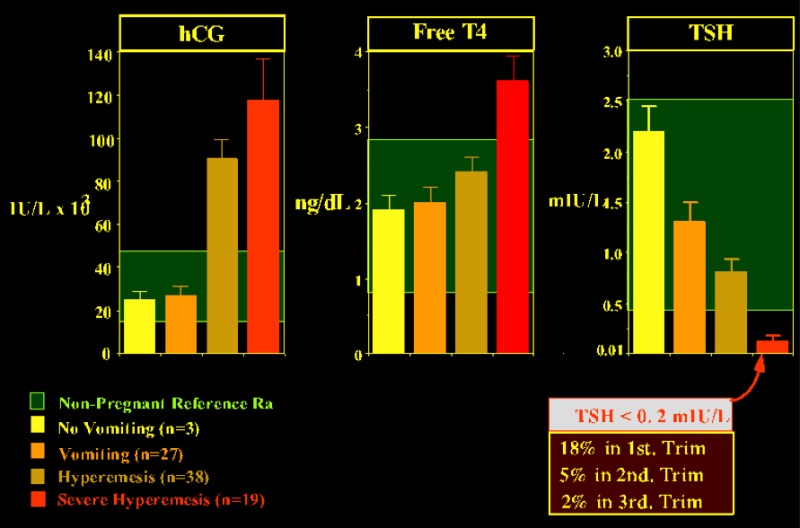
Figure 14-10
Relationship between the severity of vomiting and the mean (with SE) serum concentrations of hCG, free T4, and TSH. The inset in the lower right part of the figure shows the prevalence of suppressed TSH levels, for each trimester of gestation, in a cohort of normal pregnant women. The data were graphically adapted by Carole Spencer (thanks to Carole for allowing me to borrow the slide). The figures are based on studies by Goodwin (Ref 118)
AUTOIMMUNE THYROID DISEASE AND PREGNANCY
The whole spectrum of autoimmune thyroid disease occurs in pregnancy and the postpartum period (see table 14-5). These conditions and their relation to pregnancy are discussed in the rest of this chapter
Table 14-5Autoimmune Thyroid Disease During Pregnancy and the Postpartum Period
1. Asymptomatic autoimmune disease
a) Thyroid antibody positiive (TPOAb and/TgAb) :euthyroid
b) Subclinical hypothyroidism2 Primary hypothyroidism
a) Thyroid destruction (Hashimoto's disease)
b) Circulating TSH-receptor-blocking antibody
3. . Graves' Diseasea)Euthyroidb) Hyperthyroid
4 Postpartum Thyroid Disease
The prevalence of AITD in the pregnant population is comparable to that found in the general female population with a similar age range, i.e. between 5-15% (125). Careful study of women with thyroid antibodies during pregnancy has shown that despite the expected decrease in antibody titers during gestation, thyroid function gradually deteriorated towards hypothyroidism in a significant fraction of such women (Fig 14-11 a,b,c).
In the 1st trimester, serum TSH (albeit within the normal range) was already significantly shifted to higher values in women with AITD, compared with normal pregnant controls. Serum TSH remained higher throughout gestation and at parturition 40% of AITD-positive women had a serum TSH >3 mU/L, with almost one-half of them above 4 mU/L. Thus, while women with AITD were able to maintain a normal thyroid function in early gestation (due to sustained thyrotropic stimulation), their mean serum free T4 levels were significantly reduced to (or below) the lower limit of the normal reference range at delivery. Average reduction in serum free T4 reached 30% and almost one half of these women had free T4 values in the hypothyroid range by the time of delivery, confirming that these women have a reduced functional thyroid reserve. The risk of progression to hypothyroidism could be predicted from serum TSH levels and TPO-Ab titers measured in early pregnancy. When serum TSH was already above 2.5 mU/L and/or TPO-Ab titers above 1,250 U/mL before 20 weeks, these markers were predictive for the development of i hypothyroidism by the end of pregnancy. Practical use of these markers in early gestation can therefore identify those women who carry the highest risk.A Chinese study has confirmed this approach noting that between 7 and 12 weeks gestation the titers of TPOAb and TSH correlate positively and negatively with FT4 respectively (127). Preventive thyroxine treatment administered to avoid the potential deleterious effects of hypothyroxinemia and possibly thyroid antibodies on both maternal and fetal outcomes may then be considered. . There is also evidence from retrospective and some prospective studies that positive thyroid antibodies impacts adversely upon the course of pregnancy in several ways.
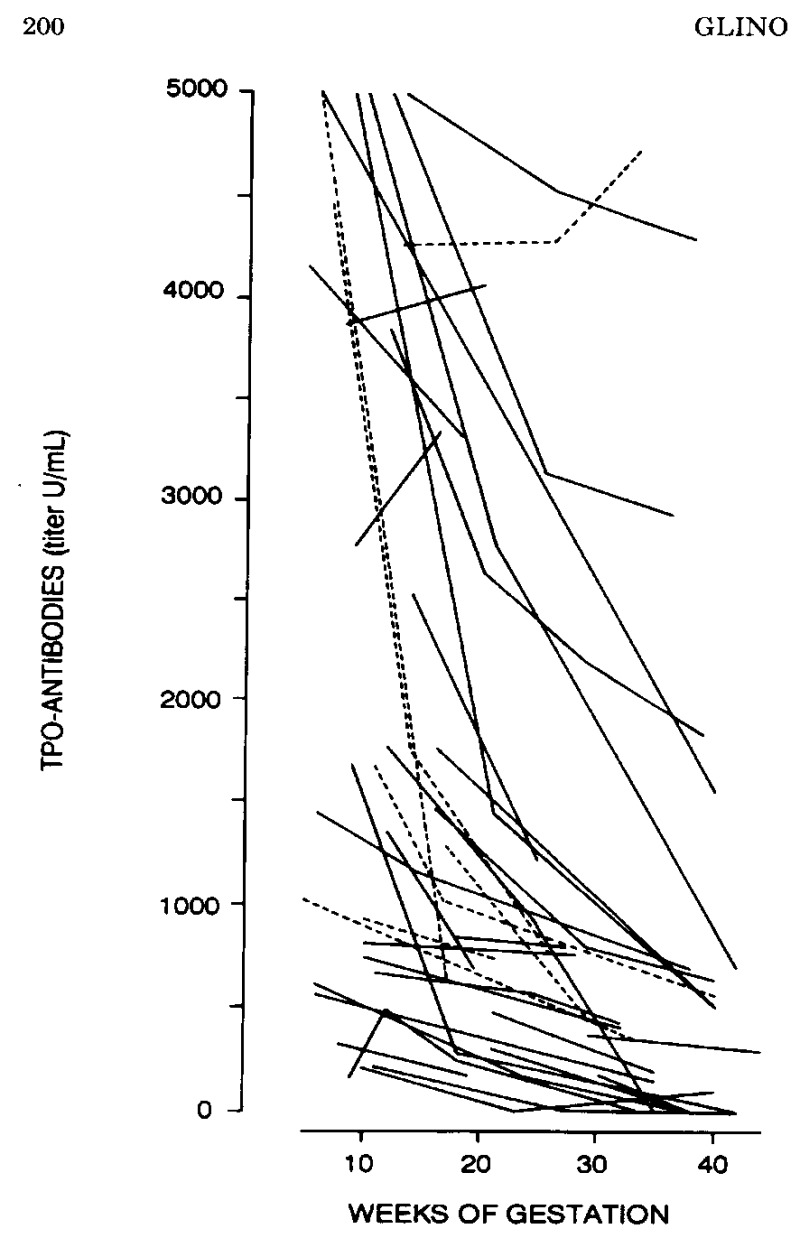
Figure 14-11a
Changes in TPO-Ab in pregnant women with AITD. There was a marked reduction in antibody titers, by 50-60% on the average (solid lines represent asymptomatic euthyroid women; dotted lines women with known hypothyroidism) (from 126).
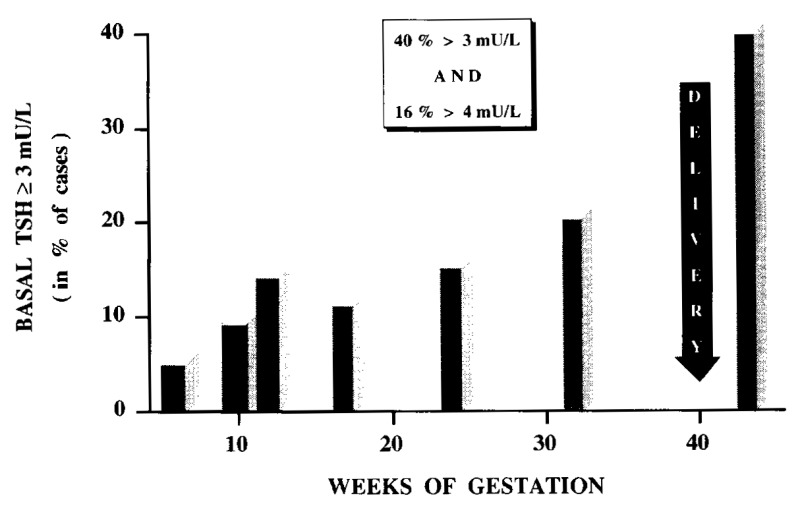
Figure 14-11bAmong women with thyroid antibodies, a progressively increasing fraction developed biochemical hypothyroidism, with 10% of them having a basal serum TSH >3 mU/L in 1st trimester, 20% in 2nd & 3rd trimesters, and finally ~40% at delivery (from 126).
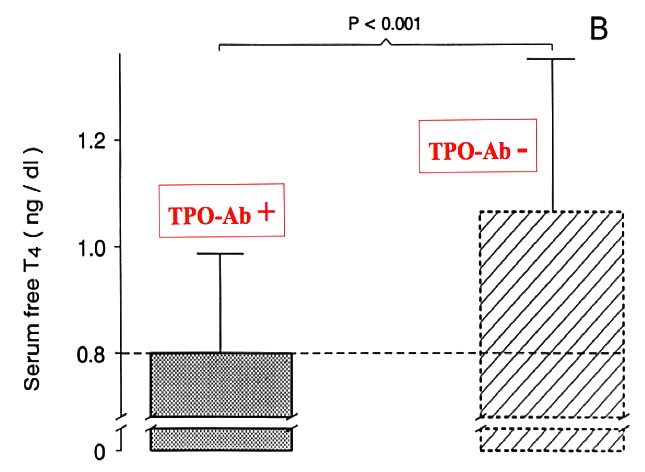
Figure14- 11c
Mean serum free T4 concentrations at delivery in women with and without thyroid immunity. In women with AITD, mean serum free T4 was not only significantly lower than in controls, but in addition, was at the lower limit of normality (from 126).
THYROID AUTOIMMUNITY AND DISORDERS OF FEMALE REPRODUCTION
Infertility
Infertility is defined as the absolute inability to conceive after one year of regular intercourse without contraception. The overall prevalence of infertility is estimated to range from 10% to 15% and has remained stable over the past few decades. The work up of infertile women usually identifies different causal factors, including male-factor infertility in 30%, female causes of infertility in 35%, a combination of both male and female infertility in 20%, and idiopathic infertility in 15%. Female causes of infertility comprise endometriosis, tubal occlusion and ovulation dysfunction. Among the factors that may negatively influence normal fertility, immunologic factors are known to play an important role in the reproduction processes of fertilization, implantation and early development of the embryo. Different investigations support the association between reproductive failure and abnormal immunological test results, including anti-phospholipid, anti-nuclear antibodies and organ specific autoimmunity, among which the presence of antithyroid antibodies (127-129). However, In women with reproductive failure the presence of autoantibodies does not appear to affect the numbers of K cells in the endometrium around the time of implantation (130). In women with repeated implantation failure the percentage of cytotoxic T cells was increase in those with thyroid autoimmunity compared to those without (131).
With regard to thyroid dysfunction, clinical hypothyroidism is clearly associated with female infertility and, in women in the reproductive age, autoimmune thyroid disease (AITD) is the most common cause of hypothyroidism (132,133). Although many of the studies relating to the association of thyroid antibodies and infertility are subject to selection bias, retrospective analysis, and different causes of infertility, they broadly confirm the association. Analysis of a large Danish population (11254 women) has shown that impaired fertility is associated with TSH, TPOAb and subclinical hypothyroidism (134). A previous large study employing control women study has documented an OR of 2.1 (1.7-2.6), p< 0.0001, in favour of the association of infertility and thyroid antibodies (135). Medically-assisted conception and onset of gestation is not hampered by AITD, but a successful outcome of the ongoing pregnancies is significantly reduced in those women with AITD due to greater early pregnancy loss (see Figure 14-23) (136)
The mechanism of the association between thyroid antibodies and infertility is not clear. A review has noted that thyroid hormone disorders and TPOAb are associated with disturbed folliculogenesis,spermatogenesis, fertilization and embryogenesis but the pathogenesis of TPOAb and reproduction is not well understood (137). It is of interest that there is an increase in infertility in women with endometriosis [RR 3.57] (138) which is known to have immune cell depression (NK cells), as well as decreased activity and cytotoxicity against autologous endometrium (139). The importance of NK cells has been emphasised (139) and impaired cellular and humoral response in women with unexplained infertility has been shown (140). The demonstration of antithyroid antibodies in ovarian follicles (141) may also suggest a critical role in infertility associated with autoimmune thyroid disease. These conclusions are strengthened by a study in mice in which it was noted that the anti TPO antibody may affect post-implantation embryo development leading to fetal loss (142). Lack of vitamin D was suggested as a predisposing factor to autoimmune diseases, and was shown to be reduced in patients with thyroid autoimmunity. In turn, its deficiency is also linked to infertility and pregnancy loss, suggesting a potential interplay with thyroid autoimmunity in the context of infertility (143)
The main practical question is whether one should give the benefit of thyroxine administration to infertile women who have positive thyroid antibodies with variable degrees of thyroid insufficiency.Screening for thyroid function in infertile women should be routinely performed (144,145) Obviously, overt thyroid dysfunction should be treated before conception or planned ART. Since SCH has a negative impact on the outcome of pregnancy after ART, thyroxine treatment should also be advised (146). It should be noted that in a study of 21 thyroxine treated women compared to 219 euthyroid women, women with hypothyroidism had a significantly decreased chance of achieving a pregnancy following IVF compared to euthyroid patients (147). The reasons are unknown and more data are required. Controlled ovarian hyperstimulation studied in 57 women led to significant elevations in TSH, often above pregnancy appropriate targets. These findings were particularly evident in women with preexisting hypothyroidism and may have important clinical implications for screening and thyroid hormone supplementation (148) Evidence on the treatment of isolated autoimmune features, but without thyroid dysfunction, was insufficiently documented until recently to advise prompt action (see later section on medical interventions).
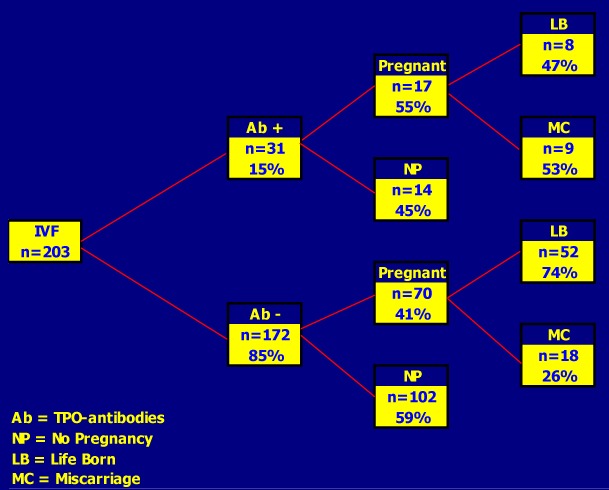
Figure 14-12
Outcome of Assisted Reproduction (IVF) in 203 women with (15%) and without (85%) thyroid autoimmunity (TAI). The rate of successfully-induced pregnancies was not decreased in TAI positive women (~50%), but miscarriages occurred twice more frequently in them (53 versus 26%; O.R for miscarriage in TAI positive cases = 3.77) (from Poppe, Ref 136).
In males, hyperthyroidism causes alterations in spermatogenesis and fertility, and most studies show that hyperthyroid male patients have abnormalities in seminal parameters, mainly sperm motility. These abnormalities tend to improve and normalize when euthyroidism is restored by treatment. Concerning hypothyroidism in males, severe and prolonged thyroid insufficiency may impair reproductive function, particularly when its onset occurs in childhood. Severe juvenile hypothyroidism may also be associated with precocious puberty. Finally, patho-zoospermia and astheno-zoospermia seem more prevalent in infertile males who present features of AITD(2). . Among 71 men with thyroid dysfunction (1/3rd with hyperthyroidism and 2/3rd with hypothyroidism), the authors found an elevated frequency of erectile dysfunction (56/71; 79%). Moreover, the restoration of a euthyroid status by thyroid treatment also restored a normal (or significantly improved) erectile function.(149)
Miscarriage
Thirty-one percent of all pregnancies end in miscarriage. Generally, women who experience a single pregnancy loss do not routinely undergo an evaluation for the cause of miscarriage. Women who experience recurrent miscarriages (i.e. 0.3%-5% of women), which is defined as three or more spontaneous miscarriages without an intervening live birth, should thoroughly be evaluated for an underlying etiology (such as infections, auto-immune disorders, exposure to drugs, etc.) (150).
Stagnaro-Green (151) reported a doubling of the spontaneous miscarriage rate in women who were Ab+ve compared with an Ab-ve cohort (17 vs 8.4%; p = 0.001). Subsequent meta analyses confirmed these associations (152,153). In a further 22 studies up to 2007 with only 6 showed no statistical correlation between the presence of antibodies and miscarriage (154). High TSH levels in women without overt thyroid dysfunction are associated with miscarriage but maternal FT4 levels and child loss were not associated (154). In 101 women with a TSH level more than 20mIU/L treated with T4 adverse pregnancy outcomes occurred no more frequently than in a control group of 205 euthyroid women. However the TSH level during pregnancy was correlated with the rate of abortion and premature delivery (155). In 216 women known to have had a miscarriage before 12 weeks gestation autoimmunity was independently associated (156). A meta analysis of 21 studies (13 cohort and 8 case control) showed a pooled odds ratio of 2.55 [CI 1.42-4.57 p=0.002] (157). A large study in which 17,298 women were screened for thyroid autoimmunity (158) showed a 3 fold increase in placental abruption in the 6% who were antibody positive (OR 3.4 CI 1.7-6.7). This 3 fold risk of placental abruption has been confirmed by a further meta analysis of 31 studies (159) involving more than 12000 women. Meta analysis of both the cohort (n=19) and case-control studies showed a positive association of thyroid antibodies with pregnancy loss (OR 3.9 CI: 2.48-6.12 p<0.001) for cohort studies and 1.8 (1.25-2.6 p,0.002) for case-control studies. A similar OR was found by a Dutch review (3.73 95% CI 1.8-7.6) (160). The association between AITD and miscarriages does not imply a causal relationship, as underlying causal mechanisms might also be attributable to a combination of factors that would potentially lead to miscarriage by themselves. In contrast an observational study of 220 women with recurrent miscarriage with TPOAbs compared to 496 women with miscarriage but no antibodies it was found that the prevalence of TPOAb in women with unexplained RM was not higher than in the general population, TPOAb-positive status did not have a prognostic value regarding the outcome of a subsequent pregnancy, and empirical thyroxine therapy in those who tested positive did not seem to improve outcome (161).However a systematic review has suggested that L Thyroxine does indeed reduce miscarriage rates (162). The American Society of Reproductive medicine asserts that there is fair evidence that thyroid autoimmunity is associated with miscarriage and that L-Thyroxine may improve pregnancy outcomes especially if TSH is > 2.5mIU/L (145).Miscarriage may be linked to a generalized immune imbalance. Women who have had multiple miscarriages have an increased number of CD5/20+ B cells compared with women who have had one or none (163).. Aberrant immune recognition of thyroglobulin (Tg) and placental antigens by antibodies to Tg has been demonstrated in mice immunized with human Tg, and resulted in decreased fetal and placental weights (164).However, evaluation of thyroglobulin expression in reproductive organs of mice showed no message in placenta, decidua or ovary suggesting that antithyroglobulin antibodies have no direct detrimental effect on such organs in patients with thyroid autoimmunity suffering from recurrent abortion (165).On the other hand Ticconi et al (166) in a case control study of 160 women with recurrent miscarriage (RM) found both TPO and Tg antibodies to be more frequently present than in 100 healthy pregnant women. Importantly, more than 90% of the RM women had evidence of other autoantibodies suggesting a more general maternal autoimmune defect in RM.
AITD may be associated with inappropriate low levels of thyroid hormones for the given gestational period, despite apparent biological euthyroidism. Only women with AITD and who experienced a miscarriage showed a difference in median serum levels of TSH and T4 compared to women without AITD (167). Women with AITD are generally older than healthy controls and increased age is an independent risk factor for miscarriage. AITD could act therefore by delaying the occurrence of conception because of its known association with infertility. Thyroid antibody-positive women would tend to become pregnant only at an older age (3-4 years older, on the average) and be more prone to pregnancy loss. There are no clear answers to the problem of thyroid autoimmunity and miscarriage and the subject has been reviewed (168).
Women Undergoing IVF
Different regimes of IVF are now frequently employed in infertile women and new approaches to ovarian stimulation are being implemented (169). A meta analysis of 4 studies on 1098 subfertile women (170) with thyroid autoimmunity and 957 controls showed an RR for miscarriage of 1.99 (CI 1.416-2.793, p<0.001) [ Fig 14-24].
Therefore, on current evidence, it does appear that the presence of thyroid autoimmunity is associated with an increased risk for spontaneous miscarriage in subfertile women achieving a pregnancy through an IVF procedure.(136,170-175); however the clinical picture is not clear cut .Negro et al. (176) found that pregnancy rates were not affected by the presence of TPOAb in euthyroid women undergoing assisted reproductive technology (ART). Intracytoplasmic sperm injection is a relatively new method of ferilisation. Studies of patients with thyroid autoimmunity and thyroid antibodies (anti TPO) concluded that these abnormalities did not affect cumulative delivery rates, fertilization, pregnancy rates, live birth rates or miscarriage rate compared to women without thyroid autoimmunity (177-179). Successful modulation of the immune system with beneficial pregnancy outcome has been reported in patients with AITD who received immunoglobulins with (or without) additional heparin or aspirin (180-182). However, the sudies were not adequately controlled, only comprising small numbers of patients who also had othe, auto-antibodies other than thyroid antibodies.
Fig 14-24
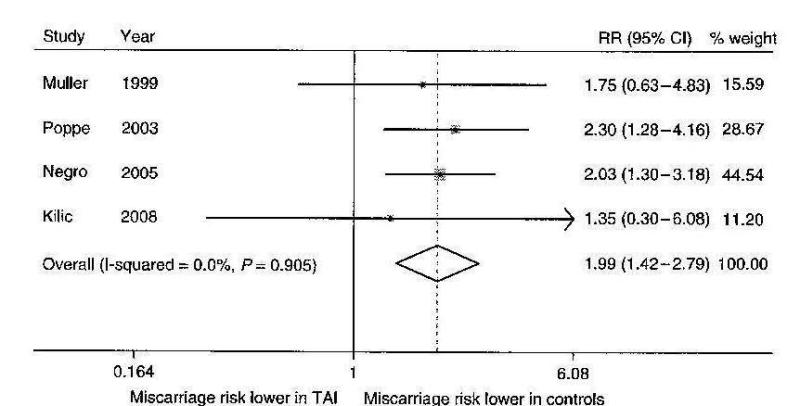
Miscarriage risk in euthyroid women with thyroid autoimmunity undergoing IVF. % weight refers to the emphasis placed on each of the 4 studies used in the analysis. From (170)
It is possible that TPOAb+ve women could have a better outcome with IVF if they also received LT4 as well as aspirin and prednisone (183) When 50 micrograms of LT4 was administered to women with subclinical hypothyroidism undergoing IVF showed an improvement in embryo quality and pregnancy outcome (184). A retrospective cohort study has confirmed that the vast majority of hypothyroid treated women who achieve pregnancy through IVF require an increase in the L-T4 dose during gestation (185). This is a similar situation to hypothyroid pregnant women not having IFV (vide infra).
These findings have implications for screening and medical intervention. For instance, if delayed conception plays a significant role to explain decreased fertility in women with AITD, it would certainly constitute an argument for screening systematically infertile women for the presence of mild thyroid underfunction that is so frequently associated with thyroid antibodies, particularly when women seek medical advice before IVF procedures. There is a high prevalence of women with elevated serum TSH levels, an association between oligo-amenorrhea and abnormally elevated serum TSH values and an overall improvement in the success rate of induced pregnancies after thyroxine administration (186). A recent study of 50 patients confirmed the link between thyroid function, forecast of conception and pregnancy, but noted that there is no recommendation on the TSH target level in patients undergoing assisted reproduction (187). Finally, women with AITD could be advised to plan for a pregnancy at a younger age, although this type of medical advice is more easily said than applicable in practice.
Although a clear association exists between thyroid autoimmunity and pregnancy loss, systematic screening cannot be universally recommended at present time, at least until adequately designed therapeutic trials will demonstrate beyond doubt a clear reduction in the rate of miscarriage with thyroxine treatment. However, many centers, in Europe and elsewhere, already routinely screen women with infertility and/or miscarriage for the presence of thyroid autoimmunity and dysfunction.
Preterm Birth
Preterm delivery (PTD) , that is birth occurring at or before 37 weeks gestation is a major cause of perinatal morbidity and mortality. It is reported to have an incidence of 12.7% (188) and an association with thyroid abnormalities was suggested (189). A subsequent review (190) concluded that autoimmune thyroid disease (positive thyroid antibodies in a euthyroid woman) is a risk factor for PTD and cited studies from Belgium, Pakistan and Italy in which PTD was observed in 16- 26.8% of TPOAb+ve women compared to 8-8.2% of antibody negative women ( all statistically significant). However, the incidence of PTD was only 4% in TPOAb+ve versus 3% in antibody negative women (p=ns) (191). Other groups have also failed to find an association between PTD and thyroid autoimmunity (192- 194). However, an increase in very preterm birth (before 34 weeks) was found in women who were TPOAb positive in the first trimester (192,195). A meta analysis of the studies defining PTD at 37 weeks showed an OR for the association of thyroid antibodies in 5 studies to be 2.07 [CI 1.17-3.68. p=0.01](159).A further meta-analysis(196) reviewed 11 prospective cohort studies involving 35, 467 participants and showed the combined RR of preterm delivery for pregnant women with thyroid antibodies compared with the reference group was 1.41 (95% CI 1.08-1.84, P=0.011). Other studies have also strengthened the association between PTD and thyroid autoimmunity (197-199). Although methodology and number of women studied varies in the different reports, current evidence suggests that the presence of TPO-Ab in pregnant women significantly increases the risk of preterm delivery. Further studies are required to evaluate other factors (eg ethnicity) associated with these findings. For example Interleukin-6 levels may also be an important factor (200). Thyroid disease is associated with systemic lupus erythematosus and pregnant patients with this disorder also have an increase in PTD (201).
Implications for Therapy
In the conditions referred to above the patients are all euthyroid. Although there may be a tendency in TPOAb+ve women to develop a raised TSH later on in pregnancy this only occurs in the minority. The Generaation R study indicated that hypothyroxinemia (RR about 3.5) as well as TPO-Antibody positivity (RR about 2.0) are risk factors for premature delivery (202) It has been suggested that L-thyroxine treatment may correct any slight deficiency in this clinical situation as well as influencing the systemic immune disturbance and the placental-decidual environment (159). Two prospective randomised trials by Negro and colleagues (176,203) support this view. In the first L-thyroxine (1mcg/kg/day)was given to women scheduled to have IVF treatment; this resulted in a 36% reduction in miscarriage rate. The later study used a mean L-T4 dose of 49.7mcg/day in women with positive antiTPOAb and noted a 75% reduction in miscarriage as well as a 69% reduction in pre-term births. More trials are awaited before a firm recommendation can be made. A systematic review (162) stated that for subclinical hypothyroidism and thyroid autoimmunity, evidence is insufficient to recommend treatment with levothyroxine. However a Cochrane review (204) stated that a reduction in preterm birth and a trend towards miscarriage with L-T4 was shown.The lack of prospective randomized controlled trials in this area of practice is currently impeding progress in high quality evidence based clinical decision making.
PRIMARY HYPOTHYROIDISM
Clinical Epidemiology
The prevalence of overt and subclinical hypothyroidism in pregnancy is estimated at 0.3-0.5% and 2-3% (or even up to 5%) respectively (205,206). Endemic iodine deficiency is the most common cause of hypothyroidism seen in pregnant women worldwide. Even mild iodine deficiency can be associated with a high prevalence of thyroid autoimmunity in the first trimester (32), and inadequate iodine status has been accompanied by a high prevalence of hypothyroidism in pregnancy (207). However the main cause of hypothyroidism in iodine-replete populations is chronic autoimmune thyroiditis (208). Other causes include post-surgical, post-radioiodine ablation and hypothyroidism secondary to pituitary disease which, although rare, can include lymphocytic hypophysitis occurring during pregnancy or postpartum (209). (Table 14-6)
Table 14-6
Etiology and Diagnosis of Hypothyroidism in Pregnancy
| Cause |
Diagnostic Feature
|
| Autoimmune thyroiditis | Positive thyroid antibody test (TPOAb TSH Receptor blocking antibodies |
| Iodine deficiency | Low urinary iodine. Goiter |
| Post surgical | History of Graves’ disease or toxic nodular goiter , Thyroid cancer, Benign goiter |
| Pituitary disease | Features of hypopituitarism |
Overt hypothyroidism in pregnancy may present classically but is oftentimes subtle and difficult to distinguish from the symptoms of normal pregnancy. A high index of suspicion is therefore required especially in women with a predisposition to thyroid disease such as a personal or family history of thyroid disease, the presence of goitre or the co-existence of other autoimmune disorders like type 1 diabetes.(210) which are all predictive factors of high risk of autoimmune thyroid disease. Thyroid antibodies are found in 5-15% of normal women in the childbearing age and, when the iodine nutrition status is adequate, the main cause of hypothyroidism during pregnancy is chronic autoimmune thyroiditis. An earlier review concluded that the overall prevalence of hypothyroidism was 2.2% to 3.4%, and the prevalence of thyroid antibodies ranged from 25% to 77% of hypothyroid pregnant women, with a mean prevalence of 46%. Thyroid autoimmunity was 5.2-fold more frequent in women with a diagnosis of hypothyroidism, compared with euthyroid controls (mean of 48.5% versus 9.2%) (211).
Clinical and Diagnostic Features
Symptoms and signs may raise clinical suspicion of hypothyroidism during pregnancy (weight increase, sensitivity to cold, dry skin, etc.) but others may go unnoticed (asthenia, drowsiness, constipation, etc.). Because many women remain asymptomatic, particular attention is required from the obstetrical care providers for this condition to be diagnosed and to evaluate more systematically thyroid function when women attend the prenatal clinic for the first time. Only thyroid function tests confirm the diagnosis. A serum TSH elevation suggests primary hypothyroidism and measurement of serum free T4 levels further distinguish between subclinical hypothyroidism (SCH) and overt hypothyroidism (OH), depending on whether free T4 is normal or clearly below normal for gestational age. Determination of thyroid antibodies, thyroperoxidase (TPO-Ab) and thyroglobulin (TG-Ab) antibodies, confirms the autoimmune origin of the disorder (212). Recently there have been a number of studies examining the relationship between ovarian reserve and thyroid autoimmunity (TA) using anti Mullerian hormone (AMH) as a marker for the former. Women with TA have a reduced ovarian follicular reserve (213). There is evidence that AMH levels are inversely correlated with TSH levels in infertile women of reproductive age (214). Although the probability of a poor response to controlled ovarian hyperstimulation (COH) is high and independent of autoimmune thyroid disease (AITD) in women with low serum AMH levels, in those women with good ovarian reserve (high AMH) the presence of AITD impairs the outcome of COH (215). In a large cross sectional retrospective study (216) it was found that a) serum thyroid hormone levels, anti-TPOAb and prevalence of subclinical hypothyroidism were no different in different ovarian reserve categories, b) a higher prevalence of subclinical and overt hypothyroidism was seen in women with a genetic cause for low ovarian reserve compared to those with unexplained cause. The relationship is clearly complex and further work is required.
Effect of Hypothyroidism on the Outcome of Pregnancy
Despite the known association between decreased fertility and hypothyroidism, the latter condition does not preclude the possibility to conceive. Gestational hypothyroidism, and particularly subclinical hypothyroidism is not rare. Abalovich at al, showed that 34% of hypothyroid women became pregnant without thyroxine treatment: 11% of them had OH and 89% SCH (217).. When hypothyroid women become pregnant and maintain the pregnancy, they carry an increased risk for early and late obstetrical complications (Table 14-7). An analysis of 223,512 singleton pregnancies from a retrospective US cohort showed that , thyroid diseases were associated with obstetrical, labor, and delivery complications (218). Unfortunately these authors had no access to treatment details. Furthermore, in a study of 92 women on T4 replacement therapy the occurrence of maternal or fetal/neonatal complications could not be predicted by maternal TSH/fT4 through pregnancy, presence of thyroid autoimmunity or dose of LT4 replacement (219).
Table 14-7
Adverse Outcomes of Hypothyroidism
MOTHER
Infertility
Miscarriage
Preterm Delivery
Anemia in pregnancy
Preeclampsia
Abruptio placenta
Postpartum haemorrhage
BABY
Increased fetal death rate
Preterm birth
Intra Uterine Growth Retardation
Low birth weight
Increased neonatal respiratory distress
Impaired neurointellectual child development
Attention Deficit Hyperactivity Disorder
Autism
The frequency of these complications depends on whether they are associated with overt (high TSH associated with low FT4) or subclinical hypothyroidism (high TSH associated with FT4 within the reference range). With regard to fetal death rates Benhadi et al (154) noted that the risk of child loss increased with higher levels of maternal TSH although maternal FT4 concentrations and child loss were not associated. Ashoor et al (220-222) however, have observed that fetal loss was associated with both an increase in TSH and a decrement in FT4 although the presence of thyroid antibodies did not affect these results. They have also shown that impaired thyroid function may predispose to the development of late pre eclampsia (221) but they found no evidence of thyroid dysfunction or maternal thyroid antibodies to be related to preterm birth (222). A definitive study by Casey et al.(223) found that subclinical hypothyroidism in pregnancy has a relative risk of 1.8 for premature birth/low birth weight.Interestingly,maternal high-normal FT4 levels in early pregnancy were associated with lower birth weight and small for gestational age in the Generation R study of more than 4000 women (224). The continuous reciprocal relationship between maternal weight and FT4 has also been noted in the 2nd trimester in more than 9000 women (225) An overview is given by Negro (226).
While the prevalence of hypothyroidism and subclinical hypothyroidism has been mentioned it should be noted that the state of isolated hypothyroxinemia (IH) occurs in around 2.5 to 10% depending on the definition employed (227). There has been controversy as to whether IH is a real entity and if so whether there are any adverse effects of the condition in gestation. The cause(s) of IH are not clear but iodine deficiency may be an important factor. IH does have adverse obstetric effects, although early studies of first trimester IH showed no adverse outcomes (228,229). Subsequent studies have documented an increase in preterm labour and macrosomia as well as gestational diabetes and increased placental abruption(230). Breech presentation,,larger fetal and infant head size and fetal distress have also been associated with IH (230-234). Despite these data guideline committees have concluded that there is not enough evidence to recommend L-T4 treatment in women with IH (13-15).
A review of the treatment of subclinical hypothyroidism SCH) in pregnancy concluded that while SCH is associated with multiple maternal and neonatal outcomes the value of L-T4 treatment remains uncertain (235). Nevertheless,adequate thyroxine treatment is critical to the outcome independent of the type of hypothyroidism (OH/SCH) A retrospective study of 150 pregnancies noted that adequate treatment of overt and SCH minimised the risks of abortion and premature delivery regardless of initial thyroid status,whereas inadequate therapy resulted in an increased rate of abortion and preterm deliveries (218) rate. A prospective randomised intervention trial also showed that even in euthyroid thyroid antibody positive pregnant women who were treated with thyroxine the rates of miscarriage and pre-term delivery were lower than euthyroid antibody positive women who did not receive thyroxine treatment (203). A retrospective study of women with SCH who were or were not prescribed L-T4 during pregnancy showed that the L-T4 group had fewer loss of pregnancies, and fewer low birth weight infants as well as better APGAR scores in the infants (236). From a practical point of view adherence to L-T4 therapy is critical and was noted to be low among 17% of women prescribed this drug in a large survey (237). Appropriate counselling is recommended.
FETAL-NEONATAL CONSEQUENCES OF MATERNAL HYPOTHYROIDISM
Role of Thyroid Hormone During Fetal Brain Development
Thyroid hormones are major factors for the normal development of the brain. Extensive animal experiments reported by Teng’s group in China have shown neurodevelopmental impairment in subclinically hypothyroid rats due to alteration of the CREB signaling pathway (238) Marginal iodine deficiency affects dendritic spine development (239) and hypothyroxinemia also inhibits brain development (240,241). Hippocampal structure and function is affected in humans and rats resulting from thyroid hormone deficiency (242,243). The mechanisms of actions of thyroid hormones in the developing brain are mainly mediated through two ligand activated thyroid hormone receptor isoforms (244). Physiological amounts of free T4 are present in coelomic and amniotic fluids surrounding the developing embryo already in first trimester. Also, specific nuclear receptors are present in fetal brain as early as ~8 weeks post-conception (245). It is known that thyroid hormone deficiency may cause severe neurological disorders resulting from the deficit of neuronal cell differentiation and migration, axonal and dendritic outgrowth, myelin formation and synaptogenesis (246). This is the situation well documented in iodine deficient areas where the maternal circulating thyroxine concentrations are too low to provide adequate fetal levels particularly in the first trimester. Even in an iodine sufficient area maternal thyroid dysfunction (hypothyroidism, subclinical hypothyroidism or hypothyroxinemia) during pregnancy results in neuro-intellectual impairment of the child; hence maternal thyroid hormones are required through gestation for proper brain development and specific effects will depend on when maternal hormone deficiency occurs during pregnancy (247) The neurobiology of fetal brain development depends on many factors including the availability of thyroxine (T4) delivery to the fetal neurones (248) . There is also an important role for the thyroid hormone transporters in one or more of these processes (249). While MCT8 facilitates thyroid hormone transport to the neurone, OATP1C1 appears to be related to thyroid hormone transport into the astrocyte. At this stage it favours the transport of T4 more than T3 but as the deiodinase II is within the astrocyte this enables conversion to occur and then allows T3 to be transferred into the neurone. Other thyroid hormone transporters are probably regulating thyroid hormone transport into the oligodendrocyte. These processes depend on maternal iodide supply, maternal T4 synthesis, maternal T4 placental transport and the conversion of T4 to T3 in the fetus by the Type II deiodinase. The discovery that children born with the Allan-Herndon-Dudely syndrome have a mutation in the thyroid transporter monocarboxylate 8 (MCT8) (250 )has accentuated the interest in many of the transporters (251).Thyroid hormone receptor development in brain occurs very early in gestation, certainly before the fetal thyroid begins to function which is around sixteen to eighteen weeks (5). In early gestation thyroid hormone effects on genes related to neurodevelopment, for example, myelin, can be recorded.
Clinical Studies on the Role of Maternal Hypothyroidism for the Psycho-Neurological Outcome in the Progeny
Man et al(252) first noted that children of mothers with inadequately treated hypothyroidism had significantly lower IQs than those born to adequately treated patients or normal controls. These pioneering data did not gain much clinical attention, probably because the prevailing dogma, at that time, was that maternal TH did not cross the placenta .
Impaired intellectual development has been reported in children born to women with non-iodine deficient hypothyroidism during pregnancy (253-255) as well as in children from hypothyroxinemic mothers (256- 261). Attention deficit disorder (262,263), autistic symptoms in offspring (264) and schizophrenia in later life (265) have been associated with maternal hypothyroxinemia. Attention deficit disorder was previously noted in offspring from mothers with thyroid autoimmunity (266). Children from mothers with anti thyroid peroxidase antibodies have been found to have intellectual impairment in early infancy (267) and a reduced childhood cognitive performance at age 4 and 7 and sensineural hearing loss at both ages (268).. Other studies have also shown suboptimal development in children exposed to hypothyroidism during pregnancy (269-271). If maternal T4 concentrations are corrected by the 20th week (272) or prior to the 3rd trimester (273,274) many of these adverse effects can be prevented..In addition, isolated hypothyroxinemia in the 2nd trimester is not associated with impaired cognitive, language and motor scores at age 2 (275). These studies emphasise the temporal nature of fetal brain development (276) and underpin the notion that women should not have an abortion if hypothyroidism is found and treated in the first trimester The seminal study of Haddow et al (253) is worthy of further comment. They found that the full IQ scores of children whose mothers had a high TSH during gestation were 7 points lower than controls (p<0.005) and that 19% of them had scores of less than 85 compared to 5% of controls (p<0.007). However there was no IQ decrement noted in the prospective double blind randomized controlled antenatal thyroid screening study (CATS) study in children of both hypothyroxinemic and high TSH mothers studied at 3 years of age who received levothyroxine therapy during pregnancy compared to children whose mothers were not treated with levothyroxine (276). As mentioned above it is possible that the timing of thyroxine administration in gestation is an important factor (241).Indeed, a more extensive replication of the CATS study has reported no difference in IQ measurements in children up to the age of 5 whose mothers received or did not receive L-T4 during gestation, but the drug was not commenced till late in the 2nd trimester (277). In a Chinese prospective population-based development study of 1017 women with singleton pregnancies clinical hypothyroidism was associated with increased fetal loss, low birth weight, and congenital circulation system malformations. Subclinical hypothyroidism was associated with increased fetal distress, preterm delivery, poor vision development, and neurodevelopmental delay. Isolated hypothyroxinemia was related to fetal distress, small for gestational age, and musculoskeletal malformations as well as spontaneous abortion (234). An important association study from the population based prospective study from Rotterdam (Generation R), which included MRI scans on the children, reported that maternal FT4 showed an inverted U shaped association with child IQ, child grey matter volume and cortex volume(278). This suggests that optimal T4 concentrations during gestation might require to be in a narrower range than previously thought. Brain morphology studies have also shown abnormal corpus callosum development in children born to women treated for hypothyroidism(279) and this maternal condition may also contribute to abnormal cortical morphology in the offspring (280). Maternal and/ neonatal thyroid function at delivery in children born at or over 37 weeks' gestation was not associated with impaired neurodevelopment at 5.5 years (281) although. lower levels of cord T4 were associated with increments in the McCarthy scales at this age. In premature infants (<34 weeks gestation) higher maternal levels of TSH at delivery were associated with significantly lower scores on the general cognitive index at 5.5 yr.(282)The neurodevelopmental impairment is similar to that seen in iodine deficient areas (see chapter on iodine deficiency) and implies that iodine status should be normalised in regions of deficiency. However, much of the USA and parts of Europe are not iodine deficient which raises the question of routine screening of thyroid function during early pregnancy or even at preconception which will be discussed below. In summary, the current weight of evidence suggests that hypothyroidism and subclinical hypothyroidism and hypothyroxinemia all have an adverse effect on neurodevelopmental outcome in the progeny. It is however the case that not all the evidence shows this and much of the evidence relates to association studies, Despite this there is a reasonable case for treatment of the woman with subclinical hypothyroidism in pregnancy to prevent these outcomes. However,treatment should be carefully monitored.
Management and Therapy of Gestational Hypothyroidism
Administration of L-thyroxine is the treatment of choice for maternal hypothyroidism, when the iodine nutrition status is adequate.
A number of studies have indicated that during pregnancy thyroxine requirements increase during gestation (283-286). The increase is due to the rapid rise in TBG levels resulting from the physiological rise in estrogen concentrations, the increased distribution volume of thyroid hormones (vascular, hepatic, and the fetal-placental unit), and finally the increased placental transport and metabolism of maternal T4(208). . If a pregnancy is planned, patients should have thyroid function tests measured soon after the missed menstrual period. If serum TSH is not increased at that time, tests should be repeated at 8-12 weeks and then again at 20 weeks, as the increase in hormone requirements may not become apparent until later during gestation. In women not receiving T4 who may have risk factors for thyroid disease (eg positive family history or other autoimmune disorder) thyroid function should be measured pre conception. If the TSH is less than 2.5mIU/L no action is required. If it is more than 3.5mIU/L thyroxine therapy may be indicated, especially if thyroid antibodies are present. If TSH is between 2.5 and 3.5mIU/L it would be prudent to check again in 4 weeks if possible. Treatment should be initiated with a dose of 100-150 µg/day or titrated according to body weight. In non pregnant women, the full replacement thyroxine dose is 1.7-2.0 µg/kg bw/day. During pregnancy, because of the increased requirements, the full replacement thyroxine dose should be increased to 2.0-2.4 mg/kg bw/day (208,287)
Women who already take thyroxine before pregnancy usually need to increase their daily dosage by 30-50%, on average, above preconception dosage; appropriate dose increments must be made once pregnancy is confirmed t The preconception thyroxine dose should be adjusted aiming to maintain serum TSH near the low-normal range (288) which should be within the trimester specific reference range ( ie approx. 2.5 mIU/L) It has been suggested that if SCH is newly diagnosed in pregnancy a T4 dose of 1.20µg/kg/day is appropriate to achieve a TSH less than 4.2 mU/L(289).
In general women who receive T4 because of previous ablative treatment (eg for thyroid cancer) require a greater increase than those receiving the drug because of Hashimoto’s thyroiditis where there still may be some reserve thyroid tissue. Therefore patients with Hashimoto’s disease require a lesser increase in T4 dose. Before pregnancy thyroid function should be checked and T4 dose adjusted to achieve a serum TSH of at least less than 2.5mIU/L. Indeed, a retrospective study has suggested that in women on T4 for hypothyroidism who are planning to become pregnant should have TSH levels not greater than about 1.2mIU/L (290) although the guidelines are not so stringent in their recommendations (10-15). The woman should be advised to increase the dose of T4 by 30-50% once pregnancy is confirmed. A convenient method for achieving this for some women would be to take 2 extra tablets of T4 per week (284). Thyroid function (T4 and TSH) should then be checked every 4 weeks as dose requirements may change during the course of gestation. It has also been suggested that The increment in thyroxine can be based on the initial degree of TSH elevation; women with a serum TSH between 5-10 mU/L, the average increment in T4 dosage is 25-50 µg/day; for those with a serum TSH between 10-20 mU/L, 50-75 µg/day; and for those with a serum TSH >20 mU/L, 75-100 µg/day (208).. The aim should be to keep the TSH around 2.5mIU/L or less remembering of course that TSH levels are difficult to interpret in the first trimester because of rising hCG concentrations. In the postpartum period most women should take their prepregnancy dose of T4. However some women with Hashimoto’s thyroiditis may require more than the pre-pregnancy dose because of possible postpartum progression of autoimmune thyroiditis (286). The question of compliance with therapy and the efforts of the physician to maintain the appropriate levels of thyroid hormone are important considerations particularly in the context of pregnancy (237). In 389 women, in the USA (185) 43% of serum TSH levels measured in 1st trimester were at or above 2.5 mU/L; In 2nd trimester, 33% of serum TSH measurements were at or above 3.0 mU/L (291). Even when the upper limit of TSH was defined as a serum TSH value above the 98th percentile of normal, 20% of values in 1st trimester and 23% in 2nd trimester were above this limit. In the UK , even in 18-45 year old pregnant women already on L-Thyroxine ,46% had a TSH level greater than 2.5 mU/L (292). These data suggest that the optimum care of pregnant women on L-thyroxine is not present despite evidence for its effect in reducing preterm birth and miscarriage (290,292). American Endocrine Society Guidelines recommend that as the potential benefits outweigh the the potential risks, women with SCH should receive T4 treatment (14,15). Women with autoimmunity (ie positive thyroid antibodies) should be carefully monitored during gestation as there is a tendency for a rise in TSH. If this occurs T4 therapy should be given.
THYROTOXICOSIS
Hyperthyroidism during pregnancy is relatively uncommon, with a prevalence estimated to range between 0.1% and 1% (0.4% clinical & 0.6% subclinical) (292). However, a population study of more than 400,000 births showed an incidence of 0.9% (293)Causes of thyrotoxicosis include those found in the general population, as well as others that occur specifically during pregnancy(294,295). While the commonest cause is Graves’ disease etiologies such as single toxic adenoma, toxic multinodular goiter, subacute or silent thyroiditis, iodide-induced thyrotoxicosis, and thyrotoxicosis factitia can occur but are uncommon during pregnancy. Molar disease should always be considered as it can potentially lead to severe thyrotoxicosis, particularly in pregnant women with a pre-existing autonomous or nodular goiter. However, since uncomplicated hydatidiform mole is easily diagnosed in early gestation, it rarely leads to severe thyrotoxicosis (52).. Other extremely rare causes of hyperthyroidism (described recently as isolated cases) include hyperplacentosis and struma ovarii (296).
In women in the childbearing age, the most common cause of hyperthyroidism is GD, as this etiology accounts for 85% of clinical hyperthyroidism in pregnancy. Another cause of hyperthyroidism is hyperemesis gravidarum. This is common and requires differentiation from Graves’ disease (2) (see section on Hyperemesis gravidarum below).
Clinical Diagnosis of Hyperthyroidism in Pregnancy
Even though the historical clues and physical findings are the same in pregnant and non pregnant patients, the diagnosis of thyrotoxicosis may be difficult to make clinically during pregnancy. Nonspecific symptoms such as fatigue, anxiety, tachycardia, heat intolerance, warm moist skin, tremor and systolic murmur may be mimicked by normal pregnancy. Alternatively, presence of goiter, ophthalmopathy and pretibial myxoedema obviously points to the suspicion of GD (see Table 14-8). A useful symptom of hyperthyroidism is that, instead of the customary weight gain, patients may report weight loss or, even more frequently perhaps, absence of weight gain despite an increased appetite (unless there is also excessive vomiting). Nausea (morning sickness) occurs frequently during normal pregnancy. However, the occurrence of hyperemesis gravidarum accompanied by weight loss must always raise the suspicion of hCG-induced hyperthyroidism.
Table 14-8Clinical features suggesting the possibility of hyperthyroidism due to Graves' disease in a pregnant patient
Historical
1. Prior history of hyperthyroidism or autoimmune thyroid disease in the patient or her family.
2. Presence of typical symptoms of hyperthyroidism including weight loss (or failure to gain weight), palpitations, proximal muscle weakness, or emotional lability.
3. Symptoms suggesting Graves' disease such as ophthalmopathy or pretibial myxedema.
4. Thyroid enlargement.
5. Accentuation of normal symptoms of pregnancy such as heat intolerance, diaphoresis, and fatigue.
6. Pruritus.Physical examination
1. Pulse rate > 100.
2. Widened pulse pressure.
3. Eye signs of Graves' disease or pretibial myxedema.
4. Thyroid enlargement especially in iodine sufficient geographical areas.
5. Onycholysis.
Laboratory Diagnosis
Patients suspected of having hyperthyroidism require measurement of serum TSH, T4 and T3 levels, and anti-TSH receptor antibodies (TRAb). Virtually all patients with significant symptoms have a serum TSH <0.1 mU/L, as well as concurrent elevations in serum free T4 and T3 levels. However, interpretation of thyroid function tests must take into account the hCG-mediated decrease in serum TSH that occurs during pregnancy. Near the end of 1st trimester, at the time of peak hCG values, serum TSH levels may be transiently lowered to values below 0.4 mU/L in ~20% of euthyroid women (297,298). Thus, the degree and duration of TSH suppression (mainly but not only) in 1st trimester must be considered in making the differential diagnosis. Concerning T4 and T3 levels, the pitfalls and necessary caution in the interpretation of serum free T4 and T3 have been discussed earlier (see the section on thyroid function parameters in normal pregnancy).
Patients with GD usually have positive thyroid antibodies (TG-Ab and TPO-Ab) and, therefore, antibody presence should alert the clinician to the possibility that autoimmune thyroid disease is the cause of symptoms evoking hyperthyroidism. Most patients with GD have detectable TRAb. Since TRAb production tends to undergo immunologic remission during the second half of pregnancy, detection of TRAb may depend upon gestational age at determination (299). Presence of TRAb in 1st trimester is highly useful in helping make the differential diagnosis between GD and other causes of gestational hyperthyroidism.
Clinical Aspects of the Management of Graves’ Disease in Pregnancy
Prepregnancy Counseling
Prepregnancy counseling plays a very important role in the care of young women with Graves’ disease. All women of childbearing age affected with Graves’ hyperthyroidism should be strongly advised to seek contraception counseling, in order to avoid pregnancy while hyperthyroid (2,295). A discussion of the different hyperthyroid therapeutic choices is important for those women planning a pregnancy: ablative therapy, by 131I or surgery, or medical therapy. Before commencing specific treatment for a woman with GH in pregnancy it is essential to provide appropriate counselling advice [300). Risks to mother, fetus, and neonate from untreated GH during gestation are compelling reasons for recommending preconception counselling (PC). PC should include discussion as to the optimum treatment of GH in women wishing to become pregnant
- If ablative therapy is chosen the following recommendations are suggested::
- Pregnancy test prior to ablation,
- Delay of conception on average of 6 months following therapy in order to adjust LT4 doses to target values for pregnancy (serum TSH 0.3-2.5).
- Determination of TSH-receptor antibody (TRAb); the gradual disappearance from the circulation post therapy depends on the type of treatment chosen; following thyroidectomy there is a gradual disappearance of TRAb titers, while following 131I therapy there is an increase in TRAb titers that may last for 12 months followed by a gradual fall in titers (301). Therefore, in patients with high TRAb titers surgery appears to be the therapy of choice in women contemplating pregnancy (302).
- B) For women on antithyroid drugs the following discussion with the patient and her family is recommended:
- a) If still in need to ATD for more than 2 years, the possibilities of remission during pregnancy are very low, the patient, most likely, will need ATD therapy through pregnancy (see section below on management of Graves’ disease in pregnancy).
- b) PTU may induce liver toxicity with potential liver failure requiring liver transplantation; therefore recommendations by the American Thyroid Association (13) limited the use of PTU to those patients allergic to MMI, in the treatment of thyroid storm and in the first trimester of pregnancy because of the rare instances of methimazole embryopathy. In a woman on MMI therapy at the time of conception or planning a pregnancy it is advisable to switch to PTU if it is deemed that ATD are required at that time and resume MMI after the first trimester.
- c) Close follow-up throughout the pregnancy for frequent blood tests and adjustment of ATD dose, since the need for dose adjustments is common.
- d) Possibility of disease aggravation in the first trimester and recurrence in the postpartum period, due to postpartum thyroiditis or recurrence of Graves’ (303).
- e) Recommendations regarding breastfeeding while on ATDs.
- f) TRAb determination for prediction of potential fetal or neonatal complications
Complications of Hyperthyroidism and Pregnancy
These are listed in table 14-9. Untreated hyperthyroidism carries a high risk of complications (295,296) The risk of complications for mother and child , is related to the duration and adequate control of maternal hyperthyroidism. In women with unrecognized maternal GD infants showed severe prematurity (mean gestational age of 30 weeks at delivery) associated with very low birth weight (<2 Kg) and neonatal hyperthyroidism requiring treatment with ATD (308). In contrast, for those patients in whom the diagnosis was made early and treatment started promptly, the outcome was excellent. In 230 pregnant women with GD in Japan, no adverse impact on the outcome of pregnancy in patients with adequately treated Graves' disease was observed (309). Rare causes of thyrotoxicosis (eg due to an activating TSH receptor gene mutation) may also result in premature delivery and low birth weight (310)
Table 14-9Complications of Graves’ Hyperthyroidism in Pegnancy
| Category | Item | Reference |
| Maternal | Adverse drug effects | |
| Left ventricular dysfunction | 304 | |
| Thyroid storm | 305 | |
| Obstetric Antepartum | Miscarriage | |
| Preeclampsia | 306 | |
| Preterm labor [PTL] | ||
| Stillbirth | ||
| Gestational Hypertension | ||
| Fetal thyroid dysfunction | ||
| Obstetric Intrapartum | Fetal distress | |
| Preterm deliveries | ||
| Primary Cesarean Section | ||
| Placental abruption | ||
| Postpartum Hemorrhage | ||
|
Neonatal Primary Outcome
| Birth weight < 2500 g | |
| Macrosomia >4000 g | ||
| Apgar scores | ||
| NCU admission | ||
| Respiratory Distress Syndrome (RDS) | ||
| Congenital abnormalities | ||
| Thyroid dysfunction | 307 |
Fetal & Neonatal Adverse Effects of Maternal Hyperthyroidism
Fetal Hyperthyroidism
Although rare, this is a preventable complication with potential severe sequelae, including death (311) Clinical evaluation at the time of maternal hyperthyroidism diagnosis, will indicate the few women at risk, namely those with: a) active Graves’ hyperthyroidism, b) previous history of Graves' disease treated with ablation therapy, either surgery or 131I, and c) mothers with active Graves’ hyperthyroidism undergoing therapeutic thyroidectomy in the second trimester of pregnancy . A determination of TRAb titer should be obtained between 22 and 26 weeks gestation, a value of 3-5 times above normal is an indication for fetal evaluation for detection of potential fetal thyrotoxicosis. The fetal thyroid TSH receptor starts responding to Thyroid Stimulating Immunoglobulin (TSI) stimulation during the second trimester. The placental transfer of IgG from mother to fetus increases by the end of the second trimester, reaching a level in the fetus similar to that of the mother around 30 weeks' gestation (2). Therefore, the symptoms of fetal hyperthyroidism are usually not evident until 22 to 26 weeks of gestation.
Evaluation of fetal hyperthyroidism can be assessed a) by fetal ultrasonographic data, showing the presence of fetal goiter, tachycardia (persistent fetal heart rate of >160 bpm);, b) fetal heart monitor tracing showing a sustained baseline of 170 to 180 beats per minute with moderate variability that exhibits acceleration with a lack of deceleration,[unique to fetal thyrotoxicosis (312)] c) growth retardation, increased fetal motility. Other signs developing later are intrauterine growth restriction (IUGR), oligohydramnios or hydrops, and accelerated bone maturation (311). This last sign is diagnosed by the presence of distal femoral ossification center before 31 weeks gestation (313) and is highly predictive of the disease. Serial cordocentesis for diagnosis and monitoring drug therapy has been proposed, but its value has been questioned, restricted to centers with expertise (314) because of a significant risk to the fetus (complications and/or fetal loss in 1% of cases) . It is generally recommended only if the information to be gained will change therapy.
When fetal hyperthyroidism is suspected in utero, it is reasonable to initiate ATD treatment with MMI (20 mg/day) combined with thyroxine administration, when required, to maintain maternal euthyroidism.
Fetal Hypothyroidism
Inhibitory TRAb production has been shown to cause hypothyroidism transiently in neonates born to mothers with GD (315)
Administration of ATD to treat maternal GD may induce fetal hypothyroidism that clearly should be avoided by maintaining maternal circulating thyroid hormone levels in the upper quartile of the normality range (295,296). Radioactive iodine has unintentionally been administered (in exceptional cases) to GD women who were unaware that they were pregnant and who decided, nevertheless, to maintain the pregnancy. Despite the risks of performing cordocentesis, it has been shown to be useful to predict the fetal outcome (316).
Fetal Goiter in Mothers with Graves’ Disease
The treatment of maternal hyperthyroidism may be associated with the presence of fetal goiter, thus raising clinical concern with regard to its etiology and management. Fetal goiter may result directly from the placental transfer of thyroid growth-stimulating effects of maternal TRAb, as well as from the inhibitory effect of ATD on the fetal gland inducing fetal hypothyroidism (317). The spectrum of neonatal thyroid dysfunction in pregnant women with GD receiving ATD can range from frank hypothyroidism (secondary to the exposure to MMI and maternal blocking TRAb) to neonatal Graves’ thyrotoxicosis (secondary to exposure to maternal stimulating TRAb) thus making the prenatal diagnosis extremely difficult ..
Of 72 mothers with past or present GD all infants from 31 pregnancies with no detectable TRAb and mothers without ATD treatment, were normal at birth. In the remaining 41 pregnancies, 30 women had positive TRAb and/or a treatment with ATD: fetal thyroid ultrasound was normal (32 wks gestation) and there was almost no evidence of fetal thyroid dysfunction. However,11 fetuses were found to have a goiter, of which 7 were hypothyroid and 4 hyperthyroid. The main risk factors for fetal hyperthyroidism were poorly controlled maternal hyperthyroidism and elevated TRAb. The risk factor for hypothyroidism was mothers being treated with ATD and having a serum T4 in the normal range (rather than upper limit of normal). The authors recommended TRAb measurement in women with current or past GD at the beginning of pregnancy, and close observation of those pregnancies with elevated TRAb or ATD treatment by performing monthly fetal ultrasonography after 20 weeks of gestation (313,318).
Neonatal Thyrotoxicosis
One to 5% of neonates of mothers with GD have hyperthyroidism (neonatal GD) due to the trans-placental passage of stimulating maternal TRAb. The overall incidence is low because of the balance between stimulatory and inhibitory antibodies, and also maternal treatment with ATD (311). The incidence of neonatal GD is not directly related to maternal thyroid function. Risk factors for neonatal thyroid dysfunction include history of a previously affected baby, prior radioiodine ablative treatment, and elevated TRAb titers at delivery (317). A higher TRAb value is associated with a higher risk of neonatal thyroid dysfunction (309).
Undetected fetal thyrotoxicosis may be followed by thyrotoxicosis at birth. Neonatal thyrotoxicosis is considered to be uncommon, occurring in ~1% of pregnancies in patients with Graves’ disease (319) . Risks appear highest in the offspring of women with not-well-controlled GD, as well as in women with the highest TRAb titers. Mothers with a prior history of bearing infants with neonatal GD are also at high risk of repeated episodes (296). Neonatal GD is usually diagnosed at or shortly following birth, after maternal ATD has been cleared from neonatal serum and thyroid gland. Signs of neonatal thyrotoxicosis include congestive heart failure, goiter, proptosis, jaundice, hyperirritability, failure to thrive, and tachycardia.. Cord serum free T4 and TSH determinations should be performed in all deliveries of mothers with a history of GD. Treatment should be initiated in conjunction with the neonatologist, and may include iodide, ATD, glucocorticoids, digoxin, and beta-adrenergic blocking agents, depending on the cardiovascular status. Neonatal hyperthyroidism may have a delayed onset in some infants, particularly those in whom both anti-TSH receptor blocking and stimulating antibodies coexist. Thus, the pediatrician should be alerted to measure serum free T4 if symptoms suggesting thyrotoxicosis appear during the first 6-8 weeks of life, even if cord serum results were normal, and especially when cord serum TSH was suppressed (318).
Sporadic cases of neonatal hyperthyroidism without evidence of the presence of circulating TSI in mother or infant are due to activating of mutations in the TSH receptor molecule (320). It is inherited as an autosomal dominant trait and, in contrast to Graves' neonatal hyperthyroidism, the condition persists indefinitely. Treatment with antithyroid medications followed by thyroid ablation therapy will eventually be needed in addition to genetic counseling.
Neonatal Central Hypothyroidism
Infants born to mothers with uncontrolled hyperthyroidism due to GD may present with central congenital hypothyroidism (296). High maternal serum T4 levels, during a prolonged period of time, cross the placental barrier leading to suppression of fetal TSH by pituitary feedback. In most cases, the diagnosis is made at birth or shortly thereafter, on the basis of a low neonatal serum total T4 contrasting with an inappropriately low serum TSH. In the majority of these infants, there is a return to euthyroidism within a few weeks or months; rarely, this condition may be due to mutation of the TSH receptor and result in a problem with neonatal screening. (321) There may also be a risk of thyroid ‘disintegration’ (i.e. abnormal ultrasound patterns found during childhood), possibly as the result of prolonged central hypothyroidism (322)...
Management of Graves’ Disease in Pregnancy
Graves' hyperthyroidism (GH) usually tends to improve gradually during gestation, although exacerbations can be observed in the first weeks. The spontaneous improvement: may be due to the partial immunosuppressive state of pregnancy (progressive decrease in TRAb production; changes in cytokine production) the rise in maternal serum TBG levels that tends to reduce serum free T4 & T3 fractions and obligatory iodine losses specific for pregnancy that may, paradoxically, constitute an advantage for women with GD. There is dispute as to whether the balance between blocking and stimulating TRAb activity may be modified in pregnancy (299). The exacerbation of thyrotoxicosis in women with GD during early pregnancy may be due in part to the stimulatory effect of high hCG levels (vide infra).
Antithyroid Drugs
Although antithyroid drugs (ATD) are the main treatment for GD during pregnancy (319,294) recent developments relating to the adverse effects of ATD in pregnancy have led to more caution in their use (323) Recommendations for use of PTU in the first trimester, and MMI later, are discussed below. The overall goal of therapy is to control maternal disease by maintaining the patient at a high euthyroid level, while minimizing the risk of fetal hyperthyroidism or hypothyroidism by using the smallest possible dose of ATD.
The initial recommended dose of PTU is 100 to 450 mg/day in 3 divided doses or MMI 10 to 20 mg/day; very seldom a larger initial dose is required. In patients with minimum symptoms, an initial dose of 10 mg of MMI daily or PTU 50 mg two or three times a day may be initiated. In most patients, clinical improvement is seen in 2 to 6 weeks, and improvement in thyroid tests occurs within the first 2 weeks of therapy, with normalization to chemical euthyroidism in 3 to 8 weeks in over 50% of patients (324). Resistance to drug therapy is unusual, most likely due to poor patient compliance. With clinical and thyroid test improvement, the dose of antithyroid medication should be reduced by half of the initial dose. The daily dose is adjusted every two to four weeks according to the results of thyroid tests. Serum TSH may remain suppressed despite the normalization of thyroid hormone levels for many weeks, frequently through pregnancy . The ATD dosage should be maintained at a minimum and should indeed be continued, in low dose if necessary, to the end of gestation, although there are differing opinions concerning this strategy. Patients should be assessed at regular intervals, every 2 to 4 weeks at the onset of treatment and every four weeks thereafter, to allow for proper medication adjustments to keep the FT4 or FT4I within target goals. Clinical clues of good therapeutic response are improvement in symptoms and weight gain. High FT4 levels (even in the mildly thyrotoxic range) and the presence of TRAb antibodies are useful indices of the fetal need for antithyroid treatment to prevent fetal goitre and maintenance of fetal euthyroid state (325) Continuing ATD to the end of gestation will prevent hyperthyroidism in labor which is undesirable and, if carefully monitored, should be a safe strategy. There is evidence from a retrospective non- randomized trial that continuing ATD throughout pregnancy substantially prevents postpartum recurrence of Graves’ hyperthyroidism without adverse effects on the fetus (326). However, postpartum recurrence of Graves’ hyperthyroidism has been documented more frequently (84 %) in patients previously treated for Graves’ disease with ATD before a successful pregnancy compared to a rate of 56 % in women similarly treated but not having a pregnancy (327). The case for postpartum monitoring is, therefore, very strong.
Combined administration of ATD and thyroxine to the mother should be avoided, since trans-placental passage of ATD is high while negligible for thyroid hormones and, hence, thyroxine will not protect the fetus from ATD-induced hypothyroidism.
Assessing TRAb concentration is essential in the management. Titers of TRAb measured after 22 weeks gestation may be slightly elevated suggesting a very low probability for the fetus to develop hyperthyroidism, and a good indicator for using lower doses of ATD. The classical course of Graves’ disease during pregnancy frequently encompasses exacerbation of hyperthyroidism during 1st trimester and a gradual improvement in the 2nd half of gestation. Maternal production of TRAb may remain elevated after thyroid ablation using radioiodine or even after a prior thyroidectomy or the apparent cure of the disease by antithyroid drug (ATD) therapy given several years before pregnancy. In euthyroid pregnant women who have previously received ATD for GD but who are currently not receiving ATD treatment, the risk of fetal/neonatal thyrotoxicosis is negligible and, therefore, systematic measurement of TRAb is not mandatory. For a euthyroid pregnant woman (with or without thyroid hormone replacement therapy) who has previously been treated with radioiodine or undergone thyroid surgery for GD, the risk of fetal/neonatal thyrotoxicosis depends upon the level of TRAb produced by the mother. As a result, TRAb should be measured in early pregnancy to evaluate this risk. If significantly elevated TRAb is detected at weeks 18-22 or the mother is taking ATD in the third trimester, a TRAb measurement should again be performed in late pregnancy (weeks 30-34) to evaluate the need for neonatal and postnatal monitoring It should be remembered that the standard TRAb assays measure displacement of binding by TSH to the TSH receptor and do not distinguish between stimulating and blocking TRAbs. Assays that do distinguish are usually only available in research settings.
PTU, MMI and CBZ (converted to MMI by the liver), are equally effective in controlling the disease (213). The risk of hepatic toxicity due to PTU has been emphasized due to the number of cases requiring liver transplantation and as a cause of death (329). MMI can also induce a milder cholestatic liver toxicity not associated with liver failure (330). While PTU can rarely cause antineutrophil cytoplasmic antibody-associated vasculitis (331) agranulocytosis and liver failure were very rare in a large population survey in pregnancy of PTU and MMI (332); birth defects were the dominant side effect in pregnancy, their relative incidence being re-evaluated recently by the late Professor Laurberg and his Danish colleagues. A detailed literature analysis concluded that both MMI and PTU use in early pregnancy may result in birth defects in 2-3% of exposed children and that the highest risk was in gestational weeks 6-10 (ie during organogenesis)(333). A meta analysis concurred with this view (334). This has therapeutic implications (vide infra). It is claimed that studies which have not found ATD associated birth defects were either not sufficiently powered or did not study outcomes at optimal ages (335). Aplasia cutis, occurred in a small group of infants born of mothers on MMI therapy (336),. It has been reported in infants from mothers receiving PTU but much less commonly (337). ”Methimazole embryopathy” includes choanal atresia and/or esophageal atresia, minor dysmorphic features and development delay An OR (odds ratio) of 18 (95% Cl 3-121) for choanal atresia among infants whose mothers received MMI in the first trimester compared to the general population was noted (338); PTU did not seem to be a major human teratogen in one study (339) but 3/47 PTU 1st trimester exposed mothers had children with congenital abnormalities (229) A retrospective review (340) of the pregnancy outcomes of 6744 pregnant women with Graves’ disease in relation to all observed congenital anomalies showed a significantly higher rate of major anomalies in the MMI group of babies (4.1%) compared to those seen in the PTU group (2.1%) [p=0.002]. However, examination of Danish records of more than 817,000 infants showed that birth defects (in the neck and face and urinary system) due to PTU do indeed occur but are generally less severe than in children exposed to MMI or CBZ; but these children did require surgical correction (341). In line with these data a meta-analysis indicated that PTU was a safer choice for treatment according to the risk of birth defects but that a shift between MMI and PTU failed to provide protection against birth defects. That is to say both drugs can be associated with birth defects (342).
An advisory committee recommended limiting the use of PTU to the first trimester of pregnancy (343). Exceptions to this are patients with MMI allergy or those with thyroid storm. It is accepted that when PTU is not available MMI can be used in the first trimester.
PTU and MMI are equipotent in the management of hyperthyroidism in pregnancy, both drugs having similar placental transfer kinetics (344). Furthermore, when the efficacies of both drugs have been compared in pregnant women, euthyroidism was achieved equally with equivalent amounts of drugs and at the same weeks of treatment (345). Obstetric and neonatal outcomes were no different in both groups.
In view of the recent information on teratogenic effects of thionamide drugs in pregnancy revised management guidelines have been suggested (13).
Women taking MMI or PTU should be instructed to confirm potential pregnancy as soon as possible and contact their physician immediately pregnancy is diagnosed. If she is on low dose ATD the physician should consider discontinuing ATDs (depending on clinical disease status) because of potential teratogenic effects. Clinical and laboratory testing should occur every 2 weeks or with longer intervals if euthyroidism persists. If ATD are required PTU should be used through 16 weeks of pregnancy. Pregnant women receiving MMI who are in need of continuing therapy during pregnancy should be switched to PTU as early as possible;
a dose ratio of approximately 1:20 should be used (e.g. methimazole 5 mg daily = PTU 100 mg twice daily). If ATD therapy is required after 16 weeks gestation, it remains unclear whether PTU should be continued or therapy changed to methimazole as both medications are associated with potential adverse effects and shifting potentially may lead to a period of less-tight control. General treatment guidelines are shown in table 14-10
Table 14-10Treatment guidelines for Graves' disease during pregnancy
| 1. | Monitor pulse, weight gain, thyroid size, serum free T4 and T3, and TSH every 2-4 weeks, and titrate ATD as necessary. |
| 2. | At pregnancy diagnosis see above paragraph.PTU recommended for 1st trimester. Then switch to MMI. |
| 3. | Use the lowest dosage of ATD that maintains the patient in a euthyroid or mildly hyperthyroid state. The ATD dose can usually be adjusted downward after 1st trimester and often (but not always) discontinued during last trimester. |
| 4. | Do not attempt to normalize serum TSH. Serum TSH concentrations between 0.1 & 0.4 mU/L are generally appropriate, but lower levels are acceptable if the patient is clinically satisfactory. |
| 5. | While as little as 100-200 mg PTU/day may affect fetal thyroid function, dosages as high as 400 mg PTU (~30 mg MMI) have been used. |
| 6. | Communicate regularly with obstetric care providers, especially with respect to fetal pulse and growth in the 2nd half of gestation. |
| 7. | Consider thyroidectomy if persistently high doses of ATD are required (PTU >600 mg/d or MMI >40 mg/d), or if the patient is not compliant or cannot tolerate the administration of ATD. |
| 8. | Beta-adrenergic blocking agents and low doses of iodine may be used peri-operatively to control hyperthyroidism. |
| 9. | ATD will often need to be reinstituted or increased after delivery. |
Beta-Adrenergic Blocking Agents
Propranolol may be used transiently to control symptoms of acute hyperthyroid disease and for pre-operative preparation, and there are no significant teratogenic effects of propranolol reported in humans or animals. If a patient requires long-term propranolol administration, careful monitoring of fetal growth is advised, because of a possible association with intrauterine growth restriction (346)
Iodides
Iodine crosses the placenta. If given in large amounts and for prolonged periods, it may induce fetal goiter and hypothyroidism. However, iodine has been used in small amounts, 6 to 40 mg/day in a group of pregnant Japanese women with mild hyperthyroidism (347). Elevation in serum TSH was observed in 2 of 35 newborns, and the mothers were slightly hyperthyroid at the time of delivery. In general however, iodine therapy is not routinely indicated in the treatment of hyperthyroidism in pregnancy.
Radioactive Iodine Administration
Radioactive iodine administration is contraindicated during pregnancy. In case of inadvertent radioiodine administration, the fetus is exposed to radiation from mother’s blood (approximately 0.5-1.0 Rad per mci administered). Since fetal thyroid uptake of radioiodine commences after the 12th week, exposure to maternal radioiodine prior to this time is not associated with fetal thyroid dysfunction (348). However, treatment with radioiodine after 12 weeks leads to significant radiation effects on the fetal thyroid. Multiple incidents of inadvertent exposure to radioiodine have been reported, causing fetal thyroid destruction, in utero hypothyroidism, and subsequent neural damage (349).
Surgery
Subtotal thyroidectomy in pregnancy is effective in managing the disease and usually should be performed in the second trimester. Unacceptable side effects of ATDs,poor patient compliance and very large goiter with potential obstruction are indications for surgery as well as patient preference .The mother should be prepared with β-blocking agents to rendered her hemodynamicaly stable and with Lugol’s solution for at least 10 days to reduce thyroid gland vascularity. A TRAb assay should also be performed as the fetus is at risk of hyperthyroidism (350).
Breast Feeding in Mothers with Treated Graves’ Disease
Lactation during ATD therapy has been discussed (351,). PTU and MMI are secreted in human milk, although PTU less so because of more extensive binding to albumin. With moderate doses of MMI or PTU (MMI: <20 mg/d; PTU: <250-300 mg/d), the risk to the infant is practically negligible.. The drug should be taken by the mother after a feeding but there is no need to monitor infant thyroid function. There is also a possibility that allergic reactions associated with ATD (agranulocytosis or rash) may occur in the infant. Wide experience has confirmed that the use of ATD in lactatin mothers does not pose a risk to the neonate and appears to be safe.
Gestational Non Autoimmune Hyperthyroidism
Thyrotoxicosis and hCG
Non autoimmune gestational hyperthyroidism or gestational transient thyrotoxicosis “GTT” is characterized by elevated serum free T4 and T3 levels, suppressed TSH, variable clinical evidence of hyperthyroidism, usually minimal thyroid enlargement, and absence of thyroid auto-antibodies and ophthalmopathy. The syndrome occurs transiently near the end of the 1st trimester of gestation, usually in hitherto healthy women who have otherwise a normal pregnancy, and it is frequently associated with excessive vomiting (296). GTT occurs in women without past history of GD and absence of TRAb. GTT is not always clinically apparent, due to its transient nature but is common in hyperemesis gravidarum (HG) up to 45%). The severity of GTT is related to serum hCG levels which are elevated. In patients with HG and GTT, thyroid function normalized by the second trimester without antithyroid treatment. GTT does not affect pregnancy outcomes (352). The prevalence of GTT is highly variable, being as low as 0.3% (Japan) or as high as 11% (Hong Kong) (353,354). A figure of around 2-3% of normal pregnancies is the usual case in Europe. (2). GTT is always transient; elevated serum free T4 values revert gradually to normal in parallel with the decrease in hCG concentrations. Serum TSH often remains partially (or totally) suppressed for several weeks after free T4 reverted to normal, i.e. until after mid-gestation (355).
Twin pregnancy is associated with sustained and high hCG concentrations Peak hCG values are significantly higher (almost double) and of a much longer duration in women with a twin pregnancy (up to 6 weeks compared to a few days in singleton pregnancy).. While peak hCG values lasted only for a few days in singleton pregnancy, peak hCG levels (>100,000 UI/L) lasted for up to six weeks in twin pregnancies (356). Hence intense vomiting is more frequently noted in women with a twin pregnancy.
Treatment
In most cases, no specific treatment is required and symptoms can be relieved by administration of beta-adrenergic blocking agents for a short period, while waiting for the spontaneous recovery of elevated thyroid hormones to occur. Hydration and antiemetics may be needed. In patients with a severe clinical presentation (clear symptomatic hyperthyroidism) it is important to rule out the presence of Graves’ disease by measurement of TRAb. Therapy with PTU for a few weeks has been suggested by some.
Pathogenic Mechanisms in GTT
The etiology of the syndrome is due to hCG itself or derivatives of hCG (52). Based on the example of GTT associated with twin pregnancy, a direct quantitative effect of elevated hCG concentrations to stimulate the thyroid gland is probably sufficient to explain hyperthyroidism in most pregnant women, provided that hCG remains above 75,000-100,000 UI/L for a sufficient period of time. Thus, GTT is directly related to both the amplitude and duration of peak hCG values (357). Human CG acts as a weak TSH agonist to increase cAMP production, iodide transport and cell growth in thyrocytes (52). It remains possible that abnormal h CG molecular variants, with a prolonged half life, are produced in these situations explaining sustained prolonged high circulating hCG levels (52). hCG molecular variants with a more potent thyrotropic activity have been detected, although these variants are more usually found in women with hydatidiform mole or choriocarcinoma (358). The hCG stimulation of the thyroid is related to the marked homology between hCG and TSH molecules, as well as between LH/CG and TSH receptors (259). Gestational non autoimmune hyperthyroidism can be considered an example of an endocrine ‘spill-over’ syndrome, a concept based on molecular mimicry between hormone ligands and their receptors (52).
TSH Receptor Mutations Hypersensitive to hCG
The thyrocyte may be a passive bystander of abnormal thyrotropic activity of hCG in GTT, or it may play an active role in its response to hCG through variable degrees of sensitivity of the TSH receptor. A woman with recurrent gestational hyperthyroidism was reported (122) who, after two miscarriages, presented with overt hyperthyroidism and hyperemesis early in pregnancy,. During her next pregnancy, she experienced a relapse of the same situation. The patient’s mother had also been diagnosed with hyperthyroidism during her 2nd and 3rd gestations, mistaken for GD. Study of the TSH receptor of this patient disclosed a single mutation in the extracellular domain of the TSH receptor (K183R), rendering the mutant receptor highly sensitive to hCG, and accounting for recurrent thyrotoxicosis during pregnancies in the presence of normal hCG levels. This finding remained unique until the same group described another case in 2016 (123). It is possible that some women who develop GTT may have an abnormality at the level of the TSH receptor, but perhaps not the same mutation as described (see Figure 14-14).
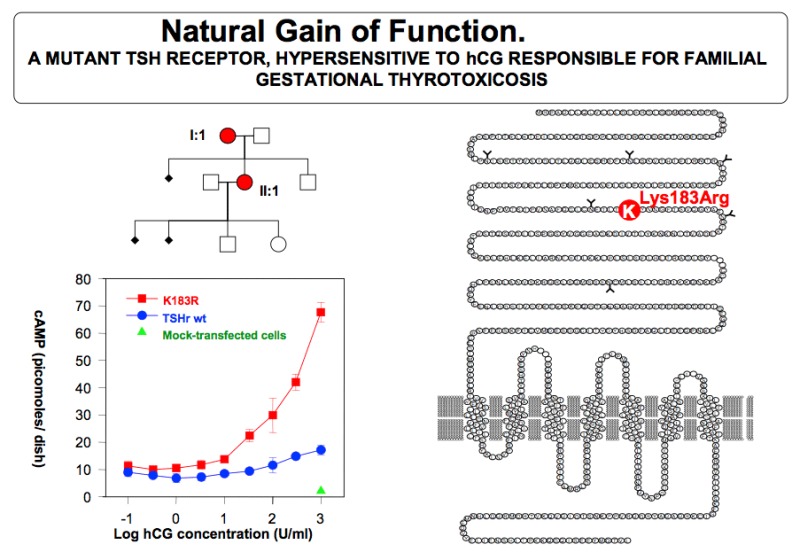
Figure14- 14
TSH receptor mutation, with a Lysine to Arginine mutation in position 183 of the ecto-domain. The graph on the left shows that the mutation confers high sensitivity to hCG (red curve), compared with wild type TSH receptor (blue curve). The family tree (upper right) shows the pedigree of the patient. (from 122).
Hyperemesis Gravidarum and Gestational Hyperthyroidism
Hyperemesis Gravidarum (HG) is reported to occur in 0.3 to 1.0% of pregnancies; it is defined as persistent nausea and vomiting in the first trimester of pregnancy, resulting in greater than 5% weight loss, ketonuria, dehydration liver and electrolyte abnormalities (hypokalemia, metabolic alkalosis, hyponatremia, hypochloremia) in severe cases (359). The onset of nausea is at about 4 to 6 week’s gestation, with worsening by 7- 9 weeks gestation, resolution by the end of the first trimester in 60% of cases, and complete resolution by 20 weeks in the vast majority of women.
The incidence of hyperthyroidism in women with HG depends on the severity of symptoms, ethnic background, perhaps dietary iodine intake, interpretation of thyroid tests and other unknown factors. The diagnosis of HG is based on the presence of clinical and physical clues: lack of hyperthyroid symptoms before conception, similar symptoms in previous pregnancies, and absence of goiter or Graves’ ophthalmopathy. Serum FT4 or Free Thyroxine Index (FT4I) are above the reference range, serum TSH is suppressed or undetectable and markers of thyroid autoimmunity (TPOAb and TRAb) are absent. In less than 20 % of affected women, serum TT3 is slightly elevated.
Ethnic variation in the incidence of HG suggesting strong evidence for a genetic component of HG. has been noted in a Norwegian study which showed 2.2% of Pakistani women; 1.9% of Turkish women and 0.5% of Norwegian women (360). A subsequent study found that, sisters and mothers were more affected than controls (361), An association between HG, hyperthyroidism and hydatidiform mole, has been documented (39). From the clinical aspect the most severely affected women have the lowest TSH and the highest FT4 and FT3 (many in the thyrotoxic range) (362)..
Antithyroid medications are not required in the vast majority of cases. In one series in which antithyroid medication was used, pregnancy outcome was not significantly different to a similar group of patients receiving no therapy (363). Occasionally, severe vomiting and hyperthyroidism may require parenteral nutrition
The differential diagnosis from Graves’ hyperthyroidism may be difficult, as vomiting may also be a presenting symptom of hyperthyroidism of Graves' disease. The diagnosis of transient hyperthyroidism of hyperemesis gravidarum should be considered in women with severe vomiting, no clinical manifestations of Graves' disease, and biochemical evidence of hyperthyroidism. Vomiting should be persistent and severe with a significant weight loss, since most women with morning sickness of pregnancy have normal thyroid function tests . Thyroid gland color flow Doppler sonography may be helpful in the diagnosis (364). Hyperemesis Gravidarum may also occur in women with Graves’ hyperthyroidism and in those with a previous history of Graves’ hyperthyroidism in remission; this is explained by the thyrotrophic action of hCG early in gestation. The differential diagnosis between the two entities may be difficult, the presence of TRAb favoring the diagnosis of Graves’ hyperthyroidism.
Trophoblastic diseases, partial and complete hydatidiform moles, and choriocarcinoma are other causes of hyperthyroidism early in pregnancy. Patients may present without symptoms in spite of chemical hyperthyroidism, or with various degrees of severity, including congestive heart failure. Evacuation of the mole eliminates the source of the excessive hCG and reverses the clinical and biochemical features of hyperthyroidism. Treatment with β-adrenergic blocking agents is effective in controlling the symptoms.
NODULAR THYROID DISEASE
Thyroid Nodule Growth During Pregnancy
Thyroid nodules can be detected in up to 10% of pregnant women. In an iodine deficient area there was no correlation between pregnancy and nodular thyroid disease (365). In a Chinese population pregnancy is associated with an increase in preexisting thyroid nodules as well as new thyroid nodule formation (366). In a retrospective study from the California Cancer Registry from 1991 to 1999, 129 cases of thyroid cancer were diagnosed during pregnancy: 3.3/100,000 diagnosed before pregnancy; 0.3/100,000 at the time of delivery and 10.8/100,000 within one year after delivery (367).
Diagnostic Evaluation and Management of a Thyroid Nodule in Pregnancy
Fine needle aspiration biopsy is the first investigation of choice and in one report yielded a malignancy/suspicious result in 35%. (368). In the presence of a single thyroid nodule detected on physical examination, or a dominant nodule in a multinodular gland, confirmed by ultrasonography, the following approach is suggested :
a) solid lesion <1cm, follow up in the postpartum;
b) nodules >1-1.5 cm, should be considered for FNA if there are suspicious findings on ultrasound,
c) in the presence of tracheal obstruction, immediate surgery;
d) if the FNA is diagnostic of malignancy or it is a suspicious lesion, some authors recommend that surgery may be postponed until after delivery, unless there are lymph node metastases or the lesion is a large primary or there is extensive lymph node involvement in a medullary cancer,
e) surgery and FNAB could both be postponed until after delivery with probable safety,
f) a woman with a malignant lesion or rapid growth should be offered surgery in the second trimester of gestation;
g) Some authors recommend that women with follicular lesions or early stage papillary carcinoma may postpone the surgery until postpartum, since these lesions are not expected to progress rapidly (369,370).
In a retrospective study of 61 women pregnant at the time of the diagnosis of differentiated thyroid carcinoma (papillary cancer in 87%, follicular cancer 13%) 14 were operated on during pregnancy, the other 47 women undergoing surgery 1 to 84 months after delivery (371). The outcome was compared with a group of 598 nonpregnant women matched for age and similar follow up (median 22.4 years and 19.5 years respectively). Treatment and outcome were similar in those operated in the postpartum period. It was concluded that both diagnostic studies and initial therapy might be delayed until after delivery in most patients. Cancer registry data compared disease-related survival in 6505 women diagnosed with thyroid cancer during pregnancy or 1 year post delivery and noted no significant difference in outcome up to 11 years compared to an age-matched non-pregnant cohort (372). The impact of pregnancy on thyroid cancer had been considered to be minimal in that there is no difference in rates of metastases or recurrence compared to non-pregnant women with the same disease.
However, an Italian study of 123 women with differentiated thyroid cancer in relation to pregnancy concluded that pregnancy had a negative impact on the outcome, by showing a poorer prognosis compared to those women diagnosed in nongravid periods (373). The role of estrogen and estrogen receptor status in this regard is still not clear as there are data showing a proliferative effect on thyroid cancer cell lines(374) but there are other reports of estrogen only stimulating adenomatous tissue but not neoplastic thyroid (375). In addition recent studies on the effect of pregnancy on prognosis of differentiated thyroid cancer (DTC) have stated that persistence /recurrence of DTC is significantly higher in pregnant patients (376) and that pregnancy was associated with increase in size of papillary microcarcinoma (377), More studies are required on follow up and mechanisms for these observations.
Pregnancy and Co-existing Thyroid Malignancy
Whether women already treated for thyroid malignancy should become pregnant is of concern, but current evidence suggests that treated differentiated thyroid cancer without evidence of residual disease should not inhibit an intended pregnancy. A meta analysis of the association of thyroid carcinoma with pregnancy concluded that multiple pregnancies and a <5 year interval were indetified as high risk factors for thyroid carcinoma but thyroid carcinoma during pregnancy was not associated with a significant risk of lymphatic and distant metastases (378). In a retrospective analysis on 36 women who became pregnant a median of 4.3 years after initial therapy for differentiated thyroid carcinoma, and were evaluated a median of 4 months after delivery (0.1-1.7 years), total thyroidectomy was performed in 80% and lobectomy in 20% (379). From the clinical progression and serum thyroglobulin (Tg) values it was concluded that pregnancy “is probably a mild stimulus to cancer growth as evidence by minor disease progression in some patients with known structural disease before pregnancy”. Hirsch et al (380) evaluated 63 consecutive women (90 births), followed from 1992 to 2009 who had delivered at least once after total thyroidectomy plus 131-Iodine (in 58 of them) for papillary thyroid cancer. Serum thyroglobulin (Tg) values and neck ultrasound were compared before and after pregnancy. Six women out of the 63 showed disease progression during the first pregnancy and two had disease progression only during the second pregnancy. Serum TSH levels during pregnancy correlated with disease persistence before pregnancy and disease progression during pregnancy. An interesting finding was that a non-suppressed TSH level during pregnancy did not stimulate disease progression during pregnancy; They concluded that pregnancy does not cause thyroid cancer recurrence in PTC survivors who have no structural or biochemical evidence of disease persistence at the time of conception. A study of 24 patients with papillary cancer suggested that PTC during pregnancy may be more locoregionally aggressive but no difference in survival or recurrence was demonstrated compared to non pregnant women (381). The conclusion from these studies indicate no progression of the disease in women free of disease before pregnancy, however there is a possibility of progression in those patients with evidence of residual cancer at the time of conception. Further studies are needed before a firm recommendation could be offered to patients
In patients who are clinically and biochemically free of disease but who present with a high risk tumor, TSH suppression should be maintained with serum TSH levels between 0.1 – 0.5 mU/L. In low-risk patients free of disease, TSH may be kept within the low normal range (0.3–1.5 mU/L). Finally, in patients who have not undergone remnant ablation, who are clinically free of disease and have undetectable suppressed serum Tg and normal neck US, the serum TSH may be allowed to remain in the low normal range (0.3–1.5 mU/L) (371). The recent ATA guidelines(13) suggest that PTC detected in early pregnancy should be monitored sonographically. If it grows substantially before 24-26 weeks gestation, or if cytologically malignant cervical lymph nodes are present, surgery should be considered during pregnancy. However, if the disease remains stable by midgestation, or if it is diagnosed in the second half of pregnancy, surgery may be deferred until after delivery.The impact of pregnancy on women with newly-diagnosed medullary carcinoma or anaplastic cancer is unknown. However, a delay in treatment is likely to adversely impact outcome. Therefore, surgery should be strongly considered, following assessment of all clinical factors.
Effect of Previous 131 Iodine Therapy
Previous 131I therapy does not result in demonstrable adverse events in subsequent pregnancies (382). One of the most common concerns in young people, male and female, is the potential side effects on future fertility and pregnancies following Iodine 131 therapy, both for Graves’ therapy and thyroid cancer. The majority of studies (383-385) concluded that in young men, following 131Iodine doses of up to 3.7 GBq, serum FSH and LH increases with some oligospermia, with normalization within 18 months following treatment. There are few cases of permanent damage, related to patient age, therefore it is recommended to perform a semen analysis before treatment. The risk of testicular dysfunction is increased after repeated or high cumulative radioiodine activity (386). No effects on the progeny have been reported. The effect on women is very consistent in the many reported series. Women may have irregular menses in the first twelve months following therapy, with restitution of normal cycling and fertility thereafter. In a review of 54 studies (387) there was no increase in miscarriages, congenital malformations, or prematurity compared to previous treatment. Early onset of menopause has been reported (387). Overall doses up to 3.7 GBq resulted only in transient menstrual cycle abnormalities lasting up to one year, but no permanent ovarian failure. Although there are no specific studies assessing the risk of pregnancies within 12 months of 131Iodine treatment, the overall consensus by experts in the field is to postpone pregnancy for one year after ablation therapy. The importance of achieving a serum TSH within target should be emphasized. Determination of serum hCG in addition to the pregnancy test on the day of radioactive therapy is recommended, since several cases of false negative pregnancy tests have been reported.
Thyroid hormone administration is justified to achieve a slightly suppressed (but detectable) serum TSH in pregnant women with an FNAB positive for or suspicious for cancer and who elect to delay surgical treatment until postpartum.
POSTPARTUM THYROID DYSFUNCTION
In addition to changes in circulating thyroid hormone concentrations observed during gestation (1), pronounced alterations in the immune system are evident (388). The cellular changes consist of a change from the so-called Th1 state to a predominance of cytokines such as IL-4 consistent with a Th2 status. On the humoral side the titre of anti thyroid peroxidase antibodies (anti TPOAb), found in 10% of pregnant women at 14-16 weeks gestation, decreases markedly during the 2nd and 3rd trimesters. At birth the Th2 status abruptly reverts back to the non pregnant Th1 position and this is accompanied by a dramatic rebound in the titre of antiTPOAb which reaches a maximum between 3 and 6 months postpartum (‘immune rebound phenomenon’). If thyrotropin receptor stimulating antibodies (TRAb) are present in early pregnancy they behave in a similar manner through gestation and the postpartum period. These immunological changes at delivery and the postpartum set the scene for the development of postpartum thyroid dysfunction. The changes in postpartum thyroid dysfunction may be transient or permanent and may be due to destructive or stimulating disease (Fig 14-15).

Figure 14-15(from 389) Patterns of Postpartum Thyroid Dysfunction
POSTPARTUM GRAVES’ DISEASE
Individual patients at high risk of postpartum onset of Graves' disease can be found in early pregnancy by the detection of TRAb. Up to 40% of women with Graves’ hyperthyroidism have been found to occur after a recent pregnancy (390) and The PP period is significantly associated with a relapse of hyperthyroidism in GD patients being in remission after ATD (391).
It is important to differentiate postpartum Graves' disease with accompanying hyperthyroidism from the other causes of postpartum hyperthyroidism. The presence of circulating TRAb , radioiodine uptake, together with clinical examination and thyroid scintiscanning will usually resolve any diagnostic difficulty. A combination of positive TRAb and high thyroid blood flow suggests the presence of Graves’ disease (392). Spectral Doppler sonography may also be useful at this time (393). However silent thyroiditis (i.e. postpartum thyroiditis) commonly develops concomitantly with the activation of Graves’ disease and may delay or mask the development of Graves’ hyperthyroidism .The serum thyroglobulin concentration, which is raised in postpartum destructive thyroiditis with hyperthyroidism, has also been shown to be useful for the differentiation of this condition from Graves’ hyperthyroidism following delivery. Therapy of postpartum Graves’ hyperthyroidism should be carried out by the usual methods remembering that radioiodine is contraindicated during breast feeding. In addition, radiation safety requirements may make it very difficult for the mother to care for her new born child. Ideally another carer should look after the child for at least a week if an activity of 400-600 MBq (app. 10-16 mci) is administered. Alternatively the patient may be treated with antithyroid drugs at this stage. Clearly, prevention of postpartum patients Graves’ hyperthyroidism may be achieved by adequate treatment of the condition before the onset of gestation (394). Screening for TRAb during pregnancy may detect patients at risk of postpartum relapse
POSTPARTUM THYROIDITIS
Postpartum patients with lassitude and other symptoms of hypothyroidism were described in 1948 and these complaints were treated successfully with thyroid extract (395). The syndrome remained generally unrecognised until the 1970s when reports from Japan and Canada rediscovered the existence of postpartum thyroid disease and established the nature of the condition to be related to the immune rebound phenomenon (see above). Several reviews are available (396-399).
Incidence
A variable incidence (from 3-17%) has been reported worldwide because of wide variations in the number of women studied, the frequency of thyroid assessment postpartum, diagnostic criteria employed and differences in hormone assay methodology. However there is a general consensus that the disease occurs in 5-9% of unselected postpartum women. Women with Type 1 diabetes have a three-fold incidence of PPT compared to non-diabetics. In these cases there is a strong association with thyroid antibodies. PPT is also more likely to occur in women who has had a previous episode.
Clinical Spectrum
PPTD is a destructive thyroiditis characterized by transient hyperthyroidism (median time of onset 13 weeks) and/or hypothyroidism (median time of onset 19 weeks) which may occur up to 9 months following delivery (400). The distribution of clinical presentation is approximately 19% hyperthyroid alone, 49% hypothyroid alone and the remaining 32% hyper followed by hypothyroidism. Although the clinical manifestations of the hyperthyroid state are not usually severe in PPT, lack of energy and irritability are particularly prominent even in antibody +ve women who do not develop thyroid dysfunction. In contrast the symptomatology of the hypothyroid phase may be profound. Raised levels of circulating thyroid peroxidase autoantibody (TPOAb) are detected in 10% of pregnant women at 16 weeks Of these, 50% develop PPTD during the first six months of the postpartum period (401) Permanent hypothyroidism is reported in as many as 30% of these cases after 3 years, and in 50 % at 7 - 10 years .An association with depression has been observed with PPTD (402); depression is also associated with thyroid antibody positive women irrespective of thyroid status (403,404). However, a study in an Australian population found no association between PPTD or thyroid antibody status with depression (405). TPOAb positivity has also been associated with dysphoric mood during and after pregnancy (406).
Diagnosis
Hyperthyroidism in the postpartum period may be due to a recurrence or the development of new Graves' disease or to the hyperthyroid phase of postpartum thyroiditis. Symptoms of hyperthyroidism are much more evident in Graves' disease .As postpartum hyperthyroidism is a destructive process radioiodine uptake will be very low at early and late times after isotope administration. TSH receptor antibodies are not seen unless there is coexisting Graves' disease. Hyperthyroidism due to postpartum thyroiditis is diagnosed by a suppressed TSH together with an elevated FT4 or FT3, or elevated FT3 and FT4, with either set of criteria occurring on more than one occasion. A report (407) of PPT with hyperthyroidism in a patient with thyroid hormone resistance noted the suppression of TSH in this state which is unusual in RTH. If possible a normal range of thyroid hormone concentrations should be derived in the postpartum period as they fall into a narrower range than the general population. Antibodies to thyroxine and T3, which may occur in autoimmune thyroid disease, may cause confusion in diagnosis but they are infrequent.
Hypothyroidism in the postpartum period is diagnosed when either TSH >3.6 mU/L together with FT4<8 pmol/l or FT3 <4.2 pmol/l or TSH 10mU/l on one or more occasion is present. The most frequent symptoms have been found to be lack of energy, aches and pains, poor memory, dry skin and cold intolerance. Thyroid ultrasonography has demonstrated diffuse or multifocal echogenicity reflecting the abnormal thyroid morphology and consistent with the known lymphocytic infiltration of the thyroid .The destructive nature of the thyroiditis is also shown by the increase in urinary iodine excretion in the hyperthyroid as well as the hypothyroid phase of the syndrome. In addition there is evidence that an early rise in serum thyroglobulin (a further indicator of thyroid destruction) may help in the identification of those women at risk of PPT.
Management of PPT
The hyperthyroid phase is relatively asymptomatic and usually requires no specific therapy. If symptoms of tachycardia and palpitations are troublesome of if other symptoms of hyperthyroidism such as sweating or anxiety are present then beta adrenoreceptor blocking agents may be used. Propanolol is the drug of choice but if nightmares develop a more cardioselective β blocker may be used. If this class of drug is contraindicated verapamil may be effective for cardiac symptoms. Antithyroid drugs are not indicated as the condition is a destructive thyroiditis. In contrast, patients experience persistent and troublesome symptoms related to the hypothyroid period and treatment with L-Thyroxine should be given starting with 0.1mg per day increasing as necessary. At this stage it will not be clear whether the patient has developed transient or permanent hypothyroidism. In this instance it is reasonable to treat with thyroxine for one year and then review the patient to determine the thyroxine requirement. This will normally mean that the patient should stop the therapy for 4-6 weeks and then have a thyroid function test. Patients who have been known to have transient thyroid dysfunction postpartum should be checked at least annually as 50% of them will develop hypothyroidism after 7 years. This is in contrast to TPOAb +ve women who have not experienced any thyroid dysfunction postpartum whose rate of hypothyroidism at 7 years follow up is only 5%. Clearly these patients require less intensive surveillance.
Follow Up and Course of the Syndrome
While the hyperthyroidism of PPT always resolves several long term studies of the hypothyroid phase have documented persistence of hypothyroidism in 20-30% of cases (397). Follow-up assessment of antiTPO positive women (at 16 weeks gestation) 9 years later has shown that the rate of development of hypothyroidism was significantly greater (48% vs 8% ) in those who had had PPT compared to those who were euthyroid antibody positive (408).. Recurrence of transient PPT has been observed by several workers and there is a 70% chance of developing recurrent PPT after a first attack and a 25% risk even if the woman was only anti- TPO positive without thyroid dysfunction during the first postpartum period.
The Etiology and Nature of PPT
Factors which predispose towards the development of PPT include the presence of thyroid antibodies (usually TPO but occasionally Tg), a previous episode, type 1 diabetes mellitus and a positive family history of thyroid disease. There is no influence of breast feeding, cigarette smoking, parity or baby gender on the development of PPT. PPT is usually only seen in women who were known to have positive TPO antibodies as determined at 16 weeks gestation although other groups have described the condition in women without thyroid antibodies and with no discernible immune abnormality (401). The destructive nature of the thyroiditis has been noted above. There is no evidence that ambient iodine concentrations affect the incidence of the disease and iodine administration to marginally iodine deficient pregnant women will not prevent the onset of PPTD.
PPTD is an organ specific syndrome which has been regarded as a model of aggravation of the autoimmune state. Abnormal thyroid morphology has been shown by multifocal echogenicity and lymphocytic infiltration and follicles suggestive of Hashimoto’s disease in thyroid biopsies. .The rapid perturbation of the immune system in the post partum year contrasts with the relatively stable situation seen in chronic Hashimoto's thyroiditis. Although the antibody response is dramatic,its precise role in the immunopathogenesis of the condition remains to be determined. Probably, the antibody titre is merely a marker of disease and the immunological damage is mediated by lymphocyte and complement associated mechanisms. A prospective study of lymphocyte sub populations in anti TPO +ve pregnant women and antibody negative controls showed a significant fall in the CD4+/CD8+ ratio in late pregnancy and into the postpartum period in the controls (409). In contrast, women who subsequently developed PPT had a significantly higher CD4+/CD8+ ratio and T cell activation than in normal TPO-ve women. In addition, a particular lymphocyte subset ( CD45RA+ T cells), was also significantly elevated in those women destined to develop PPT and it is possible that this subset serves as a marker in this respect. It has also been suggested that the immunological determinants of postpartum thyroid dysfunction may in part occur antenatally (410). HLA haplotype restriction of the type commonly seen in autoimmune thyroid disease is seen in PPT.
Quantitative examination of complement C3b in PPT patients has shown that, not only is there activation of the complement system by thyroid directed autoantibodies, but that complement activation is related to the extent of the thyroiditis and correlates with the severity of the thyroid dysfunction .
Finally an exciting development has been reported by Negro et al who showed that the administration of selenium (as sodium selenite ) to TPOAb positive women through gestation led to a reduction in the postpartum TPOAb rise and also a significant reduction in postpartum hypothyroidism (411). These data still require confirmation.
SCREENING FOR THYROID DISEASE IN PREGNANCY
Screening for Disease
Medical screening is the systematic application of a test or inquiry to identify individuals at sufficient risk of a specific disorder to benefit from further investigation or direct preventive action (412). The requirements for a justifiable screening test are shown in table 14-11.
Table 14-11
Justification for Screening Test (adapted from 412)
- Well defined disorder with known incidence/prevalence
- Medically important disorder
- Screening test simple and safe with established cut off values
- Effective treatment available
- Cost of test relative to benefit should be known
- Adequate logistics for the testing and follow up
- Patient and management acceptability
It will be apparent that screening a population must be considered very carefully in respect of the condition being screened for, the effectiveness (and safety) of any intervention and the potential anxiety of the patient. If the effectiveness is not known with certainty then evidence should be sought, usually in the form of a randomised trial. The criteria listed in table 1 will now be discussed. Some data relevant to the debate has been mentioned in this chapter.
Does Thyroid Screening in Pregnancy Meet the Above Criteria for Screening?
The prevalence of Graves’ disease is approximately 3.0/1000 with an
incidence of about 0.5/1000/year. The prevalence and incidence in women
during child bearing years is not known but thyrotoxicosis is said to occur in 2
/1000 pregnancies and Graves’ disease would be expected to account for at
least 80% of these cases. While these figures are low, Graves’ hyperthyroidism
can have a dramatic effect on the mother as well as the fetus. There are significant maternal, fetal and neonatal complications (see section on Graves’ disease). Subclinical hyperthyroidism (i.e. normal circulating concentrations of T4 and T3 but subnormal TSH levels) occurs in approximately 1.7% of pregnant women and is not associated with adverse pregnancy outcomes (413). Screening for this condition is clearly not warranted, although if a low TSH is found the establishment of the cause will improve obstetric outcome in a number of women (414)
In contrast to hyperthyroidism, hypothyroidism is quite common in pregnancy . The incidence of subclinical hypothyroidism (raised TSH and normal or low normal T4) is at least 2.5% and these women have no clinical features and are often asymptomatic, but 50–60% will have evidence of autoimmune thyroid disease (positive TPOAbs and or thyroglobulin antibodies, TgAbs) in iodine-sufficient areas. It should be noted however that endemic iodine deficiency is the most common cause of hypothyroidism seen in pregnant women worldwide. Overt hypothyroidism occurs in only about 5% of all women who have a high TSH. During the last decade, it has become apparent that untreated maternal hypothyroidism and subclinical hypothyroidism in pregnancy is associated with adverse fetal and obstetric outcomes discussed in this chapter. There is a greater prevalence of subclinical hypothyroidism in women with delivery before 32 weeks and there is even an association between thyroid autoimmunity and adverse obstetric outcome, which is independent of thyroid function.(5). Higher maternal TSH levels even within the normal reference range are associated with an increased risk of miscarriages, fetal and neonatal distress (154) as well as preterm delivery.(18) In a prospective study, euthyroid TPOAb+ve women who received interventional L-thyroxine in early pregnancy had a reduced miscarriage rate and less preterm delivery.(203). Further prospective randomised trials are required to confirm these interesting data. Of equal or even greater importance than the above is the detrimental effect of hypothyroidism during pregnancy on fetal brain development. The neurodevelopmental impairment is similar to that seen in iodine deficient areas and implies that iodine status should be normalised in regions of deficiency. However, much of the USA is not iodine deficient which raises the question of routine screening of thyroid function during early pregnancy or even at preconception. In contrast gestational iodine deficiency in Europe is not uncommon (415) and decrements in mentation can be seen in iodine deficient areas as well as iodine sufficient ones. It should be noted however that the only randomized prospective trial adequately powered to answer the question as to whether thyroxine therapy to hypothyroid or hypothyroxinemic mothers resulted in benefit to the IQ of their children was negative (276). While there may be reasons for this result, further high quality evidence based studies must be performed to assess the situation such as the one in abstract (277).
As discussed in this chapter, isolated hypothyroxinaemia (IH) (low FT4 and normal TSH) either due to iodine deficiency or autoimmune thyroid disease has been shown to result in lower IQ in infants and young children in retrospective and prospective studies. IH Although it has been found to be associated with adverse perinatal outcomes (227) and is associated with reduced motor and intelligence performance in neonates (416) and in children aged 25–30 months in a Chinese population (255). While treatment of overt hypothyroidism has been shown to prevent the obstetric and neonatal complications the evidence for treatment of subclinical hypothyroidism in prevention is less secure. However a recent screening study where women were characterised as high risk or low risk in terms of the chance of adverse obstetric outcome there was a significant reduction in these outcomes even in low risk women who were screened for subclinical hypothyroidism (417).
Evidence for Intervention in High Risk Clinical Situations
The strength of evidence relating maternal hypothyroidism to low IQ in children suggests strongly that screening thyroid function in early gestation with l-thyroxine intervention in appropriate women would be beneficial. In addition there is evidence that such a strategy would be cost-effective. A study by Thung et al (418) compared the cost effectiveness of no screening versus routine screening for subclinical hypothyroidism in pregnancy.. The decision model demonstrated a saving of approximately $8.3 million per 100,000 women screened with an increment of 589.3 quality adjusted life years. Similar results were obtained by Dosiu et al (419) using a different screening model.
The lack of sufficient class A evidence has added to the controversy. Recent studies of targeted screening have concluded that targeted screening is unsatisfactory and that the data support the case for universal screening although more studies are required (420-422). The recommendation for universal screening has been made for pregnant women in Egypt (423), and pregnant women in China (424,425). An Asian survey found variable practice in relation to screening (426). Meanwhile other workers have recommended against screening (427,428). A recent opinion stated that testing maternal TSH as part of first trimester screening does not predict adverse pregnancy outcomes because mainly mild abnormalities in thyroid function are detected (429,430).
Several organisations have issued guidelines on whether to adopt a screening strategy for thyroid function in early pregnancy (11-15), the latest being the revised version of The American Thyroid Association guidelines (13). Generally, because of the lack of class A evidence (randomized controlled trial data) the recommendations from these guidelines) do not endorse universal screening. Instead, a targeted approach was suggested in specific clinical situations, although as noted below, this approach has limitations. The published Endocrine society of America guidelines (14) included a split recommendation, some members favoring universal screening, and others favoring targeted screening.A comparison of the 2 sets of recommendations is shown in table 14-12.
Table 14-12
Recommended patient profiles for targeted thyroid disease case finding in women seeking pregnancy, or newly pregnant:
ATA guidelines
History of thyroid dysfunction or prior thyroid surgery
Age >30 years
Symptoms of thyroid dysfunction or the presence of goiter
TPOAb positivity
Type 1 diabetes or other autoimmune disorders
History of miscarriage or preterm delivery
History of head or neck radiation
Family history of thyroid dysfunction
Morbid obesity (BMI ≥40 kg/m2)
Use of amiodarone or lithium, or recent administration of iodinated radiologic contrast
Infertility
Residing in an area of known moderate to severe iodine insufficiency
Endocrine Society Guidelines
Women over age 30 years ( ? Valid see 310a)
family history of autoimmune thyroid disease or hypothyroidism
goiter
thyroid antibodies, primarily thyroid peroxidase antibodies
symptoms or clinical signs suggestive of thyroid hypofunction
type 1 diabetes mellitus, or other autoimmune disorders
infertility
prior history of preterm delivery
prior therapeutic head or neck irradiation or prior thyroid surgery
currently receiving levothyroxine replacement
A comparison between the first ATA guidelines (12) and those of The Endocrine Society (14) concluded that the data available at that time are neither for or against universal screening (431).
Althoughtargeted screening might seem a reasonable approach in relation to economic and logistic factors, there has been accruing evidence that a substantial number of women with thyroid dysfunction would not be diagnosed in these circumstances. Vaidya (432) found that targeted testing of a previously defined high risk group who had a personal history of thyroid or other autoimmune disorders or a family history of thyroid disease (413 women) failed to detect 28% of pregnant women with a TSH>4.2mIU/L. Li et al (255) found that this strategy missed 36% of women with TSH>4.0mIU/L. Overall , targeted screening may miss 33-88% of women with a thyroid abnormality (420,423,424,432-435, ). For example, screening ‘low risk women’ identified 28% with thyroid dysfunction excluding those with just positive thyroid antibodies (420). The variability seen in these data may relate to different definitions of thyroid dysfunction and different ethnicity of the populations studied. A meta analysis performed by obstetricians in The Netherlands concluded that he overall lack of evidence precludes a recommendation for universal screening and is only justified in a research setting (162). Despite a lack of consensus among professional organisation many areas of the world are in fact performing routine screening (436-438).In the USA 74% of respondents at an ATA meeting advocated universal thyroid screening with TSH (439). To date there is an ongoing disussion relating to the evidence that levothyroxine treatment of pregnant women with subclinical hypothyroidism, isolated hypothyroxinemia, or thyroid autoimmunity is beneficial (439,,13,15). Therefore, there is ongoing debate regarding the need for universal screening for thyroid dysfunction during pregnancy . Efforts are still required to provide more high quality evidence to justify screening. There is some evidence that screening (with thyroxine intervention therapy) may at least prevent or reduce some obstetric complications associated with SCH in pregnancy (440,441). Meanwhile, optimal cooperation and communication between endocrinologists and obstetricians is also necessary.
Conclusion
The screening criteria for subclinical hypothyroidism in pregnancy are largely met. The condition is not rare and several retrospective studies imply adverse obstetric and child neurodevelopmental outcomes. However there are few prospective randomized trials to substantiate the benefit of screening and the CATS study did not show a benefit in child IQ at age 3 years (276). Nevertheless there seems to be a case for screening to prevent adverse obstetric outcomes. From the child cognitive function aspect there should be further studies where intervention is initiated early in the first trimester during the course of brain development. Unfortunately the recent NIH study reported in abstract indicated that recruitment of mothers was at least midway in the second trimester (277)
From the forgoing discussion this author believes that the lack of high quality clinical epidemiological evidence base probably does not justify universal screening at the present time. Other authors note that the data on the beneficial effects of treatment for subclinical hypothyroidism remain uncertain, but that the other established benefits justify universal screening at this time. However, it is likely that more evidence will be produced which may alter this view in the future. Other authors have suggested that screening may be worthwhile rather than searching for minor abnormalities in thyroid function tests (442) and that screening could be introduced on the premise that overt hypothyroidism is the target condition and that there is agreement with regard to treatment ( Meanwhile it must be admitted that screening is occurring round the world in a pragmatic fashion.
FINAL CONCLUSIONS
Pregnancy has profound effects on the regulation of thyroid function in healthy women and patients with thyroid disorders. These effects need to be recognized, precisely assessed, clearly interpreted, and correctly managed. For healthy pregnant women who reside in areas with a restricted iodine intake, relative hypothyroxinemia & goitrogenesis occur frequently, indicating that pregnancy constitutes a challenge for the thyroidal economy.
Overt thyroid dysfunction occurs in 2-3% of pregnancies, but subclinical thyroid dysfunction (both hyper- & hypothyroidism) is probably more prevalent and frequently remains undiagnosed, unless specific screening programs are initiated to disclose thyroid function abnormalities in early gestation. Maternal alterations of thyroid function due to iodine deficiency, hypothyroidism and hyperthyroidism have important implications for fetal/neonatal outcome. In recent years, particular attention has been focused on potential developmental risks for the fetuses of women with subclinical hypothyroidism during early gestation. These include obstetric problems and the possibility of impaired neurodevelopment.
Pregnancy increases the metabolic rate, blood flow, heart rate, and cardiac output, and various subjective sensations such as fatigue and heat intolerance that may suggest the possibility of coexistent thyrotoxicosis. Other metabolic changes which also impact the hypothalamic pituitary thyroid system are the potential direct stimulation of the maternal thyroid by hCG, as well as the accelerated metabolism of thyroxine, presumably due to increased placental deiodination enzymes.
In patients with hypothyroidism, it is important to recognize that therapeutic requirements for exogenous thyroxine are increased by 50% on average during pregnancy. This should be taken into account in the management of such patients.
Main causes of thyrotoxicosis in pregnancy include Graves' disease (uncommon, but potentially pregnancy-threatening) and gestational non autoimmune transient hyperthyroidism (more common, but remaining mild usually). The natural history of Graves' disease is altered during pregnancy, with a tendency for exacerbation in 1st trimester, amelioration during 2nd & 3rd trimesters, and typically a rebound during the postpartum period. These changes are the consequences of partial immune suppression during gestation with a rebound during the postpartum period. This must be kept in mind when treating thyrotoxic patients, since all ATD cross the placenta and may affect fetal thyroid function. PTU is now recommended only for 1st trimester and MMI for the rest of pregnancy.
Fetal and neonatal hyperthyroidism is due to the transplacental transfer of maternal stimulating TSH-receptor antibodies (TRAb). The diagnosis of fetal (and neonatal) hyperthyroidism is usually made on the basis of fetal tachycardia, accelerated bone age, and intrauterine growth retardation. It may occur in infants born to women with active Graves' disease, but also to women who have had prior definitive cure of their disease by surgery or radioactive iodine, but maintain high titers of TRAb. The proper management of pregnant patients with Graves' disease remains a difficult challenge in clinical endocrinology.
Thyroid nodules discovered during pregnancy should be aspirated for cytological diagnosis. If a malignancy is diagnosed, surgery should be performed during pregnancy or shortly thereafter. Pregnancy by itself usually does not adversely affect the natural history of differentiated thyroid carcinoma.
During the postpartum period, particular attention should be given to women with thyroid autoimmunity, since hypothyroidism and hyperthyroidism are frequently exacerbated in the months following the delivery.
Antenatal screening for thyroid dysfunction is being actively discussed by the thyroid community. At present evidence based studies are very limited and do not support this strategy. However many clinics worldwide are currently screening. Dialogue between endocrinologist and obstetrician is important in this regard The results of further randomized trials are awaited.
REFERENCES
1 Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 1997;18: 404-33
2 KrassasGE, PoppeK, GlinoerD
Thyroid function and human reproductive health. Endocr Rev 2010;31:702-55
3 Weetman AP. Immunity, thyroid function and pregnancy: molecular mechanisms Nat Rev Endocrinol. 2010;6: 311-18.
4 Lazarus JH, Soldin O, Evans C. Assessing thyroid function in pregnancy. In: Brent G, ed. Thyroid Function Testing. New York : Springer, 2010: 209-33
5 John H. Lazarus Thyroid function in pregnancy British Medical Bulletin 2011; 97:137-48
6 Spencer L, Bubner T, Bain E, Middleton P Screening and subsequent management for thyroid dysfunction pre-pregnancy and during pregnancy for improving maternal and infant health. Cochrane Database Syst Rev. 2015 21:CD011263. doi: 10.1002/14651858.
7 Okosieme OE, Lazarus JH Important considerations in the management of Graves' disease in pregnant women. Expert Rev Clin Immunol. 2015;11:947-57. doi:10.1586/1744666X.2015.1054375.
8 Zimmermann MB. Iodine deficiency. Endocr Rev. 2009; 30:376-408
9 .Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C Influence of maternal thyroid hormones during gestation on fetal brain development.Neuroscience. 2015 Oct 3. pii: S0306-4522(15)00897-0. doi: 10.1016/j.neuroscience.2015.09.070.
10 Pearce EN Thyroid disorders during pregnancy and postpartum..Best Pract Res Clin Obstet Gynaecol. 2015 ;29:700-6. doi: 10.1016/j.bpobgyn.2015.04.007
11 Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot L, Glinoer D, Mandel SJ, Stagnaro-Green A: Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2007; 92 (Supplement): S1-S47),
12 Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum.Thyroid. 2011;21:1081-125
13 Erik K. Alexander, Elizabeth N. Pearce , Gregory A. Brent, Rosalind S. Brown, Herbert Chen,Chrysoula Dosiou, William A. Grobman , Peter Laurberg , John H. Lazarus, Susan J. Mandel , Robin P. Peeters , and Scott Sullivan 2016 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease during Pregnancy and the Postpartum. Thyoid 2016 in press
14 De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:2543-65
15 Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association Guidelines for the Management of Subclinical Hypothyroidism in Pregnancy and in Children Eur Thyroid J 2014;3:76-94 (DOI: 10.1159/000362597)
- 16.
Glinoer D: Pregnancy and iodine. Thyroid 2001;11:471-81
17 Liberman CS, Pino SC, Lieh Fang S, Braverman LE, Emerson CH: Circulating iodide concentrations during and after pregnancy. J Clin Endocrinol Metab 1998 ; 83:3545-9
- 18.
Halnan KE: The radioiodine uptake of the human thyroid in pregnancy. Clin Sci 1958 17:281-90,
19 Burns R, Azizi F, Hedayati M, Mirmiran P, O'Herlihy C, Smyth PPIs placental iodine content related to dietary iodine intake? Clin Endocrinol (Oxf). 2011; 75 :261-4.
- 20.
Crooks J, Tulloch MI, Turnbull AC, et al: Comparative incidence of goitre in pregnancy in Iceland and Scotland. Lancet 1969; 2:625-7,
21, Farebrother J, Naude CE, Nicol L, Andersson M, Zimmermann MB.Iodised salt and iodine supplements for prenatal and postnatal growth: a rapid scoping of existing systematic reviews. Nutr J. 2015 2;14:89. doi: 10.1186/s12937-015-0079-z.
22 WHO Secretariat, Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation.Public Health Nutr. 2007; 10(12A):1606-11
23 Charlton K, Land MA, Ma G, Yeatman H, Houweling F Iodine status similarly suboptimal in Australian women who have desirable salt intakes compared to those with excessive intakes..Nutrition. 2014;30: 234-5. doi: 10.1016/j.nut.2013.05.009
24 Vanderpump MP, Lazarus JH, Smyth PP, Laurberg P, Holder RL, Boelaert K, Franklyn JA; British Thyroid Association UK Iodine Survey Group Iodine status of UK schoolgirls: a cross-sectional survey.Lancet. 2011;377: 2007-12.
- 25.
Lee KW, Cho MS, Shin D, Song WO. Changes in iodine status among US adults, 2001-2012. Int J Food Sci Nutr. 2016 ;67:184-94. doi: 10.3109/09637486.2016.1144717
26 Zimmermann MB The effects of iodine deficiency in pregnancy and infancy.
Paediatr Perinat Epidemiol. 2012 ;26 Suppl 1:108-17.
- 27.
Glinoer D: The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. In: Best Practice & Research in Clinical Endocrinology and Metabolism: The Thyroid and Pregnancy (Editor: Glinoer D) 2004; 18:133-52
- 28.
Smyth PP, Hetherton AM, Smith DF, Radcliff M, O'Herlihy C: Maternal iodine status and thyroid volume during pregnancy: correlation with neonatal iodine intake. J Clin Endocrinol Metab 1997; 82:2840-3
29 Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, Rasmussen OS, Larsen KR, Eriksen GM, Johannesen PL. Amelioration of some pregnancy-associated variations in thyroid function induced by iodine supplementation. J Clin Endocrinol Metab 1993; 77:1078-83
- 30.
Romano R, Jannini EA, Pepe M, Grimaldi A, Olivieri M, Spennati P, Cappa F, D'Armiento M. The effects of iodoprophylaxis on thyroid size during pregnancy. Am J Obstet Gynecol 1991;164:482-5,
31 Glinoer D, Lemone M, Bourdoux P, De Nayer P, DeLange F, Kinthaert J, LeJeune B. Partial reversibility during late postpartum of thyroid abnormalities associated with pregnancy. J Clin Endocrinol Metab1992; 74:453-7
32 Moreno-Reyes R, Glinoer D, Van Oyen H, Vandevijvere S.High prevalence of thyroid disorders in pregnant women in a mildly iodine-deficient country: a population-based study.
J Clin Endocrinol Metab. 2013;98:3694-701
- 33.
Glinoer D, De Nayer P, Delange F, Lemone M, Toppet V, Spehl M, Grün JP, Kinthaert J, Lejeune B. A randomized trial for the treatment of mild iodine deficiency during pregnancy: maternal and neonatal effects. J Clin Endocrinol Metab 1995; 80:258-69
- 34.
Rotondi M, Amato G, Biondi B, Mazziotti G, Del Buono A, Rotonda Nicchio M, Balzano S, Bellastella A, Glinoer D, Carella C. Parity as a thyroid size-determining factor in areas with moderate iodine deficiency. J Clin Endocrinol Metab 2000; 85:4534-37
- 35.
Knudsen N, Bülow I, Laurberg P, Ovesen L, Perrild H, Jørgensen T. Parity is associated with increased thyroid volume solely among smokers in an area with moderate to mild iodine deficiency. Europ J Endocrinology 2002;146:39-43
36 Glinoer D, Demeester R, Lemone M, Larsimont D, Andry G. Acute increase in goiter size during a normal pregnancy: an exceptional case report. Thyroid 2003;13:881-884
37.Aloumanis K, Mavroudis K, Vassiliou I, Arkadopoulos N, et al: Urgent thyroidectomy for acute airway obstruction caused by a goiter in a euthyroid pregnant woman. Thyroid 2006; 16:85-88,
- 38.
Vermiglio F, Lo Presti VP, Castagna MG, et al: Increased risk of maternal thyroid failure with pregnancy progression in an iodine deficient area with major iodine deficiency disorders. Thyroid 1999;9:19-24
39 Report of a WHO Technical Consultation on prevention and control of iodine deficiency in pregnancy, lactation, and in children less than 2 years of age (Geneva, 24-26 January 2005) (de Benoist B, Delange F, Editors). Public Health Nutrition (Special Issue) 10 (12A):1527-1611, 2007
40 Vandevijvere S, Amsalkhir S, Mourri AB, Van Oyen H, Moreno-Reyes R.
Br J Nutr. 2013: 28;109:2276-84.
41 Technical Consultation: Salt reduction and iodine fortification strategies in public health co-sponsored by the George Institute for Global Health, AustraliaIn collaboration with the International Council for the Control of Iodine Deficiency Disorders Global Network 25 - 27 March 2013Sydney, Australia
42 The effect of iodine prophylaxis on the incidence of endemic cretinism.
Pharoah PO, Buttfield IH, Hetzel BS.Adv Exp Med Biol. 1972;30:201-21
43 Melse-Boonstra A, Gowachirapant S, Jaiswal N, Winichagoon P, Srinivasan K, Zimmermann MB. Iodine supplementation in pregnancy and its effect on child cognition.
J Trace Elem Med Biol. 2012 ;26:134-6
44 van Mil NH, Tiemeier H, Bongers-Schokking JJ, Ghassabian A, Hofman A, Hooijkaas H, Jaddoe VW, de Muinck Keizer-Schrama SM, Steegers EA, Visser TJ, Visser W, Ross HA, Verhulst FC, de Rijke YB, Steegers-Theunissen RP. Low urinary iodine excretion during early pregnancy is associated with alterations in executive functioning in children.
J Nutr. 2012;142:2167-74.
45 Bath SC, Steer CD, Golding J, Emmett P, Rayman MP.
Lancet. 2013 ;382:331-7.
46 Taylor PN, Okosieme OE, Dayan CM, Lazarus JH. Impact of iodine supplementation in mild-to-moderate iodine deficiency: systematic review and meta-analysis.
Eur J Endocrinol. 2013 2;170: R1-R15. PMID:24088547
47 Fuse Y, Ohashi T, Yamaguchi S, Yamaguchi M, Shishiba Y, Irie M Iodine status of pregnant and postpartum Japanese women: effect of iodine intake on maternal and neonatal thyroid function in an iodine-sufficient area. J Clin Endocrinol Metab. 2011;96:3846-54
48 Sang Z, Wei W, Zhao N, Zhang G, Chen W, Liu H, Shen J, Liu J, Yan Y, Zhang W.
Thyroid dysfunction during late gestation is associated with excessive iodine intake in pregnant women.J Clin Endocrinol Metab. 2012;97: E1363-9.
49 Nohr SB, Laurberg P: Opposite variations in maternal and neonatal thyroid function induced by iodine supplementation during pregnancy. J Clin Endocrinol Metab 2000; 85:623-627
50 Zimmermann MB, Aeberli I, Torresani T, Bürgi H: Increasing the iodine concentration in the Swiss iodized salt program markedly improved iodine status in pregnant women and children: a 5-y prospective national study Am J Clin Nutrition 2005; 82:388-92
- 51.
Andersson M, Takkouche B, Egli I, et al: Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull WHO 2005; 83:518-25,
- 52.
Hershman JM: Physiological and pathological aspects of the effect of human chorionic gonadotropin on the thyroid. In: Best Practice & Research in Clinical Endocrinology and Metabolism: The Thyroid and Pregnancy (Editor: Glinoer D) 2004 ; 18:249-264,
53 . Glinoer D, De Nayer P, Robyn C, Lejeune B, Kinthaert J, Meuris S.: Serum levels of intact human chorionic gonadotropin (hCG) and its free alpha and beta subunits, in relation to maternal thyroid stimulation during normal pregnancy. J Endocrinol Invest 1993; 16:881-8
54 Kraiem Z, Lahat N, Sadeh O, Blithe DL, Nisula BC: Desialylated and deglycosylated human chorionic gonadotropin are superagonists of native human chorionic gonadotropin in human thyroid follicles. Thyroid 1997;7:783-8
55 Walkington L, Webster J, Hancock BW, Everard J, Coleman RE.Hyperthyroidism and human chorionic gonadotrophin production in gestational trophoblastic disease.Br J Cancer. 2011;104:1665-9
- 56.
Glinoer D, Gershengorn MC, Dubois A, Robbins J.: Stimulation of thyroxine-binding globulin synthesis by isolated rhesus monkey hepatocytes after in vivo -estradiol administration. Endocrinology 1977;100:807-13
- 57.
Ain KB, Mori Y, Refetoff S: Reduced clearance rate of thyroxine-binding globulin (TBG) with increased sialylation: a mechanism for estrogen-induced elevation of serum TBG concentration. J Clin Endocrinol Metab1987; 65:689-96
58 Ain KB, Refetoff S: Relationship of oligosaccharide modification to the cause of serum thyroxine-binding globulin excess. J Clin Endocrinol Metab 1988;66:1037-43
- 59.
Refetoff S: Inherited thyroxine-binding globulin abnormalities in man. Endocr Rev 1989; 10:275-93,
- 60.
Zigman JM, Cohen SE, Garber JR: Impact of thyroxine-binding globulin on thyroid hormone economy during pregnancy. Thyroid 2003; 13:1169-75
61 Glinoer D: Increased TBG during pregnancy and increased hormonal requirements. Thyroid (letter to the Editor) 14:179, 2004
62 Dowling JT, Appleton WG, Nicoloff JT: Thyroxine turnover during human pregnancy. J Clin Endocrinol Metab 1964;27:1749-50
63 Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone
delivery to the fetus. Nat Clin Pract Endocrinol Metab 2009; 5:45-54.
64 Patel J, Landers K, Li H, Mortimer RH, Richard K. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 2011;22:164-70.
65 Burns R, O'Herlihy C, Smyth PP. The Placenta as a Compensatory Iodine Storage Organ.Thyroid. 2011, 21: 541-46
66 Burns R, O'Herlihy C, Smyth PP. Regulation of iodide uptake in placental primary cultures.
Eur Thyroid J. 2013;2:243-51. doi: 10.1159/000356847
67 Li H, Patel J, Mortimer RH, Richard K Ontogenic changes in human placental sodium iodide symporter expression. Placenta. 2012 ;33:946-8.
68 Andersen SL, Nøhr SB, Wu CS, Olsen J, Pedersen KM, Laurberg P
Thyroglobulin in smoking mothers and their newborns at delivery suggests autoregulation of placental iodide transport overcoming thiocyanate inhibition. Eur J Endocrinol. 2013;15:168:723-31.
69 Deng WB, Liang XH, Liu JL, Yang ZM. Regulation and function of deiodinases during decidualization in female mice.Endocrinology. 2014;155:2704-17. doi: 10.1210/en.2014-1015
70 Loubiere LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta 2010; 31:295-304.
71 Sun YN, Liu YJ, Zhang L, Ye Y, Lin LX, Li YM, Yan YQ, Chen ZP. Expression of organic anion transporting polypeptide 1c1 and monocarboxylate transporter 8 in the rat placental barrier and the compensatory response to thyroid dysfunction.PLoS One. 2014 24;9:e96047. doi: 10.1371/journal.pone.0096047.
72 Loubière LS, Vasilopoulou E, Glazier JD, Taylor PM, Franklyn JA, Kilby MD, Chan SY.
Expression and function of thyroid hormone transporters in the microvillous plasma membrane of human term placental syncytiotrophoblast.Endocrinology. 2012 ;153:6126-35.
73 Vasilopoulou E, Loubière LS, Heuer H, Trajkovic-Arsic M, Darras VM, Visser TJ, Lash GE, Whitley GS, McCabe CJ, Franklyn JA, Kilby MD, Chan SY Monocarboxylate transporter 8 modulates the viability and invasive capacity of human placental cells and fetoplacental growth in mice. PLoS One. 2013 12;8:e65402. doi: 10.1371/journal.pone.0065402.
74 Landers KA, Mortimer RH, Richard K. Transthyretin and the human placenta.
Placenta. 2013 ;34:513-7
75 Mortimer RH, Landers KA, Balakrishnan B, Li H, Mitchell MD, Patel J, Richard K. Secretion and transfer of the thyroid hormone binding protein transthyretin by human placenta.Placenta. 2012 ;33:252-6
76 Laoag-Fernandez JB, Matsuo H, Murakoshi H, Hamada AL, Tsang BK, Maruo T. 3,5,3 '-triiodothyroninedown-regulates Fas and Fas ligand expression andsuppresses caspase-3 and poly (adenosine 5 '-diphosphate-ribose) polymerase cleavage and apoptosis in early placental extravillous trophoblasts in vitro. Journal of Clinical Endocrinology and Metabolism 2004;89:4069-4077.
77 Roti E, Gnudi A, Braverman LE: The placental transport, synthesis and metabolism of hormones and drugs which affect thyroid function. Endocr Rev1983; 4:131-149
78 Vulsma T, Gons MH, De Vijlder JMM: Maternal fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect of thyroid dysgenesis. N Engl J Med , 1989;321:13-6
79 Contempre B, Jauniaux E, Calvo R, Jurkovic D, Campbell S, de Escobar GM : Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab1993; 77:1719-22
80 Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C Molecular basis of thyrotropin and thyroid hormone action during implantation and early development..Hum Reprod Update. 2014;20: 884-904. doi: 10.1093/humupd/dmu028
81 Saben J, Kang P, Zhong Y, Thakali KM, Gomez-Acevedo H, Borengasser SJ, Andres A, Badger TM, Shankar K. RNA-seq analysis of the rat placentation site reveals maternal obesity-associated changes in placental and offspring thyroid hormone signaling. Placenta. 2014;35:1013-20. doi: 10.1016/j.placenta.2014.09.015.
82 Silva JF, Ocarino NM, Serakides R.Placental angiogenic and hormonal factors are affected by thyroid hormones in rats. Pathol Res Pract. 2015;211:226-34. doi: 10.1016/j.prp.2014.11.003.
83 Vasilopoulou E, Loubière LS, Lash GE, Ohizua O, McCabe CJ, Franklyn JA, Kilby MD, Chan SY.Triiodothyronine regulates angiogenic growth factor and cytokine secretion by isolated human decidual cells in a cell-type specific and gestational age-dependent manner.Hum Reprod. 2014;29:1161-72. doi: 10.1093/humrep/deu046.
84 Korevaar TI, Steegers EA, de Rijke YB, Visser WE, Jaddoe VW, Visser TJ, Medici M, Peeters RP Placental Angiogenic Factors Are Associated With Maternal Thyroid Function and Modify hCG-Mediated FT4 Stimulation. J Clin Endocrinol Metab. 2015;100:E1328-34. doi: 10.1210/jc.2015-2553
85 Chen CY, Chen CP, Lin KH. Biological functions of thyroid hormone in placenta. Int J Mol Sci. 2015 16;16:4161-79. doi: 10.3390/ijms16024161.
86 Kahric-Janicic N, Soldin SJ, Soldin OP, West T, Gu J, Jonklaas J: Tandem mass spectrometry improves the accuracy of free thyroxine measurements during pregnancy. Thyroid 17:303-11, 2007
87 Lee RH, Spencer CA, Mestman JH, Miller EA, Petrovic I, Braverman LE, Goodwin TM. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol. 2009 ;200:260.e 1-6 doi: 10.1016/j.ajog.2008.10.042
88 Soldin, O. P. Soldin D, Sastoque M. Gestation-specific thyroxine and thyroid stimulating hormone levels in the United States and worldwide. Ther Drug Monit 2007; 29: 553-9.
89 van Deventer HE, Soldin SJ.The expanding role of tandem mass spectrometry in optimizing diagnosis and treatment of thyroid disease. Adv Clin Chem. 2013; 61:127-52
90 Fritz, K. S., R. B. Wilcox, Nelson JC. A direct free thyroxine (T4)
immunoassay with the characteristics of a total T4 immunoassay. Clin Chem 2007;
53: 911-5
91 Berta E, Samson L, Lenkey A, Erdei A, Cseke B, Jenei K, Major T, Jakab A, Jenei Z, Paragh G, Nagy EV, Bodor M. Evaluation of the thyroid function of healthy pregnant women by five different hormone assays.Pharmazie 2010 ;65:436-39.
92 Springer D, Bartos V, Zima T. Reference intervals for thyroid markers in early pregnancy determined by 7 different analytical systems. Scand J Clin Lab Invest. 2014 ;74:95-01. doi: 10.3109/00365513.2013.860617.
93 Bliddal S, Feldt-Rasmussen U, Boas M, Faber J, Juul A, Larsen T, Precht DH. Gestational age-specific reference ranges from different laboratories misclassify pregnant women's thyroid status: comparison of two longitudinal prospective cohort studies. Eur J Endocrinol. 2013 27;170:329-9. doi: 10.1530/EJE-13-0672
94 Anckaert E, Poppe K, Van Uytfanghe K, Schiettecatte J, Foulon W, Thienpont LM. FT4 immunoassays may display a pattern during pregnancy similar to the equilibrium dialysis ID-LC/tandem MS candidate reference measurement procedure in spite of susceptibility towards binding protein alterations Clin Chim Acta. 2010; 41:1348-53
95 Midgley JE, Hoermann R.Measurement of total rather than free thyroxine in pregnancy: the diagnostic implications.Thyroid. 2013 ;23:259-61
96 Gilbert, R. M., N. C. Hadlow, Walsh JP, Fletcher SJ, Brown SJ, Stuckey BG, Lim EM . Assessment of thyroid function during pregnancy: first-trimester (weeks 9-13) reference intervals derived from Western Australian women. Med J Aust 2008;189: 250-3.
97 Gong, Y. and B. R. Hoffman . Free thyroxine reference interval in each trimester of pregnancy determined with the Roche Modular E-170 electrochemiluminescent immunoassay. Clin Biochem 2008;41: 902-6.
98 Marwaha, R. K., S. Chopra, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, Singh S. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG 2008; 115: 602-6.
99 Stricker, R. Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, Stricker R. et al. (2007). Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. Eur J Endocrinol 157: 509-14.
100 Pearce, E. N. Oken E, Gillman MW, Lee SL, Magnani B, Platek D, Braverman LE. . Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr Pract 2008;14: 33-9.
101 Lambert-Messerlian, G., M. McClain, Haddow JE, Palomaki GE, Canick JA, Cleary-Goldman J, Malone FD, Porter TF, Nyberg DA, Bernstein P, D'Alton ME. First- and second-trimester thyroid hormone reference data in pregnant women: a FaSTER (First- and Second-Trimester Evaluation of Risk for aneuploidy) Research Consortium study. Am J Obstet Gynecol 2008;199: 62. e1-6. doi: 10.1016/j.ajog.2007.12.003
102 La'ulu, S. L. and W. L. Roberts . Second-trimester reference intervals for thyroid tests: the role of ethnicity. Clin Chem 2007;53:1658-64.
103 Thevarajah M, Chew YY, Lim SC, Sabir N, Sickan J. Determination of trimester specific reference intervals for thyroid hormones during pregnancy in Malaysian women.Malays J Pathol. 2009; 31:23-7.
104 Vila L, Serra-Prat M, Palomera E, et al. Reference values for thyroid function tests in pregnant women living in Catalonia, Spain. Thyroid; 2010 :20:221-5.
105 Ashoor G, Kametas NA, Akolekar R, Guisado J, Nicolaides KH. Maternal thyroid function at 11-13 weeks of gestation.Fetal Diagn Ther. 2010; 27:156-163
106 Azizi F, Ladan M, Amouzegar A, Hossein D, Maryam T, Sahar A, Hedayati M.
Establishment of the trimester-specific reference range for free thyroxine index. Thyroid. 2013 ;23:354-9.
107 Xing J, Yuan E, Li J, Zhang Y, Meng X, Zhang X, Rong S, Lv Z, Tian Y, Jia L.Trimester- and Assay-Specific Thyroid Reference Intervals for Pregnant Women in China.
Int J Endocrinol. 2016;2016:3754213. doi: 10.1155/2016/3754213.
108 Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, Li C, Xu B, Bi L, Meng T, Du J, Zhang S, Gao Z, Zhang X, Yang L, Fan C, Teng W. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. 2014; 99:73-9. doi: 10.1210/jc.2013-1674
109 Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP.Thyroid function in pregnancy: what is normal? Clin Chem. 2015;61:704-13. doi: 10.1373/clinchem.2014.236646
110 McNeil AR, Stanford PE. Reporting Thyroid Function Tests in Pregnancy.
Clin Biochem Rev. 2015;36:109-26
111 Panesar, NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38: 329-32.
112 Boas M, Forman JL, Juul A, Feldt-Rasmussen U, Skakkebaek NE, Hilsted L, Chellakooty M, Larsen T, Larsen JF, Petersen JH, Main KM. Narrow intra-individual variation of maternal thyroid function in pregnancy based on a longitudinal study on 132 women. Eur J Endocrinol. 2009;161:903-10.
113 Walker JA, Illions EH, Huddleston JF, Smallridge RC.Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstet Gynecol. 2005 ;106:1365-71.
114 Korevaar TI, Medici M, de Rijke YB, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VW, Hofman A, Ross HA, Visser WE, Hooijkaas H, Steegers EA, Tiemeier H, Bongers-Schokking JJ, Visser TJ, Peeters RP. Ethnic differences in maternal thyroid parameters during pregnancy: the Generation R study.J Clin Endocrinol Metab. 2013 ;98:3678-86.
115 Han C, Li C, Mao J, Wang W, Xie X, Zhou W, Li C, Xu B, Bi L, Meng T, Du J, Zhang S, Gao Z, Zhang X, Yang L, Fan C, Teng W, Shan Z. High Body Mass Index Is an Indicator of Maternal Hypothyroidism, Hypothyroxinemia, and Thyroid-Peroxidase Antibody Positivity during Early Pregnancy. Biomed Res Int. 2015;2015:351831. doi: 10.1155/2015/351831
116 Haddow JE, Neveux LM, Palomaki GE, Lambert-Messerlian G, Malone FD, D'Alton ME. An Inverse Relationship Between Weight and Free Thyroxine During Early Gestation Among Women Treated for Hypothyroidism.Thyroid. 2015;25:949-53. Doi
10.1089/thy.2015.0085.
117 Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG: Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11:527- 39,
118 Grjibovski AM, Vikanes A; Stoltenberg C Magnus P. Consanguinity and the risk of hyperemesis gravidarum in Norway. Acta Obstetr Gynecol Scand 2008; 87:20-5.
119 Zhang Y, Cantor RM, MacGibbon K, Romero R, Goodwin TM, Mullin PM, Fejzo MS. Familial aggregation of hyperemesis gravidarum. Am J Obstet Gynecol 2011; 204:230.e1-7 doi: 10.1016/j.ajog.2010.09.018
120.Jordan V, Grebe SK, Cooke RR, Ford HC, Larsen PD, Stone PR, Salmond CE. Acidic forms of chorionic gonadotrophin in European and Samoan women are associated with hyperemesis gravidarum and may be thyrotrophic. Clin Endocrinol1999; 50:619-27,
121 Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, Velkeniers B. Assisted reproduction and thyroid immunity: an unfortunate combination? J Clin Endocrinol Metab 2003; 88:4149-52
122 Rodien P, Brémont C, Sanson ML, Parma J, Van Sande J, Costagliola S, Luton JP, Vassart G, Duprez L.Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N EnglJ Med.1998; 17;339:1823-6.
123 Coulon AL, Savagner F, Briet C, Vernin M, Munier M, Chabre O, Rodien P. Prolonged and Severe Gestational Thyrotoxicosis Due to Enhanced hCG Sensitivity of a Mutant Thyrotropin Receptor. JClin Endocrinol Metab. 2016 ;101:10-1. doi: 10.1210/jc.2015-3670.
124 Goodwin TM, Montoro M, Mestman JH: The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J Clin Endocrinol Metab 1992; 75: 1333-37.
125. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, et al.The incidence of thyroid disorders in a community: a twenty-year follow-up of the Whickam survey. Clin Endocrinol 1995;43:55-68,
126 Glinoer D, Rihai M, Grün JP, Kinthaert J: Risk of subclinical hypothyroidism in pregnant women with autoimmune thyroid disorders. J Clin Endocrinol Metab1994; 79:197-04,
127 Lin L, Zhang XL, Long Y.Analysis of thyroid peroxidase antibody in early pregnancy. Genet Mol Res. 2014;13: 5107-14. doi: 10.4238/2014.
128 Van Steirteghem A Current status of assisted reproductive technology (ART) 30 years after the first IVF birth .in The Thyroid and Reproduction Ed John Lazarus, Valdis Pirags and Sigrid Butz 2009 Thieme p 7-14
129 Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocrine Rev 2005; 26:44-62
130 Mariee NG, Tuckerman E, Laird S, Li TC. The correlation of autoantibodies and uNK cells in women with reproductive failure. J Reprod Immunol. 2012 ;95:59-66
131 Huang C, Liang P, Diao L, Liu C, Chen X, Li G, Chen C, Zeng Y. Thyroid Autoimmunity is Associated with Decreased Cytotoxicity T Cells in Women with Repeated Implantation Failure.
Int J Environ Res Public Health. 2015 25;12:10352-61. doi: 10.3390/ijerph120910352.
132 Medenica S, Nedeljkovic O, Radojevic N, Stojkovic M, Trbojevic B, Pajovic B.Thyroid dysfunction and thyroid autoimmunity in euthyroid women in achieving fertility.Eur Rev Med Pharmacol Sci. 2015;19:977-87
133 Cho MK.Thyroid dysfunction and subfertility.Clin Exp Reprod Med. 2015;42:131-5. doi: 10.5653/cerm.2015.42.4.131
134 Feldthusen AD, Pedersen PL, Larsen J, Toft Kristensen T, Ellervik C, Kvetny J. Impaired Fertility Associated with Subclinical Hypothyroidism and Thyroid Autoimmunity: The Danish General Suburban Population Study.J Pregnancy. 2015;2015:132718. doi: 10.1155/2015/132718.
135 Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction.Clin Endocrinol 2007;66:309-21
136 Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, Velkeniers B. Assisted reproduction and thyroid autoimmunity: an unfortunate combination?J Clin Endocrinol Metab. 2003;88: 4149-52
137 Vissenberg R, Manders VD, Mastenbroek S, Fliers E, Afink GB, Ris-Stalpers C, Goddijn M, Bisschop PH.Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update. 2015;21:378-87.
doi:10.1093/humupd/dmv004
138 Matarese G, De Placido G, Nikas Y, Alviggi C. Pathogenesis of endometriosis: natural immunity dysfunction or autoimmune disease? Trends Mol Med. 2003; 9 :223-8.
139 Konova E. The role of NK cells in the autoimmune thyroid disease-associated pregnancy loss. Clin Rev Allergy Immunol. 2010; 39:176-84
140 Kim NY, Cho HJ, Kim HY, Thyroid autoimmunity and its association with cellular and humoral immunity in women with reproductive failures. Am J Reprod Immunol. 2011; 65: 78-87:
141 Monteleone P, Parrini D, Faviana P, Carletti E, Casarosa E, Uccelli A, Cela V, Genazzani AR, Artini PG. Female Infertility Related to Thyroid Autoimmunity: The Ovarian Follicle Hypothesis.Am J Reprod Immunol. 2011; 66:108-14 doi: 10.1111/j.1600-0897.2010.00961.
142 Lee YL, Ng HP, Lau KS, Liu WM, O WS, Yeung WS, Kung AW. Increased fetal abortion rate in autoimmune thyroid disease is related to circulating TPO autoantibodies in an autoimmune thyroiditis animal model. Fertil Steril. 2009;91: 2104-9 doi: 10.1016/j.fertnstert.2008.07.1704.
143 Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun. 2012;38:275-81 doi: 10.1016/j.jaut.2011.11.014.
144 Artini PG, Uccelli A, Papini F, Simi G, Di Berardino OM, Ruggiero M, Cela V. Infertility and pregnancy loss in euthyroid women with thyroid autoimmunity.
Gynecol Endocrinol. 2013;291:36-41.
145 Practice Committee of the American Society for Reproductive Medicine Subclinical hypothyroidism in the infertile female population: a guideline.Fertil Steril. 2015 ;104:545-53. doi: 10.1016/j.fertnstert.2015.05.028.
146 Yoshioka W, Amino N, Ide A, Kang S, Kudo T, Nishihara E, Ito M, Nakamura H, Miyauchi A.Thyroxine treatment may be useful for subclinical hypothyroidism in patients with female infertility. Endocr J. 2015; 62:87-92. doi: 10.1507/endocrj.EJ14-0300.
147 Scoccia B, Demir H, Kang Y, Fierro MA, Winston NJ. In vitro fertilization pregnancy rates in levothyroxine-treated women with hypothyroidism compared to women without thyroid dysfunction disorders. Thyroid 2012;226:631-6.
148 Gracia CR, Morse CB, Chan G, Schilling S, Prewitt M, Sammel MD, Mandel SJ.
Thyroid function during controlled ovarian hyperstimulation as part of in vitro fertilization. Fertil Steril. 2012 ;9: 585-91.
- 149.
Krassas GE, Tziomalos K, Papadopoulou F, Pontikides N, Perros P: Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat? J Clin Endocrinol Metab 2008; 93:1815 -9. doi: 10.1210/jc.2007-2259
- 150.
Li TC, Makris M, Tomsu M, Tuckerman E, Laird S .Recurrent miscarriage: aetiology, management and prognosis. Hum Reprod Update. 2002;8:463-81
151 Stagnaro-Green A, Roman SH, Cobin RH, el-Harazy E, Alvarez-Marfany M, Davies TF. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA. 1990; 19:1422-5.
152 Prummel MF, Wiersinga WM. Thyroid autoimmunity and miscarriage.Eur J Endocrinol. 2004;150: 751-5
153 Krassas GE Perros P Kaprara A Thyroid autoimmunity, infertility and miscarriage Expert Rev. Endocrinol Metab 2008 3 127-36
154 Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009;160:985-91
155 Hirsch D, Levy S, Nadler V, Kopel V, Shainberg B, Toledano Y Pregnancy outcomes in women with severe hypothyroidism. Eur J Endocrinol. 2013 29;169: 313-20.
156 De Vivo A, Mancuso A, Giacobbe A, Moleti M, Maggio Savasta L, De Dominici R, Priolo AM, Vermiglio F. Thyroid function in women found to have early pregnancy loss. Thyroid 2010; 20:633-37.
157 Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis.Clin Endocrinol . 2011;74:513-19
158 Abbassi-Ghanavati M, Casey BM, Spong CY, McIntire DD, Halvorson LM, Cunningham FG. . Pregnancy outcomes in women with thyroid peroxidase antibodies. Obstet Gynecol. 2010; 116 381-86.
159 Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011;9: 342:d2616. doi: 10.1136 /bmj.d2616
160 van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17:605-19. doi: 10.1093/humupd/dmr024.
161 Yan J, Sripada S, Saravelos SH, Chen ZJ, Egner W, Li TC Thyroid peroxidase antibody in women with unexplained recurrent miscarriage: prevalence, prognostic value, and response to empirical thyroxine therapy Fertil Steril. 2012 ; 98:378-82.
162 Vissenberg R, van den Boogaard E, van Wely M, van der Post JA, Fliers E, Bisschop PH, Goddijn M. Treatment of thyroid disorders before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2012;18: 360-73.
163 Roberts J, Jenkins C, Wilson R, Pearson C, Franklin IA, MacLean MA, McKillop JH, Walker JJ.: Recurrent miscarriage is associated with increased numbers of CD5/20 positive lymphocytes and an increased incidence of thyroid antibodies. Eur J Endocrinol 1996;134:84-6
164 Matalon ST, Blank M, Levy Y, Carp HJ, Arad A, Burek L, Grunebaum E, Sherer Y, Ornoy A, Refetoff S, Weiss RE, Rose NR, Shoenfeld Y. The pathogenic role of anti-thyroglobulin antibody on pregnancy: evidence from an active immunization model in mice. Hum Reprod 2003;18:1094-9
165 Moravej A, Jeddi-Tehrani M, Salek-Moghaddam AR, Dokouhaki P, Ghods R, Rabbani H, Kazemi-Sefat GE, Shahbazi M, Zarnani AH..Evaluation of thyroglobulin expression in murine reproductive organs during pregnancy.Am J Reprod Immunol. 2010; 1;64:97-103. doi: 10.1111/j.1600-0897.2010.00827.x
166 Ticconi C, Giuliani E, Veglia M, Pietropolli A, Piccione E, Di Simone N. Thyroid autoimmunity and recurrent miscarriage.Am J Reprod Immunol. 2011;66: 452-9.
167 Bagis T, Gokcel A, Saygili ES: Autoimmune thyroid disease in pregnancy and the postpartum period: relationship to spontaneous abortion. Thyroid 2001; 11:1049-53
168 Velkeniers B, Van Meerhaeghe A, Poppe K, Unuane D, Tournaye H, Haentjens P. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: systematic review and meta-analysis of RCTs. Hum Reprod Update. 2013;19:251-8. doi: 10.1093/humupd/dms052
169 Macklon NS, New Approaches to Ovarian Stimulation, In: John Lazarus Valdis Pirags Sigrid Butz, Eds. The Thyroid and Reproduction, Stuttgart: Georg Thieme Verlag, 2009:15-26
170 Toulis KA, Goulis DG, Venetis CA, Kolibianakis EM, Negro R, Tarlatzis BC, Papadimas I. Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. Eur J Endocrinol. 2010 ;162:643-52. doi: 10.1530/EJE-09-0850.
171 Muller AF, Verhoeff A, Mantel MJ, Berghout A. Thyroid autoimmunity and abortion: a prospective study in women undergoing in vitro fertilization. Fertil Steril.
1999;71: 30-4
172 Negro R, Mangieri T, Coppola L Presicce G, Casavola EC, Gismondi R, Locorotondo G, Caroli P, Pezzarossa A, Dazzi D, Hassan H.Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod. 2005; 20:1529-33
173 Shoenfeld Y, Carp HJ, Molina V, Blank M, Cervera R, Balasch J, Tincani A, Faden D, Lojacono A, Doria A, Konova E, Meroni PL. Autoantibodies and prediction of reproductive failure. Am J Reprod Immunol. 2006;56: 337-44.
174 Kilic S, Tasdemir N, Yilmaz N, Yuksel B, Gul A, Batioglu S. The effect of anti-thyroid antibodies on endometrial volume, embryo grade and IVF outcome.Gynecol Endocrinol. 2008 ;24:649-55
175 Zhong YP, Ying Y, Wu HT, Zhou CQ, Xu YW, Wang Q, Li J, Shen XT, Li J.
Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int J Med Sci. 2012;9:121-5.
176 Negro R, Formoso G, Coppola L Presicce G, Mangieri T, Pezzarossa A, Dazzi D. Euthyroid women with autoimmune disease undergoing assisted reproduction technologies: the role of autoimmunity and thyroid function.J Endocrinol Invest. 2007 ;30:3-8.
177 Tan S, Dieterle S, Pechlavanis S, Janssen OE, Fuhrer D. Thyroid autoantibodies per se do not impair intracytoplasmic sperm injection outcome in euthyroid healthy women. Eur J Endocrinol. 2014; 8;170:495-500. doi: 10.1530/EJE-13-0790.
178 Łukaszuk K, Kunicki M, Kulwikowska P, Liss J, Pastuszek E, Jaszczołt M, Męczekalski B, Skowroński K. The impact of the presence of antithyroid antibodies on pregnancy outcome following intracytoplasmatic sperm injection-ICSI and embryo transfer in women with normal thyreotropine levels.J Endocrinol Invest. 2015 ;38:1335-43
179 Unuane D, Velkeniers B, Deridder S, Bravenboer B, Tournaye H, De Brucker M. Impact of thyroid autoimmunity on cumulative delivery rates in in vitro fertilization/intracytoplasmic sperm injection patients.Fertil Steril. 2016;106:144-50. doi: 10.1016/j
180 Kiprov DD, Nachtigall RD, Weaver RC, Jacobson A, Main EK, Garovoy MR. The use of intravenous immunoglobulin in recurrent pregnancy loss associated with combined alloimmune and autoimmune abnormalities. Am J Reprod Immunol 1996; 36:228-34,
- 181.
Sher G, Maassarani G, Zouves C, Feinman M, Sohn S, Matzner W, Chong P, Ching W. The use of combined heparin/aspirin and immunoglobulin G therapy in the treatment of in vitro fertilization patients with antithyroid antibodies. Am J Reprod Immunol 1998; 39:223-5
- 182.
Stricker RB, Steinleitner A, Bookoff CN, Weckstein LN, Winger EE. Successful treatment for immunological abortion with low-dose intravenous immunoglobulin. Fertil Steril 2000;73:536-40,
183 Revelli A, Casano S, Piane LD et al. A retrospective study on IVF outcome in euthyroid patients with anti-thyroid antibodies: effects of levothyroxine, acetyl-salicylic acid and prednisolone adjuvant treatments. Reprod Biol Endocrinol. 2009; 27:7:137. doi:10.1186/1477-7827-7-137
184 Kim CH, Ahn JW, Kang SP, Kim SH, Chae HD, Kang BM Effect of levothyroxine treatment on in vitro fertilization and pregnancy outcome in infertile women with subclinical hypothyroidism undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2011;95:1650-4.
185 Busnelli A, Vannucchi G, Paffoni A, Faulisi S, Fugazzola L, Fedele L, Somigliana E Levothyroxine dose adjustment in hypothyroid women achieving pregnancy through IVF.
Eur J Endocrinol. 2015;173:417-24. doi: 10.1530/EJE-15-0151.
186 Arojoki M, Jokimaa V, Juuti A, Juuti A, Koskinen P, Irjala K, Anttila L. Hypothyroidism among infertile women in Finland. Gynecol Endocrinol 14:127-31, 2000
187 Alnot-Burette J, Nakib I, Lipere A, Delemer B, Graesslin O. Thyroid function for infertile women during ovarian hyperstimulation as part of IVF. Gynecol Obstet Fertil. 2016 ;44:156-62. doi: 10.1016/j.gyobfe.2016.01.007.
188 Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;5: 371:75-84. doi: 10.2217/17455057.4.6.625
189 Stagnaro-Green A, Chen X, Bogden JD, Davies TF, Scholl TO. The thyroid and pregnancy: a novel risk factor for very preterm delivery. Thyroid. 2005;15: 351-57
190 Stagnaro-Green A. Maternal thyroid disease and preterm delivery. J Clin Endocrinol Metab. 2009; 94: 21-25.
191 Tierney K, Delpachitra P, Grossmann M Onwude J, Sikaris K, Wallace EM, Hamilton EJ, Tong S . Thyroid function and autoantibody status among women who spontaneously deliver under 35 weeks of gestation.Clin Endocrinol. 2009 ; 71: 892-95.
192 Haddow JE, Cleary-Goldman J, McClain MR. Palomaki GE, Neveux LM, Lambert-Messerlian G, Canick JA, Malone FD, Porter TF, Nyberg DA, Bernstein PS, D'Alton ME. First- and Second-Trimester Risk of Aneuploidy (FaSTER) Research Consortium. Thyroperoxidase and thyroglobulin antibodies in early pregnancy and preterm delivery. Obstet Gynecol. 2010 ;116:58-62.
193.Lata K, Dutta P, Sridhar S, Rohilla M, Srinivasan A, Prashad GR, Shah VN, Bhansali A. Thyroid autoimmunity and obstetric outcomes in women with recurrent miscarriage: a case-control study Endocr Connect. 2013; 22;2:118-24. doi: 10.1530/EC-13-0012.
194 .Bliddal S, Boas M, Hilsted L, Friis-Hansen L, Tabor A, Feldt-Rasmussen Thyroid function and autoimmunity in Danish pregnant women after an iodine fortification program and associations with obstetric outcomes U. Eur J Endocrinol. 2015;173:709-18.
195 Negro R, Schwartz A, Gismondi R,Tinelli A, Mangieri T, Stagnaro-Green A. Thyroid antibody positivity in the first trimester of pregnancy is associated with negative pregnancy outcomes. J Clin Endocrinol Metab. 2011;96:E920-24
196 He X, Wang P, Wang Z, He X, Xu D, Wang B.
Thyroid antibodies and risk of preterm delivery: a meta-analysis of prospective cohort studies. Eur J Endocrinol. 2012;167:455-64.
197 Karakosta P, Alegakis D, Georgiou V, Roumeliotaki T, Fthenou E, Vassilaki M, Boumpas D, Castanas E, Kogevinas M, Chatzi L.Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab. 2012;97:4464-72. doi: 10.1210/jc.2012-2540
198 Saki F, Dabbaghmanesh MH, Ghaemi SZ, Forouhari S, Omrani GR, Bakhshayeshkaram M. Thyroid autoimmunity in pregnancy and its influences on maternal and fetal outcome in Iran (a prospective study).Endocr Res. 2015;40:139-45. Doi:10.3109/07435800.2014.966384
199 Kumru P, Erdogdu E, Arisoy R, Demirci O, Ozkoral A, Ardic C, Ertekin AA, Erdogan S, Ozdemir NN. Effect of thyroid dysfunction and autoimmunity on pregnancy outcomes in low risk population.Arch Gynecol Obstet. 2015;291:1047-54. doi: 10.1007/s00404-014-3533-9.
200 Oztas E, Erkenekli K, Ozler S, Aktas A, Buyukkagnıcı U, Uygur D, Danisman N. First trimester interleukin-6 levels help to predict adverse pregnancy outcomes in both thyroid autoantibody positive and negative patients. J Obstet Gynaecol Res. 2015; 41:1700-7. doi: 10.1111/jog.12799.
201 Stagnaro-Green A, Akhter E, Yim C, Davies TF, Magder LS, Petri M. Thyroid disease in pregnant women with systemic lupus erythematosus: increased preterm delivery. Lupus 2011; 20:690-99
202 Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, Hofman A, Ross HA, Hooijkaas H, Tiemeier H, Bongers-Schokking JJ, Jaddoe VW, Visser TJ, Steegers EA, Medici M, Peeters RP.Hypothyroxinemia and TPO-Antibody Positivity Are Risk Factors for Premature Delivery: The Generation R Study. J Clin Endocrinol Metab. 2013;98:4382-90.
203 Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H.Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroiddisease: effects on obstetrical complications. J Clin Endocrinol Metab 2006;91:2587-91.
204 Reid SM, Middleton P, Cossich MC, Crowther CA, Bain E. Interventions for clinical and subclinical hypothyroidism pre-pregnancy and during pregnancy..Cochrane Database Syst Rev. 2013; 31;:CD007752. doi: 10.1002/14651858.CD007752.pub3.
- 205.
Klein RZ, Haddow JE, Faix JD, Brown RS, Hermos RJ, Pulkkinen A. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol 35:41-6 1991
206 Teng W, Shan Z, Patil-Sisodia K, Cooper DS. Hypothyroidism in pregnancy.Lancet Diabetes Endocrinol. 2013 ;1:228-37. doi: 10.1016/S2213-8587(13)70109-8
207 Jaiswal N, Melse-Boonstra A, Thomas T, Basavaraj C, Sharma SK, Srinivasan K, Zimmermann MB. High prevalence of maternal hypothyroidism despite adequate iodine status in Indian pregnant women in the first trimester..Thyroid. 2014;24:1419-29. doi: 10.1089/thy.2014.0071.
208 Mandel SJ. Hypothyroidism and chronic autoimmune thyroiditis in the pregnant state: maternal aspects. Best Pract Res Clin Endocrinol Metab. 2004;18: 213-24.
209 Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev 26:599-614 2005
210 . Gallas PR, Stolk RP, Bakker K, Endert E, Wiersinga WM. Thyroid dysfunction during pregnancy and in the first postpartum year in women with diabetes mellitus type 1. Eur J Endocrinol 2002; 147:443-51
211 Glinoer D: Thyroidal and immune adaptation to pregnancy: focus on maternal hypo- and hyperthyroidism. In: Pirags V, Lazarus J, Butz S (Eds) The Thyroid and Autoimmunity. Georg Thieme Verlag; Stuttgart-New 46-58, 2008
- 212.
Krassas GE: Thyroid disease and female reproduction. Fertil Steril 2000; 74:1063-70,
213 Saglam F, Onal ED, Ersoy R, Koca C, Ergin M, Erel O, Cakir B.Anti-Müllerian hormone as a marker of premature ovarian aging in autoimmune thyroid disease. Gynecol Endocrinol. 2015;31:165-8. doi: 10.3109/09513590.2014.973391
214 Kuroda K, Uchida T, Nagai S, Ozaki R, Yamaguchi T, Sato Y, Brosens JJ, Takeda S Elevated serum thyroid-stimulating hormone is associated with decreased anti-Müllerian hormone in infertile women of reproductive age. J Assist Reprod Genet. 2015;32:243-7. doi: 10.1007/s10815-014-0397-7.
215 Magri F, Schena L, Capelli V, Gaiti M, Zerbini F, Brambilla E, Rotondi M, De Amici M, Spinillo A, Nappi RE, Chiovato L. Anti-Mullerian hormone as a predictor of ovarian reserve in ART protocols: the hidden role of thyroid autoimmunity Reprod Biol Endocrinol. 2015 21;13:106. doi: 10.1186/s12958-015-0103-3.
.216 Polyzos NP, Sakkas E, Vaiarelli A, Poppe K, Camus M, Tournaye H. Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific AMH values. Hum Reprod. 2015;30:1690-6. doi: 10.1093/humrep/dev089.
217 Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid 2002;12:63-8
218 Männistö T, Mendola P, Grewal J, Xie Y, Chen Z, Laughon SK Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J Clin Endocrinol Metab. 2013 ;98:2725-33.
219Poulasouchidou MK, Goulis DG, Poulakos P, Mintziori G, Athanasiadis A, Grimbizis G, Tarlatzis BC Prediction of maternal and neonatal adverse outcomes in pregnant women treated for hypothyroidism. Hormones (Athens). 2012;11:468-76.
220 Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death.Thyroid. 2010;20: 989-93
221 Ashoor G, Maiz N, Rotas M, Kametas NA, Nicolaides KH Maternal thyroid function at 11 to 13 weeks of gestation and subsequent development of preeclampsia .Prenat Diagn. 2010 ;30:1032-8.
222 Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11-13 weeks of gestation and spontaneous preterm delivery. Obstet Gynecol. 2011;117:293-8.
223 Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ. Subclinical hypothyroidism and pregnancy outcome. Obstet Gynecol 2005; 105:239-45
224 Medici M, Timmermans S, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VW, Hofman A, Hooijkaas H, de Rijke YB, Tiemeier H, Bongers-Schokking JJ, Visser TJ, Peeters RP, Steegers EA. Maternal thyroid hormone parameters during early pregnancy and birth weight: the Generation R Study. J Clin Endocrinol Metab. 2013;98:59-66.
225 Haddow JE, Craig WY, Palomaki GE, Neveux LM, Lambert-Messerlian G, Canick JA, Malone FD, D'Alton ME; First And Second Trimester Risk Of Aneuploidy Faster Research Consortium. Impact of adjusting for the reciprocal relationship between maternal weight and free thyroxine during early pregnancy. Thyroid. 2013 ;23: 225-30.
226 Negro R. Thyroid insufficiency during pregnancy: complications and implications for screening Expert Rev. Endocrinol. Metab. 2008: 32; 1-10
227 Furnica RM, Lazarus JH, Gruson D, Daumerie C. Update on a new controversy in endocrinology: isolated maternal hypothyroxinemia. J Endocrinol Invest. 2015 ;38:117-23. doi: 10.1007/s40618-014-0203-5.
228 Casey BM, Dashe JS, Spong CY, McIntire DD, Leveno KJ, Cunningham GF.Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol 2007;109:1129-35
229 Hamm MP, Cherry NM, Martin JW, Bamforth F, Burstyn I. The impact of isolated maternal hypothyroxinemia on perinatal morbidity. J Obstet Gynaecol Can. 2009 ; 31:1015-21.
230 Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, Luthy D, Gross S, Bianchi DW, D'Alton ME. Maternal thyroid hypofunction and pregnancy outcome.Obstet Gynecol. 2008 ;112:85-92. doi: 10.1097/AOG.0b013e3181788dd7
231 Pop VJ, Brouwers EP, Wijnen H, Oei G, Essed GG, Vader HL. Low concentrations of maternal thyroxin during early gestation: a risk factor of breech presentation? BJOG. 2004 ;111:925-30
232 Mil NH, Steegers-Theunissen RP, Bongers-Schokking JJ, El Marroun H, Ghassabian A, Hofman A, Jaddoe VW, Visser TJ, Verhulst FC, de Rijke YB, Steegers EA, Tiemeier H.Maternal hypothyroxinemia during pregnancy and growth of the fetal and infant head.van Reprod Sci. 2012;19:1315-22. doi: 10.1177/1933719112450338
233 Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, Hofman A, Ross HA, Hooijkaas H, Tiemeier H, Bongers-Schokking JJ, Jaddoe VW, Visser TJ, Steegers EA, Medici M, Peeters RP. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab. 2013;98:4382-90. doi: 10.1210/jc.2013-2855.
234 Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, Xu YH, Tao FB.Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;;96:3234-41. doi: 10.1210/jc.2011-0274
235 Maraka S, Singh Ospina NM, O'Keeffe DT, Rodriguez-Gutierrez R, Espinosa De Ycaza AE, Wi CI, Juhn YJ, Coddington CC 3rd, Montori VM, Stan MN. Effects of Levothyroxine Therapy on Pregnancy Outcomes in Women with Subclinical Hypothyroidism.Thyroid. 2016 May 16. [Epub ahead of print
236 Maraka S, Ospina NM, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, Coddington CC 3rd, Stan MN, Murad MH, Montori VM.Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid. 2016;26:580-90. doi: 10.1089/thy.2015.0418.
237 Juch H, Lupattelli A, Ystrom E, Verheyen S, Nordeng H Medication adherence among pregnant women with hypothyroidism-missed opportunities to improve reproductive health? A cross-sectional, web-based study. Patient Educ Couns. 2016 11. pii: S0738-3991(16)30158-6. doi: 10.1016/j.pec.2016.04.006.
238 Zhang Y, Fan Y, Yu X, Wang X, Bao S, Li J, Fan C, Shan Z, Teng W. Maternal Subclinical Hypothyroidism Impairs Neurodevelopment in Rat Offspring by Inhibiting the CREB Signaling Pathway.Mol Neurobiol. 2015 ;52:432-41. doi: 10.1007/s12035-014-8855-x.
239 Min H, Dong J, Wang Y, Wang Y, Yu Y, Shan Z, Xi Q, Teng W, Chen J. Marginal Iodine Deficiency Affects Dendritic Spine Development by Disturbing the Function of Rac1 Signaling Pathway on Cytoskeleton. Mol Neurobiol. 2016 Jan 7
240 Hu X Wang R, Shan Z, Dong Y, Zheng H, Jesse FF, Rao E, Takahashi E, Li W1, Teng W, Teng X. Perinatal Iron Deficiency-Induced Hypothyroxinemia Impairs Early Brain Development Regardless of Normal Iron Levels in the Neonatal Brain.Thyroid. 2016 May 27. [Epub ahead of print]
241 Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development.Neuroscience. 2015 Oct 3. pii: S0306-4522(15)00897-0. doi: 10.1016/j.neuroscience.2015.09.070
242 Willoughby KA, McAndrews MP, Rovet JF. Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid. 2014 ;24:576-84. doi: 10.1089/thy.2013.0215.
243 Gilbert ME, Sanchez-Huerta K, Wood C. Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology. 2016 ;157:774-87. doi: 10.1210/en.2015-1643
244 Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone.J Neuroendocrinol. 2008 ;20: 784-94.
245 Morreale de Escobar G, Obregon MJ, Escobar del Rey F: Maternal thyroid hormones early in pregnancy and fetal brain development. In: Best Practice & Research in Clinical Endocrinology and Metabolism: The Thyroid and Pregnancy (Editor: Glinoer D) 18:225, 2004
246 Bernal J.Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3: 249-59.
247 Rovet JF The role of thyroid hormones for brain development and cognitive function..Endocr Dev. 2014;26:26-43. doi: 10.1159/000363153
248 Schroeder AC, Privalsky ML. Thyroid hormones, t3 and t4, in the brain. Front Endocrinol (Lausanne). 2014 Mar 31;5:40. doi: 10.3389/fendo.2014.00040.
249 Bernal J, Guadaño-Ferraz A, Morte B. Thyroid hormone transporters-functions and clinical implications. Nat Rev Endocrinol. 2015;11:406-17.
250 Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet. 2005 ;77:41-53.
251 Wirth EK, Schweizer U, Köhrle J.Transport of thyroid hormone in brain.Front Endocrinol (Lausanne). 2014; 24;5:98. doi: 10.3389/fendo.2014.00098.
252 Man EB, Jones WS, Holden RH, Mellits ED: Thyroid function in human pregnancy. VIII. Retardation of progeny aged 7 years; relationships to maternal age and maternal thyroid function. Amer J Obstet Gynecol 1971;111:905-16
253 Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ.Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med.1999 19;341:549-55.
254 Smit BJ, Kok JH, Vulsma T, Briët JM, Boer K, Wiersinga WM.
Neurologic development of the newborn and young child in relation to maternal thyroid function.Acta Paediatr. 2000;89:291-5
255 Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, Teng X, Guo R, Wang H, Li J, Chen Y, Wang W, Chawinga M, Zhang L, Yang L, Zhao Y, Hua T. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months.Clin Endocrinol. 2010;72:825-9 doi: 10.1111/j.1365-2265.2009.03743.x
256 Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy.Clin Endocrinol (Oxf). 1999;50:149-55.
257 Pop VJ, Brouwers EP, Vader H, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf). 2003;59:282-8.
258 Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, de Muinck Keizer-Schrama SM, Hofman A, Jaddoe VV, Visser W, Steegers EA, Verhulst FC, de Rijke YB, Tiemeier H. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. 2010 ;95:4227-34.
259 Suárez-Rodríguez M, Azcona-San Julián C, Alzina de Aguilar V.
Hypothyroxinemia during pregnancy: the effect on neurodevelopment in the child.
Int J Dev Neurosci. 2012 ;30:435-8.
260 Finken MJ, van Eijsden M, Loomans EM, Vrijkotte TG, Rotteveel J.
J Clin Endocrinol Metab. 2013 ;98:1417-26.
261 Julvez J, Alvarez-Pedrerol M, Rebagliato M, Murcia M, Forns J, Garcia-Esteban R, Lertxundi N, Espada M, Tardón A, Riaño Galán I, Sunyer J. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology. 2013 ;24:150-7.
262 Vermiglio F, Lo Presti VP, Moleti M, Sidoti M, Tortorella G,Scaffidi G, Castagna MG, Mattina F, Violi MA, Crisà A, ArtemisiaA, Trimarchi F.Attention deficit and hyperactivity disorders in theoffspring of mothers exposed to mild-moderate iodine deficiency:a possible novel iodine deficiency disorder in developed countries.J Clin Endocrinol Metab. 2004;89:6054-60.
- 263.
Modesto T, Tiemeier H, Peeters RP, Jaddoe VW, HofmanA, Verhulst FC, Ghassabian A. Maternal Mild Thyroid HormoneInsufficiency in Early Pregnancy and Attention-Deficit/
Hyperactivity Disorder Symptoms in Children. JAMA Pediatr.2015;169:838-45.
264 Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW,Hofman A, de Rijke YB, Verhulst FC, Tiemeier H. Association of gestational maternal hypothyroxinemia and increased autism risk.Ann Neurol. 2013;74:733-42.
265 Gyllenberg D, Sourander A, Surcel HM, Hinkka-Yli-SalomäkiS, McKeague IW, Brown AS. Hypothyroxinemia During Gestation and Offspring Schizophrenia in a National Birth Cohort. BiolPsychiatry. 2015 19. pii: S0006-3223(15)00520-X.
266 Ghassabian A, Bongers-Schokking JJ, de Rijke YB, van Mil N, Jaddoe VW, de Muinck Keizer-Schrama SM, Hooijkaas H, Hofman A, Visser W, Roman GC, Visser TJ, Verhulst FC, Tiemeier H. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study.Thyroid. 2012; 22:178-86
267 Pop VJ, de Vries E, van Baar AL, Waelkens JJ, de Rooy HA, Horsten M, Donkers MM, Komproe IH, van Son MM,Vader HL. Maternal thyroid peroxidase antibodies during pregnancy: a marker of impaired child development? J Clin Endocrinol Metab. 1995; 80:3561-6.
268 Wasserman EE, Pillion JP, Duggan A, Nelson K, Rohde C, Seaberg EC, Talor MV, Yolken RH, Rose NR.Childhood IQ, hearing loss, and maternal thyroid autoimmunity in the Baltimore Collaborative Perinatal Project. Pediatr Res. 2012 ;72:525-30.
269 Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy.Pediatrics. 2006;117:161-7
270 Mirabella G, Westall CA, Asztalos E, Perlman K, Koren G, Rovet J. Development of contrast sensitivity in infants with prenatal and neonatal thyroid hormone insufficiencies.Pediatr Res. 2005 ;57:902-7.
271 Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VW, Visser TJ, Visser W, de Muinck Keizer-Schrama SM, Hooijkaas H, Steegers EA, Hofman A, Verhulst FC, van der Ende J, de Rijke YB, Tiemeier H. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study.Pediatr Res. 2011;69:454-9.
272 Momotani N, Iwama S, Momotani K. Neurodevelopment in children born to hypothyroid mothers restored to normal thyroxine (T₄) concentration by late pregnancy in Japan: no apparent influence of maternal T₄ deficiency. J Clin Endocrinol Metab. 2012;97:1104-8.
273 Downing S, Halpern L, Carswell J, Brown RS Severe maternal hypothyroidism corrected prior to the third trimester is associated with normal cognitive outcome in the offspring. Thyroid. 2012 ;22:625-30.
274 Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F Subclinical hypothyroidism in pregnancy: intellectual development of offspring Thyroid. 2011;2:1143-7.
275 Craig WY, Allan WC, Kloza EM, Pulkkinen AJ, Waisbren S, Spratt DI, Palomaki GE, Neveux LM, Haddow JEMid-gestational maternal free thyroxine concentration and offspring neurocognitive development at age two years. J Clin Endocrinol Metab. 2012 ;97:E22-8.
276 Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, Chiusano E, John R, Guaraldo V, George LM, Perona M, Dall'Amico D, Parkes AB, Joomun M, Wald NJ. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012 9;366:493-501
277 Brian Casey Effect of treatment of maternal subclinicalhypothyroidism or hypothyroxinemia on IQ in offspring American Journal of Obstetrics & Gynecology Supplement to JANUARY 2016 Abstr 2 S2
278 Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, Steegers EA, Visser TJ, White T, Tiemeier H, Peeters RP. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study.Lancet Diabetes Endocrinol. 2016 ;4:35-43. doi: 10.1016/S2213-8587(15)00327-7
279 Samadi A, Skocic J, Rovet JF. Children born to women treated for hypothyroidism during pregnancy show abnormal corpus callosum development.Thyroid. 2015;25:494-502. doi: 10.1089/thy.2014.0548
280 Lischinsky JE, Skocic J, Clairman H, Rovet J. Preliminary Findings Show Maternal Hypothyroidism May Contribute to Abnormal Cortical Morphology in Offspring. Front Endocrinol (Lausanne). 2016; 25;7:16. doi: 10.3389/fendo.2016.00016.
281 Williams FL, Watson J, Ogston SA, Visser TJ, Hume R, Willatts P. Maternal and umbilical cord levels of T4, FT4, TSH, TPOAb, and TgAb in term infants and neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab. 2013;98:829-38.
282 Williams F, Watson J, Ogston S, Hume R, Willatts P, Visser T; Scottish Preterm Thyroid Group. Mild maternal thyroid dysfunction at delivery of infants born ≤34 weeks and neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab. 2012;97: 1977-85
283 Mandel SJ, Larsen PR, Seely EW, Brent GA. Increased need for thyroxine during
pregnancy in women with primary hypothyroidism.N Engl J Med.1990;12: 323:91-6
284 Yassa L, Marqusee E, Fawcett R, Alexander EK. Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J Clin Endocrinol Metab. 2010;95: 3234-41
285 Loh JA, Wartofsky L, Jonklaas J, Burman KD. The magnitude of increased levothyroxine requirements in hypothyroid pregnant women depends upon the etiology of the hypothyroidism. Thyroid. 2009;19:269-75
286 Galofré JC, Haber RS, Mitchell AA, Pessah R, Davies TF. Increased postpartum thyroxine replacement in Hashimoto's thyroiditis. Thyroid. 2010; 20: 901-08.
287 . Arafah BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. New Engl J Med 2001; 344:1743-9,
288 Rotondi M, Precerutti S, Chiovato L: Maternal hypothyroidism during pregnancy: possible preventive strategies. Clin Endocrinol 2006; 64:599-601
289 Abalovich M, Vázquez A, Alcaraz G, Kitaigrodsky A, Szuman G, Calabrese C, Astarita G, Frydman M, Gutiérrez S. Adequate levothyroxine doses for the treatment of hypothyroidism newly discovered during pregnancyThyroid. 2013;23:1479-83.
290 Abalovich M, Alcaraz G, Kleiman-Rubinsztein Jet al. The relationship of preconception thyrotropin levels to requirements for increasing the levothyroxine dose during pregnancy in women with primary hypothyroidism. Thyroid. 2010;20: 1175-8.
291 McClain MR, Lambert-Messerlian G, Haddow JE, Palomaki GE, Canick JA, Cleary-Goldman J, Malone FD, Porter TF, Nyberg DA, Bernstein P, D'Alton ME. Sequential first- and second-trimester TSH, free thyroxine, and thyroid antibody measurements in women with known hypothyroidism: a FaSTER trial study. Am J Obstet Gynecol 2008; 199(2): el-6
292 Taylor PN, Minassian C, Rehman A, Iqbal A, Draman MS, Hamilton W, Dunlop D, Robinson A, Vaidya B, Lazarus JH, Thomas S, Dayan CM, Okosieme OE.TSH levels and risk of miscarriage in women on long-term levothyroxine: a community-based study.J Clin Endocrinol Metab. 2014;99: 3895-902. doi: 10.1210/jc.2014-1954
293 Andersen SL, Olsen J, Carlé A, Laurberg P. Hyperthyroidism incidence fluctuates widely in and around pregnancy and is at variance with some other autoimmune diseases: a Danish population-based study. J Clin Endocrinol Metab. 2015;100:1164-71. doi: 10.1210/jc.2014-3588.
294 Okosieme OE, Lazarus JH Important considerations in the management of Graves' disease in pregnant women. Expert Rev Clin Immunol. 2015;11:947-57. doi: 10.1586/1744666X.2015.1054375
295 Marx H, Amin P, Lazarus JH: Hyperthyroidism and pregnancy. Brit Med J 336:663-67, 2008
296 Mestman JH Hyperthyroidism in pregnancy.Curr Opin Endocrinol Diabetes Obes. 2012;19: 394-401
297 Haddow JE, Knight GJ, Palomaki GE, McClain MR, Pulkkinen AJ. The reference range and within-person variability of thyroid stimulating hormone during the first and second trimesters of pregnancy. J Med Screen 11:170-4 2004
298 Dorizzi RM, Ozzola G, Sommella C, Catania F, Lelli F, Migali E, Polverini G. An approach to establish reference intervals for thyrotropin in pregnancy using the ADVIA Centaur analyzer. Clin Lab. 2010; 56:417-25.
299 Barbesino G, Tomer Y. Clinical review: Clinical utility of TSH receptor antibodies.J Clin Endocrinol Metab. 2013; 98: 2247-55. doi: 10.1210/jc.2012-4309.
300 Lazarus JH Pre-conception counselling in Graves’ disease Eur Thyroid J 2012;1:24-9
301 Laurberg P, Wallin G, Tallstedt L, Abraham-Nordling M, Lundell G, Torring O: TSH receptor autoimmunity in Graves’ disease after therapy with antithyroid drugs, surgery, or radioiodine: a 5 year prospective randomized study. Eur J Endocrinol 2008; 158:69-75.
302 Lauberg P, Bournaud C, Karmmisholt J, Orgiazzi J: Management of Graves’ hyperthyroidism in pregnancy: focus on both maternal and foetal thyroid function and caution against surgical thyroidectomy in pregnancy. European Journal of Endocrinology 2009; 160:1-8.
303 Tagami T, Hagiwara H, Kimura T, Usui T, Shimatsu A, Naruse M. The incidence of gestational hyperthyroidism and postpartum thyroiditis in treated patients with Graves’ disease. Thyroid 2007; 17:767-72.
304 Sheffield JS, Cunningham FG: Thyrotoxicosis and heart failure that complicate pregnancy. Am J Obstet Gynecol 2004; 190:211-17
305 Chonh HW, See KC, Phua J: Thyroid storm with multiorgan failure. Thyroid 2010; 20:333-6.
306 Millar LK, Wing DA, Leung AS, et al: Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism Obstet Gynecol 1994; 84: 946-9
307 Kempers MJE, van Trotsenburg ASP, van Rijn RR, et al: Loss of integrity of thyroid morphology and function in children born to mothers with inadequately treated Graves’ disease. J Clin Endocrinol Metab 2007; 92:2984-91.
308 Smith C, Thomsett M, Choong C, Rodda C, McIntyre HD, Cotterill AM Congenital thyrotoxicosis in premature infants. Clin Endocrinol 2001; 54:371-6
309 Mitsuda N, Tamaki H, Amino N, Hosono T, Miyai K, Tanizawa O. Risk factors for developmental disorders in infants born to women with Graves' disease. Obstet Gynecol 1992; 80:359-64
310 Vaidya B, Campbell V, Tripp JH, Spyer G, Hattersley AT, Ellard S. Premature birth and low birth weight associated with non-autoimmune hyperthyroidism due to an activating thyrotropin receptor gene mutation. Clin Endocrinol 2004; 60:711-8
311 Polak M, Luton D. Fetal thyroïdology.Best Pract Res Clin Endocrinol Metab. 2014 ;28:161-73. doi: 10.1016/j.beem.2013.04.013.
312 Towers CV, Thomas S, Steiger RM: The fetal heart monitor tracing in pregnancies complicated by fetal thyrotoxicosis. Amer J Perinatal 2009; 26:373-7.
313 Luton D, Le Gac I, Vullard E, Castanet M, Guibourdenche J, Noel M, Toubert ME, Léger J, Boissinot C, Schlageter MH, Garel C, Tébeka B, Oury JF, Czernichow P, Polak M. Management of Graves’ disease during pregnancy: the key role of fetal thyroid gland monitoring. J Clin Endocrinol Metab 2005;90:6093-98.
314 Kilpatrick S: Umbilical cord sampling in women with thyroid disease in pregnancy: is it necessary? Am J Obstet Gynecol 2003:;189:1-2
315 Evans C, Gregory JW, Barton J, Bidder C, Gibbs J, Pryce R, Al-Muzaffar I, Ludgate M, Warner J, John R, Moat SJ Transient congenital hypothyroidism due to thyroid-stimulating hormone receptor blocking antibodies: a case series.Ann Clin Biochem. 2011;48:386-90. doi: 10.1258/acb.2011.011007
316 Calderwood C, Williams H, Campbell IW, Toft AD, Cameron A. Cordocentesis to predict fetal outcome after administration of radio-active iodine for Graves’ disease. J Obstet Gynaecol 2002; 22:217-8
317 Polak M, Le Gac I, Vuillard E, Guibourdenche J, Leger J, Toubert ME, Madec AM, Oury JF, Czernichow P, Luton D. Fetal and neonatal thyroid function in relation to maternal Graves' disease.Best Pract Res Clin Endocrinol Metab. 2004;18:289-302.
318 Luton D, Le Gac I, Noel M, Guibourdenche J, Polak M: Thyroid function during pregnancy in women with past Graves’ disease. Brit J Obstet Gynaecol 2005;112:1565-7
319 Cooper DS, Laurberg P. Hyperthyroidism in pregnancy.Lancet Diabetes Endocrinol. 2013 ;1:238-49. doi: 10.1016/S2213-8587(13)70086-X.
320 Chester J, Rotenstein D, Ringkananont U, Steuer G, Carlin B, Stewart L, Grasberger H, Refetoff S.t al.Congenital neonatal hyperthyroidism caused by germline mutations in the TSH receptor gene.J Pediatr Endocrinol Metab. 2008;21:479-86.
321 Nicholas AK, Jaleel S, Lyons G, Schoenmakers E, Dattani MT, Crowne E, Bernhard B, Kirk J, Roche EF, Chatterjee VK, Schoenmakers N. Molecular spectrum of TSHβ subunit gene defects in central hypothyroidism in the UK and Ireland. Clin Endocrinol (Oxf). 2016; 30. doi: 10.1111/cen.13149
322 . Kempers MJ, Van Trotsenburg AS, Van Rijn RR, Smets AM, Smit BJ, de Vijlder JJ, Vulsma T. Loss of integrity of thyroid morphology and function in children born to mothers with inadequately treated Graves’ disease. J Clin Endocrinol Metab 2007 92:2984-91,
323 Napier C, Pearce SH. Rethinking antithyroid drugs in pregnancy. Clin Endocrinol (Oxf). 2015 ;82:475-7. doi: 10.1111/cen.12577.
324 Wing DA, Mmiller LK, Koonings PP: A comparison of propylthiouracil versus methimazole in the treatment of hyperthyroidism in pregnancy. Am J Obstet Gynecol 1994; 170: 90-95
325 Momotani N, Noh J, Oyangi H, Ishikawa N, Ito K. Antithyroid during therapy for Graves’ disease during pregnancy: optimal regimen for fetal thyroid status. N Engl J Med 1986;315:24-8.
326 Y. Nakagawa, K. Mori, S. Hoshikawa, M. Yamamoto, S. Ito, K. Yoshida, Postpartum recurrence of Graves hyperthyroidism can be prevented by the continuation of antithyroid drugs during pregnancy. Clin Endocrinol (Oxf). 2002; 57: 467–71
327 M. Rotondi, C. Cappelli, B. Pirali, I. Pirola, F. Magri, R. Fonte, M. Castellano, E.A. Rosei, L. Chiovato, The effect of pregnancy on subsequent relapse from Graves’ disease after a successful course of antithyroid drug therapy. J. Clin. Endocrinol. Metab 2008; 93: 3985–88
328 Mandel SJ, Cooper DS: The use of antithyroid drugs in pregnancy and lactation. J Clin Endocrinol Metab 2001; 86:2354-9
329 Rivkees SA, Szarfman A: Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J Clin Endocrinol Metab 2010;95: 3260-67.
330 Akmal A, Kung J. Propylthiouracil, and methimazole, and carbimazole-related hepatotoxicity. Expert Opin DrugSaf.2014;13:1397-406. doi: 10.1517/14740338.2014.953796.
331 Kimura M, Seki T, Ozawa H, Ishihara T, Komatsu M, Tajiri S, Yanagi H, Nishina M, Noh JY, Fukagawa M, Takagi A.The onset of antineutrophil cytoplasmic antibody-associated vasculitis immediately after methimazole was switched to propylthiouracil in a woman with Graves' disease who wished to become pregnant.Endocr J. 2013;60:383-8.
332 Andersen SL, Olsen J, Laurberg P.Antithyroid Drug Side Effects in the Population and in Pregnancy. J Clin Endocrinol Metab. 2016;101:1606-14. doi: 10.1210/jc.2015-4274.
333 Therapy of endocrine disease: antithyroid drug use in early pregnancy and birth defects: time windows of relative safety and high risk?Laurberg P, Andersen SL.Eur J Endocrinol. 2014 ;171:R13-20. doi: 10.1530/EJE-14-0135
334 Li H, Zheng J, Luo J, Zeng R, Feng N, Zhu N, Feng Q Congenital anomalies in children exposed to antithyroid drugs in-utero: a meta-analysis of cohort studies. PLoS One. 2015 14;10(5):e0126610. doi: 10.1371/journal.pone.0126610
335 Laurberg P, Andersen SL. Antithyroid Drug Use in Pregnancy and Birth Defects: Why Some Studies Find Clear Associations, and Some Studies Report None.Thyroid. 2015 ;25:1185-90. doi: 10.1089/thy.2015.0182.
336 Mandel S, Brent GA, Larsen PR: Review of antithyroid drugs use during pregnancy and report of a case of aplasia cutis. Thyroid 1994; 4: 129-133.
337 Löllgen RM, Calza AM, Schwitzgebel VM, Pfister RE.Aplasia cutis congenita in surviving co-twin after propylthiouracil exposure in utero.J Pediatr Endocrinol Metab. 2011;24:215-8.
338 Barbero P, Valdez R, Rodriguez H, Tiscornia C, Mansilla E, Allons A, Coll S, Liascovich R. Choanal atresia associated with maternal hyperthyroidism treated with methimazole: A case control study. Am J Med Genet 2008;146A: 2390-95.
339 Rosenfeld H. Ornoy A, Shchtman S, Diav-Citrin O: Pregnancy outcome, thyroid dysfunction and fetal goiter after in utero exposure to PTU: a controlled cohort study. B J Clin Pharmacol 2008;68: 609-17.
340 Yoshihara A, Noh J, Yamaguchi T, Ohye H, Sato S, Sekiya K, Kosuga Y, Suzuki M, Matsumoto M, Kunii Y, Watanabe N, Mukasa K, Ito K, Ito K. Treatment of graves' disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malfor mation.J Clin Endocrinol Metab. 2012;97:2396-403.
341 Andersen SL, Olsen J, Wu CS, Laurberg P. Severity of birth defects after propylthiouracil exposure in early pregnancy Thyroid. 2014 ;24:1533-40. doi: 10.1089/thy.2014.0150
342 Li X, Liu GY, Ma JL, Zhou L. Risk of congenital anomalies associated with antithyroid treatment during pregnancy: a meta-analysis.Clinics (Sao Paulo). 2015;70:453-9. doi: 10.6061/clinics/2015(06)12
343 Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, Laurberg P, McDougall IR, Rivkees SA, Ross D, Sosa JA, Stan MN The role of propylthiouracil in the management of Graves' disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid 2009;19:673–74.
344 Mortimer RH, Cannell GR, Addison RS: Methimazole and propylthiouracil equally cross the perfused human term placenta lobule? J Clin Endocrinol Metab 1997;82: 3099-102.
345 Gianetti E, Russo L, Orlandi F, Chiovato L, Giusti M, Benvenga S, Moleti M, Vermiglio F, Macchia PE, Vitale M, Regalbuto C, Centanni M, Martino E, Vitti P, Tonacchera M. Pregnancy outcome in women treated with methimazole or propylthiouracil during pregnancy. J Endocrinol Invest. 2015;38:977-85. doi: 10.1007/s40618-015-0281-z
346 Redmond GP: Propranolol and fetal growth retardation. Semin Perinatol 1982; 6:142-7
347 Momotani N, Hisaoka T, Noh J, Ishikawa N, Ito K. Effects of iodine on thyroid status of fetus versus mother in treatment of Graves’ disease complicated by pregnancy. J Clin Endocrinol Metab 1992; 75:738-44
348 Zanzonico PB: Radiation dose to patients and relatives incident to 131-I therapy. Thyroid 1997; 7:199-204
- 349.
Berg GE, Nystrom EH, Jacobsson L Lindberg S, Lindstedt RG, Mattsson S, Niklasson CA, Norén AH, Westphal OG.Radioiodine treatment of hyperthyroidism in a pregnant woman. J Nucl Med 1998; 39:357-61
350 Abeillon-du Payrat J, Chikh K, Bossard N, Bretones P, Gaucherand P, Claris O, Charrié A, Raverot V, Orgiazzi J, Borson-Chazot F, Bournaud C.Predictive value of maternal second-generation thyroid-binding inhibitory immunoglobulin assay for neonatal autoimmune hyperthyroidism.Eur J Endocrinol. 2014;171:451-60. doi: 10.1530/EJE-14-0254
351 Karras S, Tzotzas T, Kaltsas T, Krassas GE: Pharmacological treatment of hyperthyroidism during lactation: review of the literature and novel data. Pediatr Endocrinol Rev 2010; 8: 25-33
352 Sun S, Qiu X, Zhou J. Clinical analysis of 65 cases of hyperemesis gravidarum with gestational transient thyrotoxicosis. J Obstet Gynaecol Res. 2014 ;40:1567-72. doi: 10.1111/jog.12372.
353 Tanaka S, Yamada H, Kato EH, Furuta I, Fukushi M, Takasugi N, Fujimoto S.Gestational transient hyperthyroxinaemia (GTH): screening for thyroid function in 23163 pregnant women using dried blood spots. Clin Endocrinol 1998; 49:325-9
354 Yeo CP, Khoo DH, Eng PH, Tan HK, Yo SL, Jacob E. Prevalence of gestational thyrotoxicosis in Asian women evaluated in the 8th to 14th weeks of pregnancy: correlations with total and free beta human chorionic gonadotrophin. Clin Endocrinol 2001; 55:391-8
355 Utiger RD: Some women with hyperemesis gravidarum have transient hyperthyroidism. Clinical Thyroidology 2002;14:56,
356 Grün JP, Meuris S, De Nayer P, Glinoer D: The thyrotropic role of human chorionic gonadotropin (hCG) in the early stages of twin (versus single) pregnancy. Clin Endocrinol 1997;46:719-25
357 Yoshikawa N, Nishikawa M, Horimoto M,et al. Thyroid-stimulating activity in sera of normal pregnant women.J Clin Endocrinol Metab. 1989 ;69:891-5
358 Talbot JA, Lambert A, Anobile CJ, McLoughlin JD, Price A, Weetman AP, Robertson WR. The nature of human chorionic gonadotrophin glycoforms in gestational thyrotoxicosis. Clin Endocrinol 2001; 55:33-9
359 Vassart G, Dumont JE: The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 1992;13: 596-611
360 Grjibovski AM, Vikanes A; Stoltenberg C Magnus P. Consanguinity and the risk of hyperemesis gravidarum in Norway: Acta Obstetr Gynecol Scan 2007; 87: 20-5
361.Zhang Y, Cantor RM, MacGibbon K, Romero R, Goodwin TM, Mullin PM, Fejzo MS.Familial aggression of hyperemesis gravidarum. Am J Obstet Gynecol 2011; 204:230.e1-230.e7.
362 Goodwin TM. Hyperemesis gravidarum. Obstet Gynecol Clin North Am. 2008;35:401-17, viii. doi: 10.1016/j.ogc.2008.04.002
363 Bober SA, McGill AC, Tunbridge WMG: Thyroid function in hyperemesis gravidarum. Acta Endocrinologica 1986; 111:404-10.
364 Kari Kumar KVS, Vamsikrishna P, Verma A, Muthukrishnan J, Meena U, Modi KD: Evaluation of thyrotoxicosis during pregnancy with color doppler sonography. International Journal of Gynecology and Obstetrics 2008; 102:152-5.
365 Karger S, Schötz S, Stumvoll M, Berger F, Führer D. Impact of pregnancy on prevalence of goitre and nodular thyroid disease in women living in a region of borderline sufficient iodine supply. Horm Metab Res. 2010;42:137-42
366 Kung AW, Chau MT, Lao TT, Tam SC, Low LC The effect of pregnancy on thyroid nodule formationJ Clin Endocrinol Metab. 2002;87:1010-114.
- 367.
Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: Results of linkage with California cancer registry. Am J Obstet Gynecol 2003;189:1128-35
- 368.
Hay I. Nodular thyroid disease diagnosed during pregnancy: How and when to treat. Thyroid 1999;9:667-70.
369 Pacini F, Schlumberger M, Dralle H Ilisea R, Smith Y, Wiersinga W. European consensus of the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006; 154:787-803
370 Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-1214.
371 Moosa M, Mazzaferri EL Outcome of differentiated thyroid cancer diagnosed in pregnant women. J Clin Endocrinol Metab1997; 82:2862-6
372 Yasmeen S, Cress R, Romano PS, Xing G, Berger-Chen S, Danielsen B, Smith LH Thyroid cancer in pregnancy. Int J Gynaecol Obstet 2005; 91:15-20
373 Vannucchi G, Perrino M, Rossi S, Colombo C, Vicentini L, Dazzi D, Beck-Peccoz P, Fugazzola L. Clinical and molecular features of differentiated thyroid cancer diagnosed during pregnancy.Eur J Endocrinol. 2010;162:145-51.
374 Lee ML, Chen GG, Vlantis AC, Tse GM, Leung BC, van Hasselt CA. Induction of thyroid papillary carcinoma cell proliferation by estrogen is associated with an altered expression of Bcl-xL. Cancer J. 2005;11:113-21.
375 Hishinuma A, Yamanaka T, Kasai K, So S, Tseng CC, Bamba N, Ohtake H, Shimoda S.Different growth control of the two human thyroid cell lines of adenomatous goiter and papillary carcinoma.Thyroid. 1995;5:41-6.
376 Messuti I, Corvisieri S, Bardesono F, Rapa I, Giorcelli J, Pellerito R, Volante M, Orlandi F.Impact of pregnancy on prognosis of differentiated thyroid cancer: clinical and molecular features.Eur J Endocrinol. 2014;10:170:659-66. doi: 10.1530/EJE-13-090311;doi:10.4061/2011/549609
377 Ito Y, Miyauchi A, Kudo T, Ota H, Yoshioka K, Oda H, Sasai H, Nakayama A, Yabuta T, Masuoka H, Fukushima M, Higashiyama T, Kihara M, Kobayashi K, Miya A. Effects of Pregnancy on Papillary Microcarcinomas of the Thyroid Re-Evaluated in the Entire Patient Series at Kuma Hospital. Thyroid. 2016 ;26:156-60. doi: 10.1089/thy.2015.0393
378 Zhou YQ, Zhou Z, Qian MF, Gong T, Wang JD. Association of thyroid carcinoma with pregnancy: A meta-analysis. Mol Clin Oncol. 2015 ;3:341-6
379 .Leboeuf R, Emerick LE, Martorella AJ, Tuttle RM Impact of pregnancy on serum thyroglobulin and detection of recurrent disease shortly after delivery in thyroid cancer survivors. Thyroid 2007;17:543-7
380 Hirsch D, Levy S, Tsvetov G, Weinstein R, Lifshitz A, Singer J, Shraga-Slutzky I, Grozinski-Glasberg S, Shimon I, Benbassat C.Impact of pregnancy on outcome and prognosis of survivors of papillary thyroid cancer. Thyroid 2010; 20:1179- 85. doi:10.1089/thy.2010.0081.
381 Lee JC, Zhao JT, Clifton-Bligh RJ, Gill AJ, Gundara JS, Ip J, Sywak MS, Delbridge LW, Robinson BG, Sidhu SB Papillary thyroid carcinoma in pregnancy: a variant of the disease? Ann Surg Oncol. 2012;19: 4210-6
382 Lin JD, Wang HS, Weng HF, Kao PF. Outcome of pregnancy after radioactive iodine treatment for well differentiated thyroid carcinomas. J Endoc Invest 1998;21 662-7.
383 Sawka AM, Lakra DC, Lea J Alshehri B, Tsang RW, Brierley JD, Straus S, Thabane L, Gafni A, Ezzat S, George SR, Goldstein DP. 2008 A systematic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female thyroid cancer survivors. Clin Endocrinol (Oxf) 2008:69:479-90
384 Garsi JP, Schlumberger M, Rubino C Ricard M, Labbé M, Ceccarelli C, Schvartz C, Henri-Amar M, Bardet S, de Vathaire F. Therapeutic administration of 131I for differentiated thyroid cancer: radiation dose to ovaries and outcome of pregnancies.J Nucl Med. 2008;49: 845-52.
385 Sioka C, Fotopoulos A: Effects of I-131 therapy on gonads and pregnancy outcome in patients with thyroid cancer. Fertility Sterility 2011; 95: 1552-9
386 Sawka AM, Lea J, Alshehis B, Straus S, Tsang RW, Brierley JD, Thabane L, Rotstein L, Gafni A, Ezzat S, Goldstein DP. A systemic review of the gonadal effects of therapeutic radioactive iodine in male thyroid cancer survivors. Clin Endocrinol 2008: 68:610-17
387 Cecarelli C, Bencivelli W, Morciano D, Pinchera A, Pacini F. I 131 therapy for differentiated thyroid cancer leads to an earlier onset of menopause: results of a retrospective study. J Clin Endocr Metab 2001; 86: 3512-15
388 Weetman AP.Immunity, thyroid function and pregnancy: molecular mechanisms.Nat Rev Endocrinol. 2010;6:311-8
389 Amino N, Tada H. Hidaka Y. Thyroid disease after pregnancy: postpartum thyroiditis. In: Wass JAH, Shalet SM (eds), Oxford textbook of Endocrinology and Diabetes. Oxford, UK: Oxford University press, 2002: 527-32
390 Tada H, Hidaka Y, Tsuruta E, Kashiwai T, Tamaki H, Iwatani Y, Amino N. Prevalence of postpartum onset of disease within patients with Graves’ disease of child-bearing age. Endocr J 1994; 41: 325-27
391 Rotondi M, Cappelli C, Pirali B,et al. The effect of pregnancy on subsequent relapse from Graves' disease after a successful course of antithyroid drug therapy Rotondi M, Cappelli C, Pirali B, Pirola I, Magri F, Fonte R, Castellano M, Rosei EA, Chiovato LJ Clin Endocrinol Metab. 2008;93:3985-8
392 Ide A, Amino N, Kang S, Yoshioka W, Kudo T, Nishihara E, Ito M, Nakamura H, Miyauchi A. Differentiation of postpartum Graves' thyrotoxicosis from postpartum destructive thyrotoxicosis using antithyrotropin receptor antibodies and thyroid blood flow.Thyroid. 2014 ;24:1027-31. doi: 10.1089/thy.2013.0585.
393 Gaberšček S, Osolnik J, Zaletel K, Pirnat E, Hojker S. An Advantageous Role of Spectral Doppler Sonography in the Evaluation of Thyroid Dysfunction During the Postpartum Period.
J Ultrasound Med. 2016 ;35: 1429-36. doi: 10.7863/ultra.15.07033
394 Lazarus JH, Ludgate ME. Prevention and treatment of postpartum Graves’ disease. In: Bailliere’s Clinical Endocrinology and Metabolism. New Aspects of Clinical Graves’ Disease. Bailliere Tindall, London. Vol 11(3): pp 549-560; 1997
395 Roberton HEW: Lassitude, coldness and hair changes following pregnancy, and their treatment with thyroid extract. BMJ ii: 2275, 1948
396 Stagnaro-Green A Postpartum thyroiditis..Best Pract Res Clin Endocrinol Metab. 2004 ;18:303-16
397 Lazarus JH, Postpartum Thyroid Disease in The Thyroid and Reproduction Eds John Lazarus Valdis Pirags Sigrid Butz. Georg Thieme Verlag, Stuttgart 2009 p 105-113
398 Landek-Salgado MA, Gutenberg A, Lupi I, Kimura H, Mariotti S, Rose NR, Caturegli P.Pregnancy, postpartum autoimmune thyroiditis, and autoimmune hypophysitis: intimate relationships.Autoimmun Rev. 2010 ;9:153-7.
399 Pearce EN.Thyroid disorders during pregnancy and postpartum.Best Pract Res Clin Obstet Gynaecol. 2015;29:700-6. doi: 10.1016/j.bpobgyn.2015.04.007
400 Muller AF, Drexhage HA, Berghout A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev. 2001;22:605-30.
401 Lazarus JH, Hall R, Othman S Parkes AB, Richards CJ, McCulloch B, Harris B. The clinical spectrum of postpartum thyroid disease. QJM 1996; 89:429-35.
402 Harris B, Fung H, Johns S Kologlu M, Bhatti R, McGregor AM, Richards CJ, Hall R. Transient post-partum thyroid dysfunction and postnatal depression. J Affect Disord. 1989; 17: 243-9.
403 Harris B, Othman S, Davies JA Weppner GJ, Richards CJ, Newcombe RG, Lazarus JH, Parkes AB, Hall R, Phillips DI. Association between postpartum thyroid dysfunction and thyroid antibodies and depression. BMJ. 1992; 305:152-6.
404 Pop VJ, de Rooy HA, Vader HL van der Heide D, van Son M, Komproe IH, Essed GG, de Geus CA. Postpartum thyroid dysfunction and depression in an unselected population. N Engl J Med. 1991; 324:1815–6.
405 Kent GN, Stuckey BG, Allen JR Lambert T, Gee V. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol (Oxf). 1999; 51: 429-38.
406 Groer MW, Vaughan JH Positive thyroid peroxidase antibody titer is associated with dysphoric moods during pregnancy and postpartum J Obstet Gynecol Neonatal Nurs. 2013 ;42:E 26-32.
407 Paragliola RM, Concolino P, De Rosa A, Mello E, Zuppi C, Pontecorvi A, Capoluongo E, Corsello SM The first case of association between postpartum thyroiditis and thyroid hormone resistance in an Italian patient showing a novel p.V283A THRB mutation.
Thyroid. 2013; 23: 506-10.
408 Premawardhana LDKE, Parkes AB, Ammari F, John R, Darke C, Adams H, Lazarus JH. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000; 85:71-5.
409 Stagnaro-Green A Roman SH, Cobin RH, el-Harazy E, Wallenstein S, Davies TF. A prospective study of lymphocyte-initiated immunosuppression in normal pregnancy: evidence of a T-cell etiology for postpartum thyroid dysfunction. J Clin Endocrinol Metab1992:;74: 645-53
410 Kokandi AA Parkes AB, Premawardhana LD, John R, Lazarus JH. Association of postpartum thyroid dysfunction with antepartum hormonal and immunological changes. J Clin Endocrinol Metab 2003; 88:1126-32
411 Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H.The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies.J Clin Endocrinol Metab. 2007;92:1263-8.
412 Wald N, Law M Medical Screening in Oxford Textbook of Medicine 2010 5th Edition Eds Warrell DA, Cox TM and Firth JD. Oxford University Press, Oxford, New York Vol 1 pp94 – 108
413 Casey BM, Dashe JS, Wells CE, McIntire DD, Leveno KJ, Cunningham FG. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol 2006; 107:337-41
414 Lazarus JH, Kaklamanou M. Significance of low thyroid-stimulating hormone in pregnancy. Curr Opin Endocrinol Diabetes Obes. 2007;14: 389-92
415 Lazarus JH. Iodine status in Europe in 2014. Eur Thyroid J. 2014; 3:3-6. doi: 10.1159/000358873.
416 Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics.2006; 117:161-7.
417 Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A.Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy.J Clin Endocrinol Metab. 2010; 95: 1699-707
418 Thung SF, Funai EF, Grobman WA. The cost-effectiveness of universal screening in pregnancy for subclinical hypothyroidism. Am J Obstet Gynecol. 2009; 200: 267.e1-7.
419 Dosiou C, Sanders GD, Araki SS, Crapo LM. Screening pregnant women for autoimmune thyroid disease: a cost-effectiveness analysis. Eur J Endocrinol. 2008; 158: 841-51
420 Granfors M, Åkerud H, Skogö J, Stridsberg M, Wikström AK, Sundström-Poromaa I. Targeted thyroid testing during pregnancy in clinical practice. Obstet Gynecol. 2014;124:10-5. doi: 10.1097/AOG.
421 Nazarpour S, Tehrani FR, Simbar M, Tohidi M, AlaviMajd H, Azizi F. Comparison of universal screening with targeted high-risk case finding for diagnosis of thyroid disorders.
Eur J Endocrinol. 2016 ;174:77-83. doi: 10.1530/EJE-15-0750.
422 Jouyandeh Z, Hasani-Ranjbar S, Qorbani M, Larijani B. Universal screening versus selective case-based screening for thyroid disorders in pregnancy.Endocrine. 2015;48:116-23. doi: 10.1007/s12020-014-0385-9.
423 Ahmed IZ, Eid YM, El Orabi H, Ibrahim HR. Comparison of universal and targeted screening for thyroid dysfunction in pregnant Egyptian women.Eur J Endocrinol. 2014 ;171: 285-91. doi: 10.1530/EJE-14-0100..
424 Yang H, Shao M, Chen L, Chen Q, Yu L, Cai L, Lin Z, Zhang C, Lu X. Screening strategies for thyroid disorders in the first and second trimester of pregnancy in China.
PLoS One. 2014 12;9:e99611. doi: 10.1371/journal.pone.0099611
425 Ma L, Qi H, Chai X, Jiang F, Mao S, Liu J, Zhang S, Lian X, Sun X, Wang D, Ren J, Yan Q. The effects of screening and intervention of subclinical hypothyroidism on pregnancy outcomes: a prospective multicenter single-blind, randomized, controlled study of thyroid function screening test during pregnancy. J Matern Fetal Neonatal Med. 2016; 29:1391-4. doi: 10.3109/14767058.2015.1049150.
426 Azizi F, Amouzegar A, Mehran L, Alamdari S, Subekti I, Vaidya B, Poppe K, San Luis T Jr, Akamizu T.Screening and management of hypothyroidism in pregnancy: results of an Asian survey.Endocr J. 2014; 61:697-704.
427 Casey B1, de Veciana M2. Thyroid screening in pregnancy.Am J Obstet Gynecol. 2014 ;211:351-3.e1. doi: 10.1016/j.ajog.2014.08.013.
428 Spencer L1, Bubner T, Bain E, Middleton P. Screening and subsequent management for thyroid dysfunction pre-pregnancy and during pregnancy for improving maternal and infant health. Cochrane Database Syst Rev. 2015 21;:CD011263. doi:
10.1002/14651858.CD011263.pub2.
429 Ong GS, Hadlow NC, Brown SJ, Lim EM, Walsh JP. Does the thyroid-stimulating hormone measured concurrently with first trimester biochemical screening tests predict adverse pregnancy outcomes occurring after 20 weeks gestation?J Clin Endocrinol Metab. 2014;99:E2668-72. doi: 10.1210/jc.2014-1918.
430 Taylor PN, Okosieme OE, Premawardhana L, Lazarus JH. Should all women be screened for thyroid dysfunction in pregnancy? Womens Health (Lond). 2015;11:295-307. doi: 10.2217/whe.15.7
431 Amouzegar A, Mehran L, Sarvghadi F, Delshad H, Azizi F, Lazarus JH.
Comparison of the American Thyroid Association with the Endocrine Society practice guidelines for the screening and treatment of hypothyroidism during pregnancy.
Hormones (Athens). 2014;13:307-13. doi: 10.14310/horm.2002.1486
432 Vaidya B, Anthony S, Bilous M et al. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding J Clin Endocrinol Metab. 2007;92:203-07.
433 Horacek J, Spitalnikova S, Dlabalova B, Malirova E, Vizda J, Svilias I, Cepkova J, Mc Grath C, Maly J. Universal screening detects two-times more thyroid disorders in early pregnancy than targeted high-risk case finding. Eur. J. Endocrinol. 2010; 163:645-65
434 Jiskra J, Bartáková J, Holinka Š, Límanová Z, Springer D, Antošová M, Telička Z, Potluková Low prevalence of clinically high-risk women and pathological thyroid ultrasound among pregnant women positive in universal screening for thyroid disorders. E.Exp Clin Endocrinol Diabetes. 2011;119:530-5.
435 .Wang W, Teng W, Shan Z, Wang S, Li J, Zhu L, Zhou J, Mao J, Yu X, Li J, Chen Y, Xue H, Fan C, Wang H, Zhang H, Li C, Zhou W, Gao B, Shang T, Zhou J, Ding B, Ma Y, Wu Y, Xu H, Liu W. The prevalence of thyroid disorders during early pregnancy in China: the benefits of universal screening in the first trimester of pregnancy Eur J Endocrinol. 2011;164: 263-8.
436 Haddow JE, McClain MR, Palomaki GE, Kloza EM, Williams J. Screening for thyroid disorders during pregnancy: results of a survey in Maine. American Journal of Obstetrics and Gynecology. 2006; 194: 471-4.
437 Vaidya B, Hubalewska-Dydejczyk A, Laurberg P, Negro R, Vermiglio F, Poppe K. Treatment and screening of hypothyroidism in pregnancy: results of a European survey. European Journal of Endocrinology. 2012 ;166: 49-54
438 Vila L, Velasco I, González S, Morales F, Sánchez E, Torrejón S, Soldevila B, Stagnaro-Green A, Puig-Domingo M.L Vila, Barcelona, Spain Controversies in Endocrinology: On the need of universal thyroid screening in pregnant women. Eur J Endocrinol. 2013; 29;170(:R17-30. doi: 10.1530/EJE-13-0561
439 Srimatkandada P, Stagnaro-Green A, Pearce EN. Attitudes of ATA survey responden.ts toward screening and treatment of hypothyroidism in pregnancy.Thyroid. 2015;25:368-9. doi: 10.1089/thy.2014.0322.
440 Reid SM, Middleton P, Cossich MC, Crowther CA, Bain E.
Interventions for clinical and subclinical hypothyroidism pre-pregnancy and during pregnancy. Cochrane Database Syst Rev. 2013 May 31;5:CD007752. doi: 10.1002/14651858.
441Taylor PN, Lacey A, Thayer D, Yusof M, Tabasum A, Muller I, Marsh L, Ludgate M Rees A, Boelaert K, Chan S, Nelson S, Rees A Lazarus JH, Dayan CM, Vaidya B, Okosieme O.Controlled Antenatal Thyroid Study: Obstetric Outcomes.British Thyroid Association 2016; Abstr. No 01 Newcastle-on-Tyne.
442 Laurberg P, Andersen SL, Pedersen IB, Andersen S, Carlé A.Screening for overt thyroid disease in early pregnancy may be preferable to searching for small aberrations in thyroid function tests.Clin Endocrinol (Oxf). 2013 ;79:297-304. doi: 10.1111/cen.12232
443 Pop VJ Pregnancy, postpartum and the thyroid: isn't it time to offer women optimal care?
Facts Views Vis Obgyn. 2014;6:166-70.
- ABSTRACT
- INTRODUCTION
- MATERNAL THYROID PHYSIOLOGY
- AUTOIMMUNE THYROID DISEASE AND PREGNANCY
- THYROID AUTOIMMUNITY AND DISORDERS OF FEMALE REPRODUCTION
- PRIMARY HYPOTHYROIDISM
- FETAL-NEONATAL CONSEQUENCES OF MATERNAL HYPOTHYROIDISM
- THYROTOXICOSIS
- NODULAR THYROID DISEASE
- POSTPARTUM THYROID DYSFUNCTION
- POSTPARTUM THYROIDITIS
- SCREENING FOR THYROID DISEASE IN PREGNANCY
- FINAL CONCLUSIONS
- REFERENCES
- 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum.[Thyroid. 2017]2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, et al. Thyroid. 2017 Mar; 27(3):315-389.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Thyroid disease in the perinatal period.[Aust Fam Physician. 2012]Thyroid disease in the perinatal period.Forehan S. Aust Fam Physician. 2012 Aug; 41(8):578-81.
- Review Iodine supplementation for women during the preconception, pregnancy and postpartum period.[Cochrane Database Syst Rev. 2017]Review Iodine supplementation for women during the preconception, pregnancy and postpartum period.Harding KB, Peña-Rosas JP, Webster AC, Yap CM, Payne BA, Ota E, De-Regil LM. Cochrane Database Syst Rev. 2017 Mar 5; 3(3):CD011761. Epub 2017 Mar 5.
- Review Thyroid disorders associated with pregnancy: etiology, diagnosis, and management.[Treat Endocrinol. 2005]Review Thyroid disorders associated with pregnancy: etiology, diagnosis, and management.Lazarus JH. Treat Endocrinol. 2005; 4(1):31-41.
- Thyroid Regulation and Dysfunction in the Pregnant Patient - EndotextThyroid Regulation and Dysfunction in the Pregnant Patient - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
