This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008-. doi: 10.3824/stembook.1.3.1
Controversy over whether pancreatic islet cells arise from adult stem or progenitor-like cells actually predates the discovery of insulin, and the recent use of islet transplantation to treat diabetes has only intensified interest in this question. Recent breakthroughs, particularly those based on Cre-loxP lineage-tracing in the mouse, have resolved some aspects of this controversy, but not all. We now know that insulin-producing β-cells and other islet cells derive from multipotent progenitors in the embryo, but that their maintenance and expansion in postnatal life is driven primarily by proliferation of existing differentiated cells. This appears to be true even during regeneration, and seems to apply to the exocrine acinar cells as well as islets. Following pancreatic duct ligation, however, islet precursors re-appear in the injured pancreas, arising from ducts and differentiating into new islet cells. Thus, while the pancreas does not normally rely on classical stem cells, a stem cell-like mechanism for new islet differentiation may be inducible under specific circumstances. Understanding the signals that promote β-cell formation in the embryo and adult should facilitate efforts to derive clinically-useful β-cells in vitro, either from adult ducts or embryonic stem cells.
Introduction
Type I diabetes is caused by the autoimmune destruction of pancreatic β-cells, and has emerged as a case study for stem cell-based “regenerative medicine.” Its selling point is the idea that the location of β-cells within the pancreas is irrelevant to their ability to regulate blood sugar through insulin release. Moved elsewhere, so long as they have access to the circulation, β-cells should function to maintain glucose homeostasis – a hypothesis amply supported by animal studies (Ballinger and Lacy, 1972), and now the basis for clinical islet transplantation in humans (Naftanel and Harlan, 2004).
Islet transplantation confronts formidable hurdles as a treatment for type I diabetes, such as blocking autoimmunity and preserving graft function. When these difficulties are overcome, however, the approach will still be hampered by the scarcity of cadaver-derived islets. Three potential solutions have been proposed: first, to enhance replication of islet cells in vitro, “stretching” the limited supply; second, to isolate adult stem cells from the pancreas that can expand and produce new β-cells; third, to manipulate embryonic stem (ES) cells so that they adopt a β-cell identity.
The first of these approaches, recently discussed elsewhere (Dhawan et al., 2007; Nir and Dor, 2005), is beyond the scope of this review, although we will discuss the contribution of β-cell proliferation to islet regeneration following injury. With respect to the second approach, we will consider the existence of stem cells in the adult pancreas, and discuss how they might be exploited clinically. Finally, we will discuss recent advances toward β-cell derivation from ES cells, and consider how this approach might benefit both basic and clinical researchers. As any of these strategies will depend on understanding normal pancreas development and homeostasis, it is here that we will begin our review.
The normal pancreas: embryonic development and adult homeostasis
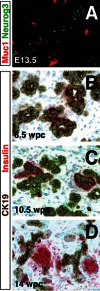
The mammalian pancreas arises from two evaginations in the posterior foregut, one dorsal and one ventral, which expand into the tail and head, respectively, of the mature organ. The first evidence of pancreas development, at embryonic day 8.5 (E8.5) in the mouse, is expression of the homeodomain transcription factor Pdx1 within the cells that will evaginate to form the pancreatic buds (Ohlsson et al., 1993). Shortly thereafter, these undifferentiated progenitor cells begin to express the bHLH transcription factor Ptf1a (Burlison et al., 2008; Kawaguchi et al., 2002), as well as the digestive enzyme Cpa1 (Zhou et al., 2007). Genetic lineage-tracing, using the Cre-loxP system, indicates that essentially all mature pancreatic cells, including acini, ducts and islets, arise from these Pdx1+/Ptf1a+ progenitor cells (Gu et al., 2002; Kawaguchi et al., 2002). Moreover, in both mice and humans, pancreas growth and differentiation are nearly abolished when either of these genes is mutated (Jonsson et al., 1994; Kawaguchi et al., 2002; Krapp et al., 1998; Sellick et al., 2004; Stoffers et al., 1997). Although there have been few thorough studies of human embryonic and fetal pancreas development, the weight of evidence indicates overall similarity to that of rodents (Castaing et al., 2005; Piper et al., 2004; Sarkar et al., 2008).
Space does not permit a full discussion of the complexities of pancreas development, which have been reviewed elsewhere (Collombat et al., 2006; Dhawan et al., 2007; Murtaugh, 2007). We will focus instead on the issue of pancreatic lineages, as it relates directly to the question of pancreatic stem cells. Figure 1 summarizes a number of mouse lineage-tracing experiments, the important elements of which are as follows:
- Transient expression of the bHLH transcription factor Neurog3/Ngn3 marks specification of these progenitors to the islet lineage (Gu et al., 2002).
These last findings represent only the latest word in a debate about adult “neogenesis,” i.e. the differentiation of new islets from stem or precursor cells, that has been active since the late 19th-century (Bensley, 1911; Bonner-Weir and Weir, 2005). Fueling this argument are two long-standing observations: first, that islet (and β-cell) mass continues to increase after birth, and second, that islet cells and their precursors in the embryo are found to reside in or near ductal structures (see Figure 2). If embryonic ducts can differentiate into islets, why not those of the adult? On the other hand, the rates of postnatal islet proliferation and death are generally low, and there is no obvious proliferative niche such as is found in the skin or intestines. As the differentiated cells of those tissues are short-lived, they must be replenished by a classical stem cell compartment; the slow turnover of islet cells may not require such a mechanism. On the other hand, as we will discuss, β-cells exhibit some capacity for regeneration after injury, and it is possible that some of this capacity reflects the activity of “facultative stem cells” similar to those proposed to exist in the liver (Michalopoulos, 2007).
The field is therefore divided into camps that we term “expansionist,” believing that β-cell mass increases via replication of existing β-cells, and “neogenicist,” arguing that at least some of this increase reflects differentiation of islet precursor cells. Two lines of evidence support the expansionist position. The first concerns Neurog3, the expression of which marks the precursors of all islet cells (Gu et al., 2002), and which is necessary and sufficient for endocrine specification in vivo (Apelqvist et al., 1999; Gradwohl et al., 2000; Johansson et al., 2007; Schwitzgebel et al., 2000). Neurog3 expression is thus the sine qua non of islet development, yet it is expression is undetectable in the adult pancreas, including ducts (Gradwohl et al., 2000; Lee et al., 2006; Schwitzgebel et al., 2000). These findings, or more precisely the lack thereof, suggest that adult islet precursor cells do not exist.
Neogenicists might argue that the slow postnatal increase in β-cell mass requires very few islet precursors at any one time, perhaps easy to overlook. Furthermore, as we discuss below, it may be that neogenesis “ramps up” only when β-cell mass is lost to injury or disease. The strongest expansionist counter-argument is the seminal lineage-tracing study of Dor and colleagues (Dor et al., 2004), the results of which are schematized in Figure 3A. Briefly, these researchers used an inducible Cre-loxP system to heritably mark a fraction of islet cells (the “pulse,” indicated as purple nuclei on the left side of Figure 3A). This marking was performed while the mice were young (6-8 weeks), and a subset of the mice were immediately sacrificed and analyzed for labeling index. The remaining mice were allowed to age for up to a year (the “chase”), during which time β-cell numbers increased several fold. If this increase was due to neogenesis, the β-cells labeling index should have fallen due to influx of unmarked islet precursor cells. Instead, the labeling index remained constant during the chase (see Figure 3A, right), supporting the expansionist model (Dor et al., 2004).
This model has since acquired independent support from alternative pulse-chase paradigms, using novel lineage-tracing techniques (Brennand et al., 2007) or sensitive measurement of proliferation kinetics (Teta et al., 2007). These studies present a powerful contrast to those based mainly on histology. For example, small β-cell clusters, or solitary β-cells adjacent to ducts, are frequently interpreted as products of neogenesis (Bouwens and Pipeleers, 1998; Wang et al., 1993). Analysis of these structures by lineage-tracing (Dor et al., 2004) and cell proliferation kinetics (Teta et al., 2007), however, strongly suggests that the majority of them originate from pre-existing β-cells.
Even if neogenesis does not make a major contribution for normal β-cell maintenance and growth, the process may be activated following damage to the pancreas or to the β-cells themselves. We will examine this possibility in the next sections, but we should first note that there is no evidence for such compensatory behavior by progenitor cells of the embryonic pancreas. Instead, if a subset of progenitors is destroyed in utero, by expression of a toxic transgene, the organ remains proportionally smaller in mass (Stanger et al., 2007). If compensation for lost cells does occur in adults, the underlying phenomenon is unlikely to simply recapitulate embryonic organogenesis.
Pancreatic regeneration: re-growth or re-differentiation?
The pancreas is no liver – if half is excised, the remnant will not double in size. The same applies to β-cells, which can be targeted for experimental destruction by various means. Nonetheless, both the pancreas and its β-cells are capable of at least modest regeneration following surgical, chemical or transgenic injury, in some cases sufficient to reverse hyperglycemia (reviewed in Bouwens and Rooman, 2005). Do these situations, in which the normally slow mitotic engine of the adult organ shifts into high gear, reveal a role for pancreatic stem cells?
For several decades, researchers have generated rodent models of diabetes by poisoning β-cells with relatively selective compounds, most commonly streptozotocin (Bouwens and Rooman, 2005; Lenzen, 2008). As a rule, the effects of these treatments are irreversible, although development of diabetes is more gradual with lower doses and younger animals (Junod et al., 1969; Like and Rossini, 1976). Nonetheless, β-cell numbers do appear to rebound moderately following high-dose streptozotocin (STZ) treatment of young rats, or low-dose treatment of adult mice (Bonner-Weir et al., 1981; Dutrillaux et al., 1982; Krishnamurthy et al., 2006; Teta et al., 2005). This system exemplifies a problem in the field: β-cell regeneration occurs only after less-than-complete destruction of existing cells. It is therefore hard to know if new β-cells arise via neogenesis or expansion of residual survivors. While analysis of proliferation kinetics supports the latter model (Teta et al., 2005; Wang et al., 1994), there have not yet been any genetic marking experiments in which pre-existing β-cells are marked and traced through the regeneration process.
The fact that normal animals almost never recover from STZ-induced diabetes makes it a questionable model for regeneration. As an alternative, Nir et al (Nir et al., 2007) developed a bi-transgenic mouse strategy in which the diphtheria toxin A (DTA) subunit is expressed, in a regulateable fashion, within mature β-cells. The potent and cell-autonomous lethality of DTA produces an all-or-nothing model of β-cell killing, in which individual cells either express DTA and die, or fail to express the gene and survive. In this system, transgene activation causes rapid development of diabetes, which is reversible upon transgene deactivation. The return to normoglycemia takes several weeks, and is accompanied by an almost complete restoration of β-cell mass. Importantly, simultaneous genetic lineage-tracing reveals that the new β-cells arise via mitotic expansion of pre-existing β-cells (Nir et al., 2007).
What about more extreme models of β-cell and pancreatic damage and regeneration? Partial pancreatectomy has been a favorite model of pancreatic regeneration for many years, and rodents can restore lost β-cell mass following removal of the majority of the organ (Brockenbrough et al., 1988). Several lines of evidence, however, have converged to indicate that this process – which is slow and often incomplete – is driven by expansion rather than neogenesis. First, when existing β-cells are genetically marked prior to pancreatectomy, identical labeling indices are found before and after regeneration (Dor et al., 2004). Second, proliferation kinetic studies indicate enhanced β-cell mitosis post-pancreatectomy, and no sign of differentiation from ductal progenitors (Teta et al., 2007). Finally, Neurog3 expression is not re-activated during regeneration, as might be expected for a process that recapitulates fetal neogenesis (Lee et al., 2006).
One caveat to these mouse studies is that they have involved 50-70% pancreatectomy, rather than the subtotal (90-95%) removal that has been reported to produce neogenesis in rats, with histological evidence of duct-to-islet differentiation (Bonner-Weir et al., 1993). Whether Neurog3 is re-expressed in the rat model is not yet known, but a positive result would be especially compelling given recent evidence, discussed below, that Neurog3-dependent neogenesis can be induced in adult mouse ducts (Xu et al., 2008). A further caveat, possibly more important, is that the mouse studies might not have had sufficient statistical power to exclude a minor contribution from ductal neogenesis. For this reason, it would be extremely helpful to have an inducible, duct-specific Cre line, analogous to that used by Dor and colleagues (Dor et al., 2004) to mark pre-existing β-cells. By marking duct cells prior to injury, one should be able to detect even a minor contribution on their part to regenerated β-cells. Until such experiments have been performed, it seems prudent to conclude that regeneration of lost β-cells generally involves expansion of those cells left behind, rather than neogenesis (see Figure 3A). β-cell proliferation is known to accelerate under physiological conditions of high insulin demand, such as pregnancy, and this same mechanism may be employed during regeneration (Bouwens and Rooman, 2005).
Before moving on, we should pause to consider the question – less well studied – of whether stem cells exist for the exocrine pancreas. The exocrine pancreas can also regenerate following partial pancreatectomy, although the extent appears to be less than that of the β-cells (Brockenbrough et al., 1988; Desai et al., 2007). Other injury models exist for acinar cells, such as treatment with high levels of the secretagogue caerulein, in which acinar cells are initially destroyed and later recover (Willemer et al., 1992). Remnant duct and/or acinar cells appear to revert to a more progenitor-like state in these models; for example, Pdx1 expression is highly upregulated, and their proliferation rate increases (Jensen et al., 2005; Sharma et al., 1999). Lineage-tracing studies indicate that the recovery in acinar mass in these models occurs not via differentiation of new acini from ductal or other progenitors, however, but by expansion of those acinar cells that survived injury (Desai et al., 2007; Strobel et al., 2007). The acinar cells themselves generally do not change fate during this process, although after repeated caerulein treatment a small number of acinar cells seem to adopt a duct-like phenotype (Desai et al., 2007; Strobel et al., 2007). Proliferation of existing differentiated cells therefore appears to be the rule for growth and regeneration in the adult pancreas, endocrine and exocrine.
The exception that proves the rule: pancreatic duct ligation
All doubt regarding duct-to-islet neogenesis could be resolved with a duct-specific inducible Cre transgenic mouse. While no such mouse is yet described, the impetus for its development has become overwhelming in light of recent work providing very strong evidence of in vivo β-cell neogenesis (Xu et al., 2008). This study made use of pancreatic duct ligation (PDL), in which the main duct of the splenic lobe is tied off, causing massive death of distal acinar cells. In rats, PDL induced β-cell mass within the ligated portion to double within a week, while the unligated portion remains unchanged (Wang et al., 1995). This increase is too rapid to be accounted for by the observed proliferation rate of existing β-cells, and the model presents histological evidence of neogenesis from ducts (Wang et al., 1995). Importantly, PDL differs from the injury models described above in that it does not involve loss of pre-existing β-cells, nor even transient hyperglycemia (Wang et al., 1995; Xu et al., 2008). Indeed, Frederick Banting exploited the acinar deletion caused by PDL, and the resulting elimination of contaminating proteases, in his initial efforts to isolate insulin (Banting, 1925). We are therefore reluctant to desribe the PDL model as “regeneration” per se, as it involves production of extra β-cells rather than the replacement of β-cells lost to injury. In addition, the signals involved are probably distinct from those that couple β-cell mass to physiological insulin demand, as there is no hyperglycemia, and the unligated portion of the organ is completely unaffected. Presumably some very local signal is activated after duct ligation, perhaps triggered by acinar cell death or inflammation, and perhaps more similar to the signals that regulate embryonic islet development than those normally involved in maintaining adult β-cell mass.
Neurog3 expression studies provide the best evidence for neogenesis following PDL (Xu et al., 2008). As noted above, Neurog3 is not normally expressed in the adult pancreas, even after pancreatectomy. Following PDL, however, several assays indicated its re-expression: realtime PCR analysis of total pancreatic RNA, immunostaining with a monoclonal anti-Neurog3 antibody, and expression of three independent Neurog3-driven reporter genes (both transgenic and knock-in). As the perdurance of these reporters exceed that of Neurog3 itself, Xu and colleagues were able to show that their expression initiated in duct cells and persisted into new islet cells, including β-cells. Furthermore, lentiviral knockdown of Neurog3 in the ligated pancreas blocked the doubling of β-cell mass, consistent with the need for Neurog3 in fetal islet development (Gradwohl et al., 2000; Xu et al., 2008).
Together, the results of this study strongly indicate that duct ligation induces β-cell neogenesis. Furthermore, if the β-cell “pulse-chase” paradigm was to be applied to this system (Dor et al., 2004), we would predict a large increase in the proportion of unlabeled β-cells, reflecting a doubling of β-cell mass due primarily to neogenesis (see Figure 3B). This experiment has not yet been performed, although the reagents clearly exist. As noted above, many uncertainties regarding neogenesis could be clarified with a duct-specific inducible Cre driver line, which should robustly confirm the ductal origin of new β-cells formed after PDL. Assuming that the conclusions of Xu and colleagues (Xu et al., 2008) are confirmed by lineage-tracing of duct cells, it raises three immediate questions: first, what signals are responsible for evoking Neurog3 re-expression and islet neogenesis; second, is there a special subset of duct cells that can undergo islet differentiation, or does the abnormal environment, post-PDL, trigger stochastic conversion of normal duct cells into islet precursors; third, can these signals can be used to induce β-cell differentiation from human duct cells?
Although there is no obvious answer to the first question, it should be noted that islet neogenesis appears to occur in the ducts of transgenic mice expressing interferon-γ in β-cells; as in the PDL model, the pancreata of these mice exhibit inflammation and immune cell infiltration, but not hyperglycemia (Gu et al., 1994; Gu and Sarvetnick, 1993). (Whether Neurog3 is upregulated in these mice is unknown.) An inflammatory signal may therefore comprise part of the trigger for ductal neogenesis. The second question is equally hard to answer; interestingly, a similar issue has been raised regarding the role of astrocytes as adult neural stem cells: are the neurogenic astrocytes intrinsically unique, or is their stem cell behavior determined by their niche (Riquelme et al., 2008)?
The third question, of whether human duct cells can be converted into β-cells, is the subject to which we now turn.
Duct-to-islet differentiation: in vitro veritas?
The possibility of deriving β-cells in culture, via differentiation of ductal or other adult progenitor cells, has been intensely studied over the past several years, with successes reported using both mouse and human tissue (Bonner-Weir et al., 2000; Gao et al., 2005; Ramiya et al., 2000; Seaberg et al., 2004; Suzuki et al., 2004; Zulewski et al., 2001). None of these methods has yet achieved widespread use, however, and two general problems seem to bedevil studies in this area. First, not knowing the signals that trigger endocrine differentiation, in utero or in adults (e.g. following PDL), makes it hard to design rational culture conditions to optimize the process. Second, most pancreatic cell preparations – even those from which intact islets have been separated – are contaminated by pre-existing β-cells. Survival and expansion of those β-cells during culture will make it hard to detect true neogenesis. Indeed, one group has reported that prior removal of rare islet cells from a duct preparation, by sorting against the islet cell surface marker NCAM, almost completely eliminated in vitro neogenesis (Gao et al., 2005). This might imply that a phenomenon widely interpreted as neogenesis actually represents the expansion of pre-existing islet cells in vitro, or else that islet precursors express a marker, NCAM, generally assumed to be specific to differentiated endocrine cells.
Two other recent studies, meanwhile, do provide strong evidence for islet differentiation in culture from adult non-islet cells; although their approaches differ, they converge on one intriguing point. Hao and colleagues (Hao et al., 2006) found that culturing adult human pancreas cells with the aminoglycoside antibiotic G418 would eliminate both mesenchymal cells as well as pre-existing β-cells. The remaining non-endocrine pancreatic epithelial cells (NEPECs) did not form β-cells in culture, or upon transplantation to the mouse kidney capsule. When co-transplanted with human fetal islet cell clusters (a mix of islet, epithelial and mesenchymal tissue), however, 10-20% of NEPECs differentiated into insulin+ β-cells (Hao et al., 2006). In separate work, Yatoh and colleagues (Yatoh et al., 2007) purified duct cells from adult human pancreas by sorting for the duct cell surface antigen CA19-9. The purified duct cells did not form β-cell differentiation in culture, or when transplanted to the mouse kidney capsule; when aggregated with pancreatic stromal fibroblasts prior to transplantation, however, ∼1% of the duct cells underwent β-cell differentiation (Yatoh et al., 2007).
Although these studies used different methods to isolate starting material (and report very different efficiencies of β-cell neogenesis), they share the fact that neogenesis occurred only following transplantation to the kidney capsule, and required co-transplantation with “helper cells.” The kidney capsule is known to be a favorable environment for differentiation of transplanted fetal islets (Castaing et al., 2005; Hayek and Beattie, 1997), and the expansion of embryonic islet progenitors is promoted by association with mesenchymal cells (Duvillie et al., 2006; Miralles et al., 2006). Thus, the progenitor-like behavior observed in these studies parallels that seen in utero, including the unfortunate fact that it works best under conditions that are not easily mimicked in vitro.
Together with the duct ligation work described above (Xu et al., 2008), these studies suggest that the short supply of donor islets could be “stretched” by expansion and differentiation of progenitor-like cells within the leftover exocrine tissue. (A shortcoming of the culture studies, however, is that they have not yet documented Neurog3 expression by the putative precursor cells.) The fact that neogenesis only occurs after transplantation also raises concerns about its eventual clinical applicability; something critical happens in vivo that cannot be reproduced under controlled conditions in vitro. As we discuss in the next and final section, this in vivo/in vitro dichotomy also applies to another proposed source of new β-cells, embryonic stem cells.
From embryonic stem cell to beta-cell
This review has focused on what goes on inside the pancreas, and in particular whether one can obtain stem cells – or cells with similarly useful properties – from the pancreas. In this penultimate section, we will take a detour to consider the opposite question: can one obtain pancreas from stem cells, specifically embryonic stem (ES) cells? A full consideration of the ES cell-to-pancreas literature is outside the scope of this review; we refer interested readers to several other, excellent reviews that treat the topic at length, including its numerous pitfalls and false starts (Madsen, 2005; Murry and Keller, 2008; Spence and Wells, 2007).
The attraction of ES cells as a potential source of pancreatic tissue, including islets, is their capacity to differentiate into any cell type. This is proven for mouse ES cells, which can reconstitute an entire embryo (Nagy et al., 1993), and correlative evidence suggests that their human counterparts (hESCs) are similarly pluripotent (Odorico et al., 2001). The fact that ES cells can become pancreas in a mouse, however, does not mean that they are easily compelled to do so in a dish. Work in a number of “directed differentiation” paradigms, including pancreas, suggests that success depends on recapitulating, in vitro, the steps that occur during normal development in vivo (Murry and Keller, 2008; Spence and Wells, 2007). Among the first steps of pancreas development is the induction of definitive endoderm, which requires signaling by transforming growth factor-β (TGFβ) proteins of the activin or Nodal families (Gamer and Wright, 1995; Henry et al., 1996; Tremblay et al., 2000). Applying this insight to ES cell differentiation, it has recently been shown that both mouse and human ES cells can be robustly converted to endoderm by treatment with activin or Nodal (D'Amour et al., 2005; Kubo et al., 2004; Yasunaga et al., 2005).
Several groups have since exploited additional known regulators of pancreas development, such as FGF10 and retinoic acid, to push ES cells further down the path of β-cell differentiation (D'Amour et al., 2006; Jiang et al., 2007; Micallef et al., 2005; Nakanishi et al., 2007). The work of D’Amour and colleagues (D'Amour et al., 2006) is particularly compelling, as it was performed entirely in monolayer culture, and resulted in highly efficient generation of cells expressing pancreatic progenitor markers. Although insulin-producing cells did develop in these cultures, however, they appeared to be immature, and were not glucose-responsive. More recently, this group and another have transplanted hESC-derived pancreatic progenitors into the mouse kidney capsule or fat pad, and shown that these environments can promote differentiation of mature, functional human β-cells (Kroon et al., 2008; Shim et al., 2007). The nature of the signals provided at the site of transplantation are unknown, although, as noted above, very similar findings were made with cells derived from the adult pancreatic duct (Hao et al., 2006; Yatoh et al., 2007). One potentially important element present in vivo, and absent from in vitro cultures, is the host endothelium. Endothelial cells have been shown to promote insulin expression and β-cell proliferation (Lammert et al., 2001; Nikolova et al., 2006), and could contribute a critical signal for the final differentiation of β-cells from both embryonic and adult stem cells.
Even before all the steps are worked out for converting ES cells to β-cells, they should provide a useful test platform for basic research on pancreas and islet development. Mouse knockout and transgenic studies have provided a long list of genes important for various steps of pancreas development, yet the underlying circuitry through which these genes interact has proven hard to decipher from simple knockout and overexpression studies. A reproducible ES cell differentiation system – even if not pristine enough for clinical use – should permit quick testing of existing hypotheses as well as generation of new ones. Critical insights gained in ES cells can then be definitively tested in vivo, establishing a virtuous circle in which data from one system informs experiments in the other.
Conclusions
The past several years have seen a wealth of new data on the origin of differentiated cells in the embryonic and adult pancreas, and we have been obliged to revise our opinions about pancreatic stem cells and β-cell neogenesis accordingly. Prior to the widespread use of genetic lineage-tracing, we were inclined to accept histological evidence of neogenesis, particularly following injury (Murtaugh and Melton, 2003). Once lineage-tracing studies revealed that division of pre-existing β-cell could account for both growth and regeneration, however (Dor et al., 2004), our view of neogenesis became more skeptical (Murtaugh, 2007). Today, with the recent evidence that pancreatic duct ligation can induce bona fide, Neurog3-dependent islet neogenesis (Xu et al., 2008), we are left with a more mixed view, as follows:
- The embryo does contain self-renewing, multipotent progenitor cells, co-expressing Pdx1, Ptf1a and Cpa1. These are not classical stem cells, however, as they do not undergo continued self-renewal through adulthood, but differentiate prior to birth (see Figure 1). Furthermore, when a subset of these cells is destroyed, the others are unable to expand to replace them.
- There is a mechanism to replace lost β-cells in the adult, but it depends primarily on expansion of pre-existing β-cells rather than differentiation of stem or precursor cells (see Figure 3A). A similar expansionist mechanism seems to be in place for acinar cells as well.
- When the exocrine pancreas is injured by duct ligation, a remarkably rapid and robust process of neogenesis is initiated, in which Neurog3-expressing islet precursors arise in the ducts and differentiate into new β-cells (and other endocrine cell types, Figure 3B). This response is not quite the same as what we typically call “regeneration”: duct ligation does not destroy endogenous β-cells, nor does the animal experience hyperglycemia. Instead, duct ligation seems to evoke the generation of supernumerary β-cells, by mechanisms that remain unknown.
Although the ducts currently appear to be the source of new β-cells following duct ligation, it is unclear if they contain a small number of dedicated stem cells, held in emergency reserve, or if the abnormal environment induces facultative progenitor-like behavior in otherwise normal ducts. Determining whether all duct cells are potential islet precursors, and identifying the signals governing their behavior, will be critical to any clinical use of ducts as a source of new β-cells. Such studies will require the creation of transgenic mice in which mature duct cells can be marked and traced, the absence of which now leaves a major gap in the field. The signals that promote islet development in utero are also poorly understood, and their further characterization should improve efforts to differentiate β-cells from ES cells. We are hopeful that further dialogue between embryology, regeneration and stem cell biology will lead to insights that cross disciplines, and promote the goal of producing islet cells useful for treatment of patients.
Acknowledgements
Our work is supported by the NIH (R01DK075072), the Searle Scholars Program and the Boehringer Ingelheim Fonds. We thank Neil Hanley for permission to use his images of human fetal pancreas. We are grateful to the members of the Murtaugh lab, and to all of our colleagues in the pancreas development community, for many helpful discussions.
References
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson D. J, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. [PubMed: 10476967] [CrossRef]
- Ballinger W. F, Lacy P. E. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175–186. [PubMed: 4262169]
- Banting F. G. 1925. Nobel lecture.
- Bensley R. R. Studies on the pancreas of the guinea pig. Am. J. Anat. 1911;12:297–388. [CrossRef]
- Bonner-Weir S, Baxter L. A, Schuppin G. T, Smith F. E. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42:1715–1720. [PubMed: 8243817] [CrossRef]
- Bonner-Weir S, Taneja M, Weir G. C, Tatarkiewicz K, Song K. H, Sharma A, O'Neil J. J. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7999–8004. [PMC free article: PMC16659] [PubMed: 10884429] [CrossRef]
- Bonner-Weir S, Trent D. F, Honey R. N, Weir G. C. Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes. 1981;30:64–69. [PubMed: 6112177] [CrossRef]
- Bonner-Weir S, Weir G. C. New sources of pancreatic beta-cells. Nat. Biotechnol. 2005;23:857–861. [PubMed: 16003374] [CrossRef]
- Bouwens L, Pipeleers D. G. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia. 1998;41:629–633. [PubMed: 9662042] [CrossRef]
- Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol. Rev. 2005;85:1255–1270. [PubMed: 16183912] [CrossRef]
- Brennand K, Huangfu D, Melton D. All beta Cells Contribute Equally to Islet Growth and Maintenance. PLoS Biol. 2007;5:e163. [PMC free article: PMC1877817] [PubMed: 17535113] [CrossRef]
- Brockenbrough J. S, Weir G. C, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes. 1988;37:232–236. [PubMed: 3292318] [CrossRef]
- Burlison J. S, Long Q, Fujitani Y, Wright C. V, Magnuson M. A. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev. Biol. 2008;316:74–86. [PMC free article: PMC2425677] [PubMed: 18294628]
- Castaing M, Duvillie B, Quemeneur E, Basmaciogullari A, Scharfmann R. Ex vivo analysis of acinar and endocrine cell development in the human embryonic pancreas. Dev. Dyn. 2005;234:339–345. [PubMed: 16134081] [CrossRef]
- Collombat P, Hecksher-Sorensen J, Serup P, Mansouri A. Specifying pancreatic endocrine cell fates. Mech. Dev. 2006;123:501–512. [PubMed: 16822656] [CrossRef]
- D'Amour K. A, Agulnick A. D, Eliazer S, Kelly O. G, Kroon E, Baetge E. E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23:1534–1541. [PubMed: 16258519] [CrossRef]
- D'Amour K. A, Bang A. G, Eliazer S, Kelly O. G, Agulnick A. D, Smart N. G, Moorman M. A, Kroon E, Carpenter M. K, Baetge E. E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006;24:1392–1401. [PubMed: 17053790] [CrossRef]
- Desai B. M, Oliver-Krasinski J, De Leon D. D, Farzad C, Hong N, Leach S. D, Stoffers D. A. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J. Clin. Invest. 2007;117:971–977. [PMC free article: PMC1838936] [PubMed: 17404620] [CrossRef]
- Dhawan S, Georgia S, Bhushan A. Formation and regeneration of the endocrine pancreas. Curr. Opin. Cell Biol. 2007;19:634–645. [PMC free article: PMC2695413] [PubMed: 18061427] [CrossRef]
- Dor Y, Brown J, Martinez O. I, Melton D. A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. [PubMed: 15129273] [CrossRef]
- Dutrillaux M. C, Portha B, Roze C, Hollande E. Ultrastructural study of pancreatic B cell regeneration in newborn rats after destruction by streptozotocin. Virchows Arch. B. Cell Pathol. Incl. Mol. Pathol. 1982;39:173–185. [PubMed: 6123190] [CrossRef]
- Duvillie B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582–589. [PubMed: 16505219] [CrossRef]
- Gamer L. W, Wright C. V. Autonomous endodermal determination in Xenopus: regulation of expression of the pancreatic gene XlHbox 8. Dev. Biol. 1995;171:240–251. [PubMed: 7556900] [CrossRef]
- Gao R, Ustinov J, Korsgren O, Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005;48:2296–2304. [PubMed: 16193291] [CrossRef]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1607–1611. [PMC free article: PMC26482] [PubMed: 10677506] [CrossRef]
- Gu D, Lee M. S, Krahl T, Sarvetnick N. Transitional cells in the regenerating pancreas. Development. 1994;120:1873–1881. [PubMed: 7523055]
- Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development. 1993;118:33–46. [PubMed: 8104143]
- Gu G, Dubauskaite J, Melton D. A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. [PubMed: 11973276]
- Hao E, Tyrberg B, Itkin-Ansari P, Lakey J. R, Geron I, Monosov E. Z, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat. Med. 2006;12:310–316. [PubMed: 16491084] [CrossRef]
- Hayek A, Beattie G. M. Experimental transplantation of human fetal and adult pancreatic islets. J. Clin. Endocrinol. Metab. 1997;82:2471–2475. [PubMed: 9253320] [CrossRef]
- Henry G. L, Brivanlou I. H, Kessler D. S, Hemmati-Brivanlou A, Melton D. A. TGF-beta signals and a pattern in Xenopus laevis endodermal development. Development. 1996;122:1007–1015. [PubMed: 8631246]
- Jensen J. N, Cameron E, Garay M. V, Starkey T. W, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. [PubMed: 15765408] [CrossRef]
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar A. S. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. [PubMed: 17510217] [CrossRef]
- Johansson K. A, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell. 2007;12:457–465. [PubMed: 17336910] [CrossRef]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. [PubMed: 7935793] [CrossRef]
- Junod A, Lambert A. E, Stauffacher W, Renold A. E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J. Clin. Invest. 1969;48:2129–2139. [PMC free article: PMC297467] [PubMed: 4241908] [CrossRef]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald R. J, Wright C. V. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 2002;32:128–134. [PubMed: 12185368] [CrossRef]
- Krapp A, Knofler M, Ledermann B, Burki K, Berney C, Zoerkler N, Hagenbuchle O, Wellauer P. K. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752–3763. [PMC free article: PMC317250] [PubMed: 9851981] [CrossRef]
- Krishnamurthy J, Ramsey M. R, Ligon K. L, Torrice C, Koh A, Bonner-Weir S, Sharpless N. E. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. [PubMed: 16957737] [CrossRef]
- Kroon E, Martinson L. A, Kadoya K, Bang A. G, Kelly O. G, Eliazer S, Young H, Richardson M, Smart N. G, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008;26:443–452. [PubMed: 18288110] [CrossRef]
- Kubo A, Shinozaki K, Shannon J. M, Kouskoff V, Kennedy M, Woo S, Fehling H. J, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. [PubMed: 14998924] [CrossRef]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. [PubMed: 11577200] [CrossRef]
- Lee C. S, De Leon D. D, Kaestner K. H, Stoffers D. A. Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes. 2006;55:269–272. [PubMed: 16361411]
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. [PubMed: 18087688] [CrossRef]
- Like A. A, Rossini A. A. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. [PubMed: 180605] [CrossRef]
- Madsen O. D. Stem cells and diabetes treatment. APMIS. 2005;113:858–875. [PubMed: 16480455] [CrossRef]
- Micallef S. J, Janes M. E, Knezevic K, Davis R. P, Elefanty A. G, Stanley E. G. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301–305. [PubMed: 15585742] [CrossRef]
- Michalopoulos G. K. Liver regeneration. J. Cell. Physiol. 2007;213:286–300. [PMC free article: PMC2701258] [PubMed: 17559071] [CrossRef]
- Miralles F, Lamotte L, Couton D, Joshi R. L. Interplay between FGF10 and Notch signalling is required for the self-renewal of pancreatic progenitors. Int. J. Dev. Biol. 2006;50:17–26. [PubMed: 16323074] [CrossRef]
- Murry C. E, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. [PubMed: 18295582] [CrossRef]
- Murtaugh L. C. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–438. [PubMed: 17185316] [CrossRef]
- Murtaugh L. C, Melton D. A. Genes, signals, and lineages in pancreas development. Annu. Rev. Cell Dev. Biol. 2003;19:71–89. [PubMed: 14570564] [CrossRef]
- Naftanel M. A, Harlan D. M. Pancreatic islet transplantation. PLoS Med. 2004;1:e58; quiz e75. [PMC free article: PMC539048] [PubMed: 15630467] [CrossRef]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8424–8428. [PMC free article: PMC47369] [PubMed: 8378314] [CrossRef]
- Nakanishi M, Hamazaki T. S, Komazaki S, Okochi H, Asashima M. Pancreatic tissue formation from murine embryonic stem cells in vitro. Differentiation. 2007;75:1–11. [PubMed: 17244017] [CrossRef]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber H. P, Ferrara N, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev. Cell. 2006;10:397–405. [PubMed: 16516842] [CrossRef]
- Nir T, Dor Y. How to make pancreatic beta cells–prospects for cell therapy in diabetes. Curr. Opin. Biotechnol. 2005;16:524–529. [PubMed: 16084716] [CrossRef]
- Nir T, Melton D. A, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 2007;117:2553–2561. [PMC free article: PMC1957545] [PubMed: 17786244] [CrossRef]
- Odorico J. S, Kaufman D. S, Thomson J. A. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. [PubMed: 11359944] [CrossRef]
- Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. [PMC free article: PMC413720] [PubMed: 7901001]
- Pictet R, Rutter W. J. Development of the embryonic endocrine pancreas. In: Steiner D, Freinkel N, editors. In Handbook of Physiology, Section 7. Baltimore: Williams & Williams; 1972. pp. 25–66.
- Piper K, Brickwood S, Turnpenny L. W, Cameron I. T, Ball S. G, Wilson D. I, Hanley N. A. Beta cell differentiation during early human pancreas development. J. Endocrinol. 2004;181:11–23. [PubMed: 15072563] [CrossRef]
- Ramiya V. K, Maraist M, Arfors K. E, Schatz D. A, Peck A. B, Cornelius J. G. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 2000;6:278–282. [PubMed: 10700229] [CrossRef]
- Riquelme P. A, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:123–137. [PMC free article: PMC2605490] [PubMed: 17322003] [CrossRef]
- Sarkar S. A, Kobberup S, Wong R, Lopez A. D, Quayum N, Still T, Kutchma A, Jensen J. N, Gianani R, Beattie G. M, et al. Global gene expression profiling and histochemical analysis of the developing human fetal pancreas. Diabetologia. 2008;51:285–297. [PubMed: 18094957] [CrossRef]
- Schwitzgebel V. M, Scheel D. W, Conners J. R, Kalamaras J, Lee J. E, Anderson D. J, Sussel L, Johnson J. D, German M. S. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. [PubMed: 10903178]
- Seaberg R. M, Smukler S. R, Kieffer T. J, Enikolopov G, Asghar Z, Wheeler M. B, Korbutt G, Van Der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 2004;22:1115–1124. [PubMed: 15322557] [CrossRef]
- Sellick G. S, Barker K. T, Stolte-Dijkstra I, Fleischmann C, Coleman R. J, Garrett C, Gloyn A. L, Edghill E. L, Hattersley A. T, Wellauer P. K, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat. Genet. 2004;36:1301–1305. [PubMed: 15543146] [CrossRef]
- Sharma A, Zangen D. H, Reitz P, Taneja M, Lissauer M. E, Miller C. P, Weir G. C, Habener J. F, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. [PubMed: 10078550] [CrossRef]
- Shim J. H, Kim S. E, Woo D. H, Kim S. K, Oh C. H, McKay R, Kim J. H. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. [PubMed: 17457565] [CrossRef]
- Spence J. R, Wells J. M. Translational embryology: Using embryonic principles to generate pancreatic endocrine cells from embryonic stem cells. Dev. Dyn. 2007;236:3218–3227. [PubMed: 17973329] [CrossRef]
- Stanger B. Z, Tanaka A. J, Melton D. A. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. [PubMed: 17259975] [CrossRef]
- Stoffers D. A, Zinkin N. T, Stanojevic V, Clarke W. L, Habener J. F. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat. Genet. 1997;15:106–110. [PubMed: 8988180] [CrossRef]
- Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo C. F, Warshaw A. L, Thayer S. P. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. [PMC free article: PMC2254582] [PubMed: 18054571] [CrossRef]
- Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004;53:2143–2152. [PubMed: 15277399] [CrossRef]
- Teta M, Long S. Y, Wartschow L. M, Rankin M. M, Kushner J. A. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. [PubMed: 16123343] [CrossRef]
- Teta M, Rankin M. M, Long S. Y, Stein G. M, Kushner J. A. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell. 2007;12:817–826. [PubMed: 17488631] [CrossRef]
- Tremblay K. D, Hoodless P. A, Bikoff E. K, Robertson E. J. Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development. 2000;127:3079–3090. [PubMed: 10862745]
- Wang R. N, Bouwens L, Kloppel G. Beta-cell proliferation in normal and streptozotocin-treated newborn rats: site, dynamics and capacity. Diabetologia. 1994;37:1088–1096. [PubMed: 7867880] [CrossRef]
- Wang R. N, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. [PubMed: 8786013] [CrossRef]
- Wang T. C, Bonner-Weir S, Oates P. S, Chulak M, Simon B, Merlino G. T, Schmidt E. V, Brand S. J. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J. Clin. Invest. 1993;92:1349–1356. [PMC free article: PMC288276] [PubMed: 8376589] [CrossRef]
- Willemer S, Elsasser H. P, Adler G. Hormone-induced pancreatitis. Eur. Surg. Res. 1992;24(Suppl 1):29–39. [PubMed: 1601022] [CrossRef]
- Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. [PubMed: 18243096] [CrossRef]
- Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt L. M, Nishikawa S, Chiba T, Era T, Nishikawa S. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat. Biotechnol. 2005;23:1542–1550. [PubMed: 16311587] [CrossRef]
- Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir G. C, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802–1809. [PubMed: 17473224] [CrossRef]
- Zhou Q, Law A. C, Rajagopal J, Anderson W. J, Gray P. A, Melton D. A. A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell. 2007;13:103–114. [PubMed: 17609113] [CrossRef]
- Zulewski H, Abraham E. J, Gerlach M. J, Daniel P. B, Moritz W, Muller B, Vallejo M, Thomas M. K, Habener J. F. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. [PubMed: 11246871] [CrossRef]
- *
Edited by: Alexander F. Schier. Last revised September 6, 2008. Published July 11, 2008. This chapter should be cited as: Murtaugh, L.C. and Kopinke, D., Pancreatic stem cells (July 11, 2008), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.3.1, http://www.stembook.org.
- Introduction
- The normal pancreas: embryonic development and adult homeostasis
- Pancreatic regeneration: re-growth or re-differentiation?
- The exception that proves the rule: pancreatic duct ligation
- Duct-to-islet differentiation: in vitro veritas?
- From embryonic stem cell to beta-cell
- Conclusions
- Acknowledgements
- References
- Review Can pancreatic duct-derived progenitors be a source of islet regeneration?[Biochem Biophys Res Commun. 2009]Review Can pancreatic duct-derived progenitors be a source of islet regeneration?Xia B, Zhan XR, Yi R, Yang B. Biochem Biophys Res Commun. 2009 Jun 12; 383(4):383-5. Epub 2009 Mar 24.
- Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth.[Proc Natl Acad Sci U S A. 2008]Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Proc Natl Acad Sci U S A. 2008 Dec 16; 105(50):19915-9. Epub 2008 Dec 3.
- Review Pancreatic progenitors: The shortest route to restore islet cell mass.[Islets. 2011]Review Pancreatic progenitors: The shortest route to restore islet cell mass.Venkatesan V, Gopurappilly R, Goteti SK, Dorisetty RK, Bhonde RR. Islets. 2011 Nov-Dec; 3(6):295-301. Epub 2011 Nov 1.
- Matters arising: Insufficient evidence that pancreatic β cells are derived from adult ductal Neurog3-expressing progenitors.[Cell Stem Cell. 2023]Matters arising: Insufficient evidence that pancreatic β cells are derived from adult ductal Neurog3-expressing progenitors.Magenheim J, Maestro MA, Sharon N, Herrera PL, Murtaugh LC, Kopp J, Sander M, Gu G, Melton DA, Ferrer J, et al. Cell Stem Cell. 2023 Apr 6; 30(4):488-497.e3.
- Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes.[Diabetes. 2001]Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Diabetes. 2001 Mar; 50(3):521-33.
- Pancreatic stem cells - StemBookPancreatic stem cells - StemBook
Your browsing activity is empty.
Activity recording is turned off.
See more...