NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990.

Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition.
Show detailsDefinition
The thyroid gland normally lies just caudal to the thyroid cartilage in the anterior neck. This location allows an examiner to inspect and palpate this bilobed structure, which in the adult human being weighs from 15 to 25 g. Physical examination of the thyroid gland enables the experienced clinician to construct a rather narrow differential of its anatomical pathology, whereas diagnostic testing (Chapter 142) is frequently necessary to establish the thyroid's functional status.
Technique
The patient should hold a glass of water and be seated. There should be room for the examiner on all sides of the seated patient. Place the patient's head in slight hyperextension with good crosslight falling on the anterior neck and then ask the patient to swallow. The outline of the thyroid gland in thin individuals can be observed frequently as a protuberance on both sides of the trachea moving cephalad in tandem with but 2 cm below the crest of the thyroid cartilage (Figure 138.1). Look for abnormal enlargement, contour, asymmetry, and masses while the patient swallows repeatedly. The neck should also be inspected for abnormal masses and prominent pulsations.
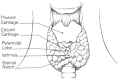
Figure 138.1
The deep structures of the anterior neck. These anatomic landmarks properly orient the examiner.
The art of thyroid gland palpation has spawned a number of distinct attitudes, and each examiner should, through practice, adopt a comfortable technique. Frequently it is advantageous to examine the gland while you stand behind as well as on each side of the patient. Identify the thyroid cartilage, the thyrocricoid membrane, and the cricoid cartilage, a horizontal structure 5 mm wide that marks the superior border of the isthmus. Palpate the isthmus (frequently impalpable unless enlarged), and if standing to the side of the patient, slide the tips of your fingers so that their palmar surfaces rest on the trachea with the dorsal surface medial to the sternocleidomastoid muscle. A frequent mistake is to move the fingers too laterally and trap the body of the muscle between your fingers and the trachea. The ipsilateral lobe can be palpated simultaneously with your thumb or with the other hand from the opposite direction. When you stand behind the patient, identify the landmarks and isthmus with one hand, and when in position to feel the thyroid lobe on that side, place the fingers of your other hand symmetrically on the other side of the trachea. Again identify each lobe while the patient swallows. Feel the gland's surface, note any asymmetry, texture, and estimate the size of each lobe (normally 7 to 10 g). When goiter is present, measure any discrete masses as well as the neck's greatest circumference. A penciled tracing of the goiter's outline provides a reliable record for future comparison. One should also palpate the neck for lymphadenopathy and search for masses (especially in the midline for abnormalities of the thyroglossal duct) and surgical scars.
Transillumination is helpful only in confirming the nature of a superficial thin-walled cyst. Occasional patients with Graves" disease present with an auscultable bruit and palpable thrill over a diffusely enlarged goiter.
Basic Science
The anlage of the thyroid gland, recognizable by the third week of gestation, develops from an endodermal thickening at the base of the tongue and migrates caudally, with the ultimobranchial bodies from the fourth pharyngeal pouch. This fusion accounts for the two distinct cell types that constitute the mature gland and possess quite distinct functions: the follicular cells, which concentrate and organify iodine and are involved in thyroid hormone synthesis; and the parafollicular, or "c," cells, which secrete calcitonin, a polypeptide that has a minor role in the regulation of serum calcium concentrations. The gland is first able to concentrate iodine by week 12 of gestation.
The substance of the gland is arranged into secretory units called follicles, which are surrounded by epithelial cells participating in thyroid hormone synthesis. The follicular lumens are filled with colloid-storing thyroglobulin, the macromolecular glvcoprotein precursor of the circulating hormones.
The fully developed thyroid has the shape of a butterfly with two lobes connected by a thin isthmus overlying the trachea. The body is encompassed by a thin fibrous capsule and normally moves cephalad with deglutition. The parathyroid glands usually lie posterolateral to the thyroid lobes, whereas the recurrent laryngeal nerves run down grooves between the trachea and esophagus. These anatomic relationships are crucial to thyroid surgeons, since the major complications of thyroidectomy involve the accidental disturbance of these two structures. The pyramidal lobe, an embryonic vestige of the thyroglossal duct, usually arises from the medial aspect of the right lobe and extends cephalad as a small, fingerlike projection. Occasionally, the thyroid gland can be found in ectopic locations (e.g., behind the sternum and at the base of the tongue).
The gland elaborates two major hormones, thyroxine (T4) and triiodothyronine (T3) in a molar ratio of approximately 10 : 1. The major thyromimetic activity belongs to T3, the 5′-monodeiodinated product of T4. The latter compound may function mainly as a prohormone, and whether it has intrinsic activity remains controversial. Once released from the gland, T4 arid T3 circulate in the serum bound in large part to proteins: thyroid binding globulin (TBG), prealbumin, and albumin. Over 99% of each is bound, and most of the evidence to date suggests that the free hormone is the biologically available portion. Once delivered to the target tissue, T4 apparently enters the cell, is deiodinated to T3, and translocates to the cell nucleus where it binds to endogenous proteinaceous receptors. The nature of these receptors has recently been elucidated. They are apparently encoded by isoforms of the c-erb A proto-oncogene and are related to nuclear receptors for steroid hormones and retinoic acid. Through its interaction with the cell nucleus, thyroid hormone is an important regulator of protein synthesis and may have direct actions on the mitochondrion and the plasma membrane. One important effect of thyroid hormone is to stimulate cellular respiration, a process that apparently derives from the induction of Na+-K+-ATPase, the sodium pump.
A major determinant of thyroid gland function is thyrotropin (TSH), a glycoprotein synthesized and released from thyrotropic cells in the anterior pituitary. TSH is regulated in turn by the hypothalamus through its elaboration of thyrotropin-releasing hormone (TRH), a tripeptide. TSH stimulates the thyroid gland to increase iodine uptake and thyroid hormone synthesis and release. Thyroid hormone then feeds back on the pituitary and provides negative modulation of TSH production.
Clinical Significance
Enlargement of the thyroid most commonly results from increased pituitary secretion of TSH or lymphocyte production of TSH-like immunoglobulins. In addition, a number of inflammatory, infiltrative, and neoplastic diseases can cause goiter. Physical examination enables the clinician to differentiate among these possibilities.
The pituitary most commonly secretes an excess amount of TSH to compensate for a deficiency in thyroid hormone biosynthesis. Initially TSH causes a symmetrical enlargement of the thyroid gland. With time the gland can become asymmetrical and multinodular. A dominant nodule in a multinodular gland can resemble a neoplastic process. Immunoglobulins that mimic TSH stimulation also cause a symmetrical goiter, but because hormone biosynthesis is not defective, thyrotoxicosis can result. These goiters become quite vascular and can manifest a bruit and thrill. When "destructive" immunoglobulin predominates in autoimmune thyroid disease, one can feel a characteristic finely nodular ("cobblestone") texture. Autoimmune thyroid disease can also produce uninodular or multinodular lesions indistinguishable from neoplasia. Although autoimmune goiter is rarely painful, inflammatory diseases characteristically present with a tender goiter. Subacute thyroiditis, the most common inflammatory thyroid disease, usually enlarges the whole gland, but can also present with a dominant mass. Acute bacterial infections, while more focal, are easily distinguishable by their exquisite tenderness and the warmth and redness of the overlying skin.
Although a dominant nodule in a diffusely abnormal gland can harbor a malignancy, the physician most frequently entertains this diagnostic possibility with a solitary nodule in an otherwise normal gland. These palpable abnormalities mandate further evaluation, which might include sonography, radionuclide scanning, or, preferably, microscopic tissue examination. The relative merits of each of these diagnostic maneuvers have been described in detail elsewhere.
The finding of goiter per se does not necessarily imply that the gland is supplying the body with a disordered amount of thyroid hormone. When thyroid function is so altered, the patient presents with systemic manifestations as a result of excessive or deficient peripheral actions of thyroid hormone. Table 138.1 lists the common symptoms and signs resulting from excessive amounts of circulating thyroid hormone. Many patients will have several of these symptoms, and it is rare for all to be absent in clinically significant thyrotoxicosis. Hyperthyroidism most commonly results from autoimmune production of thyroid-stimulating immunoglobulins and is known in America as Graves" disease. Nodular goiters can also cause hyperthyroidism as a result of an excessive "autonomous" production of thyroid hormone. A solitary toxic nodule typically suppresses the remainder of the gland and appears as a unilateral goiter, often with no palpable gland contralaterally. The ingestion of pharmacologic amounts of exogenous thyroid hormone suppresses the entire gland, leaving no palpable tissue in the face of thyrotoxicosis. Granulomatous and lymphocytic thyroiditis can disrupt the thyroid's follicular architecture, releasing sufficient amounts of stored hormone to cause transient thyrotoxicosis.
Table 138.1
Symptoms and Physical Signs of Hyperthyroidism.
Hypothyroidism occurs when the gland is unable to produce enough hormone to satisfy the metabolic requirements of the body. Primary hypothyroidism in association with goiter is caused by iodine deficiency, enzymatic defects in thyroid hormone biosynthesis, autoimmune destruction of the glandular parenchyma, as in Hashimoto's thyroiditis, and in individuals with underlying thyroid disease by the ingestion of goitrogens such as lithium, sulfonamides, and large quantities of iodine. Nongoitrous hypothyroidism results from idiopathic thyroid atrophy, iatrogenic ablation, and dysfunction of the pituitary or hypothalamus. The latter secondary and tertiary forms of hypothyroidism are important to recognize and distinguish from primary hypothyroidism because concomitant dysfunction of the hypothalamic—pituitary—adrenal axis is commonly present. The symptoms and signs of hypothyroidism are listed in Table 138.2. They may be quite subtle when hypothyroidism is mild and of short duration. Conversely, these manifestations may be flagrant and profound in patients who have gone undiagnosed for several years. Severe, long-standing hypothyroidism is characterized by the deposition of glycosaminoglycans in the skin and other organs, a process known as myxedema.
Table 138.2
Symptoms and Physical Signs of Hypothyroidism.
References
- DeGroot L, Refetoff S, Stanbury JB. The thyroid and its diseases. New York: Wiley, 1986.
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. [PMC free article: PMC6159881] [PubMed: 3283939]
- Ingbar, SH. The thyroid gland. In: Wilson JD, Foster DW, eds. Textbook of endocrinology. Philadelphia: W.B. Saunders, 1985;682–815.
- Ingbar SH, Braverman LE, eds. The thyroid. Philadelphia: JB Lippincott, 1986.
- Oppenheimer JH. Thyroid hormone action at the cellular level. Science. 1979;203:971–79. [PubMed: 218285]
- Oppenheimer JH, Schwartz HL, Mariash CN, Kinlaw WB, Wong NCW, Freake HC. Advances in our understanding of thyroid hormone action at the cellular level. Endocrine Rev. 1987;8:288–308. [PubMed: 3308445]
- Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocrine Rev. 1989;10:366–91. [PubMed: 2673756]
- Sterling K. Thyroid hormone action at the cell level. N Engl J Med 1979;300:117.173–77. [PubMed: 215907]
- Cytologically undetermined thyroid's follicular lesions: surgical procedures and histological outcome in 472 cases.[Ann Ital Chir. 2013]Cytologically undetermined thyroid's follicular lesions: surgical procedures and histological outcome in 472 cases.Conzo G, Troncone G, Docimo G, Pizza A, Sciascia V, Bellevicine C, Napolitano S, Della Pietra C, Palazzo A, Signoriello G, et al. Ann Ital Chir. 2013 May-Jun; 84(3):251-6.
- The effect of aging on the anatomic position of the thyroid gland.[Clin Anat. 2017]The effect of aging on the anatomic position of the thyroid gland.Bann DV, Kim Y, Zacharia T, Goldenberg D. Clin Anat. 2017 Mar; 30(2):205-212. Epub 2016 Nov 27.
- Variation of counting efficiency in determination of (131)I activity in the thyroid gland as a result of its position relative to the detector.[J Radioanal Nucl Chem. 2014]Variation of counting efficiency in determination of (131)I activity in the thyroid gland as a result of its position relative to the detector.Kierepko R, Janowski P, Grochowska M. J Radioanal Nucl Chem. 2014; 300(2):825-828. Epub 2014 Mar 9.
- Review Ectopic thyroid tissue: anatomical, clinical, and surgical implications of a rare entity.[Eur J Endocrinol. 2011]Review Ectopic thyroid tissue: anatomical, clinical, and surgical implications of a rare entity.Noussios G, Anagnostis P, Goulis DG, Lappas D, Natsis K. Eur J Endocrinol. 2011 Sep; 165(3):375-82. Epub 2011 Jun 29.
- Review [Sonography of the thyroid].[Praxis (Bern 1994). 2006]Review [Sonography of the thyroid].Wiesner W, Engel H, Steinbrich W, Oertli D. Praxis (Bern 1994). 2006 Apr 12; 95(15):575-80.
- Neck and Thyroid Examination - Clinical MethodsNeck and Thyroid Examination - Clinical Methods
Your browsing activity is empty.
Activity recording is turned off.
See more...