NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Technological Innovation in Medicine; Gelijns AC, Halm EA, editors. The Changing Economics of Medical Technology. Washington (DC): National Academies Press (US); 1991. (Medical Innovation at the Crossroads, No. 2.)

The Changing Economics of Medical Technology.
Show detailsSUSAN BARTLETT FOOTE
As a nation, we have come to expect innovation in drugs, devices, and clinical procedures. This chapter examines innovation in the medical device industry. Although traditional models of innovation are relevant to an understanding of the industry, they do not tell the whole story. Public policies have wrapped themselves around the innovative process at virtually every stage. Numerous government institutions intervene in the process to accomplish a variety of public goals. I have coined the term “polyintervention” to describe this type of policy environment. 1 The challenge is to determine how this constellation of policies affects innovation in the medical device industry.
Polyintervention is analogous to polypharmacy—a problem familiar to health care professionals. Polypharmacy can occur when a patient takes a number of prescription drugs. Each may have been prescribed for a legitimate ailment, but over time medications can conflict in purpose, thus producing harmful interactions. Some drug interactions may be unexpected, some may be predictable and tolerable, and some can be fatal. Doctors often will request that patients bring all their medications into the office for a global evaluation of the patient's medication regimen. The rationale for each drug and the appropriateness of each dose can be checked, as well as the presence of unwanted side effects or unanticipated and potentially dangerous interactions. Polyintervention in the arena of medical devices requires similar scrutiny.
Let us pursue the medical analogy by characterizing the medical device industry as the patient. The goal is to assess the effect of the prescriptions, that is, public policies, on innovation in the industry. This paper presents a framework for evaluation of polyintervention in the medical device industry, with a specific focus on the two important prescriptions of government regulation and reimbursement. After an evaluation of current interventions, treatment options will be discussed.
Although drugs, devices, and procedures all are forms of medical technology, the policy environment for each category is very different. The device environment is perhaps the most complex. The primary policy hurdle for drugs is Food and Drug Administration (FDA) regulation. For procedures, there is no federal regulation, but much depends on the payment policies of third parties. Devices are subject to both influences to varying degrees. Although this paper discusses devices only, the framework for policy analysis could be used to evaluate drugs and procedures as well.
THE ASSESSMENT: PUBLIC POLICIES AND MEDICAL DEVICE INNOVATION
The Limits of the Traditional Models of Innovation
The medical device industry is subject to many of the same economic forces that affect all highly innovative industries. Device producers must make reasonable profits, ever vigilant of the commercial strategies and technological advances of competitors. A rich scholarly literature on innovation has developed models of the innovative process. Scholars have identified essential stages of innovation, which appear in Figure 5.1 (1).

FIGURE 5.1
The stages of innovation.
The process begins with pure science—the systematic study of phenomena to add to the total of human knowledge. The technology stage is directed toward use and includes the process of invention and development. Invention is the first confidence that something should work and the first tests to demonstrate that it does. Development involves a wide range of activities that measure the chances of success of the product. Finally, the market stage includes adoption of the innovation and its diffusion into the stream of commerce. The steps can be distilled into two categories: the early stages affect the research and development (R&D) of innovative products; the later stages seek to influence adoption and diffusion of such products.
Much of the medical device industry can be understood by referring to the traditional innovation literature, which describes the links between these stages of innovation; how technology is transferred from one stage to another; how firms are organized to facilitate the transfer of technology; and other issues of competitiveness, strategy, and profitability. However, this literature takes a limited view of the environment in which innovation occurs. In particular, it often ignores the role of government in the process. Traditional theorists often assume that innovation is driven primarily by the play of free-market forces in the private sector. And, by traditional measurements, the medical device industry is highly innovative. First, the industry reinvests a high percent of sales in R&D (7.5 percent). The total number of patents issued to innovators has increased, and shipments of medical devices are growing, with sales growth projected into the 1990s (2). But medical device innovation does not take place in a vacuum. Public policies impact the process at every stage. Conventional measures of innovation—industry R&D spending, number of patents issued, annual sales and shipments—may not tell us much about the role and impact of government intervention.
The Pivotal Role of Public Policy
Public policies such as federal regulation, product liability statutes, reimbursement rules, and government funding for basic research have had a significant impact on the production and diffusion of new medical devices.
Figure 5.2 notes some of the many federal and state institutions and activities that can affect innovation in the medical device industry.
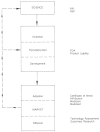
FIGURE 5.2
The stages of innovation: impact of institutions.
This chapter emphasizes federal regulation and reimbursement, two of the most important policies that affect medical device producers. As Figure 5.2 illustrates, there are other important policies as well, and reference is made to them throughout. For example, the National Institutes of Health (NIH) funds intramural and extramural projects that deeply affect the quality and quantity of biomedical scientific research. Product development and marketing strategies inevitably weigh the legal product liability environment, and government's actual or proposed interest in technology assessment may affect adoption and diffusion in the marketplace.
Such a diversity of government influences almost guarantees polyintervention, but before turning to an evaluation of this condition, the unique characteristics of the patient—that is, the medical device industry—must be explored.
THE MEDICAL DEVICE INDUSTRY AS PATIENT
A comprehensive policy analysis is complicated by the diversity of the medical device industry. An estimated 7,000 firms make over 1,700 types of medical products. In contrast, the pharmaceutical industry is composed of considerably fewer large, established companies. The firms within the device industry vary greatly. Some are single-product firms or have a small product line, such as IOPTEX Research, an innovative intraocular lens firm. Others are giants in computers and electronics, such as Hewlett-Packard, known for its sophisticated monitoring equipment, and General Electric, with its eight medical product subsidiaries, some of which are leaders in diagnostic imaging modalities. Still others are billion-dollar pharmaceutical firms with medical product divisions. For example, Pfizer, Inc., one of the world's largest drug firms, has a small laser subsidiary (Pfizer Laser Systems, with 50 employees and 3 products), a subsidiary producing hospital supplies and orthopedic implants (Howmedica), and a dental supply producer (Austenal). Medical device companies draw upon diverse fields of science and technology, and their products range from big-ticket capital equipment, such as lithotripters to crush kidney stones, with price tags over $1 million, to more mundane hospital supplies, such as surgical gloves and syringes. Finally, the commercial markets themselves cover a broad spectrum—from hospitals to physicians' offices, laboratories, and the home.
An assessment of the impact of government policies on device innovation must note that the medical device patient is many patients. Indeed, as we look at public policies, we must understand that the impact of policy prescriptions may vary significantly from segment to segment of this industry. Despite this diversity, however, I will not shy away from efforts to generalize in pursuit of greater understanding of the total policy environment.
THE EVALUATION: THE IMPACT OF POLYINTERVENTION
How does one analyze the impact of policies on innovation when there is so much diversity and fragmentation, both in products and firms in the private sector and among the public policies? One familiar approach is the case study. A case study examines how a policy or several policies affect the invention, development, adoption, and diffusion of a particular product or procedure. Case studies make up a large portion of the work on medical devices, provide essential data for the analyst (3-5), and can be used to illustrate specific relationships and interactions. This paper takes a different approach. Our analysis starts on the policy side of the equation. The search is for patterns, relationships, and interactions among the policies. The result is a more systematic examination of the total policy environment.
The matrix in Figure 5.3 aggregates the public policies on two dimensions: (1) whether they affect R&D of devices or adoption and use of devices, thus situating them along the innovation continuum (the vertical axis), and (2) whether the policy goal is to promote or to inhibit innovation (the horizontal axis). The boxes are numbered and represent a rough chronology based on the time when the relevant policies were introduced (6,7). In developing this matrix, several important themes emerge. First, while the thrust of policies in the 1950s and 1960s was to promote innovation, policies initiated in the 1970s and 1980s tended to inhibit them. Second, the matrix illustrates that the number of these policies has built up over time. Although some policies have been modified in response to changing social and political forces, very few are discontinued. New policies are rarely substituted for older ones. Rather, policies accumulate, and the effect is a stratification or layering of them. These policies are intended to influence all stages of the innovation process, and they have different goals. Some promote innovation; others inhibit it. By focusing on these trends in the policy environment, we can begin to see the collective effects on the industry. This global view helps identify the potential for conflict or duplication and leads the way to rational policy reform.
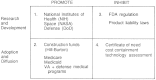
FIGURE 5.3
Public policies that promote or inhibit innovation.
The first box in the matrix focuses on how government policy promotes R&D. The primary vehicle for promotion is support for basic scientific research by NIH (8-10). Much NIH support involves funds for basic science rather than product development and may not have a direct impact on specific device technologies. However, NIH activities, such as the Artificial Heart Program, have targeted specific devices. The National Aeronautics and Space Administration's (NASA's) R&D programs have also supported technologies with device-related applications, primarily products that rely on electronic monitoring, control, and miniaturization. Research at the Defense Department encouraged work on ultrasound and lasers, technologies with valuable medical applications. The heyday of federal biomedical research support occurred in the 1950s and 1960s. More recently, support for biomedical science has leveled off, while funds for defense-related R&D have expanded (11). In addition, when the federal and state governments began to support health services through Medicare and Medicaid programs, political interest in research support began to wane. Politicians could point to support for health services, which had more immediate and direct benefits to constituents than the less direct and long-range research goals.
Box 2 identifies government promotion of demand for medical devices. The passage of Medicare and Medicaid in 1965 ushered in the biggest boom the medical device market had ever known. Although these programs did not support the device industry directly, they greatly expanded demand for its products. The impact was dramatic. Government programs injected billions of dollars into the medical marketplace every year, financing the adoption of medical technology. As federal spending accounted for an ever-increasing share of health expenditures, the acquisition of capital-intensive medical devices soared. This was especially true in the hospital sector, which was insulated from price in its decision making because payment policies allowed for capital cost pass-through to the federal government. The incentives under federal programs created price insensitivity, leading to near-maximum growth rate. By some estimates the government pays for over 40 percent of all new medical technologies (12). Government policy has changed the face of medical care. For example, capital- and technology-intensive facilities such as intensive care units, composed of sophisticated and expensive life-support and monitoring equipment, were virtually unknown in 1960; by 1984 they comprised 8 percent of all hospital beds. In the 1980s there has been dramatic growth in capital- and technology-intensive diagnostic imaging facilities.
Government policies that promote use of such devices continue. NIH maintains support for basic science—over $7.5 billion in 1990—which provides a strong foundation for some aspects of new device development. However, the amount in constant dollars has leveled off from the heyday of federal support in the 1950s and 1960s. Private spending now accounts for a much larger share of total R&D in the industry (11). Some special programs to promote small innovative businesses, as well as efforts to target product development through NIH, such as the Artificial Heart Program, have continued to support medical device innovation in its early stages.
In contrast, the policies of the late 1970s and the 1980s attempted to constrain the production and use of medical devices. Box 3 includes the two primary inhibitory policies: federal regulation and product liability statutes. Although regulation of food and drugs dates back to the turn of the century, and the FDA had some jurisdiction over medical devices as early as 1938, it was not until 1976 that the FDA acquired extensive pre-market and post-market regulatory authority over medical devices.
The stated goal of the Medical Device Amendments of 1976 was to “provide for the safety and effectiveness of medical devices intended for human use” (13). The law created a three-tiered classification scheme based on the degree of health risk presented by a device. Devices that pose the most significant safety risks are placed in Class III, the most restrictive classification, and must receive pre-marketing approval. Class II devices must meet performance standards, and all devices are subject to the general controls of Class I. These include reporting of defects, plant inspections, and other post-marketing restraints (14). This system acknowledges the variety of medical devices and is quite different from the requirement by the FDA that all new drugs must undergo pre-marketing approval.
FDA's implementation of the complex device law has been controversial. FDA has subjected only a tiny fraction of the thousands of medical devices to pre-marketing approval; the vast majority of devices have entered the marketplace virtually unregulated. Congress regularly has expressed dissatisfaction with the FDA's implementation of the law (15). Recent General Accounting Office (GAO) reports have criticized the FDA for shortcomings in both pre-marketing review and post-marketing surveillance (16,17). For the last several years the subcommittees of the House Committee on Energy and the Environment noted “regulatory gaps and loopholes” and “inadequate agency scrutiny” of new medical devices (18).
In the final hours of the last Congress, new medical device legislation was enacted (19). The new law streamlines the device classification process and expands FDA's authority to track devices, recall defective products, and impose civil penalties on the industry. It also extends reporting requirements to hospitals and other facilities.
Although it had antecedents in early common law rules, there was an expansion of product liability law in the 1970s. The goal of liability law is to compensate injured users for product-related harm and to deter the production of unsafe products. Judgments imposing liability even in the absence of fault on the part of manufacturers (known as strict liability) and huge jury awards, including the imposition of punitive damages, led to proliferation of lawsuits and immense legal uncertainty for innovators (20). This uncertainty is exacerbated by the lack of uniformity in the separate state jurisdictions. Because each state develops its own set of liability policies in its legislature and courts, the legal environment is complex and relatively unpredictable. Many medical devices have been caught up in the liability environment— most notably, intrauterine devices, heart valves, pacemakers, and anesthesia equipment (21,22).
Box 4 identifies government policies that inhibit the adoption and diffusion of medical devices. Although concerns about costs date back almost to the advent of Medicare and Medicaid, cost containment became a central focus in the late 1970s. From 1975 to 1980 the percentage of Gross National Product (GNP) spent on health care rose from 8.4 to 9.2 percent. This is relatively small compared with the post-1980 changes: from 10.7 percent in 1985 to 11.1 percent in 1987, with projections to over 11.5 percent for 1990 (23). Medicare payments to hospitals nearly quadrupled from 1980 to 1987, rising from $25.9 billion to $100.9 billion.
In 1982 Congress passed legislation that began the process of payment reform for Medicare (24). In 1983 the final plan established the Prospective Payment System (PPS), replacing the cost-plus payment rules that allowed providers to set the amounts for reimbursement. Under PPS, payment rates are set in advance, with all procedures placed in 467 separately priced diagnosis-related groups. The hope was that this type of payment system would make expenditures more predictable and hospitals more efficient, ultimately controlling escalating costs.
Although these and other efforts to control costs have not succeeded in reducing overall expenditures, they have had an impact on how and where health care dollars are spent. The incentives of this complicated system have slowed the growth of inpatient hospital costs, although the increase in outpatient costs has outpaced inflation. There is an extensive and growing literature on how PPS has changed resource allocation, locus of care, and health care spending generally (12,24).
As one might expect, PPS also has had an idiosyncratic effect on medical technology. The system appears to discourage big-ticket items, especially those used in hospitals. Under the old rules a portion of capital expenditures could be passed through to the Medicare system—that is, hospitals bore very little of the true costs of the purchased equipment. PPS intends to limit or eliminate this pass-through provision, but that policy has not yet been implemented. When it is, there will be even greater constraints on hospital technology purchases. Given the financial pressures for hospitals to control spending, PPS has been hard on technologies that require costly support systems. Innovations such as angioplasty equipment (lasers and other catheters to unplug arteries) require highly trained specialists and a high-cost hospital setting. To date, PPS applies only to hospitals (Part A of Medicare); thus, there has been an incentive to produce new technologies that can be used outside the hospital, leading to growth of mobile units and freestanding diagnostic and surgical centers.
There is no doubt that efforts to control spending are here to stay. The question is how those efforts will affect the market for medical devices. At the very least, the marketplace will be uncertain as new or additional proposals appear in the challenging process of cost control. Uncertainty alone may deter innovators. At the very most, these efforts, coupled with limits on market size or constraints on profits through payment policies, will dampen innovation in this industry significantly.
Each public policy that has been described, standing alone, has the potential to inhibit innovation in medical devices. However, their effects are exacerbated by polyintervention. Uncertainty is magnified when changes in a number of public policies can alter the incentives to produce or market a product. As we have seen, these policies have different goals, emanate from different agencies and institutions, involve different decision-making processes, and change at different times, generally without consultation or coordination. In addition, stratification of rules and regulations can lead to redundancy, conflicts, and deleterious interactions. Managing this complex environment requires constant, careful, and costly vigilance. Two case studies illustrate the interactions between innovators and the policy environment.
Case Study: Lithotripsy
The introduction of extracorporeal shock wave lithotripsy (ESWL) in 1984 illustrates dynamic innovation in the private sector and its relationship to public policies (25-28). Kidney stones in the urinary tract (urolithiasis) develop when minerals, primarily calcium and oxalate, form crystals rather than being diluted and passed out of the body. More than 300,000 patients a year, 70 percent of them young to middle-aged males, develop kidney stones. For many, treatment with fluids and painkillers is sufficient; in 20 to 40 percent of cases, however, the stones cause secondary infections, impaired kidney function, or severe pain, warranting more aggressive intervention, but until the past decade, surgery was the only form of medical help in most cases.
The first major advance included percutaneous endoscopic techniques developed in the early 1980s that permitted stone extraction or disintegration and reduced the morbidity associated with conventional surgery. The second major advance was ESWL. Its most exciting feature was that it offered a noninvasive way to treat kidney stones. The first ESWL devices required the patient to be placed in a water bath. After X-ray monitors positioned the patient, intense sound waves were generated by a high-voltage underwater spark. The resultant sound waves disintegrated the stone into fine bits of sand that could easily pass out of the body. Subsequent technological modifications eliminated the need for the water bath, and mobile units were developed. Currently, devices that use optical fibers as conduits for pulses of laser light that fragment stones are in experimental stages of development.
Although ESWL is an exciting innovation, several factors might have led to skepticism about its likely commercial success. The equipment was very expensive (early models cost at least $1.5 million), there was a viable surgical alternative, and the patient base was small and likely to remain so. When the constraints of public policy—regulation and cost containment—are added, the successful adoption of the technology becomes even more doubtful. The device was subject to the highest form of FDA regulation—Class III— requiring pre-marketing approval by the FDA. In addition, payment incentives seemed to be against its rapid adoption by hospitals. Only a small percentage of kidney stone patients are covered by Medicare, and current Medicare payment levels for outpatient ESWL do not adequately cover the costs of the treatment for those patients who receive it.
Despite these barriers, the product took only 13 months to receive FDA approval and then diffused rapidly. There were 200 lithotripters in operation within 2 years of introduction. The market now includes 220 devices and is basically saturated. There are 10 firms in the market. Only four have received FDA approval, and the others have devices in investigational stages. The market leader is Dornier Medical Systems, the first to receive a pre-marketing application (PMA), along with Medstone, Diasonics, Technomed, and Northgate Research.
The next generation of machines is already in development. In a relatively short period there have been major improvements in the original device; other designs, such as the use of laser technology, are on the horizon. There have been a number of creative marketing solutions to the problems of high cost and low patient volume. Entrepreneurs have put together joint ventures with physicians and hospitals that ensure a broad patient base, lower the unit cost of treatment, and amortize the cost of the device. Some freestanding centers have developed symbiotic relationships with providers of other forms of kidney stone treatment so that comprehensive services and alternative treatments to lithotripsy are all available in one center.
What lessons about innovation in the device industry can be learned from this case? How can we explain the success of this expensive, highly regulated technology? One explanation is that truly useful technologies usually succeed despite the barriers placed in their paths. There is, no doubt, truth to this conclusion. However, it may be that the dynamism and creativity of the industry are based on the expectation of enormous market expansion through the application of this technology to patients with gallstones, a much more prevalent clinical condition.
There are 20 million gallstone patients in the United States, with 487,000 gallbladder removals in hospitals every year. The treatment of gallbladder disease is a $5 billion market (28). If lithotripsy could be applied to many of these patients, hospitals could avoid much of the costs associated with surgery, and the firms could compete for this greatly expanded market.
Whether that expansion will occur is now in doubt. Here is where the policy process reenters. In October of 1989, an FDA advisory panel recommended that the agency disapprove the PMAs filed by Dornier Medical Systems and Medstone International for biliary (gallstone) lithotripters. The panel members expressed concern about the safety data in the PMAs. Questions also were raised about the effectiveness of lithotripsy for destroying all gallstones. Preliminary evaluations reveal that only a small percentage of patients with gallstones may benefit from EWSL (29). The delay in marketing approval will allow competitors to catch up with the two leaders, although the ultimate clinical usefulness of biliary lithotripsy remains uncertain. Manufacturers have been slow to gather data because the lack of any third-party reimbursement for this new procedure has limited the number of patients who have received it. In addition, because the drugs used in conjunction with the treatment work slowly, studies often take a long time to complete.
The failure to receive FDA approval may be only a temporary and minor delay. It may also mean that the technology is inappropriate for the proposed use and that the FDA sagely is placing safety concerns ahead of the desires of the innovative firms to rush to market. Or we may be seeing a regulatory failure in that the FDA is inappropriately obstructing the entry of a valuable innovation into the marketplace. FDA's decision delays reimbursement from third-party payers, including Medicare, which will rarely pay for unapproved technologies, further burdening the innovators. Nor does FDA approval necessarily guarantee Medicare coverage of the procedure. The Health Care Financing Administration (HCFA), Medicare's payment authority, makes its own assessments of new technologies for coverage and payment decisions, often independent of FDA findings.
At this point, the lithotripsy industry remains dynamic, highly innovative, and very competitive. However, the market for kidney stone treatment is not expanding. No improved technology to date has left the others outmoded. Whether the expansion for use in gallstone treatment will occur depends upon the public sector—the FDA and third-party payers (primarily Medicare). The marketplace must wait for the policy process to resolve the debate.
Case Study: Intraocular Lenses
Millions of Americans, particularly the elderly, suffer from eye diseases causing impaired vision. Cataracts, opacities of the lens of the eye, are often a result of degenerative changes in old age or of such diseases as diabetes. The symptoms include gradual loss of vision. The usual treatment is removal of the diseased lens and implantation of an intraocular lens (IOL) (30-34).
Ophthalmology in general, and IOLs in particular, represent one of the largest and most dynamic health care markets. IOLs are regulated by the FDA. Because most of the implant candidates are elderly, the market is strongly tied to Medicare policy as well. The interaction of regulation and reimbursement has the potential for significant impact on the industry.
IOLs are one of the few ophthalmic products that the FDA has placed in Class III. Regulated since 1979, IOLs are subject to special requirements imposed by Congress and enforced by the FDA. FDA reviews data on safety and efficacy for all Class III devices in the PMA stage. During the experimental stage, Class III products may receive an investigational device exemption (IDE) that allows them to be used in controlled studies while the manufacturer gathers and evaluates data about safety and efficacy. The collection of data supporting a PMA is expensive and time consuming and may represent a significant barrier to entry for innovative firms. For IOLs, however, a special exception was made. Producers could charge for the costs of the implanted lenses in the IDE stage. This exemption facilitated the development of IOLs during the 2 or more years of device testing required in clinical practice.
The vast majority of IOL implants are done on elderly patients with cataracts or other degenerative eye problems. Indeed, the availability of Medicare payment guaranteed a large, stable market for lens removal and IOL implantation. The average cataract patient is 68 years old, so Medicare is virtually the only payer for cataract surgery. The numbers of implants grew rapidly in the 1980s. There were 177,000 implants in 1979 and 888,000 in 1986. In 1987 there were 1.1 million implants in the United States and another 1 million internationally. IOL sales have been estimated at $400 million annually.
Cataract surgery costs the federal government close to $1.5 billion a year. It is the largest item in the Medicare program. During the 1980s, much of the treatment shifted from hospital to outpatient surgery centers or physicians' offices, which are covered under Part B of Medicare. Medicare provides the funding; growth can be attributed to advances in technology, including cataract management, anesthesia, surgical technique, and postoperative care. New IOL technology includes soft lenses that can be implanted with smaller incisions (the one-stitch lens is popular), bifocal implants, and other specialty lenses.
There are a number of companies in the IOL market, ranging from large firms such as Johnson & Johnson (IOLAB), Coopervision (acquired by Alcon/ Nestle), and Allergan (purchased by Smith Kline in 1989), to such smaller firms as IOPTEX Research, a small, privately held industry leader, and Chiron Ophthalmics, a subsidiary of Chiron Corporation, a biotechnology firm. There are foreign IOL makers from West Germany, France, Belgium, Israel, and Japan as well.
Changes in Medicare policy as well as FDA regulations present some threats to the market for IOLs. Congress has reduced federal Medicare payments for cataract surgery twice since 1986. HCFA has lowered the amount of payment to physicians for the procedure, which had increased 61 percent in a 3-year period. HCFA also plans to establish a new payment rate for an IOL implanted during the cataract extraction. This proposed rate of $200 would mean an average decrease of at least $100 on the former IOL rate. Some have advocated a tiered rate to accommodate the higher costs of newer specialty lenses. To date, the proposed flat rate of $200 stands.
The FDA has also imposed new requirements for data collection. For new bifocal and multifocal products, 50 implants have to be studied for a year, and then the studies can be expanded to 500 implants. Overall, it is likely to take nearly 4 years before a new lens will receive its pre-market approval. The guidelines have made it difficult for new companies to introduce competitive products.
This longer period for approval is less onerous if the innovator receives at least partial payment for experimental lens implants. Rumors have been circulating that the exemption allowing payment for IOLs under IDEs soon will be rescinded. The HCFA officials say that this will encourage producers to move from the IDE to PMA stage. They assert that companies have been allowing products to languish under IDEs because the exception reduces the economic incentive to go to market. The device is paid for in either case. However, the longer testing requirements and threatened withdrawal of payment during the investigational period probably will have an inordinate impact on newer, less well-capitalized entrants.
The changes in Medicare payment rates and FDA regulations could each have an adverse effect on innovation. The collective impact, however, is likely to be even more significant, particularly on smaller or newer firms. Clearly, cost containment and safety are important considerations. There have been serious concerns about overcharging for the implant procedures and the products as well as suspicions about unnecessary implants and concerns about the safety of some designs. However, it remains to be seen how the pursuit of safety, efficacy, and cost containment will affect innovation in this dynamic segment of the device industry.
TREATMENT OPTIONS: IMPROVING THE PUBLIC POLICY ENVIRONMENT
The public wants innovations that improve the quality of medical care. Many government policies have promoted innovation, including Medicare payments and federal support for biomedical research. However, the public supports other values, such as safety, in medical technology. Although it is hard medicine to swallow, there is a recognition that cost containment in some form is also necessary. Can we have it all? Probably not. However, an understanding of the policy environment can prevent unnecessary constraints on innovation, particularly those caused by the interaction among the many overlapping policy interventions. The following discussion suggests some ways of approaching policy reform. It is meant to be illustrative and thought provoking; it is beyond the scope of this chapter to map a detailed blueprint of comprehensive change.
Fine-tuning Individual Policies
As policy makers implement their goals, they should be sensitive to the potential impact on innovation. A brief look at regulation and reimbursement illustrates this point. If proposed reforms make regulation more stringent, as pending bills appear to do, certain precautions are essential. The well-documented drug lag occurs when regulation delays entry of innovative pharmaceuticals onto the market (35). Recent efforts by the FDA to speed up the regulatory process, particularly for drugs for life-threatening conditions such as AIDs and cancer, should be applauded. Similarly, tightening controls on medical devices must never be allowed to create a “device lag.” Congress should consider this possibility and make appropriate legislative accommodations, including adequate resources for timely device evaluation. Congress should also consider better use of post-marketing controls to monitor devices in use. These regulations do not delay entry into the market and are less burdensome on innovation than pre-marketing regulation. However, given the intense criticism of FDA's post-marketing surveillance system, Congress is skeptical of FDA's commitment to this form of regulation. The general point to be made is that the FDA should balance the desire for innovation with its goal of promoting safety and efficacy in medical devices. Lessons learned from the pharmaceutical experience should help develop an appropriate balance between safety and innovation.
Policy makers in the business of containing costs should also be sensitive to the value of innovation. Many have expressed concerns about stifling innovation, but because it is an elusive concept to measure and costs are spiraling ever higher, it is easy to overlook this value in pursuit of another. Proposals that support innovation include allowances for scientific advances or pass-throughs for certain new technologies during some designated trial period; automatic temporary approval for coverage by third-party payers of all FDA-approved technology for designated periods of time; and allowances for experimental treatments and technologies in designated centers of excellence to gather information on costs and benefits of innovations. Innovation need not increase costs, although many innovations do, particularly when first introduced. Premature decisions about the value of innovations may inhibit more thoughtful and accurate evaluations that can come only with experience. The system must permit more flexibility in order to protect innovations, particularly when public payment policies to do otherwise influence such a sizeable share of the potential market.
Understanding Polyintervention
It is also possible to conceptualize reform among the various policies, particularly to control duplication and overlap in the polyinterventionist environment. To illustrate, Figure 5.4 takes another view of the policy matrix.
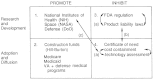
FIGURE 5.4
Interaction of public policies that affect innovation.
Three types of interactions are denoted by lowercase letters: “a” represents the interactions within boxes, “b” represents interactions between boxes on the vertical axis, and “c” represents interactions across boxes on the horizontal axis. Again, a cautionary note is appropriate. The discussion that follows is meant to highlight how the analysis might proceed; it is not intended to propose comprehensive policy reform.
Policies within one box on the matrix, “a,” presumably intend to accomplish similar goals (to inhibit supply or promote demand, for example). In some cases there is policy duplication and overlap, often imposing unnecessary costs and constraints on innovators. I have written a detailed discussion of the interaction between regulation and product liability with suggested proposals for reform (21). The general point of that article can be summarized succinctly. Both regulation and liability are systems designed to promote safety. Although their goals overlap, the institutions that carry out these policies employ vastly different methods and evaluate safety in different ways. The value of device safety can be protected without two elaborate institutions. Reform of the system depends on a wide range of factors. The ultimate goal would be to preserve or improve the pursuit of safe medical devices while eliminating overlapping, inconsistent, and irrational attributes of both systems. Similarly, one can evaluate more rational coordination of policies in box 4—for example, linking technology assessment mechanisms to HCFA's cost controls.
It is also possible to coordinate policies between boxes on the vertical axis, represented by “b.” These policies share common goals—to inhibit or to promote innovation—albeit at different stages of the innovative process. Coordination might make the policy environment more coherent for manufacturers and might reduce costs of federal intervention in the process.
An illustration of such cooperative efforts by the FDA and HCFA is the Cardiac Pacemaker Registry. Following controversies about unnecessary implantation of pacemakers and serious safety violations by some pacemaker companies, the FDA and HCFA proposed a joint rule to establish a registry. Physicians and providers requesting or receiving Medicare payment for implantation or removal of a pacemaker must provide product safety and performance information to the pacemaker registry database. The final rule permits HCFA to deny Medicare payment to providers who fail to submit required information to the registry (36). The FDA's independent jurisdiction extends only to manufacturers, not providers. By developing a cooperative scheme, the FDA can acquire information about pacemaker performance to which providers, not manufacturers, have direct access. The link to payment is a strong incentive for compliance.
It is also important that the two agencies do not duplicate evaluations. There is a current controversy about whether the HCFA should engage in safety evaluations of products that have received FDA approval. Some believe that these efforts are redundant and that safety determinations need to be made only once by one federal agency; FDA approval should be sufficient for other agencies. Others accuse the HCFA of using safety inappropriately as a pretense to deny coverage and reimbursement for medical technologies that it does not want to pay for. Regardless of the merits of these accusations, it is legitimate to question federal duplication of safety evaluations.
It is also possible to coordinate policies even when the goals are very different—that is, promotion of R&D and inhibition of diffusion and adoption. This is represented by “c” on the matrix. For example, NIH, or other sources of federal research money, could be used to promote products that would specifically reduce health care costs. It is legitimate to ask, for example, why NIH promotes artificial heart research when it is unlikely that Medicare would ever be able to pay for the procedure for all the elderly that might want or need it. It is interesting to note that some NIH officials tried to curtail the program in 1988, but Congress prevented that action. The fight to save the program was led by Senator Orrin Hatch (R-Utah), in whose state much of the funded research takes place.
CONCLUSION
Polyintervention describes an environment in which there are many different policies imposed by a variety of institutions. This multivalent policy environment is rooted in our federal system, with the separation of powers across the branches of the federal government and the existence of 50 sovereign states. Moreover, it is inevitable that important products such as medical devices will attract many levels of scrutiny because of the great social costs and benefits associated with health care.
This diversity and complexity of policies can be frustrating to innovators, particularly if they do not appreciate the intricate history of health policy in the United States. It must not be forgotten that multiple sources of public policy may be beneficial as well. The system permits experimentation and flexibility and prevents the likelihood that one institution or individual could unilaterally ban or promote a technology. Nevertheless, polyintervention is particularly acute for the medical device industry and can have an adverse impact on innovation. While innovation is important, other values, including safety, universal access, and cost controls, cannot be overlooked. Some of these values may compete with innovation. Our ultimate goal should be to reconcile the competing societal values wherever possible. When it is not possible, we must be explicit about the conflicts and make the hard policy choices fairly and rationally. Innovators and patients deserve no less.
REFERENCES
- 1.
- Jewkes J, Sawers D, Sillerman R. The Sources of Innovation. London : Macmillan, 1969.
- 2.
- Pollard MR, Persinger GS. Investment in health care innovation. Health Affairs 1987 ; 6:93-106. [PubMed: 3114113]
- 3.
- Rettig R. The politics of organ transplantation: a parable of our time. Journal of Health Politics, Policy and Law 1989 ; 14: 191-227. [PubMed: 2654279]
- 4.
- Rettig R. Lessons learned from end stage renal disease experience. In Egdahl RH, editor; , Gertman P , editor. (eds). Technology and the Quality of Health Care. Germantown, MD : Aspen Systems; 1978 : 153-174.
- 5.
- Plough A. Borrowed Time: Artificial Organs and the Politics of Extending Lives. Philadelphia : Temple University Press, 1986.
- 6.
- Foote SB. From crutches to CT scans: business-government relations and medical product innovation. In Post JE , editor. (ed). Research in Corporate Social Performance and Policy. Greenwich, Conn. : JAI Press, 1986.
- 7.
- Foote SB. Coexistence, conflict, cooperation: public policies toward medical devices. Journal of Health Politics, Policy and Law 1986 ; 11: 501-523. [PubMed: 3794266]
- 8.
- Shannon JA. Advancement of medical research—a twenty year view of the role of the National Institutes of Health. Journal of Medical Education, 1967. [PubMed: 5334672]
- 9.
- Harden V. Inventing the NIH: Federal Biomedical Research Policy 1887-1937. Baltimore : Johns Hopkins University Press, 1976.
- 10.
- Spingarn N. Heartbeat: The Politics of Health Research. Washington, D.C. : Robert B. Luce, 1976.
- 11.
- Brooks HR. National science policy and technology innovation. In: Landau, R, editor; , Rosenberg, N , editor. (eds). The Positive Sum Strategy. Washington, D.C. : National Academy Press, 1986: 119-167.
- 12.
- Garrison LP, Wilensky GR. Cost containment and incentives for technology. Health Affairs 1986; summer : . [PubMed: 3527916]
- 13.
- Preamble to MDA Pub. L. no. 94-295, 90 Stat. 539.
- 14.
- Foote SB. Loops and loopholes: hazardous device regulation under the 1976 medical device amendments to the Food, Drug and Cosmetic Act. Ecology Law Quarterly 1978 ; 7: 101-135.
- 15.
- United States House of Representatives, Committee on Energy and Commerce, Subcommittee on Oversight and Investigations. Medical Device Regulation: The FDA's Neglected Child. Washington, D.C. : U.S. Government Printing Office, 1983.
- 16.
- General Accounting Office. Medical Devices: The FDA's Implementation of the Medical Device Reporting Regulation. Washington, D.C. : U.S. Government Printing Office, 1989. (GAO/PEMD 89-10).
- 17.
- General Accounting Office. Medical Device Recalls: An Overview and Analysis 1983-1988. Washington, D.C. : U.S. Government Printing Office, (GAO/PEMD 89-15BR).
- 18.
- United States House of Representatives, Committee on Energy and Commerce, Subcommittee on Health and Environment. Hearings on health and safety issues related to medical devices. Testimony of the Honorable John D. Dingell. Washington, D.C. : U.S. Government Printing Office, November 6, 1989.
- 19.
- Pub. L. no. 101-629, 1990.
- 20.
- Mahoney RJ, Littlejohn SE. Innovation on trial: punitive damages v. new products. Science 1989 ; 146: 1395-1399. [PubMed: 17755994]
- 21.
- Foote SB. Product liability and medical device regulation: proposal for reform. In : Ekelman K , editor. (ed). New Medical Devices: Invention, Development, and Use. Washington, D.C. : National Academy Press, 1988:73-92. [PubMed: 25032312]
- 22.
- Mastroianni L, editor; , Donaldson PJ, editor; , Kane TT , editor. (eds). Developing New Contraceptives: Obstacles and Opportunities. Washington, D.C. : National Academy Press, 1990. [PubMed: 25144076]
- 23.
- Reinhart, UE. Somber clouds on the horizon. Health Week Forecast '88, December 23, 1987.
- 24.
- Russell L. Medicare's New Hospital Payment System. Washington, D.C. : The Brookings Institution, 1989.
- 25.
- Biomedical Business International, July 15, 1988 ; 11: 98-100.
- 26.
- Alder HC, Murray ML. Operating characteristics of U.S. lithotripter facilities. In American Hospital Association, Hospital Technology Series Special Report, July 1987. [PubMed: 10304474]
- 27.
- Weiss M, Freiherr G. Romancing the market for stones. Healthweek, December 4, 1989: 18-26.
- 28.
- Citrin DB. Extracorporeal shock wave lithotripsy. Arthur D. Little Decision Resources, 1987 ; Sec. 2: 85-88.
- 29.
- Costly shock wave machines fare poorly on gallstones, disappointing hospitals. Wall Street Journal, February 9, 1990: B1, B6.
- 30.
- United States House of Representatives, Committee on Ways and Means. Medicare Reimbursement for Cataract Surgery. Washington, D.C. : U.S. Government Printing Office, 1985 ; 35: 99-37.
- 31.
- Editorial. The role of the Food and Drug Administration in ophthalmology. Archives of Ophthalmology, August 1986 ; 104: 1145-1148. [PubMed: 3755590]
- 32.
- Worthen DM, Boucher JA, Buxton J, Lowther G, Talbott M. Update report on intraocular lenses. In American Academy of Ophthalmology 1981 ; 88: 381-385. [PubMed: 7267010]
- 33.
- McCarthy E, Pokras R, Moien M. National trends in lens extraction 1965-1984. Journal of the American Optometric Association 1988 ; 59: 31-35. [PubMed: 3278043]
- 34.
- Biomedical Business International, May 16, 1989; 12: 69-72.
- 35.
- Kaitin K, Mattison N, Northington F. The drug lag: an update of new drug introduction in the United States and the United Kingdom, 1977 through 1987. Clinical Pharmacology and Therapeutics 1989 ; 46: 121-138. [PubMed: 2758722]
- 36.
- Federal Register. Vol. 52 , No. 141, July 23, 1987, 27756-27765. [PubMed: 10301665]
Footnotes
1. The medical device industry is not the only one subject to polyinterventionary effects. Others include the oil industry, the automobile industry, and the nuclear power industry. For each, the configuration of public policies varies according to the history of the industry, the values of the public, and the perceived importance of the issues at stake.
- PubMedLinks to PubMed
- The Impact of Public Policy on Medical Device Innovation: A Case of Polyinterven...The Impact of Public Policy on Medical Device Innovation: A Case of Polyintervention - The Changing Economics of Medical Technology
Your browsing activity is empty.
Activity recording is turned off.
See more...