“This lesion (the atheroma), undoubtedly the most dangerous of all prethrombotic processes, has often been called an atheromatous abscess.”
Meyer Friedman, [60]
The Necrotic Core
Atherosclerosis is characterized by the proliferation, then destruction of intimal fibrous tissue, resulting in the formation of an atheroma, as originally proposed by Virchow [39]. The presence of a necrotic core is objective evidence that atherosclerosis is ultimately a destructive, not an FP disease, and the necrotic core is a component, not a complication of atherosclerotic disease. The precise mechanism of atheroma formation has not been fully worked out [12,37], but apparently the IA, directly or indirectly, is responsible for cell death, leading to non-viable degenerative tissue and eventual necrosis [58]. The overall cell count within a plaque decreases as the plaque enlarges, indicating that the lost cells are not being replaced, and that plaque enlargement is due primarily to the growth of the necrotic core rather than to the growth of fibrous tissue [58]. The mechanism of cell death is believed to be either coagulation necrosis or apoptosis, possibly related to the toxic effects of oxidized LDL or pro-apoptotic mechanisms [25,37,39,59]. Both of these responses, active tissue proliferation and progressive enlargement of the necrotic core, result in increasing luminal stenosis and ultimately, if unchecked, to obstruction of coronary flow.
As stated in Chapter 3, plaque size, as reflected by the severity of luminal stenosis, is directly related to the magnitude, severity, and extent of injury caused by the IA, as shown in the magnitude of the T lymphocyte response in the adventitia. The same may be true of the formation and growth of atheromas. If plaque size is directly related to the magnitude of the injury caused by the IA, then it follows that the size of the atheroma, also produced as a result of activity of the IA, is also related to the magnitude of the injury. The formation and growth of an atheroma may also be directly related to the toxicity, virulence, concentration, or dose of the IA causing atherosclerosis, and/or to the susceptibility of the patient to the ongoing, progressive, destructive activity of the IA.
Size of Atheroma and Luminal Stenosis
Table 2 illustrates the relationship between luminal stenosis and the presence of an atheroma in 83 patients who died of ACD. Atheromas were present in 2,223 (32%) of 7,056 coronary segments taken from these 83 patients, with 88% in plaques with more than 50% luminal stenosis. There was a significant difference in frequency, p=<0.001, of atheromas in those segments with >80% stenosis compared with segments having <50% stenosis. These results show that the frequency of atheromas increases as the plaque enlarges.
Table 2
Comparison of luminal stenosis and size of atheroma in 83 patients who died of acute coronary disease.
The circumferential extent of intimal involvement with an atheroma, also determined by a review of each microscopic section, was graded in the following manner: Each artery segment was divided into four, 90° quadrants, then graded on how many of the four quadrants contained an atheroma. Grade I indicated one quadrant contained an atheroma; Grades II, III, and IV indicated involvement of two, three, or four additional quadrants contained an atheroma. Table 2 shows the relationship between luminal stenosis and the severity and extent of atheromas in each coronary segment. The relationship between luminal stenosis and the circumferential extent of the necrotic core showed Grades III and IV atheromas were significantly more common, p=<0.001, in those segments with >80% luminal stenosis than in those with <50% stenosis. Therefore, a direct relationship exists between plaque size, as reflected in the severity of luminal stenosis, and the circumferential extent to which each coronary segment is involved with an atheroma. Progressive and expanding injury caused by the IA results in a progressive increase in plaque size and a progressive increase in the proportion of that plaque that is atheroma.
Atheromas Are Similar to Bacterial Abscesses
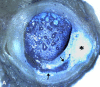
Atheromas are abscess-like in the sense that they are associated with inflammatory infiltrates, grow in size, are composed of necrotic, amorphous material, are acellular, and contain toxic, irritating, and antigenic material [37,60,61]. Atheromatous degeneration is similar to caseation observed with tuberculosis in that both have a high lipid content and appear “cheesy” on gross appearance. Figures 6A, 6B, illustrate the gross and microscopic appearance of atheromas, showing a yellow, acellular, lipid-laden, cheese-like necrotic core with a semi-solid consistency. These photos show the tendency of atheromas to grow circumferentially from a central focus toward the plaque shoulder, and are frequently associated with adventitial inflammatory infiltrates (Figures 5B-5E).
The natural course of events for most bacterial abscesses is to point and drain spontaneously, removing the offending organism and the toxic core material. The spontaneous rupture of a bacterial abscess is related to increasing volume and pressure within the necrotic core, plus the action of digestive, proteolytic enzymes on the surrounding tissue, particularly on the overlying cap. Resolution and healing of such abscesses commonly follows this spontaneous drainage, provided the necrotic core is sufficiently emptied of necrotic material to allow resolution to take place. The over-all inflammatory response associated with localization and encapsulation of the necrotic tissue and of the offending agent that characterizes a bacterial abscess is an important component. This is thought to be a physiologic defense against the growth and spread of a necrotizing, destructive organism. The development of a pathologic condition or disease as a result of this spontaneous drainage does not mean these physiologic defenses, per se, are at fault. For example: Rupture of a bacterial bowel abscess that results in peritonitis does not mean the defensive responses leading to spontaneous rupture and drainage of the abscess are pathologic, or that these defensive responses can or should be prevented. Proper treatment is to focus on and treat the IA, not the defensive responses. The same may be true of atheromas.
Proteolytic Enzymes
Atheromas contain a number of proteolytic enzymes, particularly matrix metalloproteinases (MMPs), derived primarily from monocyte-derived macrophages present in and around the necrotic core of atheromas [62,63]. These digestive enzymes, because of their ability to digest injured or degenerated tissue, play a major role in the enlargement and growth of atheromas, but they are also necessary for the repair of injury [62]. Therefore, the digestion and destruction of dead or injured tissue by MMPs is a component of active atherosclerotic disease and constitutes one method of converting dead and damaged tissue to a semisolid form, presumably for the purpose of removing it.
Figures 6B-6F illustrate digestion of fibrous tissue, taking place within different atheromas in different patients. The fibrous tissue undergoing digestion is acellular with loss of normal tissue substance and architecture, and it stains differently from neighboring, viable cellular tissue. If these proteolytic enzymes are essential to the physiologic resolution of injury, then the action of such enzymes in the formation of atheromas is, in the final analysis, also a physiologic response, not a complication of the disease. In other words, these enzymes may provide a key defense by promoting the removal of degenerating tissue and toxic agents from the artery wall.
Some of the fibrous fragments are quite large, have been completely separated from the surrounding wall, and are free-floating within the lipid core (Figure 6F). Should a major PU occur, these tissue fragments would constitute a sizable embolus to the distal circulation. Because of their fibrous structure, they may be difficult to remove by circulating enzymes and may cause ischemia and/or focal infarction [64]. The digestion of these fragments by MMPs, while the atheroma is still intact and before rupture or ulceration, may reduce the pathogenetic potential of such tissue emboli.
“Exit” Tracts
Another potential action of MMPs is the creation of communicating channels or tracts between deep-lying atheromas and the artery lumen. Figures 7A-7C illustrate an example of a tiny, narrow, long, serpiginous tract that connects the lumen with an underlying necrotic core. The presence of red blood cells and injection mass within these tracts proves their in-vivo existence and excludes the possibility that they are cutting or post-mortem artifacts. Furthermore, the presence of fibrin, red staining material on Martius Scarlet Blue (MSB) stain, (Figures 7A, 7B), shows pre-mortem communication with the lumen, activation of the clotting system, and formation of intraintimal thrombus. These tracts pass through acellular, degenerated fibrous tissue (Figure 7C). They are presumably formed by the action of MMPs secreted by macrophages that line the tracts, and possibly with other digestive enzymes as well [63], providing a route for plaque contents to enter circulating blood. Specifically, these tracts are NOT formed by the splitting and disruption of normal, cellular, viable fibrous tissue by external forces, such as hemodynamic stresses [63], but are due to actions and metabolic activities taking place within the core [65]. Such tracts may be called “exit” tracts because they contribute to core decompression and reverse lipid transport. These communicating tracts are similar to the sinus tracts observed in chronic osteomyelitis and may open and close at recurrent intervals, depending on factors contained within the atheroma. Perhaps all plaques, at some time in their existence, will develop such tracts that serve to reduce plaque size.
Cleavage Planes
Atherosclerotic plaques often present a layered appearance, both on gross and histologic examination (Figures 7D–F). Stary, et al, suggest this layered appearance may be due to repeated disruptions of the lesion surface associated with hematomas or thrombotic deposits [15]. However, the layered appearance on gross examination to which we are referring, (Figures 7D–7F), is due to alternating layers of fibrous tissue and necrotic core tissue, caused by different light-absorbing properties of these two major tissue types. Tracing this layering through subserial sections often shows these seemingly separate foci of necrosis are actually caused by extensions of a single necrotic core, located either proximal or distal to this site. The layered appearance may be the result of a proximal/distal expansion or burrowing of an atheroma, probably facilitated by MMPs, growing and expanding in a longitudinal direction within the plaque at different depths [32]. Also, adjacent atheromas may communicate and fuse with one another to form interconnecting necrotic cores that can extend long distances throughout the intimal layer and be associated with multiple cleavage planes or tracts within a given atheroma or between atheromas [66]. The cleavage planes are often oriented parallel to the fibrous cap, with the necrotic core generally directed or oriented toward to plaque shoulder (Figures 7D–7F). Some of these cleavage planes may represent a previous exit tract, now healed (Figure 7E).
If this is the correct assessment of these cleavage planes, is the presence or formation of such tracts an important feature of atherosclerosis? The cleavage planes may be important if blood enters the core at one site, as through an UP, and then extends along cleavage planes, taking the path of least resistance through the atheroma, with resulting plaque swelling and an increase in luminal stenosis. The planes may also be important from the standpoint of drainage of plaque contents in that an exit tract at one point may serve to drain a necrotic core some distance away.
Plaque Shoulder Ulcerations
It is well known that macrophage foam cells and the MMPs they produce are heavily concentrated at the plaque shoulder [63], and that there is a propensity for the plaque to ulcerate at this site. Ridolfi, et al., noted a fibrous cap that was eroded on the under surface over a necrotic core, supporting the view that the fibrous cap is eroded from within [67].
Figures 8A–8C, illustrate ulcerations involving the shoulder of the plaque in different patients. The fibrous cap is thin and attenuated at the point of ulceration. The free end of the fibrous cap appears to be valve-like in structure, (Figures 8A–8C), so it could allow the extrusion of plaque contents in diastole, then close in systole, preventing the ingress of blood from the lumen. If this is correct, nature may provide a mechanism for plaque contents to be extruded, at the same time preventing or reducing the inflow of large amounts of blood into the core area. The force of arterial pulsation may pump or “milk” plaque contents into the lumen in this manner. Thus, the necrotic core may be intermittently drained or partially drained in this manner.
None of these UPs are associated with significant luminal or occlusive thrombosis. Therefore, spontaneous PU and debulking, early in plaque development prior to the development of significant luminal stenosis, may be beneficial. Ulceration and drainage of the necrotic core early in plaque development are rarely associated with thrombosis and may be beneficial if resolution, reendothelialization, and fibrotic scarring stabilize the plaque and reduce luminal stenosis [57]. These observations lead us to question the prevailing opinion that PU is a pathologic event that must be prevented if we are to prevent acute coronary disease [63].
Incision and drainage followed by resolution and healing are the proper treatment for all bacterial abscesses. Incision and drainage may also be the proper treatment for atheromas. PTCA is basically a form of incision and drainage because the atheroma is split, its contents drained by this procedure. Follow-up studies after PTCA show a reduction in luminal stenosis consistent with resolution and healing [68]. As long as the PTCA is accomplished without such complications as thrombosis or restenosis, this may be the preferred method of treatment for a large atheroma or a vulnerable plaque [69]. The use of coronary stents may contribute to the success of the PTCA by assisting in complete drainage, preventing or reducing post-PTCA dissection along cleavage planes, and promoting healing and subsequent resolution.
Increased Intraplaque Pressure
Evidence for increased pressure within an atheroma comes from observation of the actions of cholesterol crystals within the necrotic core. Figures 8D-F, illustrate parallel alignment of cholesterol crystals at the site of PU, suggesting that these crystals are being actively and forcibly extruded under pressure from within the necrotic core. This alignment of cholesterol crystals may be a marker of impending PU in the intact atheroma.
Figure 8F illustrates an explosion-like eruption of a relatively small atheroma, preceded by parallel orientation of cholesterol crystals within an exit tract. If disruption of the fibrous cap were caused by external hemodynamic stress, we would not expect to see such uniform orientation, but rather a total disorganization of the cholesterol crystals. These findings provide further support for, and are consistent with, increased intraplaque pressure and spontaneous rupture or ulceration of the necrotic core [66,70].
We propose actively growing atheroma result in increased pressure within the necrotic core. This increased pressure contributes to spontaneous ulceration and drainage of plaque contents, similar to that observed with bacterial abscesses.
The parallel orientation of the cholesterol crystals in Figures 8D and 8E, raises further questions about these crystals. Are they actually sharp and needle-like as they appear, and could they act like a battering ram to pierce or damage tissue, thereby facilitating or promoting PU? If so, then this is another mechanism to consider in the pathogenesis of PU.
Thermal Heterogeneity
Further evidence that atheromas are actively growing, inflammatory abscesses is provided by studies of thermal heterogeneity. These studies show active, progressive, growing, inflammatory atheromas are “hot” compared with adjacent normal tissue. Increasing vascularity is associated with this inflammation and is similar to the increased temperature associated with an inflamed and swollen bacterial abscess [65,71]. Although virtually all UP are associated with inflammation and could therefore be considered “hot,” there are many plaques with associated severe inflammation that have not ulcerated [57]. Therefore the use of this tool to identify the vulnerable plaque ready to ulcerate may be difficult. At present, the determination of thermal heterogeneity is an invasive technique, but its potential for identifying the most active plaques would be a major step forward.
In Review
Atherosclerosis is initially an FP disease, but it evolves into a destructive process that leads to luminal stenosis, caused primarily by the growth and expansion of lipid-laden, necrotic atheromas. PU and subsequent drainage of plaque contents are components of and a natural consequence of the growth of an atheroma, not, per se, a pathologic event. Attempts to prevent PU may not be appropriate. Atheromas grow primarily by the digestion of degenerated, lipid-rich, fibrous tissue, through the action of proteolytic digestive enzymes, particularly MMPs. The growth and expansion of atheromas result in increased pressure within the necrotic core, leading to the formation of cleavage planes that communicate with adjacent atheromas and to the formation of exit tracts and/or UP, primarily at the shoulder of the plaque. Decompression and/or debulking of atheromas through PU, early in plaque development, prior to significant luminal stenosis, may be beneficial in stabilizing the plaque, reducing luminal stenosis, and halting active progression of the disease process at that site.
Unanswered Questions
What is the natural history of an UP that is not completely drained of necrotic contents at the time of plaque rupture or ulceration? Do such partially drained atheromas act like an incompletely drained bacterial abscesses? Do they continue to fester as a chronic, indolent lesion that intermittently drains and releases plaque contents, as in chronic osteomyelitis? Do all plaques with a necrotic core ulcerate and drain, not just once, but repeatedly during their development? What are the implications, in terms of potential complications, of a single necrotic core extending long distances along the course of the coronary artery? Is PU beneficial if it can be accomplished without pathologic sequelae? Could the alignment of cholesterol crystals or intraintimal fibrin be detected by Magnetic Resonance Imaging (MRI) or other modalities and be used as a means of identifying the vulnerable plaque with impending rupture [72,73]? These are some of the questions that must be considered in dealing with the pathogenesis of atherosclerosis and the subsequent sequelae.
Publication Details
Copyright
Publisher
Heart Research Foundation, Sacramento (CA)
NLM Citation
Frink RJ. Inflammatory Atherosclerosis: Characteristics of the Injurious Agent. Sacramento (CA): Heart Research Foundation; 2002. Chapter 4, Atheromas Are Caseous Abscesses.