NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press/Taylor & Francis; 2014.
5.1. INTRODUCTION
Uremic pruritus, now better named “chronic kidney disease-associated pruritus” (CKD-aP), remains a frequent and compromising symptom in patients with advanced or end-stage renal disease (Mettang et al. 1996). Most therapeutic trials have shown only limited success. Several times in the past a new treatment option has been reported to be effective, but very soon thereafter conflicting results appear (De Marchi et al. 1992; Balaskas and Uldall 1992; Peer et al. 1996; Pauli-Magnus et al. 2000b). The main obstacle in the effort to create effective treatment modalities is the incomplete knowledge of the underlying pathophysiological mechanisms. Furthermore, given the great clinical heterogeneity of patients with kidney failure, systematically performed studies are hard to undertake and therefore sparse.
5.2. CLINICAL FEATURES OF CKD-aP
The intensity and spatial distribution of pruritus vary significantly over time, and some patients are affected to a varying degree throughout the duration of their renal disease. The intensity of CKD-aP ranges from sporadic discomfort to complete restlessness during day- and nighttime. The skin of hemodialysis patients with chronic itch looks quite similar to that of patients without itch. However, there is evidence of secondary skin changes, most likely due to scratching. Excoriation by scratching with or without impetigo can occur as a secondary phenomenon and rarely even prurigo nodularis is observed (Figure 5.1a through c). There are interindividual differences in the spatial distribution of CKD-aP: 25%–50% of patients with CKD-aP complain about generalized pruritus (Morvay and Marghescu 1988; Ponticelli and Bencini 1992). In the remaining patients, CKD-aP seems to affect predominantly the back, the face, and the forearm, respectively (Gilchrest et al. 1982). In about 25% of patients with CKD-aP, pruritus was most severe during or immediately after dialysis (Dawn and Yosipovitch 2006). Once patients develop CKD-aP, it can last for months or years (Mathur et al. 2010).
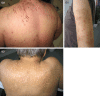
FIGURE 5.1
Typical skin changes due to uremic pruritus. (a) Scratch marks on the back with excoriations. (b) Typical hyperkeratotic partly excoriated nodules (prurigo nodularis) on the arm. (c) Deep scars on the shoulders and back of a female patient on hemodialysis. (more...)
5.2.1. Epidemiology
Whereas during the early days of dialysis treatment, CKD-aP was a very common problem, it appears that its incidence has declined over the past 20 years. In the early 1970s, Young et al. (1973) reported that about 85% of patients were affected by CKD-aP. This number decreased to 50% to 60% in the late 1980s (Mettang et al. 1990). An investigation in Germany showed that only 22% of all dialysis patients complained about moderate to severe pruritus at the time they were studied (Pauli-Magnus et al. 2000b). On the basis of a large-scale investigation published a few years ago, more than 40% of hemodialysis patients suffer from chronic pruritus (Pisoni et al. 2006) (Figure 5.2).
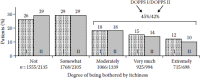
FIGURE 5.2
Prevalence and intensity of uremic pruritus according to DOPPS-data from 1996–1999 (I) and 2002–2003 (II); DOPPS, Dialysis Outcome and Practice Patterns Study. (After Pisoni, R. L. et al., Nephrol. Dial. Transplant, 21, 3495–3505, (more...)
Interestingly, severe pruritus is very rare in pediatric patients on dialysis. This could be shown by a systematic review of all German pediatric dialysis centers involving 199 children, where only 9.1% of the children on dialysis complained of pruritus (Figure 5.3). Moreover, the intensity was not very severe in the affected patients (Schwab et al. 1999).
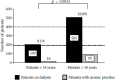
FIGURE 5.3
Prevalence of uremic pruritus in children on dialysis (18 years or younger) and in adult dialysis patients (older than 18 years). Prevalence of uremic pruritus in children is significantly lower than in adult patients (chi-squared test). (From Schwab, (more...)
Data on the prevalence of CKD-aP in peritoneal dialysis patient are rather scarce. The few reports available, however, permit the conclusion that patients undergoing peritoneal dialysis are affected as much by pruritus as hemodialysis patients are (Tessari et al. 2009).
The prevalence of CKD-aP is obviously underestimated by German nephrologists. We were able to corroborate this by a recent poll among German nephrologists. There are many reasons for this. First, the great variation in the intensity of symptoms may be responsible, and second, it is classified as a minor problem (not acutely life-threatening) (Weisshaar et al. 2009).
5.2.2. Etiopathology
So far, there have been no clear ideas regarding the pathogenesis of CKD-aP (Mettang et al. 2002). Among others, parathyroid hormone and histamine were investigated more closely as presumed pruritogenic factors. Parathyroid hormone is believed to be a possible pathogenetic factor based on multiple observations that persistent pruritus in patients with secondary hyperparathyroidism was substantially improved after parathyroidectomy (Massry et al. 1968; Hampers et al. 1968). However, substantial data argue against parathyroid hormone being the likely trigger of CKD-aP (Stahle-Bäckdahl et al. 1989).
It has also been hypothesized that histamine, which accumulates in renal failure and classically mediates pruritus, might also account for the itching in patients with end-stage renal failure (Stockenhuber et al. 1990). Nevertheless, the literature contains contradictory data as to the impact of histamine (Mettang et al. 1990). The concept of histamine playing a significant part in the generation of pruritus is challenged by the absence of typical skin changes such as wheals and by the usually observed therapeutic failure of antihistaminics (Weisshaar et al. 2004).
The impact of xenobiotic agents and uremic toxins has not yet been established. There is also some controversy regarding the influence of other factors, i.e., increased tissue concentrations of vitamin A and metastatic microcalcifications caused by calcium and magnesium salts (Blachley et al. 1985). In a study including 10 hemodialysis patients with or without pruritus, we wanted to find out whether there might be a difference regarding the calcium-binding protein fetuin. This admittedly small study population revealed no discrepancy in the serum levels of fetuin, calcium, or 25-hydroxy-vitamin D (Mettang et al. 2010).
Xerosis of the skin, described in a large number of patients with renal failure, was at least partially ruled out as pathogenetically relevant (Szepietowski et al. 2004).
Special attention has focused not only on neuropathic disorders but also on receptor proliferation of pruritus-mediating cells and on central nervous alterations. Enhanced stimulation of central µ-opioid receptors by accumulated endorphins or endogenous morphine-like compounds is possibly responsible for increased pruritus. This hypothesis is supported by the observation that uremic patients will experience a substantial amelioration of itching after the oral application of naltrexone (a µ-opioid receptor antagonist) (Peer et al. 1996). The positive action of this substance, however, could not be demonstrated in a large-scale study by our group on dialysis patients presenting with pruritus (Pauli-Magnus et al. 2000b; see Treatment Options section). According to another hypothesis, pruritus in patients with reduced kidney function is brought about by an imbalance between the activities of the mostly antagonistic acting µ- and κ-opioid receptors in favor of µ-receptor activation. For this reason, the application of a κ-agonist was recommended for treatment (see Treatment Options section) (Umeuchi et al. 2003).
More recent research points to another etiopathology of CKD-aP, namely, a microinflammation in skin and probably at the systemic level. Proof of elevated CRP values was established in hemodialysis patients with chronic pruritus (Virga et al. 2002; Kimmel et al. 2006); a relative increase in proinflammatory-relevant TH 1-cells and raised IL-6 levels were detected as well by our study group (Kimmel et al. 2006).
5.2.3. Treatment Options
Therapeutic options in CKD-aP are limited. Most studies on this subject are not truly valid as a result of inadequate documentation of the basics, of concomitant diseases and therapeutic measures taken, and of very low case numbers. The most consequential approaches to treatment are presented herewith:
- Topical treatment
- Systemic treatment with µ-opioid receptor antagonists and κ-agonists
- Gabapentin
- Drugs with an anti-inflammatory action
- Ultraviolet phototherapy
- Acupuncture
5.3. TOPICAL TREATMENT
5.3.1. Tacrolimus Ointments
Treatment with tacrolimus-containing unguents or creams for skin lesions in atopic dermatitis have in a number of cases resulted in complete healing and remission of pruritus (Gianni and Sulli 2001). Back in the late 1990s, we had three patients with particularly bothersome CKD-aP, and within the framework of an individual treatment trial, we gave them a 0.03% tacrolimus-containing unguent that was to be applied twice daily. The patients were also instructed to keep an “itch diary” with daily documentation of the intensity of their pruritus (visual analog scale 1–10) to define the degree of itching 1 week before, during and after treatment. Two of these patients felt they were completely cured, and the third patient reported substantial relief (reduction of VAS score from 7 to 3). Unfortunately, pruritus relapsed in all three of them right after the termination of treatment. Major side effects under this short-term treatment were not observed (Pauli-Magnus et al. 2000a) (Figure 5.4).
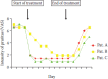
FIGURE 5.4
Course of symptoms on treatment with tacrolimus ointment 0.03% in three patients with intractable uremic itch. (After Pauli-Magnus, C. et al., Perit. Dial. Int., 6, 802–803, 2000a.)
A few years later, Kuypers and coworkers initiated a “proof of concept” study enrolling 25 patients afflicted with CKD-aP and were able to prove the efficacy of tacrolimus ointments (Kuypers et al. 2004). A visual analog scale (VAS) was used again for the assessment of pruritus and the prospective outcome of treatment. At the end of the 6 weeks course of treatment, they found a mean reduction of itching by 42.9%.
The results of a double-blind, placebo-controlled study conducted in the US on 22 hemodialysis patients with pruritus (Duque et al. 2005) are difficult to interpret. Treatment with both the active agent as well as vehicle turned out to be highly effective (itch was reduced by 80% versus baseline). A difference between tacrolimus and vehicle could not be demonstrated. However, the authors were at loss to explain the surprising result that the vehicle itself was highly effective. It should be noted that serious adverse reactions were not observed in this study.
5.3.2. Unguents Containing Gamma Linolenic Acid
In a study published a few years ago, Chen and coworkers tested the efficacy of topical treatment with gamma linolenic acid at high concentrations (essential fatty acid that also contains various plant seeds, evening primrose oil, etc.).
Seventeen patients with severe refractory CKD-aP were studied for 6 weeks in a crossover study (washout period 2 weeks). A VAS (score of 0–100) was used. The intensity of pruritus could be brought down from 75 to 30 when the active agent was applied, whereas the pruritus scores merely dropped from 72.5 to 67.5 in the patients receiving placebo (Chen et al. 2006). The mechanism of action of this therapeutic approach remains obscure. It can be speculated that gamma linolenic acid may serve as a “prodrug” in the formation of anti-inflammatory-acting prostaglandins. Considering that no side effects were encountered, this substance might be a valuable add-on in the treatment of patients with CKD-aP.
5.4. µ-OPIOID RECEPTOR ANTAGONISTS
5.4.1. Naltrexone
In a double-blind crossover study, Peer et al. (1996) were able to demonstrate that one week of treatment with the orally available µ-receptor antagonist naltrexone had brought on nearly complete remission of itching in 15 patients with severe hemodialysis-related pruritus. Subsequently, our group tried to reproduce these results in a similarly designed study (placebo controlled, double blind, crossover trial). Twenty-three patients with CKD-aP in whom other approaches had failed thus far received 50 mg naltrexone or placebo for a total of 4 weeks. Sixteen of these 23 patients completed the study. During the naltrexone treatment period, pruritus was eased by 29% (according to ratings using a VAS of 1–10) and by 17.6% according to a pruritus score (based on a questionnaire focusing on the frequency, intensity, and nocturnal occurrence of itching). Compared to this, pruritus declined by 16.9% (VAS) and by 22.3% (pruritus score) during the period of placebo treatment (Pauli-Magnus et al. 2000b). The differences between the naltrexone and the placebo periods were statistically insignificant for both VAS ratings and pruritus scores.
The results of these two studies are contradictory and confusing. The disparate results are neither explained by dissimilarities in compliance, the doses of naltrexone, or the study design, which was crossover, double blind, and placebo controlled in both cases. The studies merely differed as to the intensity of pruritus in the evaluated groups. Whereas the trial by Peer and coworkers exclusively concentrated on patients most seriously afflicted with pruritus (mean VAS 10), the mean intensity of pruritus in our patients was found to be VAS 6. This and other differences, for instance, regarding hemodialysis treatment, the material used, divergent lifestyle, eating habits, and environmental circumstances in various parts of the world may well have been involved in producing these contradictory results.
5.5. κ-OPIOID RECEPTOR AGONISTS
Because κ-opioid receptors primarily mediate µ-opioid receptor-antagonistic effects and because κ-agonistic drugs are known to suppress morphine-induced itch, the assumption that these substances might be capable of alleviating pruritus did not seem far-fetched (Umeuchi et al. 2003).
5.5.1. Nalfurafine
A meta-analysis of two randomized double-blind and placebo-controlled studies on a total of 144 hemodialysis patients with CKD-aP corroborated the antipruritic effect of nalfurafine, a potent κ-receptor agonist. The substance was administered as a short infusion, 3 times weekly for a period of 4 weeks following hemodialysis. The rate of side effects was low. Compared to placebo, a significant effectiveness could be confirmed (Wikström et al. 2005).
In a randomized prospective placebo-controlled phase III study with a total of 337 hemodialysis patients with pruritus, a Japanese work group applied nalfurafine hydrochloride orally, the dose being 2.5 and/or 5 µg daily over 2 weeks (Kumagai et al. 2010). The effects on itching were registered by a horizontal VAS scale from 0 to 100 mm. On treatment with the test substance, the intensity of pruritus decreased by 22 (5 µg) and 23 mm (2.5 µg), respectively, after 7 days of application, while the intensity of itching dropped by only 13 mm in the placebo group. The differences were statistically significant. However, the incidence of undesired drug actions (insomnia in particular) was substantially higher in both treatment groups (35.1% with 5 µg and 25% with 2.5 µg) than in the placebo group (16.2%). Moreover, the effect of medication wore off quickly once treatment was completed.
5.6. OTHER MEDICAL TREATMENT OPTIONS
5.6.1. Pentoxiphylline
Pentoxiphylline is a weak TNF-alpha inhibitor; its immunomodulating action has been verified in various studies.
Under the presumption that CKD-aP had a (micro-)inflammatory origin, we infused seven hemodialysis patients exhibiting pronounced pruritus with 600 mg pentoxiphylline for 4 weeks, subsequent to each hemodialysis treatment that they underwent (Mettang et al. 2007). Only three out of these seven patients were able to tolerate treatment for the entire duration of the study. Because of side effects or concomitant development of other illnesses, four patients withdrew from the trial. The patients who completed the entire course of therapy reported considerable amelioration of itching.
This effect was sustained until several weeks after the end of treatment. Considering the rather modest tolerance of the agent (at least with the dose chosen), this approach can only be recommended in exceptional cases.
5.6.2. Thalidomide
Thalidomide is a substance used for immune modulation in the treatment of graft versus host reactions after bone marrow transplantation or in the treatment of multiple myeloma, and which—among other things—is capable of reducing the lymphocytic TNF-alpha production. It could be shown that thalidomide also helps in the management of CKD-aP (Silva et al. 1994).
In a placebo-controlled randomized double-blind crossover study on the management of CKD-aP resistant to treatment, thalidomide proved to lower the pruritus scores by approximately 50%. The precise mechanism of action of this substance has not yet been elucidated. Aside from the previously described suppression of TNF-alpha production, a central abating effect might be involved as well (Daly and Shuster 2000).
5.6.3. Gabapentin
The substance gabapentin was originally developed as an anticonvulsant. Researchers later discovered that this agent exerts a pain-modulating effect, especially in patients with diabetic neuropathy. In a randomized, placebo-controlled study with hemodialysis patients, Gunal et al. (2004) showed that the 3 times weekly oral application of 300 mg gabapentin led to a dramatic diminution of pruritus in these patients (reduction of the intensity of itch, determined by a VAS of 0–10, from 8.4 prior to treatment to 1.2 at the end of the 4 weeks’ study period).
Razeghi et al. (2009) performed another double-blind crossover study with 34 hemodialysis patients who received 100 mg gabapentine 3 times weekly with similar results.
The rate of side effects in these two studies was trivial. Against this background, gabapentin appears to be a highly interesting, promising substance for the management of CKD-aP and might come to be one of the most important tools in the combat against pruritus in patients on dialysis.
5.6.4. Ultraviolet Phototherapy
Back in the 1970s, Gilchrest et al. (1979) had already reported on the efficacy of UV-phototherapy in patients with CKD-aP. Whereas the treatment by long-wave ultraviolet radiation remained largely ineffective, UVB radiation resulted in significantly improved itching in nine out of 10 patients.
A series of studies followed suit to determine the efficacy of UV radiation in patients with CKD-aP. According to a meta-analysis by Tan et al. (1991), UVB therapy was the most encouraging mode of treatment, whereas UVA therapy turned out to be a failure. More recent research suggests that narrowband UVB radiation is just as effective as treatment with broadband UV rays, with less frequent side effects (Baldo et al. 2002). In a recently published study, however, this effect could not be verified (Ko et al. 2011).
The fear that UVB radiation enhances the development of malignant skin tumors could not be totally dispelled. Some caution is thus required in the treatment of patients who are scheduled on long-term immunosuppressive therapy, e.g., after kidney transplantation.
5.6.5. Acupuncture
A very interesting approach to treat CKD-aP is acupuncture. In a study by Duo (1987), electro-acupuncture or sham-electro-stimulation was applied to six patients on hemodialysis in a blinded manner. Patients receiving acupuncture showed a significantly higher reduction in pruritus score than the sham-treated patients. In another study a few years ago, 40 patients with CKD-aP were treated with acupuncture either at the Quchi (LI11) acupoint or at a nonacupoint 2 cm lateral, three times weekly for 1 month. Patients treated at the Quchi acupoint revealed a substantial reduction in pruritus score regarding severity, distribution, and sleep disturbance ending up with maximum 45 points (38.3 ± 4.3, 17.3 ± 5.5, and 16.5 ± 4.9 start versus 4 weeks versus 12 weeks later), whereas pruritus in patients receiving sham-acupuncture did not change substantially (38.3 ± 4.3, 37.5 ± 3.2, and 37.1 ± 5 start versus 4 weeks versus 12 weeks later) (Che-yi et al. 2005).
Given these results, acupuncture in experienced hands might be a useful tool in the treatment of CKD-aP.
Figure 5.5 shows a potential therapeutic algorithm of CKD-aP. Considering that pruritus is generally not immediately life-threatening and that its treatment is often associated with considerable adverse reactions, the decision should always be carefully balanced as to benefits and risks. Preference should be given to therapeutic procedures with a favorable or benign profile of side effects. In desperate cases, patients principally eligible for a kidney transplant may be declared “high urgency,” which will decrease their waiting time. In most cases, a successful kidney transplantation will stop CKD-aP in patients treated (Altmeyer et al. 1986).
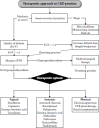
FIGURE 5.5
Therapeutic algorithm in uremic pruritus: Kt/V, urea clearance in relation to urea distribution volume; iPTH, intact parathormone. (After Mettang, T. and Weisshaar, E., Skin Therapy Lett., 15, 1–5, 2010.)
REFERENCES
- Altmeyer P, Kachel H. G, Schäfer G, Faßbinder W. Normalisierung der urämischen Hautveränderungen nach Nierentransplantation. Hautarzt. 1986;37:217–221. [PubMed: 3516928]
- Balaskas E. V, Uldall R. P. Erythropoietin does not improve uremic pruritus. Perit. Dial. Int. 1992;12:330–331. [PubMed: 1511055]
- Baldo A, Sammarco E, Plaitano R, Martinelli V, Monfrecola G. Narrowband (TL-01) ultraviolet B phototherapy for pruritus in polycythaemia vera. Br. J. Dermatol. 2002;147:979–981. [PubMed: 12410710]
- Blachley J. D, Blankenship D. M, Menter A, Parker T. F III, Knochel J. P. Uremic pruritus: Skin divalent ion content and response to ultraviolet phototherapy. Am. J. Kidney Dis. 1985;5:237–241. [PubMed: 4003393]
- Chen Y. C, Chiu W. T, Wu M. S. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am. J. Kidney Dis. 2006;48:69–76. [PubMed: 16797388]
- Che-yi C, Wen C. W, Min-Tsung K, Chiu-Ching H. Acupuncture in haemodialysis patients at the Quchi (LI11) acupoint for refractory uraemic pruritus. Nephrol. Dial. Transplant. 2005;20:912–915. [PubMed: 15985509]
- Daly B. M, Shuster S. Antipruritic action of thalidomide. Acta Derm. Venereol. 2000;80((1)):24–25. [PubMed: 10721827]
- Dawn A. G, Yosipovitch G. Butorphanol for treatment of intractable pruritus. Am. Acad. Dermatol. 2006;54:527–531. [PubMed: 16488311]
- De Marchi S, Cecchin E, Villalta D, Sepiacci G, Santini G, Bartoli E. Relief of pruritus and decreases in plasma histamine concentrations during erythropoietin therapy in patients with uremia. New Engl. J. Med. 1992;326:969–974. [PubMed: 1545849]
- Duo L. J. Electrical needle therapy of uremic pruritus. Nephron. 1987;47((1987)):179–183. [PubMed: 3500424]
- Duque M. I, Yosipovitch G, Fleischer A. B, Willard J, Freedman B. I. Lack of efficacy of tacrolimus ointment 0.1% for treatment of hemodialysis-related pruritus: A randomized, double-blind vehicle-controlled study. J. Am. Acad. Dermatol. 2005;52:519–521. [PubMed: 15761435]
- Gianni L. M, Sulli M. M. Topical tacrolimus in the treatment of atopic dermatitis. Ann. Pharmacother. 2001;35:943–946. [PubMed: 11485148]
- Gilchrest B. A, Rowe J. W, Brown R. S, Steinman T. I, Arndt K. A. Ultraviolet phototherapy of uremic pruritus. Long-term results and possible mechanisms of action. Ann. Intern. Med. 1979;91:17–21. [PubMed: 464448]
- Gilchrest G. A, Stern R. S, Steinman T. I, Brown R. S, Arndt K. A, Anderson W. W. Clinical features of pruritus among patients undergoing maintenance hemodialysis. Arch. Dermatol. 1982;118:154–156. [PubMed: 7065661]
- Gunal A. I, Ozalp G, Yoldas T. K, Gunal S. Y, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: A randomized, placebo-controlled, double-blind trial. Nephrol. Dial. Transplant. 2004;19:3137–3139. [PubMed: 15575002]
- Hampers C. L, Katz A. I, Wilson R. E, Merrill J. P. Disappearance of uremic itching after subtotal parathyroidectomy. New Engl. J. Med. 1968;279:695–697. [PubMed: 5670910]
- Kimmel M, Alscher D. M, Dunst R, Braun N, Machleidt C, Kiefer T, Stülten C. et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol. Dial. Transplant. 2006;21:749–755. [PubMed: 16249205]
- Ko M. J, Yang J. Y, Wu H. Y, Hu F. C, Chen S. I, Tsai P. J, Jee S. H, Chiu H. C. Narrowband ultraviolet B phototherapy for patients with refractory uremic pruritus: A randomized controlled trial. Br. J. Dermatol. 2011;165:633–639. [PubMed: 21668425]
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: A phase III, randomized, double-blind, placebo-controlled study. Nephrol. Dial. Transplant. 2010;25:1251–1257. [PubMed: 19926718]
- Kuypers D. R, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. A prospective proof of concept study of the efficacy of tacrolimus ointment on uraemic pruritus (UP) in patients on chronic dialysis therapy. Nephrol. Dial. Transplant. 2004;19:1895–1901. [PubMed: 15150348]
- Massry S, Popovzer M. M, Coburn J. M, Mokoff D. L, Maxwell M. H, Kleeman C. R. Interactable pruritus as a manifestation of secondary hyperparathyroidism in uremia. New Engl. J. Med. 1968;279:697–700. [PubMed: 5670911]
- Mathur V. S, Lindberg J, Germain M, Block G, Tumlin J, Smith M, Grewal M, McGuire D. ITCH National Registry Investigators. A longitudinal study of uremic pruritus in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010;5:1410–1419. [PMC free article: PMC2924419] [PubMed: 20558560]
- Mettang T, Fischer F. P, Kuhlmann U. Urämischer Pruritus—Pathophysiologische und therapeutische Konzepte. Dtsch. Med. Wschr. 1996;121:1025–1031. [PubMed: 8801075]
- Mettang T, Fritz P, Weber J, Machleidt C, Hübel E, Kuhlmann U. Uremic pruritus in patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). The role of plasma histamine and skin mast cells. Clin. Neprol. 1990;34:136–141. [PubMed: 1699691]
- Mettang T, Krumme B, Bohler J, Roeckel A. Pentoxifylline as treatment for uraemic pruritus—An addition to the weak armentarium for a common clinical symptom? Nephrol. Dial. Transplant. 2007;22:2727–2728. [PubMed: 17556413]
- Mettang T, Matterne U, Roth H. J, Weisshaar E. Lacking evidence for calcium-binding protein fetuin-A to be linked with chronic kidney disease-related pruritus (CKD-rP). NDTPlus. 2010;3:104–105. [PMC free article: PMC4421544] [PubMed: 25949421]
- Mettang T, Pauli-Magnus C, Alscher D. M. Uraemic pruritus—New perspectives and insights from recent trials. Nephrol. Dial. Transplant. 2002;17:1558–1563. [PubMed: 12198205]
- Mettang T, Weisshaar E. Pruritus: Control of itch in patients on dialysis. Skin Therapy Lett. 2010;15:1–5. [PubMed: 20361169]
- Morvay M, Marghescu S. Hautveränderungen bei Haemodialysepatienten. Med. Klin. 1988;83:507–510. [PubMed: 3221807]
- Pauli-Magnus C, Klumpp S, Alscher D, Kuhlmann U, Mettang T. Short-term efficacy of tacrolimus ointment in severe uremic pruritus. Perit. Dial. Int. 2000a;6:802–803. [PubMed: 11216583]
- Pauli-Magnus C, Mikus G, Alscher D. M, Kirschner T, Nagel W, Gugeler N, Risler T, Berger E. D, Kuhlmann U, Mettang T. Naltrexone does not relieve uremic pruritus: Results of a randomized, placebo-controlled crossover-study. J. Am. Soc. Nephrol. 2000b;11:514–515. [PubMed: 10703675]
- Peer G, Kivity S, Agami O, Fireman E, Silverberg D, Blum M, Iaina A. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet. 1996;348:1552–1554. [PubMed: 8950882]
- Pisoni R. L, Wikström B, Elder S. J, Akizawa T, Asano Y, Keen M. L, Saran R, Mendelssohn D. C, Young E. W, Port F. K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2006;21:3495–3505. [PubMed: 16968725]
- Ponticelli C, Bencini P. L. Uremic pruritus: A review. Nephron. 1992;60:1–5. [PubMed: 1738396]
- Razeghi E, Eskandari D, Ganji M. R, Meysamie A. P, Togha M, Khashayar P. Gabapentin and uremic pruritus in hemodialysis patients. Ren. Fail. 2009;31:85–90. [PubMed: 19212903]
- Schwab M, Mikus G, Mettang T, Pauli-Magnus C, Kuhlmann U. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Urämischer Pruritus im Kindes- und Jugendalter. Monatszeitschrift Kinderheilkunde. 1999;147:232.
- Silva S. R, Viana P. C, Lugon N. V, Hoette M, Ruzany F, Lugon J. R. Thalidomide for the treatment of uremic pruritus: A crossover randomized double-blind trial. Nephron. 1994;67((3)):270–273. [PubMed: 7936015]
- Stahle-Bäckdahl M, Hägermark O, Lins L. E, Törring O, Hilliges M, Johansson O. Experimental and immunohistochemical studies on the possible role of parathyroid hormone in uremic pruritus. J. Intern. Med. 1989;225((1989)):411–415. [PubMed: 2746157]
- Stockenhuber F, Kurz R. W, Sertl K, Grimm G, Balcke P. Increased plasma histamine in uremic pruritus. Clin. Sci. 1990;79((5)):477–482. [PubMed: 2174315]
- Szepietowski J. C, Reich A, Schwartz R. A. Uraemic Xerosis. Nephrol. Dial. Transplant. 2004;19:2709–2712. [PubMed: 15328388]
- Tan J. K. L, Haberman H. F, Coldman A. J. Identifying effective treatments for uremic pruritus. J. Am. Acad. Dermatol. 1991;25:811–824. [PubMed: 1839393]
- Tessari G, Dalle Vedove C, Loschiavo C, Tessitore N, Ruguiu C, Lupo A, Girolomoni G. The impact of pruritus on the quality of life of patients undergoing dialysis: A single centre cohort study. J. Nephrol. 2009;22((2)):241–248. [PubMed: 19384842]
- Umeuchi H, Togashi Y, Honda T, Nakao K, Okano K, Tanaka T, Nagase H. Involvement of central mu-opioid system in the scratching behavior in mice, and the suppression of it by the activation of κ-opioid system. Eur. J. Pharmacol. 2003;477:29–35. [PubMed: 14512095]
- Virga G, Visentin I, La Milia V, Bonadonna A. Inflammation and pruritus in haemodialysis patients. Nephrol. Dial. Transplant. 2002;17:2164–2169. [PubMed: 12454228]
- Weisshaar E, Dunker N, Röhl F. W, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp. Dermatol. 2004;13:298–304. [PubMed: 15140020]
- Weisshaar E, Matterne U, Mettang T. How do nephrologists in haemodialysis units consider the symptom of itch? Results of a survey in Germany. Nephrol. Dial. Transplant. 2009;24:1328–1330. [PubMed: 19155533]
- Wikström B, Gellert R, Ladefoged S. D, Danda Y, Akai M, Ide K, Ogasawara M. et al. Kappa-opioid system in uremic pruritus: Multicenter, randomized, double-blind, placebo-controlled clinical studies. J. Am. Soc. Nephrol. 2005;16:3742–3747. [PubMed: 16251241]
- Young A. W, Sweeney E. W, David D. S, Cheigh J, Hochgelerent E. L, Sakai S, Stenzel K. H, Rubin A. L. Dermatologic evaluation of pruritus in patients on hemodialysis. N. Y. State J. Med. 1973;73:2670–2674. [PubMed: 4519082]
- Effect of topical vitamin D on chronic kidney disease-associated pruritus: An open-label pilot study.[J Dermatol. 2015]Effect of topical vitamin D on chronic kidney disease-associated pruritus: An open-label pilot study.Jung KE, Woo YR, Lee JS, Shin JH, Jeong JU, Koo DW, Bang KT. J Dermatol. 2015 Aug; 42(8):800-3. Epub 2015 Apr 28.
- The impact of underlying diseases-related drugs on the chronic kidney disease-associated pruritus in hemodialysis patients.[J Res Med Sci. 2022]The impact of underlying diseases-related drugs on the chronic kidney disease-associated pruritus in hemodialysis patients.Azimi SZ, Alizadeh N, Ramezanzadeh E, Monfared A, Leili EK. J Res Med Sci. 2022; 27:86. Epub 2022 Nov 25.
- Review Pruritus Associated With Chronic Kidney Disease: A Comprehensive Literature Review.[Cureus. 2019]Review Pruritus Associated With Chronic Kidney Disease: A Comprehensive Literature Review.Swarna SS, Aziz K, Zubair T, Qadir N, Khan M. Cureus. 2019 Jul 28; 11(7):e5256. Epub 2019 Jul 28.
- Review An update on pruritus associated with CKD.[Am J Kidney Dis. 2007]Review An update on pruritus associated with CKD.Patel TS, Freedman BI, Yosipovitch G. Am J Kidney Dis. 2007 Jul; 50(1):11-20.
- Review Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges.[Int J Nephrol Renovasc Dis. 2017]Review Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges.Shirazian S, Aina O, Park Y, Chowdhury N, Leger K, Hou L, Miyawaki N, Mathur VS. Int J Nephrol Renovasc Dis. 2017; 10:11-26. Epub 2017 Jan 23.
- Pruritus in Renal Disease - ItchPruritus in Renal Disease - Itch
Your browsing activity is empty.
Activity recording is turned off.
See more...
