NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Williams M, Todd GD, Roney N, et al. Toxicological Profile for Manganese. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2012 Sep.
3.1. INTRODUCTION
The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and other interested individuals and groups with an overall perspective on the toxicology of manganese. It contains descriptions and evaluations of toxicological studies and epidemiological investigations and provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health.
A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile.
Manganese is a naturally occurring element found in rock, soil, water, and food. In humans and animals, manganese is an essential nutrient that plays a role in bone mineralization, protein and energy metabolism, metabolic regulation, cellular protection from damaging free radical species, and formation of glycosaminoglycans (Wedler 1994). Manganese acts as both a constituent of metalloenzymes and an enzyme activator. Enzymes that contain manganese include arginase, pyruvate carboxylase, and manganese-superoxide dismutase (MnSOD) (Keen and Zidenberg-Cher 1990; NRC 1989; Wedler 1994). Manganese, in its activating capacity, can bind either to a substrate (such as adenosine triphosphate, ATP), or to a protein directly, thereby causing conformational changes (Keen and Zidenberg-Cher 1990). Manganese has been shown to activate numerous enzymes involved with either a catalytic or regulatory function (e.g., transferases, decarboxylases, hydrolases) (Wedler 1994). The nutritional role of manganese is discussed in Section 3.4. Although manganese is an essential nutrient, exposure to high levels via inhalation or ingestion may cause some adverse health effects.
It has been suggested that these adverse health effects, especially neurologic effects, are occurring on a “continuum of …dysfunction” that is dose-related (Mergler et al. 1999). In other words, mild or unnoticeable effects may be caused by low, but physiologically excessive, amounts of manganese, and these effects appear to increase in severity as the exposure level or duration of exposure increases. Case reports and occupational studies address this continuum of nervous system dysfunction and help to characterize the apparent dose-response relationship. It is clear that chronic exposure to manganese at very high levels results in permanent neurological damage, as is seen in former manganese miners and smelters. Chronic exposure to much lower levels of manganese (as with occupational exposures) has been linked to deficits in the ability to perform rapid hand movements and some loss of coordination and balance, along with an increase in reporting mild symptoms such as forgetfulness, anxiety, or insomnia.
Chemical Forms of Concern. Manganese can exist in both inorganic and organic forms. This profile will discuss key manganese compounds in both forms, with inorganic compounds discussed first.
The inorganic forms include manganese chloride (MnCl2), manganese sulfate (MnSO4), manganese acetate (MnOAc), manganese phosphate (MnPO4), manganese dioxide (MnO2), manganese tetroxide (Mn3O4), and manganese carbonate (MnCO3). Emphasis has been placed on the health effects of compounds containing inorganic manganese in the Mn(II), Mn(III), or Mn(IV) oxidation states, since these are the forms most often encountered in the environment and the workplace. There is evidence in animals and humans that adverse neurological effects can result from exposure to different manganese compounds; much of this information on toxicity differences between species of manganese is from reports and experiments of acute exposures to very high doses. Results from animal studies indicate that the solubility of inorganic manganese compounds can influence the bioavailability of manganese and subsequent delivery of manganese to critical toxicity targets such as the brain; however, the influence of manganese oxidation state on manganese toxicity is not currently well understood. Manganese in the form of permanganate produces toxic effects primarily through its oxidizing capacity. However, because of its tendency to oxidize organic material, the permanganate ion is not stable in the environment; thus, the probability of exposure to this species around waste sites is considered very low. For this reason, data on exposures to permanganate are only briefly discussed.
The organic compounds that will be discussed are methylcyclopentadienyl manganese tricarbonyl (MMT) and mangafodipir. The latter is a chelate of Mn(II) and an organic ligand, dipyridoxyl diphosphate (MnDPDP; Mn(II) N,N’-dipyridoxylethylenediamine-N,N’-diacetate 5,5'bis(phosphate)). These compounds were chosen for this profile because their toxicity is expected to be mediated by excess exposure to elemental manganese. Organic fungicides containing manganese, such as maneb, were not chosen for discussion in this profile, because their critical toxic effects are expected to be mediated by the organic moities of their chemical structure, not by excessive elemental manganese.
MMT is a fuel additive developed in the 1950s to increase the octane level of gasoline and thus improve the antiknock properties of the fuel (Davis 1998; Lynam et al. 1999). Additional information on the chemical, physical, and environmental properties of MMT is included in Chapter 4. Exposure to MMT is expected to be primarily through inhalation or oral pathways, although occupational exposure for gasoline attendants or mechanics may be more significant via dermal absorption. Engines using MMT-containing gasoline and equipped with catalytic converters primarily emit manganese in inorganic phosphate and sulfate forms and smaller amounts of manganese dioxides can be detected (Mölders et al. 2001; Ressler et al. 2000; Zayed et al. 1999a, 1999b). These findings and observations that MMT is very unstable in light and degrades quickly in air (Garrison et al. 1995) suggest that human exposure to manganese from the use of MMT in gasoline is most likely to occur in inorganic forms as a result of the combustion of MMT, with the exception of people occupationally exposed to uncombusted gasoline containing MMT. However, despite this evidence, there are some reports that MMT levels in the environment increase with traffic density (Garrison et al. 1995; Zayed et al. 1999a, 1999b); therefore, inhalation and/or ingestion exposures to the parent compound are possible. Exposure and resultant toxicity from MMT’s inorganic combustion products are covered under the inorganic subsections, while toxicity attributable to MMT is covered under the organic subsections.
Mangafodipir is a contrast agent for magnetic resonance imaging (MRI) used primarily (after intravenous administration) to detect and characterize neoplastic liver lesions; it has also been found to aid in the identification of kidney and pancreatic tumors (Federle et al. 2000; Grant et al. 1997a, 1997b; Ni et al. 1997). The compound is only used in the diagnosis of organ-specific cancers and is found exclusively in a clinical setting. Mangafodipir is injected intravenously; therefore, inhalation, oral, and dermal pathways of exposure are not a concern. Because exposure to this compound is pathway-specific and the exposure population is inherently limited, toxicity arising from exposure to mangafodipir will be discussed in a separate subsection to Section 3.2.4, Diagnostic Uses.
3.2. DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE
To help public health professionals and others address the needs of persons living or working near hazardous waste sites, the information in this section is organized first by route of exposure (inhalation, oral, and dermal) and then by health effect (death, systemic, immunological, neurological, reproductive, developmental, genotoxic, and carcinogenic effects). These data are discussed in terms of three exposure periods: acute (14 days or less), intermediate (15–364 days), and chronic (365 days or more).
Levels of significant exposure for each route and duration are presented in tables and illustrated in figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest-observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies. LOAELs have been classified into "less serious" or "serious" effects. "Serious" effects are those that evoke failure in a biological system and can lead to morbidity or mortality (e.g., acute respiratory distress or death). "Less serious" effects are those that are not expected to cause significant dysfunction or death, or those whose significance to the organism is not entirely clear. ATSDR acknowledges that a considerable amount of judgment may be required in establishing whether an end point should be classified as a NOAEL, "less serious" LOAEL, or "serious" LOAEL and that, in some cases, there will be insufficient data to decide whether the effect is indicative of significant dysfunction. However, the Agency has established guidelines and policies that are used to classify these end points. ATSDR believes that there is sufficient merit in this approach to warrant an attempt at distinguishing between "less serious" and "serious" effects. The distinction between "less serious" effects and "serious" effects is considered to be important because it helps the users of the profiles to identify levels of exposure at which major health effects start to appear. LOAELs or NOAELs should also help in determining whether or not the effects vary with dose and/or duration and place into perspective the possible significance of these effects to human health.
The significance of the exposure levels shown in the Levels of Significant Exposure (LSE) tables and figures may differ depending on the user's perspective. Public health officials and others concerned with appropriate actions to take at hazardous waste sites may want information on levels of exposure associated with more subtle effects in humans or animals (LOAELs) or exposure levels below which no adverse effects (NOAELs) have been observed. Estimates of levels posing minimal risk to humans (Minimal Risk Levels or MRLs) may be of interest to health professionals and citizens alike.
A User's Guide has been provided at the end of this profile (see Appendix B). This guide should aid in the interpretation of the tables and figures for Levels of Significant Exposure and the MRLs.
3.2.1. Inhalation Exposure
Inorganic manganese compounds are not volatile, but they can exist in the air as aerosols or suspended particulate matter. Table 3-1 and Figure 3-1 summarize the available quantitative information on the health effects that have been observed in humans and animals following inhalation exposure to various inorganic manganese compounds. All exposure levels are expressed as milligrams of manganese per cubic meter (mg manganese/m3).
Table 3-1
Levels of Significant Exposure to Inorganic Manganese - Inhalation.
Figure 3-1
Levels of Significant Exposure to Inorganic Manganese - Inhalation.
Many of the studies, especially those dealing with occupational exposures, make the distinction between respirable and total manganese dust. Respirable dust is usually defined by a particular dust particle size that varies from study to study. It is typically defined as those particles ≤5 microns; these smaller dust particles can enter the lower areas of the lungs, including the bronchioles and the alveoli. These particles can be absorbed by the lung and will enter the bloodstream immediately, thus avoiding clearance by the liver. Total dust represents larger particles that cannot travel as deeply into the lungs as respirable dust, and will largely be coughed up and swallowed. Although many of the recent occupational studies have provided information on the size of the respirable particles that are associated with the exposure levels documented, some of the occupational studies and historical studies in miners only measure total dust. The profile provides, where possible, the different exposure levels in terms of respirable and total dust, but does not make a further distinction between particle sizes of the respirable dust.
3.2.1.1. Death
No conclusive studies have been located that show inhalation exposure of humans to manganese resulting in death. Hobbesland et al. (1997a) investigated nonmalignant respiratory diseases as a cause of death in male ferromanganese and silicomanganese workers. The authors found a slight excess in the numbers of deaths caused by pneumonia for manganese furnace workers, but could not discount other work-related exposures as potential causes of the pneumonia.
In analyses performed several years ago, MMT in gasoline was found to combust primarily to manganese tetroxide, but in the low levels currently used in gasolines, it is primarily combusted to manganese phosphate and manganese sulfate (Lynam et al. 1999). Therefore, inhalation exposures to exhaust from gasoline containing MMT will be discussed with inorganic manganese exposures. No deaths were observed in male outbred albino rats and male golden hamsters exposed to the exhaust (either irradiated or non-irradiated) from automobiles that were fueled with MMT-containing gasoline (Moore et al. 1975).
No other studies were located regarding death in humans or animals after inhalation exposure to inorganic manganese.
MMT has been used in very few inhalation studies due to the photolability of the compound; its short half-life in air makes it a very difficult compound to administer to laboratory animals in exposure chambers or nose-cones. Hinderer (1979) evaluated the toxicity of various unspecified MMT concentrations administered to 10 male Sprague-Dawley rats per exposure group during 1- and 4-hour exposure periods. The inhalation LD50 was determined to be 62 mg manganese/m3 (247 mg MMT/m3*55 mg manganese/218.1 mg MMT=62 mg manganese/m3) for a 1-hour exposure and 19 mg manganese/m3 for a 4-hour exposure. No mention was made in the report of steps taken to prevent MMT photodegradation during the experiment.
3.2.1.2. Systemic Effects
The highest NOAEL values and all LOAEL values from each reliable study for systemic effects in each species and duration category are recorded in Table 3-1 and plotted in Figure 3-1.
Respiratory Effects. In humans, inhalation of particulate manganese compounds such as manganese dioxide or manganese tetroxide can lead to an inflammatory response in the lung. This is characterized by an infiltration of macrophages and leukocytes, which phagocytize the deposited manganese particles (Lloyd Davies 1946). Damage to lung tissue is usually not extensive, but may include local areas of edema (Lloyd Davies 1946). Symptoms and signs of lung irritation and injury may include cough, bronchitis, pneumonitis, and minor reductions in lung function (Abdel-Hamid et al. 1990; Akbar-Khanzadeh 1993; Boojar and Goodarzi 2002; Lloyd Davies 1946; Roels et al. 1987a); occasionally, pneumonia may result (Lloyd Davies 1946). These effects have been noted mainly in people exposed to manganese dust under occupational conditions, although there is some evidence that respiratory effects may also occur in residential populations near ferromanganese factories (Kagamimori et al. 1973; Nogawa et al. 1973; WHO 1987). The frequency of effects has been shown to decrease in at least one population when concentrations of total manganese in falling dust declined (Kagamimori et al. 1973). It is likely that the inflammatory response begins shortly after exposure and continues for the duration of the exposure.
It is important to note that an inflammatory response of this type is not unique to manganese-containing particles, but is characteristic of nearly all inhalable particulate matter (EPA 1985d). This suggests that it is not the manganese per se that causes the response, but more likely the particulate matter itself.
An increased prevalence of pneumonia has also been noted in some studies of workers with chronic occupational exposure to manganese dust (Lloyd Davies 1946) and in residents near a ferromanganese factory (WHO 1987). It seems likely that this increased susceptibility to pneumonia is mainly secondary to the lung irritation and inflammation caused by inhaled particulate matter, as discussed above.
Inhalation of particulate manganese compounds such as manganese dioxide or manganese tetroxide also leads to an inflammatory response in the lungs of animals, although inhalation of manganese chloride did not cause lung inflammation in rabbits (Camner et al. 1985). Several acute- and intermediate-duration studies in animals report various signs of lung inflammation following periods ranging from 1 day to 10 months at manganese concentrations ranging from 0.7 to 69 mg/m3 (Bergstrom 1977; Camner et al. 1985; Shiotsuka 1984; Suzuki et al. 1978; Ulrich et al. 1979a, 1979b). Bergstrom (1977) and Ulrich et al. (1979a, 1979b) determined NOAELs, which are reported in Table 3-1. Increased susceptibility to lung infection by bacterial pathogens following inhalation of manganese dusts has been noted in acute animal studies (Maigetter et al. 1976). Conversely, Lloyd Davies (1946) reported no increase in the susceptibility of manganese-treated mice to pneumococci or streptococci. Bredow et al. (2007) reported that nose-only inhalation exposure to 2 mg manganese/m3 as manganese chloride aerosols 6 hours/day for 5 consecutive days did not cause lung lesions in female GVB/N mice, but induced a 2-fold increase in pulmonary levels of mRNA for vascular endothelial growth factor (VGEF), a regulator of proliferation, migration, and formation of new capillaries. Elevated levels of VGEF have been associated with respiratory diseases, but current understanding is inadequate to know if this pulmonary gene expression response to manganese is adverse or benign.
Moore et al. (1975) exposed male golden hamsters and outbred albino rats to automobile exhaust from a car that burned MMT-containing fuel. The animals were exposed to non-irradiated exhaust or irradiated exhaust; the irradiation served to convert hydrocarbon gases and vapors to particulate form. Controls for each species were exposed to clean air. The animals were exposed for 8 hours/day for 56 consecutive days. While the hamsters were fed a diet containing an adequate amount of manganese for normal development, the rats were divided into two groups: one group was fed a manganese-sufficient diet (42.2 μg manganese/g diet) and the other group was fed a manganese-deficient diet (5 μg manganese/g diet). After the exposure, the authors observed a thickening of the cuboidal epithelium at the level of the terminal bronchiole in the golden hamsters. The lesion was not classified as severe and only affected one to two sites per lung section. Further, the lesions did not increase with length of exposure to the exhaust products (from 1 to 9 weeks). The incidence of lesions in the lung was 21% after exposure to irradiated exhaust, 14% after exposure to non-irradiated exhaust, and 6% after exposure to clean air.
More recently, reversible inflammation (pleocellular inflammatory infiltrates and fibrinonecrotic debris) in the nasal respiratory epithelium (but not the olfactory epithelium) was observed in young adult male Crl:CD(SD)BR rats following 13 weeks of inhalation exposure to 0.5 mg manganese/m3 as manganese sulfate, but not in rats exposed to 0.1 mg manganese/m3 as manganese sulfate or manganese phosphate (hureaulite) (Dorman et al. 2004b). The lesions were not apparent in groups of rats assessed 45 days after the end of exposure, indicating their transient nature. In studies with young male Rhesus monkeys exposed to 0, 0.06, 0.3, or 1.5 mg manganese/m3 as manganese sulfate 6 hours/day, 5 days/week for 65 days, no nasal histological effects were found in exposed monkeys, but the high exposure level induced lesions in the lower respiratory tract (mild subacute bronchiolitus, alveolar duct inflammation, and proliferation of bronchus-associated lymphoid tissue) (Dorman et al. 2005b). The lower airway lesions from intermediate-duration exposure appear to have been transient, because they were not found in monkeys assessed 45 days after the end of exposure (Dorman et al. 2005b). These findings in rats and monkeys are consistent with the understanding that inflammation of respiratory tissues from high-level exposure to inhaled manganese particulates is likely a consequence of the inhaled particulate matter.
No studies were located concerning respiratory effects in humans following inhalation exposure to MMT.
Male rats exposed to high concentrations of MMT (exposure doses not reported) via inhalation exhibited labored breathing during and after 1- and 4-hour exposures (Hinderer 1979). Gross necropsy or histopathological analyses on these animals were not performed.
Cardiovascular Effects. Three studies reported adverse cardiovascular effects after occupational exposure to manganese. Saric and Hrustic (1975) observed a lower mean systolic blood pressure in male workers at a ferromanganese plant. Manganese concentrations in the plant ranged from 0.4 to 20 mg/m3, but specific data on exposure levels were lacking. More recently, Jiang et al. (1996a) studied the potential cardiotoxicity of manganese dioxide exposure in 656 workers (547 males, 109 females) involved in manganese milling, smeltering, and sintering. The authors took 181 samples of airborne manganese (not specified if respirable or total dust), with a geometric mean of 0.13 mg/m3. The workers, whose work tenure ranged from 0 to 35 years, had a greater incidence of low diastolic blood pressure. The incidence of this effect was highest in young workers with the lowest tenure in the plant. There was no increase of abnormal electrocardiograms between workers and their matched controls. The authors surmised that manganese’s ability to lower the diastolic blood pressure weakens with age as the elasticity of the blood vessels deteriorates.
Hobbesland et al. (1997b) reported a significantly increased incidence in sudden death mortality for workers in ferromanganese/silicomanganese plants during their employment period (standardized mortality ratio [SMR]=2.47). The sudden deaths included cardiac deaths and other natural causes. More specifically, among furnace workers, who are more likely to be exposed to manganese fumes and dusts than non-furnace workers, the mortality during active-person time was statistically significantly elevated (38.7%) compared to non-furnace workers (23.3%; p<0.001). However, the authors caution that the association of increased death and manganese exposure is speculative and the increase in sudden death could also be caused by common furnace work conditions (heat, stress, noise, carbon monoxide, etc.).
No studies on cardiovascular effects from inhalation exposure to MMT in humans or animals were located.
Gastrointestinal Effects. There are no reports of gastrointestinal effects following inhalation exposure to inorganic manganese in humans or animals.
There are no reports concerning the gastrointestinal effects following inhalation exposure to MMT in humans or animals.
Hematological Effects. Examination of blood from persons chronically exposed to high levels of manganese in the workplace has typically not revealed any significant hematological effects (Mena et al. 1967; Roels et al. 1987a; Smyth et al. 1973; Whitlock et al. 1966). The effect of manganese exposure on erythrocyte superoxide dismutase activity remains inconsistent; some investigators observed increased activity among male manganese smelters (Yiin et al. 1996), while others reported decreased activity in male welders (Li et al. 2004). However, an increased plasma malondialdehyde level is consistent between manganese-exposed smelters (Yiin et al. 1996) and welders (Li et al. 2004). Malondialdehyde is a product of lipid peroxidation; lipid peroxidation is believed to be a mechanism for cell toxicity. The authors observed that plasma malondialdehyde and manganese levels were strongly correlated in exposed workers and interpreted this response to be an indicator of manganese toxicity via lipid peroxidation.
No studies on hematological effects from inhalation exposure to MMT in humans or animals were located.
Hepatic Effects. Even though the liver actively transports manganese from blood to bile (see Section 3.4.4), there is no information to indicate that the liver is adversely affected by manganese; however, there are few specific studies on this subject. In a study by Mena et al. (1967), workers chronically exposed to manganese dust in the workplace exhibited no abnormalities in serum levels of alkaline phosphatase. Of 13 patients who were hospitalized with chronic manganese poisoning, 1 had a 20% sulfobromophthalein (SBP) retention and 1 had a 12% SBP retention, although histological examination of a liver biopsy from the latter revealed no abnormal tissue (Mena et al. 1967). No significance was ascribed to the elevated SBP retention.
Rats exposed to manganese tetroxide dusts for 9 months exhibited no adverse effects or histopathological lesions; however, slight increases in liver weights were noted (Ulrich et al. 1979b). These data, although limited, indicate that the liver is not significantly injured by manganese.
No studies on hepatic effects from inhalation exposure to MMT in humans or animals were located.
Musculoskeletal Effects. No studies were located concerning musculoskeletal effects from inhalation exposure to inorganic manganese.
No studies were located concerning musculoskeletal effects from inhalation exposure to MMT in humans or animals.
Renal Effects. The kidney is not generally considered to be a target for manganese, but specific studies are rare. No abnormalities in urine chemistry were detected in workers chronically exposed to manganese dusts in the workplace (Mena et al. 1967), but other more sensitive tests of renal function were not performed.
No studies were located regarding renal effects in animals after inhalation exposure to inorganic manganese.
No studies on renal effects from inhalation exposure to MMT in humans or animals were located.
Endocrine Effects. Few studies have measured endocrine effects in humans exposed to inorganic manganese. Two studies measured hormonal levels after exposure to manganese. The first study (Alessio et al. 1989) involved chronic exposure of foundry workers to manganese for approximately 10 years. The exposure levels were reported as 0.04–1.1 mg manganese/m3 (particulate matter) and 0.05–0.9 mg/m3 as fumes. These levels overlap the current American Congress of Governmental Industrial Hygiene (ACGIH) threshold limit value-time weighted average (TLV-TWA) of 0.2 mg/m3 for particulate, but are less than the limit of 1 mg/m3 for manganese fumes. The study reported both elevated prolactin levels and elevated cortisol levels; however, no changes in the levels of follicle stimulating hormone (FSH) and luteinizing hormone (LH) were noted.
Smargiassi and Mutti (1999) reported effects in a group of workers from a ferroalloy plant who were exposed occupationally to elevated levels of airborne manganese. Serum prolactin levels in these workers were evaluated in a 1992 study and again in a 1997 study. Serum prolactin levels, which were significantly elevated in the earlier analysis, had also increased significantly over the earlier measurement (p<0.001). This difference was especially noticeable in those with abnormally high prolactin levels. During the five year period between studies, exposure levels were consistent and were not reduced; therefore, it is unclear whether prolactin levels reflect current or cumulative exposure.
Other elements of endocrine function (reproductive function, etc.) are discussed elsewhere in the text.
No studies were located regarding endocrine effects in animals after inhalation exposure to inorganic manganese.
No studies on endocrine effects from inhalation exposure to MMT in humans or animals were located.
Dermal Effects. No studies have been located concerning dermal effects in humans or animals following inhalation exposure to inorganic or organic manganese.
Ocular Effects. No studies have been located concerning ocular effects in humans or animals following inhalation exposure to inorganic manganese.
There are no studies reporting ocular effects following inhalation exposure of humans to MMT. One- and 4-hour exposures to doses of MMT used in lethality studies resulted in conjunctivitis in rats (Hinderer 1979).
Body Weight Effects. No studies were located regarding body weight effects in humans following exposures to inorganic manganese.
No studies were located regarding body weight effects in humans following inhalation exposure to MMT. Hinderer (1979) observed a decrease in weight gain in Sprague-Dawley rats during the first 7 days following a 1- or 4-hour exposure to unspecified MMT concentrations in an acute toxicity test. The rats resumed their normal weight gain by 14 days post-exposure.
Metabolic Effects. No studies were located concerning metabolic effects from inhalation of inorganic manganese in humans or animals.
No studies were located concerning metabolic effects following inhalation exposure to MMT in humans or animals.
3.2.1.3. Immunological and Lymphoreticular Effects
One study on immunological effects in humans following inhalation to inorganic manganese was located. Male welders exposed to manganese (0.29–0.64 mg/m3 for an unspecified duration), vibration, and noise exhibited suppression of the T and B lymphocytes characterized by reductions in serum immunoglobin G (IgG) and total E-rosette-forming cells (Boshnakova et al. 1989). However, the welders in this study were exposed to numerous other compounds, including cobalt, carbon dioxide, and nitric oxide. Therefore, it is impossible to determine whether exposure to manganese caused the effects. It is not known whether any of these changes are associated with significant impairment of immune system function. No studies were located on lymphoreticular effects in humans exposed to manganese by the inhalation route.
No studies were located on immunological or lymphoreticular effects in animals exposed to inorganic manganese by the inhalation route.
As noted above, inhalation exposure to particulate manganese compounds can lead to an inflammatory response in the lung (i.e., pneumonitis), and this is accompanied by increased numbers of macrophages and leukocytes in the lung (Bergstrom 1977; Lloyd Davies 1946; Shiotsuka 1984; Suzuki et al. 1978). However, this is an expected adaptive response of the immune system to inhaled particulates, and these data do not indicate that the immune system is injured. Conflicting data are reported concerning increased susceptibility to bacterial infection after exposure to airborne manganese. Lloyd Davies (1946) indicated that manganese exposure did not increase the susceptibility of mice to bacterial infection, whereas Maigetter et al. (1976) reported that exposure to aerosolized manganese dioxide altered the resistance of mice to bacterial and viral pneumonias.
No studies on immunological or lymphoreticular effects from inhalation exposure to MMT in humans or animals were located.
3.2.1.4. Neurological Effects
Overview. Neurological effects from repeated inhalation exposure to manganese are well recognized as effects of high concern based on case reports and epidemiological studies of groups of occupationally exposed and environmentally exposed people and results from animal inhalation studies. The highest NOAEL values and all LOAEL values from each reliable study for neurological effects in each species and duration category are recorded in Table 3-1 and plotted in Figure 3-1.
There is conclusive evidence from studies in humans that inhalation exposure to high levels of manganese compounds (usually manganese dioxide, but also compounds with Mn(II) and Mn(III)) can lead to a disabling syndrome of neurological effects referred to as ‘manganism’.
Studies estimating the impact of low-level exposure to manganese on neurological health have employed a number of sensitive tests designed to detect signs of neuropsychological and neuromotor deficits in the absence of overt symptoms (Iregren 1990, 1994, 1999). These analyses allow the comparison of discrete performance values that are associated with either biological levels of manganese or approximations of exposure levels. Thus, they allow for the comparison of one exposure group to another without the subjective description of neurological symptoms that were prevalent in the studies with miners and others with frank manganism. A number of epidemiological studies have used these techniques to study the psychological or neurological effects of exposure to low levels of manganese in the workplace (Bast-Pettersen et al. 2004; Beuter et al. 1999; Blond and Netterstrom 2007; Blond et al. 2007; Bouchard et al. 2003, 2005, 2007a, 2007b; Chia et al. 1993a, 1995; Crump and Rousseau 1999; Deschamps et al. 2001; Gibbs et al. 1999, Iregren 1990; Lucchini et al. 1995, 1999; Mergler et al. 1994; Myers et al. 2003a, 2003b; Roels et al. 1987a, 1992, 1999; Summers et al. 2011; Wennberg et al. 1991) or in environmental media close to manganese-emitting industries (Hernández-Bonilla et al. 2011; Lucchini et al. 2007; Kim et al. 2011; Menezes-Filho et al. 2011; Mergler et al. 1999; Riojas-Rodríguez et al. 2010; Rodríguez-Agudelo et al. 2006; Solís-Vivanco et al. 2009; Standridge et al. 2008). Some of these studies have found statistically significant differences between exposed and non-exposed groups or significant associations between exposure indices and neurological effects (Bast-Pettersen et al. 2004; Chia et al. 1993a; Iregren 1990; Lucchini et al. 1995, 1999; Mergler et al. 1994; Roels et al. 1987a, 1992; Wennberg et al. 1991), whereas others have not found significant associations (Deschamps et al. 2001; Gibbs et al. 1999, Myers et al. 2003a, 2003b; Summers et al. 2011; Young et al. 2005). The neurological effects associated with prolonged low-level manganese exposure generally have been subtle changes, including deficits in tests of neuromotor or cognitive functions and altered mood states; they have been referred to by various authors as preclinical or subclinical neurological effects. As shown in Table 3-1 and Figure 3-1, manganese air concentrations associated with these effects in chronically exposed workers range from about 0.07 to 0.97 mg manganese/m3 (manganese in total or inhalable dust measurements). For several of these work environments, values of concentrations of manganese in respirable dust (generally particulate diameters <10 µm) represented <20–80% of the total dust values.
Manganism from High-Level Occupational Exposure to Inorganic Manganese. There is conclusive evidence from studies in humans that inhalation exposure to high levels of manganese compounds (usually manganese dioxide, but also compounds with Mn(II) and Mn(III)) can lead to a disabling syndrome of neurological effects referred to as ‘manganism’. Manganism is a progressive condition that usually begins with relatively mild symptoms, but evolves to include dull affect, altered gait, fine tremor, and sometimes psychiatric disturbances. Some of these symptoms also occur with Parkinson’s disease, which has resulted in the use of terms such as “Parkinsonism-like disease” and “manganese-induced Parkinsonism” to describe those symptoms observed with manganese poisoning. Despite the similarities, significant differences between Parkinsonism and manganism do exist (Barbeau 1984; Calne et al. 1994; Chu et al. 1995). Barbeau (1984) reported that the hypokinesia and tremor present in patients suffering from manganism differed from those seen in Parkinson’s disease. Calne et al. (1994) noted other characteristics that distinguish manganism from Parkinson’s disease: psychiatric disturbances early in the disease (in some cases), a “cock-walk,” a propensity to fall backward when displaced, less frequent resting tremor, more frequent dystonia, and failure to respond to dopaminomimetics (at least in the late stages of the disease).
Manganism and Parkinson’s disease also differ pathologically. In humans and animals with chronic manganese poisoning, lesions are more diffuse, found mainly in the pallidum, caudate nucleus, the putamen, and even the cortex with no effects on the substantia nigra and no Lewy bodies (Pal et al. 1999; Perl and Olanow 2007). In people with Parkinson’s disease, lesions are found in the substantia nigra and other pigmented areas of the brain (Barbeau 1984). Moreover, Lewy bodies are usually not found in substantia nigra in manganism cases, but are almost always found in cases of Parkinson’s disease (Calne et al. 1994; Perl and Olanow 2007). Manganese appears to affect pathways that are post-synaptic to the nigrostriatal system, most likely the globus pallidus (Chu et al. 1995). MRI of the brain reveals accumulation of manganese in cases of manganism, but few or no changes in people with Parkinson’s disease; fluorodopa positron emission tomography (PET) scans are normal in cases of manganism, but abnormal in people with Parkinson’s disease (Calne et al. 1994). Other studies suggest that manganese produces a syndrome described as parkinsonism, distinct from Parkinson’s Disease or manganism (Lucchini et al. 2007, Racette et al. 2005). It is likely that the terms Parkinson-like-disease and manganese-induced-Parkinsonism will continue to be used by those less knowledgeable about the significant differences between the two. Nonetheless, comparison with Parkinson’s disease and the use of these terms may help health providers and health surveillance workers recognize the effects of manganese poisoning when encountering it for the first time.
Typically, the clinical effects of high-level inhalation exposure to manganese do not become apparent until exposure has occurred for several years, but some individuals may begin to show signs after as few as 1–3 months of exposure (Rodier 1955). The first signs of the disorder are usually subjective, often involving generalized feelings of weakness, heaviness or stiffness of the legs, anorexia, muscle pain, nervousness, irritability, and headache (Mena et al. 1967; Nelson et al. 1993; Rodier 1955; Tanaka and Lieben 1969; Whitlock et al. 1966). These signs are frequently accompanied by apathy and dullness along with impotence and loss of libido (Abdel-Hamid et al. 1990; Emara et al. 1971; Mena et al. 1967; Nelson et al. 1993; Rodier 1955; Schuler et al. 1957). Early clinical symptoms of the disease include a slow or halting speech without tone or inflection, a dull and emotionless facial expression, and slow and clumsy movement of the limbs (Mena et al. 1967; Nelson et al. 1993; Rodier 1955; Schuler et al. 1957; Shuqin et al. 1992; Smyth et al. 1973; Tanaka and Lieben 1969). In a study by Wolters et al. (1989), 6-fluorodopa (6-FD) and 18F-2-fluoro-2-deoxyglucose (FDG) PET were used to investigate the neurochemistry of four patients with "early manganism." FDG PET demonstrated decreased cortical glucose metabolism. No anomalies were noted in the 6-FD scans. This led the authors to suggest that, in early manganism, damage may occur in pathways that are postsynaptic to the nigrostriatal system, and most likely involve striatal or pallidal neurons.
As the disease progresses, walking becomes difficult and a characteristic staggering gait develops. Muscles become hypertonic, and voluntary movements are accompanied by tremor (Mena et al. 1967; Rodier 1955; Saric et al. 1977a; Schuler et al. 1957; Smyth et al. 1973). Few data are available regarding the reversibility of these effects. They are thought to be largely irreversible, but some evidence indicates that recovery may occur when exposure ceases (Smyth et al. 1973). Manganism has been documented in welders and in workers exposed to high levels of manganese dust or fumes in mines or foundries. Extreme examples of psychomotor excitement have been observed in manganese miners and, to a lesser extent, in industrial workers (Chu et al. 1995; Mena et al. 1967; Nelson et al. 1993). The behavior, known as “manganese madness” (Mena 1979) includes nervousness, irritability, aggression, and destructiveness, with bizarre compulsive acts such as uncontrollable spasmodic laughter or crying, impulses to sing or dance, or aimless running (Emara et al. 1971; Mena et al. 1967; Rodier 1955; Schuler et al. 1957). Patients are aware of their irregular actions, but appear incapable of controlling the behavior.
The reports of frank manganism (Rodier 1955; Schuler et al. 1957; Smyth et al. 1973) observed in manganese miners clearly indicate that the onset of manganism results from chronic exposure to high concentrations of the metal. Documented cases indicate that the most important route of exposure is inhalation of manganese dusts or fumes, while other pathways such as ingestion of the metal from mucociliary transport of larger particles and hand-to-mouth activity, may contribute a smaller amount. Based on the data provided by Rodier (1955) and Schuler et al. (1957), it appears that the frequency of manganism cases increased with prolonged exposure, suggesting that the seriousness of the symptoms presented increases with cumulative exposure. For example, Rodier (1955) reports that the highest percentage of manganism cases (28, or 24.4%) occurred in miners with 1–2 years experience. Only six cases of manganism (5.2%) were reported in males with 1–3 months exposure, and 68% of the cases reported occurred after exposures >1–2 years in length. Rodier did not present statistics on the number of men in the mine who were employed for comparable durations who did not suffer from manganism. Schuler et al. (1957) studied fewer manganism cases, but showed that the number of men with manganism increased with time spent mining, with the average time delay before onset of the disease being 8 years, 2 months. In fact, the minimum duration of exposure to the metal was 9 months before signs of manganism became recognizable, and the maximum exposure was 16 years. However, Schuler et al. (1957) point out that their study was not intended to “suggest incidence rates” and of the 83 miners selected for examination of potential manganism, only 9 were chosen as actually suffering from manganese poisoning. As with the Rodier (1955) study, the Schuler et al. (1957) study did not discuss the exposure duration or symptomatology of those men not displaying “frank manganism;” therefore, these collective data, although suggestive of a cumulative effect of manganism neurotoxicity, must be interpreted with caution.
Huang et al. (1998) documented the progression of clinical symptoms of manganism in five surviving workers (from an original six) chronically exposed to manganese in a ferroalloy plant. These men were exposed from 3 to 13 years and were examined 9–10 years after manganese exposure had ceased. Neurologic examination revealed a continuing deterioration of health exhibited in gait disturbance, speed of foot tapping, rigidity, and writing. The men had high concentrations of manganese in blood, urine, scalp, and pubic hair at the time of the original neurologic evaluation. Follow-up analyses revealed a drastic drop in manganese concentrations in these fluids and tissues (e.g., 101.9 μg/g manganese in blood from patient 1 in 1987; 8.6 μg/g manganese in blood in 1995). Further, T1-weighted MRI analysis did not reveal any high-signal intensity areas. These data support the progression and permanence of clinical effects from excess manganese exposure, even when tissue levels of the metal had returned to normal. Further, it shows that this neurotoxicity can continue in the absence of continuing manganese exposure and that a spectrum of responses to excess manganese exposure can be seen depending upon dose, duration of exposure, and timing of the observation. While some subclinical manifestations of manganese neurotoxicity will resolve, once neuropathology has occurred (in the form of loss of dopaminergic neurons), then reversal becomes more limited and is restricted to functional compensation.
As shown in Table 3-1 and Figure 3-1, cases of frank manganism have been associated with workplace exposure levels ranging from about 2 to 22 mg manganese/m3 (Cook et al. 1974; Rodier 1955; Saric et al. 1977; Schuler et al. 1957; Tanaka and Lieben 1969; Whitlock et al. 1966). For example, Tanaka and Lieben (1969) reported that no cases of frank manganism were diagnosed in 38 workers from Pennsylvania industrial plants in which estimated air concentrations were below 5 mg manganese/m3, whereas 7 cases were diagnosed in 117 workers from plants with air concentrations exceeding 5 mg/m3. Whitlock et al. (1966) reported on two cases of frank manganism in workers who were exposed to estimated air concentrations ranging from 2.3 to 4.7 mg manganese/m3.
Neurological Assessments of Workers Exposed to Low Levels of Inorganic Manganese. In a cross-sectional epidemiological study of 141 male workers in a manganese dioxide and salt producing plant, Roels et al. (1987a) detected preclinical neurological effects (alterations in simple reaction time, audioverbal short-term memory capacity, and hand tremor) in workers exposed to 0.97 mg manganese (median concentration in total dust)/m3 as manganese dioxide, manganese tetroxide, manganese sulfate, manganese carbonate, and manganese nitrite for a group average of 7.1 years. End points in exposed workers were compared with end points in a matched control group of 104 non-exposed male workers from a nearby chemical plant. The prevalences of subjective symptoms were similar in exposed and control workers, except for the elevation of nonspecific symptoms (such as fatigue, tinnitus, trembling of fingers, and increased irritability) in the exposed workers. Statistically significant mean deficits were found in exposed workers (compared with controls) in tests of simple reaction time (visual), audioverbal short-term memory capacity, eye-hand coordination, and hand steadiness. The prevalence of abnormal values in the neurological tests were not statistically significantly correlated with manganese levels in blood or urine or duration of employment, with the exception that blood levels of manganese were correlated with prevalence of abnormal responses in tests of eye-hand coordination and hand steadiness.
Iregren (1990) used neurobehavioral tests (simple reaction time, digit span, finger tapping, verbal ability, hand dexterity, and finger dexterity tests from the Swedish Performance Evaluation System, SPES) to study adverse effects in 30 male workers from two different manganese foundries exposed to an estimated median concentration of 0.14 mg manganese (in total dust)/m3 as manganese dioxide for 1–35 years (mean, 9.9 years). The exposed workers had below-average scores on a number of the tests, such as reaction time and finger tapping, when compared to matched controls with no occupational manganese exposure.
Roels et al. (1992) provided similar results to these earlier reports. Workers in a dry alkaline battery factory exhibited impaired visual reaction time, hand-eye coordination, and hand steadiness when exposed to concentrations of manganese dioxide in total dust ranging from 0.046 to 10.840 mg manganese/m3 and in respirable dust from 0.021 to 1.32 mg manganese/m3 (exposure ranged from 0.2 to 17.7 years). A lifetime integrated exposure (LIE) for both total manganese dust and respirable manganese was estimated for each of the exposed workers (LIE=∑((Cjob 1 × T1) + (Cjob 2 × T2) + … (Cjob n × Tn)), where C is concentration, T is years of exposure, and LIE is expressed as mg manganese/m3 times year). Based on the analysis of data by a logistic regression model, it was suggested that there was an increased risk (odds ratio [OR]=6.43, 95% confidence interval [CI]=0.97–42.7) of decreased hand steadiness at a lifetime integrated exposure level of 3.575 mg/m3*year for total dust or 0.730 mg/m3*year for respirable dust. It should be noted that the LIE at which an increased risk of abnormal neurological function occurs is based on exposures in an occupational setting. Therefore, periods of exposures would be followed by periods that would be relatively free of manganese inhalation. Presumably, during these “rest” periods the homeostatic mechanism would excrete excess manganese to maintain the manganese concentration within physiologic limits. Further, the LIE for deleterious neural effects may be biased in favor of a higher concentration due to the “healthy worker effect” (i.e., the most susceptible individuals are not incorporated into the study).
Crump and Rousseau (1999) performed a follow-up study of 213 men occupationally exposed to manganese, 114 of whom were subjects in the Roels et al. (1987a, 1987b) studies. Exposure data were unavailable during the 11 years of study (1985–1996) during which blood and urine samples were taken and neurological tests (short-term memory, eye-hand coordination, and hand steadiness) were administered as in the Roels studies. Yearly blood and urine manganese levels remained fairly consistent throughout the study period, and were comparable to the levels reported in the previous studies. The authors suggest that the consistency of these data on manganese levels indicates that the airborne manganese concentrations to which the subjects were exposed during the study period were likely comparable to those at the time of the Roels studies. The average age and exposure duration of the subjects increased from 36 and 7 years, respectively, in 1985, to 41 and 14 years, respectively, in 1996. Variations in year-to-year test results were observed that were not attributable to age of the subject or exposure to manganese. The authors observed decreases in errors in the short-term memory test (number of repeated words and number of errors). During 1987, 1988, and 1989, the average number of words remembered on the memory test was lower than in any other year. However, there was a progressive improvement in percent precision and percent imprecision on the eye-hand coordination test during 1985– 1988 (after 1991, the design of the test was modified and percent imprecision was lower in that year and all subsequent testing years). The authors suggest several reasons for the inter-year variability in test results (Crump and Rousseau 1999), including variations in test conditions, different groups of workers being tested in different years, the mood of the workers following a plant restructuring, and increased caution on the part of the subjects when answering test questions. When data analysis was controlled for year of testing, older workers performed significantly worse than younger workers on total words recalled in the memory test, and on percent precision and percent sureness in the eye-hand coordination test. Further, blood and urine manganese levels were not significantly associated with performance on memory or eye-hand coordination tests, but blood manganese was negatively associated with performance on the hand steadiness test (p<0.05). Age was not a factor in hand steadiness when the year of test was controlled for in the analysis. Crump and Rousseau (1999) investigated whether individual test scores worsened with time by studying the group of 114 men from the original Roels et al. (1987a, 1987b) studies and a subset of 44 long-term employees who had been given both memory and hand steadiness tests on two occasions, 8 years apart. These analyses revealed decreases in performance over time for a particular hole in the hand steadiness test and improvements in repetitions and errors on the memory test, both of which were statistically significant. The authors suggest that the improvements in the memory test were likely the result of increased caution on the part of the subject. The changes in performance over time could not be attributed solely to manganese exposure because it was impossible to control for age and year of testing in all of the analyses. The authors noted the lack of an age-matched control group with which to compare test results and the absence of data caused by workers ending their terms of employment. Some have questioned whether inter-year variability in test results, potentially caused by different test administrators over time, would affect interpretation of the findings. While this may contribute to the changes in performance over time seen in the Crump and Rousseau (1999) study, this factor will potentially impact any study of this type. The lack of a control group precludes the determination of a reliable NOAEL or LOAEL based on the results of this study.
A study by Mergler et al. (1994) also supports the work of Iregren and Roels. This epidemiologic study included 115 (95% of the total) male workers from a ferromanganese and silicomanganese alloy factory who were matched to other workers from the region with no history of exposure. The groups were matched on the following variables: age, sex, educational level, smoking, and number of children. These workers were exposed to both manganese dioxide dusts and manganese fumes. Environmental levels of manganese in total dust were measured at 0.014–11.48 mg/m3 (median, 0.151 mg/m3; arithmetic mean, 1.186 mg/m3, geometric mean, 0.225 mg/m3), while manganese levels in respirable dust were 0.001–1.273 mg/m3 (median, 0.032 mg/m3; arithmetic mean, 0.122 mg/m3; geometric mean, 0.035 mg/m3), and mean duration of exposure was 16.7 years. The exposed workers had significantly greater blood manganese levels, but urinary manganese did not differ between groups. Manganese workers showed decreased performance on tests of motor function (including those from the SPES) as compared to matched control workers with no manganese exposure. Using test results obtained from performance of the groups on the Luria-Nebraska Neuropsychological Battery and other tests, the authors reported that manganese-exposed workers performed more poorly than controls on tests of motor function, particularly on tests that required alternating and/or rapid hand movements and hand steadiness. The exposed workers also differed significantly from the controls in cognitive flexibility and emotional state. They also exhibited significantly greater levels of tension, anger, fatigue, and confusion. Further, these workers had a significantly lower olfactory threshold than controls; this is the first study to report this effect following inhalation exposure to manganese. Several follow-up studies of the workers from this manganese alloy plant are described later in this section (Bouchard et al. 2005, 2007a, 2007b).
Similar effects to those observed in the Mergler et al. (1994) study were observed by Chia et al. (1993a). Workers in a manganese ore milling plant exposed to 1.59 mg manganese (mean concentration in total dust)/m3 exhibited decreased scores in several neurobehavioral function tests including finger tapping, digit symbol, and pursuit aiming. Further, the workers exhibited an increased tendency for postural sway when walking with their eyes closed (Chia et al. 1995).
An epidemiologic study (Lucchini et al. 1995) also supports findings of these studies concerning the preclinical neurological effects of manganese exposure. This study, which evaluated performance on neuromotor tests (seven tests from the SPES, including simple reaction time, finger tapping, digit span, additions, symbol digit, shapes comparison, and vocabulary) involved 58 male workers from a ferroalloy plant. The workers had been exposed for 1–28 years (mean, 13; standard deviation [SD], 7) to geometric mean airborne concentrations of manganese, as manganese dioxide, in total dust as high as 0.070–1.59 mg/m3 (geometric means in different areas). These concentrations had decreased in the last 10 years to a range of 0.027–0.270 mg manganese (in total dust)/m3. At the time of the study, the exposed workers were undergoing a forced cessation from work of 1–48 days. Blood and urine manganese levels were analyzed. A cumulative exposure index (CEI) was calculated for each subject by multiplying the average annual airborne manganese concentration in respirable dust characteristic of each job by the number of years for which this activity was performed. Significant correlations were found between the log value of blood manganese concentrations in exposed workers and the tests of additions, digit span, finger tapping, and symbol digit (log values for the last two tests); between the log value of urinary manganese levels and the performance on the additions test; and between the log value of the CEI and the log value of the symbol digit score. Further, a significant correlation on an individual basis was found between external exposure, represented by CEI, and blood and urine manganese levels. These results are unique in that they are the first to suggest that blood and urine manganese concentrations are indicative of exposure on an individual basis. As suggested by Lucchini et al. (1995), the correlations may be observable in this study, when they have not existed in past studies (Roels et al. 1987a, 1992), because the workers were assessed at a time when they were not currently being exposed to manganese. In support of this possibility, the correlation coefficients between the urine and manganese levels and the CEI increased with time elapsed since the last exposure to airborne manganese (Lucchini et al. 1995).
Roels et al. (1999) performed an 8-year prospective study with 92 subjects exposed to manganese dioxide at a dry-alkaline battery plant (Roels et al. 1992) to determine if poor performance on tests measuring visual reaction time, eye-hand coordination, and hand steadiness could be improved if occupational manganese exposure were decreased. The workers were divided into “low” (n=23), “medium” (n=55), and “high” (n=14) exposure groups depending on location within the plant and job responsibility. At the end of the 1987 study, technical and hygienic improvements had been implemented within the plant to decrease atmospheric manganese concentrations. Yearly geometric mean values for airborne total manganese dust (MnT) in the “low,” “medium,” and “high” exposure areas decreased in the following manner, respectively: ~0.310–~0.160; ~0.900–~0.250; and ~3–~1.2 mg/m3. The cohort decreased from 92 subjects in 1987 to 34 subjects in 1995 due to turnover, retirement, or dismissal, but no worker left due to neurological signs or symptoms. A separate group of workers was selected who had prior manganese exposure (ranging from 1.3 to 15.2 years). These subjects had left the manganese processing area of the plant prior to the end of 1992, and therefore, their exposure to manganese had ceased at that time; these workers were still employed in other areas of the plant. The control group consisted of 37 workers employed at the same polymer factory that had provided the control population in the previous study (Roels et al. 1992). This group, with an average age of 38.5 (range, 32–51 years) allowed for the analysis of age as a confounder. Exposure data (respirable manganese and total manganese dust, MnT) were taken with personal air samplers. Time-trend analysis of air sampler data revealed a significant decrease in total manganese from 1987 to 1995, with a more pronounced decline from 1992 forward. From 1987 to 1990, the authors observed that the precision of the hand-forearm movement (PN1) in the eye-hand coordination test for the whole cohort worsened, but then got progressively better. Hand steadiness and visual simple reaction time variables were inconsistent over time, and time-trends were not observed. When the cohort was divided into exposure groups, and analyzed for performance on the eye-hand coordination test, it was revealed that in general, the performance on the PN1 aspect of the test improved from 1987 to 1995, especially after 1991. The performance of the “low-dose” group was comparable to that of the control group in 1987 (Roels et al. 1992) and to that of the control group in 1997. The performance of the “medium-dose” group was intermediate between the “low-dose” and “high-dose” group. The only significant differences in performance were in the “high-dose” group as compared to the “low-dose” group during the years 1988–1990 (test scores of 49–51 for the high-dose group and 63–65 for the “low-dose” group). However, it was noted that performance on the eye-hand coordination test for the “medium” and “high-dose” groups was considerably poorer than the controls.
Significant differences were noted in variables in the hand steadiness test between the exposure groups during 1987–1992 (data not reported), when manganese concentrations were at their highest. However, no readily identified temporal changes in performance among the groups on this test was found, nor with the visual reaction time test. When the authors performed separate time-trend analysis on MnT levels and PN1 (eye-hand coordination test) values, a significant time effect was present for each variable. An analysis of covariance was performed for each exposure group (low, medium, and high) in which log MnT was considered as covariate in order to adjust for estimation of PN1 variations as log MnT changed over time. The resultant data suggested that a reduction in log MnT was associated with an improvement in PN1 for each group. The authors also found that when time was also considered with log MnT as an interaction term, it did not influence PN1 variations over the years and the effect of time on PN1 values disappeared when log MnT was maintained as an ordinary covariate. The authors interpreted this to mean that performance on the eye-hand coordination tests were only related, and inversely so, to the exposure to manganese. In other words, when manganese exposure was increased, test performance decreased and vice versa (Roels et al. 1999). However, in the high-exposure group, the performance increased from 71 to 83% of that of the control group, and leveled off at this point, despite decreased manganese exposure occurring from 1991/1992 with most dramatic improvements occurring in 1994. The authors suggest that this leveling off of performance by the high-exposure group may be indicative of a permanent effect of manganese on eye-hand coordination. The authors tested PN1 values in exposed subjects 3 years following a cessation of exposure. They found that in 20/24, the PN1 values were below the mean PN1 values of the control group, but 16 of these individuals showed an improvement in 1996 (percent improvement unspecified). The remaining four subjects (three “low-exposure” and one “medium-exposure” subjects) had PN1 values that exceeded the mean value of the control group. However, these data indicate that although there was improvement in performance on the coordination test, the vast majority of the exposed group still could not perform to the level of an unexposed worker 3 years after manganese exposure ceased. In addition, the exposed workers who did perform as well or better than the control subjects were among the least exposed workers while at the plant. As discussed previously, performance of the “low-exposure” group on eye-hand coordination tests during 1992–1995 was comparable to that of the control groups from 1987 and 1997, indicating that manganese exposure of these individuals during that time did not severely impact their ability to perform this neurobehavioral test. Comparable performance on the tests by the same control group in 1987 and 10 years later, in 1997, indicates that age was not a confounder in this study. None of the variables except visual reaction time was significantly correlated with age, and the existing correlation in the visual reaction time test only represented a 3% difference (Roels et al. 1999).
Lucchini et al. (1999) also investigated differences in neurobehavioral test performance over time as exposure to manganese (manganese dioxide and manganese tetroxide) decreased. The study group consisted of 61 men who worked in different areas of a ferroalloy plant. The plant was divided into three exposure areas with total manganese dust (geometric mean) values decreasing from 1981 to 1995: “high-exposure” values decreased from 1.6 to 0.165 mg/m3; “medium-exposure” values decreased from 0.151 to 0.067 mg/m3; and “low-exposure” values decreased from 0.57 to 0.012 mg/m3. The authors estimated that the annual average manganese concentration in the “medium-exposure” group was 0.0967 mg manganese in total dust/m3. Respirable dust constituted 40–60% of the total dust value. Control subjects consisted of 87 maintenance and auxiliary workers from a nearby hospital who had not been exposed to neurotoxins. The study and control groups were well matched except for years of education and the percentage of subjects working night shifts. The study groups answered a questionnaire concerning neuropsychological and Parkinsonian symptoms and underwent testing to determine the effect of manganese on neuromotor performance. Four tests were from the SPES (addition, digit span, finger tapping, symbol digit) and five timed tasks were from the Luria Nebraska Neuropsychological Battery (open-closed dominant hand--Luria 1, open-closed non-dominant hand—Luria 2, alternative open-closed hands—Luria 3, thumb-fingers touch dominant hand—Luria 4, and thumb-fingers touch non-dominant hand—Luria 5). Individual scores were taken from these subtests, and the sum of the Luria tests was taken (Luria sum). Postural tremor was also measured, as was visual reaction time and coordination ability via the hand pronation/supination test. Manganese levels in blood and urine, as well as blood lead levels were analyzed prior to each neurobehavioral test. Manganese levels in both blood and urine were significantly elevated in exposed workers compared to controls (p<0.0001). Blood lead levels were also significantly higher in the ferroalloy workers (p=0.0002). The authors noted that the study groups did not report as many complaints as those reported in the Mergler et al. (1994) study.
After correcting for age, education, alcohol, smoke, coffee, shift work, and blood lead levels, an analysis of test results indicated that performance of the exposed workers was significantly different than that of controls on all tests except for Luria 5 and Luria sum (Lucchini et al. 1999). A comparison of SPES test results from workers tested in 1990 or 1991 and those from this study did not indicate any difference in paired t-test values; this indicates that performance did not improve over time or with decreasing exposure to manganese. CEI values were calculated (in the same manner as in Lucchini et al. [1995]) for each exposure group and performance on the neurobehavioral tests was analyzed for correlation to these values and to manganese levels in body fluids. Significant differences were found between those with low CEI values of <0.5 mg/m3*years, mid CEI values of 0.5–1.8 mg/m3*years, and high CEI values of >1.8 mg/m3*years and performance on the following tests: symbol digit, finger tapping, dominant and non-dominant hand, and digit span. A positive correlation was observed between the log CEI value and these tests, indicating that performance decreased as exposure increased. No correlations were found between CEI values and manganese levels in blood and urine; these results differ from the correlation between CEI and manganese levels in fluids from the previous study (Lucchini et al. 1995). Lucchini et al. (1995) estimated a manganese dose (total dust) that would represent the annual airborne manganese concentration indicative of neurobehavioral deficit in this study by dividing the geometric mean CEI of the mid-exposure subgroup, 1.1 mg/m3*years, by the geometric mean value of years of exposure for this same subgroup, 11.51, yielding a value of 0.096 mg/m3. A comparable respirable dust value would be 0.038 mg/m3 (0.096*0.40).
Gibbs et al. (1999) studied a population of workers in a U.S. plant that produces electrolytic manganese metal. These 75 workers and a well-matched group of control workers with no manganese exposure were administered a computerized questionnaire concerning neurological health issues (including mood, memory, fatigue, and other issues) and were analyzed for performance on several neurobehavioral tests including hand steadiness (Movemap steady, Movemap square, and tremor meter), eye-hand coordination (orthokinisimeter), and rapidity of motion (four-choice reaction time and finger tapping). The Movemap test is a relatively recent test that has not undergone widespread use, and it has not been validated by other researchers. Further, although technically sophisticated, the test has not been observed to discriminate between exposure groups any better than simpler current methods (Iregren 1999). Airborne levels of total and respirable manganese were obtained using personal samplers and were not available for years prior to 1997. Using the arithmetic mean of samples collected in 12 different job categories, exposure was estimated for the years prior to 1997. Cumulative exposure values for each worker were estimated for the 30-day and 12-month exposure periods just prior to neurobehavioral testing. Multiple regressions of the test scores were performed using age and each of the following manganese exposure variables individually as explanatory variables: duration of exposure; 30-day cumulative exposure; 1-year cumulative exposure; and cumulative occupational exposure to either respirable or total manganese. Shift work was also used as a variable in conjunction with age and cumulative 30-day exposure to respirable or total manganese. The authors threw out outlying data points if they were >3 times the SD of the residual after a model fit. Exposures to respirable and total dust were highly correlated (r2, 0.62–0.75), as were cumulative exposures over the previous 30 days and the previous year (r2, 0.72–0.82); however, lifetime integrated exposure was not correlated with either 30-day or 12-month exposure values. The average exposure value for manganese-exposed workers was estimated at 0.066±0.059 mg/m3 (median, 0.051 mg/m3) for respirable dust, and 0.18±0.21 mg/m3 for total dust.
Responses to the questionnaire and performance on the neurobehavioral tests did not differ significantly between exposed and control groups (Gibbs et al. 1999). Cumulative years of exposure had an effect on tapping speed—speed increased with increased exposure, but only when outliers were included in the analysis. The authors also reported an inverse correlation between age and performance on tests measuring eye-hand coordination but positively correlated between age and complex reaction time. The study by Gibbs et al. (1999) is the first to report a lack of poorer performance on neurobehavioral tests by workers chronically exposed to manganese. Interestingly, the median exposure estimates for respirable dust in this population (0.051 mg/m3) is slightly higher than the lowest level of respirable dust at which preclinical neurological effects have been seen (0.032 mg/m3) as reported by Mergler et al. (1994).
Gorell et al. (1999) noted a high OR of 10.51 for the development of Parkinson’s disease in individuals >50 years old who were occupationally exposed to manganese for >20 years, but not for those exposed for <20 years. However, the numbers of individuals with a >20-year exposure was rather small (n=4), and occupational exposures to other metals (copper, and lead-iron, lead-copper, and iron-copper combinations) for >20 years were also associated with increased risk for the disease.
In a cross-sectional study of 138 (114 male and 34 female) enamels-production workers, Deschamps et al. (2001) administered a questionnaire about neurological symptoms; evaluated performance on psychological tests of similarity recognition, vocabulary (oral word association), geometrical figure recognition (visual gestalts), and short-term memory (digit span); and measured levels of manganese in blood samples. Results were compared with a control group of 137 nonexposed workers matched for age, educational level, and ethnic group. Exposed workers were employed for a mean duration of 19.87 years (SD±9) in enamels production. Mean manganese levels in 15 personal air samples and 15 stationary air samples collected at the plant during the year preceding the tests were 2.05 mg manganese/m3 (SD 2.52; range 0.5–10.2) for total dust and 0.035 mg manganese/m3 for respirable manganese (SD 0.063; range 0.01–0.293). Symptoms of asthenia, sleep disturbance, and headache were significantly elevated in exposed workers, compared with controls, but no significant differences in blood levels of manganese or performance on the administered tests were found between the exposed and control groups of workers. Clinical examination of the exposed subjects revealed no cases of obvious neurological impairment, but sensitive psychomotor tests of simple reaction times and motor functions were not administered in this study.
In a cross-sectional study, Myers et al. (2003a) evaluated results from a health questionnaire and a battery of neurobehavioral tests administered to 489 workers employed as office workers, miners, surface processors, engineers, and other service workers from two South African manganese mines. Cumulative exposure indices for each subject were calculated based on total dust measurements and job history. Workers were employed in the mines for a mean of 10.8 years (SD=5.5 years; range 1–41 years), had an average cumulative exposure index of 2.2 (mg manganese/m3 per year, SD=2.2; range=0–20.8), an average exposure intensity of 0.21 mg manganese/m3 (SD=0.14; range, 0–0.99), and an average blood manganese concentration of 8.5 µg/L (SD=2.8; range, 2.2–24.1). Neurobehavioral end points included three tests of motor function in the Luria-Nebraska battery (tests 1, 2, and 23), mean reaction time in the SPES, and three cognitive tests (forward and backward digit span and digit-symbol score). Multiple linear regression analysis revealed no significant (p<0.05) associations between any measure of exposure and questionnaire or test battery outcomes.
In another cross-sectional study, Myers et al. (2003b) evaluated neurobehavioral end points in a group of 509 workers at a South African manganese smelter, compared with a group of unexposed workers from an electrical fittings assembly plant (remote from the manganese smelter). Workers were employed for a mean of 18.2 years (SD 7.6), compared with 9.4 years (SD 7.0) in the control group. Exposure was assessed from manganese determinations in dust from personal air samples, blood samples, and urine samples. Cumulative exposure indices were calculated for each exposed worker based on manganese concentrations in “inhalable” dust from personal air samples and job histories. Mean values for exposed workers were 16.0 mg manganese/m3 per year (SD 22.4) for cumulative exposure index, 0.82 mg manganese/m3 (SD 1.04) for average intensity of exposure, 12.5 µg manganese/L (SD 5.6) for blood manganese, and 10.5 µg manganese/L (SD 20.3) for urine manganese. Control workers had mean values of 6.4 µg manganese/L (SD 1.7) for blood manganese and 0.96 µg manganese/L (SD 0.81) for urine manganese. Neurobehavioral end points included the Swedish nervous system questionnaire and the following neurobehavioral test batteries: World Health Organization (WHO) neurobehavioral core test battery, SPES, Luria-Nebraska tests, and Danish product development tests (tests of hand steadiness, tremor, and body sway). Information collected for potential confounders included age, educational level, alcohol and tobacco consumption, neurotoxic exposures in previous work, past medical history, and previous head injury. Multiple linear and logistic regression analyses were conducted to examine possible exposure-response relationships. Several tests showed significant (p<0.05) differences between exposed and control workers, but no evidence of exposure-response relationships including the following: the Santa Ana, Benton and digit span WHO tests; hand tapping and endurance tapping SPES tests; one Luria-Nebraska test (item 2L); several self-reported symptoms (e.g., tiredness, depressed, irritated); and increased sway under two conditions (eyes open with or without foot insulation). Results from two other tests (WHO digit-symbol test and Luria-Nebraska item 1R) showed differences between exposed and control groups and some evidence for increased deficits with increasing exposure, but the change with increasing exposure was greater at lower exposure levels than at higher exposure levels. Results from all of the remaining tests showed no significant adverse differences between the exposed and control groups. The authors concluded that “the most likely explanation for few, weak and inconsistent findings with implausible or counterintuitive exposure-response relationships is chance, and it is concluded that this is essentially a negative study.”
Young et al. (2005) reanalyzed the data collected by Myers et al. (2003b) on the basis of estimated exposures to manganese in “respirable” dust. Exposure estimates for each worker (cumulative exposure indices in mg manganese/m3 per year and average intensity of exposure in mg manganese/m3) were recalculated based on manganese determinations in personal air samplings of respirable dust (collected on 37 mm, 5 µm MCEP membrane filters, as opposed to inhalable dusts of larger particle sizes used to estimate exposure in the earlier analyses by Myers et al. [2003b]). Results from comparisons of mean performances of exposed and control groups in the neurobehavioral tests and regression analyses to assess exposure-response relationships were similar to results from the earlier analyses by Myers et al. (2003b) based on manganese determinations in inhalable dust. The authors concluded that the results did not provide evidence that exposure estimates based on respirable dust provide a more sensitive method to detect manganese neurobehavioral effects.
A cross-sectional study by Summers et al. (2011) supports the work of Myers et al. (2003a, 2003b). Neuropsychological tests of attention, short-term memory span, information-processing speed, and executive functioning (Digit Symbol Coding, Controlled Oral Word Association Test, Trail Making Test, Matrix Reasoning, and the Stroop Neuropsychological Screening Test) were used to study adverse effects in 143 employees in a smelting plant exposed to estimated mean concentrations of 0.384 mg/m3 inhalable manganese dust or 0.123 mg/m3 respirable manganese dust (as per Australian standards) for 1–29 years (mean, 10.6 years). Cumulative exposure indices for both inhalable and respirable manganese dust were calculated for each subject based on yearly air-sampling data for each occupational position and job history. Correlational and hierarchical linear regression analysis was conducted to assess associations of these exposure metrics, along with age, education, and intellectual ability (estimated IQ), with performance on the neuropsychological tests. In hierarchical analysis of performance and respirable manganese cumulative exposure (including age, estimated IQ, and education as explanatory variables), statistically significant relationships were found for decreasing performance with increasing exposure on the Trail Making (Part A), Matrix Reasoning, and Stroop color-word tests (measures of attention and executive function), and for increasing performance with increasing exposure on the Digit Symbol Coding test. The magnitude of the effects on performance was small, as reflected by the percentages of the variance in test scores explained by respirable cumulative manganese exposure (ranging from 0.5 to 3.7, depending on the test). In contrast, estimated IQ and education explained 3.2–24.5% of the variance. Summers et al. (2011) concluded that the decrements in performance associated with cumulative respirable manganese exposure were small and “not of clinical significance”, because the magnitudes of these effects were smaller than the standard error of measurement in the tests.
Bast-Pettersen et al. (2004) cross-sectionally examined neurobehavioral end points in a group of 100 male workers in manganese alloy plants and a group of 100 control workers (paired matched for age) from two plants, one producing silicon metal and microsillica and another titanium oxide slag and pig iron. Manganese alloy workers were employed for a mean of 20.2 years (SD 8.6; range 2.1– 41.0 years); comparable statistics were not reported for the control workers. Exposure was assessed from manganese determinations in dust from personal air samples (collected on 3 days for each subject closely before the neurobehavioral assessment), blood samples, and urine samples. Arithmetic means for manganese workers were 0.753 mg manganese/m3 inhalable dust for work room air (geometric mean 0.301; range 0.009–11.5 mg manganese/m3), 189 nmol manganese/L in blood (range 84–426 nmol/L), and 3.9 nmol manganese/mmol urine creatinine (range 0.1–126.3). The Institute of Occupational Medicine (IOM) personal samplers used in this study are expected to provide estimates that are approximately 2-fold higher than estimates using 25- or 37-mm plastic Millipore personal air samplers used in many earlier studies to measure “total dust”. Mean levels of manganese in blood (166 nmol manganese/L) and urine (0.9 nmol manganese/mmol creatinine) of control workers were significantly lower than levels in exposed workers. Neurobehavioral end points included: two self-administered neuropsychiatric questionnaires; six tests of cognitive functions (Weschlers adult intelligence scale, digit symbol, trail making test, Stroop color-word recognition, digit span, and Benton visual retention); and eight tests of motor functions (static hand steadiness, “TREMOR” test, finger tapping, foot tapping, supination/pronation of hand, Luria-Nebraska thumb/finger sequential touch, simple reaction time, and hand-eye coordination). Information collected for potential confounders included age, years of education, alcohol and tobacco consumption, and prevalence of previous brain concussions. Multiple linear regression analyses were conducted to examine the influence of potential confounders and exposure-response relationships for test results. No significant (p<0.05) effect of exposure was found in tests for cognitive functions, reaction time, or symptom reporting. No statistically significant (p<0.05) differences were found in tests of motor speed, grip strength, or reaction time. Postural tremor as measured in the hand steadiness test was significantly (p<0.05) increased in the exposed group compared with the controls and showed an exposure-response relationship when the exposed group was regrouped into three groups of increasing duration of employment. Results from an alternative test of tremor (“TREMOR”) did not distinguish between the manganese alloy group and the control group. The results indicate that the manganese-exposed group of workers had increased hand tremor compared with the control group, but were indistinguishable from the control group in other tests of motor function, cognitive function, or symptom reporting.
Bouchard et al. (2005) reanalyzed results from neurobehavioral tests administered by Mergler et al. (1994) to 74 male workers in a manganese alloy plant to examine the influence of age on the tests. At the time of testing, workers had been employed an average of 19.3 years (range 1–27 years) and 71 of the workers were employed for >10 years. Based on personal air and stationary air samples 8-hour time-weighted average manganese concentrations ranged from 0.014 to 11.48 mg manganese/m3 total dust (geometric mean=0.225 mg manganese/m3) and from 0.001 to 1.273 mg manganese/m3 respirable dust (geometric mean=0.035 mg manganese/m3). The referent group contained 144 workers with no history of occupational exposure to neurotoxicants who were matched for age, educational level, smoking status, and number of children. Mean blood manganese levels were 11.9±5.3 µg/L (range 4.4–25.9 µg/L) in exposed workers and 7.2±0.3 µg/L (range 2.8–15.4 µg/L) in controls. Paired differences between exposed and control workers increased significantly (p<0.05) with age for one of nine tests of neuromotor domain (nine-hole hand steadiness test); 3 of 12 tests of cognitive domain (trail making B [test of visual conception and visuomotor tracking], delayed word recall [test of learning, recall and attention], and cancellation H [test of visuomotor tracking and concentration]); and 1 of 4 sensory domain tests (vibratometer–vibrotactile perception of the index and toe). The results suggest that older workers may be more slightly more susceptible to the neurological effects of low-level manganese exposure than younger workers.
Bouchard et al. (2007a) examined neuropsychiatric symptoms in a group of 71 male workers in a manganese alloy plant, 14 years after cessation of exposure, and in a group of 71 unexposed referents of similar age and education levels from the same geographical region. Based on personal air and stationary air samples during the operation of the plant, 8-hour time-weighted average manganese concentrations were 0.014–11.48 mg manganese/m3 total dust (geometric mean=0.225 mg manganese/m3) and 0.001–1.273 mg manganese/m3 respirable dust (geometric mean=0.035 mg manganese/m3). The mean number of years of occupational exposure to manganese was 15.7 (range, 7.4–17.3 years). The exposed workers were participants in the earlier study by Mergler et al. (1994). Neuropsychiatric symptoms were assessed by a self-administered questionnaire, the Brief Symptom Inventory, from which scores were determined for somatization (psychological distress from perception of bodily dysfunction), obsessive-compulsive behavior, interpersonal sensitivity (feeling of personal inadequacy), depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. Former, manganese workers showed significantly (p<0.05) higher scores (after adjustment for age, education, and alcohol consumption) for two of the nine neuropsychiatric symptoms (depression, anxiety), compared with controls.
In a follow-up to the Mergler et al. (1994) study, Bouchard et al. (2007b) evaluated neurobehavioral end points in a group of 77 male former workers in a manganese alloy plant, 14 years after cessation of employment, and in a group of 81 nonexposed referents group-matched for age, education and alcohol consumption. The groups were initially assessed in 1990 and, for the present study in 2004, in five neuromotor tests, nine cognitive tests, and six mood state tests. Based on personal air and stationary air samples during the operation of the plant, 8-hour time-weighted average manganese concentrations were 0.014–1.48 mg manganese/m3 total dust (geometric mean=0.225 mg manganese/m3) and 0.001–1.273 mg manganese/m3 respirable dust (geometric mean=0.035 mg manganese/m3). Mean years of occupational exposure to manganese was reported as 15.3 years (maximum=17.3 years). In the 1994 assessment, significant (p<0.05) differences between exposed and control workers were found in scores for one of five neuromotor tests (Luria Motor Scale), three of nine cognitive tests (cancellation H, digit span, color-word test), and one (tension-anxiety) of six mood state tests. In 2004, significant (p<0.05) differences between the exposed and control workers persisted for one (Luria Motor Scale) of five neuromotor tests, none of the nine cognitive tests, and one (confusion-bewilderment) of the six mood states. These results indicate that exposure-related effects observed initially in the manganese alloy workers did not progress in a 14-year period following cessation of employment.
Neurological Assessments of Environmentally Exposed Populations Exposed to Inorganic Manganese. Mergler et al. (1999) studied environmental exposure to manganese and its possible effect on mood (Bowler et al. 1999), neuromotor function (Beuter et al. 1999), and levels of the metal in biological fluids (Baldwin et al. 1999). The study group was a community in southwest Quebec, Canada, near which a former manganese alloy production plant served as a point source for environmental manganese pollution. Due to the presence of MMT in gasoline in Canada, inhaled manganese from car exhaust is a potential contributor to manganese exposures experienced in the population studied. A total of 273 persons comprised the test population. These individuals were selected using a stratified random sampling strategy from the Quebec Health Plan Register, which includes all residents. This strategy helped to ensure that no selection bias was introduced. These individuals were administered a test battery including a computerized neuromotor test, blood sampling, visual function tests from the Neurobehavioral Evaluation System-2, an extensive neuropsychological test battery, and diverse tests covering such areas as olfactory threshold, finger tapping, digit span, and postural sway. Blood sampling data for the study subjects (Baldwin et al. 1999) indicated that manganese levels in women (geometric mean=7.5 μg/L) were significantly higher than in men (6.75 μg/L). No relationship was found between the overall level of manganese in blood and those of lead or iron in serum. However, blood manganese levels were negatively correlated with serum iron in women and had a tendency to decrease with increasing age. Serum iron levels in men were higher than in women. The authors analyzed manganese in drinking water from the study subjects’ residences and analyzed air samples from four different locations for total manganese particulates and PM10 values. The geometric mean value for manganese in drinking water was 4.11 μg/L; there was no correlation between individual values in drinking water manganese and manganese blood levels. Intersite differences in manganese values in total particulate were not observed in the air samples, but intersite differences did exist for manganese in PM10 values. Two geographical areas were identified where manganese in air contributed to blood manganese levels; serum iron was negatively related to blood manganese levels in this analysis (Baldwin et al. 1999).
The Profile of Moods State and Brief Symptom Inventory self-report scales were used to assess condition of mood in the study population (Bowler et al. 1999). The results from these analyses indicated that men who are older (>50 years) and have higher blood manganese levels (≥7.5 μg/L) showed significant disturbances in several mood symptoms with significantly increased values for anxiety, nervousness, and irritability; emotional disturbance; and aggression and hostility when compared to those with lower levels of blood manganese. Neuromotor, neurological, and neurobehavioral analyses revealed that subjects with higher blood manganese levels (≥7.5 μg/L) performed significantly worse on a test for coordinated upper limb movements, with poorest performance in older men (Mergler et al. 1999). Also in men, proximal events on the qualified neurological examination, involving arm movements were significantly slower for those with higher blood manganese, and hand movements (distal events) tended to be in the same direction. No correlation was observed in women. Other measures of motor performance (e.g., hand-arm tremor and tapping movements) were not related to blood manganese levels, although a significant decrease in tremor frequency dispersion was observed with log MnB (manganese blood level). For both men and women, performance on the learning and memory tests was inversely correlated with manganese blood level values, although performance on individual portions of the overall test varied significantly with gender. For men, higher levels of manganese in blood were associated with poorer performance on list acquisition, delayed auditory recall, and visual recognition following a distracter. Females, in contrast, tended to recall fewer geometric shapes, made more errors on the visual reproduction test, but remembered more numbers on the digit span forward test. This study is unique in that it is the first to study both males and females in an exposed population, and it shows an association between elevated manganese blood levels linked to elevated environmental manganese and poor performance on neurobehavioral and neuropsychiatric tests. This study also reported that neurological effects associated with higher levels of blood manganese were more likely to be observed in persons >50 years of age. In contrast, Roels et al. (1999) reported that age was a significant factor only in performance of the visual reaction time test, but not for the eye-hand coordination test or the measure of hand steadiness used in their longitudinal studies. However, Crump and Rousseau (1999) reported that older age was a significant factor in poor performance in tests of short-term memory and eye-hand coordination. Although there were no statistically significant neurological effects associated with manganese exposure among workers of a metal-producing plant evaluated by Gibbs et al. (1999), these investigators also noted that test performance in eye-hand coordination and reaction time decreased with increasing age.
Rodríguez-Agudelo et al. (2006) examined neurobehavioral end points in 168 women and 120 men from eight communities at various distances from manganese extraction or processing plants in the district of Molango, Mexico. Manganese levels in PM10 dust in air samples collected from 28 houses were determined, and the values obtained from the closest monitor were assigned to each of the 288 participants (values ranged from 0 to 5.86 µg manganese/m3). Concentrations of manganese in samples of drinking water and maize grain were mostly below detection limits, whereas soil concentrations ranged from about 6 to 280 mg manganese/kg, with the largest concentrations noted in samples collected close to the manganese industrial sites. Blood samples were collected from each participant and used for manganese and lead determinations. Neuromotor tests (which were a Spanish adaptation of Luria diagnostic procedures) were administered, and odds ratios (ORs) were calculated for 24 different end points involving hand motor functions using dichotomous assessments of performance (e.g., normal and poor) after grouping the participants based on associated manganese concentrations in air or blood manganese levels. No associations were found between neuromotor performance and blood levels of manganese or lead. After grouping the participants into those associated with air concentrations between 0 and 0.1 µg manganese/m3 and those with concentrations between 0.1 and 5.86 µg manganese/m3 (approximate midpoint=3 µg manganese/m3), significantly (p<0.05) elevated ORs for poor performance were calculated for only 3 of the 24 neuromotor end points (two movement coordination, left hand performance [OR=1.99, 95% CI 1.15–3.43]; change of hand position, left hand performance [OR=1.98, 95% CI 0.99–3.95], and conflictive reaction, a test of verbal regulation of movement [OR=2.08. 95% CI 1.17–3.71]). Although the authors concluded that the results indicate that “there is an incipient motor deficit in the population environmentally exposed to large manganese levels,” a more likely explanation for the few and inconsistent findings is chance. This explanation is supported by the finding that no statistically significant associations were found between any neuromotor function end points and blood manganese levels. In addition, the lack of air monitoring data for individual participants in the study precludes assigning the “high” air concentration exposure level as a reliable LOAEL or NOAEL.
Solís-Vivanco et al. (2009) evaluated the same group of subjects with a battery of neuropsychological tests for cognitive function (general cognitive state, attention, semantic and phonological fluency, construction, verbal memory, visual memory coding and recall, and depression). Using logistic regression analysis with air manganese concentration as an exposure variable, no risk of poor performance was found with a 0.05 μg/m3 cut-off point. When using a 0.1 μg/m3 cut-off point, only 1 of 10 cognitive measures had a significantly increased risk of poor performance (attention as measured by the digit span test, OR = 1.75, CI 1.01–3.06). Solís-Vivanco et al. (2009) concluded that the attention impairments associated with high levels of air manganese exposure are evidence of cognitive impairment in the exposed population. However, similar to the study by Rodríguez-Agudelo et al. (2006), the finding on this one measure could be due to chance, as there was no association between blood manganese levels and cognitive performance.
In the same Molango mining district in central Mexico, a cross-sectional study assessed intellectual function in 79 children (ages 7–11 years) exposed to an average manganese air concentration of 0.13 μg/m3 for at least 5 years (Riojas-Rodríguez et al. 2010). The children received a medical exam, and their height and weight were measured. Intellectual function was assessed with the revised Wechsler Intelligence Scale for Children. Maternal intelligence was assessed with the Progressive Matrices of the Raven test. Blood and hair samples were collected from the children to measure manganese concentrations, along with blood concentrations of lead and hemoglobin. A control group was comprised of 93 unexposed children (ages 7–11 years) from socioeconomically-matched communities from the Aqua Blanca district 80 km southeast from the manganese source. Children in the exposed communities had significantly elevated mean blood (9.71 μg/L) and hair (12.13 μg/g) manganese concentrations compared with controls (8.22 μg/L and 0.57 μg/g, respectively). Statistically significant (p<0.05) negative associations were found between hair manganese concentrations and verbal and full scale scores. Blood manganese concentration was inversely, but nonsignificantly, associated with verbal and full scale scores. After adjusting for age and sex, the strongest inverse association between hair concentration and intellectual function was in young girls, with little evidence of associations in boys at any age. Associations with blood concentration were not modified by sex, but age adjustment suggested that the inverse relationship was limited to younger participants. Riojas-Rodríguez et al. (2010) concluded that findings suggest that air-borne manganese exposure is inversely associated with intellectual function in young school-age children. However, manganese exposure from other sources (groundwater, dietary) was not considered, and association between air concentration and test results were not explored.
Hernádez-Bonilla et al. (2011) evaluated the same groups of children for motor impairments. Parameters assessed were manual dexterity, (fine) motor coordination, and motor speed (using the grooved pegboard, finger tapping, and Santa Ana tests). There was a significant inverse relationship between execution of the finger tapping test with blood, but not hair, manganese concentration. Additionally, exposed children made significantly more errors in the grooved pegboard test than controls, but this effect was not associated with blood or hair manganese levels. There was no correlation between manganese concentration in blood or hair in any of the other motor function tests. Hernádez-Bonilla et al. (2011) concluded that there was only subtle evidence of adverse effects on motor speed and coordination.
Similar cognitive findings were reported in a cross-sectional study by Menezes-Filho et al. (2011), in which intellectual function was assessed in 83 children from 55 families (ages 6–12 years) and their primary caregivers from the village of Cotegipe in Brazil, which is within a 2-km radius from a ferromanganese alloy plant that has been emitting high levels of manganese into the air for 4 decades. The height and weight of each child was recorded, and blood and hair samples were collected to measure manganese levels. Blood levels of lead and iron were also measured. Intellectual function was assessed in children using the Wechsler Intelligence Scale for Children, version III. To assess intellectual function in primary caregivers (94% mothers), the Raven Progressive Matrix was administered. Caregivers also provided hair samples for manganese level testing and responded to a questionnaire on sociodemographics and birth history. The mean blood and hair manganese concentrations in children were 8.2 and 5.83 μg/L, respectively. The mean hair manganese concentration in caregivers was 3.5 μg/L, and levels correlated with their children's hair manganese concentration. After adjusting for maternal education and nutritional status, there was a significant (p<0.05) negative association between hair manganese levels in children and their verbal and full scale scores. In addition, after adjusting for education years, family income, and age, there was a significant (p<0.05) negative association between caregiver’s hair manganese levels and performance on the Raven Progressive Matrix. Menezes-Filho et al. (2011) concluded that high manganese exposure, likely via air emissions from the plant, had detrimental effects on cognition in both adults and children, especially in the verbal domain. However, they state that poor cognitive development in children may also be due in part to lower caregiver IQs. Additionally, this study bears the limitations of a cross-sectional design, and causal inferences cannot be made on the relationship of manganese exposure and cognitive defects.
In a community-based study, Lucchini et al. (2007) examined possible associations between prevalence of Parkinsonian disorders and levels of manganese in settled dust collected from communities in the vicinities of manganese ferroalloy industrial plants in the province of Brescia, Italy. Parkinsonian patients were identified from clinical registers from local hospitals, area neurologists, and records of exemption from prescription payments, as well as from records of L-Dopa prescriptions; a total of 2,677 Parkinsonian cases were identified among 903,997 residents. SMRs for each of 206 municipalities were calculated based on national rates standardized for age and gender. Municipalities with the highest SMRs were located within 20 km and/or downwind of three manganese alloy industrial plants in the Valcamonica region of Brescia. An average standardized prevalence of 492 cases/100,000 residents was observed in the 37 municipalities of the Valcamonica region. Crude and standardized prevalence rates for the Valcamonica municipalities were significantly (p<0.05) higher than rates for the other 169 municipalities of Brescia. Municipality-based SMRs for Parkinsonian disorders were significantly (p<0.05) associated with manganese levels in settled dust, and manganese levels in settled dust samples from the 37 municipalities in Valcamonica were significantly (p<0.05) higher than levels in samples for the other 169 municipalities. The results suggest that prolonged environmental exposure to excessive manganese in the Valcamonica region of Brescia may increase the risk for Parkinsonian disorders, but the results do not identify a reliable NOAEL or LOAEL that can be expressed in units of manganese air concentrations. The authors speculated that, even though manganese-induced and Parkinsonian neurological disorders are expected to have two distinct target areas in the brain (the globus pallidus and the substantia nigra, respectively), structural and chemical interconnections between the brain areas may interact to cause increased risk for Parkinsonian disorders as suggested by Weiss (2006).
In a preliminary cross-sectional study, Standridge et al. (2008) evaluated postural balance in 22 residents (13 females and 9 males; ages 20–59 years old) from a manganese-exposed Ohio community where a large ferro-and silico-manganese smelter has been active for >50 years. Subjects had been living within 10 miles of the refinery for at least 3 years, were known not to have a history of manganese occupational exposure, and had been exposed to estimated mean daily ambient manganese concentrations between 0.1 and 2.0 μg/m3. The control group was comprised of 22 military subjects (10 females and 12 males; ages 24–57 years old) who were considered to be unexposed to occupational and environmental neurotoxicants. Results from a postural sway analysis, along with blood and hair manganese levels, were compared with unexposed controls. Several covariates (age, gender, height, weight, alcohol intake, tobacco usage, and blood lead levels) were also recorded. Postural analysis measures of manganese-exposed residents were significantly larger than controls in five out of eight postural balance outcomes (sway area for eyes open on the platform, sway area for eyes open or closed on foam, sway length for eyes open or closed on the foam). After adjustment for covariables, a significant positive association was found between hair manganese levels and sway area and length (eyes open or closed on the platform). Standridge et al. (2008) concluded that these preliminary findings suggest subclinical impairment in postural balance in manganese-exposed residents.
Kim et al. (2011) conducted a cross-sectional study evaluating motor function in 100 residents from the same manganese-exposed Ohio community. Subjects had been living in the community for 10–65 years and had been exposed to 0.04–0.96 μg/m3 of respirable manganese particulate (mean, 0.18 μg/m3; based on U.S. EPA dispersion modeling). Results from the Unified Parkinson's Disease Rating Scale, a postural sway test, and a comprehensive questionnaire exploring demographics and general health were compared to 90 unexposed residents from a demographically similar comparison town in Ohio. Blood samples were collected from all subjects for ferritin, alanine transpeptidase, gamma-glutamyl transferase, manganese, mercury, lead, and cadmium levels. There were no significant differences between the exposed and comparison groups in regards to manganese blood levels, demographics, or major health outcomes. However, when adjusted for covariates (presence of other neurotoxic metals, factors aggravating susceptibility to manganese or motor performance, demographics), the manganese-exposed residents had a significantly increased risk of abnormal performance on the Unified Parkinson's Disease Rating Scale and showed significantly higher postural sway scores. Kim et al. (2011) concluded that these subclinical findings may possibly reflect early subtle effects of chronic, low-level manganese exposure, but alternatively might be due to chance due to the cross-sectional study design, the small to medium effect size, and the lack of association between air or blood manganese levels and motor function performance.
Neurological Studies of Animals Exposed by Inhalation to Inorganic Manganese. In several early animal studies, intermediate or chronic inhalation exposure of monkeys and rats to manganese dusts has not produced neurological signs similar to those seen in humans (Bird et al. 1984; EPA 1983c; Ulrich et al. 1979a, 1979b). For example, Ulrich et al. (1979a) reported that monkeys continually exposed for 9 months to aerosols of manganese dioxide at concentrations as high as 1.1 mg manganese/m3 showed no obvious clinical signs of neurotoxicity, no histopathological changes in brain tissues, and no evidence for limb (leg) tremor or electromyographic effects on flexor and extensor muscles in the arm. However, in a chronic study with Rhesus monkeys, decreased levels of dopamine were found in several regions of the brain (caudate and globus pallidus) (Bird et al. 1984). Behavioral tests detected signs of neurological effects in mice (increased open-field activity and decreased maternal pup retrieval latency), although these are only seen at relatively high exposure levels (60–70 mg manganese/m3) (Lown et al. 1984; Morganti et al. 1985).
Several studies provide evidence for associations between decreased neuronal cell counts in the globus pallidus and neurobehavioral changes (increased locomotor activity) in rats exposed by inhalation for 13 weeks to a mixture of manganese phosphate/sulfate (at 1.05 mg manganese/m3) or manganese sulfate alone (at concentration between 0.009 and 0.9 mg manganese/m3), but not to manganese phosphate alone at concentrations up to 1.1 mg manganese/m3 (Normandin et al. 2002; Salehi et al. 2003, 2006; Tapin et al. 2006). Other 13-week rat inhalation exposure studies reported increased brain manganese concentrations and increased locomotor activity after exposure to 3.75 mg manganese/m3 as metallic manganese (St-Pierre et al. 2001) and increased brain manganese concentrations with no increases in olfactory bulb, cerebellar, or striatal concentrations of glial fibrillary acidic protein (GFAP) after exposure to 0.5 mg manganese/m3 as manganese sulfate or 0.1 mg manganese/m3 as manganese phosphate (Dorman et al. 2004b). GFAP is a widely acknowledged marker of damage to astrocytes.
In male Sprague-Dawley rats, increased locomotor activity (increased distance traveled, but no change in resting time) was observed after up to 13 weeks of exposure to 0.03 or 3 mg of a manganese phosphate/sulfate mixture/m3 (6 hours/day, 5 days/week), but not at 0.3 mg/m3 (Salehi et al. 2003). These exposure concentrations correspond to 0.01, 0.11, and 1.05 mg manganese/m3. Assessment of brain manganese levels, hind limb tremor, and neuropathology of the brain (counts of neuronal cells) found no evidence for tremor at any exposure level, but rats at the highest exposure level showed significantly (p<0.05) increased concentrations of manganese in the frontal cortex, globus pallidus, and caudate putamen, as well as significantly (p<0.05) decreased neuronal cell counts in the globus pallidus and caudate putamen, compared with control values or to values for rats in the lower exposure groups (Salehi et al. 2006).
In similar experiments with male Sprague-Dawley rats exposed to 0, 0.03, 0.3, or 3 mg manganese sulfate/m3 (Tapin et al. 2006) or 0, 0.03, 0.3, or 3 mg manganese phosphate/m3 (Normandin et al. 2002) for 13 weeks by the same exposure protocol, some differences in results were obtained. These exposure levels correspond to 0.009, 0.09, or 0.9 mg manganese/m3 for manganese sulfate and 0.01, 0.11, or 1.1 mg manganese/m3 for manganese phosphate. With exposure to manganese phosphate, manganese levels were significantly (p<0.05) elevated (at 3 mg/m3) in the olfactory bulb, frontal cortex, globus pallidus, caudate putamen, and cerebellum regions of the brain, but no exposure-related effects were found on neuronal cell counts or locomotor activity (Normandin et al. 2002). In contrast, manganese sulfate exposure significantly (p<0.05) increased manganese levels in all regions of the brain, and decreased neuronal counts in the globus pallidus at 0.3 and 3 mg manganese sulfate/m3, compared with controls (Tapin et al. 2006). In addition, the two highest exposure levels of manganese sulfate were associated with significantly (p<0.05) increased locomotor activity (distance traveled), increased resting time, and decreased total ambulatory counts; the lowest exposure level, 0.03 mg manganese sulfate/m3 also increased the distance traveled end point of locomotor activity (Tapin et al. 2006). As with the manganese phosphate/sulfate mixture, neither manganese phosphate nor manganese sulfate exposure was associated with hind limb tremors in the rats. Earlier studies by the same research group, found that Sprague-Dawley rats exposed to 3.75 mg aerosols of metallic manganese/m3 (6 hours/day, 5 days/week for 13 weeks) showed significantly (p<0.05) higher manganese concentrations in various regions of the brain, and higher distance traveled and lower resting time in locomotor tests, compared with controls; neuronal counts were not assessed in this earlier study (St-Pierre et al. 2001).
Several studies have examined the influence of inhalation exposure to manganese sulfate on biochemical end points associated with oxidative stress or inflammation in the brain of rats (Erikson et al. 2005, 2006; HaMai et al. 2006; Taylor et al. 2006) and monkeys (Erikson et al. 2007, 2008). Erikson et al. (2005, 2006) exposed neonatal rats to manganese sulfate (0, 0.05, or 1 mg manganese/m3) during gestation and postnatal days (PNDs) 1–18 and examined five brain regions for several biochemical end points associated with oxidative stress either on PND 19 (Erikson et al. 2006) or after 3 weeks without exposure (Erikson et al. 2005). End points included levels of glutamine synthase (GS) protein and mRNA, metallothionein (MT) mRNA, tyrosine hydroxylase (TH) protein and mRNA, and total reduced glutathione. At PND 9, increased manganese concentrations in the striatum (the most consistently affected region) were associated with decreases in GS, MT, and TH mRNA, and significantly decreased levels of glutathione (Erikson et al. 2006), but these were not apparent 3 weeks after cessation of exposure (Erikson et al. 2005). However, other end points (such as decreased GS protein) were changed, compared with control values, 3 weeks after cessation of exposure (Erikson et al. 2005). Similar end points, as well as levels of mRNA and protein for glutamate transporters, were examined in six brain regions of young male Rhesus monkeys exposed to 0, 0.06, 0.3, or 1.5 mg manganese/m3 as manganese sulfate for 65 days (Erikson et al. 2007). Exposure-related changes included decreased MT mRNA in most regions, decreased TH protein levels in the caudate and globus pallidus, increased GSH in the frontal cortex, and decreased GSH in the caudate. In a follow-up study, Erikson et al. (2008) examined similar end points in groups of four Rhesus monkeys exposed to 1.5 mg manganese/m3 for 15 or 33 days or 65 days with 45 or 90 days of recovery. The previously reported alterations (MT mRNA, TH protein, GSH) were confirmed after 33 days of exposure, and all but the increased GSH levels in the frontal cortex persisted at least 90 days after treatment cessation. Additional persistent effects include decreased GS protein and glutamate transporter (GLT-1) mRNA and protein in various brain regions and decreased glutamate transporter GLAST protein in globus pallidus. In another study, HaMai et al. (2006) exposed three groups of rats to 0 or 0.71 ng manganese/m3 (2 hours/day) as manganese sulfate on gestation days (GDs) 9 and 10, on PNDs 37–47, or on GDs 9 and 10 plus PNDs 37–47 and measured brain levels of mRNA for gene products related to oxidative stress or inflammation. Gestational exposure was associated with decreased mRNA for amylid precurson (APP), cyclooxygenase-2 (COX-2), neuronal nitric oxide synthetase (nNOS), and GFAP, whereas adult exposure was associated with greater transcriptional decreases for the same gene products as well as transcriptional growth factor beta (HaMai et al. 2006). The results from these studies indicate that acute-or intermediate-duration inhalation exposure to manganese sulfate concentrations ranging from about 0.1 to 1 mg manganese/m3 can differentially affect brain biochemical markers of neurotoxicity, but understanding of the neurotoxic mechanism of manganese is inadequate to confidently define any one of the observed changes as biologically adverse.
No studies on neurological effects from inhalation exposure to MMT in humans or animals were located.
3.2.1.5. Reproductive Effects
As discussed earlier (see Section 3.2.1.4), impotence and loss of libido are common symptoms in male workers afflicted with clinically identifiable signs of manganism attributed to occupational exposure to manganese for 1–21 years (Emara et al. 1971; Mena et al. 1967; Rodier 1955; Schuler et al. 1957). These symptoms could lead to reduced reproductive success in men. Impaired fertility (measured as a decreased number of children/married couple) has been observed in male workers exposed for 1–19 years to manganese dust (0.97 mg/m3) at levels that did not produce frank manganism (Lauwerys et al. 1985). This suggests that impaired sexual function in men may be one of the earliest clinical manifestations of manganism, but no dose-response information was presented so it is not possible to define a threshold for this effect. Jiang et al. (1996b) performed a reproductive epidemiological study on 314 men in a manganese plant. The men, from six different factories, performed milling, smeltering, and sintering duties for up to 35 years. The geometric mean airborne manganese concentration (assumed to be total dust) was 0.145 mg/m3 as manganese dioxide. The researchers found no significant differences in reproductive outcomes between exposed and control workers (controls were matched for several factors, including age, smoking, personal hygiene, living habits, and cultural background). The incidences of sexual dysfunction were evaluated through researchers’ questions and judged by the occurrence of two positive responses to three potential conditions: impotence, abnormal ejaculation (early ejaculation or nonejaculation), and lack of sexual desire. Impotence and lack of sexual desire were higher in the exposed group than in the controls (Jiang et al. 1996b). Wu et al. (1996) reported increased semen liquefaction time and decreased sperm count and viability in three groups of men occupationally exposed to manganese: 63 miners or ore processors, 38 electric welders in mechanical fields, and 110 electric welders in shipbuilding. Matched controls consisted of 99 men who were employed in the same occupation and from the same area, but were not exposed to manganese or other reproductive toxins. The men had been exposed to manganese for ≥1 year. Geometric means of total manganese dust (as manganese dioxide) ranged from 0.14 mg/m3 for mining operations to 5.5 mg/m3 for manganese powder processing. Manganese fume concentrations varied; the mechanical welders were exposed to a concentration of 0.25 mg/m3 (geometric mean), while the shipbuilding area concentrations ranged from geometric means of 6.5–82.3 mg/m3, depending on the location within the ship. The miners had a significant percentage (14.3%; p<0.01) of samples with increased liquefaction time, decreased sperm count (34.9%; p<0.01), and decreased percentage of total viable sperm (33.3% had abnormal counts; p<0.01) compared to controls. Welders in shipbuilding had decreased sperm viability levels that were significantly different from controls (p<0.01). Manganese concentrations in semen were significantly increased compared to controls in the mechanical welders; copper, nickel, chromium, and iron concentrations were also elevated in semen in welders in both mechanical and shipbuilding careers. Further, stepwise regression analysis of the impact of these other metals on the measured reproductive parameters indicated that the higher the nickel concentration, the lesser the semen volume and the greater the number of deformed sperm. Copper in the seminal fluid was also positively linked with the viable sperm percentage, sperm viability and number of sperm. Although this study indicates that manganese exposure can cause sperm toxicity, the presence of other metals prevents any conclusive statements concerning its importance. Gennart et al. (1992) performed a reproductive study on 70 male workers exposed to manganese dioxide at a median concentration of 0.71 mg manganese/m3 in total dust for an average of 6.2 years in a dry alkaline battery plant. Results from a questionnaire answered by the workers and controls in the study and from analysis of birthrates of exposed and control workers revealed no difference in birthrates between the groups.
These results in human studies reveal conflicting evidence for whether occupational exposure to manganese causes adverse reproductive effects. Effects reported may occur as a secondary result of neurotoxicity but do not provide information on any direct effect manganese may have on the reproductive organs. No information was found regarding reproductive effects in women.
Intratracheal instillation studies in rabbits indicate that single high doses of manganese (158 mg/kg, as manganese dioxide) can cause severe degenerative changes in the seminiferous tubules and lead to sterility (Chandra et al. 1973; Seth et al. 1973). This effect did not occur immediately, but developed slowly over the course of 4–8 months following the exposure. Direct damage to the testes has not been reported in humans occupationally exposed for longer periods, suggesting that this effect may not be of concern under these exposure circumstances. However, it is unclear if specific studies to investigate possible testicular damage have been performed.
None of the studies located reported adverse effects in female animals following inhalation exposure to manganese. In a study with female mice (Lown et al. 1984), the average number of pups born to exposed females was increased when dams were exposed to manganese dioxide before conception through gestation. In a report of a study of tissue manganese concentrations in lactating rats and their offspring following exposure to manganese sulfate aerosols at 0, 0.05, 0.5, or 1 mg manganese/m3 starting 28 days prior to breeding through PND 18, no mention was made of reproductive performance variables such as the percentage of dams that delivered or the number of pups per litter (Dorman et al. 2005a).
The highest NOAEL values and all LOAEL values from each reliable study for reproductive effects in each species and duration category are recorded in Table 3-1 and plotted in Figure 3-1.
No studies were located concerning reproductive effects following inhalation exposure to organic manganese compounds in humans or animals.
3.2.1.6. Developmental Effects
Very little information is available on the developmental effects of inorganic manganese from inhalation exposure. The incidences of neurological disorders, birth defects, and stillbirths were elevated in a small population of people living on an island where there were rich manganese deposits (Kilburn 1987). However, no conclusions could be reached on the causes of either the neurological effects or the increased incidence of birth defects and stillbirths because there were insufficient exposure data. Control data were not provided, and the study population was too small for meaningful statistical analysis. Although inhalation exposure was not ruled out, the route of exposure was assumed to be primarily oral.
As discussed in Section 3.2.1.4, two studies reported statistically significant inverse relationships between an index of exposure to manganese in air (manganese concentration in hair) and intellectual function in children living in communities near manganese industries (Menezes-Filho et al. 2011; Riojas-Rodríguez et al. 2010). Additionally, Hernández-Bonilla et al. (2011) reported that children living in a manganese mining area had higher manganese hair concentrations than children from a non-mining area, but did not show clear performance deficits on several tests of motor skills (grooved pegboard, finger tapping, and Santa Ana test), compared with the control group of children. No statistically significant associations were found for increasing performance deficits with increasing hair concentrations, but a statistically significant association was found for finger tapping deficits with increasing manganese blood concentrations. The results provide suggestive evidence of an association between environmental exposure of children to manganese and impaired cognitive abilities, but are inadequate to establish causal relationships due to the cross-sectional design and inability to control for possible confounding factors. The study of motor function did not find clear and consistent evidence for motor function deficits in these children.
Lown et al. (1984) evaluated the developmental effects of inhaled manganese in mice. The study involved exposing dams and non-pregnant female mice to either filtered air or manganese at an average concentration of 61 mg/m3 (as manganese dioxide) 7 hours/day, 5 days/week, for 16 weeks prior to conception. The authors then exposed the mice to either air or manganese post-conception, irrespective of preconception exposure. Once delivered, six pups (three of each sex) were distributed to foster mothers and then nursed in the absence of exposure to manganese. The pups were then evaluated on postpartum day 7 for weight gain and gross locomotor activity and on day 45 for different behavioral parameters and learning performance. The authors observed that pups raised by foster mothers that had been exposed to manganese preconception and filtered air postconception had reduced weights compared to pups raised by foster mothers exposed only to filtered air. The activity data indicated that there were no observable differences in activity between pups who had been exposed to manganese in utero and those that had not. Therefore, the data did not provide evidence that manganese exposure resulted in adverse neurological developmental effects.
No studies were located concerning developmental effects in humans or animals following inhalation exposure to organic manganese.
3.2.1.7. Cancer
No studies were located regarding carcinogenic effects in humans or animals after inhalation exposure to inorganic or organic manganese.
3.2.2. Oral Exposure
Although humans are often exposed to significant quantities of inorganic manganese compounds in food and water (see Sections 6.4 and 6.5), reports of adverse effects in humans from ingestion of excess manganese are limited. Most information on the effects of oral exposure to inorganic manganese is derived from studies in animals. These studies are summarized in Table 3-2 and Figure 3-2, and the findings are discussed below. All doses are expressed as mg manganese/kg/day.
Table 3-2
Levels of Significant Exposure to Inorganic Manganese - Oral.
Figure 3-2
Levels of Significant Exposure to Inorganic Manganese - Oral.
Health effects following oral exposure to the organic manganese compound, MMT, were observed in animals. Studies involving oral exposure of animals to MMT are summarized in Table 3-3 and Figure 3-3. As discussed previously, because inhalation, oral, and dermal pathways are not a concern regarding exposure to mangafodipir, this compound’s studies are not presented in an LSE table or figure; instead, they are discussed in Section 3.2.4.
Table 3-3
Levels of Significant Exposure to MMT - Oral.
Figure 3-3
Levels of Significant Exposure to MMT - Oral.
3.2.2.1. Death
Three studies have been located in which death in humans may have been caused by the ingestion of manganese-contaminated water (Hafeman et al. 2007; Kawamura et al. 1941; Spangler and Spangler 2009). Kawamura et al. (1941) reported death from "emaciation" in two adults who ingested drinking water contaminated with high levels of manganese. Hafeman et al. (2007) reported high mortality among infants <1 year of age in a Bangladesh population where the drinking water supplied by certain local wells contained high levels of manganese. As discussed in detail in Sections 3.2.2.4 (Kawamura et al. 1941) and 3.2.2.6 (Hafeman et al. 2007; Spangler and Spangler 2009), several aspects of these three reports suggest that factors other than, or in addition to, high levels of manganese in drinking water may have been responsible for the deaths.
In animals, most studies indicate that manganese compounds have low acute oral toxicity when provided in feed. In rats, daily doses of 1,300 mg manganese/kg/day (as manganese sulfate in the feed) for 14 days did not affect survival (NTP 1993). Survival was decreased in male rats fed 200 mg manganese/kg/day (as manganese sulfate) for 2 years (NTP 1993). The cause of death was attributed to increased severity of nephropathy and renal failure; however, female rats fed 232 mg manganese/kg/day (as manganese sulfate) for 2 years were not affected in this manner (NTP 1993). Similarly, doses as high as 2,251 mg manganese/kg/day (as manganese chloride) in the diet were tolerated by male mice (females were not tested) for 6 months without lethality (Gianutsos and Murray 1982). The survival of both male and female mice was also unaffected by feeding as much as 731 mg manganese/kg/day (as manganese sulfate) for 2 years (NTP 1993).
In contrast to these studies, when exposure is by gavage (usually as highly concentrated solutions of manganese chloride in water), measured LD50 values for 1–21 days of exposure range from 225 to 1,082 mg manganese/kg/day in mice and rats (Holbrook et al. 1975; Kostial et al. 1978, 1989; Rehnberg et al. 1980; Singh and Junnarkar 1991; Smyth et al. 1969). These results suggest that gavage dosing with a bolus of a concentrated soluble manganese compound in water may not be a good model for determining the toxic effects of manganese ingested by humans from environmental sources. Bolus dosing produced death in animals at concentrations near the daily dose levels tolerated in food or drinking water by the same strains and species of animals subjected to longer durations of exposure. It is possible that bolus dosing circumvents the homeostatic control of manganese absorption. It should be noted that the concentrations used in the bolus dosing studies are much higher than even excess levels to which certain humans are typically exposed.
In a study where young pigs were fed a diet moderately high (1.7 mg manganese/kg/day) in manganese but deficient in magnesium, all eight pigs consuming the high manganese diet died within 5 weeks following convulsive seizures; only two of the pigs in a group without supplemental manganese died (Miller et al. 2000). Further studies suggested that high dietary manganese could exacerbate magnesium deficiency in heart muscle, thus creating a complicating factor in the deaths of the magnesium-deficient pigs (Miller et al. 2000).
In conclusion, route of exposure and animal species and strain differences, as well as sex, may account for some of the observed variations in the lethality of manganese. In addition, deficiencies in certain essential nutrients, such as magnesium, may increase the lethal potential of excess manganese.
No studies were located concerning death in humans following ingestion of MMT.
MMT, dissolved in oil and administered by gavage, was found to have LD50 values of 15 mg manganese/kg in the male and female Sprague-Dawley rat and 58 mg manganese/kg in the adult female CD-1 mouse (Hinderer 1979).
Hysell et al. (1974) administered via gavage increasing amounts of MMT (dissolved in oil) to adult COBS rats, 10 animals/group. No lethality was observed at the lowest two doses of 3.8 and 7.5 mg manganese/kg, but 5/10 rats died within 2–6 days postdosing at a dose of 11.3 mg manganese/kg. Increasing numbers of rats died at higher doses, with decreasing times of death post-dosing; complete mortality occurred at the highest dose of 37.5 mg manganese/kg. The survivors appeared normal by 14 days. The LD50 (14-day) was estimated at 14.6 mg manganese/kg.
Hanzlik et al. (1980a) determined the 14-day LD50 for purified MMT administered in corn oil via gavage to adult male Sprague-Dawley rats to be 12.5 mg manganese/kg (95% confidence interval, 9.5–16.8 mg manganese/kg). The animals survived similar times post-dosing as those in the Hysell et al. (1974) study.
All LD50 values from each reliable study for death in each species and duration category are recorded in Table 3-3 and plotted in Figure 3-3.
3.2.2.2. Systemic Effects
In general, there is a lack of data concerning systemic toxic effects in humans who have ingested manganese. This is likely due to the strong homeostatic control the body exerts on the amount of manganese absorbed following oral exposure; this control protects the body from the toxic effects of excess manganese. Studies in humans and animals provide limited data regarding the effects of manganese ingestion on systemic target tissues. This information is discussed below and is organized by target tissue. Table 3-3 and Figure 3-3 present the highest NOAEL and all LOAEL values from each reliable study for these effects for each species and each duration category.
Respiratory Effects. No studies were located regarding respiratory effects in humans after oral exposure to inorganic manganese.
No respiratory effects were reported in mice fed up to 3,900 mg manganese/kg/day (as manganese sulfate) or rats fed 1,300 mg manganese/kg/day (as manganese sulfate) for 14 days (NTP 1993). Male rats fed manganese sulfate for 13 weeks showed no respiratory effects at 520 mg manganese/kg/day; however, females exhibited decreased lung weight at 40–618 mg manganese/kg/day (NTP 1993). No respiratory effects were noted in mice of either sex fed 122–1,950 mg manganese/kg/day (as manganese sulfate) for 13 weeks (NTP 1993), in rats fed up to 232 mg manganese/kg/day (as manganese sulfate), or in mice fed up to 731 mg manganese/kg/day (as manganese sulfate) for 2 years (NTP 1993).
The lungs of adult male Sprague-Dawley rats administered one dose of MMT via gavage in corn oil (31.25 mg manganese/kg) showed signs of hemorrhage and alveolar and perivascular edema, with an accumulation of proteinaceous material in the alveoli. As early as 12 hours following gavage administration of this same dose, the lung/body weight ratio increased to 2.5 times the control value (Hanzlik et al. 1980). Hinderer (1979) observed dark red lungs in Sprague-Dawley rats and CD-1 mice administered sublethal doses (values unspecified) of MMT in an acute toxicity study. Gross necropsy of the lungs of COBS rats administered one dose of MMT in Wesson oil (dose range, 20–37.5 mg manganese/kg) revealed severe congestion and the release of a serosanguinous fluid upon sectioning; histopathology of lungs from rats dying within 24 hours post-exposure showed severe congestion, perivascular and alveolar edema, and alveolar hemorrhage (Hysell et al. 1974). Sections of lungs from rats surviving until 14 days post-exposure revealed extensive areas of consolidation, thickened alveolar septa and focal areas of alveolar macrophage activity.
Cardiovascular Effects. No studies were located regarding cardiovascular effects in humans after oral exposure to inorganic manganese.
In a 1993 National Toxicology Program (NTP) study, no cardiovascular effects (pathological lesions) were observed in mice or rats fed 3,900 or 1,300 mg manganese/kg/day, respectively, for 14 days. No cardiovascular effects were observed in rats or mice exposed for 13 weeks to doses as high as 1,950 mg manganese/kg/day (as manganese sulfate) or for 2 years to doses as high as 731 mg manganese/kg/day (as manganese sulfate) (NTP 1993).
In a study of weanling male Sprague-Dawley rats provided with a diet supplemented with 55 mg manganese/kg/day for 14 weeks, Kalea et al. (2006) found that the level of uronic acid in aortas of the manganese-supplemented group was significantly (p<0.05) higher than in a group of rats fed a diet with adequate manganese (5.5 mg manganese/kg/day). Among heparan sulfate glycosaminoglycans, aortas from manganese-supplemented rats contained higher concentrations of total galactosaminoglycans and decreased concentration of hyaluronan and heparan sulfate (50% less heparan sulfate) when compared to aortas from rats consuming diets with adequate manganese. Heparan sulfate chains of aortas from manganese-supplemented rats contained 41% higher concentration of non-sulfated units compared to those of rats fed the adequate manganese diet (Kalea et al. 2006). These results raise concern about the potential for manganese to influence vascular chemistry in deleterious ways, creating increased vulnerability to cardiovascular events.
In the course of investigating a mechanism to explain the sudden deaths in pigs from high doses of manganese (Miller et al. 2000), studies were conducted in which pigs were fed either low (3.4 mg/kg/day) or adequate dietary magnesium (6.8 mg/kg/day) along with high (55 mg/kg/day) or low doses (5.5 mg/kg/day) of manganese (Miller et al. 2004). No differences in heart muscle ultrastructure were observed; however, marked myocardial necrosis and mitochrondrial swelling were observed in pigs fed high dietary manganese in combination with low magnesium (13.9 mg magnesium/kg/day; Miller et al. 2004). In pigs fed high manganese and adequate magnesium, no swelling of myocardial mitrochondria was observed. These results suggest that high manganese, when fed in combination with low magnesium, disrupts mitochondrial ultrastructure (Miller et al. 2004). In another related study, when rats were provided with high dietary manganese (13.8 mg manganese/kg/day as manganese carbonate) for 8 weeks, heart muscle oxygen consumption was depressed, although no effects of manganese on hematologic variables were observed (Miller et al. 2006). No effects of manganese were observed on heart muscle activities for Ca+2 ATPase, liver glutathione peroxidase, or brain glutathione peroxidase at doses as high as 55 mg manganese/kg/day (Miller et al. 2006). The depression in heart muscle oxygen consumption produced by high dietary manganese presents yet another possible mechanism by which high doses of manganese can produce adverse cardiovascular events.
No studies were located regarding the cardiotoxic effects of MMT in either humans or animals following oral exposure.
Gastrointestinal Effects. No studies were located regarding gastrointestinal effects in humans after oral exposure to manganese, except for one case report of a child who accidentally ingested some potassium permanganate (Southwood et al. 1987). This led to severe local corrosion of the mouth, esophagus, and stomach due to the caustic effects of potassium permanganate on the tissue, but there was no evidence of systemic toxicity.
Adverse gastrointestinal effects have been reported in guinea pigs and mice but not in rats. Guinea pigs administered 4.4 mg manganese/kg/day (as manganese chloride by gavage) did not suffer any gross abnormalities in either the stomach or small or large intestines as a result of treatment but did have patchy necrosis and decreased adenosine triphosphatase and glucose 6-phosphatase levels in both the stomach and small intestine (Chandra and Imam 1973). This study differs from the others in its delivery of manganese (by gavage); the gavage treatment may have partially or completely contributed to the adverse effects seen in the stomach and small intestine of the guinea pigs. No gastrointestinal effects were observed in female mice fed 1,950 mg manganese/kg/day (as manganese sulfate in food) or rats fed up to 618 mg manganese/kg/day (as manganese sulfate in food) for 13 weeks, but male mice exhibited mild hyperplasia and hyperkeratosis of the forestomach at 1,950 mg manganese/kg/day, also in food (NTP 1993).
In a 1993 NTP study, rats fed as much as 232 mg manganese/kg/day (as manganese sulfate) for 2 years showed no gastrointestinal effects; however, mice treated with manganese sulfate for 2 years exhibited hyperplasia, erosion, and inflammation of the forestomach at 585 mg manganese/kg/day for males and 731 mg manganese/kg/day for females. The acanthosis was judged by the authors to be a result of direct irritation of the gastrointestinal epithelium and to be of minor consequence.
No studies were located concerning gastrointestinal effects following oral exposure to MMT in humans. Hinderer (1979) observed discolored intestinal tracts in Sprague-Dawley rats and fluid-filled intestines and spotting of the intestine in CD-1 mice dosed by gavage with high concentrations (values not provided) of MMT in a 14-day toxicity study. Hysell et al. (1974) observed that single lethal doses of 20–37.5 mg manganese/kg (as MMT, given by gavage) produced small intestines that were distended with clear watery contents and thin, friable walls.
Hematological Effects. In a dietary study with female subjects (Davis and Greger 1992), no changes in hematocrit, serum transferrin, or serum ferritin were reported following supplementation with 0.25 mg manganese/kg/day for 119 days. Vieregge et al. (1995) found no effects on hemoglobin, ceruloplasmin, or copper and iron levels in serum for a population of 40-year-old people who had ingested at least 0.3 mg manganese/L in drinking water for a minimum of 10 years. These data indicate that exposure to increased manganese in water did not result in observable hematological toxicity.
Alterations in hematological parameters have been reported in rats and mice, although they were found to vary depending on species, duration, and the form of manganese administered. No conclusive evidence regarding a significant functional deficit has been reported. In mice fed 284 mg manganese/kg/day for 100 days, red blood cell count was decreased by manganese acetate and manganese chloride; white blood cell count was decreased by manganese acetate, manganese chloride, and manganese dioxide; and hematocrit was decreased by manganese carbonate (Komura and Sakamoto 1991). However, manganese carbonate had no effect on red blood cells or white blood cells, manganese dioxide had no effect on red blood cells or total hematocrit, and manganese acetate and manganese chloride had no effect on total hematocrit. It has been suggested that the manganese-related effects on red blood cells may be related to the displacement of iron by manganese. The significance of the other hematological effects was not noted. In a study in rats and mice dosed with manganese sulfate for 14 days, 13 weeks, or 2 years, minor changes in hematology parameters were reported; these changes varied depending on species, dose, and duration, and the study authors did not consider them to be clearly related to compound administration (NTP 1993). No significant hematological effects were observed in mice exposed to 180 mg manganese/kg/day (as manganese tetroxide) for 224 days (Carter et al. 1980). In a study where male Sprague-Dawley rats were fed 55 mg manganese/kg/day as manganese carbonate for 8 weeks, significantly decreased hematocrit and hemoglobin levels were observed (Miller et al. 2006). However, an even lower level of dietary manganese carbonate (35.8 mg manganese/kg/day) fed to male Sprague-Dawley rats in a diet containing a relatively low concentration of magnesium (200 mg magnesium/kg feed/day) for 4 weeks also produced significantly decreased hematocrit and hemoglobin levels (Miller et al. 2006). Thus, the potential for dietary manganese to produce adverse effects on red blood cells may be further modulated by the relative availability of magnesium in the diet.
No studies were located concerning hematological effects following oral exposure to MMT in humans or animals.
Musculoskeletal Effects. No studies were located regarding musculoskeletal effects in humans after oral exposure to inorganic manganese.
In young rats, high concentrations of manganese chloride in the diet (218–437 mg manganese/kg/day) led to rickets (Svensson et al. 1985, 1987); however, this was found to be due to a phosphate deficiency stemming from precipitation of manganese phosphate salt (MnHPO4) in the intestine rather than to a direct biological effect of manganese on bone formation. No significant musculoskeletal effects were observed in mice or rats fed up to 731 mg manganese/kg/day for 2 years (NTP 1993).
No studies were located concerning musculoskeletal effects following oral exposure to MMT in humans or animals.
Hepatic Effects. A single study of human oral exposure of manganese investigated potential hepatotoxicity by analyzing liver enzymes in serum. Vieregge et al. (1995) reported no effects on bilirubin, alkaline phosphatase, glutamic pyruvic transaminase, glutamic oxalacetic transaminase, or gamma glutamyl transferase in humans, ≥40 years old, who had ingested well water containing ≥0.30 mg/L for at least 10 years. These limited data indicate that chronic exposure to elevated levels of manganese did not result in observable liver toxicity in this population.
In animals, a variety of histological changes in subcellular organelles (e.g., rough and smooth endoplasmic reticulum, Golgi apparatus) were observed in the livers of rats exposed to 12 mg manganese/kg/day for 10 weeks (as manganese chloride) (Wassermann and Wassermann 1977). However, these changes were not considered to be adverse but to be adaptive, possibly in response to increased manganese excretion in the bile (see Section 3.4.4). Reductions in liver weight have also been reported in male Fischer 344 rats fed 1,300 mg manganese/kg/day (as manganese sulfate) for 14 days. However, these effects were not seen in B6C3F1 mice fed dosages up to 3,900 mg manganese/kg/day (as manganese sulfate) for 14 days (NTP 1993). Similarly, no treatment-related evidence of liver damage based upon organ weight, histology, or liver function tests were found in male Wistar rats dosed with 271 mg manganese/kg/day (as manganese chloride) in drinking water for 2 or 4 weeks (Rivera-Mancía et al. 2009). In rats fed up to 618 mg manganese/kg/day (as manganese sulfate) for 13 weeks, decreased liver weights were reported in males at ≥33 mg manganese/kg/day and females at 618 mg manganese/kg/day (NTP 1993). When mice were fed 122–1,950 mg manganese/kg/day (as manganese sulfate) for 13 weeks, the females showed no hepatic effects; however, the males exhibited both relative and absolute reduced liver weights at 1,950 mg manganese/kg/day (NTP 1993). In CD-1 mice, no hepatic changes were seen in males fed 205 mg manganese/kg/day (as manganese tetroxide) (Gray and Laskey 1980). No significant hepatic histological changes were observed in either mice or rats exposed for 2 years, with rats fed up to 232 mg manganese/kg/day (as manganese sulfate), and mice fed up to 731 mg manganese/kg/day (as manganese sulfate) (NTP 1993). Additionally, Avila et al. (2008) reported no evidence of increased oxidative stress in the liver in Wistar rats, as measured by thiobarbituric acid reactive substance (TBARS) production, δ-aminolevulinate-dehydratase (δ-ALA-D) activity, and protein carbonylation, at doses up to 1,730 mg manganese/kg/day (as manganese chloride in drinking water) for 30 days.
There are no studies concerning hepatic effects following oral exposure to MMT in humans.
Hinderer (1979) observed mottling of the liver in CD-1 mice administered high doses (unspecified) of MMT via gavage in a 14-day acute toxicity study. Histological evaluation of livers of adult male Sprague-Dawley rats administered 31.3 mg manganese/kg/day (as MMT) revealed scattered hepatocytes throughout the lobule that contained cytoplasmic vacuoles (Hanzlik et al. 1980b). Twelve hours after administration of the same dose, no changes in plasma glutamic pyruvic transaminase (GPT) or liver glucose 6-phosphatase (G6P) activities were observed. After the death of 8/14 animals at this dose level (24 hours post-dosing), there were still no changes in plasma GPT, liver G6P, or hepatic triglycerides (Hanzlik et al. 1980b). Hysell et al. (1974) observed that COBS rats that were gavage-dosed with 20–37.5 mg manganese/kg (as MMT) once and died within 24 hours post-dosing had livers with acute centrolobular passive congestion. This damage progressed to hepatic parenchymal necrosis and leukocytic infiltration in those rats surviving 48–72 hours (15–37.5 mg manganese/kg/day), and extensive cytoplasmic vacuolar change in rats surviving to 14 days.
Renal Effects. No studies were located regarding renal effects in humans after oral exposure to inorganic manganese.
In animal studies, no significant renal histopathological changes were observed in any of the following: mice and rats fed up to 3,900 or 1,300 mg manganese/kg/day (as manganese sulfate) for 14 days (NTP 1993); mice exposed to 205 mg manganese/kg/day (as manganese tetroxide) in their diet for 90 days (Gray and Laskey 1980); mice or rats fed up to 1,950 mg manganese/kg/day for 13 weeks (NTP 1993); or mice fed up to 731 mg manganese/kg/day for 2 years and female rats fed 232 mg manganese/kg/day (as manganese sulfate) (NTP 1993). Additionally, Avila et al. (2008) reported no evidence of increased oxidative stress in the kidney in Wistar rats, as measured by TBARS production, δ-ALA-D activity, and protein carbonylation, at doses up to 1,730 mg manganese/kg/day (as manganese chloride in drinking water) for 30 days. Contrary to these findings, increased severity of chronic progressive nephropathy was noted in male rats fed 200 mg manganese/kg/day (as manganese sulfate) for 2 years (NTP 1993). In addition, glomerulosclerosis/nephritis and urolithiasis (kidney stones) were observed in male, but not female, Sprague-Dawley rats exposed to dietary doses ≥87 mg manganese/kg/day for 63 days (Ponnapakkam et al. 2003b).
No studies were located concerning renal effects in humans following oral exposure to MMT.
Hanzlik et al. (1980b) observed occasional vacuolar degeneration of proximal convoluted tubules of the kidney in Sprague-Dawley rats administered a single gavage dose of 31.3 mg manganese/kg (as MMT). Histopathologic renal effects observed within 24 hours of a gavage dose of 20–37.5 mg manganese/kg (Hysell et al. 1974) included hyaline droplet change, cytoplasmic vacuolation of the proximal convoluted tubules, and distention of the glomerular space and tubule lumens with a finely granular material that stained lightly basophilic. Within 48 hours post-dosing, there was severe tubular degeneration in the form of nuclear pyknosis and cell lysis. Animals surviving the administration of 3.75–25 mg manganese/kg did not have any adverse renal effects.
Endocrine Effects. No studies were located regarding endocrine effects in humans after oral exposure to inorganic manganese; however, other elements of endocrine function (e.g., reproductive effects) following oral exposure to inorganic manganese are discussed elsewhere.
In mice fed up to 3,900 mg manganese/kg/day (as manganese sulfate) and rats fed 1,300 mg manganese/kg/day (as manganese sulfate) for 14 days, no endocrine effects (pathological lesions) were observed (NTP 1993). The adrenal gland was assessed for atypical cells and hyperplasia. In the pituitary gland, the pars distalis was assessed for cyst, hyperplasia, and hypertrophy. The pars intermedia was checked for cysts. C-cells and hyperplasia were examined in the thyroid gland. No endocrine effects were observed in mice or rats fed up to 1,950 mg manganese/kg/day (as manganese sulfate) for 13 weeks. A 2-year study in rats fed up to 232 mg manganese/kg/day (as manganese sulfate) reported no endocrine effects (NTP 1993). However, in a 2-year mouse study, thyroid follicular hyperplasia and dilatation were observed in males fed 584 mg manganese/kg/day, and thyroid follicular hyperplasia was observed in females fed 64 mg manganese/kg/day (NTP 1993).
No studies were located regarding endocrine effects in humans or animals following oral exposure to MMT.
Dermal Effects. No studies were located regarding dermal effects in humans after oral exposure to inorganic manganese.
In animals, no significant dermal histopathological changes were observed in mice or rats exposed for 2 years to doses up to 731 or 232 mg manganese/kg/day, respectively, (NTP 1993).
No studies were located regarding dermal effects following oral exposure to organic manganese.
Ocular Effects. No studies were located regarding ocular effects in humans after oral exposure to inorganic manganese.
In animals, no significant ocular histopathological changes were observed in mice or rats exposed for 2 years to average oral doses of 731 or 232 mg manganese/kg/day (as manganese sulfate), respectively (NTP 1993).
No studies were located regarding ocular effects in humans or animals after oral exposure to organic manganese.
Body Weight Effects. No studies were located regarding body weight effects in humans after oral exposure to inorganic manganese.
In some animal studies, lower body weights were observed in rats and mice in manganese-dosed groups. For example, an NTP study (1993) reported decreases in body weight gain of 57% in male rats and 20% in female rats fed 1,300 mg manganese/kg/day (as manganese sulfate in food) for 14 days. Similarly, Avila et al. (2008) reported decreases in body weight gain of 50% (sex unspecified) in Wistar rats fed 760 mg manganese/kg/day (as manganese chloride in drinking water). Exon and Koller (1975) reported that rats fed daily doses of manganese tetroxide as low as 6 mg manganese/kg/day (mean ingestion value over the duration of the experiment) for 28 days gained only 44% as much weight over the course of the study as control rats. No changes in eating habits in this lowest dose group were observed, although rats in the highest dose group at 4,820 mg manganese/kg/day did exhibit decreased weight gain due to starvation and the effects of the manganese. No histopathological changes were reported in the exposed animals. The authors suggested that the decrease in weight gain might have been due to manganese interference in metabolism of calcium, phosphorous, and iron.
In chronic studies, a similar sex-related difference in the response to this effect was reported. By the end of a 2-year exposure to the maximum daily dose of 200 mg manganese/kg/day (as manganese sulfate in food), male rats had a final mean body weight that was 10% lower than that of controls; however, females’ mean body weights were not significantly different from those of controls throughout the study at all dose levels (232 mg manganese/kg/day was the maximum dose for female rats) (NTP 1993). Food intake (as mg/kg/day) was similar for exposed groups and control groups and for males and females (NTP 1993).
Laskey et al. (1982) investigated body weight changes in a study of adverse reproductive toxicity in male and female Long-Evans rats exposed to manganese. Pregnant dams were fed 0, 350, 1,050, and 3,500 mg manganese/kg/day (in conjunction with a low-iron diet [20 mg iron/kg/day] or a diet adequate in iron [200 mg iron/kg/day]); the pups were continued on their respective diets from day 14 to 15 postpartum to the end of the study (224 days). Manganese treatment did not have any effect on body weight, in either sex fed adequate iron. In iron-deficient male rats, however, body weights were significantly decreased from controls at 24 days postpartum in the 1,050 mg manganese/kg/day diet and at all doses at 40- and 60-day time points. Interestingly, body weight was not significantly different in iron-deficient male rats fed manganese at 350 mg/kg/day at 100 days and at 224 days (no dose group had weight values significantly different from control at day 224). Female body weights were only significantly different in the highest dose at day 24 and in the remaining two manganese doses at day 60. Body weights were not significantly different from controls for the remainder of the study. Significant mortality in both sexes from the highest manganese group fed an iron-deficient diet limited the available data.
In a study designed to evaluate developmental effects of manganese exposure, groups of pregnant Sprague-Dawley rats were exposed to 4.79 mg manganese/mL (as manganese chloride in drinking water) from GD 1 through PND 24 (Molina et al. 2011). Mean body weights of exposed dams were decreased by 15 and 28% at the end of gestation and lactation, respectively, compared with controls; the difference was statistically significant (p<0.05) at the end of lactation, but not at the end of gestation. Water consumption was significantly decreased in treated animals during gestation and lactation (24 and 29% lower than controls, respectively). Based on body weight and water intake, the study authors calculated daily manganese doses during gestation and lactation as 565 and 1,256 mg manganese/kg/day, respectively.
No studies were located concerning body weight effects following oral exposure to MMT in humans. Hanzlik et al. (1980b) observed no significant differences in acutely exposed rats at a dose of 31.3 mg manganese/kg as MMT. Hinderer (1979) also observed normal weight gain in surviving Sprague-Dawley rats and CD-1 mice administered doses of MMT ranging from 7 to 159 mg manganese/kg in a one-dose 14-day lethality study.
In a chronic study, Komura and Sakamoto (1992b) administered 11 mg manganese/kg/day (as MMT) in chow to male ddY mice for 12 months. A 12% decrease in weight gain was observed at 9 months between exposed mice and mice fed unmodified chow, increasing to a 17% difference at 12 months. All differences in these time points were statistically significant. There was no observed difference in food intake between the exposed and control groups.
Metabolic Effects. No studies were located regarding metabolic effects following oral exposure to inorganic manganese in humans or animals.
No studies were located regarding metabolic effects following oral exposure to MMT in humans or animals.
3.2.2.3. Immunological and Lymphoreticular Effects
No studies were located regarding immunological or lymphoreticular effects in humans after oral exposure to inorganic manganese.
Alterations in white blood cell counts have been reported in rats and mice following oral exposure to manganese. One NTP study reported immunological effects in rodents treated for 13 weeks, but not in those treated for 2 years (NTP 1993). Mice were fed 122–1,950 mg manganese/kg/day (as manganese sulfate) for 13 weeks. Males exhibited decreased leukocyte counts at ≥975 mg manganese/kg/day; however, these effects may not have been treatment-related; females were unaffected. For 13 weeks, rats were fed 33–520 mg manganese/kg/day (males) and 40–618 mg manganese/kg/day (females); neutrophil counts were increased in males at ≥33 mg manganese/kg/day, lymphocytes were decreased in males at ≥130 mg manganese/kg/day, and total leukocytes were decreased in females at ≥155 mg manganese/ kg/day (NTP 1993). Rats fed up to 232 mg manganese/kg/day (as manganese sulfate) and mice fed up to 731 mg manganese/kg/day (as manganese sulfate) for 2 years exhibited no gross or histopathological changes or organ weight changes in the lymph nodes, pancreas, thymus, or spleen (NTP 1993). Komura and Sakamoto (1991) reported decreased white blood cell counts in mice following dosing at 284 mg manganese/kg/day with manganese acetate, manganese chloride, or manganese dioxide for 100 days. It is not known if any of these changes are associated with significant impairment of immune system function.
No studies were located regarding immunological or lymphoreticular effects following oral exposure to MMT in humans or animals.
3.2.2.4. Neurological Effects
Manganism Effects in Humans—Oral Exposure to Inorganic Manganese. Although inhalation exposure to high levels of manganese is known to result in a syndrome of profound neurological effects in humans (see Section 3.2.1.4, above), there is only limited evidence that oral exposure leads to the severe neurological effects associated with high-level occupational exposure to manganese.
An outbreak of a disease with manganism-like symptoms was reported in a group of six Japanese families (about 25 people) exposed to high levels of manganese in their drinking water (Kawamura et al. 1941). Noted symptoms included a masklike face, muscle rigidity and tremors, and mental disturbance. Five people were severely affected (2 died), 2 were moderately affected, 8 were mildly affected, and 10 were not affected. These effects were postulated to be due to the contamination of well water with manganese (14 mg/L) that leached from batteries buried near the well. Although many of the symptoms reported were characteristic of manganese toxicity, several aspects of this outbreak suggest that factors in addition to manganese may have contributed to the course of the disease. First, symptoms appeared to have developed very quickly. For example, two adults who came to tend the members of one family developed symptoms within 2–3 weeks. Second, the course of the disease was very rapid, in one case progressing from initial symptoms to death in 3 days. Third, all survivors recovered from the symptoms even before the manganese content of the well had decreased significantly after removal of the batteries. Thus, while there is no doubt that these people were exposed to manganese, there is considerable doubt that all of the features of this outbreak (particularly the deaths) were due to manganese alone.
A manganism-like neurological syndrome has been noted in an aboriginal population living on an island near Australia where environmental levels of manganese are high (Kilburn 1987). Symptoms included weakness, abnormal gait, ataxia, muscular hypotonicity, and a fixed emotionless face. Although it seems likely that excess manganese exposure is an etiologic factor in this disease (based on occupational exposure data from a study where exposure was assumed to be primarily by inhalation although oral exposure was not ruled out), absence of data on dose-response correlations and absence of data from a suitable control group preclude a firm conclusion on the precise role of manganese (Cawte et al. 1987). It is possible that other factors besides manganese exposure may have contributed to the neurological effects, including genetic factors, dietary deficiencies in antioxidants and calcium, and excess alcohol consumption (Cawte et al. 1989). Also, it should be noted that if manganese intake is a causal factor for neurological damage, exposure of the population evaluated in this study could occur not only through the oral route (e.g., food, water, soil), but also by inhaling manganese-containing dusts in environmental or workplace air (Cawte et al. 1987).
Other Neurologic Effects in Adults—Oral Exposure to Inorganic Manganese. Kondakis et al. (1989) reported that chronic intake of drinking water containing elevated levels of manganese (1.8–2.3 mg/L) led to an increased prevalence of neurological signs in the elderly residents (average age, 67 years) of two small towns in Greece. Effects in these residents were compared with effects in similarly aged residents in a town where manganese levels were 0.004–0.015 and 0.082–0.25 mg/L. These levels are within and slightly above levels found in U.S. drinking water, respectively (see Section 6.4.2). Over 30 different neurological signs and symptoms were evaluated, each being weighted according to its diagnostic value for Parkinsonism. Based on this system, the average neurological scores for the residents of the control town (0.004–0.015 mg manganese/L), the town with mid-range levels (0.08–0.25 mg manganese/L), and the town with elevated manganese (1.8–2.3 mg manganese/L) were 2.7, 3.9, and 5.2, respectively. Results from this study suggest that higher-than-usual oral exposure to manganese might contribute to an increased prevalence of neurological effects in the aged population.
However, there are a number of limitations to this study that make this conclusion uncertain. First, no details were reported regarding which neurological signs or symptoms were increased, so it is difficult to judge if the differences were due to effects characteristic of manganism or to nonspecific parameters. Second, the weighting factors assigned to each neurological symptom were based on the symptom’s diagnostic value for Parkinsonism; however, there are clinically significant differences between manganism and Parkinsonism. Therefore, the weighting scheme should have placed more weight on those symptoms (e.g., sleep disorders, emotional lability, weakness, fatigue, and irritability) reported in humans with manganism, such as manganese-exposed miners. The report does not indicate whether efforts were made to avoid bias in the examiner or in the study populations. Nonetheless, the use of the weighting scheme does strengthen the authors' assertion of an association between elevated manganese concentration in the water source and increased susceptibility to neurological symptoms in older populations. Although the subjective parameters included in this scoring are indicative of alterations in mood or emotional state, and affective disorders often accompany other more objective nervous system effects, the authors did not state whether individuals who experienced neurological signs did, in fact, ingest higher levels of manganese than unaffected individuals. The authors reported that the populations in the towns were very similar to each other, but they provided few data to substantiate this. In this regard, even small differences in age, occupational exposures, or general health status could account for the small differences observed. Thus, this study suggests, but does not prove, that chronic oral intake of high levels of manganese can lead to neurological changes in humans.
A study by Vieregge et al. (1995) reported no difference in performance on neurological function studies by people who had ingested well water with high concentrations of manganese. These individuals (high-exposure group), ages ≥40 years, were exposed to manganese at a minimum concentration of 300 μg manganese/L in water for at least 10 years. The controls consisted of a matched group of people who ingested well water with a manganese concentration no higher than 0.05 mg/L. Mean blood manganese concentrations in the high-concentration group were 8.5±2.3 μg/L compared to the control value of 7.7±2.0 μg/L. Performance on motor coordination tests in the ‘high-exposure’ group was no different than the performance of the control group. The authors noted that they could not control for the ingestion of water from sources other than the wells described. Ingestion of manganese in food is also a major contributor, but the authors did not report an estimate of manganese levels ingested from foodstuffs. However, these possible confounders were considered negligible because no differences between groups were revealed in a risk factor analysis for nutritional factors performed by the authors and because manganese concentrations in the blood were not statistically different between the two groups. Manganese drinking water levels for the ‘control group’ in this study were within the range of levels reported in U.S. drinking water (see Section 6.4.2). As with the report by Kondakis et al. (1989), a limitation of this study is the use of a neurological assessment scale for ‘Parkinsonian signs’ rather than an evaluation of symptoms associated with manganism, though the authors observed no ‘detectable’ neurological impairment.
Goldsmith et al. (1990) investigated a cluster of Parkinson's disease in the southern region of Israel. They reported an increased prevalence of Parkinsonism particularly among those 50–59 years old, which suggested early onset of the disease. The authors believed that a potential environmental cause was the water source common to residents in the region where the cluster of Parkinson’s disease was observed. Although the authors reported that the water samples examined showed a “substantial excess of aluminum and a smaller excess of iron and manganese,” the concentrations were not reported. Soil samples were reported to contain excess concentrations of manganese as well as beryllium, chromium, europium, and ytterbium, though no quantitative values were provided. The residents were connected to a national water system, so it could not be determined when the water supply may have become contaminated with excess levels of manganese and other metals. Moreover, there was no clear evidence that persons living in the region were actually exposed to a contaminated water supply. Although identified as a cluster of Parkinson’s disease rather than manganism, the authors suggested that the disease cluster might be related to an environmental source. However, the limitations in this study make it difficult to make any clear association between chronic oral intake of excess levels of manganese and the prevalence of neurological disease.
Iwami et al. (1994) studied the metal concentrations in rice, drinking water, and soils in Hohara, a small town on the Kii peninsula of Japan. This town reportedly had a high incidence of motor neuron disease. The researchers observed that a significantly increased manganese content in local rice and a decreased concentration of magnesium in drinking water were positively correlated with the incidence of motor neuron disease in Hohara (r2=0.99).
Evidence of neurological effects following oral manganese exposure has been noted in case studies of adults, as well. For example, in a case report of a man who accidentally ingested low doses of potassium permanganate (about 1.8 mg manganese/kg/day) for 4 weeks, the man began to notice weakness and impaired mental capacity after several weeks (Holzgraefe et al. 1986). Although exposure was stopped after 4 weeks, the authors reported that a syndrome similar to Parkinson's disease developed after about 9 months. Though suggested by the appearance of a syndrome resembling Parkinsonism, it is difficult to prove that these neurological effects were only caused by exposure to the manganese compound. The authors speculated that the ingested MnO4− was reduced to Mn(II) or Mn(III); however, while this would be expected, it was not measured. Since MnO4− is a corrosive agent, it seems likely that it may have caused significant injury to the gastrointestinal tract (the patient did experience marked stomach pain), perhaps leading to a larger-than-normal gastrointestinal absorption of manganese.
In another study, Banta and Markesbery (1977) reported on a case involving a 59-year-old man with no occupational or environmental exposure to manganese. The man exhibited dementia and neuromuscular deficiencies including bradykinesia, shuffling gait, retropulsion, and rigidity in the upper extremities. Masked faces with infrequent blinking and stooped posture were also observed. Manganese concentrations were significantly elevated in serum, urine, hair, feces, and cerebrum. Although the authors posit that the man may have had Alzheimer’s disease as well as manganese toxicity, they question how the individual could build up significant body stores of manganese in the absence of occupational exposure or any other known source of excess manganese. The authors suggest that the manganese overload may have been caused by abuse of vitamins and minerals.
An association has been suggested between violent behavior and excess manganese exposure; this was investigated by measuring the correlation between the manganese content in hair and violent behavior in prison subjects and controls (Gottschalk et al. 1991). The prisoners did have significantly higher hair manganese content than controls, but further research was indicated to determine whether manganese was a causative factor in violent behavior. The highest concentrations of manganese demonstrated in the hair samples (1.8–2.5 ppm) were, however, within the control ranges reported by Kondakis et al. (1989) (0– 13 ppm) and Huang et al. (1989) (0.1–2.2 ppm for scalp and 0.3–9.8 ppm for pubic hair). Another factor to be considered in the interpretation of these results is the hair color composition within the samples evaluated. At least one study (Cotzias et al. 1964) has reported that manganese content was greater in dark hair when compared to that found in lighter colored hair. Another study showed that manganese accumulated in melanin-containing tissues including the melanin from human hair (Lydén et al. 1984). In their study of inhabitants living in Angurugu on Groote Eylandt, Australia, Stauber et al. (1987) found that samples of grey hair from one elderly Aborigine participant had the same manganese content as the individual’s black hair. The white hairs of a local dog also had the same manganese content as the dog’s black hairs. Based on this evidence, these investigators stated that there was no evidence to support previous reports that dark colored hair concentrated more manganese than light hair. The average manganese content in scalp hair among male and female Aborigine residents was 3.5–5-fold greater than the average scalp hair manganese in male and female Caucasian residents, respectively. The authors cautioned that interpretation of data on manganese content in scalp hair should take into consideration endogenous as well as potential exogenous sources. Moreover, long-term manganese exposure that may be associated with adverse effects may not be represented by manganese content in hair growth from only a few months (Stauber et al. 1987). Thus, further investigations are needed to determine whether manganese content can vary significantly due to hair color pigment alone.
Manganese has also been associated with amyotrophic lateral sclerosis (ALS). In a human study, spinal cord samples from ALS patients were found to have higher manganese concentrations in the lateral fasciculus and anterior horn than in the posterior horn (Kihira et al. 1990). Also, ALS patients exhibited a positive correlation between manganese and calcium spinal cord content, whereas controls exhibited a negative correlation. It was suggested that an imbalance between manganese and calcium in ALS patients plays a role in functional disability and neuronal death. There was also some indication from previous studies that an excess intake of manganese in drinking water may have caused this imbalance, although data to support this were not presented. While this is suggestive of an association between manganese and ALS, it is equally plausible that ALS leads to an imbalance in manganese-calcium metabolism.
No neuropsychological effects were found in a study by Finley et al. (2003) of healthy, nonsmoking, premenopausal women were studied in a research project using a crossover design to determine the combined effects of very low or high dietary manganese with foods containing either saturated or unsaturated fats on measures of neuropsychological and basic metabolic function. Women were fed for 8 weeks at one of two doses of manganese (0.01 or 0.3 mg manganese/kg/day), with one-half of the subjects receiving 15% energy as cocoa butter and the other half receiving 15% energy as corn oil. Blood draws and neuropsychological tests (involving tests of steadiness and ability to control muscular tremor, signs of Parkinson's and related neurologic diseases, as well as tests to determine a range of components related to hostility and anger) were given at regular intervals during the dietary periods. Manganese intake did not affect any neurological measures and only marginally affected psychologic variables.
Neurologic Effects in Children—Oral Exposure to Inorganic Manganese. A number of studies have examined the potential for adverse neurological outcomes from childhood exposure to manganese-contaminated drinking water and/or food (Bouchard et al. 2007c, 2011; Claus Henn et al. 2010, 2011; Farias et al. 2010; He et al. 1994; Kim et al. 2009; Wasserman et al. 2006, 2011; Zhang et al. 1995).
Two early studies (He et al. 1994; Zhang et al. 1995) reported adverse neurological effects in children (aged 11–13) who were exposed to excess manganese in well water and in foods fertilized with sewage water. However, these two studies have several flaws that preclude their use as substantial support for the link between ingestion of excess manganese and the incidence of preclinical neurological effects in children. These studies utilized a group of 92 children pair-matched to 92 controls who lived in a nearby region. The pairs were matched for age, sex, grade, family income level, and parental education level; in addition, all children lived on farms. Although the groups were well matched, the duration and amount of manganese uptake from the flour (from wheat fertilized with sewage) and drinking water containing excess levels was not well characterized. Moreover, the studies did not indicate if nutritional status, such as low iron or calcium intake, which could greatly enhance manganese uptake, were evaluated as potential confounding factors.
The exposed population drank water with average manganese levels of 0.241 mg/L (He et al. 1994; Zhang et al. 1995). The control group drank water containing 0.04 mg manganese/L. These values were measured over 3 years, although it was not stated if the children were exposed during the entire 3 years, or what the children’s daily manganese intakes were. The exposed children performed significantly more poorly (p<0.01) in school and on neurobehavioral exams than control students. School performance was measured as mastery of the native language and other subjects; neurobehavioral performance was measured using the WHO core test battery. However, the report did not state what measures, if any, were taken to ensure that the individuals administering the tests were blind to the exposure status of the subject. Such safeguards would be necessary to prevent the introduction of bias in measurement and analysis of the performance data of the subjects. The exposed children’s hair, blood, and urine manganese levels were significantly increased relative to controls. A simple correlation analysis indicated the performance of exposed children on five of the six of the neurobehavioral tests administered (digit span, Santa Ana manual dexterity, digit symbol, Benton visual retention test, and pursuit aiming test) was inversely correlated with hair manganese levels. Although the authors reported that iron, copper, and zinc were measured in blood and hair, no other metals were measured in these tissues. Because the exposed group presumably ingested food from sources irrigated with sewage, the children may have been exposed to increased levels of other metals, such as lead or mercury. The authors indicate that the children were exposed to increased manganese in their diet from excess levels in foodstuffs and drinking water. Of the foodstuffs evaluated (cabbage, spinach, potatoes, eggplant, sorghum, and flour), only wheat flour contained excess manganese compared to that from the control area. Although the total amount of manganese ingested from the wheat flour and drinking water was not estimated, the authors suggest that the elevated manganese level in drinking water was the key factor contributing to the observed effects. The authors report that children ingesting food and water containing elevated manganese showed poor performance in neurobehavioral tests and poorer school performance when compared to children from a control area. Because exposure levels and duration were not well defined, these studies as reported are not rigorous enough to establish causality between ingestion of excess manganese and preclinical neurological effects in children. Nonetheless, these studies are strongly suggestive that subclinical neurobehavioral effects often seen in industrial workers exposed to excess manganese via inhalation are observed in children.
Wasserman et al. (2006) conducted a cross-sectional investigation of intellectual function on 142 10-year-old children in Araihazar, Bangladesh, who had consumed tube-well water with an average concentration of 793 µg manganese/L and 3 µg arsenic/L. The children received a medical examination and their weight, height, and head circumferences were measured. Intellectual function was assessed on tests drawn from the Wechsler Intelligence Scale for Children, version III, by summing weighted items across domains to create verbal, performance, and full scale raw scores (the tests were adapted for use in this particular population). Maternal intelligence was assessed with Raven's Standard Progressive Matricies, a non-verbal test considered relatively free of cultural influences. Children provided urine specimens for measuring urinary arsenic and creatinine and provided blood samples for measuring blood lead, arsenic, manganese, and hemoglobin concentrations. To assess the dose-response relationship between manganese in well water and intellectual function, children were stratified into four approximately equal sized groups, based on well water manganese levels. The results of the intelligence tests are displayed in Table 3-4.
Table 3-4
Scores on Intelligence Tests.
The results indicated that, unadjusted for other contributors, children in group 1 (i.e., those with estimated mean dose of 0.006 mg manganese/kg bw/day), when compared with the other three groups, had higher full scale scores; groups 2 (estimated mean dose of 0.02 mg manganese/kg bw/day) and 4 (estimated mean dose of 0.07 mg manganese/kg bw/day) were significantly different. The unadjusted result for performance scores revealed that group 2 had a significantly lower score than group 1. In the verbal test, group 4 had a significantly lower unadjusted score than group 1.
After adjustment for sociodemographic factors, groups 1 and 4 were significantly different on all three tests, with group 4 performing more poorly (Table 3-4). Although groups 2 and 3 (estimated mean dose of 0.04 mg manganese/kg bw/day) performed more poorly on average than group 1, the averages from groups 2 and 3 were not statistically significantly different from group 1. Therefore, children consuming the largest amounts of manganese from well water, estimated to be on average 0.07 mg manganese/kg bw/day, are considered to have shown significant decrements in all forms of intellectual performance tested.
Wasserman et al. (2011) conducted a similar epidemiological study in Araihazar, Bangladesh, evaluating the intellectual function of 151 8–11-year-old children using the updated 4th edition of the Intelligence Scale for Children, from which raw scores for verbal comprehension, perceptual reasoning, working memory, processing speed indices, and full scale were calculated (the tests were adapted for use in this particular population). Maternal intelligence was measured on a population-adapted Wechsler Abbreviated Scale of Intelligence. The weight, height, and head circumferences of each child was measured, as were concentrations of lead, manganese, arsenic, and selenium in blood samples. To assess the relationship between manganese in well water and intellectual function, children were divided into two approximately equal sized groups, with either "low" or "high" (>500 μg/L) well water manganese levels (estimated daily manganese doses from water consumption are 0.015 and 0.081 mg/kg/day for the “low” and “high” groups, respectively). Average manganese concentrations in blood for the low and high groups were 14.58 and 15.49 μg/L, respectively. Before adjustment for other confounders, blood concentrations of manganese and arsenic were significant (p<0.05) explanatory variables for deficits in full scale scores, verbal comprehension scores, working memory scores, and perceptual reasoning scores (the latter only for manganese), but not in processing speed scores; the magnitudes of these effects were small, reflected by the finding that blood concentrations of arsenic and manganese together explained <5% of the variances in test scores. After adjustment for sociodemographic factors, negative associations between blood manganese concentration and both working memory and perceptual reasoning domains remained statistically significant.
A pilot study conducted by Bouchard et al. (2007c) found significant associations between hair levels of manganese and certain behavioral end points. The study involved a group of children (24 boys and 22 girls) from Quebec, Canada whose homes received drinking water from one of two wells; one well provided water with a relatively high level of manganese (610 μg/L; W1) and the second well provided water with a much lower level of manganese (160 μg/L; W2). The children, aged 9–13, had estimated average exposure levels of 0.02 mg manganese/kg/day (W1) and 0.007 mg manganese/kg/day (W2). The children with exposure to water from the high-manganese well had significantly higher (p<0.05) levels of manganese in their hair than those children exposed to water from the low-manganese well. Moreover, the children with high concentrations of manganese in their hair demonstrated significantly more (p<0.05) oppositional behaviors (e.g., breaking rules, getting annoyed or angered) and more hyperactivity than children with lower manganese hair concentrations (after adjustment of scores for age, sex, and income). No manganese-related differences were observed for tests related to cognitive problems (disorganization, slow learning, lack of concentration). Although this report is a pilot study, it nonetheless suggests the possibility that exposure to relatively high levels of manganese in water can influence behavior in children.
More recently, Bouchard et al. (2011) conducted a cross-sectional study assessing the intellectual function of 362 school-aged children (6–13 years old) from eight municipalities in southern Quebec exposed to 1– 2,700 μg/L of manganese in their home tap water (median value, 34 μg/L). For each child, total manganese intake was estimated from both the diet and water consumption (including direct water ingestion and for water incorporated in food preparations) using a food frequency questionnaire orally administered to the mother. Estimated manganese intakes from water consumption ranged from 0 to 0.03 mg/kg/day (50th percentile, 0.0003 mg/kg/day), while estimated manganese intakes from dietary sources were >2 orders of magnitude higher than estimated water intakes ranging from 0.01 to 0.44 mg/kg/day (50th percentile, 0.08 mg/kg/day). Mothers also provided information on covariates, including socioeconomic status indicators, home cognitive stimulation, and maternal depression. Cognitive abilities in children were assessed with the Wechsler Abbreviated Scale of Intelligence and maternal nonverbal intelligence was assessed with the Raven’s Progressive Matrices Test. Hair samples were collected from each child for measurement of manganese concentration. In unadjusted analyses, there were significant negative associations between full scale, verbal, and performance scores, and both tap water and hair manganese concentrations. The estimated dietary manganese intake was not significantly (p>0.05) correlated with intellectual performance scores. After adjusting for covariates, negative associations remained statistically significant (p<0.05) between tap water manganese concentrations or estimated manganese water intake and full scale and performance scores (not verbal scores) and between hair manganese concentration and full scale scores. Using a fully adjusted regression model, predicted IQ scores for children with 1 and 216 µg manganese/L tap water concentrations differed by 6.2 Full Scale IQ points. The results demonstrated associations between manganese concentrations in tap water or estimated manganese intakes from water and intellectual impairment in children, but no associations between estimated manganese intakes from diet and intelligence scores. Bouchard et al. (2011) concluded that the findings support the hypothesis that low-level, chronic exposure in drinking water is associated with intellectual impairments in children, acknowledged that inferences that can be drawn from the study are limited due to the cross-sectional design, and suggested that the findings should be replicated in another study.
Other studies have evaluated possible associations between manganese blood levels and cognitive function in school-aged children (Kim et al. 2009), attention-deficit/hyperactivity disorder (ADHD) in school-aged children (Farias et al. 2010), and mental and psychomotor development scores in children from 12 to 36 months of age (Claus Henn et al. 2010). The children in these studies were not known to have been exposed to any particularly high levels of manganese in the environment, and were expected to have been exposed to manganese principally via the oral route, as expected for the general population.
In a cross-sectional study, Kim et al. (2009) evaluated the intellectual function of 261 school-aged Korean children (mean age 9.7 years) with mean blood manganese and lead concentrations of 14.3 μg/L (range 5.3–29.02 μg/dL) and 1.73 μg/dL (range 0.42–4.91 μg/dL), respectively. Children included in the study were recruited from four different Korean cities. Cognitive function was assessed with the abbreviated form of the Korean Educational Development Institute-Wechsler Intelligence Scales, which individually scores vocabulary and, arithmetic (verbal IQ), picture arrangement and block design (performance IQ), and full scale IQ. Caregivers (e.g., mothers or fathers) completed an extensive questionnaire about demographics and other potential covariates for cognitive development. Linear regression analysis, both before and after adjustment for covariates, showed a significant (p<0.05) inverse association between blood manganese and full scale and verbal IQs. The same results were found for blood lead levels. The effect was small, as the blood manganese and blood lead levels explained only 4% of the variances in the full scale IQ and 5% of the variances in the verbal IQ. However, there was an increase in the percentages of the variances explained when the blood levels of both metals were entered as predictive variables, suggesting a joint action of lead and manganese concentrations on full scale and verbal IQs in the children. Further analysis separated the children into two groups: low manganese (blood concentrations <14 μg/L, n=131) and high manganese (blood concentrations >14 μg/L, n=130). There was no difference in mean blood lead concentration between the high and low manganese groups. Linear regression analysis showed lead to be a significant predictive variable for full scale and verbal IQ scores in the high manganese group, but not in the low manganese group. The results are consistent with joint toxic action of lead and manganese on full scale and verbal IQ scores in these children, but the design of the experiment is inadequate to conclude whether the joint action is additive, greater than additive, or less than additive.
Another study in school-aged children (ages 7–15 years) investigated the potential relationship between ADHD and manganese exposure by comparing blood manganese levels in 96 students diagnosed with ADHD and 35 controls (Farias et al. 2010). Treatment-naive students diagnosed either ADHD-combined type (n=50) or ADHD-inattentive type (n=24) had significantly (p<0.05) elevated mean serum manganese levels (4.5 and 5.2 μg/L, respectively), compared with controls (3.5 μg/L). Students with ADHD-combined type (n=21) or ADHD-inattentive type (n=11) who were currently being treated with stimulants had manganese levels that were significantly lower than treatment-naïve students with ADHD (2.9 and 2.8 μg/L, respectively), and not significantly different from controls. No differences were noted in whole-blood iron, magnesium, calcium, or potassium between groups. The results provide evidence for an association between increased manganese blood levels and ADHD, but provide inadequate evidence to establish a causal relationship with this disorder.
In a prospective study, Claus Henn et al. (2010) examined possible associations between early postnatal manganese blood levels and developmental scores in 486 infants from Mexico City. Blood samples were obtained at 12 months (n= 296) and 24 months (n=475), and analyzed for manganese and lead concentrations. Child neurodevelopment was assessed at 6-month intervals from 12 to 36 months using the Mental and Psychomotor Development Indices (MDI, PDI) from the Bayley Scales of Infant Development-II, Spanish version. Twelve- and 24-month manganese concentrations were correlated and declined over time (24.3 and 20.3 μg/L, respectively), and 24-month blood lead concentration was positively associated with 24-month manganese blood concentration. A statistically significant association was found between 12-month MDI scores and 12-month blood manganese concentrations, adjusting for potential confounding variables including blood lead, gender, and maternal IQ and education. The data were consistent with an inverted U-shaped regression model: 12-month MDI scores increased with 12-month manganese concentrations up to about 25 μg/L and decreased at higher concentrations. The highest 12-month MDI score of 97.7 points was predicted to occur at 24.4 μg/L; lower scores of 93.9 and 94.2 points were predicted for the 5th and 95th percentile values of 18.1 and 32.5 μg/L, respectively. No statistically significant associations were found between MDI scores at 24 or 36 months and 24-month manganese blood concentrations or between PDI scores and blood manganese concentrations at any time point. The results suggest that the mental development of infants at 12 months of age is more susceptible to either deficient or excessive intakes of manganese than at 24 or 36 months. In a companion study, Claus Henn et al. (2011) evaluated manganese-lead interactions in the same group of children. At 12 months, but not 24 months, there was a significant manganese-blood interaction among children in the highest manganese exposure group (5th quintile). In this quintile, MDI scores and PDI scores were decreased 2.23 and 1.24 points per μg/dL increase in lead, respectively, compared with 0.07 and 0.27 points per μg/dL increase in lead in the lower four quintiles of manganese exposure. Claus Henn et al. (2011) concluded that the results suggest a possible synergism between lead and excessive manganese to impair development of mental and psychomotor skills during the first year of life. The study design, however, is inadequate to discern if the possible interaction is additive or greater than additive.
There are also individual case reports that supply further evidence of potential neurological effects in children from exposure to drinking water contaminated with high levels of manganese. Sahni et al. (2007) report a case history of a previously healthy Canadian 6-year-old girl who lived with her family in an urban center in Canada. Since 2000, the child’s family had spent summers at their nearby cottage, characterized as weekend visits in June, followed by full-time residence in July and August. While the municipal water used at the primary residence of the family had non-detectable levels of manganese, the cottage well used between 2000 and 2003 was found to have manganese concentrations of 1.7–2.4 mg/L. A neighboring cottage well used in 2004 had 1.7–2.2 mg manganese/L, while spring water used in 2004 had non-detectable levels. The child’s estimated intake from well water exposure was 0.103 mg manganese/kg/day. In 2005, municipal water was brought to the cottage for drinking, but well water was used for washing and cooking. A food history demonstrated that the family consumed more manganese-rich foods, such as pineapples and leafy green vegetables, than a typical Canadian family. However, the family was not vegetarian. The patient and her 7-year-old, asymptomatic sister had very similar diets, with the exception that the sister consumed soy milk due to lactose intolerance. No inhalation exposures to manganese were identified. No industrial releases of manganese were reported in the vicinity of either residence. No other possible source of manganese involving occupational exposures, hobbies among family members, etc., was identified. The patient presented with pica and emotional lability in August 2004. Over the following months, she developed progressive behavioral and neurologic symptoms. She became withdrawn and less verbal, with stuttered and slurred speech. Her balance, coordination, and fine motor skills declined; eventually (in November 2004), she could no longer stand independently, tended to fall backward, and demonstrated a high steppage "cock-like" gait. An MRI indicated hyperintensity in the basal ganglia, indicative of high manganese accumulation. The patient demonstrated high levels of manganese in whole blood (39.7 µg/L). The patient also had severe iron deficiency and polycythemia, which was attributed to elevated cobalt. Her blood levels of lead were normal. While her liver manganese was elevated, her liver function was normal, as was her blood copper level. Other members of the family had elevated blood levels of manganese (1.9–2.8 µg/L) when tested between March and June 2005. The patient's symptoms abated to a large degree when she was treated with phlebotomies for the polycythemia and ethylene-diamine tetraacetic acid chelation for the manganese overload and iron therapy. These treatments occurred from November 2004 through July 2005, when her iron supplementation stopped. By August 2005, the patient’s condition had deteriorated, with her pica returning; she fell frequently and needed assistance where she was previously independent. Phlebotomies and oral iron therapy were resumed in October 2005. The authors concluded that a metabolic disorder involving divalent metals (manganese, iron, and cobalt) interacting with environmental exposures was the most likely explanation for the patient's symptoms.
Brna et al. (2011) describe a very similar case in a previously healthy 5-year-old girl, whose primary exposure was also determined to be from elevated well-water manganese levels (1.7–2.4 mg/L) at her family’s country vacation home. The child’s estimated intake from well water exposure was 0.104 mg manganese/kg/day. The child presented with a recent history of intermittent urinary incontinence, pica, behavioral changes, speech difficulties, social withdrawal, and gross and fine motor incoordination. Upon admission, she could not walk independently. Neurological examination revealed a narrow-based, high stepping gait, retropulsion with preserved strength, deep tendon reflexes, and sensation. She had mild truncal ataxia and subtle action tremor. Clinical chemistry revealed polycythemia without abnormalities in the bone marrow. She had elevated hemoglobin, decreased mean cell volume, increased red blood cell distribution, decreased serum iron, and elevated total iron binding capacity. She also had elevated serum cobalt levels and profoundly elevated whole-blood and serum manganese levels (723 and 38 nmol/L, respectively). Cranial MRI revealed bilateral symmetric hyperintense signals in the basal ganglia, brainstem, and cerebellum on T1-weighted imaging, consistent with a diagnosis of hypermanganism. Like the previous case, her parents also had elevated serum manganese levels (29.5–42.8 nmol/L), but their whole-blood levels were normal and they were clinically well. Her 7-year-old sister had normal manganese levels and no symptoms. Treatment with phlebotomy (for polycethemia), iron infusions, a manganese-free diet, and calcium ethylenediaminetetraacetic acid (EDTA) chelation therapy improved her condition somewhat. At age 10, she was able to walk 40 m unaided with an improved stepping gait, but regularly used an electric wheelchair for mobility. Her neurological status was stable, with improved speech, behavioral, and fine motor skills.
Woolf et al. (2002) describe the case of a 10-year-old boy whose sole source of drinking water at home over a 5-year period was from a well on the family's property in a Boston, Massachusetts suburb. The well water tested after 5 years of use had a manganese concentration of 1.21 ppm (estimated intake: 0.06 mg manganese/kg/day). The child had elevated blood levels of manganese (serum concentration of 0.90 µg/100 mL, compared to reference normal of <0.265 µg/100 mL) and whole-blood manganese concentration of 3.82 µg/100 mL (reference normal: <1.4 µg/100 mL). The child’s urinary excretion of manganese was found to be 8.5 µg/L over a 24-hour period (reference normal: <1.07 µg/L). Although no other member of the family exhibited elevated blood concentrations of manganese, the child and his brother each had elevated manganese levels in hair samples (the patient's level was 3,091 ppb; the brother's was 1,988 ppb; reference normal: <260 ppb hair). At this time, the family switched to bottled drinking water, but continued to use the well water for other purposes (bathing, etc.). The child exhibited no evidence of illness or tremors. A detailed neurologic examination was normal. His balance with his eyes closed was good, but he did not coordinate rapid alternating motor movement well. His fine motor skills were normal and he had no sensory deficits. A battery of neuropsychologic tests revealed that while the child's global cognitive skills were intact, he had striking difficulties in both visual and verbal memory (14th and 19th percentiles, respectively), suggesting a deficit in free retrieval skills, and had a general memory index at the 13th percentile and learning index at the 19th percentile. The child was in 5th grade at the time of testing and had no history of learning problems, although teachers had persistently reported difficulties with listening skills and following directions. The authors report that the findings from the neuropsychological testing are consistent with the toxic effects of manganese, although the authors indicate that a causal relationship cannot be inferred in this case.
Though limited, these case reports also provide further evidence for a link between ingestion of elevated levels of manganese and learning or behavioral problems in children. Other studies have found that manganese levels in hair are higher in learning-disabled children than in normal-functioning children (Collipp et al. 1983; Pihl and Parkes 1977). The route of excess exposure is not known, but is presumed to be mainly oral. These observations are consistent with the possibility that excess manganese ingestion could lead to learning or behavioral impairment in children as suggested by the results from other epidemiological studies (Bouchard et al. 2007c, 2011; Claus Henn et al. 2010; Farias et al. 2010; He et al. 1994; Kim et al. 2009; Wasserman et al. 2006, 2011; Zhang et al. 1995). However, an association of this sort is not sufficient to establish a cause-effect relationship because a number of other agents, including lead, might also be involved (Pihl and Parkes 1977). Moreover, other potentially confounding factors (e.g., health and nutritional status) must be taken into consideration in interpreting such studies.
Neurologic Effects in Humans with Liver Dysfunction—Oral Exposure to Inorganic Manganese. Several studies report the link between hepatic encephalopathy and an increased manganese body burden following chronic liver disease in adults (Hauser et al. 1994; Pomier-Layrargues et al. 1998; Spahr et al. 1996) and children (Devenyi et al. 1994) and in individuals with surgically-induced portacaval shunts (PCS) (Hauser et al. 1994). The manganese exposure in these studies was assumed to originate from a normal diet. Hepatic encephalopathy comprises a spectrum of neurological symptoms commonly occurring in individuals with chronic liver disease; these symptoms include varying degrees of mental dysfunction, although extrapyramidal symptoms may also be identified during a clinical examination (Spahr et al. 1996).
In the Hauser et al. (1994) study, two men aged 49 and 65 years, both with chronic liver disease, and one 56-year-old man with cirrhosis of the liver and a portacaval shunt, showed a variety of neurological symptoms including bradykinesia, postural tremor of the upper extremities, and gait disturbances, as well as a decrease in cognitive function. These men all had significant elevations (p<0.05) in blood manganese as compared to healthy male and female controls, and had hyperintense signals in the basal ganglia bilaterally as measured by T1-weighted MRI. Similar elevations of blood manganese were reported in a population of 57 cirrhotic patients with an absence of clinical encephalopathy (Spahr et al. 1996). Blood manganese was elevated in 67% of the patients and was significantly higher in those patients with previous portacaval anastomoses or transjugular intrahepatic portosystemic shunt. MRI signal hyper intensity was observed in the globus pallidus; the elevated blood manganese levels were significantly correlated with the intensity of the signal in affected patients. Neurological evaluation of extrapyramidal symptoms using the Columbia rating scale indicated a significant incidence of tremor, rigidity, or akinesia in ~89% of the patients, although there was no significant correlation between blood manganese level and these symptoms.
Similar results were observed in a young girl with Alagille’s syndrome (involving neonatal cholestasis and intrahepatic bile duct paucity) with end-stage cholestatic liver disease who exhibited several neurological dysfunctions including dystonia, dysmetria, propulsion, retropulsion, and poor check response bilaterally (Devenyi et al. 1994). The girl had elevated blood manganese (27 μg/L compared to normal value of ~9.03 μg/L) and exhibited hyperintense MRI signal in the basal ganglia. After a liver transplant, the MRI signal abated and the blood manganese level returned to normal. This study and those in adults indicate that the increased manganese body burden (as evidenced by increased manganese blood and brain levels) may contribute to the resultant neurological symptoms and encephalopathy in individuals with cirrhosis or chronic liver disease.
Rose et al. (1999) evaluated brain manganese levels in 12 autopsied cirrhotic individuals who died from hepatic coma and 12 control subjects with no history of hepatic, neurological, or psychiatric disorders at time of death. Neutron activation analysis of the brain tissue revealed an increase in manganese content in the cirrhotic individuals, particularly in the globus pallidus, which had 186% more manganese than that of controls (significant at a level of p<0.001). Significant, although less extreme, increases in manganese were also found in the putamen and caudate nucleus from cirrhotic patients. However, the increased brain manganese did not correlate with patient age, the etiology of the cirrhosis, or the history of recurrent hepatic encephalopathy (reported in 6 patients).
Neurologic Effects in Adult Animals—Oral Exposure to Inorganic Manganese. A few animal studies have observed effects that are comparable to clinical signs seen in people with manganism. Gupta et al. (1980) reported that monkeys given 25 mg manganese/kg/day (as manganese chloride) for 18 months developed weakness and muscular rigidity (however, no data were provided to support these observations). Rats dosed with 150 mg manganese/kg/day (as manganese chloride) developed a rigid and unsteady gait after 2–3 weeks, but this was a transient condition that was not apparent by 7 weeks (Kristensson et al. 1986). In addition, in two separate studies, the authors reported a decrease in spontaneous activity, alertness, muscle tone, and respiration in mice dosed once with 58 mg manganese/kg/day by gavage (Singh and Junnarkar 1991) and staggered gait and histochemical changes in two third-generation mice treated with 10.6 mg manganese/kg/day (as manganese chloride) in drinking water (Ishizuka et al. 1991).
Most other early studies in animals reported changes in brain chemical end points, including concentrations of neurotransmitters or alterations in motor activity with both hypo- and hyperactivity reported. As shown in Table 3-3 and Figure 3-3, changes of this sort have been reported at oral exposure levels that ranged from about 1 to >2,000 mg manganese/kg/day (as manganese chloride, manganese acetate, or manganese tetroxide) (e.g., Bonilla 1978b; Bonilla and Prasad 1984; Chandra 1983; Eriksson et al. 1987a; Gianutsos and Murray 1982; Gray and Laskey 1980; Komura and Sakamoto 1991, 1992b; Lai et al. 1984; Nachtman et al. 1986; Subhash and Padmashree 1991).
More recent studies have continued investigations of brain chemistry alterations in animals following acute-to intermediate-duration oral exposure to manganese (Avila et al. 2008; Calabresi et al. 2001; Desole et al. 1997; Lipe et al. 1999; Liu et al. 2006; Morello et al. 2007; Ranasinghe et al. 2000). In particular, studies have focused on the dopaminergic system due to observed motor dysfunction following manganese exposure and similarities between manganism and parkinsonism (Calabresi et al. 2001; Desole et al. 1997; Ranasinghe et al. 2000). Additionally, a few studies have reported neuropathology following manganese exposure, as evidenced by neuronal damage and/or increased oxidative stress (Avila et al. 2008; Liu et al. 2006; Spadoni et al. 2000). Studies of the effects of manganese on a variety of behavioral assessments in rats also have been conducted; these studies have found changes in measures related to fear, locomotor activity, and cognitive performance (Calabresi et al. 2001; Shukakidze et al. 2003; Torrente et al. 2005; Vezér et al. 2005, 2007). In some of these studies, electrophysiological changes in the brain were associated with behavioral changes (Calabresi et al. 2001; Vezér et al. 2005, 2007).
In a study by Lipe et al. (1999), groups of 30-day-old and 90-day-old male Sprague-Dawley rats were exposed to10 or 20 mg manganese/kg/day as manganese chloride for 30 days. A dose-dependent decrease in body weight gain was found in the adult, but not the weanling rats. Significant (p<0.05) increases were observed in concentrations of aspartate, glutamate, glutamine, taurine, and gamma-aminobutyric acid (GABA) in the cerebellum of the adult rats dosed with 20 mg manganese/kg/day; this increase also appeared to be dose dependent. A significant (p<0.05) decrease in the concentration of glutamine was observed in caudate nucleus and hippocampus of weanling rats dosed with 10 mg manganese/kg/day. A significant (p<0.05) increase in GABA concentration in the caudate nucleus of weanlings was observed in the 20 mg manganese/kg/day group. A significant (p<0.05) decrease in the concentration of glutamine in the caudate nucleus and hippocampus was found in weanlings of the 10 mg manganese/kg group.
In a study by Morello et al. (2007), groups of adult male Wistar rats had free access to either normal drinking water or to a water solution providing 611 mg manganese/kg/day as manganese chloride, with treatment lasting for 13 weeks. A significant reduction in the number of immunoreactive cells for glutamine synthetase was observed in the globus pallidus for manganese-treated animals compared with controls (33% reduction). No effect of manganese was observed in the sensorimotor cortex or striatum, nor was there any effect observed for other manganoproteins tested.
In a study by Ranasinghe et al. (2000), groups of male Sprague-Dawley rats were provided daily with 0 (n=2), 74.9 (n=4), or 149.8 mg (n=4) mg manganese/kg/day, administered as manganese sulfate; another control group of two rats received 20 mg sodium/day. All animals were treated for 50 days. Mean manganese concentrations in liver, brain, heart, and kidney were elevated in the low- and high-dose groups, compared with untreated sodium controls, but statistical analyses of these data were not performed. A decrease was observed in dopamine serum levels in manganese-treated rats compared to controls; the sulfated form was increased in both dose groups compared to controls (12–13 times; from 0.014 nmol/mL in controls to 0.179 nmol/mL in the 20 mg manganese group). Increases were also observed in L-dopa and L-dopa sulfate in both treatment groups. No treatment-related differences were observed in serum levels of L-P tyrosine or its L-P tyrosine sulfate.
In a study by Desole et al. (1997), groups of 3-month-old male Wistar rats were given gavage doses of 0 or 8.8 mg manganese/kg/day as manganese chloride in water for 6 days. Other groups of control or manganese-treated rats received 20 mg/kg buthionine (S,r) sulfoximine0ethyl ester (BSO-E) by intraperitoneal injection twice daily (1 hour before gavage treatment) on days 4, 5, 6, and 7. Rats were sacrificed on day 7, and brainstem samples were extracted for determination of concentrations of dopamine, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and noradrenaline (NA), as well as concentrations of reduced glutathione, ascorbic acid, dehydroascorbic acid, and uric acid (the latter being indicators of oxidative stress potential). Compared with controls, manganese treatment alone increased concentrations of GSH (10–14%) and uric acid (28–45%) in striatum and brainstem, without affecting ascorbic acid concentrations, increased concentrations of DOPAC and HVA in striatum, without affecting dopamine, and decreased brainstem concentrations of dopamine. As expected, BSO-E treatment alone decreased GSH concentrations in striatum (23%) and brainstem (35%), without affecting striatal or brainstem concentrations of ascorbic acid, dehydroasocrbic acid, or uric acid or striatal concentrations of dopamine, DOPAC, or HVA; however, brainstem concentrations of dopamine were decreased by this treatment. Compared with controls, manganese plus BSO-E treatment decreased concentrations of GSH and ascorbic acid in striatum (42 and 22%, respectively) and brainstem (23 and 22%, respectively) and increased concentrations of dihydroxyascorbic acid and uric acid; these results are indicative of a heightened oxidative stress condition. In addition, manganese plus BSO-E treatment decreased striatal concentrations of dopamine, DOPAC, and HVA and brainstem concentrations of dopamine and noradrenaline. The magnitude of the manganese plus BSO-E treatment changes were mostly larger than changes seen in all other experimental groups. The results indicate that the manganese treatment decreased brainstem concentrations of dopamine without affecting neurochemical indicators of oxidative stress and that a glutathione depleted condition potentiated the effects of manganese on brainstem and striatal concentrations of dopamine, DOPAC, and HVA.
Calabresi et al. (2001) measured locomotor activity, fear, and learning and memory in male Wistar rats treated with either tap water as drinking water or a solution of manganese chloride (1,310 mg manganese/kg/day) as drinking water for 10 weeks. Brain manganese levels ranged from 3 to approximately 4 times higher than controls. Manganese-treated rats were significantly (p<0.001) more active than control rats in the open field. Manganese-treated rats showed progressively and significantly more interest in the "novel" object over three trials than the control rats (p<0.001; an average of four contacts for manganese-treated animals compared to an average of <2 for controls on the third trial). Manganese-treated animals also produced significantly (p<0.05) more fecal boluses (indicative of heightened fearfulness) in the open field than control rats over the three trials. No major differences were observed between treatment groups in the eight-arm radial maze test, with the manganese-treated animals taking significantly (p<0.01) more 45 degree angle turns than the control rats. An enhanced dopaminergic inhibitory control of the corticostriatal excitatory transmission via presynaptic D2-like dopamine receptors in corticostriatal slices obtained from the manganese-treated rats was observed. The use of agonists acting on presynaptic purinergic, muscarinic, and glutamatergic metabotropic receptors revealed normal sensitivity. Membrane responses recorded from single dopaminergic neurons following activation of D2 dopamine autoreceptors were also unchanged following manganese intoxication. The authors suggest that the behavioral symptoms described in the "early" clinical phase of manganism may be produced by an abnormal dopaminergic inhibitory control on corticostriatal inputs (Calabresi et al. 2001).
Spadoni et al. (2000) studied groups of male, PND 20 Wister rats provided with either access to drinking water or 3311 mg manganese/kg/day in drinking water, with treatment lasting for 13 weeks. No neuronal loss or gliosis was detected in the globus pallidus with either treatment. However, the majority of GP neurons from manganese-treated rats died following brief incubation in standard dissociation media. Patch-clamp recordings in the whole-cell configuration were not tolerated by surviving GP neurons from manganese-treated rats. Manganese-treated GP cells, but not striatal cells, demonstrated an unusual response to glutamate, since repeated applications appeared to produce irreversible cell damage.
Liu et al. (2006) studied 12-week-old female C57Bl/6 mice, paired as littermates from timed pregnant dams, that received by gavage either water or 43.7 mg manganese/kg/day as manganese chloride for 8 weeks prior to sacrifice. Manganese-treated mice had significantly (p<0.05) increased levels of manganese in the striatum and decreased locomotor activity and striatal dopamine content. Neuronal injury in the striatum and globus pallidus was observed, especially in regions proximal to the microvasculature. Neuropathological assessment revealed marked perivascular edema, with hypertrophic endothelial cells and diffusion of serum albumin into the perivascular space. Immunofluorescence studies revealed the presence of apoptotic neurons expressing neuronal NOS choline acetyltransferase, and enkephalin in both the striatum and globus pallidus. Soma and terminals of dopaminergic neurons were morphologically unaltered in either the substantia nigra or striatum. Regions with neuronal injury contained increased numbers of reactive astrocytes that coexpressed inducible NOS2 and localized with areas of increased neuronal staining for 3-nitrotyrosine protein adducts, a marker of NO formation. The data suggest a possible role for astrocyte-derived NO in injury to striatal-pallidal interneurons from manganese intoxication.
Avila et al. (2008) investigated open field behaviors and orofacial dyskinesia in adult Wistar rats (5/group of unspecified gender) exposed to drinking water containing 0, 2.8, or 6.9 mg manganese/mL (0, 10, or 25 mg MnCl2/mL) for 30 days. Using an allometric equation for drinking water consumption (EPA 1988) and averages of mean body weights reported for exposure day 0 and 30, estimated doses are: 0, 760, or 1,730 mg manganese/kg/day. Behavioral tests (open field, orofacial dyskinesia measures) were performed on days 0 and 30. On day 30, animals were sacrificed and the striatum and hippocampus were dissected for slice preparation. Some striatum slices were used to measure calcium influx. The remaining striatum tissue and the hippocampus were homogenized for biochemical analyses measuring oxidative stress indices (TBARS production, δ-ALA-D activity, protein carbonylation). In both exposed groups, animals exhibited significantly (p<0.05) decreased motor activity in the open-field test and decreased tongue protrusion frequency. Frequency of vacuous chewing movement was significantly (p<0.05) decreased only in the high-dose group. There were no differences in rearing frequency in either treated group compared with controls. Calcium influx in the striatum was significantly (p<0.05) decreased in both treatment groups compared with controls. TBARS levels were significantly elevated and ALA activity was significantly decreased in the striatum, but not hippocampus, of animals in the high-dose group. Protein carbonylation in exposed groups did not differ from controls in either region. The results indicate an association between manganese-induced decreases in motor activity in rats and increased markers of oxidative stress in the striatum.
Vezér et al. (2005, 2007) evaluated multiple neurobehavioral end points in young adult male Wistar rats treated by water gavage with 0, 6.5, or 25.9 mg manganese/kg/day for 10 weeks. Rats were tested in an eight-arm radial maze test (spatial learning and memory test) and an open field test (locomotor ability).
Rats were also tested for amphetamine-induced locomotor activity, acoustic startle response, and prepulse inhibition. At 5 and 10 weeks of treatment, as well as at the end of the post-treatment period 8 weeks later, electrophysiological testing was performed, including recording of cortical evoked potentials as well as spontaneous electrical activity in the hippocampus. Immunohistochemistry was performed to detect changes in density of glial fibrillary acid protein (GFAP) immunoreactive structures in the hippocampal CA1region. Blood and tissue samples (from the cortex and hippocampus) were collected in the 5th and 10th treatment and 12 post-treatment week. Blood and tissue levels of manganese were determined. Manganese accumulation was first seen in blood and then in brain of high-dose rats. Decreased short-and long-term spatial memory performance (at least p<0.05) and decreased spontaneous open field activity (p<0.05) were observed in both low- and high-dose groups, compared with controls. The number of acoustic startle responses, as well as their associated prepulse inhibition of the acoustic startle responses, were decreased in manganese-treated animals. The latency of sensory evoked potentials increased and their duration decreased. Manganese levels returned to normal at the end of the post-treatment period, but impairment of long-term spatial memory remained, as well as the decrease in number of acoustic startle responses in high-dose rats. Prepulse inhibition responses returned to normal. Open field activity returned to normal at the end of post-treatment, but a residual effect could be observed under the influence of D-amphetamine. The electrophysiological effects partially returned to normal during post-treatment. Significantly (p<0.05) high percentages of area showing GFAP immunoreactivity were observed in the dentate gyrus (but not in the striatum radiatum or striatal oriens) in the low- and high-dose groups, compared with controls.
Another factor that could potentiate the neurotoxicity of manganese was explored by Torrente et al. (2005), with rats subjected to restraint stress along with manganese exposure. Groups of 15 adult male Sprague-Dawley rats (250–300 g) were dosed for 2 weeks with either plain drinking water or drinking water providing 38.2 mg manganese/kg/day as manganese chloride. The manganese chloride group was then split into two groups, with drinking water doses of 76 and 153 mg manganese/kg/day provided for another 19 weeks. One-half of the animals in each group were subjected to restraint stress for 2 hours daily by placing them in metacrilate cylindrical holders. Animals treated with 153 mg manganese/kg/day with restraint traveled a significantly shorter distance than control restraint animals (38% decrease; p<0.05). Manganese concentrations in brain and cerebellum were significantly elevated in exposed groups, compared with controls. Body weight and food consumption were significantly decreased (p<0.05) in the exposed groups, compared with control values. Terminal body weights were 86 and 51% of control values in the low- and high-dose unrestrained groups and 90 and 56% in the respective restrained groups. Open field activity was significantly decreased (p<0.05) in the high-dose restrained groups. Spatial learning was also impaired in high-dose rats with or without restraint); for example, unrestrained high-dose rats showed significantly (p<0.05) increased latency to find a hidden platform in the water maze test on days 1, 2, 3, 4, and 5 of testing.
In a study by Shukakidze et al. (2003), groups of white rats were tested for cognitive performance in a multipath maze. Group I served as a control group, which was trained in the maze for 10 days, fed normal feed for 30 days, and then retested. Groups II and III, instead of receiving normal feed, received dosed feed at 5.6 or 13.9 mg manganese/kg/day (as manganese chloride). Groups IV and V were dosed the same doses as Groups II and III, respectively, but received the doses for 30 days prior to maze training. Groups II and III received normal feed for the next 90 days prior to retesting for 10 days. An additional group of animals received a single dose (undefined route) prior to 10 days of training in the maze. Both groups of rats dosed after training (Groups II and III) showed moderate disruption of their acquired skill in the maze compared to controls. Group III also demonstrated increased "aggressivity". Both groups that were exposed prior to training (Groups IV and V) were entirely unable to learn the maze. When these rats were reassessed after a 3-month period without excess manganese, they remained unable to learn the maze. After training, 8/12 rats in the group with the single dose (Group VI) mastered the maze; 4/12 required assistance from the experimenter to orient themselves. Groups of 9 (control) and 10 (manganese-treated) rats were tested in an active avoidance of conditioned and unconditioned stimuli paradigm. Manganese-treated rats received by mouth 13.9 mg manganese/kg/day (as manganese chloride) in water 1 hour prior to the experiment on day 1. Rats were tested over 16–17 days. Manganese treatment resulted in significant and reversible behavioral change, with manganese exposure leading to worsened acquisition of the avoidance reaction in response to unconditioned and conditioned stimuli, increased latent period of conditioned reflex activity, and increased numbers of errors and time taken to navigate a maze, beginning on day 5 of the experimental period and lasting until day 10–15, depending on the end point.
Neurological Effects in Young Animals—Oral Exposure to Inorganic Manganese. Numerous studies have evaluated the effect of early postnatal and juvenile manganese exposure on neurodevelopment in animals. Several have reported biochemical changes in the brain, including alterations in the dopaminergic, noradrenergic, serotonergic, or gabaergic systems; increased monoamine oxidase; and decreased iron levels (Anderson et al. 2007a, 2009; Chandra and Shukla 1978; Deskin et al. 1981; Dorman et al. 2000; Kern et al. 2010; Kern and Smith 2011; Kristensson et al. 1986; Moreno et al. 2009; Reichel et al. 2006; Tran et al. 2002a, 2002b). Additionally, many studies have reported altered behavior following developmental manganese exposure, including hyperactivity, altered social interactions, transient ataxia, altered acoustic startle, impaired learning, and increased stereotypic behaviors (Dorman et al. 2000; Golub et al. 2005; Kern and Smith 2011; Kern et al. 2010; Kristensson et al. 1986; Moreno et al. 2009; Tran et al. 2002a, 2002b). While results from these studies are varied, taken together, they indicate that excess manganese exposure during early postnatal development can lead to alterations in brain chemistry and behavioral development.
Studies of manganese in Rhesus monkeys by Golub et al. (2005) were prompted by the observation that soy-based formulas provided to human infants contain relatively high levels of manganese and thus may pose a potentially toxic hazard to early neurological development. Groups of eight male infant Rhesus monkeys were fed a commercial cow's milk based formula (Similac containing 50 µg manganese/L as control, providing 17.5 mg manganese/kg/day), a commercial soy protein based formula (soy containing 300 µg manganese/L, providing 107.5 mg manganese/kg/day), or the same soy formula with added manganese chloride for a final concentration of 1,000 µg manganese/L (soy plus manganese, providing 328 mg manganese/kg/day). Formulas were exclusively fed to infants starting on the day of birth and extending through 4 months of age, at which time monkeys were transitioned to standard laboratory diet. A behavioral test battery was administered over an 18-month period. The battery included measures of motor, cognitive, and social skills, as well as tests related to the dopamine system (reward delay, fixed interval dopamine drug response). Infants that did not generate sufficient data in each test to permit evaluation were excluded from data analyses. Growth and levels of the dopamine metabolite HVA and the serotonin metabolite 5-hydroxyindolacetic acid (5-HIAA) in CSF at 4, 10, and 12 months of age were also measured. No significant differences between groups were observed for body weights and levels of dopamine and serotonin metabolites in cerebrospinal fluid.
Monkeys fed soy supplemented with manganese were consistently more active during 12 weekly 7-minute observation periods, compared with control and soy monkeys. "Motor behaviors" were observed in seven of eight soy plus manganese monkeys, compared with three of eight in soy monkeys and three of eight in control monkeys. Assessment of gross motor maturation during these observation periods did not detect clear differences between the groups. Both soy and soy plus manganese groups showed some changes in activity/sleep patterns. Compared with controls at 4 months, the 4-month monkeys fed soy plus manganese showed 50% less activity (p<0.05) during the sleep portion of the sleep/wake cycle (this change was not seen at 8 months). At 8 months (but not at 4 months), both soy and soy plus manganese monkeys showed significantly (p<0.05) longer sleep periods and shorter longest time inactive during awake periods than controls. Social interactions were assessed during 16 sessions in which each monkey was paired with another monkey in the study. In these sessions, both soy and soy plus manganese monkeys demonstrated ~66% less time (p<0.05) in chase or rough play and more time in clinging activity compared with control monkeys.
Significant group differences were not consistently observed in more highly structured tests to assess cognitive functions including learning, memory, and attention than controls (p<0.05 for 328 mg manganese/kg/day and p<0.01 for 107.5 mg manganese/kg/day). For example, a response latency decrease was observed in a reward delay response task in the soy group by 50% compared to control, but no significant difference (although a 20% reduction) was observed in the soy plus manganese group. The authors noted that more formal tests of cognitive functions would be most appropriately administered at more mature ages.
Other studies in neonatal animals have detected neurostructural and neurochemical changes at supplementary doses similar to or slightly above dietary levels (1–10 mg manganese/kg/day) (Chandra and Shukla 1978; Deskin et al. 1980), suggesting that young animals might be more susceptible to manganese than adults.
Kristensson et al. (1986) investigated the developmental effects of manganese chloride on 3-day-old male rat pups. The authors dosed the pups with 150 mg manganese/kg/day by gavage in water for 41 days. The pups developed a transient ataxia on days 15–22, which was resolved by the end of the dosing period. The exposed pups also had increased levels of manganese in the blood and the brain (7–40-fold increase in 15- and 20-day-old rats, with cortex and striatum concentrations being relatively equal). In 43-day-old rats, the increases in brain manganese levels were less than those observed in younger rats (i.e., approximately 3 times the control levels), but the striatal levels were higher than in the cortex. Manganese treatment decreased the concentration of homovanillic acid (metabolite of dopamine) in the striatum and the hypothalamus, but not in other brain regions. No other monoamines and metabolites were affected. In a similar study, neonatal rats given bolus doses of manganese chloride in water of 0.31 mg manganese/kg/day for 60 days suffered neuronal degeneration and increased brain monoamine oxidase on days 15 and 30 of the study, but did not show any clinical or behavioral signs of neurotoxicity (Chandra and Shukla 1978).
Deskin et al. (1980, 1981) also found changes in brain chemistry in rat pups dosed with manganese. In the first study, male rat pups were administered 0, 1, 10, or 20 mg manganese/kg/day (as manganese chloride) via gavage in 5% sucrose solution for 24 days postnatal. The authors observed that the two highest doses resulted in decreased dopamine levels in the hypothalamus, while the highest dose resulted in a significant decrease in brain tyrosine hydroxylase activity and a significant increase in monoamine oxidase activity in the hypothalamus. Hypothalamic norepinephrine was unaffected by any manganese dose, and no significant changes in neurochemistry were noted in the corpus striatum. The authors suggested that the observed effects were probably due to decreased activity of tyrosine hydroxylase and increased levels of monoamine oxidase.
The second study (Deskin et al. 1981) involved dosing male rat pups with 0, 10, 15, or 20 mg manganese/kg/day (as manganese chloride) via gavage in 5% sucrose solution, for 24 days starting at birth. The authors performed neurochemical analyses of hypothalamus and corpus striatum as before and observed that serotonin was increased in the hypothalamus at the highest dose, but was not elevated significantly in the striatum. Acetylcholinesterase levels were significantly decreased in the striatum at the highest dose, but were unchanged in the hypothalamus. The authors believed that the decrease in acetylcholinesterase to be of minor functional significance given that other mechanisms can also regulate acetylcholine metabolism.
A study by Kontur and Fechter (1988) reported no difference in levels of monoamines and related metabolites in neonatal rats at 22 mg manganese/kg/day as manganese chloride (14–21 days), although Dorman et al. (2000) reported elevated striatal DA and DOPAC in 21-day-old rats administered the same high daily dose used by Kontur and Fechter (1988) from PND 1 to 21. Effect of manganese treatment on neurobehavior was also evaluated in this study. There was a significant decrease in body weight gain in pups at the highest manganese exposure dose. Although there were no statistically significant effects on motor activity or performance in the passive avoidance task in the neonates, manganese treatment induced a significant increase in amplitude of the acoustic startle reflex at PND 21. However, in adult rats, the amplitude of the acoustic startle reflex was significantly decreased compared to the control at the lowest dose tested.
Reichel et al. (2006) studied the effects of manganese in male Sprague-Dawley rats that were born and dosed daily with an oral dose of 0, 4.4 or 13.1 mg manganese/kg/day as manganese chloride on postpartum days 1–21. Locomotor activity was assessed (distance traveled horizontally; PNDs 10–14), as was olfactory orientation (PNDs 9–13), negative geotaxis (PNDs 8–12) and balance and coordination (PND 90). Day of eye opening, pinna detachment, and incisor eruptions was also evaluated. Mean body weights at PND 21 were decreased by about 2 and 3% in the low- and high-dose groups, respectively, compared with controls. Manganese concentrations in striatum were elevated in the high dose group, compared with control, at PND 14 (~4-fold) and PND 21 (~2-fold), but not at PND 90. Manganese levels were not measured in the low-dose group. No exposure-related effects were noted on developmental landmarks (eye opening, pinna detachment, incisor eruption), basal motor activity during the neonatal period (PNDs 10–14) and adulthood (PND 90), or olfactory discrimination of home cage bedding during the neonatal period. The only behavioral end point affected during the neonatal period was a significant (p<0.05) increase in mean latencies to rotate 180° on the inclined plane of a negative geotaxis task. At PND 90, dopamine transporter binding sites in the striatum were decreased by about 20 and 60% in the low- and high-dose groups, respectively; only the high-dose value was significantly different (p<0.05) from the control. At PND 90, the locomotor activating effects of 20 mg/kg cocaine were significantly (p<0.05) decreased in the neonatally exposed manganese high dose group, compared with controls. The results indicate that neonatal exposure of rats to excess manganese caused subtle behavioral effects (altered balance in the neonatal period and diminished locomotor response to cocaine in adulthood) and neurochemical effects in adulthood (decreased dopamine binding sites in the striatum).
In a study by Tran et al. (2002a), Sprague-Dawley PND 1 litters were culled to 10–12 pups per dam and then were supplemented from PNDs 1–20 with 0, 0.7, 3.8, or 7.5 mg manganese/kg/day as manganese chloride provided by mouth. Male and female pups were used. Righting test (PND 6), homing test (olfactory discrimination; PND 10), and passive avoidance (PND 32) were performed. Striatal dopamine levels were also determined after sacrifice on PND 40. Brain tissue analyses for iron, copper, zinc, and manganese content were performed on animals sacrificed on PNDs 14, 21, and 40. Animals were not dosed after PND 20. The two highest dose groups of rats took approximately twice as long (2 seconds) as control and 0.7 mg manganese/kg/d (approximately 1 second) to right themselves; this result was not statistically significant. In the homing test of olfactory discrimination, the 7.5 mg manganese/kg/day group took significantly longer to reach their goal compared to controls and the 3.8 mg manganese/kg/day group (the 0.7 mg manganese/kg/day group performed similarly to the control). The control group required approximately 40 seconds; the high-dose group required 75 seconds (an 88% increase in the high-dose group over the control). In the passive avoidance task, there was a positive linear trend, with the highest dose group showing a 3-fold increase in the number of footshocks received over the control. The 3.8 mg manganese/kg/d group showed a 2-fold increase in the number of footshocks over the control. A negative linear relationship was also observed in striatal dopamine concentrations, with the high-dose group having approximately half the dopamine concentration of the control. No dose-related trends over time points were observed in manganese content of tissues. The highest dose group showed some statistically significant (p<0.05) increases in manganese in brain tissue. No changes were seen in iron, copper, or zinc tissue concentration. Both males and females were used in behavioral tests since ANOVAs showed no interactive effects of treatment or sex.
In a companion study, Tran et al. (2002b) explored whether there were lasting behavioral and neurochemical changes following manganese exposure. Again, Sprague-Dawley rat pups received dietary supplementation in the form of 0, 0.7, 3.8, or 7.5 mg manganese/kg/day (as manganese chloride). Male and female pups were sacrificed during infancy and at weaning (18–24 per treatment group) for tissue analyses of trace elements. Twenty-four rats were sacrificed at PND 35 for dopamine analysis (Tran et al. 2002a). The 32 remaining rats, all males, no longer received treatment. Behavioral testing began with a burrowing detour test (PNDs 50–56) and ended with a passive avoidance test (PNDs 60–64). No statistically significant results for any individual treatment group for any behavioral task or striatal dopamine levels. A statistically significant positive trend was observed for passive avoidance (approximately 50% more footshocks in highest dose group, compared with control). The control had approximately 2 times the striatal dopamine levels of the two highest dose groups on animals sacrificed on PND 65.
Kern et al. (2010) evaluated Sprague-Dawley rat pups administered 0, 25, or 50 mg manganese/kg/day as manganese chloride in 25% sucrose vehicle via micropipette from PND 1 to 21. Dose groups were balanced across sex within each of 26 litters, each culled to 10 pups with an approximate 2:1 male to female ratio. On PND 23, 15– 20 and 7 males per group were tested in open area and the elevated plus maze activities, respectively. Groups of 15–20 males were evaluated on PNDs 27–46 on the radial arm maze. Blood and tissue samples were collected on PND 24 (8–12/sex/group) and PND 36 (females only, number unspecified) for measurement of hematocrit and blood and brain manganese levels. On PND 24, an additional 4–7 males/group were sacrificed for immunohistochemical analysis of dopamine transporter (DAT) and dopamine D1 and D2 receptors in several brain regions. Following treatment, manganese blood levels were increased 2–3-fold at PND 24 (p<0.05), but hematocrit levels were not altered. Males in the 50 mg/kg/day group had significantly (p<0.05) increased activity in the open field and significantly impaired spatial learning in the radial arm maze. All treated males had increased stereotypical behavior in the radial arm maze (p<0.05). There were no differences in behavior during the elevated plus maze test. Additionally, there were significant decreases in dopamine D1 and D2 receptors and dopamine transporter in multiple brain regions from males from the 50 mg/kg/day group.
In a follow-up study, Kern and Smith (2011) evaluated the effects of preweaning manganese exposure on the adult dopaminergic system, behavior, and astrocytic activation. Sprague-Dawley rat pups were exposed to 0, 25, or 50 mg manganese/kg/day using the experimental design described above. On PNDs 97–98, 15–20 adult males per group were evaluated in the open area test. Blood and tissue samples were collected on PND 24 (8–12/sex/group) and PND 107 (10 males/group) for measurement of blood and brain manganese levels. Additionally, 4–7 males per group were sacrificed for immunohistochemical analysis of dopamine transporter, dopamine D1 and D2 receptors, and GFAP levels in the brain were sacrificed on PNDs 24 and 107. Preweaning exposure to manganese did not lead to significantly (p>0.05) elevated manganese levels in the blood or brains of adult rats. However, exposure at 50 mg/kg/day led to significantly (p≤0.05) increased densities of dopamine D2 receptors in the prefrontal cortex of adult brains (in contrast to decreased dopamine receptor and transporter densities in weanling brains). Astrocyte activation was significantly (p<0.05) increased in both weanling (prefrontal cortex) and adult rats (medial striatum) with preweaning exposure at 25 or 50 mg/kg/day. At 50 mg/kg/day, additional regions in the adult brain had significantly increased atrocytic activation (prefrontal cortex, nucleus accumbens). In the open field, there were no measureable differences in activity in adults (compared to increased activity in weanlings reported in the previous study). However, a residual effect could be observed under the influence of D-amphetamine. Kern et al. (2011) concluded that preweaning manganese exposure leads to lasting molecular and functional impacts in multiple brain regions of adult animals, long after brain manganese levels return to normal. However, it appears that behavioral effects may be reversible.
In order to determine if developing animals are more susceptible to the neurochemical and neurobehavioral effects of manganese exposure, Moreno et al. (2009) exposed mice as juveniles, as adults, or as both juveniles and adults. Littermates from timed-pregnant C57Bl/6 mice were paired in control and manganese-exposed groups, receiving 0, 4.4, or 13.1 mg manganese/kg/day as manganese chloride via gavage from PND 20 to 24 and again from week 12 to 20. Additional animal groups were exposed only from PND 20 to 24 or from week 12 to 20. Open field activity was measured every other day during early exposure (11–18 animal/group), and every other week thereafter (8–10 animals/group). Following treatment, animals were sacrificed. Brain levels of dopamine and its metabolite DOPAC, and serotonin and its metabolite 5-HIAA, were measured in the striatum of 3–4 animals per group. Brain levels of manganese, iron, and copper were also measured in three animals per group. In all treated animals, brain regions with the highest levels of manganese were striatum, substantia nigra, and cortex. Only modest increases in copper and iron brain levels were detected. In the open field, juvenile-only exposed males, but not females, spent significantly (p<0.05) less time in the margins of the open field (increased novelty-seeking behavior). Adult-only exposed mice did not display any open field behavioral alteration. However, male mice exposed at both ages spent significantly (p<0.05) more time in the margins of the open field (decreased novelty seeking behavior). Additionally, males exposed to 13.1 mg/kg/day at both ages made significantly (p<0.05) fewer movements. Several alterations in the dopaminergic system were reported in all groups. In juveniles exposed to 13.1 mg/kg/day, dopamine levels were significantly (p<0.05) increased, but DOPAC levels were significantly decreased. Therefore, the DOPAC:dopamine ratio (an indicator of dopamine turnover) was increased in this group. In contrast, mice with adult-only exposure at 13.1 mg/kg/day and all manganese-treated mice exposed at both ages had significantly (p<0.05) decreased dopamine and DOPAC levels. In the serotonergic system, the only significant (p<0.05) finding was increased 5-H1AA in juvenile-only exposed mice at 13.1 mg/kg/day. These results indicate that developing mice are more sensitive to neurobehavioral and neurochemical effects of manganese exposure than adult animals, and that previous juvenile exposure increases susceptibility to these effects from manganese exposure in adults.
Weber et al. (2002) evaluated indicators of oxidative stress in Charles River CD rat pups that were dosed (by mouth with micropipette) according to average pup weight for each litter starting on PND 1 and continuing until PND 21 at doses of 0 (nanopure water vehicle), 6.9, or 138 mg manganese/kg/day. Pups were sacrificed on PND 21, and samples of cerebellum and cerebral cortex were collected and frozen in liquid nitrogen, with manganese concentrations evaluated in brain tissue. Also evaluated were cerebrocortical and cerebellar metallothionein (MT) mRNA levels, glutamine synthetase (GS) activity, GS protein levels, and total glutathione (GSH) levels. High-dose manganese exposure significantly increased (p<0.05) total cerebrocortical GSH when compared to control without changes observed in any of the other measures. The same change was apparent with the high-dose manganese exposure on cerebellar GSH, although slight differences in the standard error of the mean prevented reaching statistical significance. However, it should be noted that these measures actually decreased with respect to the control in the low dose manganese group. Overall, data do not appear to support an effect of manganese exposure on measured biochemical variables indicative of oxidative stress.
Neurologic Effects in Animals with Liver Dysfunction—Oral Exposure to Inorganic Manganese. Several animal studies have evaluated the potential for hepatic dysfunction to enhance the neurotoxicity of manganese (Montes et al. 2001, 2006; Rivera-Mancia et al. 2009; Rose et al. 1999).
Rose et al. (1999) reported the effects on manganese body burden (exclusively from the diet) in rats with either induced cirrhosis of the liver, acute liver failure (induced by portacaval anastomosis followed by hepatic artery ligation), or a surgically-administered portacaval shunt (PCS). Brain manganese levels in these three groups of rats were compared to control rats and sham-operated rats. PCS and sham-operated rats were evaluated 4 weeks following surgery, while cirrhotic rats were studied 6 weeks following surgery. Rats with acute liver failure were studied 15–18 hours following devascularization at coma stage of encephalopathy. Manganese levels were statistically significantly increased as compared to non-treated controls and sham-operated controls in both cirrhotic and PCS rats in the frontal cortex, globus pallidus, and caudate/putamen; manganese levels were highest in the globus pallidus. For example, in the globus pallidus, brain manganese was increased 57% in the PCS rats as compared to the control rats (p<0.0001). However, the level of manganese in the globus pallidus in the PCS rats was significantly elevated as compared to cirrhotic rats, indicating that shunting is a strong determinant of manganese deposition in the brain.
Montes et al. (2001) also explored the potential for hepatic disease to potentiate the toxic effects of manganese by observing effects on levels of specific neurotransmitters. Groups of male Wistar rats were assigned to one of six treatments: (1) sham operated; (2) bile duct ligated (BDL); (3) sham operated with 15.1 mg manganese/kg/day supplied as manganese chloride in drinking water; (4) BDL with 15.1 mg manganese/kg/day in drinking water; (5) sham operated with 26.7 mg manganese/kg/day in drinking water; or (6) BDL with 26.7 mg manganese/kg/day in drinking water. The BDL condition models a cirrhotic-type condition in the rats. Rats received this treatment for 4 weeks beginning at surgery. At the end of treatment, rats were weighed and killed. Total bilirubins (as well as conjugated and unconjugated forms) increased over control in all BDL groups, but there was no significant effect of manganese treatment. There was also no effect of manganese on alanine aminotransferase levels or on collagen, although these measures were significantly increased by BDL. However, the combination of BDL and manganese exposure produced 2- and 4-fold increases (p<0.001) of striatal manganese content at the 15.1 and 26.7 mg manganese/kg/day doses, respectively, while BDL alone did not produce changes. Striatal DA content was significantly decreased compared to control in BDL rats; the addition of 26.7 mg manganese/kg/day to BDL produced an approximate 33% increase in dopamine (DA) content over BDL alone. The highest dose of manganese produced 2-fold striatal HVA increases over control in both sham-operated and BDL rats. BDL and manganese treatment at 15.1 mg manganese/kg/day each individually produced 2-fold increases over control levels in striatal DA turnover, measured as HVA/DA; the combination of BDL with manganese at 15.1 mg manganese/kg/day produced the same result as each condition individually. The sham-operated and BDL high dose rats each had HVA/DA levels of nearly 3 times the control level; all of these differences were significant (p<0.05). These results suggest that hepatic dysfunction can, indeed, potentiate the neurotoxicity of manganese.
In another study, Montes et al. (2006) explored the potential role of hepatic dysfunction as a potentiator of the toxic effects of manganese on neuronal damage produced by oxidative stress. Groups of male Wistar rats were assigned to one of four treatments (n=6–9 in each group): (1) sham operated; (2) BDL; (3) sham operated with 26.7 mg manganese/kg/day (as manganese chloride) in drinking water; or (4) BDL with 26.7 mg manganese/kg/day in drinking water. Rats received this treatment for 4 weeks beginning at time of surgery. Compared with sham-operated controls, BDL treatment with or without manganese caused significant (p<0.05) increases (>2-fold) in gamma glutamyltranspeptidase and alanine aminotransferase activities, collagen, and glycogen levels, but manganese alone did not increase these indices of liver damage. Manganese or BDL treatments alone caused moderate, statistically significant (p<0.05) increases (~20%) in manganese content in the striatum and globus pallidus. Manganese contents in both regions were further and markedly increased by the BDL and manganese treatment (300–400% increase). Levels of nitric oxide (NO) were not consistently changed in either brain region in manganese-alone or BDL plus manganese-treated rats compared with sham-operated controls, with the exception that the NO levels in the globus pallidus were decreased (p<0.05) by ~25% in BDL and BDL plus manganese rats. Constitutive nitric oxide synthetase (NOS) activities in the globus pallidus were decreased (but not to a statistically significant degree) in BDL and BDL plus manganese-treated rats.
In a similar study, Rivera-Mancía et al. (2009) investigated alterations in brain astocytes in manganese-exposed male Wistar rats with and without liver damage. Similar experimental groups were used (number of rats/group not specified: (1) sham operated; (2) BDL; (3) sham operated with 1 mg manganese/mL (as manganese chloride) in drinking water; or (4) BDL with 1 mg manganese/mL (as manganese chloride) in drinking water. Rats received this treatment for 2 or 4 weeks beginning at time of surgery. Using an allometric equation for drinking water consumption (EPA 1988) and average reported body weight, the estimated dose in manganese-exposed groups is 271 mg/kg/day. Brain levels of manganese were measured in the cortex, striatum, and globus pallidus. Altered and normal astrocytes were counted in the same regions. Manganese or BDL treatment, alone or in concert, led to significantly (p<0.05) elevated manganese levels in all three brain regions tested. The number of damaged astrocytes was significantly (p<0.05) increased in animals from both BDL groups compared to the sham-operated control. However, manganese exposure in either sham operated or BDL animals did not increase the extent of astrocyte damage. This indicates that short-term manganese exposure alone, either with or without liver damage, did not induce gliosis in the cortex, striatum, or globus pallidus in rats.
Neurological Effects in Iron-Deficient Animals—Oral Exposure to Inorganic Manganese. Studies reporting competition between iron and manganese in absorption indicate the impact an iron-poor diet will have on manganese uptake in the human (Chandra and Tandon 1973; Davis et al. 1992a, 1992b; Diez-Ewald et al. 1968; Mena et al. 1969; Rehnberg et al. 1982; Thomson et al. 1971). Further, competition between manganese and iron at the blood-brain barrier has been reported (Aschner and Aschner 1990), indicating that excesses of either metal will affect the brain distribution of the other.
A recent study in rats has been conducted to determine the mechanism by which iron is regulated at the blood-brain barrier and the blood-cerebrospinal fluid (B-CSF) barrier and how manganese may alter these processes (Li et al. 2006). Additionally, two studies by Anderson et al. (2007a, 2009) have explored the interplay between iron deficiency and manganese supplementation and its ultimate potential for modulating neurotransmission in the neonatal rat brain. These studies, together with gestational exposure studies evaluating altered iron metabolism (Garcia et al. 2006, 2007; Jarvinen and Ahlström 1975; Molina et al. 2011; see Section 3.2.2.6), suggest that manganese-mediated alterations in iron pharmacokinetics may, at least in part, underlie some of the observed adverse neurological effects associated with elevated manganese exposure.
Li et al. (2006) dosed groups of 7–8-week-old male Sprague-Dawley rats were with sterile saline (control) or manganese chloride dissolved in sterile saline at 2.2 or 6.6 mg manganese/kg/day; rats were dosed daily via gavage for 5 consecutive days/week (weekdays only) for 30 days. Serum iron concentrations were found to be significantly decreased (p<0.05) at 2.2 and 6.6 mg manganese/kg/day (50 and 66% of control value, respectively. In contrast, iron concentrations in the cerebrospinal fluid (CSF) were significantly (p<0.05) increased at 2.2 and 6.6 mg manganese/kg/day (136 and 167% of control values). Manganese produced a dose-dependent increase of binding of IRP1 to iron-responsive element-containing RNA in (percentage increase of high-dose group over control indicated in parentheses): the choroid plexus (+70%); in capillaries of striatum (+39%), hippocampus (+56%), and frontal cortex (+49%); and in brain parenchyma of striatum (+67%), hippocampus (+39%), and cerebellum (+28%). Manganese exposure significantly increased the expression of TfR mRNA in choroid plexus and striatum with a reduction in the expression of Ft mRNA. The results indicate that intermediate-duration oral exposure to excess manganese decreased serum iron concentrations and increased iron concentrations in the CSF. These changes were associated with: (1) increased binding of iron regulatory proteins and mRNA containing iron responsive element in several brain regions and (2) upregulation of transferritin receptor mRNA and down-regulation of ferritin mRNA in choroid plexus and striatum.
In a study by Anderson et al. (2007a), male and female PND 1 Sprague-Dawley rats were divided into groups receiving either a control diet (35 mg iron/kg, 10 mg manganese/kg diet and drinking water) or a diet with manganese supplementation (same as control diet with 1 g/L of manganese chloride added to drinking water for a final dose of 71.1 mg manganese/kg/day). Rats were sacrificed after 6 weeks of treatment. Additional females and males (n=6 per group) were provided with an iron-deficient diet (4 mg/kg iron, 10 mg manganese/kg diet and drinking water) and an iron deficient/manganese supplemented diet (same iron-deficient diet plus 1 g manganese chloride/L water). Manganese exposure significantly (p<0.05) reduced iron concentrations in the caudate putamen and the substantia nigra from male and female rats. In female rats, manganese exposure also significantly reduced iron levels in the caudate putamen. The largest decrease was seen in the female caudate putamen, where iron levels dropped by approximately 66% compared to controls and the female substantia nigra, where iron levels dropped by approximately 75% compared to controls. Manganese concentrations in the brain were seen to increase over controls most prominently in the female globus pallidus (approximately 60%). A significant negative correlation (p<0.05) was observed between synaptosomal manganese concentration and 3H-GABA uptake in rats of both sexes. 3H-GABA levels were significantly reduced from controls in both males and females (by approximately 50%). In rats provided with an iron-deficient diet, few differences were observed between the iron-deficiency condition and the iron-deficiency plus manganese condition. In males, iron levels were approximately 10 times higher in the caudate putamen of iron-deficient animals than in the animals that were iron-deficient and manganese-supplemented.
In a more recent study by Anderson et al. (2009), a similar paradigm was used to evaluate the effect of manganese exposure and iron deficiency on the noradrenergic system. Groups of male weanling Sprague-Dawley rats were divided into the four groups described above (24/group), with the exception that a final dose of 68.3 mg manganese/kg/day was attained in manganese-exposed animals (the previous study used an average dose between males and females). Rats were sacrificed after 6 weeks of treatment. Again, manganese exposure significantly (p<0.01) reduced extracellular iron concentrations and increased extracellular manganese concentrations in the caudate putamen. A significant negative correlation (p<0.01) was observed between synaptosomal manganese concentration and 3H-NE uptake in the locus coeruleus. In manganese-treated animals, extracellular NE concentrations were significantly (p<0.01) decreased, and, in general, NE transporter and receptor proteins and mRNA levels were decreased across the brain. Again, in rats provided with an iron-deficient diet, few differences were observed between the iron-deficiency condition and the iron-deficiency plus manganese condition.
Neurological Effects—Oral Exposure to MMT. No studies regarding neurological effects following oral exposure to MMT by humans were identified.
Komura and Sakamoto (1992b) administered 11 mg manganese/kg/day (as MMT) to ddY mice in food for 12 months. To measure differences in behavior between exposed and control mice that were fed normal chow, spontaneous motor activity was measured at regular intervals during exposure to determine differences in behavior between exposed and control mice fed normal chow. The authors observed a significant increase in spontaneous activity at day 80; no other significant differences were noted. In a separate study (Komura and Sakamoto 1994), the authors analyzed brain levels of different neurotransmitters and metabolites after identical MMT treatment. MMT resulted in a 66% decrease in dopamine (DA; p<0.05) and a 95% decrease in normetanephrine (NMN; p<0.01) in the hypothalamus; in the hippocampus, DA was unchanged, while the level of DOPAC was reduced 41% (p<0.05), and the 3-methoxytyramine (3MT) level increased 3.5-fold (p<0.01). In the midbrain, the only significant changes noted were an almost 6-fold increase in 3MT (p<0.01) and a 1.75-fold increase of HVA, a metabolite of DOPAC via conjugation by catechol-o-methyl transferase (p<0.05). In the cerebral cortex, HVA was decreased by 61%, norepinephrine (NE) by 64%, and epinephrine by 43% (all were p<0.05) due to MMT administration. In the cerebellum, DOPAC was decreased 51% (p<0.05), while NMN was increased 7.7-fold (p<0.01). Finally, in the medulla oblongata, DOPAC was decreased by 45% (p<0.05), HVA was decreased by 55% (p<0.01), and serotonin (5HT) was decreased 81% (p<0.01); metanephrine was increased approximately 2.75-fold in the medulla (p<0.05).
Through analysis of the distribution of manganese in the different brain regions of the mice, the authors observed relationships between manganese content and neurotransmitter levels. For example, a weak relationship was found between the manganese level in the corpus striatum and the level of NE. There was no relationship between the increase in HVA and the manganese levels in this same region. The relationship between the increase in 3MT and manganese levels in the midbrain was weak, as was the relationship between DOPAC and manganese levels in the cerebellum. There were no relationships between amines and manganese levels in the hippocampus, cerebral cortex, or medulla oblongata, although some changes were found. A significant correlation was found between the level of NMN and manganese in the cerebellum. As discussed more fully in Section 3.4.2, the cerebellum contained the most manganese of any brain region following MMT administration (Komura and Sakamoto 1994).
3.2.2.5. Reproductive Effects
There are no available studies evaluating reproductive effects in humans following oral manganese exposure.
In a 14-day study in rats, no changes in testicular weight were reported at 1,300 mg manganese/kg/day (NTP 1993). However, several intermediate-duration studies in rats and mice indicate that manganese ingestion can lead to delayed maturation of the reproductive system in males. One study investigated the effect of 1,050 mg manganese (as manganese tetroxide)/kg/day, provided to weanling mice and their dams starting when the pups were 15 days old (Gray and Laskey 1980). On day 30, the mice were weaned and maintained on the high-manganese diet until killed for analysis at 58, 73, or 90 days old. The growth and general appearance of the weanling rats appeared normal. At time of death, preputial gland, seminal vesicle, testes, and body weights were measured. The high-manganese diet resulted in a significant decrease in growth of these reproductive tissues but no growth retardation of the body and no change in liver or kidney weights.
A later study by Laskey et al. (1982) evaluated the reproductive functioning of male and female Long-Evans rats that had been exposed to 0, 350, 1,050, and 3,500 mg manganese/kg/day (in conjunction with a low-iron diet [20 mg iron/kg/day] or a diet adequate in iron [200 mg iron/kg/day]) while in utero (dams were fed the described diets during gestation) and from day 14 to 15 postpartum. The rats were maintained on the diet throughout the remainder of the study (224 days). The rats were mated at 100 days postpartum and the reproductive success of these matings was evaluated.
In males, manganese treatment resulted in decreased testes weights (testes weights analyzed with body weight as a covariable) observed at 40 days (at the 1,050 and 3,500 mg manganese/kg/day dose levels) and 100 days (at the 1,050 mg manganese/kg/day dose level) of age, only when administered with the low-iron diet. Hormone levels in male rats were also evaluated. No treatment-related effect was seen in 40-day-old males. At 60 and 100 days of age, however, dose-related decreases in serum testosterone were observed, while serum LH (luteinizing hormone) levels remained relatively unchanged. Luteinizing hormone (LH) is secreted by the pituitary to stimulate testosterone production in the Leydig cells. Testosterone levels control LH production through a negative feedback loop. An increase in testosterone would normally be associated with a subsequent decrease in LH. The decrease in testosterone simultaneous with a stable LH levels suggests that manganese is targeting the Leydig cells. Manganese treatment in both iron regimens prevented the normal decrease in serum follicle-stimulating hormone (FSH) from 60 to 100 days. In addition, manganese only negatively affected epididymal sperm counts at 100 days in the iron-deficient group. When serum concentrations of LH, FSH, and testosterone and epididymal sperm counts from the 60- and 100-day-old rats were used to predict the reproductive age of the males, the 60-day-old animals were predicted correctly. Of the 100-day-old animals, 2/12 controls, 7/12 at 350 mg manganese/kg, and 12/12 at 1,050 mg manganese/kg were classified as 60 days old. These data indicate that manganese induced a significant maturational delay in the reproductive organs of the male rat (Laskey et al. 1982).
To further assess the mechanism of toxicity of manganese in the pre-weanling rat, Laskey et al. (1985) dosed rats from birth to 21 days of age with particulate manganese tetroxide in 50% sucrose solution by gavage at doses of 0, 71, or 214 mg manganese/kg/day. They then assessed the hypothalamic, pituitary, and testicular functions in the rat by measuring the endogenous or stimulated serum concentrations of FSH, LH, and testosterone at 21 or 28 days of age. LH-releasing hormone (LH-RH) was used to stimulate the pituitary-testicular axis to secrete FSH, LH, and subsequently testosterone; human chorionic gonadotropin (hCG) was used to stimulate acutely (2-hour time period) the testicular secretion of testosterone and repeatedly (7-day time period) to assess the ability of the Leydig cells to maintain maximal testosterone synthesis and secretion. Some rats from both control and manganese-dosed groups were castrated to determine the effect this would have on the study end points. Manganese treatment had only a slight effect on body and testes weights, while no effects were observed on unstimulated or stimulated FSH or LH serum levels. In addition, manganese did not affect endogenous or acute hCG-stimulated serum testosterone concentrations, but did decrease serum testosterone level following repeated hCG stimulation. Liver manganese at the 71 mg/kg/day manganese dose was significantly elevated over controls in both castrated (8.42±7.23 mg/kg for treated vs. 1.96±0.22 mg/kg for controls) and noncastrated (3.36±0.91 mg/kg for treated vs. 1.81±0.11 mg/kg for controls) rats. In addition, hypothalamic manganese concentrations were significantly increased at the 71 mg/kg/day dose in both castrated (6.10±3.0 mg/kg in treated vs. 0.59±0.11 mg/kg in controls) and noncastrated (3.73±1.18 mg/kg in treated vs. 0.65±0.057 mg/kg in controls) rats. The authors speculate that since their earlier results had shown changes in male reproductive development in postpubertal animals with minimal manganese concentrations in tissues (Gray and Laskey 1980; Laskey et al. 1982), it seemed likely that the changes in this later study (Laskey et al. 1985) would result from high manganese concentrations in the hypothalamus, pituitary, or testes, with the tissue with the highest manganese concentration being the site of the toxic reproductive effect. However, the results from this latest study reveal that manganese had no effect on the hypothalamus or pituitary to produce LH or FSH in pre-weanling rats, despite the increased manganese concentrations. Rather, the data indicate that it is delayed production of testosterone, shown by the inability of the Leydig cells to maintain maximum serum concentrations of the hormone, which results in the delayed sexual maturation. This delay in testosterone was not significant enough, however, to impair rodent fertility at manganese doses as high as 1,050 mg/kg/day (Laskey et al. 1982).
A slight decrease in pregnancy rate was observed in rats exposed to 3,500 mg manganese/kg/day (as manganese tetroxide) in the diet for 90–100 days prior to breeding (Laskey et al. 1982). Since both sexes were exposed, it is not possible to conclude whether the effect was in males, females, or both. However, this exposure regimen did not have significant effects on female reproductive parameters such as ovary weight, litter size, ovulations, or resorptions (Laskey et al. 1982).
Manganese was found to affect sperm formation and male reproductive performance in other intermediate-duration oral studies (Elbetieha et al. 2001; Joardar and Sharma 1990; Ponnapakkam et al. 2003a, 2003c). Joardar and Sharma (1990) administered manganese to mice, as potassium permanganate or manganese sulfate, at 23–198 mg/kg/day by gavage for 21 days. The treatment resulted in sperm head abnormalities, and the percentage of abnormal sperm was significantly elevated in all exposed mice as compared to controls. Increased incidences of testicular degeneration occurred in male Sprague-Dawley rats exposed for 63 days to doses ≥137.2 mg manganese/kg/day as manganese acetate, but not at 68.6 mg/kg/day (Ponnapakkam et al. 2003c). Impaired male fertility was observed in male mice exposed to manganese chloride in drinking water for 12 weeks before mating with unexposed females at a daily dose level of 309 mg manganese/kg/day, but not at doses ≤154 mg manganese/kg/day (Elbetieha et al. 2001). In the 309-mg/kg/day group, 17 pregnancies occurred in 28 mated females, compared with 26 pregnancies out of 28 females mated with controls. At lower dose levels in another study, decreased sperm motility and sperm counts were observed in male CD-1 mice after 43 days of exposure to manganese acetate at doses of 4.6 or 9.6 mg manganese/kg/day, but these doses did not impair the ability of these males to impregnate unexposed females (Ponnapakkam et al. 2003a).
In another intermediate feeding study, Jarvinen and Ahlström (1975) administered varying doses of manganese sulfate, from nutritionally deficient levels to excess amounts, to Sprague-Dawley female rats for 8 weeks prior to mating. The rats were continued on manganese diet (0.75, 4.5, 10, 29, 94, or 187 mg manganese/kg/day) until GD 21. The authors found no effect of manganese on maternal weight gain, implantation number, resorptions, or percentage of dead fetuses. The authors did observe that manganese doses of 94 mg manganese/kg/day and higher resulted in significant increases in liver manganese concentrations, whereas nonpregnant females had liver manganese concentrations that were unchanged, irrespective of dose. These data suggest that pregnancy allows the female to develop significant liver manganese stores, and it is possible these stores may be mobilized during gestation or at a future time. The authors also noted that pregnant rats had consistent liver iron concentrations, whereas nonpregnant rats developed a dose-dependent decrease in liver iron concentrations. Further, the highest dose in dams caused a significant increase in fetal manganese content.
Szakmáry et al. (1995) studied the reproductive effects of manganese chloride, administered by gavage to pregnant rabbits and rats at concentrations of 0, 11, 22, and 33 mg manganese/kg/day on GDs 6–20 in the rabbit and throughout gestation in the rat. Manganese did not result in any reproductive effect in the rabbit, but the highest manganese dose did cause an increase in postimplantation loss in the rat. In 13-week dietary studies, no gross or histopathological lesions or organ weight changes were observed in reproductive organs of rats fed up to 618 mg manganese/kg/day or mice fed 1,950 mg manganese/kg/day, but the reproductive function was not evaluated (NTP 1993).
More recent oral studies indicate that ingested manganese does not result in female reproductive toxicity when rat dams were exposed during pregnancy, but impaired female fertility was observed when female mice were exposed to manganese in drinking water for 12 weeks before mating with unexposed males. The first study involved a dose of 22 mg manganese/kg/day administered as manganese chloride by gavage to female rats on days 6–17 of gestation (Grant et al. 1997a). No treatment-related mortality, clinical signs, changes in food or water intake, or body weights were observed in the dams. In the second study (Pappas et al. 1997), manganese chloride was provided to pregnant rats in drinking water at doses up to 620 mg manganese/kg/day throughout gestation. The manganese did not adversely affect the health of the dams, litter size, or sex ratios of the pups. More extensive analyses of female reproductive organs were not performed. Similarly, Kontur and Fechter (1985) found no significant effect on litter size in female rats exposed to manganese chloride in drinking water except at concentrations so high (1,240 mg manganese/kg/day) that water intake by the dams was severely reduced. In contrast, Elbetieha et al. (2001) reported that decreased numbers of implantations and viable fetuses were observed in female Swiss mice exposed to manganese chloride in drinking water at a dose level of 277 mg manganese/kg/day for 12 weeks before mating with unexposed males.
In a 2-year NTP study, no adverse reproductive effects (lesions in reproductive organs) from manganese sulfate exposure were reported for rats at up to 232 mg manganese/kg/day or mice at up to 731 mg manganese/kg/day (NTP 1993).
The highest NOAEL values and all LOAEL values from each reliable study for reproductive effects in each species and duration category are recorded in Table 3-3 and plotted in Figure 3-3.
No studies were located regarding reproductive effects in humans or animals following oral exposure to MMT.
3.2.2.6. Developmental Effects
Developmental Effects in Humans—Oral Exposure to Inorganic Manganese. Very little information is available on the developmental effects of manganese in humans. The incidences of neurological disorders and the incidences of birth defects and stillbirths were elevated in a small population of people living on an island where there were rich manganese deposits (Kilburn 1987); however, the lack of exposure data, the small sample sizes, and the absence of a suitable control group preclude ascribing these effects to manganese. The route of exposure was assumed to be primarily oral, but inhalation exposure was not ruled out.
Potential developmental effects of manganese were suggested by the results of a study by Hafeman et al. (2007), where high infant mortality in a Bangladesh community was reported in conjunction with the presence of a local drinking water supply containing high levels of manganese. The Health Effects of Arsenic Longitudinal Study (HEALS) was conducted on 11,749 participants 18–70 years of age living in Araihazar, Bangladesh. Data on the reproductive history of the 6,707 women in this population were collected and samples were taken of drinking water from all of the wells in the study region. Manganese concentrations were determined for a total of 1,299 wells, representing the drinking water supply of 3,824 infants <1 year old. Eight-four percent of infants were exposed, directly or through maternal intake, to water manganese levels above 0.4 mg/L with manganese concentrations ranging from 0 to 8.61 mg/L, for an average calculated daily intake of 0.26 mg manganese/kg/day. Of the 3,837 children born to women who reported to drink from the same well for most of their childbearing years, 335 of them died before reaching 1 year of age. Infants exposed to greater than or equal to the 0.4 mg/L WHO (2004b) standard for manganese in drinking water had an elevated mortality risk during the first year of life compared to unexposed infants (OR=1.8; 95% CI, 1.2–2.6). Adjustment for water arsenic indicators of social class and other variables and potential confounders did not appreciably alter the results. When the population was restricted to infants born to recently married parents (marriage year 1991 or after), the elevation was larger (OR=3.4; 95% CI, 1.5–7.9). Although the results of the study suggest that the presence of high levels of manganese in the water may be responsible for the high infant mortality observed here, information provided by the authors on mechanism of manganese exposure suggests that infant exposure to the high levels of manganese in the water may be complex (i.e., would likely require direct rather than indirect or fractionated exposure, such as that occurring through breast milk or by in utero exposure). The authors also indicate that it is not possible to infer that the manganese is solely responsible for the high rate of infant mortality documented in this study
Similarly, results from a pilot ecologic study in North Carolina suggest an association between increased risk for infant mortality and increased groundwater manganese concentration (Spangler and Spangler 2009). County-level infant mortality rates and the percent of low birth weight births were obtained from the North Carolina Center for State Health Statistics database, combined for the years 1997–2001. Groundwater concentration values ranging from 0.003 to 0.346 mg/L (mean, 0.078 mg/L) were obtained from the North Carolina Geological Survey groundwater database, which contains 5,778 samples from all 100 counties in North Carolina from 1973 and 1979. Analysis revealed that county-level infant mortality rates were significantly (p<0.05) associated with logarithmically transformed groundwater manganese concentration. With every log increase in concentration, there was a 2.074 increase in county level infant deaths per 1,000 live births. Percent of babies born with low birth weight was not associated with manganese groundwater concentration. However, as in Hafeman et al. (2007), potential sources of manganese exposure (dietary, inhalation) and potential confounders were not examined. Taken together, these two studies provide inadequate evidence to establish a causal relationship between elevated manganese exposure in drinking water and increased infant mortality rates.
As discussed in Section 3.2.2.4, several studies have evaluated adverse neurological results in children with increased oral exposure to manganese and/or elevated hair or blood concentration of manganese (where the route of exposure is presumed to be mainly oral). Several studies have reported an inverse relationship between manganese exposure in school-aged children and intellectual function (Bouchard et al. 2011; Kim et al. 2009; Wasserman et al. 2006, 2011). Elevated manganese exposure has also been associated with poor performance on the WHO neurobehavioral core tests (the emotional status test was omitted) (He et al. 1994; Zhang et al. 1995), increased oppositional behavior and hyperactivity (Bouchard et al. 2007c), and ADHD (Farias 2010). Additionally, elevated manganese levels in 12-month-old infants were associated with decreased mental development scores (Claus Henn et al. 2010). Although observed effects in these studies cannot be causally linked to manganese exposure exclusively, taken together, they support the hypothesis that oral exposure to elevated manganese may be detrimental to neurodevelopment.
Standard Developmental Studies in Animals—Oral Exposure to Inorganic Manganese. In animals, standard developmental toxicity studies have not found distinct effects on fetal survival, gross fetal malformations, or skeletal or visceral malformations or alterations. For example, acute administration of manganese chloride by gavage to pregnant rats at a dose of 22 mg manganese/kg/day on GDs 6– 17 resulted in no adverse fetal developmental effects, measured as weight gain, gross malformations, or skeletal malformations (Grant et al. 1997a). In another study, Szakmáry et al. (1995) studied the developmental toxicity of manganese in the rabbit and rat. The metal, as manganese chloride, was administered by gavage during the whole period of gestation in the rat, and during organogenesis (day 6– 20) in the rabbit at concentrations of 0, 11, 22, and 33 mg/kg/day. In the rabbit, manganese treatments did not result in decreases in fetal weights, skeletal retardation, or extra ribs, or in an increase in fetuses afflicted with major anomalies. In the rat, the highest dose resulted in retardation of development of the skeleton and internal organs. In addition, manganese at the highest dose caused a significant increase in external malformations, such as clubfoot. However, when pups from dams treated at the same dose were allowed to grow for 100 days after birth, no external malformations were observed, indicating that these effects were self-corrected. No significant differences were found in any of the groups concerning the development of the ears, teeth, eyes, forward motion, clinging ability, body posture correction reflex, or negative geotaxis reflex.
Reproductive Development Studies in Animals—Oral Exposure to Inorganic Manganese. Several animal studies of the effects of manganese on reproductive development show developmental effects (Gray and Laskey 1980; Laskey et al. 1985, 1982).
One study involved pre-weanling mice (Gray and Laskey 1980) that were fed 1,050 mg manganese/ kg/day (as manganese tetroxide) beginning on PND 15. On days 58, 73, and 90, mice were sacrificed and reproductive organ (preputial gland, seminal vesicle, and testes) weights and body weights were measured. The manganese decreased the growth of these reproductive organs, but had no effect on body growth or liver or kidney weights.
In another study, Laskey et al. (1982) evaluated the effect of dietary manganese exposure on rats during gestation and continued during nursing and after weaning at doses of 0, 350, 1,050 or 3,500 mg manganese/kg/day. The manganese was given in combination with either 20 or 200 mg iron/kg/day (the former is deficient in iron, the latter is adequate). Manganese treatment was lethal at the highest dose in the iron-deficient diet, but had no effect on male or female body weight at any age in animals receiving an iron-sufficient diet. In the iron-poor diet, body weights of males were significantly depressed (p<0.05) through day 100 of the study, whereas the females’ body weights were depressed only through day 60. Select females and males were mated at day 90–100 of the study and the reproductive outcomes were analyzed. The manganese treatment did not have any significant adverse effects at any dose except to significantly decrease the number of pregnancies at the highest dose (p<0.05). Litter size, ovulations, resorptions, preimplantation deaths, and fetal weights were unaffected by the metal. Testes weights in males were significantly decreased from controls only when administered manganese in conjunction with an iron-poor diet: at day 40 at 1,050 and 3,500 mg manganese/kg/day and at day 100 at 1,050 mg/kg/day. Hormone levels in male rats were also evaluated. No effect was seen from manganese treatment in 40-day-old male rats. At 60–100 days of age, however, dose-related decreases in serum testosterone were observed, when age-related increases were expected and no increase in serum LH was observed. Manganese given in both iron regimens prevented the normal decrease in serum follicle-stimulating hormone (FSH) from 60 to 100 days. Manganese decreased epididymal sperm count only when given with the iron-poor diet as measured at 100 days.
A third study involved gavage administration of 0, 71, or 214 mg manganese/kg/day (as manganese tetroxide) to pre-weanling rats from birth to 21 days of age (Laskey et al. 1985). Functioning of the hypothalamus, pituitary, and testicular tissues were measured by assaying endogenous or stimulated serum concentrations of FSH, LH, and testosterone at days 21 or 28. No manganese-related effects were observed on unstimulated or stimulated FSH or LH serum levels. In addition, manganese did not affect endogenous or acute hCG-stimulated serum testosterone concentrations but did decrease serum testosterone level following chronic hCG stimulation. Liver and hypothalamic manganese concentrations were significantly increased in treated rats given the 71 mg/kg/day dose over controls. The authors hypothesized that the manganese had an unknown affect on the testicular Leydig cell that resulted in the delayed production of testosterone. This delayed production was presumably causing the delayed reproductive maturation seen in the earlier study (Gray and Laskey 1980), but was not enough to affect fertility outcomes at doses as high as 1,050 mg/kg/day (Laskey et al. 1982).
Neurodevelopmental Studies in Animals—Oral Exposure to Inorganic Manganese. As discussed in Section 3.2.2.4, numerous studies have reported altered neurochemistry and/or neurobehavior following neonatal or juvenile manganese exposure (Anderson et al. 2007a, 2009; Chandra and Shukla 1978; Deskin et al. 1981; Dorman et al. 2000; Golub et al. 2005; Kern and Smith 2011; Kern et al. 2010; Kristensson et al. 1986; Moreno et al. 2009; Reichel et al. 2006; Tran et al. 2002a, 2002b). Similarly, many animal studies have examined neurological end points in animals repeatedly exposed during gestation or through gestation into early postnatal development. End points evaluated include neurochemistry (Lai et al. 1984), neurobehavior (Ali et al. 1983a; Pappas et al. 1997), and neuropathology (Lazrishvilli et al. 2009; Pappas et al. 1997). Additionally, two studies have evaluated the potential relationship between altered iron metabolism following manganese exposure and changes in neurochemistry (Garcia et al. 2006) and neurobehavior (Molina et al. 2011). While results from these studies are varied and inconsistent, taken together, the weight-of-evidence suggests that excess manganese exposure during development can lead to alterations in brain chemistry and behavioral development.
Lai et al. (1984) studied the effect of chronic dosing of 40 mg manganese/kg/day (as manganese tetroxide given in drinking water) to neonatal rats that were exposed from conception, throughout gestation, and up to 2 years of age. The authors found that manganese treatment led to small decreases in choline acetyltransferase activities in cerebellum and midbrain of 2-month-old rats. The regional distribution of glutamic acid decarboxylase or acetylcholinesterase was unchanged.
Ali et al. (1983a) conducted a gestational study investigating the neurological effects of excess manganese in drinking water on rats maintained on either a normal or low-protein diet. Manganese exposure originated 90 days prior to mating and continued throughout gestation and nursing. The offspring of rats who drank the equivalent of 240 mg manganese as manganese chloride/kg/day had pups with delayed air righting reflexes. No treatment-related effects were observed in body weight or brain weight in pups from dams fed the normal amount of protein. Significant delays in age of eye opening and development of auditory startle were observed only in the pups of dams fed protein-deficient diets.
An intermediate drinking water study in pregnant rats (Pappas et al. 1997) investigated the developmental neurotoxicity of manganese chloride doses of either 120 or 620 mg manganese/kg/day given on GDs 1–
21. Following birth, the dams were continued on manganese until weaning at PND 22. When the dams were removed, the pups were continued on the same manganese doses until PND 30. Male pups were observed on several days subsequent to exposure in a number of behavioral tests that measured spontaneous motor activity, memory, and cognitive ability. The manganese-treated rats’ performance was not significantly different from control rats. Pups from the highest-dose group exhibited a significantly decreased weight gain on several days post-dosing, as well as an increased activity level on PND 17 that was no longer evident by PND 30. The high-dose rats were not overactive on other days, and the decreased weight gain was resolved by PND 90. Neurochemical analyses of the brains from treated pups indicated that brain manganese concentrations were significantly elevated in the high-dose group, as compared to controls. Brain enzyme and dopamine concentrations were not significantly different between groups, but cortical manganese concentrations were significantly elevated in the high-dose group. Cortical thickness was significantly different in several areas of the brains of pups in the high-dose group but was only found to be significantly different in one area of the low-dose group. The significance of the cortical thinning is not clear.
Lazrishvilli et al. (2009) reports gliosis following developmental exposure to manganese in three groups of rat pups (12/group) from mothers receiving 0, 10, or 20 mg manganese chloride/kg/day (0, 4.4, or
8.7 mg manganese/kg/day) in feed for 15–20 days before pregnancy, during pregnancy, and for 1 month after parturition. Pups were sacrificed on postnatal day 40, and brains were removed and fixed for histological and morphological assessment and estimations of manganese content. Manganese was significantly elevated in cerebral cortex in both treatment groups, but not other brain regions (striatum, diencephalon, mesencephalon, or medulla oblongata). There was no difference in the number of neurons, and only a small proportion (7–10%) of neurons showed significant damage (pyknotic and swollen cells). However, there was a significant (p<0.05), dose-related increase in the number of glial cells and the glial index throughout the brain (gliosis). This is in contrast to negative findings for gliosis in adult rats following exposure to 147 mg manganese/kg/day for 2 or 4 weeks (Rivera-Mancía et al. 2009, see Section 3.2.2.4).
Developmental Effects on Iron Metabolism in Animals—Oral Exposure to Inorganic Manganese. In a longer-duration intermediate study, Jarvinen and Ahlström (1975) fed female rats up to 187 mg manganese/kg/day (as manganese sulfate) for 8 weeks prior to conception. The rats were continued on manganese treatment until the 21st day of gestation. The unborn pups from dams administered 94 mg manganese/kg/day had significantly decreased weights as compared to the other groups. No gross malformations were observed in the fetuses, and alizarin-stained bone preparations revealed no abnormalities in any dose group. However, fetuses from dams fed the highest manganese dose had significantly higher concentrations of manganese in their bodies than fetuses from the other groups. These data indicate that a level of 187 mg manganese/kg/day overwhelmed the rat’s homeostatic control of manganese and the metal accumulated in the fetus. The highest manganese dose also resulted in a significant decrease in the iron content of the fetuses.
Garcia et al. (2006, 2007) studied the relationship between dietary manganese and dietary iron on brain chemistry and neurotransmission. In one study, groups of 5–7 dams were fed diets containing 35 ppm iron (control) or 8 mg manganese/kg/day and 35 ppm iron (manganese-supplemented) from GD 7 through PND 7 (Garcia et al. 2006). On PND 4, pups born to control dams were pooled and randomly cross-fostered to dams fed one of the two diets such that initial mean litter weights were equivalent. Pups were exposed to each of these diets via maternal milk from PND 4 to 21 as well as via direct ingestion of chow (beginning around PND 11) and were euthanized on PND 21. In the dams, the high manganese diet induced changes in hematological parameters similar to those seen with iron-deficiency: 50% decrease in plasma iron (without significant decreases in hemoglobin) and increased plasma transferrin and total iron binding capacity. Compared with controls, manganese-exposed pups showed decreased hemoglobin (about 20%), decreased plasma levels of iron (about 70%), increased plasma tranferrin and total iron binding capacity (about 10%), increased brain concentrations of manganese, chromium, and zinc, decreased brain iron levels, increased protein expression of divalent metal transporter-1 (DMT-1) and transferrin receptor (TfR) in all brain regions, increased GABA concentrations, and increased ratios of GABA to glutamate concentrations. Because GABA is an inhibitory amino acid and glutamate is an excitatory amino acid, the authors suggested that the manganese treatment induced enhanced inhibitory transmission in the brain of the pups. The results indicate that manganese treatment altered transport and distribution of iron in developing rat pups and induced perturbations in brain levels of the neurotransmitter, GABA.
In a further study by Garcia et al. (2007), groups of 5–7 GD 7 timed-pregnant Sprague-Dawley rats were fed one of three experimental diets: control (35 mg Fe/kg diet; 10 mg manganese/kg diet), low iron (3 mg Fe/kg diet; 10 mg manganese/kg diet), or low iron with supplemented manganese (3 mg Fe/kg diet, 100 mg manganese/kg diet). On PND 4, pups born to the control dams were pooled and randomly cross-fostered to dams fed one of the two iron-deficient diets, such that initial mean litter weights were approximately equivalent. The pups received these diets via maternal milk from PND 4 to 21, at which time the pups were sacrificed. Levels of essential metals in the brain were measured (in cerebellum, cortex, hippocampus, striatum, and midbrain) by inductively coupled plasma-mass spectrometry. Increases in brain levels in low iron/manganese-treated rats (compared to control and low iron) were seen for the following metals: copper, manganese (~50%), chromium (~150%), cobalt (~150%), molybdenum (~25%), zinc (~130%), aluminum (~130), and vanadium (~150%). A decrease in brain iron levels was observed for low iron animals; low iron/manganese-treated rats had iron levels significantly higher than the low iron animals.
Molina et al. (2011) investigated the effect of manganese exposure during development on iron metabolism. Pregnant Sprague-Dawley rats (3/group) were exposed to 0 or 4.79 mg manganese/mL (as manganese chloride in drinking water) from GD 1 through PND 24. Based on body weight and water intake, study authors calculated daily manganese doses in the exposed group to be 565 and 1,256 mg/kg/day during gestation and lactation, respectively. Offspring were culled to 12 per dam at PND 2, with approximate equal male to female ratio. Iron pharmacokinetics were measured in all pups on PND 25, after which they were sacrificed to evaluate the intestinal expression of divalent metal transporter 1 (DMT1), blood and brain levels of manganese, liver and brain levels of non-heme iron, and blood zinc protoporphyrin levels. Overall tissue uptake of iron was lower and zinc protoporphyrin levels were significantly decreased in manganese-exposed pups, compared with controls. Intestinal absorption of iron was not altered, nor was expression of duodenal DMT1 by manganese exposure. Hematocrit and non-heme iron levels were not altered in exposed pups. Additionally, before sacrifice, 4 pups/sex/dam were tested on the elevated plus apparatus on PND 24. Pups demonstrated significantly (p<0.05) lower anxiety-related behavior on several measures from this paradigm. These finding indicate that developmental exposure to manganese leads to alterations in anxiety-like behaviors, which may be mediated through manganese-induced changes in iron metabolism.
Developmental Studies in Animals—Oral Exposure to MMT. No studies of developmental effects following oral exposure to MMT in humans or animals were located.
3.2.2.7. Cancer
No studies were located regarding carcinogenic effects in humans after oral exposure to inorganic manganese.
Chronic (2-year) feeding studies in rats and mice have yielded equivocal evidence for the carcinogenic potential of manganese. For example, rats exposed to up to 232 mg manganese/kg/day as manganese sulfate for 2 years showed no increases in tumor incidence (NTP 1993). Mice fed up to 731 mg manganese/kg/day as manganese sulfate for 2 years had a marginally increased incidence of thyroid gland follicular cell adenomas (high-dose animals) and a significantly increased incidence of follicular cell hyperplasia (NTP 1993); this was considered by NTP to be "equivocal evidence of carcinogenic activity of Mn(II) sulfate monohydrate in male and female B6C3F1 mice" (there was "no evidence of carcinogenic activity" in rats in this study).
No studies were located regarding carcinogenic effects in humans or animals following oral exposure to MMT.
3.2.3. Dermal Exposure
For inorganic manganese compounds, dermal exposure is not a typical pathway of exposure because manganese does not penetrate the skin readily. For organic manganese, dermal exposure is a possibility with all compounds discussed in this profile. This exposure pathway is most likely, however, with MMT, where occupational workers (mechanics, workers in the gasoline industry, pesticide manufacturers and sprayers) are likely to handle large quantities of these compounds.
No studies were located regarding the any health effects in humans or animals after dermal exposure to inorganic manganese.
3.2.3.1. Death
No studies were located regarding death in humans from dermal exposure to MMT.
Hinderer (1979) reported LD50 values for rabbits (strain and sex were unreported) that were administered varying doses of “neat” commercial MMT on abraded skin in the trunk area for 24 hours. These values, generated by four different laboratories, ranged from 140 to 795 mg/kg. Although this dose range is wide, the author reported that it was analogous to the wide oral LD50 range given for the compound in other reports.
3.2.3.2. Systemic Effects
Respiratory Effects. No studies were located regarding respiratory effects in humans or animals following dermal exposure to MMT.
Cardiovascular Effects. No studies concerning cardiovascular effects following dermal exposure to MMT in humans or animals were located.
Gastrointestinal Effects. No studies were located regarding gastrointestinal effects in humans following dermal exposure to MMT. Hinderer (1979) observed bloody diarrhea in rabbits exposed dermally to MMT; the compound was obtained as commercial grade, “neat,” and applied to shaved skin for 24 hours. No histopathology was performed to ascertain the presence of lesions on the gastrointestinal tract.
Hematological Effects. No studies were located regarding hematological effects in humans or animals following dermal exposure to MMT.
Musculoskeletal Effects. No studies regarding musculoskeletal effects in humans or animals following dermal exposure to MMT were located.
Hepatic Effects. Hinderer (1979) observed that rabbits that underwent dermal application of a commercial “neat” solution of MMT for 24 hours on shaved skin had discoloration of the liver and swollen liver. No histopathology was performed.
Renal Effects. Hinderer (1979) observed that rabbits that underwent dermal application of a commercial “neat” solution of MMT for 24 hours on shaved skin had discoloration of the kidneys and swollen and congested kidneys. No histopathology was performed.
Endocrine Effects. No studies were located regarding endocrine effects in humans or animals following dermal exposure to MMT.
Dermal Effects. No studies were located regarding dermal effects in humans following dermal exposure to MMT. Hinderer (1979) observed that rabbits exposed dermally to commercial “neat” MMT on shaved skin for 24 hours developed edema and erythema. Further dermal irritation tests performed showed that MMT is a moderate skin irritant. Campbell et al. (1975) exposed male albino rats dermally to MMT for 24 hours on closely clipped dorsolateral aspects of the trunk that were either abraded or allowed to remain intact; skin reactions were evaluated and scored at 24 hours and again 48 hours later. By comparing skin reactions following exposure to a test rating that categorized irritancy levels, MMT was determined to be safe for intact or abraded skin contact. However, the authors note that MMT in concentrated form is absorbed through the skin, and dermal absorption or interactions with other materials or factors were not incorporated into their study.
Ocular Effects. No studies were located regarding ocular effects in humans or animals following dermal exposure to inorganic manganese.
Hinderer (1979) performed a standard Draize irritation test with commercial “neat” MMT in rabbits and found the compound not to be an eye irritant.
Body Weight Effects. No studies were located regarding body weight effects in humans or animals following dermal exposure to inorganic manganese.
Rabbits exposed dermally to commercial “neat” MMT exhibited slight body weight loss, although the actual amount was not reported (Hinderer 1979).
Metabolic Effects. No studies were located regarding metabolic effects in humans or animals following dermal exposure to inorganic manganese.
No studies were located regarding metabolic effects in humans or animals following dermal exposure to MMT.
3.2.3.3. Immunological and Lymphoreticular Effects
No studies were located regarding immunological and lymphoreticular effects following dermal exposure to inorganic manganese in either humans or animals.
No studies regarding immunological and lymphoreticular effects following dermal exposure to MMT in humans or animals were located.
3.2.3.4. Neurological Effects
No studies were located regarding neurological effects following dermal exposure to inorganic manganese in either humans or animals.
Rabbits exposed to “neat” commercial grade MMT on shaved areas of their trunks for 24 hours experienced the following reported symptoms: polypnea, vocalization, excitation, ataxia, tremors, cyanosis, and convulsions (Hinderer 1979).
3.2.3.5. Reproductive Effects
No studies were located regarding reproductive effects in humans or animals following dermal exposure to inorganic manganese.
No studies were located regarding reproductive effects in humans or animals following dermal exposure to organic manganese.
3.2.3.6. Developmental Effects
No studies were located regarding reproductive effects in humans or animals after dermal exposure to inorganic manganese.
No studies were located in humans or animals concerning developmental effects following dermal exposure to MMT.
3.2.3.7. Cancer
No studies were located regarding carcinogenic effects in humans or animals after dermal exposure to inorganic manganese.
No studies were located regarding carcinogenic effects in humans or animals after dermal exposure to MMT.
3.2.4. Diagnostic Uses
Manganese is a paramagnetic element that can contain up to five unpaired electrons in its ionic form. The unpaired electrons can facilitate T1 relaxation (in MRI) by interacting with hydrogen nuclei of water molecules (Earls and Bluemke 1999). This T1 relaxation provides a contrast in signal during MRI from normal cells and tumor cells because normal cells will take up the metal, whereas the cancerous cells take up little or no manganese (Toft et al. 1997a). The Mn2+ ion is the ion of choice because it is most readily found in the body. However, because increased amounts of other sources of Mn2+, especially manganese chloride, were found to have a high acute toxicity (as discussed in the previous sections), it is necessary to chelate the Mn2+ ion with another molecule that might decrease the toxic nature of the free ion. One such chelate is the fodipir molecule, or dipyridoxal diphosphate. The result is mangafodipir, Mn(II)-N,N’-dipyridoxylethylendiamino-N,N;-diacetate-5,5'-bis(phosphate), or manganese dipyridoxal diphosphate (MnDPDP). This clinical imaging agent is primarily used in the detection of hepatobiliary tumors, as it is preferentially taken up by parenchymatous cells. However, as other organs have parenchymatous cells, the compound is also useful in the detection of kidney, pancreas, and adrenal gland tumors (Earls and Bluemke 1999).
This section will discuss the adverse effects of administration of mangafodipir. This section will not discuss the efficacy of mangafodipir as a contrast agent in the identification of abdominal cancer. Because this compound is used primarily in the detection of liver and other parenchymatous tumors, it is found exclusively in hospitals and other clinical settings. It is only administered intravenously; therefore, all subsequent studies discussed entail an intravenous exposure route. Because the toxicity of mangafodipir is mediated by manganese, the doses will be in mg manganese/kg body weight, rather than in terms of the parent compound.
3.2.4.1. Death
There are no reports of lethality in humans following administration of mangafodipir.
Administration of mangafodipir can occur either all at once (bolus) or over a specific timed period necessary to give the entire amount of a precalculated dose (slow infusion). The latter method has been found to be better tolerated in a clinical setting (Bernardino et al. 1992; Lim et al. 1991; Padovani et al. 1996).
Mangafodipir was found to cause lethality in both sexes of Swiss-Webster mice with an LD50 of 2,916 mg manganese/kg after slow infusion of 15 seconds (Larsen and Grant 1997). The compound had an LD50 of 103 mg/kg in both sexes of the same rodent when administered in a bolus dose (Larsen and Grant 1997), showing the increased toxicity in the bolus administration. When given as a slow infusion over 5 minutes in both sexes of the CD-1 mouse, the compound had an LD50 value of 157 mg/kg, and when given at a rate of 1.2 mL/second in BOM:NMRI male mice, the LD50 was 211 mg/kg. In another study, the LD50 in both sexes of the Swiss-Webster mouse was found to be 290 mg/kg, when given as a slow infusion over approximately 2.5 minutes (Elizondo et al. 1991). One male and one female beagle dog given a single slow infusion (lasting ~110 seconds) of 160 mg/kg mangafodipir, as well as the one male given 120 mg/kg, died prior to the second day of the experiment; the remaining female given 120 mg/kg was sacrificed due to a moribund condition on day 3 of the experiment (Larsen and Grant 1997). Dogs of both sexes given 83 or 99 mg/kg survived the 14-day observation period. A single slow infusion (lasting 5 minutes) at a dose of 160 mg/kg did not result in lethality in the Sprague-Dawley rat (Larsen and Grant 1997).
Death was not observed in Sprague-Dawley rats administered nine doses of 16 mg manganese/kg/day (as mangafodipir) given over 3 weeks (Elizondo et al. 1991; Larsen and Grant 1997). Moribund condition prompted the sacrifice of one male and one female beagle dog on days 12 and 21, respectively, of a 21-day exposure period in which the animals were administered 5.4 mg/kg/day manganese (as mangafodipir), whereas a lower dose of 1.6 mg/kg/day did not result in death or sacrifice of any treated dogs (Larsen and Grant 1997). Moribund condition also prompted the sacrifice of a single male Cynomolgus monkey on day 18 of a mangafodipir-dosing regimen involving 16 mg manganese/kg/day doses also given 3 times/week for 3 weeks (Larsen and Grant 1997). The authors did not indicate the precise cause of lethality in the sacrificed dogs; however, they noted the dogs’ livers showed histological signs of cholangiohepatitis, fibroplasia, bile duct proliferation, and hepatocyte necrosis, with cortical tubular necrosis in the kidneys. The sacrificed monkey had a serum chemistry profile indicative of renal failure and associated liver toxicity.
3.2.4.2. Systemic Effects
Respiratory Effects. No reports were located concerning respiratory effects in humans following dosing with mangafodipir.
A single dose of 160 mg manganese/kg as mangafodipir in Sprague-Dawley rats of both sexes resulted in dyspnea (Larsen and Grant 1997).
Cardiovascular Effects. Mangafodipir, when administered to humans in timed doses in clinical studies has resulted in transient facial flushing and increased blood pressure at doses as low as 0.2 mg manganese/kg (facial flushing) (Bernardino et al. 1992; Lim et al. 1991; Padovani et al. 1996; Wang et al. 1997).
Slow infusion of mangafodipir at doses of 16.5 mg manganese/kg resulted in no cardiotoxicity in mongrel dogs of either sex (Karlsson et al. 1997). The dogs suffered from medically-induced acute ischaemic heart failure; cardiotoxicity was measured as the depression of cardiovascular function, with specific measured end points being aortic pressure, pulmonary artery pressure, right atrial pressure, cardiac output, and heart rate (Karlsson et al. 1997). Sprague-Dawley rats suffered no cardiotoxicity (as measured by histomorphological evaluation) after a single administration of mangafodipir at doses as high as 63 mg/kg (Larsen and Grant 1997).
Rats administered nine doses (3 times/week for 3 weeks) of 16 mg manganese/kg did not suffer any adverse cardiovascular effects as measured by histomorphological analyses (Larsen and Grant 1997). Twenty-one days of daily administration of 5.4 mg manganese/kg in beagle dogs resulted in reduced heart rate by the end of the treatment (Larsen and Grant 1997). Cynomolgus monkeys administered 16 mg/kg for 3 days/week for 3 weeks resulted in flushing of the face, but no other measured cardiovascular effects (Larsen and Grant 1997).
Gastrointestinal Effects. Incidences of gastrointestinal effects in humans following injection with mangafodipir have been limited to rare complaints of nausea or vomiting that are short-lived (15 seconds to 5 minutes in length) and not dose-or administration rate-dependent (bolus vs. infusion) (Bernardino et al. 1992; Lim et al. 1991; Padovani et al. 1996; Wang et al. 1997). A dose of 81 mg manganese/kg as mangafodipir in beagle dogs of both sexes resulted in vomiting, diarrhea, and decreased food consumption (Larsen and Grant 1997).
Vomiting was observed in Cynomolgus monkeys of both sexes after administration of nine doses of 16 mg manganese/kg, given 3 times/week for 3 weeks (Larsen and Grant 1997). No other gastrointestinal effects in animals were reported.
Hematological Effects. No hematological changes (versus pretreatment values) were noted in three different studies that included 13 healthy males (Wang et al. 1997), 54 healthy males (Lim et al. 1991), or 96 human volunteers of both sexes with known or suspected focal liver tumors (Bernardino et al. 1992) administered up to 1.4 mg manganese/kg as mangafodipir (either via bolus or slow infusion).
A single dose of 63 mg manganese/kg as mangafodipir in both sexes of Sprague-Dawley rats resulted in no adverse hematological effects (Larsen and Grant 1997). Intermediate studies of adverse effects were also negative. Doses as high as 16 mg/kg given 3 times/week for 3 weeks to Sprague-Dawley rats (Elizondo et al. 1991; Larsen and Grant 1997) or Cynomolgus monkeys, or 5.4 mg/kg in beagle dogs dosed daily for 21 days, failed to induce any adverse hematological effects (Larsen and Grant 1997).
Musculoskeletal Effects. No reports of musculoskeletal effects in humans or animals following mangafodipir administration were located.
Hepatic Effects. Blood chemistry analyses revealed no significant changes in liver enzymes in several volunteers, either with or without tumors, given mangafodipir at doses up to 1.4 mg manganese/kg (Bernardino et al. 1992; Lim et al. 1991; Wang et al. 1997). Three individuals dosed with 0.55 mg manganese/kg and one dosed with 1.4 mg/kg had increased serum alanine aminotransferase; however, there was no dose response with these results and the maximum increase in the enzyme was to 70 International Units (IU)/l (the upper limit of the normal range is 45 IU/l) (Lim et al. 1991).
A single dose of up to 63 mg manganese/kg administered to both sexes of Sprague-Dawley rats did not produce any adverse hepatic effects as observed by histomorphological analyses (Larsen and Grant 1997).
The administration of nine total doses of mangafodipir, three per week, at 16 mg manganese/kg/day per dose, resulted in an increased incidence (relative amount unreported) in hepatic microgranulomas in female Sprague-Dawley rats, but no effect on liver enzymes as measured by serum chemistry (Elizondo et al. 1991; Larsen and Grant 1997). Twenty-one daily doses of 1.6 mg/kg/day resulted in an increase in serum enzymes (alanine aminotransferase, ornithine carbamyl transferase, glutamine dehydrogenase, alkaline phosphatase, gamma-glutamyl transferase), as well as bilirubin and cholesterol, in both sexes of beagle dogs, while a higher dose of 5.5 mg/kg/day resulted in increased liver enzymes and liver weight and changes in liver pathology (cholangiohepatitis, fibroplasia, bile duct proliferation, and hepatocyte necrosis) (Larsen and Grant 1997). The authors noted that altered serum albumin:globulin ratios and increased prothrombin time were indicative of decreased liver protein synthesis. When dogs at this high dose were allowed a 4-week recovery period, healing of the liver was observed; specific measures of healing were not provided, although resolution of lesions in other affected organs, such as the kidneys, was mentioned. The authors also noted that increased serum levels of liver enzymes and decreased liver protein synthesis were reversible effects in dogs allowed a recovery period. Doses of 0.54 mg/kg/day did not have any effect on the liver (Larsen and Grant 1997). In both sexes of the Cynomolgus monkey, nine total doses of 16 mg/kg/day given 3 times/week for 3 weeks, resulted in increases in liver enzymes (alanine aminotransferase, gamma-glutamyl transferase), as well as increases in bilirubin and relative liver weights in males, and focal hepatitis/cholangiolitis in one male at the end of the dosing period. When the monkeys were given a 2-week recovery period following a 3-week administration of the highest dose, only one male had a liver lesion, which was in the process of healing. Doses of 1.6 mg/kg/day in this primate did not cause any adverse hepatic effects (Larsen and Grant 1997).
Renal Effects. Administration of mangafodipir at up to 1.4 mg manganese/kg in a few human studies has not resulted in any adverse renal effects as measured by blood chemistry or urinalysis (Bernardino et al. 1992; Wang et al. 1997).
Single doses of mangafodipir up to 63 mg manganese/kg given to Sprague-Dawley rats did not cause renal effects as measured by blood chemistry, urinalysis, gross necropsy, and histopathology (Larsen and Grant 1997). Sprague-Dawley rats of both sexes given nine doses (thrice weekly for 3 weeks) of 16 mg/kg manganese did not show any adverse renal effects as measured by urinalysis, blood chemistry, and histomorphological analysis (Elizondo et al. 1991; Larsen and Grant 1997). Daily administration of mangafodipir over 21 days in both sexes of the beagle dog at concentrations up to 6 mg/kg resulted in cortical tubular necrosis of the kidneys at this highest dose, as well as decreased glomerular filtration rate, as indicated by high serum carbamide and creatinine levels. There were no measurable effects at ≤1.6 mg/kg (Larsen and Grant 1997). Administration of nine doses of mangafodipir, also given thrice weekly for 3 weeks, at individual concentrations of 16 mg manganese/kg to Cynomolgus monkeys of both sexes resulted in increased kidney weights and enzymes, as well as creatinine, urea, and other inorganic ions. Doses of 1.6 mg/kg over the same time period did not result in any adverse effect (Larsen and Grant 1997).
Endocrine Effects. No studies were located regarding endocrine effects in humans or animals following administration of mangafodipir.
Dermal Effects. No studies were located regarding dermal effects in humans or animals following intravenous administration of mangafodipir.
Ocular Effects. No studies were located concerning ocular effects in humans following administration of mangafodipir.
Cynomolgus monkeys administered nine individual doses at 16 mg/kg over 3 weeks and beagle dogs given up to 6 mg/kg daily for 21 days did not have any adverse ocular effects from the mangafodipir treatment (Larsen and Grant 1997).
Body Weight Effects. No reports were located concerning body weight effects in humans following mangafodipir dosing.
Mice given acute doses of mangafodipir as high as 275 mg manganese/kg and rats administered a dose of 160 mg/kg did not suffer any body weight effects (Larsen and Grant 1997).
Rats (Elizondo et al. 1991; Larsen and Grant 1997) and monkeys (Larsen and Grant 1997) administered nine doses of mangafodipir over 3 weeks at doses as high as 16 mg manganese/kg did not have any treatment-related effects on body weight. Dogs administered 21 daily doses of the compound suffered decreased body weight (unspecified decrease) at 5.4 mg/kg, but no effect at 1.6 mg/kg (Larsen and Grant 1997). There were no significant treatment-related adverse effects on body weight of male and female rats or female rabbits used in reproductive studies with mangafodipir (Blazak et al. 1996; Grant et al. 1997a; Treinen et al. 1995), except for a transient decrease in body weight during weeks 2–5, 9, and 10 in male rats administered 6 mg manganese/kg/day for 85 days (Grant et al. 1997a). The authors noted that the decrease was significant when compared to controls, but did not report actual data.
Metabolic Effects. No studies were located regarding metabolic effects in humans or animals following administration of mangafodipir.
3.2.4.3. Immunological and Lymphoreticular Effects
No studies were located regarding immunological or lymphoreticular effects in humans following exposure to mangafodipir.
Injection of mangafodipir 3 times/week for 3 weeks in Sprague-Dawley rats at doses of 1.6, 6.3, or 16 mg manganese/kg resulted in eosinophilia in females only at the highest dose, but had no effect in males. The authors stated they are unsure of the clinical importance of this effect as it was only seen at repeated high doses (Larsen and Grant 1997). Daily dosing of mangafodipir in beagle dogs of both sexes at doses of
1.6 mg manganese/kg for 21 days resulted in a decrease in eosinophils and an increase in toxic neutrophils (absolute amounts not reported) (Larsen and Grant 1997). A lower dose of 0.54 mg/kg had no immunological effect.
3.2.4.4. Neurological Effects
No statistically significant increases in adverse neurological effects in humans following mangafodipir administration were reported. In one study, four subjects given doses ranging from a low of 0.17 mg/kg to a high of 1.4 mg/kg complained of light-headedness or dizziness (Lim et al. 1991). Five of 96 patients administered mangafodipir complained of a headache following dosing; only two of these five, given varying doses of mangafodipir ranging from 0.17 to 1.4 mg manganese/kg, could be attributed to the contrast agent (Bernardino et al. 1992). No other neurological effects were reported in human studies.
Single doses of mangafodipir ranging from 8.3 to 275 mg manganese/kg in mice and a single dose of 160 mg/kg in rats, resulted in decreased activity and abnormal gait and stance (Larsen and Grant 1997). Mongrel dogs infused once with mangafodipir at doses of 0.55, 3.3, or 16.5 mg manganese/kg did not have any treatment-related changes in plasma catecholamines or physiological signs of sympathetic activation as compared to the undosed controls (Karlsson et al. 1997). In a separate study, beagle dogs receiving either single doses ranging from 83 to 160 mg/kg or 21 daily doses at 5.4 mg manganese/kg suffered decreased appetite as measured by decreased food consumption; when the dogs were allowed a recovery period following the repeated dosing, the food consumption normalized within the first 2–3 days (Larsen and Grant 1997).
Rats and monkeys administered nine doses of up to 16 mg/kg each did not have any observable neurotoxic effects (Larsen and Grant 1997).
Grant et al. (1997a) did observe behavioral changes in the pups of Sprague-Dawley dams exposed to 0, 0.6, 1.1, or 2.2 mg manganese/kg on GDs 6–17. Although no significant effects were observed at the lowest dose, the exposed pups suffered a significant decrease in grasp/holding time and a 10–11% decrease in body weight at PNDs 4 and 7 at the 1.1 mg/kg dose. At the highest dose, pup weight was significantly decreased at PNDs 4, 7, 14, and 21; performance on grasp/holding, negative geotaxis, and surface righting tests was also significantly impaired. In addition, postnatal survival was decreased on days 0–4 (56 vs. 95.9% in the control group) and 4–21 (78.9 vs. 100% in the control group) at the highest dose (Grant et al. 1997a).
Current studies do not provide evidence on the potential for neurotoxicity following clinical exposure to mangafodipir. In general, studies on neurological effects in humans or animals following mangafodipir exposure did not involve a long observation period. Because deposition of manganese in the brain can be significantly delayed following exposure, it is possible that the studies to date were terminated prior to the onset of potential neurotoxicity. However, neurotoxicity in humans or animals has not been reported following single exposures to manganese, even at high doses. Studies on toxicokinetics of other manganese compounds also indicate that a single exposure is not likely to result in significant neurological effects. For further information on distribution, refer to Section 3.4 Toxicokinetics.
3.2.4.5. Reproductive Effects
No studies were located regarding reproductive effects in humans following administration of mangafodipir.
A single dose of 160 mg/kg in male Sprague-Dawley rats resulted in no adverse effects in testes as measured by organ weight and histomorphological analysis (Larsen and Grant 1997).
Male Sprague-Dawley rats dosed nine times in 3 weeks with 16 mg manganese/kg as mangafodipir suffered a decrease in absolute testes weights, but no relative decrease in weight and no histomorphological effects (Larsen and Grant 1997).
Injection of pregnant Sprague-Dawley rats with up to 4.4 mg manganese/kg as mangafodipir, on GDs 6– 8, 9–11, 12–14, or 15–17 (all during organogenesis) resulted in no evidence of reproductive toxicity as measured by pregnancy rate, numbers of corpora lutea, implantations or resorptions (Treinen et al. 1995). Further, daily intravenous administration of doses up to 2.2 mg manganese/kg throughout GDs 6–17 did not result in any significant changes in pregnancy rate, corpora lutea, implantations, or resorptions (Treinen et al. 1995). However, Grant et al. (1997a) observed a >50% rate of post-implantation loss in pregnant Sprague-Dawley rats administered 2.2 mg manganese/kg as mangafodipir during GDs 6–17. Doses of 0.6 and 1.1 mg/kg resulted in postimplantation loss rates that were similar to that of the control group. There were no obvious differences in compound administration or animal husbandry between the two studies that would indicate why such disparate results would occur. Intravenous dosing of New Zealand white rabbits with up to 1.1 mg manganese/kg/day on GDs 6–17 did not cause reproductive toxicity in one study (Grant et al. 1997a), but a dose of 3.3 mg manganese/kg/day during GDs 6–18 in the same species resulted in a significant increase (3-fold) in post-implantation loss (Blazak et al. 1996). This latter dose corresponds to a 12-fold increase over the one-time human clinical dose (Earls and Bluemke 1999).
Mangafodipir dosing in female Sprague-Dawley rats for 22 total days, starting prior to conception and ending on the 7th day of gestation at a dose of up to 6 mg manganese/kg, did not result in any adverse reproductive effects (Grant et al. 1997a).
Male Sprague-Dawley rats dosed for 84–85 days with 0, 0.6, 2, or 6 mg manganese/kg as mangafodipir did not show any signs of reproductive toxicity as measured by histomorphological analyses. Although absolute testes weights in the intermediate dose group were reduced compared to controls, relative weights were not, and in the absence of histopathological findings, this reduction is not considered an adverse effect. The treated rats were bred with females to determine if mangafodipir dosing had any effect on fertility. Pregnancy rates, and the number of corpora lutea, implantations, or resorptions were unaffected by parental treatment (Grant et al. 1997a).
3.2.4.6. Developmental Effects
No studies were located regarding developmental effects in humans following intravenous exposure to mangafodipir.
Treinen et al. (1995) tested the sensitivity of different gestational periods to the administration of mangafodipir in Sprague-Dawley rats. Pregnant rats were dosed with 0, 1.1, 2.2, or 4.4 mg manganese/kg on 3 consecutive days: GDs 6–8, 9–11, 12–14, or 15–17. The 1.1 mg/kg dose given on days 15–17 resulted in a significant increase in skeletal malformations in fetuses (10/113 fetuses vs. 0/106 in the control group; p<0.05). A higher dose of 2.2 mg/kg also caused a significant increase in malformations when given on GDs 12–14 (10 out of 104 fetuses affected) and days 15–17 (21/143) (both p<0.05), and the 4.4 mg/kg dose caused increases in malformations when given on days 9–11(5/83), 12–14 (45/128), and 15–17 (98/129) (all p<0.05). The malformations seen in this study included angulated or irregularly shaped clavicle, femur, fibula, humerus, ilium, radius, tibia, ulna, and/or scapula (Treinen et al. 1995).
The offspring of Sprague-Dawley rats dosed with 0, 0.1, 0.3, or 1 mg manganese/kg as mangafodipir daily throughout GDs 6–17 had a significant increase (p<0.05) in abnormal limb flexures (38/270 fetuses affected) and skeletal malformations (141/270 fetuses affected) only at the highest dose (Treinen et al. 1995). These malformations included the same ones listed for the segmented teratology study above. In a separate experiment evaluating the teratology of mangafodipir administration on GDs 6–17 in pregnant Sprague-Dawley rats, Treinen et al. (1995) observed a significant increase (p<0.05) in skeletal malformations in offspring of rats dosed with 2.2 mg manganese/kg (86/92 fetuses affected) compared to controls. In both the segmented and continuous teratology studies, no maternal toxicity was observed.
Fetuses from Sprague-Dawley females dosed with 0, 0.6, 1.1, or 2.2 mg manganese/kg on GDs 6–17 exhibited a statistically significant increase in wavy ribs at 0.6 mg/kg (20.5% of the viable fetuses impacted vs. 0.7% at the control dose; p<0.05). At the intermediate dose, there was a statistically significant increase in the number of fetuses with abnormalities (20 out of 159 viable fetuses) including distortion or misshaping of one or more of the following bones: humerus, radius, ulna, scapula, clavicle, femur, tibia, and fibula; in addition, 56.6% of the viable fetuses had wavy ribs and the fetuses weighed 14% less than controls (p<0.05). At 2.2 mg/kg, there was a significant decrease in fetal viability (56% decrease; p<0.05), a greater increase in fetuses with abnormalities (45 out of 64 viable fetuses,) and a greater percentage (85.9%) with wavy ribs (Grant et al. 1997a). These effects were observed in the absence of maternal toxicity. By contrast, when the mangafodipir was administered for 22 days prior to conception and up to GD 7 in the same species at doses of 0, 0.6, 2, and 6 mg manganese/kg/day, no adverse effects on the number of viable fetuses, fetal weight, or the number of fetuses with abnormalities were reported (Grant et al. 1997a). These teratogenic studies indicate that developmental toxicity resulting from mangafodipir dosing is highly dependent on the time-frame of administration.
Grant et al. (1997a) also observed behavioral changes in the offspring of Sprague-Dawley dams administered 0, 0.6, 1.1, or 2.2 mg manganese/kg on GDs 6–17. The exposed pups suffered a significant decrease in grasp/holding time and a 10–11% decrease in body weight at PNDs 4 and 7 at the 1.1 mg/kg dose, but no significant effects at the lower dose (Grant et al. 1997a). At the highest dose, pup weight was significantly decreased at PNDs 4, 7, 14, and 21, and performance on grasp/holding, negative geotaxis, and surface righting tests was significantly impaired. In addition, postnatal survival was decreased on days 0–4 (56 vs. 95.9% in the control group) and 4–21 (78.9 vs. 100% in the control group) at the highest dose (Grant et al. 1997a). These effects occurred at doses that did not cause observable maternal toxicity.
Mangafodipir administration in New Zealand white rabbits at doses of 0, 0.3, 0.55, or 1.1 mg manganese/kg on GDs 6–18 resulted in incomplete ossification of the sternebrae at 1.1 mg/kg in one study (Grant et al. 1997a), but no significant effects on fetotoxicity or fetal weight; this dose did not result in any maternal toxicity. In a separate study, mangafodipir at doses as high as 3.3 mg manganese/kg in the same strain of rabbit for the same period of exposure did not result in any significant increases in external, skeletal, or visceral malformations in a separate teratology study (Blazak et al. 1996). This dose did result in an 11% decrease in fetal weight (although this value was not statistically significant in the study, it is considered a significant developmental effect) and a 20% decrease in the number of viable fetuses (also not statistically significant). It is not readily apparent why two studies with similar dosing regimens would obtain such conflicting results. A comparison between rat and rabbit gestational studies indicates that the rabbit is a much less sensitive model for reproductive and developmental toxicity induced by mangafodipir.
3.3. GENOTOXICITY
There is some evidence from a study on occupationally exposed welders that manganese may cause chromosomal aberrations; the welders were exposed to other potentially toxic compounds including nickel (known to cause chromosomal aberrations) and iron; therefore, the observed increase in chromosomal aberrations cannot be attributed solely to manganese (Elias et al. 1989). Mutagenicity studies in both bacteria and mammalian strains are equivocal. While manganese sulfate was shown to not be mutagenic to Salmonella typhimurium strains TA97, TA98, TA100, TA1535, or TA1537 either in the presence or absence of S9 from Aroclor 1254-induced liver from rats or Syrian hamsters (Mortelmans et al. 1986), it was shown to be mutagenic to strain TA97 elsewhere (Pagano and Zeiger 1992). In yeast (Saccharomyces cerevisiae strain D7), a fungal gene conversion/reverse mutation assay indicated that manganese sulfate was mutagenic (Singh 1984). Manganese chloride was reportedly not mutagenic in S. typhimurium strains TA98, TA100, and TA1535, but it was mutagenic in strain TA1537, and conflicting results were obtained for TA102 (De Méo et al. 1991; Wong 1988).
In vitro assays in mammalian cells also gave conflicting results concerning manganese mutagenicity. Manganese chloride produced gene mutations in cultured mouse lymphoma cells (Oberly et al. 1982). Manganese chloride caused DNA damage in vitro using human lymphocytes at a concentration of 25 µm without metabolic activation, but not at the lower tested concentrations of 15 and 20 µm (Lima et al. 2008). The compound also caused DNA damage in human lymphocytes using the single-cell gel assay technique in the absence of metabolic activation, but caused no DNA damage when S9 was present (De Méo et al. 1991). Manganese sulfate induced sister chromatic exchange in Chinese hamster ovary (CHO) cells in both the presence and absence of S9 from Aroclor 1254-induced rat liver (Galloway et al. 1987). In a separate assay, manganese sulfate also induced chromosomal aberrations in CHO cells in the absence of S9 but not in its presence (Galloway et al. 1987). Manganese chloride caused chromosome aberrations in human lymphocytes without metabolic activation, but only when treated in the G2 phase of the cell cycle; treatment in the G1, G1/S, and S1 phases of the cell cycle did not result in chromosome aberrations (Lima et al. 2008). The compound was also found to be clastogenic in root tip cells of Vicia faba (Glass 1955, 1956), but not in cultured FM3A cells in the absence of metabolic activation (Umeda and Nishimura 1979). Potassium permanganate caused chromosomal aberrations in FM3A cells (Umeda and Nishimura 1979), but not in a primary culture of cells from Syrian hamster embryos when tested in the absence of metabolic activation (Tsuda and Kato 1977). Manganese chloride caused cell transformation in Syrian hamster embryo cells (Casto et al. 1979). A list of in vitro study results is given in Table 3-5.
Table 3-5
Genotoxicity of Manganese In Vitro.
Manganese chloride did not produce somatic mutations in Drosophila melanogaster fruit flies in one study (Rasmuson 1985), and manganese sulfate did not induce sex-linked recessive lethal mutations in germ cells of male D. melanogaster (Valencia et al. 1985).
In vivo assays in mice showed that oral doses of manganese sulfate or potassium permanganate caused micronuclei and chromosomal aberrations in bone marrow (Joardar and Sharma 1990). In contrast, oral doses of manganese chloride did not cause chromosomal aberrations in the bone marrow or spermatogonia of rats (Dikshith and Chandra 1978). A list of in vivo study results is given in Table 3-6.
Table 3-6
Genotoxicity of Manganese In Vivo.
The results of in vitro studies show that at least some chemical forms of manganese have mutagenic potential. However, as the results of in vivo studies in mammals are inconsistent, no overall conclusion can be made about the possible genotoxic hazard to humans from exposure to manganese compounds.
Genotoxicity data concerning MMT was not available.
One study was located regarding genotoxic effects in humans following inhalation exposure to manganese. In this study, the incidences of chromosomal aberrations in three groups of welders with occupational exposures (10–24 years) to metals including manganese, nickel, and chromium were examined (Elias et al. 1989). An increase in chromosomal aberrations was found in the group working with the metal active gas welding process; however, since their exposures included nickel as well as manganese, the authors could not attribute the results to any one metal exposure (nickel is known to cause chromosomal aberrations by the inhalation route). The median manganese concentrations during the survey were 0.18 mg/m3 for respirable dust and 0.71 mg/m3 for total dust. No information was available regarding the genotoxicity of manganese alone.
No studies were located regarding genotoxic effects in humans after oral exposure to inorganic manganese.
In male Swiss albino mice, manganese sulfate and potassium permanganate have both been found to be clastogenic, and their effects were found to be dependent primarily on the concentration (not duration) of exposure (Joardar and Sharma 1990). In this in vivo study, oral doses were administered at varying levels over a 3-week period. The manganese sulfate doses were 10.25, 20.25, and 61 mg/100 g body weight, and the potassium permanganate doses were 6.5, 13, and 38 mg/100 g body weight. Sperm head abnormalities and the frequency of chromosomal aberrations in bone marrow cells and micronuclei were significantly increased. In male rats, repeated oral doses of 0.014 mg manganese/kg/day (as manganese chloride) for 180 days did not produce any significant chromosomal damage in either bone marrow or spermatogonial cells (Dikshith and Chandra 1978).
No studies were located regarding genotoxic effects in animals after inhalation exposure to inorganic manganese.
No studies were located concerning genotoxic effects in humans or animals following inhalation or exposure to MMT.
3.4. TOXICOKINETICS
Manganese is required by the body and is found in virtually all diets. As discussed in Chapter 6, adults consume between 0.7 and 10.9 mg of manganese per day in the diet, with higher intakes for vegetarians who may consume a larger proportion of manganese-rich nuts, grains, and legumes than non-vegetarians (WHO 2004b). Manganese intake from drinking water is substantially lower than intake from food. Exposure to manganese from air is considered negligible as compared to intake from diet, although persons in certain occupations may be exposed to much higher levels than the general public (see Section 6.7).
Even though daily dietary intake of manganese can vary substantially, adult humans generally maintain stable tissue levels of manganese through the regulation of gastrointestinal absorption and hepatobiliary excretion (Andersen et al. 1999; Aschner and Aschner 2005; Aschner et al. 2005; Roth 2006). Following inhalation exposure, manganese can be transported into olfactory or trigeminal presynaptic nerve endings in the nasal mucosa with subsequent delivery to the brain, across pulmonary epithelial linings into blood or lymph fluids, or across gastrointestinal epithelial linings into blood after mucociliary elevator clearance from the respiratory tract (Aschner and Dorman 2006; Dorman et al. 2006a; Roth 2006). Manganese is found in the brain and all other mammalian tissues, with some tissues showing higher accumulations of manganese than others. For example, liver, pancreas, and kidney usually have higher manganese concentrations than other tissues (Dorman et al. 2006a). The principal route of elimination of manganese from the body is fecal elimination via hepatobiliary excretion; contributions from pancreatic, urinary, and lactational elimination are expected to be small (Dorman et al. 2006a). Excess manganese is expected to be eliminated from the body rapidly. For example, following the intravenous bolus injection of manganese chloride in rats, manganese concentrations in plasma return to normal levels within 12 hours (Zheng et al. 2000).
3.4.1. Absorption
3.4.1.1. Inhalation Exposure
No studies were located regarding the absolute amount of manganese that is absorbed by humans or animals after inhalation exposure to manganese dusts.
In general, the extent of inhalation absorption is a function of particle size, because size determines the extent and location of particle deposition in the respiratory tract. Manganese from smaller particles that are deposited in the lower airway is mainly absorbed into blood and lymph fluids, while manganese from larger particles or nanosized particles deposited in the nasal mucosa may be directly transported to the brain via olfactory or trigeminal nerves. Alternatively, particles deposited in the upper or lower respiratory tract may be moved by mucociliary transport to the throat, where they are swallowed and enter the stomach. The latter process is thought to account for clearance of a significant fraction of manganese-containing particles initially deposited in the lung. Thus, manganese may be absorbed in the nasal mucosa, in the lung, and in the gastrointestinal tract following inhalation of manganese dust. However, the relative amounts absorbed from each site are not accurately known.
Absorption of manganese deposited in the lung is expected to be higher for soluble forms of manganese compared with relatively insoluble forms of manganese (Aschner et al. 2005). Evidence in support of this hypothesis comes from studies in which 3-month-old male Sprague-Dawley rats were given intratracheal doses (1.22 mg manganese/kg) of relatively soluble (manganese chloride) or insoluble (manganese dioxide) forms of manganese (Roels et al. 1997). Peak concentrations of manganese in blood were observed earlier after manganese chloride intratracheal administration (0.5 hour) compared with manganese dioxide (168 hours after administration). Peak concentration of manganese in blood were about 4-fold higher in rats exposed to manganese chloride than in rats exposed to manganese dioxide (Roels et al. 1997). Confirmatory evidence has been presented by Dorman et al. (2001a, 2004b). For example, rats exposed to manganese sulfate (0.1 mg manganese/m3, 6 hours/day, 5 days/week for 13 weeks) showed higher olfactory bulb and striatum manganese concentrations than rats exposed to 0.1 mg manganese/m3 manganese phosphate (hureaulite) (Dorman et al. 2004b).
Results consistent with nasal uptake of manganese and transport to the brain along neuronal tracts have been obtained in several animal studies (Brenneman et al. 2000; Dorman et al. 2001a, 2002a; Elder et al. 2006; Fechter et al. 2002; Henriksson et al. 1999; Lewis et al. 2005; Normandin et al. 2004; Thompson et al. 2011; Tjälve and Henriksson 1999; Tjälve et al. 1996; Vitarella et al. 2000). For example, following intranasal administration of 4 μg/kg 54Mn (as manganese chloride) to weanling Sprague-Dawley rats, whole-body autoradiography showed that the olfactory bulb contained the vast majority of measured manganese at 1, 3, and 7 days post-dosing (90, 69, and 47%, respectively) with values decreasing to a low of 16% at 12 weeks (Tjälve et al. 1996). Significant uptake of manganese by other brain regions was not observed until the third day, when the basal forebrain, cerebral cortex, hypothalamus, and striatum had 21, 2, 3, and 1% of the measured label, respectively (Tjälve et al. 1996). Subsequent experiments with varying doses of manganese chloride showed that the uptake of manganese into the olfactory epithelium and the transfer to the brain olfactory bulb leveled off at the highest doses, indicating that these are saturable processes (Henriksson et al. 1999). Following single, 90-minute, nose-only inhalation exposures of 8-week old male CD rats to aerosols of manganese chloride (0.54 mg 54Mn/m3; Brenneman et al. 2000) or manganese phosphate (0.39 mg 54Mn/m3; Dorman et al. 2002a), peak concentrations of radioactivity in the brain olfactory bulb (at 1–3 days after exposure) were about 20- or 4-fold higher, respectively, than peak concentrations in the striatum at 21 days after exposure. Results consistent with transport of manganese to the brain along olfactory neurons have also been obtained in rats exposed to manganese phosphate aerosols in inhalation chambers (0, 0.03, 0.3, or 3 mg manganese/m3) 6 hours/day for up to 14 days (Vitarella et al. 2000). Elevated concentrations of manganese were observed in the olfactory bulb, striatum, and cerebellum at the 0.3 and 3 mg manganese/m3 exposure levels, compared with control levels, and concentrations in the olfactory bulb were about 1.4–2.4-fold higher than concentrations in the striatum (Vitarella et al. 2000). Elevated manganese concentrations were also found in the olfactory bulb, striatum, and cerebellum, following 90 days of inhalation chamber exposure (6 hours/day, 5 days/week) of young (6 weeks old at start) male or female CD rats or aged (16 months old at start) male CD to aerosols of either manganese sulfate or manganese phosphate (“hureaulite”) at an exposure concentration of 0.1 mg manganese/m3 (Dorman et al. 2004b). Regardless of age or gender, the olfactory bulb showed the highest elevation in manganese concentration, compared with other brain tissues, and concentrations in the olfactory bulb were higher in rats exposed to soluble manganese than in rats exposed to relatively insoluble manganese phosphate (Dorman et al. 2004a). Following 12 days of inhalation exposure of rats to ultrafine manganese oxide particles (30 nm diameter; about 0.5 mg manganese dioxide/m3), Elder et al. (2006) reported that manganese concentrations in the olfactory bulb were increased by 3.5-fold over controls, compared with 2-fold increased concentrations in lungs. Lung lavage analysis showed no signs of pulmonary inflammation following 11 days of exposure, but several markers of inflammation were noted in the olfactory bulb including increase tumor necrosis factor-α mRNA and protein. Elder (2006) argued that these results are consistent with the direct transport of the nanosized particles from the nasal mucosa via the olfactory neuronal tract to the olfactory bulb, noting that when the right nares were occluded, manganese only accumulated in the left olfactory bulb.
Elevated concentrations of manganese have also been observed in the trigeminal ganglia of rats and mice at 0, 7, and 14 days following nose-only inhalation exposure to aerosols of manganese chloride at a concentration of about 2 mg manganese/m3, 6 hours/day, 5 days/week for 2 weeks (Lewis et al. 2005). The latter results are consistent with uptake of manganese in the nasal respiratory epithelium and subsequent transport to the brain via trigeminal neurons. In Rhesus monkeys exposed to 1.5 mg manganese/m3 manganese sulfate for 65 days, olfactory epithelium, olfactory bulb, and trigeminal nerve manganese concentrations were increased by about 17-, 8-, and 2-fold over concentrations in air control monkeys (Dorman et al. 2006a). These results are consistent with the hypothesis that the nasal olfactory transport route may be more important than the trigeminal neuron transport route. Support for this hypothesis comes from studies showing that in rats with more than 90% of neurons and supporting cells destroyed in the olfactory epithelium, transport of radiolabelled manganese to the brain was markedly inhibited, compared with normal rats, 7 days following intranasal instillation of a single dose of 54MnCl2 (7.5 µCi/kg); brain concentrations of radiolabel in rats with damaged olfactory epithelium were about 90% lower than concentrations in normal rats (57.22 versus 5.58 nCi/g; Thompson et al. 2011). The relative importance of the nasal route of manganese absorption (and delivery to the brain) in humans (versus lung absorption and transport across the blood-brain barrier) has not been quantified, but it may be less important in humans than in rats because the olfactory bulb accounts for a larger part of the central nervous system and the olfactory epithelium accounts for a larger proportion of the nasal mucosa in rats compared with humans (Aschner et al. 2005; Dorman et al. 2002a). Using a pharmacokinetic model describing the olfactory transport and blood delivery of manganese in rats, Leavens et al. (2007) calculated that 21 days or 8 days following acute inhalation exposure of rats to 54MnCl2 or 54MnHPO4, respectively, direct olfactory transport accounted for the majority of label in the olfactory bulb, but only a small percentage (≤3%) of the label in the striatum. In normal rats 7 days after nasal instillation of a single dose of 54MnCl2, the mean concentrations of radiolabel in the olfactory bulb were about 10-, 12-, 9-, 25-, 25-, and 41-fold higher than concentrations in the cortex, hippocampus, basal ganglia, substantia nigra, brain stem, and cerebellum, respectively (Thompson et al. 2011). These results indicate that the olfactory bulb is the principal site of accumulation for manganese absorbed and transported to the brain via the nasal route, and that distribution to other brain regions is restricted.
Absorption of manganese deposited in the lung or nasal mucosa of rats is expected to be influenced by iron status, with enhanced absorption under iron-deficient conditions and diminished absorption under iron-excess conditions. Following intratracheal administration of 54Mn-manganese chloride, 54Mn blood concentrations were lower in male Sprague-Dawley rats fed a high-iron diet (about 10,000 ppm Fe), compared with concentrations in rats fed a control iron (210 ppm Fe) diet (Thompson et al. 2006). These results are consistent with diminished pulmonary absorption of manganese under iron-loaded conditions. Supporting this interpretation, 4 hours after 54Mn administration, levels of 54Mn (expressed as a percentage of the instilled dose) were higher in the lungs of high-iron rats, compared with control rats, but generally lower in other tissues in high-iron versus control rats (Thompson et al. 2006). In rats fed the high-iron diet, mRNA levels for divalent metal transporter 1 (DMT1—a transport protein that facilitates membrane transport of divalent iron and manganese) were decreased in the bronchus-associated lymphatic tissue of high-iron rats, compared with control rats (Thompson et al. 2006). In Belgrade rats, homozygous (b/b) for a mutation in DMT1 that impairs transport function and fed 500 ppm Fe in the diet, 54Mn blood levels following intranasal administrations of 54Mn-manganese chloride were markedly (2– 5-fold) lower than those in blood of anemic heterozygous (+/b) rats fed a 20 ppm Fe diet (Thompson et al. 2007). For example, levels of 54Mn remaining in the blood 4 hours after administration were 0.022 and 0.115% of the instilled dose in the homozygous (b/b) and anemic heterozygous (+/b) rats, respectively (a 5-fold difference). Intermediate levels of 54Mn in blood were found in heterozygous (+/b) rats fed the 500 ppm Fe diet (Thompson et al. 2007). In Sprague-Dawley rats, levels of DMT1 protein in the olfactory epithelium were 1.5- to 2.5-fold greater under anemic conditions (20 ppm Fe in diet for 3 weeks), compared with iron-sufficient conditions, 200 ppm Fe in diet for 3 weeks (Thompson et al. 2007). These results are consistent with the hypothesis that up- and down-regulation of DMT1 plays a role in enhanced nasal absorption of manganese under iron-deficient conditions and diminished absorption under iron-excess conditions, respectively.
No studies were located regarding the absorption of organic manganese compounds following inhalation exposure in either humans or animals.
3.4.1.2. Oral Exposure
The amount of manganese absorbed across the gastrointestinal tract in humans is variable, but typically averages about 3–5% (Davidsson et al. 1988, 1989a; Mena et al. 1969). Data were not located on the relative absorption fraction for different manganese compounds, but there does not appear to be a marked difference between retention of manganese ingested in food (5% at day 10) or water (2.9% at day 10) (Davidsson et al. 1988, 1989a; Ruoff 1995). In humans, manganese absorption tends to be greater from manganese chloride (in demineralized water) than from foods (labeled intrinsically or extrinsically with 54Mn); however, the biological half-life of manganese from either manganese chloride or food is the same (EPA 1995b; Johnson et al. 1991). In human adults, supplementation of the diet with manganese sulfate for 12–35 weeks at a level approximately 2 times the normal dietary intake caused a 30–50% decrease in absorption of a tracer dose of 54MnCl2 (Sandstrom et al. 1990).
Results from animal studies indicate that the gastrointestinal absorption of manganese is rapid and expected to be higher for soluble forms of manganese compared with relatively insoluble forms of manganese. Following a single gavage dose of 6 mg manganese/kg as manganese chloride to rats, maximal plasma concentrations were attained rapidly (Tmax=15 minutes) (Zheng et al. 2000). From analysis of time course of plasma concentrations following oral and intravenous administration, the oral bioavailability for manganese was calculated to be 13.9% (Zheng et al. 2000). Roels et al. (1997) noted that in 3-month-old male rats, gavage administered manganese chloride (24.3 mg manganese/kg) reached a maximal level in blood, 7.05 μg/100 mL, within the first 30 minutes post-dosing (first time point measured), whereas manganese from manganese dioxide, administered in the same fashion, did not reach a maximal level in blood of 900 ng/100 mL until 144 hours (6 days) post-dosing. Following four weekly gavage doses of manganese chloride at 24.3 mg manganese/kg per dose, significant increases in manganese concentration were observed in blood and the cerebral cortex, but not cerebellum or striatum, as compared to controls; for identical doses of manganese dioxide, manganese levels were significantly increased only in blood. The lack of significant increase in manganese levels in any brain region following administration of the dioxide is likely due to the delayed uptake of manganese in the blood.
One study showed that, in full-term infants, manganese is absorbed from breast milk and cow’s milk formulas that were either unsupplemented or supplemented with iron, copper, zinc, and iodine (Dorner et al. 1989). Manganese intake was greater in the formula-fed infants than in the breast-fed infants due to the higher manganese content of the formula. However, breast-fed infants retained more of their daily intake of manganese (40%) than did the formula-fed infants (20%). It must be noted that the full-term infants evaluated in this study were 2–18 weeks old, and the data did not stratify intake and retention amounts by age. Further, the data did not indicate if there were similar proportions of manganese taken up from breast milk as compared to the formulas. A study by Davidson and Lönnerdal (1989) demonstrated the in vitro receptor-mediated uptake of manganese from lactoferrin; the authors speculated that this may lead to the absorption of manganese from breast milk in human infants.
There is some evidence to suggest that gastrointestinal absorption of manganese is age-dependent. Dorner et al. (1989) have shown that infants, especially premature infants, retain a higher proportion of manganese than adults. Animal studies also support this finding. For example, Rehnberg et al. (1980, 1981, 1982) dosed 1-day-old rat pups with up to 214 mg manganese/kg/day (as manganese tetroxide) for up to 224 days, then measured manganese concentrations in tissues. The authors noted that intermediate and chronic exposure of rats to manganese tetroxide in water or food resulted in much larger increases in tissue levels in young rats (1–15 days in intermediate studies, 24–40 days in chronic study) than in older rats. These increases in neonates were judged to be due to the neonates' greater absorption of manganese as a result of a slower rate of transport through the gut (Rehnberg et al. 1985). Similar results have been reported in rats exposed to manganese chloride (Kostial et al. 1978). However, such age-dependent differences in tissue retention of manganese could also be due to differences in excretory ability (Cotzias et al. 1976; Miller et al. 1975) or to age-related changes in dietary intake levels of iron and manganese (Ballatori et al. 1987). Dorner et al. (1989) found that both pre-term and full-term infants had active excretion of manganese; in fact, some infants had negative manganese balances. Animal studies show that absorption and/or retention of manganese is higher in neonates, but returns to the level of older animals at approximately post-GD 17–18 (Kostial et al. 1978; Lönnerdal et al. 1987; Miller et al. 1975; Rehnberg et al. 1981). Available studies (Dorner et al. 1989) do not provide adequate data to determine when this transition takes place in human infants.
One of the key determinants of absorption appears to be dietary iron intake, with low iron levels leading to increased manganese absorption. Mena et al. (1969) administered oral 54Mn and 39Fe to subjects with iron-deficiency anemia (ranging in age from 13 to 44 years old) and measured manganese and iron uptake with whole-body autoradiography. The uptake of manganese by anemic subjects was 7.5% while in non-anemic subjects, it was 3.0%. This is probably because both iron and manganese are absorbed by the same transport system in the gut. The activity of this system is inversely regulated by dietary iron and manganese intake levels (Chandra and Tandon 1973; Diez-Ewald et al. 1968; Rehnberg et al. 1982; Thomson et al. 1971). Interaction between iron and manganese occurs only between nonheme iron and manganese. Davis et al. (1992a) demonstrated that increasing dietary intakes of nonheme iron, but not heme iron, depressed biomarkers of manganese status (i.e., serum manganese concentrations and lymphocyte manganese-dependent superoxide dismutase activity).
Studies of oral absorption of manganese in animals have yielded results that are generally similar to those in humans. Manganese uptake in pigs, which have similar gastrointestinal tracts to humans, has been measured using labeled manganese administered orally (Finley et al. 1997). The mean absorption rates for different times post-dosing were 5% 1–6 hours post-dosing, 7% 6–12 hours post-dosing, and 3.8% 12–24 hours post-dosing. Gastrointestinal uptake of manganese chloride in rats has been estimated to be 2.5–8.2% (Davis et al. 1993; Pollack et al. 1965). Uptake is increased by iron deficiency (Pollack et al. 1965) and decreased by preexposure to high dietary levels of manganese (Abrams et al. 1976a; Davis et al. 1992b). In a rat study, the intestinal transfer of the calcium ion and manganese ion was found to be competitive, and the authors suggested that there is a common mechanism for their transfer in the intestines (Dupuis et al. 1992). High dietary intakes of phosphorus (Wedekind et al. 1991) and calcium (Wilgus and Patton 1939) have also been demonstrated to depress manganese uptake in chicks.
Manganese absorption has also been found to vary according to manganese intake; in rats with manganese-deficient diets, absorption was at least 2-fold higher than in rats whose diets contained an adequate amount of manganese (as manganese carbonate) (Davis et al. 1992b).
Two studies in suckling rat pups found differing absorptions of manganese from different milks and formulas. The first study (Lönnerdal et al. 1987) found that the percent of 54Mn (added to the food source as an extrinsic label) retained (measured as whole-body retention) in 14-day-old pups fed breast milk, cow milk, cow milk formula, and soy formula, was 82, 90, 77, and 65%, respectively.
The latter study (Lönnerdal et al. 1994) found that 13-day-old rat pups fed 54Mn (from manganese chloride that was incubated with the food for at least 24 hours prior to feeding) in breast milk, cow milk, and several different manufacturers’ cow milk formulas had similar absorption values. These pups absorbed (measured as whole-body retention) 80% of the label from breast milk, 83% from cow milk, and 63–90% from the cow milk formulas, with the two lowest retention values being significantly lower than the others. In this latter study, manganese absorption from soy formulas was significantly lower than the other milks and formulas tested, ranging from 63 to 72%.
The inherent concentration of manganese in each of these food sources from the first study was 0.01, 0.04, 0.05, and 0.30 μg/mL, respectively (Lönnerdal et al. 1987). Therefore, when the retention of the label was multiplied by the actual manganese concentration of the food, the total amounts of absorbed manganese were 0.004, 0.018, 0.019, and 0.097 μg/dose fed, respectively. These data indicate that infants fed cow milk formula may retain 5 times more manganese, and infants fed soy formula may retain 25 times more manganese than breast-fed infants. Although the latter results differ significantly from those observed earlier, the researchers report that the similar relative values for manganese absorption were indicative of significant efforts made to optimize both the relative concentrations and the bioavailability of minerals and trace elements in the manufactured formulas.
No studies were located regarding absorption of manganese following oral exposure to MMT in humans. Several studies (Hanzlik et al. 1980a, 1980b; Hinderer 1979; Hysell et al. 1974; Komura and Sakamoto 1992a, 1992b) indicate that absorption is occurring because toxicity is observed following MMT exposure; however, no absorption rates or relative amounts were provided in these studies. The plasma temporal pattern of manganese following oral administration of MMT has been studied in male Sprague-Dawley rats (Zheng et al. 2000). Following oral gavage of 20 mg MMT/kg, manganese appears in the plasma with a Cmax between 2 and 12 hours after dosing. When nearly equivalent oral doses of MMT (5.6 mg manganese/kg) or manganese chloride (6 mg manganese/kg) were administered, the Cmax (0.93 mg manganese/mL) following oral MMT was about 3-fold higher than that following oral manganese chloride (0.30 mg manganese/mL) (Zheng et al. 2000).
3.4.1.3. Dermal Exposure
The only available human study regarding dermal exposure to manganese discussed a case report of a man burned with a hot acid solution containing 6% manganese. The authors speculated that manganese absorption had occurred across the burn area (Laitung and Mercer 1983) because the man had slightly elevated urinary manganese levels (11–14 vs. 1–8 mg/L). In most cases, manganese uptake across intact skin would be expected to be extremely limited.
No studies were located regarding absorption of organic manganese in humans or animals following dermal exposure.
3.4.2. Distribution
Manganese is a normal component of human and animal tissues and fluids. In humans, most tissue concentrations range between 0.1 and 1 μg manganese/g wet weight (Sumino et al. 1975; Tipton and Cook 1963), with the highest levels in the liver, pancreas, and kidney and the lowest levels in bone and fat (see Table 3-7). Manganese levels in the blood, urine, and serum of healthy, unexposed subjects living in the Lombardy region of northern Italy were 8.8±0.2, 1.02±0.05, and 0.6±0.014 μg/L, respectively (Minoia et al. 1990). Serum manganese concentrations in healthy males and females in Wisconsin were 1.06 and
Table 3-7
Manganese Levels in Human and Animal Tissues.
0.86 μg/L, respectively (Davis and Greger 1992; Greger et al. 1990). Although precise inhalation exposure data were not available for humans, chronic occupational exposure studies have shown that higher levels of inhalation exposure generally correspond with higher blood or urine manganese levels for groups, but that individual measurements may not correspond to individual exposure or be reliable exposure predictors (Abdel-Hamid et al. 1990; Alessio et al. 1989; Jarvisalo et al. 1992; Roels et al. 1992; Siqueira et al. 1991).
Studies investigating manganese levels in human fetal tissues or fluids are very few. Widdowson et al. (1972) measured manganese in fetal livers from 29 unborn infants (ranging in gestational age from 20 to 41 weeks) and from 5 adults. The fetal manganese levels ranged from 0.09 to 0.23 mg/100 g wet weight with a mean of 0.14 mg/100g wet weight, while the mean of the five adults was 0.18 mg/100 g wet weight (range of values not reported). The highest fetal manganese value of 0.23 mg/100 g wet weight was from one of the two infants at 41 gestational weeks of age when analyzed. The data indicate that fetal liver manganese levels throughout the latter half of gestation are comparable to those in the adult.
Concentrations of manganese also have been measured in the blood of pregnant women, as well as in the plasma of cord blood of preterm and full-term infants (Wilson et al. 1991). Manganese concentrations in full-term (37–42 weeks gestation) infants were 5.5±1.5 μg/L, slightly higher than the preterm (27– 36 weeks gestation) infants’ values of 5.0±1.1 μg/L, but the difference was not statistically significant. There were no correlations between the levels in infants and mothers. The higher manganese levels in cord blood of gestationally older infants, along with the higher manganese level in the oldest fetus from the Widdowson et al. (1972) study, suggest that manganese levels may rise slightly as the fetus approaches birth; however, there are inadequate data points to make a strong argument for this possibility. Serum manganese values of 180 healthy Venezuelan infants decreased consistently from a high value of 0.45 μg/L (mean of 22 infants) at 5 days of age to a low value of 0.29 μg/L (mean of 40 infants) at 12 months of age (Alarcón et al. 1996). The level of manganese at 12 months was the only measurement that was statistically different than the 5-day value. The values were not statistically different between the sexes. Rükgauer et al. (1997) obtained very different results in their analyses of serum manganese levels in German children, adolescents, and adults. The authors evaluated 137 children (aged 1 month– 18 years); the mean serum manganese level for all children was 1.4 μg/L (range 0.17–2.92 μg/L). When the children were separated by age, the serum manganese values were found to decrease from a mean value of 2.12 μg/L (age 0–1 year) to a minimum of 0.98 μg/L (age 14–18 years). Adults (age 22– 75 years) had a mean value of 0.79 μg/L. These data indicate that children had much higher manganese levels in serum than those levels shown by the other studies. It is unknown why this latter study indicates results that are vastly different from those reported in the earlier studies. Rükgauer et al. (1997) took precautions to prevent manganese contamination of their experimental materials during sampling and analysis. Also, the authors reported that the subjects were healthy and were not suffering from nutritional diseases or metabolic disorders and were not taking medicines containing trace elements. However, the children and adolescent subjects were chosen from a pediatric hospital after seeking medical attention on non-nutrition related matters. Therefore, this population may not be a representative sample of the general population. Animal studies, by contrast, suggest that distribution of manganese in the infant and young child may be very different from the adult.
Levels in tissues from animals fed a normal diet are generally similar but, perhaps are slightly higher than those in humans (Fore and Morton 1952; Rehnberg et al. 1982). Levels of manganese in the milk of rats fed a normal diet averaged 0.054 μg/g (Miller et al. 1975). Data on changes in tissue levels following acute exposures to excess manganese are presented in exposure-specific subsections later in this chapter.
Manganese is also found in breast milk for the continuing metabolic nutrition of the infant. One study reported manganese concentrations from 82 normal, healthy French women of 12±5.6 μg/L at postpartum day 2 in human colostrum decreasing to 3.4±1.6 μg/L at postpartum day 6 in breast milk (Arnaud and Favier 1995). Another study reported an average manganese concentration in breast milk of 6.2 μg/L using 2,339 samples from mothers of 20 full-term and 6 preterm infants (Dorner et al. 1989). Collipp et al. (1983) have reported concentrations of manganese in breast milk of 10 μg/L. These reports, however, did not address the dietary manganese intake of the nursing mothers. It is unknown whether mothers exposed to increased concentrations of manganese have higher-than-usual levels of the metal in breast milk.
Manganese is distributed throughout all cells in the body; therefore, it is present in germ cells. However, existing studies in humans and animals are not sufficient to predict if distribution of excess manganese into germ cells might result in heritable genetic changes. Manganese is constantly present in human tissues and, therefore, is able to enter germ cells. One human study involving inhalation exposure to nickel and manganese observed chromosomal aberrations in welders working with these metals (Elias et al. 1989). However, the presence of nickel is a confounding factor, as it is known for causing chromosomal changes. Studies in animals are equivocal; there are not enough data to make predictions as to the likelihood for excess exposures of manganese to cause heritable genetic changes.
Concentrations of manganese in select human and animal tissues are presented in Table 3-7 and concentrations of manganese in plasma and serum in infants of differing ages and adults are presented in Table 3-8.
Table 3-8
Manganese Levels in Human Serum/Plasma.
3.4.2.1. Inhalation Exposure
Following inhalation exposure of mice to manganese dust, for a short period of time the concentration of manganese in the lung is approximately proportional to the concentration of manganese in the air (Adkins et al. 1980c). However, as noted earlier, some of the particles that are deposited in the lung are transported to the gastrointestinal tract (Mena et al. 1969). The rate of particle transport from the lungs has not been quantified in humans, but half-times of elimination in animals range from 3 hours to 1 day (Adkins et al. 1980c; Bergstrom 1977; Newland et al. 1987).
The relative increases in tissue levels of manganese following inhalation exposure to inorganic forms of manganese have received considerable investigation in animals.
Increases of 20–60% in manganese levels in the kidney and spleen were noted in mice 24–48 hours after exposure to manganese dioxide (Adkins et al. 1980c). Rats exposed to an aerosol containing 0.0003 mg 54Mn/m3 for 1 hour had manganese levels in the liver, lung, kidney, and brain of 0.0495, 0.1366, 0.0141, and 0.0014 ng 54Mn/organ, respectively, 5 days after exposure (Wieczorek and Oberdörster 1989b). Sheep exposed to welding fumes for 3 hours exhibited a 40-fold increase in lung manganese content (Naslund et al. 1990). Preferential accumulation of manganese in specific locations of the brain (including the caudate nucleus, globus pallidus, and substantia nigra) was noted in one monkey exposed to an aerosol of manganese chloride (20–40 mg/m3) several hours/day for 3–5 months (Newland et al. 1989). This preferential uptake could play a role in the characteristic neurological effects of manganese (see Section 3.5).
Roels et al. (1997) investigated the distributional differences in rats exposed to manganese in two forms (manganese chloride and manganese dioxide) administered via intratracheal injection (intended to simulate inhalation), by gavage (oral administration) and via intraperitoneal injection. When administered intratracheally once a week for 4 weeks, 1.22 mg manganese/kg as manganese chloride resulted in a 68% steady-state increase in blood manganese concentration after the dosing period. This dose also resulted in significantly increased concentrations of manganese in the rat cerebellum (27% increase that approached statistical significance), striatum (205% increase), and cortex (48% increase), compared with control rats.
When rats were administered the same amount of manganese under the same dosing regimen, with manganese in the form of manganese dioxide, similar, but less striking, results were observed (Roels et al. 1997). Manganese concentrations in the blood were increased by 41%, and in the cerebellum, striatum, and cortex by 31, 48, and 34%, respectively, over the control rats.
Tjälve et al. (1996) investigated the distribution of manganese in brain tissues, liver, and kidneys of young male rats following intranasal injection of 54MnCl2. Radiography data indicated that 1 day after dosing, the olfactory bulb contained 90% of the manganese (measured as μg/100g wet weight) in the measured tissues, while the basal forebrain contained 6% of the manganese. Concentrations of manganese in the basal forebrain increased to 21 and 28% of the measured total at 3 and 7 days post-dosing, respectively. Manganese in the cerebral cortex, hypothalamus, striatum, and hippocampus were also maximal at 7 days post-dosing. Manganese values in liver and kidneys were approximately 1% of the total measured for the first 7 days, and then decreased steadily until 12 weeks. These results were compared to distribution of manganese following intraperitoneal injection, in which no brain region showed preferential distribution at 1, 7, or 21 days post-dosing (Tjälve et al. 1996). In another study, Gianutsos et al. (1997) found a dose-dependent accumulation of manganese in the olfactory bulb and tubercle following intranasal injection of manganese chloride into one nostril. Injection of 200 μg manganese resulted in maximally elevated levels in the olfactory bulb (400% higher than the uninjected side), with levels in the tubercle half that in the bulb within 12 hours post-exposure; these levels remained elevated for 3 days. Two injections of 200 μg manganese doubled the level of manganese in the striatum compared to saline-injected controls; single doses did not increase tissue manganese levels. No other brain regions were noted and blood manganese levels were not changed with any treatment. These data indicate that the olfactory mucosa is an important pathway for distribution of manganese into the brain.
Vitarella et al. (2000) exposed adult rats to airborne doses of particulate manganese, as manganese phosphate, at 0, 0.03, 0.3, 3 mg manganese/m3. The particles had a mean diameter of 1.5 μm. Exposures lasted for 6 hours/day for either 5 days/week (10 exposures) or 7 days/week (14 exposures). The following tissues were analyzed for manganese content using neutron activation analysis: plasma, erythrocytes, olfactory bulb, striatum, cerebellum, lung, liver, femur, and skeletal muscle. Increased manganese concentrations were reported in olfactory bulb, lung, femur, and skeletal muscle following exposure to 3 mg/m3 (after either dosing regiment); a lower dose of 0.3 mg/m3 resulted in increased manganese concentrations in olfactory bulb, and lung (14-day dose regimen only). Striatal manganese levels were increased at the two highest doses only after 14 days of exposure. However, concentrations in the cerebellum were similarly elevated, which was interpreted by the authors to indicate that accumulation of manganese was not selective for the striatum. Red blood cell and plasma manganese levels were increased only in rats exposed to the highest dose for the 10-day exposure period. These data indicate that even at lower doses manganese can accumulate in the olfactory bulb and that the neuronal pathway to the brain is significant for inhaled manganese in rodents.
Thompson et al. (2011) reported that in normal rats 7 days after nasal instillation of a single dose of 54MnCl2, the mean concentration of radiolabel in the brain olfactory bulb was about 9–41-fold higher than concentrations in other brain regions. As discussed in Section 3.4.1, these results indicate that the olfactory bulb is the principal site of accumulation for manganese absorbed and transported to the brain via the nasal route, and that distribution from the olfactory bulb to other brain regions may be restricted.
Although the results from the studies by Tjälve et al. (1996), Vitarella et al. (2000), and Thompson (2011) indicate that manganese can be transported via the olfactory neural pathway from the nasal mucosa to the olfactory bulb of the brain and, to a limited degree, to other brain regions in rodents, the relative importance of this pathway to the delivery of manganese to basal ganglia sites of neurotoxicity is uncertain. Statistical mapping of functional olfactory connections in rat brains using MRI following nasal administration of manganese chloride could readily detect connections to the olfactory bulb, but could not detect connections to other brain regions (Cross et al. 2004). Mainstream manganese entry into the brain from blood occurs through capillary endothelial cells of the blood-brain barrier and through the cerebral spinal fluid via the choroid plexuses (Bock et al. 2008; Crossgrove and Yokel 2005). A number of transport mechanisms (including facilitated diffusion, active transport, transferrin-mediated transport, divalent metal transporter-1 mediation, store-operated calcium channels) have been proposed to transport manganese across the blood barrier, but current understanding is inadequate to determine the predominant mechanism of transport (Aschner et al. 2005; 2007; Crossgrove and Yokel 2004, 2005; Roth 2006).
A concern that inhaled manganese, compared with ingested manganese, may more readily result in manganese accumulation in the brain, a principal toxicity target of manganese, has led to recent detailed investigations of manganese concentrations in various brain regions and in other tissues following inhalation exposure of animals to environmentally relevant forms of manganese. These studies have investigated manganese concentrations in tissues of young male and female CD rats exposed by inhalation to manganese sulfate or manganese tetroxide for 14 days at concentrations of 0, 0.03, 0.3, or 3 mg manganese/m3 (Dorman et al. 2001a), young male CD rats given low- (2 ppm), sufficient- (10 ppm), or high-manganese (100 ppm) diets for 67 days, followed by inhalation exposure to manganese sulfate for 14 days at concentrations of 0, 0.092, or 0.92 mg manganese/m3 (Dorman et al. 2001b), young male and female CD rats or aged male CD rats after 90 days of inhalation exposure to manganese sulfate at 0.01, 0.1, or 0.5 mg manganese/m3 or manganese phosphate at 0.1 mg manganese/m3 (Dorman et al. 2004a), maternal CD rats and offspring after inhalation exposure to manganese sulfate at 0, 0.05, 0.5, or 1.0 mg manganese/m3 starting 28 days prior to breeding through PND 18 (Dorman et al. 2005a, 2005b), and young male Rhesus monkeys after inhalation exposure to manganese sulfate at 0.06, 0.3 or 1.5 mg manganese/m3 for 15, 33, or 65 exposure days (Dorman et al. 2006a).
The results from these animal studies indicate that tissue manganese concentrations in the brain depended on aerosol concentration, exposure duration, and brain region. Tissue manganese concentrations generally increased with increasing air concentrations and durations of exposure. With repeated exposures at the highest air concentrations (≥0.92 mg manganese/m3), manganese concentrations in brain regions were elevated, compared with control animals, showing the following order: olfactory bulb>striatum>cerebellum. Illustrative data for maternal CD rats (Dorman et al. 2005a) and young Rhesus monkeys (Dorman et al. 2006a) exposed to manganese sulfate are shown in Tables 3-9 and 3-10, respectively. Comparison of manganese concentrations across tissues shows the following order in exposed maternal rats: liver > pancreas > olfactory bulb > lung > striatum ≈ femur > milk > cerebellum >> whole blood (Table 3-9). In young Rhesus monkeys after 65 days of exposure, the order was: bile > olfactory epithelium > pituitary > liver > pancreas ≈ globus pallidus > olfactory bulb > kidney > putamen > caudate > cerebellum > heart >skeletal muscle > frontal cortex > lung > parietal bone ≈ femur >> blood (Table 3-10).
Table 3-10
Mean (±Standard Error on the Mean) Tissue Manganese Concentrations (µg Manganese/g Tissue Wet Weight) in Young Male Rhesus Monkeys Exposed to Aerosols of Manganese Sulfate (1.5 mg Manganese/m3) 6 Hours/Day, 5 Days/Week for Up to 65 Days. (more...)
Brain tissues from the monkeys were dissected into more regions than the rat brains and, immediately following 65 days of exposure to the highest exposure concentration, showed the following order of elevated manganese concentrations: pituitary>globus pallidus>olfactory bulb>putamen>caudate> cerebellum>frontal cortex>trigeminal nerve (see Table 3-10). These results are consistent with the evidence that the human striatum, globus pallidus, and substantia nigra are the primary neurotoxicity target for manganese (Aschner et al. 2005; Pal et al. 1999). Three- to 5-fold increases (over air control values) in mean manganese tissue concentrations were found in the globus pallidus, putamen, and caudate in monkeys exposed to 1.5 mg manganese/m3 manganese sulfate for 65 days, but levels were <3-fold increased in the frontal cortex and cerebellum, two brain regions not generally associated with manganese neurotoxicity (Dorman et al. 2006a; Table 3-10).
Comparison with the rat results in Table 3-9 suggests that rodents do not accumulate manganese in the basal ganglia (i.e., the collection of deep regions of the brain including the striatum [comprised of the caudate and putamen]) to the same relative degree as primates, a difference that may be related to findings that overt signs of manganese neurotoxicity are more readily detected in nonhuman primates than rodents (Aschner et al. 2005; Bock et al. 2008; Newland 1999). Recent corroborative findings showed that marmosets, a nonhuman primate, accumulated more manganese in the brain (especially in the basal ganglia and the visual cortex) than rats following intravenous injection of equivalent mg/kg body weight doses of manganese chloride (Bock et al. 2008). The mechanisms by which manganese accumulates in the basal ganglia of primates are poorly understood (Aschner et al. 2005; Bock et al. 2008; Brenneman et al. 1999; Dorman et al. 2006b), but Bock et al. (2008) have hypothesized that primates may accumulate relatively more manganese in the basal ganglia than rodents because of a relatively larger cerebral spinal fluid space in lateral ventricles adjacent to the basal ganglia.
The high concentrations of manganese in bile sampled from manganese-exposed monkeys (compared with air control values in Table 3-10) are reflective of the hepatobiliary excretion of manganese. It is currently unknown whether or not the high manganese concentrations attained in the pituitary glands of these monkeys has any effect on normal pituitary function; in this study, exposed monkeys showed no difference in serum levels of luteinizing hormone (LH), a hormone that stimulates production of testosterone by the Leydig cells of the testes (Dorman et al. 2006a).
In pregnant rats repeatedly exposed to inhaled manganese, the placenta appears to partially limit the transport of manganese to the developing fetus (Dorman et al. 2005b). After inhalation exposure to manganese sulfate at 0, 0.05, 0.5, or 1.0 mg manganese/m3 starting 28 days prior to breeding through PND 18, samples of maternal tissues (whole blood, lung, pancreas, liver, brain, femur, and placenta) and fetal tissues (whole blood, lung, liver, brain, and skull cap) were collected and analyzed for manganese concentrations. Elevated (p<0.05) manganese concentrations were observed in exposed maternal rats (compared with air control rats) in the following tissues: brain and placenta at 0.5 and 1.0 mg manganese/m3 and lung at 0.05, 0.5, and 1.0 mg manganese/m3. In contrast, statistically significant elevations of manganese concentrations in sampled fetal tissues were observed only in the liver at 0.5 and 1.0 mg manganese/m3. In pups born and allowed to live up to PND 19 (and sampled for tissue evaluations at PNDs 1, 14, and 19), statistically significant (p<0.05) elevated manganese concentrations (compared with air control values) were observed in blood, liver, and bone samples from exposed neonatal rats at concentrations ≥0.05 mg manganese/m3, starting at PND 1 (Dorman et al. 2005a). As shown in Table 3-11, elevated brain manganese concentrations were observed in exposed neonates starting at PND 14 (but not at earlier time points); tissue concentrations increased with increasing exposure concentration (Dorman et al. 2005a). At PND 19, mean manganese concentration in the striatum was about 2.6-fold higher in offspring exposed to 1 mg manganese/m3, compared with air control means (Table 3-11). In contrast, the mean striatum concentration at PND 19 in maternal rats exposed to 1 mg manganese/m3 was about 1.7-fold increased, compared with controls (Table 3-11). At the lowest concentration tested, 0.05 mg/m3 (50 µg/m3), no statistically significant increase in manganese concentrations in maternal striatum or cerebellum occurred, and increases in manganese concentrations in brain regions of offspring at PND 19, were modest compared with controls (1.4–1.7-fold, Table 3-11). The results from this study suggest that the brain in developing fetuses and neonates is partially protected from excess manganese by the placenta, and that the neonatal period, compared with adulthood, is relatively more susceptible to increased manganese concentration in brain tissues with inhalation exposure to manganese sulfate aerosol concentrations between 0.05 and 1 mg manganese/m3.
Table 3-11
Manganese Concentrations in Brain Tissues of Lactating CD Rats and Offspring Exposed to Aerosols of Manganese Sulfate.
Consistent with the empirical observations in Table 3-11, PBPK model predictions of manganese concentrations in striatum, olfactory bulb, and cerebellum in PND 19 offspring of rat dams exposed by inhalation under the exposure scenarios used by Dorman et al. (2005a, 2005b) indicated that brain concentrations did not begin to increase in offspring until air concentrations exceeded 0.05–0.1 mg/m3 (Yoon et al. 2009a, 2009b). A human PBPK model developed to predict average daily AUC for manganese concentrations in the globus pallidus of the fetus, suckling neonate, and 3-year-old child from airborne manganese concentrations indicated that globus pallidus concentrations progressively increased beyond 10% of baseline concentrations in fetuses and 3-year-old children when air concentrations exceeded 0.01 mg/m3 (10 µg/m3) and in suckling neonates when air concentrations exceeded 0.001 mg/m3 (Yoon et al. 2011).
In an examination of the distribution of manganese in young adult male and female CD rats (28 days at start) and aged male CD rats (16 months at start) following 90-day inhalation exposure to manganese sulfate or manganese phosphate, no evidence was found for a gender or age effect on delivery of manganese to the striatum or on the order of manganese concentrations in tissues (pancreas> olfactory bulb > femur > testes), but gender or age-related differences in tissue manganese concentrations in other brain regions, as well as in the lung, pancreas, femur, and testis, were noted (Dorman et al. 2004a). Following a 90-day inhalation exposure to 0.5 mg manganese/m3 manganese sulfate, young adult male rats had significantly (p<0.05) higher olfactory bulb, blood, femur, and pancreas manganese concentrations than aged male rats, and aged male rats had significantly higher testis manganese concentrations than young male rats. Young male rats exposed to 0.5 mg manganese/m3 had significantly higher olfactory bulb, blood, and lung manganese concentrations than similarly exposed female rats, and female rats exposed to 0.5 mg manganese/m3 had significantly higher cerebellum manganese concentrations than control females. Young male and female rats exposed to 0.5 mg manganese/m3 for 90 days had increased 54Mn clearance rates than air-exposed controls, but similarly-exposed aged male rats did not display increased 54Mn clearance rates, compared with controls (Dorman et al. 2004a). No age-related effects were observed on the order of manganese concentrations in the various tissue.
Manganese concentrations in striatum of young male rats exposed to 0.1 mg/m3 manganese sulfate were about 1.7-fold higher than concentrations in young male rats identically exposed to manganese phosphate (Dorman et al. 2004a). These results are consistent with results from 14-day inhalation studies (Dorman et al. 2001a) and intratracheal instillation studies (Roels et al. 1997) indicating that inhalation of more soluble forms of manganese (e.g., manganese sulfate and manganese chloride) results in higher manganese concentrations in the brain than inhalation of less soluble forms, such as manganese phosphate, manganese tetroxide, or manganese dioxide. Olfactory bulb and striatal concentrations were about 2.5- and 3-fold higher, respectively, in rats exposed for 14 days by inhalation to 3 mg/m3 manganese sulfate, compared with rats exposed identically to manganese phosphate (Dorman et al. 2001a).
No studies were located regarding distribution of manganese in human or animals following inhalation exposure to MMT or mangafodipir.
3.4.2.2. Oral Exposure
Excess manganese uptake has occurred in humans following oral exposure, presumably via the diet, when the individuals suffered from chronic liver disease or some other liver dysfunction (cirrhosis, portacaval shunt, etc.). In these instances, excess manganese was shown to accumulate in certain regions of the brain, as determined by T1-weighted MRI or neutron activation analysis (Devenyi et al. 1994; Fell et al. 1996; Hauser et al. 1994, 1996; Pomier-Layrargues et al. 1998; Rose et al. 1999; Spahr et al. 1996). These studies show that manganese preferentially accumulates in the basal ganglia, especially the globus pallidus, and the substantia nigra.
Rats given a single oral dose of 416 mg manganese/kg body weight (as manganese chloride tetrahydrate) exhibited little tissue accumulation of manganese 14 days later (Holbrook et al. 1975). Studies in animals indicate that prolonged oral exposure to manganese compounds results in increased manganese levels in all tissues, but that the magnitude of the increase diminishes over time (Kristensson et al. 1986; Rehnberg et al. 1980, 1981, 1982). Table 3-12 provides illustrative data based on rats exposed to 214 mg manganese/kg(body weight)/day (as manganese tetroxide) for up to 224 days. As the data reveal, large increases in tissue levels of manganese compared with the controls occurred in all tissues over the first 24 days, but levels tended to decrease toward the control levels as exposure was continued. This pattern is thought to be due to a homeostatic mechanism that leads to decreased absorption and/or increased excretion of manganese when manganese intake levels are high (Abrams et al. 1976a; Ballatori et al. 1987; Mena et al. 1967). Davis et al. (1992b) and Malecki et al. (1996b) demonstrated that rats fed elevated levels of manganese for several weeks had increased tissue manganese concentrations, despite increased gut endogenous losses of manganese, as biliary manganese. This reflected several factors. Although the percentage of manganese absorbed decreased, the total amount of manganese absorbed increased when higher levels of manganese were fed. Moreover, although the total amount of manganese lost in bile increased when manganese intake increased, the percentage of manganese intake lost in bile remained constant at ~1% of manganese intake (Malecki et al. 1996b).
Table 3-12
Manganese Levels in Rat Tissue After Oral Exposure.
A study measuring the retention of a single oral dose of radiolabeled manganese in adult and neonatal rats indicated that retention of the label 6 days after exposure was much greater in pups (67%) than in adults (0.18%); the addition of manganese to the animals' drinking water decreased radiolabel retention in pups and adults (Kostial et al. 1989).
The distributional differences in rats exposed to either manganese chloride or manganese dioxide by gavage were investigated by Roels et al. (1997). After administration of 24.3 mg manganese/kg body weight (as manganese chloride) once weekly for 4 weeks, the authors analyzed blood and brain concentrations of the metal. Manganese concentrations were significantly elevated in the blood (approximately 83% increase over controls) and the cortex of the brain (approximately 39% increase over controls). Gavage administration of manganese dioxide, by contrast, did not significantly increase the amount of manganese in blood or any section of the brain. In addition, administration of manganese as manganese chloride by gavage caused roughly the same amount of increased manganese in the blood as intratracheal administration of manganese in the same form; it did not cause as significant an increase of manganese in the cortex (Roels et al. 1997). These data indicate that inhalation exposure to manganese in the form of manganese chloride or manganese dioxide causes accumulation of manganese in the brain more readily than oral exposure.
Acute manganese exposure in drinking water was found to alter brain regional manganese levels in neonatal rats; after 5 days of exposure, the highest level was in the striatum (12.05 μg/g wet weight) and the lowest level was in the cerebral cortex (0.85 μg/g wet weight) (Chan et al. 1992). After 10 days, the highest concentrations were in the pons and medulla and the lowest were in the hypothalamus. Regional manganese differences were less pronounced in weanling and adult rats. A study by Lai et al. (1991) confirms that intermediate exposure to manganese in drinking water increases brain manganese concentrations; rats exposed from conception to 120 days at 0.04 or 0.4 mg manganese/kg/day had mean brain manganese levels of 0.36–0.72 μg/g in the low-dose animals and 0.62–1.35 μg/g in the high-dose animals, compared to 0.21–0.38 μg/g in controls.
In a dietary study, elevated manganese levels were found in the organs of male mice fed manganese chloride, manganese acetate, manganese carbonate or manganese dioxide at 284 mg manganese/kg/day for 100 days; levels of manganese in the liver and kidney were significantly higher in the animals exposed to manganese acetate or manganese carbonate than in those exposed to manganese chloride or manganese dioxide (Komura and Sakamoto 1991). In a 1993 NTP study, mice and rats chronically fed manganese sulfate generally exhibited elevated tissue levels of manganese; the manganese levels in the liver and kidney were higher than the levels in the brain.
No studies were located concerning disposition of manganese in humans or animals following oral exposure to MMT or mangafodipir.
3.4.2.3. Dermal Exposure
No studies were located regarding tissue distribution of manganese in humans or animals after dermal exposure to inorganic manganese.
No studies were located regarding tissue distribution of manganese in humans or animals after dermal exposure to organic manganese.
3.4.2.4. Other Routes of Exposure
No studies were located regarding tissue distribution of inorganic manganese in humans after exposure via other routes of exposure.
A number of studies have been conducted that investigated various facets of the distribution of inorganic manganese in animal models. The studies utilized a number of routes of administration, and the results suggested that route may play an important role in distribution. In an intraperitoneal study performed in monkeys, manganese was reported in all tissues studied. The highest levels were found in the pancreas, liver, and kidney, and the lowest levels were found in the blood; levels in the central nervous system were found to decrease more slowly than those in other tissues (Dastur et al. 1971). Calves injected intravenously with 54Mn were found to have 3-fold higher liver manganese concentrations and 13-fold higher pancreatic manganese concentrations than calves fed manganese (Carter et al. 1974). Davis et al. (1993) observed that rats injected intraportally with free 54Mn or 54Mn complexed with transferrin and rats injected intraperitoneally with free 54Mn accumulated more manganese in the pancreatic tissue and less in the liver than those rats that were either fed 54Mn or injected intravenously in the portal vein with an albumin-54Mn complex. The similarity in the distribution of the injected manganese-albumin complex and the free manganese in the diet when compared to the distribution of manganese when it was administered by other routes or complexed with other proteins suggests that the route of administration and type of complexed protein may cause differences in the transport of manganese in the sera.
Roels et al. (1997) studied the effect of intraperitoneal administration of manganese chloride and manganese dioxide on distributional differences of manganese in rats. Doses of 1.22 mg manganese/kg as manganese chloride given once per week for 4 weeks resulted in significant increases (when compared to controls) in blood (approximately 60%), striatum (34%), and cortex (36%) concentrations of manganese; no changes were observed in the cerebellum. Identical dosing of rats with manganese dioxide resulted in significant increases in manganese levels in blood (79%), cerebellum (40%), striatum (124%), and cortex (67%) over those in controls. These data indicate that administration of manganese dioxide by this route resulted in greater accumulation of manganese in the brain than did manganese chloride.
The distribution of manganese in the brain was investigated using Cebus (Newland and Weiss 1992; Newland et al. 1989) and Macaque (Newland et al. 1989) monkeys given intravenous injections of manganese chloride that reached a cumulative dose of 10–40 mg manganese/kg. Magnetic resonance images indicated hyper-intensity of the globus pallidus and substantia nigra consistent with an accumulation of manganese in these areas (Newland and Weiss 1992; Newland et al. 1989). Substantial accumulation of manganese was also noted in the pituitary at low cumulative doses (Newland et al. 1989). London et al. (1989) reported a rapid localization of manganese in the choroid plexus observed on MRI; similarly, radiotracer studies of manganese injected into the intracerebroventricular space revealed that radiolabeled manganese was located in the choroid plexus within 1 hour and was located in the rat dentate gyrus and CA3 of the hippocampus 3 days post-dosing (Takeda et al. 1994).
No studies were located regarding disposition of MMT in humans following other routes of exposure, but toxicokinetics of MMT following parenteral administration has received some research attention in animals.
Young adult male rats were administered MMT dissolved in propylene glycol via subcutaenous injection at a dose of 1 mg manganese/kg (McGinley et al. 1987). Control rats received vehicle alone. The rats were sacrificed 1.5, 3, 6, 12, 24, 48, or 96 hours post-injection. Levels of manganese in the control animals were measured in the blood (0.09±0.01 mg/kg), lung (1.51±0.22 mg/kg), liver (2.49±0.36 mg/kg), kidney (1.29±0.23 mg/kg), and brain (0.45±0.01 mg/kg). These values were assumed by the authors to originate from the feed given to the rats and were subtracted from similar values analyzed for MMT-treated rats to determine the amount of manganese in these tissues and fluids that originated from MMT. Maximum accumulation of MMT-derived manganese was measured 3 hours after dosing and was found primarily in the following four tissues: lung (~9 mg/kg); kidney (3.9 mg/kg); liver (2.75 mg/kg); and blood (~0.75 mg/kg). Concentrations of manganese in these four tissues was still elevated (~1 mg/kg) at 96 hours post-dosing. Brain manganese concentrations were not significantly elevated over control levels in MMT-treated animals (McGinley et al. 1987).
Gianutsos et al. (1985) administered 0, 11, or 22 mg manganese/kg as MMT (dissolved in propylene glycol) to male adult mice via subcutaneous injection to determine distribution of manganese. Control mice received vehicle alone. Mice were sacrificed at different time points after dosing. The experiment was divided into an acute study (one dose) or a “chronic study” (ten doses). The brain manganese level 24 hours after the single dose of MMT at 11 mg/kg was 0.93±0.07 μg/g; the value after 22 mg/kg was 1.35±.09 μg/g. Both values were significantly different from the control value of 0.61±0.08 μg/g. The brain manganese level in the mice administered 10 doses of 11 mg/kg each was 1.37±0.27 μg/g; after 10 doses of 22 mg/kg, the value was 3.33±0.15 μg/g; both were significantly greater than the control value of 0.64±0.06 μg/g, and were significantly different than the levels reported after the acute exposure. Manganese levels in the brains of mice given a single dose of MMT at 22 mg manganese/kg were compared with those following injection of the same manganese dose as manganese chloride; mice were sacrificed at different time points from 1–24 hours post-dosing. The brain manganese levels following MMT exposure increased from a low at 1 hour to a maximum at 24 hours of ~1.4 μg/g wet weight. The manganese level in brain after manganese chloride exposure followed the same increasing trend over the 24 hour analysis period, but was higher at each time point, with a maximum value of >2.0 μg/g wet weight (Gianutsos et al. 1985).
Clinical studies involving cancer patients or healthy volunteers have analyzed the usefulness of mangafodipir as a contrast agent for the identification of certain abdominal tumors. Although these studies do not necessarily quantify the amount of manganese, or mangafodipir, in particular tissues, they are useful tools in identifying the location of the metal; also relative proportions of manganese among two or more tissues that contain the metal can be observed by differences in signal from these imaging studies.
Several studies have shown the qualitative presence of manganese in the liver due to increased signal in that organ following mangafodipir administration of 0.17–0.83 mg manganese/kg upon T1-weighted MRI (Bernardino et al. 1992; Lim et al. 1991; Padovani et al. 1996; Wang et al. 1997). Two studies show that the human liver takes up more of the manganese from mangafodipir than any other organ: the signal from the liver was roughly 2 times the amount from the spleen after dosing with 0.55 mg manganese/kg (Lim et al. 1991); the liver signal after dosing with 0.55 mg manganese/kg had reached a 100% increase over baseline signal by 20 minutes following post-dosing, whereas the maximal signal from other organs was only 80% in the pancreas, ~30% in the spleen, ~90% in the renal medulla, and 50% in the choroid plexus, all at the same dose. The renal cortex was the only other tissue to reach a 100% increase over baseline signal at 0.55 mg manganese/kg. Dosing with 0.25 mg manganese/kg (the clinically used dose for current MRI testing of patients) resulted in a similar distribution pattern, although the signal was decreased compared to the higher dose. The signal from the renal cortex at the lower dose had a maximum of 80% over baseline, whereas the signal in the liver at this dose was ~75% of the baseline value (Wang et al. 1997).
Several studies have determined the distribution of manganese in tissues of animals following intravenous administration of mangafodipir. Grant et al. (1994) reported that in rats injected with 2 times the clinical dose of [54Mn] mangafodipir (0.55 mg manganese/kg), the carcass retained 8% of the label and the tissues retained 7% of the label; individual tissue concentrations of manganese were not reported.
Gallez et al. (1997) injected adult male mice once with 0.25 mg manganese/kg as [54Mn] mangafodipir (clinical dose) and determined the tissue manganese content at time points ranging from 15 minutes to 3 months post-dosing. Brain concentration of 54Mn did not reach a maximum value of 0.26±0.04 (value is the percent of injected dose/g tissue) until 24 hours post-dosing; this value was not different than the brain manganese content of mice injected with manganese chloride. This maximum value was still observed in the brain 2 weeks post-dosing, but measurements taken at 1 and 3 months post-dosing were below the detection limit. By contrast, manganese from manganese chloride was still detectable, although not at maximal levels, at 3 months’ time. Liver manganese reached a maximum value of 7.5±1.4 (percent dose/g tissue) 15 minutes post-dosing and then decreased to below the detection limit 1 month later.
Male and female Sprague-Dawley rats injected with [54Mn] mangafodipir at a dose of 5.5 mg manganese/kg had the following distribution of labeled manganese 30 minutes post-dosing (values are given in percent injected dose/g tissue): liver, 1.3; kidney, 1.2; heart, 0.25; spleen, 0.2; blood, 0.3; small bowel, 1.3; large bowel, 0.5; muscle, 0.1; and brain, negligible. Distribution of manganese in tissues of rats injected with labeled manganese chloride was compared to the previous results, and for all tissues, the label was greater after administration with the chloride than from the mangafodipir, with the exception of kidney and large bowel, but these differences were not significant (Elizondo et al. 1991).
The distribution of label in male and female Sprague-Dawley rats injected with either [54Mn] or [14C] mangafodipir at a dose of 0.39 or 0.55 mg manganese/kg, respectively, was studied by Hustvedt et al. (1997). The plasma concentration of labeled manganese reached a peak of 10.2 μg/mL at 5 minutes post-dosing and was quickly distributed into the following organs (values given as μg equivalents of compound/g): pancreas, 10.2; liver, 4.0; kidneys, 3.6; testes/ovaries, 1.7; spleen, 1.0; heart, 0.9; and brain, 0.69. When the bile duct was cannulated, the distribution of an equivalent dose of mangafodipir showed an increased retention of labeled manganese in all organs but the brain (0.62): pancreas, 17.2; liver, 12.3; kidneys, 10.1; testes/ovaries, 5.6; small intestine, large intestine and heart, 2.1; and spleen, 1.9. By contrast, tissue retention of 14C from radiolabeled mangafodipir was very low: pancreas, 0.016; liver, 0.045; kidneys, 0.067; testes/ovaries, 0.015; spleen, 0.023; small intestine, 0.012; large intestine, 0.019; heart, 0.017; and brain, 0.009. These data indicate that manganese dissociates from the fodipir moiety after mangafodipir administration and partitions into the tissues listed above.
The tissue distribution of normal and bile-cannulated dogs following administration of [54Mn] or [14C] mangafodipir was also studied (Hustvedt et al. 1997). Doses of 0.55 manganese/kg were used except for the normal dogs when the manganese was labeled; the dose in this case was 0.38 mg/kg. The general pattern of distribution of manganese and carbon was similar to that seen with rats, except the concentrations were increased in the dog. The values for normal dogs were taken 168 hours post-dosing for both forms of labeled mangafodipir; the bile-cannulated dogs were analyzed 24 hours post-dosing. The maximum concentration of 54Mn in the plasma following dosing was 13.1 μg/mL at the end of the infusion period. The plasma concentrations declined rapidly with a terminal half-life of approximately 15 minutes. In the normal dog and bile-cannulated dog, the tissue distribution was as follows (the values for the bile-cannulated dog are given in parentheses; all values are in μg equivalents of compound/g): liver, 8.7 (79.8); pancreas, 8.1 (2.5); kidneys, 6.6 (37.5); bile, 5.9 (no sample); testes/ovaries, 2.2 (3.2); brain, 0.79 (1.1); spleen, 0.65 (26.6); and heart, 0.62 (3.1). The distribution of labeled carbon in normal (or bile-cannulated dogs) was the following: kidneys, 0.79 (4.1); liver, 0.13 (0.48); bile, 0.059 (no sample); testes/ovaries, 0.05 (0.079); pancreas, 0.015 (0.11); heart, 0.015 (0.035); spleen, 0.007 (0.15); and brain, not detected (not detected). These data indicate that in the dog, as in the rat, the manganese cation is retained by the tissues, but the fodipir moiety is not.
Distribution of 54Mn and 14C following mangafodipir administration was also studied in the pregnant rat (Hustvedt et al. 1997). Whole-body autoradiography of a section of the rat made at different time points revealed that the kidney had retained the highest amount of labeled manganese; later time points showed a distribution similar to those seen in the rat and dog studies mentioned previously with the pancreas and liver causing the most intense signal upon autoradiography. By 24 hours, fetal livers and bones were clearly seen, but placental radioactivity had decreased substantially. Fat deposits also contained a significant amount of the radioactivity at 24 hours. By contrast, radioactivity from labeled carbon in the mangafodipir was relatively uniformly distributed throughout the pregnant rat at 5 minutes and 1 hour post-dosing, with the highest levels in the kidneys. At 24 hours, virtually all tissues were indistinguishable from background.
The human distribution studies have involved much shorter observation times than the animal studies, with maximal increase in MRI signal in human studies observed in minutes following administration. These studies have shown the liver to accumulate the highest amount of manganese from the administered dose of mangafodipir. This is an important limitation since the brain, the primary target of manganese neurotoxicity, may not accumulate a significant amount of manganese until much later, possibly after the current experiments in humans and animals were truncated. Experiments in rats and dogs, both normal and bile-cannulated, indicate that the brain does not accumulate a significant amount of manganese following administration of mangafodipir at levels much higher than the recommended clinical dose of the agent (Hustvedt et al. 1997), even at 168 hours post-dosing in the dog. Gallez et al. (1997) reported that manganese accumulation in the brain of adult mice following injection of a clinical dose of mangafodipir did not reach maximal levels until 24 hours post-dosing. This would indicate that the human distribution studies were terminated prematurely. However, while brain accumulation of manganese following mangafodipir administration is similar to that from manganese chloride, the manganese is not present after 2 weeks, whereas manganese from the inorganic compound was present, although at a decreased amount, 3 months following dosing (Gallez et al. 1997). These data indicate that single, clinical doses of mangafodipir are not likely to cause persistent accumulation of manganese in the brain.
3.4.3. Metabolism
Manganese is capable of existing in a number of oxidation states, and limited data suggest that inorganic manganese may undergo changes in oxidation state within the body. Circumstantial support for this hypothesis comes from the observation that the oxidation state of the manganese ion in several enzymes appears to be Mn(III) (Leach and Lilburn 1978; Utter 1976), while most manganese intake from the environment is either as Mn(II) or Mn(IV) (see Chapter 6). Another line of evidence is based on measurements of manganese in tissues and fluids using electron spin resonance (ESR), which detects the unpaired electrons in Mn(II), Mn(III), and Mn(IV). When animals were injected with manganese chloride, levels of manganese increased in bile and tissues, but only a small portion of this was in a form that gave an ESR signal (Sakurai et al. 1985; Tichy and Cikrt 1972). This suggests that Mn(II) is converted to another oxidation state (probably Mn(III)), but it is also possible that formation of complexes between Mn(II) and biological molecules (bile salts, proteins, nucleotides, etc.) results in loss of the ESR signal without oxidation of the manganese ion.
Evidence by Gibbons et al. (1976) suggests that oxidation of manganese occurs in the body. It was observed that human ceruloplasmin led to the oxidation of Mn(II) to Mn(III) in vitro, and although the process was not studied in vivo, it is a likely mechanism for manganese oxidation in the blood. These authors also noted that manganese oxidation led to a shift in manganese binding in vitro from α2-macroglobulin to transferrin and that in vivo clearance of Mn(II)-α2-macroglobulin from cows was much more rapid than the clearance of Mn(III)-transferrin (Gibbons et al. 1976). This suggests that the rate and extent of manganese reduction/oxidation reactions may be important determinants of manganese retention and toxicity in the body.
As demonstrated in a study by Komura and Sakamoto (1991), tissue levels of manganese in rats were affected by the form in which the manganese was administered in the diet; levels of manganese were significantly higher in animals fed manganese acetate or manganese carbonate than in animals fed manganese chloride or manganese dioxide.
Reaney et al. (2006) compared brain concentrations of manganese, dopamine, and gamma amino butyric acid in female retired breeder Long Evans rats exposed to cumulative intraperitoneal doses of 0, 30, or 90 mg manganese/kg of Mn(II) chloride or Mn(III) pyrophosphate. Rats were given intraperitoneal doses of 0, 2, or 6 mg manganese/kg, 3 times/week for 5 weeks. In Mn(III)-treated rats, brain manganese concentrations (analyzed in the striatum, globus pallidus, thalamus, and cerebrum regions) and blood concentrations were higher than brain concentrations in Mn(II)-treated rats. The only other marked changes in end points between the two treatment groups was that the highest Mn(III) exposure group showed a 60% increased dopamine level in the globus pallidus (compared with controls), whereas the comparably treated Mn(II) rats showed a 40% decrease in globus pallidus dopamine level. These results suggest that manganese valence state can influence tissue toxicokinetic behavior, and possibly toxicity.
MMT. Following intravenous administration in the male rat, MMT was metabolized to hydroxylmethylcyclopentadienyl manganese tricarbonyl (CMT-CH2OH) and carboxycyclopentadienyl manganese tricarbonyl (CMT-COOH), both of which are present in urine (Hanzlik et al. 1980a). Metabolites are also present in the bile, as indicated by the fecal recovery of 3H from the ring structure in MMT following intravenous or intraperitoneal administration of radiolabeled compound to rats (Hanzlik et al. 1980a, 1980b). After intravenous dosing of MMT in rats, 11% of the radiolabel was recovered in feces within 30 minutes (Hanzlik et al. 1980b). These metabolites have not been characterized; however, the administration of phenobarbitol to the rat doubled the biliary excretion of the metabolite (Hanzlik et al. 1980a).
In vitro studies showed that rat liver microsomes activated with NADPH and molecular oxygen metabolized MMT (Hanzlik et al. 1980b). Preliminary studies with pooled liver microsomes from 5 to 6 normal or phenobarbital-induced rats showed that reaction rates of metabolism were linear for the first 20 minutes. MMT and aminopyrine, a positive control compound that is metabolized exclusively by cytochrome P450, showed parallel responses to changes in incubation conditions (i.e., NADPH dependence, inhibition by carbon monoxide, induction by phenobarbital). Liver microsomes metabolized MMT with an estimated KM of 78 μM and a Vmax of 3.12 nmol/mg protein/minute. When the studies were done with liver microsomes from phenobarbital-treated rats, the KM remained the same, but the Vmax doubled (Hanzlik et al. 1980b). Lung microsomes were equally capable of metabolizing MMT, but phenobarbital induction did not enhance the response.
In humans, an infusion of the clinical dose of MnDPDP (5 μmol/kg or 0.25 mg/kg) is rapidly dephosphorylated to manganese dipyridoxyl monophosphate (MnDPMP). This metabolite has been measured in human blood as quickly as 18 minutes after the beginning of infusion of the contrast agent, and is still measurable 1.3 hours after the start of the infusion (Toft et al. 1997a). MnDPMP was not observed in the blood after the first 18 minutes. The monophosphate is then fully dephosphorylated to manganese dipyridoxyl ethylenediamine (MnPLED); this compound has been isolated in blood from 18 minutes after the start of an infusion until 40 minutes after the start. Transmetallation of either MnDPDP, MnDPMP, or MnPLED with zinc can occur, forming ZnDPDP, ZnDPMP, or ZnPLED. ZnDPDP has been identified in the bloodstream during the first 18 minutes of an infusion of 0.25 mg manganese/kg as MnDPDP. ZnDPMP has been detected in the blood from 18 to 40 minutes following the start of the infusion, and ZnPLED has been measured in the blood from 18 minutes to 8.33 hours following the start of the infusion. The major metabolite detected in urine was ZnPLED (Toft et al. 1997a). Figure 3-4 depicts the metabolism of mangafodipir in the human.
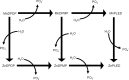
Figure 3-4
Metabolism of MnDPDP. Source: Toft et al. 1997c
To study mangafodipir metabolism in the dog, Toft et al. (1997c) injected three male and female beagles with 0.55, 1.7, or 5.5 mg manganese/kg and took timed blood samples post-dosing to analyze for the presence of metabolites. Mangafodipir was rapidly metabolized by dephosphorylation and transmetallation at all three doses. After infusion with 0.55 mg/kg, MnPLED was the primary metabolite observed in the bloodstream 1 minute after the end of the infusion period, and MnDPDP was present at a concentration lower than the five metabolites. At 30 minutes post-dosing, ZnPLED was the main metabolite. However, at 5.5 mg/kg, MnPLED was the main metabolite at all sampling times (1, 5, and 30 minutes). The authors estimated that the ratios of manganese metabolites to zinc metabolites were 1, 2, and 3.5 at doses of 0.55, 1.7, or 5.5 mg manganese/kg, respectively; these data are consistent with the authors’ hypothesis that the limited availability of free or loosely bound plasma zinc governs the initial transmetallation reaction (Toft et al. 1997c).
In vitro experiments with radiolabeled MnDPDP and whole blood or plasma from human donors indicate that mangafodipir undergoes a rapid transmetallation with zinc that is nearly complete within 1 minute after the start of incubation, followed by a relatively slow dephosphorylation process. The primary metabolite after a 90-minute incubation of whole blood with MnDPDP was MnDPMP, followed by CaDPDP/DPDP, Mn(III)DPDP (suggested as an artifact due to high pH and oxygen), and MnPLED. Experiments using 14C-DPDP indicate that this chelate cannot enter red blood cells; therefore, the zinc contained within the cells is unavailable for binding to this compound. Binding of manganese ion to serum proteins was observed as well, indicating that dissociation of the metal from the chelate had occurred during incubation (Toft et al. 1997b).
3.4.4. Elimination and Excretion
In humans, absorbed manganese is removed from the blood by the liver where it conjugates with bile and is excreted into the intestine. Biliary secretion is the main pathway by which manganese reaches the intestines where most of the element is excreted in the feces (Bertinchamps et al. 1965; Davis et al. 1993; Malecki et al. 1996). However, some of the manganese in the intestine is reabsorbed through enterohepatic circulation (Schroeder et al. 1966).
Small amounts of manganese can also be found in urine, sweat, and milk (EPA 1993b). Urinary excretion of manganese by healthy males was 7.0 nmole/g creatinine (7.0 nmole=385 ng=0.385 μg) (Greger et al. 1990). Similarly, urinary manganese excretion by women was 9.3 nmole/day. Moreover, urinary excretion of manganese was not responsive to oral intake of manganese (Davis and Greger 1992). Dorner et al. (1989) showed that some infants fed breast milk and formula suffered negative manganese balances due to high fecal excretion. However, animal studies indicate that in the young, excretion is not well-developed and may result in increased retention of the element. For example, in mice, rats, and kittens, there is an almost complete absence of excretion during the neonatal period (Cotzias et al. 1976). However, data in neonatal rats indicate that manganese retention rates decrease to rates observed in adult animals. This is indirect evidence that excretion may mature during the end of the neonatal period though the exact time frame across species is unknown.
3.4.4.1. Inhalation Exposure
In humans who inhaled manganese chloride or manganese tetroxide, about 60% of the material originally deposited in the lung was excreted in the feces within 4 days (Mena et al. 1969). Chronically exposed male workers were reported to have urine manganese levels that were significantly higher than unexposed persons; for example, male foundry workers had a mean manganese level of 5.7 μg/L compared to 0.7 μg/L in unexposed controls (Alessio et al. 1989). Other studies have reported significantly increased levels of urinary manganese in men occupationally exposed to airborne manganese dusts and fumes (Lucchini et al. 1995; Roels et al. 1987a, 1992). Mergler et al. (1994) did not report a significant difference in urinary manganese levels between the exposed and control groups in their occupational study. The differences in urinary excretion may be due to differences in duration or extent of exposure. A listing of these occupational studies that measured exposure levels of manganese and the resultant levels of the metal in biological samples is provided in Table 3-13.
Table 3-13
Levels of Manganese in Exposed and Non-Exposed Workers.
Rats exposed to either manganese chloride or manganese tetroxide by intratracheal instillation excreted about 50% of the dose in the feces within 3–7 days (Drown et al. 1986). Monkeys exposed to an aerosol of 54MnCl2 excreted most of the manganese, with a half-time of 0.2–0.36 days (Newland et al. 1987). However, a portion of the compound was retained in the lung and brain. Clearance of this label was slower, occurring with half-times of 12–250 days. These data do not provide information on how much of the manganese excreted in the feces after inhalation exposure was first absorbed and then excreted via the bile versus the amount simply transported directly from the lung to the gastrointestinal tract where it may have been absorbed. In addition, because these investigators measured manganese using gamma spectrometry techniques, the relatively long elimination half-times from the brain may have been influenced by manganese present in skull bones. In monkeys exposed to 1.5 mg manganese/m3 manganese sulfate for 65 days, manganese concentrations were elevated (compared with air control values) in many brain regions and other tissues; 45 days following cessation of exposure, concentrations remained elevated in the olfactory cortex, globus pallidus, putamen, pituitary gland, and blood, but returned to air control values by 90 days after exposure (Dorman et al. 2006a). Based on these data, Dorman et al. (2006a) calculated elimination half-lives of about 15–16 days for the globus pallidus and putamen, suspected neurotoxicity targets of manganese.
Rat studies have demonstrated that urinary excretion of manganese 1 day following inhalation exposure was increased 200- and 30-fold when the animals were treated with the chelating agents 1,2-cyclohexylene-aminetetraacetic acid (CDTA) and diethylene triamine pentaacetic acid (DTPA), respectively, but fecal excretion was not altered (Wieczorek and Oberdörster 1989b).
No studies were located regarding excretion of manganese in either humans or animals following inhalation exposure to organic manganese.
3.4.4.2. Oral Exposure
Humans who ingested tracer levels of radioactive manganese (usually as manganese chloride) excreted the manganese with whole-body retention half-times of 13–37 days (Davidsson et al. 1989a; Mena et al. 1969; Sandstrom et al. 1986). The route of manganese loss was not documented, but was presumed to be mainly fecal after biliary excretion. Serum manganese concentrations in a group of healthy men and women in Wisconsin were 1.06 and 0.86 μg/L, respectively (Davis and Greger 1992; Greger et al. 1990). Urinary excretion of manganese by men was 7.0 nmole/g creatinine (Greger et al. 1990). Similarly, urinary manganese excretion of women was 9.3 nmole/day. Moreover, urinary excretion of manganese was not responsive to oral intake of manganese (Davis and Greger 1992).
In a more recent study, young rats fed 45 mg manganese/kg/day were found to absorb 8.2% of the manganese ingested and to lose approximately 37% of the absorbed manganese through endogenous gut secretions (Davis et al. 1993).
The daily excretion of manganese from mice ingesting 11 mg manganese/kg as MMT in their daily diet was 5.4% of their daily intake (Komura and Sakamoto 1992b). In a comparison of plasma manganese kinetics following oral administration of MMT or manganese chloride in male rats, MMT-derived manganese was eliminated extremely slowly, having an average elimination half-time of 55.2 hours, compared with 4.56 hours for manganese chloride (Zheng et al. 2000). Rats receiving MMT showed an apparent oral clearance (CL/F) of 0.09 L.hours−1.kg−1, which was about 37-fold less than the oral clearance of manganese chloride (CL/F = 3.2 L.hours−1.kg−1). Accordingly, the AUC in MMT rats was about 37-fold higher than that in manganese chloride rats who received equivalent dose of manganese. A gender difference in manganese toxicokinetics following oral MMT exposure was also observed; female rats showed higher mean AUC and longer half times of plasma manganese than male rats (93.1 versus 51.8 mM hours and 68.4 versus 42.0 hours, respectively (Zheng et al. 2000).
No other studies were located regarding excretion of manganese from organic manganese compounds in either humans or animals.
3.4.4.3. Dermal Exposure
No studies were located regarding excretion of inorganic or organic manganese in humans or animals after dermal exposure to manganese.
3.4.4.4. Other Routes of Exposure
No studies were located regarding excretion of manganese by humans after exposure to inorganic manganese via other routes of exposure.
Rats exposed to manganese chloride by intravenous injection excreted 50% of the dose in the feces within 1 day (Klaassen 1974) and 85% by day 23 (Dastur et al. 1971), indicating that biliary excretion is the main route of manganese clearance. Only minimal levels were excreted in urine (>0.1% of the dose within 5 days) (Klaassen 1974). Direct measurement of manganese levels in bile revealed concentrations up to 150-fold higher than in plasma, indicating the existence of either an active transport system (Klaassen 1974) or some sort of trapping mechanism (Tichy and Cikrt 1972). Based on the difference in blood levels following portal or femoral injection, Thompson and Klaassen (1982) estimated that about 33% of the manganese burden in blood is removed in each pass through the liver. Apparently, some manganese can cross directly from the blood to the bile (Bertinchamps et al. 1965; Thompson and Klaassen 1982), but most appears to be secreted into the bile via the liver (Bertinchamps et al. 1965).
The chemical state of manganese in bile is not known, but a considerable fraction is bound to bile components (Tichy and Cikrt 1972). This material is apparently subject to enterohepatic recirculation, since biliary manganese is reabsorbed from the intestine more efficiently than free Mn(II) (Klaassen 1974). The amount of manganese that contributes to total body burden following reabsorption from enterohepatic recirculation is not known.
While biliary secretion appears to be the main pathway by which manganese is excreted into the intestines, direct transport from blood across the intestinal wall may also occur (Bertinchamps et al. 1965; Garcia-Aranda et al. 1984). The relative amount of total excretion attributable to this pathway was not quantified by Bertinchamps, but it appears to be only a fraction of that attributable to biliary secretion (Bertinchamps et al. 1965).
Manganese originating from mangafodipir administered at clinical (0.25 mg/kg) and more than twice the clinical dose (0.55 mg/kg) is primarily excreted in the feces via the bile in both humans and animals (Grant et al. 1994; Hustvedt et al. 1997; Toft et al. 1997a; Wang et al. 1997). In contrast to the chelate, DPDP, manganese is incompletely cleared from the body 24 hours after administration, and roughly 7– 8% of a dose is still retained in the body after 1 week (Hustvedt et al. 1997).
3.4.5. Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models
Physiologically based pharmacokinetic (PBPK) models use mathematical descriptions of the uptake and disposition of chemical substances to quantitatively describe the relationships among critical biological processes (Krishnan et al. 1994). PBPK models are also called biologically based tissue dosimetry models. PBPK models are increasingly used in risk assessments, primarily to predict the concentration of potentially toxic moieties of a chemical that will be delivered to any given target tissue following various combinations of route, dose level, and test species (Clewell and Andersen 1985). Physiologically based pharmacodynamic (PBPD) models use mathematical descriptions of the dose-response function to quantitatively describe the relationship between target tissue dose and toxic end points.
PBPK/PD models refine our understanding of complex quantitative dose behaviors by helping to delineate and characterize the relationships between: (1) the external/exposure concentration and target tissue dose of the toxic moiety, and (2) the target tissue dose and observed responses (Andersen and Krishnan 1994; Andersen et al. 1987). These models are biologically and mechanistically based and can be used to extrapolate the pharmacokinetic behavior of chemical substances from high to low dose, from route to route, between species, and between subpopulations within a species. The biological basis of PBPK models results in more meaningful extrapolations than those generated with the more conventional use of uncertainty factors.
The PBPK model for a chemical substance is developed in four interconnected steps: (1) model representation, (2) model parameterization, (3) model simulation, and (4) model validation (Krishnan and Andersen 1994). In the early 1990s, validated PBPK models were developed for a number of toxicologically important chemical substances, both volatile and nonvolatile (Krishnan and Andersen 1994; Leung 1993). PBPK models for a particular substance require estimates of the chemical substance-specific physicochemical parameters, and species-specific physiological and biological parameters. The numerical estimates of these model parameters are incorporated within a set of differential and algebraic equations that describe the pharmacokinetic processes. Solving these differential and algebraic equations provides the predictions of tissue dose. Computers then provide process simulations based on these solutions.
The structure and mathematical expressions used in PBPK models significantly simplify the true complexities of biological systems. If the uptake and disposition of the chemical substance(s) are adequately described, however, this simplification is desirable because data are often unavailable for many biological processes. A simplified scheme reduces the magnitude of cumulative uncertainty. The adequacy of the model is, therefore, of great importance, and model validation is essential to the use of PBPK models in risk assessment.
PBPK models improve the pharmacokinetic extrapolations used in risk assessments that identify the maximal (i.e., the safe) levels for human exposure to chemical substances (Andersen and Krishnan 1994). PBPK models provide a scientifically sound means to predict the target tissue dose of chemicals in humans who are exposed to environmental levels (for example, levels that might occur at hazardous waste sites) based on the results of studies where doses were higher or were administered in different species. Figure 3-5 shows a conceptualized representation of a PBPK model.
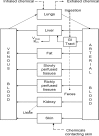
Figure 3-5
Conceptual Representation of a Physiologically Based Pharmacokinetic (PBPK) Model for a Hypothetical Chemical Substance. Note: This is a conceptual representation of a physiologically based pharmacokinetic (PBPK) model for a hypothetical chemical substance. (more...)
If PBPK models for manganese exist, the overall results and individual models are discussed in this section in terms of their use in risk assessment, tissue dosimetry, and dose, route, and species extrapolations.
PBPK models for manganese are discussed below, including descriptions of an initial conceptual PBPK model for manganese (Andersen et al. 1999) and the development of whole-body adult rat and monkey PBPK models (Nong et al. 2008, 2009; Teeguarden et al. 2007a, 2007b, 2007c), a PBPK model for manganese transport from the olfactory mucosa to the striatum (Leavens et al. 2007), a whole-body PBPK model for gestation and lactation in the rat (Yoon et al. 2009a, 2009b), and human whole-body PBPK models for adults and for fetal and neonatal exposures (Schroeter et al. 2011; Yoon et al. 2011).
Initial Conceptual PBPK Model for Manganese (Andersen et al. 1999). A qualitative PBPK model for manganese disposition in humans and animals was initially developed by Andersen et al. (1999). This model represented the current understanding of manganese nutrition and toxicology, and because several data gaps existed concerning manganese pharmacokinetics, this model was anticipated to change with time (Andersen et al. 1999). The model, shown in Figure 3-6, was not designed to be quantitative in nature. The authors indicated that several data gaps prevented such an evaluation of manganese uptake, distribution, and excretion. For instance, there were inadequate data concerning oxidation rates for manganese in blood, uptake rates of protein-bound forms by the liver, neuronal transfer rates within the central nervous system, and quantitative data on systems controlling manganese uptake via the intestines and liver (such as transport mechanism in the intestines) (Andersen et al. 1999). Andersen et al. (1999) suggested that an approach to setting acceptable exposure levels for an essential, but neurotoxic, nutrient such as manganese could be based on predicting exposure levels by any route that would increase brain manganese concentrations to a small fraction (e.g., 10–25%) of the variation observed in the general human population. Reliable and validated multiple-route PBPK models for multiple species, including humans, are needed to take this approach to setting acceptable exposure levels. Efforts to develop such models in rats, monkeys, and humans have been recently described (Leavens et al. 2007; Nong et al. 2009, 2008; Schroeter et al. 2011; Teeguarden et al. 2007a, 2007b, 2007c; Yoon et al. 2011, 2009a, 2009b).
Figure 3-6
Qualitative PBPK Model for Manganese. Source: Andersen et al. 1999
Whole-Body Rat PBPK Models (Nong et al. 2008; Teeguarden et al. 2007a, 2007b, 2007c). Utilizing pharmacokinetic and tissue manganese concentration data from several published studies of manganese in rats and mice, recent efforts have developed PBPK models for manganese in rats that include processes involved in homeostatic regulation of tissue levels of manganese taken up by ingestion and by inhalation (Nong et al. 2008; Teeguarden et al. 2007a, 2007b, 2007c). Two PBPK model structures were developed and evaluated for their ability to account for kinetics of manganese in the liver and brain striatum following inhalation and dietary administration of soluble forms of inorganic manganese. The data sets used to evaluate the models were: (1) tissue manganese concentrations in rats receiving diets containing 2, 10, or 100 ppm manganese for 13 weeks and elimination kinetics for an intravenous tracer dose of 54Mn-manganese chloride (Dorman et al. 2001b); (2) tissue manganese concentrations and tracer kinetics in rats fed a 100-ppm diet and exposed to 0, 0.03, 0.3 or 3 mg manganese/m3 manganese sulfate 6 hours/day for 14 consecutive days (Dorman et al. 2001a); and (3) tissue manganese concentrations (sampled at 0, 45, and 90 days after exposure) in rats fed a 10-ppm diet and exposed to 0, 0.1, or 0.5 mg manganese/m3 for 6 hours/day, 5 days/week for 90 days (Dorman et al. 2004b).
Structures of the models are shown in Figure 3-7. Model A is based on regulation of tissue concentrations by simple partitioning with slow inter-compartmental transfer from free manganese in tissues to deeper tissue stores of manganese (“diffusion-controlled tissue partitioning”; Nong et al. 2008; Teeguarden et al. 2007a, 2007b, 2007c). Model B features saturable binding of manganese in liver and brain with equilibrium binding constants defined by slow association and dissociation rate constants (Nong et al. 2008). Both models contain a submodel for deposition and absorption in the nose and lung shown schematically in Figure 3-8 (Teeguarden et al. 2007c).

Figure 3-7
Schematic Structures of Nong et al. (2008) PBPK Models A and B for Manganese in CD Rats*. *Values and descriptions of model parameters are in Tables 3-14, 3-15, and 3-16. Source: Nong et al. 2008
Figure 3-8
Schematic of Models for Nasopharyngeal and Lung Deposition of Manganese and Transport to Blood in the Nong et al. (2008) PBPK Models A and B for Manganese in CD Rats. Source: Teeguarden et al. 2007c
Nong et al. (2008) Model A Description and Development. Model A contains six compartments: the respiratory tract, brain striatum, liver, kidneys, bone, and slowly perfused tissues (Figure 3-7). The respiratory tract is divided into two subcompartments: nasopharyngeal tissues and lung (Figure 3-8). Table 3-14 lists parameters of Model A as described by Teeguarden et al. (2007c). Each of the six compartments is subdivided into a conventional flow-limited compartment connected to the blood and tissue stores that are not readily equilibrated with blood moving through the tissue compartment. First-order clearance rate constants (e.g., kInBrnC and koutBrnC) determine the transfer of manganese from the flow-limited compartment to the deep compartment of each tissue. The clearance rate constants, together with the blood flow to the tissue (e.g., QBrnC) and the tissue partition coefficients (e.g., PBrn), determine the steady-state concentrations and the rate of change manganese in each of the tissues, according to differential equations that are described in detail by Teeguarden et al. (2007c).
Table 3-14
Parameter Values in the Teeguarden et al. (2007c) PBPK Model for Manganese in CD Rats (Nong et al. 2008) Model A.
Physiological parameters were taken from the literature and included values for blood flows, organ volumes, and food intake rate (Table 3-14). The initial (basal) concentrations of manganese in the tissues (Table 3-14) were taken from literature values as described by Teeguarden et al. (2007c). Remaining model parameters were estimated by fitting the model to experimental data. Fractions of manganese in the shallow versus deep compartments of each tissue (e.g., fBrn and FDBrn, Table 3-14) were calibrated to obtain the best fit to intraperitoneal 54Mn clearance data collected by Furchner et al. (1966). Partition coefficients (e.g., PBrn, Table 3-14) and clearance rate constants into and out of deep compartments (e.g., kInBrnC, kOutBrnC) were calibrated with 54Mn kinetic data collected by Furchner et al. (1966) and steady-state tissue manganese concentration data collected by Wieczorek and Oberdörster (1989c). The fraction of manganese absorbed from the gut (FDietUp) was assumed to be 0.8%. The rate of biliary excretion from liver (kBile0C) was determined by matching the rate of manganese excreted from liver against the amount of manganese taken up from the diet, while maintaining steady-state levels of manganese in all tissues and matching the turnover of 54Mn for each tissue (Teeguarden et al. 2007c). For inhaled manganese, fractional depositions in the nasopharyngeal (fDepNP = 0.2), tracheobronchial (fDepTB = 0.21), and pulmonary (fDeppu = 0.07) regions were taken from the EPA (1994a) respiratory tract deposition model for 1.1-µm aerosols. The model assumed that deposited aerosols dissolved immediately and that there was no clearance from the airway lumen to the gut via mucociliary transport; this assumption is valid for soluble manganese forms such as manganese chloride and manganese sulfate, but would not be valid for less-soluble forms of manganese such as manganese phosphate (Nong et al. 2008; Teeguarden et al. 2007c).
Nong et al. (2008) described further refinements to model A parameters shown in Table 3-15. Daily manganese dietary intake (FDietUp) and biliary elimination rate constants (kBileC) were first calibrated for different levels of manganese in the diet (2, 10, 100, and 125 ppm; Table 3-15) by fitting the model to the observed steady-state tissue manganese concentration data for rats exposed to 2, 10, or 100 ppm manganese in the diet for 13 weeks (Dorman et al. 2001b). After this refinement, clearance rates for the liver and brain striatum (kIn and kOut values shown in Table 3-15) were refined by fitting the model to tissue manganese concentration data from the 14-day inhalation study by Dorman et al. (2001a).
Table 3-15
Refined Parameter Values in Nong et al. (2008) Model A.
Nong et al. (2008) Model B Description and Development. Model B contains a similar structure to Model A, except that manganese concentrations in the liver and brain striatum are dependent on capacity-limited binding of manganese (Figure 3-7). In addition, uptake from striatal blood to striatal tissues is described with diffusion terms (PA12 and PA21, Figure 3-7). The diffusion terms were included to account for observations of preferential increases in some brain regions compared with other tissues, such as liver or blood, following inhalation exposure to manganese (see Dorman et al. 2006a for review). The diffusion terms are thought to reflect movement of manganese across the blood-brain barrier (Nong et al. 2008). In Model B, the total amounts of manganese in the liver and brain striatum tissues are dependent on concentrations of free circulating manganese, the binding capacity of the tissue, and the concentrations of bound manganese in tissue stored (Nong et al. 2008). Differential equations to describe changes (with time) in amounts of free or bound manganese in the liver and the brain striatum are described in detail by Nong et al. (2008). Table 3-16 lists binding rate constants (e.g., kaBrnC, kdBrnC), binding capacities (Bmax,Brain, Bmax.Liver), brain diffusion constants (PA12 and PA21), and partition coefficients in Model B. Liver and brain striatum binding capacity levels were first determined by fitting the model to steady-state tissue concentration data from the 13-week dietary study by Dorman et al. (2001b), using starting values for the tissue binding parameters that were estimated based on clearance rate values (kIn and kout) for liver and brain striatum in Model A. Tissue binding parameters (e.g., kaBrnC, kdBrnC) and brain diffusion constants (PA12 and PA21) were then refined by fitting the model to the 14-day-inhalation tissue concentration data from Dorman et al. (2001a).
Table 3-16
Parameter Values in Nong et al. (2008) Model B.
Evaluation of Nong et al. (2008) Models A and B. Nong et al. (2008) compared the abilities of Models A and B to predict: (1) whole-body elimination kinetics of 54Mn in rats fed a 100-ppm diet for 13 weeks (data from Dorman et al. 2001b); (2) liver and brain striatum manganese concentration data in rats exposed to 0.03, 0.3, or 3 mg manganese/m3 for 6 hours/day for 14 consecutive days (Dorman et al. 2001a); (3) whole-body elimination kinetics of 54Mn in rats following 14-day inhalation exposure to 3 mg manganese/m3; and (4) liver and brain striatum manganese concentrations in rats during and following a 90-day inhalation exposure period to 0.1 or 0.5 mg manganese/m3 (Dorman et al. 2004b). Both models adequately predicted observed 54Mn elimination kinetics data, but Model B much more accurately predicted liver and brain striatum manganese concentration data during and following 14- or 90-day inhalation exposures. Model A consistently overestimated liver and brain striatum manganese concentration, particularly at concentrations of 0.1, 0.3, or 0.5 mg manganese/m3 (as shown in Figures 4 and 7 of Nong et al. 2008). Nong et al. (2008) concluded that the evaluation of the models “highlighted the importance of tissue binding in maintaining relatively constant tissue concentrations across a wide range of inhaled concentrations.”Nong et al. (2008) mentioned that the next steps in model development would be to extend tissue binding in Model B to all other tissues in the models for which appropriate data are available for calibrating tissue-specific binding rate constants.
PBPK Model for Manganese Transport from the Olfactory Mucosa to Striatum (Leavens et al. 2007).
Leavens et al. (2007) developed a pharmacokinetic model describing the olfactory transport and blood delivery of manganese to the striatum in rats following acute inhalation exposure to manganese chloride or manganese phosphate. Figure 3-9 shows the structure of the model, which presumes that manganese undergoes axonal transport from the olfactory mucosa (OM) to the olfactory bulb (OB), followed by serial transport to the olfactory tract and tubercle (OTT) and then to the striatum (S). Tables 3-17 and 3-18 list values of the model parameters for soluble manganese chloride and relatively insoluble manganese phosphate, respectively. Each of the compartments in the model (containing a left and right nasal cavity) is connected by blood and each is comprised of pools of free and bound manganese. The rates of transport between tissue compartments and between bound and free pools are modeled as first-order transport processes. Tables 3-17 and 3-18 show measured values for compartment volumes, values for blood clearance into olfactory compartments (e.g., ClOM/blood), values for rate constants for efflux from compartments to blood (e.g., kblood/OM), values for transport rate constants between compartments (e.g., kOM/el), and binding rate constants in the olfactory compartments (e.g., OM free to bound, kOM/f.b and OM bound to free, kOM/b.f). Equations for mass balance, clearance, and free concentrations of manganese for each of the compartments are described in detail by Leavens et al. (2007).
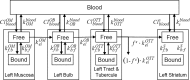
Figure 3-9
Schematic of the Leavens et al. (2007) Model to Describe Olfactory and Blood Delivery of Manganese to the Left Side of the Brain Isilateral to the Olfactory Mucosa (OM) in the Left Nasal Cavity*. *The model structure for the right side is identical. Values (more...)
Table 3-17
Parameter Values for Manganese Chloride in the Leavens et al. (2007) PBPK Model for Olfactory Transport of Manganese in Rats.
Table 3-18
Parameter Values for Manganese Phosphate in the Leavens et al. (2007) PBPK Model for Olfactory Transport of Manganese in Rats.
Model parameters were estimated by optimization procedures using kinetics data from rats exposed nose-only for 90 minutes to 54Mn-manganese chloride (Brenneman et al. 2000) or 54Mn-manganese phosphate (Dorman et al. 2002a). In each experiment, one group was exposed with both nostrils unplugged, while a second group was exposed with the right nostril plugged. Blood concentrations were not measured in either of these studies, but 54Mn concentrations in the kidney, liver, and pancreas were measured and reported. The mean concentration in these three organs is used to represent blood concentration in the model, and the data were used to obtain parameters for equations describing first-order absorption and elimination into a single compartment; values for the parameters under plugged and unplugged conditions, obtained through model optimization procedures, are listed in Table 3-19. The optimized model was used to predict the percentage of 54Mn that was transported into each compartment either via direct olfactory transport or blood delivery. For manganese chloride, olfactory transport was predicted to deliver >97–99% of the tracer in the left or right olfactory bulbs, 40–76% of the tracer in the left or right olfactory tract and tubercle, and only 4–8% of the tracer in the left or right striatum under plugged or unplugged conditions. For manganese phosphate, the respective predictions were 38–59% in the olfactory bulbs, 86–90% in the olfactory tract and tubercle and 77–83% in the striatum. Leavens et al. (2007) cautioned against the predictions for the striatum, since the model overpredicted striatum concentrations at the later time points for the plugged exposures to manganese chloride or manganese phosphate and the unplugged exposures to manganese phosphate (Figures 4–7 in Leavens et al. 2007).
Table 3-19
Parameter Values for Describing Blood Concentrations in the Leavens et al. (2007) PBPK Model for Olfactory Transport of Manganese in Rats.
Whole-body Rat and Monkey PBPK Models (Nong et al. 2009). Nong et al. (2009) modified the Nong et al. (2008) rat Model B by adding: (1) saturable binding to all tissues with association and dissociation rate constants; (2) preferential accumulation of manganese in brain regions, such as the striatum and globus pallidus; (3) respiratory and olfactory uptake based on regional particle deposition within the respiratory tract; (4) inducible biliary excretion; and (5) variable dietary absorption depending on the manganese content in food substances. The model structure contains compartments for liver, bone, lung, nasal cavity, blood, and brain (cerebellum, olfactory bulb, striatum and pituitary and manganese intakes from the diet and by inhalation. In the model, inhaled manganese is absorbed following deposition of particles on the nasal and lung epithelium. In the nose, absorbed free manganese is largely absorbed into the systemic blood and a smaller portion is transported directly into the olfactory bulb. Free manganese is transported in the blood and stored as bound manganese in each tissue, as determined by a binding capacity (Bmax) and association and dissociation rate constants for each tissue (ka, kd; Figure 3-10). Each compartment contains influx and efflux diffusion rate constants (kin and kout) allowing for differential increases for different tissues (Figure 3-10). Differential equations to describe changes in amounts of free or bound manganese in the tissue compartments, as well as numerical values of final model parameters, are described in detail by Nong et al. (2009). Model parameters were first calibrated with steady-state tissue concentrations measured by Dorman et al. (2001b) in rats fed two diets differing in manganese concentrations (10 and 125 ppm) to arrive at dose-dependent fractional gastrointestinal absorption and biliary excretion. Model parameters (including tissue:blood partition coefficients, binding rate constants and influx and efflux diffusional rate constants) were then refined by fitting the model to the 14-day inhalation tissue concentration data from Dorman et al. (2001a), including data for striatum, cerebellum, and olfactory bulb. Model simulations were consistent with empirical observations of brain tissue concentrations following 14-day inhalation exposures to 0.03, 0.3, or 3 mg manganese/m3 collected by Dorman et al. (2001a). The model was used to predict tissue concentrations following 90-day exposures of rats and compared with empirical tissue concentrations measured in two different 90-day inhalation studies (Dorman et al. 2004; Tapin et al. 2006). Model predictions for the highest exposure concentration (3 mg/m3) overestimated measured tissue concentrations, but when the rate constant for biliary excretion was increased about 2-fold, better fit to the 90-day high-concentration data was obtained.
Figure 3-10
Physiologically Based Pharmacokinetic Model Structure Describing Tissue Manganese Kinetics in Adult Rats*. *Schematic A is the structure of the full model. Schematic B describes substructures for deposition and absorption in the nose and lung. Manganese (more...)
To develop the monkey model, physiological parameters were scaled to adult monkey values and model parameters were adjusted to fit the model to manganese tissue concentrations collected by Dorman et al. (2006a) from monkeys exposed by inhalation to manganese sulfate aerosols at concentrations up to 1.5 mg/m3 for 90 days. Simulations from the final monkey model for 90-day inhalation exposure to 1.5 mg/m3 followed by 80 days after exposure were consistent with time-dependent rises in measured concentrations in liver, pituitary, globus pallidus during exposure, and post-exposure declines in pituitary and globus pallidus measured concentrations, but the model overestimated measured concentrations in the lung (during and after exposure) and in the liver after exposure.
Whole-body PBPK Model in Pregnant Rats and Fetuses (Yoon et al. 2009a). The adult rat model developed by Nong et al. (2009) was extended to develop a PBPK model that would predict fetal manganese dose and manganese disposition in rat dams and fetuses following maternal exposures (dietary and inhalation) to manganese. The tissue concentration data used to parameterize the model were those for rats exposed to 10 ppm in the diet and exposed by inhalation to manganese sulfate aerosols at 0, 0.05, 0.5, or 1 mg manganese/m3, starting from 28 days before breeding and continuing through a 14-day mating period until GD 20 (Dorman et al. 2005a, 2005b). The model structure for the dams contains compartments as in the Nong et al. (2009) adult rat model, plus a placenta through which fetal exposure occurs via two separate pathways operating simultaneously: a bidirectional diffusion process described by a maternal to fetal diffusion rate (ktrans1) and a fetal blood to placenta diffusion rate constant (ktrans 2C) and a saturable, active transport, maternal to fetal process described by a Vmax and Michaelis-Menten constant (Km) (see Figure 3-11). Compartments in the fetus included the lung, liver, brain blood, whole brain, bone, and rest of body, each with association and dissociation rate constants (Figure 3-11). Differential equations to describe changes in amounts of free or bound manganese in the tissue compartments, as well as numerical values of final model parameters, are described in detail by Yoon et al. (2009a). In general, parameters from the original adult model were modified to accommodate different life stages (i.e., pregnancy and fetal development), first using control group tissue concentration data to estimate gestation-specific parameters for dietary only exposure, and then refined by fitting to inhalation-exposure data for pregnancy and fetal development periods. Model simulations of maternal and fetal tissue concentrations on GD 20 were visually similar to empirical measurements made by Dorman et al. (2005a).

Figure 3-11
Model Structure for Simulating Manganese Exposure During Gestation in the Rat*. *Manganese is present in the body as either free (Mnf) or bound form (Mnb). Every tissue (dam and fetus) has a binding capacity (B) and dissociation rate constants (ka, kd). (more...)
Whole-body PBPK Model in Lactating Rat Dams and Fetuses (Yoon et al. 2009b). This model was developed in parallel to the development of the gestation and fetal rat model (Yoon et al. 2009a). The lactating maternal model had the same compartments as the Nong et al. (2009) adult rat model, plus a mammary gland compartment partitioned from the “rest of body” compartment in the adult model. The mammary gland compartment was assigned the same tissue binding parameters, maximum binding capacity, and partition coefficient as the “rest of body” compartment (see Figure 3-12). The model included a milk compartment described as a mass of manganese transferred from the dam to the nursing pup, with a variable rate of milk production over the lactational period (Figure 3-12). Manganese transfer from the mammary gland to milk was described as a first-order clearance process. In pups, daily dose was determined by three intake sources: diet, milk, and inhalation, and compartments were the same as those in the original adult rat model (see Figure 3-12). As described in more detail by Yoon et al. (2009b), the model incorporated time- and dose-dependent changes in the dams and offspring in manganese-specific kinetic parameters for specific tissues (e.g., maternal gastrointestinal uptake and biliary excretion, maximal binding capacities in growing pup compartments, and developmental changes in gastrointestinal uptake and biliary elimination), as well as in physiological parameters, such as maternal and fetal body weight, tissue volumes, and cardiac outputs. Manganese-specific kinetic parameters were calibrated using tissue concentration data collected by Dorman et al. (2005a, 2005b) from lactating rat dams and offspring exposed to 10 ppm manganese in the diet, and 0, 0.05, 0.5, or 1 mg manganese/m3 from PND 1 to 18 for dams and PND 19 for pups. The calibration process was described in more detail by Yoon et al. (2009b). Model simulations of tissue concentrations in rat dams (at the end of lactation) and offspring (at PND 19 at the end of exposure, and at PNDs 45 and 63) were visually similar to tissue concentrations reported by Dorman et al. (2005b). The model simulations and empirical results indicated that at the end of the inhalation exposure period (PND 19), concentrations in the striatum and olfactory bulb of offspring began to increase (compared with control values) when air concentrations exceeded 0.05–0.1 mg/m3; maternal concentrations in these brain regions began to increase at somewhat higher air concentrations between 0.1 and 0.3 mg/m3. These results indicate that at given air concentrations above about 0.05–0.1 mg/m3, brain concentrations in neonates may be elevated, compared with controls, to a greater degree than in lactating dams, but the age-specific difference in the tested air concentration range does not appear to be large. Yoon et al. (2009b) concluded that these results indicate that neonates are not at an especially increased risk to striatal manganese due to differences in pharmacokinetic factors.

Figure 3-12
Model Structure for Predicting Manganese Tissue Levels in Lactating Rat Dams and Pups*. *The diagram describes manganese kinetics in rat dams and pups. Manganese is present in the body as either free (Mnf) or bound form (Mnb). Every tissue (dams and pups) (more...)
Whole-body PBPK Model in Adult Monkeys and Humans (Schroeter et al. 2011). The PBPK model developed for monkeys (Nong et al. 2009) was scaled to humans to predict inhalation exposure conditions associated with increased brain manganese concentrations and extended to include intravenous, intraperitioneal, and subcutaneous exposure routes and a series of gastrointestinal compartments consistent with the physiology of manganese absorption and elimination (see Figure 3-13 for model structure). The models were extended to allow comparative analysis of kinetic data from studies of nonhuman primates and humans exposed by these routes to soluble, carrier-free, radiolabeled 54Mn. Modifications from the Nong et al. (2009) monkey model included adjustments of dissociation rate constants and maximal binding capacities so that each brain region contained about 60% bound manganese under diet-only exposure, refinement of diffusion parameters to simulate the rise in brain manganese concentrations during inhalation exposure and subsequent decline during post-exposure displayed by the data collected by Dorman et al. (2006a), and refinement of a dose-dependent influx term for the globus pallidus and pituitary regions. Physiological parameters in the human model were either scaled from the monkey model or obtained from the literature. Dietary absorption and biliary excretion were calibrated using human whole-body elimination kinetic data from earlier studies of humans given intravenous tracer amounts of 54Mn. Initially, diffusion rate constants and tissue binding capacities were scaled from monkey values, and association and dissociation rate constants were adjusted to attain 80% bound manganese in brain regions. Additional refinements to model parameters were necessary to maintain tissue levels within expected values with only dietary manganese exposure. More details concerning final parameters for the human and monkey models are provided by Schroeter et al. (2011). Model simulations adequately described whole-body elimination kinetic data for monkeys given 54Mn by intraperitoneal (Dastur et al. 1971), intravenous (Furchner et al. 1966), or oral administration (Furchner et al. 1966), fecal excretion data for monkeys following subcutaneous or inhalation exposures to 54Mn (Newland et al. 1987), and whole-body retention data in humans following intravenous injection (Mahoney and Small, 1968; Mena et al. 1967) or ingestion of 54Mn (Davidsson et al. 1988; Mahoney and Small 1968). Model simulations of manganese concentrations in the globus pallidus in adult humans with a normal diet following 90 days of inhalation exposure to air concentrations ranging from 0.0001 to >10 mg manganese/m3 indicated that concentrations increased slightly by about 5% over background levels at 0.1 mg/m3 and dramatically increased at higher air concentrations (see Figure 3-14).
Figure 3-13
Physiologically Based Pharmacokinetic Model Structure Describing Manganese Tissue Kinetics in Adult Monkeys and Humans*. *In each tissue department, the amount of bound manganese is in equilibrium with the assumed binding capacity (Btissue) and free manganese. (more...)

Figure 3-14
Simulated End-of-Exposure Tissue Total Manganese Levels in Rat Striatum and Monkey and Human Globus Pallidus*. The simulated rat striatal manganese levels are from Nong et al. (2009) and are compared with data (mean ± standard error) from Dorman (more...)
Whole-body PBPK Model in Humans during Gestation and Neonatal Periods (Yoon et al. 2011). Yoon et al. (2011) developed a series of PBPK models to describe manganese kinetics during fetal and neonatal development in humans and to predict internal manganese concentrations in the developing brain. The models were based on the basic structure of the rat gestation and lactation model developed by Yoon et al. (2009a, 2009b), with modifications based on cross-species extrapolations in developing monkey (Nong et al. 2009) and adult human models (Schroeter et al. 2011). The human models incorporated:
- pertinent physiological parameters in human females during gestation and lactation from previously published human pregnancy and lactation PBPK models;
- female-specific functional residual capacity, breathing frequency, and tidal volume in the adult human male model developed by Schroeter et al. (2011) describing manganese particle deposition;
- higher basal levels of absorption and biliary excretion of manganese in adult females compared with males;
- characteristics of the placental transfer of manganese in the rat model with parameter modifications based on observations of placental and fetal manganese concentrations in humans;
- a diffusional clearance from free manganese in mammary tissues to describe manganese milk secretion as in the Yoon et al. (2009b) rat model, with adjustment of the diffusion rate constant with data for human manganese milk concentrations during lactation from several published studies;
- higher fractional gut absorption of manganese in suckling neonates, compared with adults;
- inducible biliary excretion of manganese in neonates, at rates lower than in adults;
- transitions of neonatal characteristics of gut absorption and biliary excretion to those of adults;
- enhanced brain uptake of manganese during fetal and postnatal development; and
- adjustment of tissue binding parameters in fetal tissue to be consistent with published manganese concentration data from human fetal autopsy tissues and in neonatal tissues to be consistent with observed neonatal and adult human tissue concentration data.
More details on model structure, model equations, and model parameters and their development are provided by Yoon et al. (2011).
Model simulations of placental and fetal tissue manganese concentrations in the absence of airborne manganese were consistent with published data for humans with background air manganese exposure; with increasing air concentrations of manganese, placental and fetal globus pallidus concentrations began to rise at 0.01 mg/m3 (18 and 11% increase, respectively, over 0 mg/m3 values). Model simulations of human milk manganese concentrations at various lactation stages were consistent with published data for humans with background air manganese exposure; simulated milk concentrations rose with increasing air concentration, showing <10% increase at 0.01 mg/m3. Simulated average daily AUC for manganese concentrations in the globus pallidus of the fetus, suckling neonate, and 3-year-old child from manganese air concentrations increased beyond 10% of background concentrations in fetuses and 3-year-old children when air concentrations exceeded 0.01 mg/m3 (10 µg/m3) and in suckling neonates when air concentrations exceeded 0.001 mg/m3 (1 µg/m3) (Yoon et al. 2011).
3.5. MECHANISMS OF ACTION
3.5.1. Pharmacokinetic Mechanisms
Absorption. Manganese absorption occurs primarily through the diet; however, absorption via the lungs can be significant for occupationally exposed persons or for those exposed to excess levels of airborne manganese, such as downwind of a manganese point source. Manganese absorption through the gut may occur through a nonsaturable simple diffusion process through the mucosal layer of brush border membranes (Bell et al. 1989) or via an active-transport mechanism that is high-affinity, low-capacity, and rapidly saturable (Garcia-Aranda et al. 1983). Manganese particles that are too large to enter the alveoli (>10 microns in diameter) remain in the upper respiratory tract, where they are coughed up by mucociliary transport and swallowed. Differences in solubility of manganese compounds deposited in the alveolar regions may impact the rate at which manganese will be absorbed, but manganese is bioavailable when deposited in these regions (Drown et al. 1986).
Diets high in iron have been shown to suppress manganese absorption, and conversely, iron-poor diets increase manganese uptake (Lönnerdal 1997, Lönnerdal et al. 1994). Phosphorus (Wedekind et al. 1991) and calcium (Wilgus and Patton 1939) have also been found to decrease manganese uptake.
Distribution. Review articles by Aschner and Aschner (1991) and Aschner et al. (2005, 2007) summarize some of the available data regarding the distribution of manganese. Dietary manganese, thought to be absorbed as Mn(II), enters portal circulation from the gastrointestinal tract and is bound to α2-macroglobulin or albumin in the plasma. After delivery to the liver, the major portion of Mn(II) is secreted in the bile, but some may be oxidized by ceruloplasmin to Mn(III). The Mn(III) enters systemic circulation conjugated with plasma transferrin; once this complex enters a neuron, it dissociates, and from there, the manganese is transported to axon terminals. For example, Sloot and Gramsbergen (1994) observed that radiolabeled manganese injected into the striatum or substantia nigra of rat brain is transported in an anterograde direction through both γ-amino-butyric acid-producing striato-nigral and dopaminergic nigro-striatal fibers.
Other studies, however, argue for the transport of Mn(II) into the brain. For example, Murphy et al. (1991) measured the kinetics of manganese transport in the brains of adult male rats using a perfusion technique. The rats were infused with increasing concentrations of [54Mn]Cl2; blood and brain samples were analyzed for manganese at varying time points. The data indicated a saturable mechanism for transporting Mn(II) into the choroid plexus, and influx into the cerebral cortex was also near saturation at the highest plasma concentration of manganese used. Influx into other brain regions (e.g., caudate nucleus, hippocampus, hypothalamus) and cerebrospinal fluid (CSF) showed non-saturable transport of the cation. The authors suggested that the non-saturable transport into these brain regions resulted from passive diffusion of manganese down a concentration gradient from ventricular cerebrospinal fluid because some of these brain regions have components adjacent to the ventricles and manganese concentrations in these regions were below levels in the CSF. The authors also noted that at all plasma manganese concentrations tested (from 0.8 to 78 nmol/mL), the transfer coefficient for manganese uptake into the choroid plexus was significantly higher than in any other area of the central nervous system. For example, at 0.08 nmol/mL, the transfer coefficients for the CSF and the choroid plexus were 16.2±2.43×10−6 mL/second*g and 23,800±2,910×10−6 mL/second*g, respectively. Even after correcting for differences in compartment size, influx of manganese into the choroid plexus was an order of magnitude greater than influx into CSF.
Rabin et al. (1993) also measured transport of [54Mn]Cl2 in adult rats using a similar technique. In this study, the authors used three perfusates (whole blood, plasma/serum, and saline) to determine brain uptake in environments that facilitated or prevented protein binding of the metal. The authors reported that uptake of manganese into the cortex, hippocampus, caudate nucleus, and choroid plexus was greater and more rapid when saline was used rather than with whole blood. When EDTA-saline was used as the perfusate, uptake was not significantly different than zero, indicating that divalent manganese was the form taken up by the brain. The transfer coefficients of Mn(II) from saline in the different regions of the brain (frontal, parietal, and occipital cortex regions; hippocampus; caudate nucleus; and thalamus-hypothalamus) ranged from 5 to 10×10−5 mL/second*g, whereas that of the choroid plexus was 727×10−3 mL/second*g. The authors noted that the transfer coefficients were greater than that expected for passive diffusion and suggested a facilitated blood-brain barrier transport by a channel or carrier mechanism (Rabin et al. 1993). These findings of a rapid uptake mechanism and concentrated uptake into the choroid plexus are consistent with results reported by Murphy et al. (1991). Separate binding studies performed by the authors determined that albumin, transferrin, α2-macroglobulin added to the manganese during perfusion significantly decreased brain uptake of the cation in all brain regions. The authors were uncertain whether Mn(II) in the form of low-molecular mass solutes was taken up at the blood-brain barrier. However, based on other literature and their own unpublished results, they suggest that the free ion is the species transported.
Other studies have also revealed the rapid appearance of manganese in the choroid plexus. Ingersoll et al. (1995) demonstrated that manganese levels in the lateral choroid plexus were 44 and 24 times higher than levels in CSF, and blood, respectively, 4 hours after intraperitoneal injection of 10 mg manganese/kg. However, manganese concentration in the choroid plexus did not change significantly following intrathecal administration of this same dose. This demonstrated that manganese in the blood could be sequestered by the choroid plexus, whereas little to no transfer of manganese from CSF to the choroid plexus occurred. Intrathecal administration of manganese increased manganese concentrations in all brain regions examined while there were only slight changes in brain manganese concentrations after intraperitoneal administration. Moreover, intrathecal administration of manganese decreased spontaneous motor activity with no effect on motor activity following intraperitoneal dosing. The authors suggested that these results indicated that the brain is protected from high concentrations of manganese through sequestering in the choroid plexus, but this mechanism could become overwhelmed with rising levels of blood manganese such that manganese could then “leak’ from the choroid plexus into CSF and thereby enter the brain. This interpretation appears to be consistent with the findings of London et al. (1989). In these studies, 50 and 100 mg/kg manganese was administered intraperitoneal doses 5 and 10 times that used by Ingersoll et al. (1995). Using MRI images, these doses were shown to concentrate in the ventricles, the pineal gland, and the pituitary gland and the authors indicated that this high concentration of manganese appeared in the ventricular CSF because it crossed the barrier of the choroid plexus. Takeda et al. (1994) used autoradiography to also show that manganese in selected brain regions was taken up via the CSF from the choroid plexus. Moreover, Zheng et al. (1998) observed that, in a subchronic manganese intoxication rat model, the increases in manganese concentrations observed in targeted brain regions were closely related in magnitude to that of CSF manganese, but not to that of serum manganese. The observations of Takeda et al. (1994) and Zheng et al. (1998) support the view that manganese in the CSF serves as the main source for manganese distribution in brain tissues.
Recent reviews of the state of the science have emphasized that manganese can enter the brain via three pathways: (1) from the nasal mucosa to the brain olfactory bulb via olfactory neural connections; (2) from the blood through capillary endothelial cells of the blood-brain barrier; and (3) from the blood through the cerebral spinal fluid via the choroid plexuses (Aschner et al. 2005; Bock et al. 2008; Crossgrove and Yokel 2005). Current understanding is inadequate to determine which of these pathways may predominate in cases of severe manganism or cases of subtle neurological impairment in nonhuman primates or humans. A number of transport mechanisms (including facilitated diffusion, active transport, transferrin-mediated transport, divalent metal transporter-1 mediation, store-operated calcium channels) have been proposed to transport manganese across the blood barrier or into the choroid plexus, but current understanding is inadequate to determine the predominant molecular mechanism of transport in either of the pathways (Aschner et al. 2005, 2007; Crossgrove and Yokel 2004, 2005; Roth 2006).
3.5.2. Mechanisms of Toxicity
The central nervous system is the primary target of manganese toxicity. Although it is known that manganese is a cellular toxicant that can impair transport systems, enzyme activities, and receptor functions, the principal manner in which manganese neurotoxicity occurs has not been clearly established (Aschner and Aschner 1991; Aschner et al. 2007).
Mn(III) has been found to be more cytotoxic to human neural cells as a manganese pyrophosphate complex (MnPPi) than as a manganese-transferrin complex (MnTf) (Suarez et al. 1995). Specifically, human neuroblastoma cells (cell line SH-SY5Y) grown in culture showed effects of cytotoxicity from 30 μM MnPPi but did not show the same signs of cytoxicity from MnTf (membrane damage and cell granulation and aggregation) until concentrations of 60 μM were reached (Suarez et al. 1995). Both manganese complexes inhibited mitochondrial enzyme activity, but MnTf was slightly more toxic than MnPPi in this respect (Suarez et al. 1995).
Neuropathological changes are detectable in the basal ganglia of humans with manganism, and the specific area of injury appears to be primarily in the globus pallidus; the substantia nigra is sometimes affected, but generally to a lesser extent (Katsuragi et al. 1996; Yamada et al. 1986). Studies in nonhuman primates have produced similar findings (Newland and Weiss 1992; Newland et al. 1989). Limited evidence suggests that dopamine levels in the caudate nucleus and putamen are decreased in manganism patients (Bernheimer et al. 1973). Similarities in the behavior of manganism patients to those with Parkinson’s disease have prompted some to refer to manganism as "manganese-induced Parkinsonism" or "Parkinson-like disease." Further, the two diseases do affect functional related regions of the brain, but Parkinsonism is believed to be due to the selective loss of subcortical neurons whose cell bodies lie in the substantia nigra and whose axons terminate in the basal ganglia (which includes the caudate nucleus, the putamen, the globus pallidus, and other structures). These nigral neurons use dopamine as their neurotransmitter, and treatment of Parkinson patients with levo-dopa (the metabolic precursor to dopamine) often relieves some of the symptoms of Parkinson's disease (Bernheimer et al. 1973). Some investigators have reported that oral levo-dopa can temporarily improve symptoms of manganese-induced neurotoxicity (Barbeau 1984). However, most studies show that manganism patients typically do not respond to levo-dopa treatment (Calne et al. 1994; Chu et al. 1995; Huang et al. 1989), indicating that they have likely suffered degeneration of the receptors and neurons that normally respond to this neurochemical (Chu et al. 1995).
The precise biochemical mechanism by which manganese leads to this selective destruction of dopaminergic neurons is not known, but many researchers believe that the manganese ion, Mn(II), enhances the autoxidation or turnover of various intracellular catecholamines, leading to increased production of free radicals, reactive oxygen species, and other cytotoxic metabolites, along with a depletion of cellular antioxidant defense mechanisms (Barbeau 1984; Donaldson 1987; Garner and Nachtman 1989b; Graham 1984; Halliwell 1984; Liccione and Maines 1988; Parenti et al. 1988; Verity 1999). Oxidation of catechols is more efficient with Mn(III), than with Mn(II) or Mn(IV) (Archibald and Tyree 1987). Formation of Mn(III) may occur by oxidation of Mn(II) by superoxide (O2−) In cases of exposure to Mn(VII), it is likely that a reduction to the Mn(II) or Mn (III) state occurs (Holzgraefe et al. 1986), but this has not been demonstrated.
Hussain et al. (1997) studied the effects of chronic exposure of manganese on antioxidant enzymes, including manganese superoxide dismutase (MnSOD). MnSOD is an antioxidant enzyme located primarily in the mitochondria that contains manganese as a functional component. MnSOD protects against oxidative injury by catalyzing the dismutation of O2−, Hussain et al. (1997) found that administration of 0, 1.1, and 2.2 mg manganese/kg/day (as manganese chloride), 5 days/week for 3 months, resulted in increased MnSOD in the hippocampus, cerebellum, and brainstem. Other areas of the brain were not affected and other antioxidant enzymes, such as Cu,ZnSOD and glutathione peroxidase (GPx), were not increased. The researchers suggest that since a critical role of MnSOD is to protect against oxidative injury, the increase of this enzyme after manganese exposure may reduce the risk of oxidative stress induced by that exposure. Thus, this protective mechanism would have to be overwhelmed in cases of manganese toxicity. Additionally, the authors suggest that, since MnSOD was altered while Cu,ZnSOD and Gpx were unchanged, manganese may not affect cytosolic enzymes like Cu,ZnSOD. In support of this point, the authors also mention other reports that suggest that these antioxidant enzymes are independently regulated (Mossman et al. 1996; Warner et al. 1993; Yen et al. 1996).
Supporting evidence for the hypothesis that high levels of manganese exert neurotoxicity through oxidation is provided by Desole et al. (1994). The authors observed that 22 mg manganese/kg/day (as manganese chloride) administered orally in 6-month-old rats resulted in increased concentrations of DOPAC (an oxidation product of DA) and uric acid, but left DA levels unchanged. Daily doses of 44 or 66 mg manganese/kg/day resulted in significantly decreased concentrations of DA, glutathione, ascorbic acid, and DOPAC, and increased concentrations of uric acid in the rat striatum when compared to controls. The researchers also measured levels of these metabolites in the rat striatal synaptosomes, which were used as a model for neuronal terminals. Here, DA levels were unchanged at 22 mg manganese/kg/day but were decreased at the two highest doses. DOPAC levels remained constant at all three dose levels. Thus, the DOPAC/DA ratio was significantly increased at 44 and 66 mg manganese/kg/day in the synaptosomes. While the authors suggest that these data support other findings that manganese oxidizes dopamine (Segura-Aguilar and Lind 1989), the decrease in DA could be the result of decreased production or release of the chemical, rather than increased oxidation. Catabolism of adenosine triphosphate (ATP) forms xanthine and hypoxanthine, both of which are metabolized by xanthine oxidase. The products of this metabolism are uric acid and superoxide radical anion (Desole et al. 1994). The increase in uric acid production in rat striatum following oral dosing with 44 or 66 mg manganese/kg (as manganese chloride) suggests that manganese induces oxidative stress mediated by xanthine oxidase. Desole et al. (1995) expanded their studies to investigate the protective effect of allopurinol, a xanthine-oxidase inhibitor, to 3-month-old rats exposed to manganese. In this study, allopurinol was administered by gavage at a dose of 300 mg/kg/day for 4 days. Manganese (87 mg/kg/day) was also administered by gavage, for 7 days, either alone or with allopurinol; the allopurinol decreased the striatal ratio of DOPAC and homovanillic acid (HVA) to dopamine. When given in conjunction with manganese, allopurinol antagonized the manganese-induced increase in DOPAC levels and the (DOPAC + HVA)/DA ratio. Together, the two studies suggest that manganese-induced oxidative stress through the formation of reactive oxygen species may be a mechanism for manganese neurotoxicity, and allopurinol may protect against this oxidative stress in the striatum and brainstem of young rats.
Experiments such as the one by Desole et al. (1994) indicate that overexposure of rats to manganese results in increased dopamine turnover in the rat striatum. However, patients with basal ganglia dysfunction caused by manganese had normal striatal fluorodopa uptake on PET scan, indicating that the nigrostriatal pathway was intact (Wolters et al. 1989). Seven intravenous injections of manganese chloride into Rhesus monkeys resulted in an extrapyramidal syndrome characterized by bradykinesia, facial grimacing, and rigity, with gliosis of the globus pallidus and the substantia nigra par reticularis (Olanow et al. 1996). These intravenous injections, however, would have resulted in a highly elevated but transient increase in blood manganese levels. Striatal dopamine and homovanillic acid levels were within normal ranges; yet, there was clear evidence of manganese-induced neurotoxicity. Interestingly, none of the symptoms improved after levo-dopa administration, supporting findings in humans that manganism does not respond to levo-dopa treatment (Chu et al. 1995; Huang et al. 1989).
While there are a number of studies that support the hypothesis that manganese exerts its neurotoxicity through oxidation, a study by Sziráki et al. (1999) has demonstrated atypical antioxidative properties of manganese in iron-induced brain lipid peroxidation and copper dependent low density lipoprotein conjugation. However, the underlying mechanisms of the antioxidant effects are not clear. Brenneman et al. (1999) measured reactive oxygen species (ROS) in the brains of neonatal rats administered up to 22 mg manganese/kg/day for up to 49 days (dosing was only 5 days/week from day 22 to 49). On PND 21, no increase in ROS was seen in the striatum, hippocampus, or hindbrain of exposed rats at any dose, compared to controls administered water only. In the cerebellum, ROS levels were significantly increased to the same extent at both dose levels, as compared to controls. Manganese levels were not increased significantly in the cerebellum at any dose level, but were increased in the striatum, and the rest of the brain at the high dose level, when measured at PND 49. Mitochondrial manganese was not significantly elevated in the cerebellum or striatum, but was elevated in the rest of the brain at this high dose level, also at PND 49. These data do not support the hypothesis that oxidative damage is a mechanism of action in manganese-induced neurotoxicity in the rat.
As reviewed Taylor et al. (2006), the available literature contains results both in support of and inconsistent with oxidative stress involvement in manganese neurotoxicity. Recent support for oxidative stress involvement includes the finding that co-treatment of rats with the antioxidant, N-acetylcysteine, and intraperitoneal injections of high doses of manganese chloride (50 mg/kg, once or daily for 4 days) prevented the development of pathological changes observed following injection of manganese chloride alone (Hazell 2006). Likewise, mouse catecholaminergic cells (CATH.a) were protected from the cytotoxicity of 50–1000 µM manganese by supplementation of the culture media with 5 mM glutathione or 10 mM N-acetylcysteine (Stredrick et al. 2004). In contrast, in a series of studies of neonatal rats, adult male and female rats, or senescent male rats exposed by inhalation to manganese sulfate or manganese phosphate at concentrations up to 3 mg manganese/m3 with acute exposure durations or 1 mg manganese/m3 with subchronic exposure durations (Dorman et al. 2001a, 2004a, 2005a), no consistent exposure-related changes were found in the following markers of oxidative stress in various brain regions: glutathione, metallothionein, and glutamine synthetase (Taylor et al. 2006).
Mn(II) may also be involved in neurotoxicity. The neurotoxicity of Mn(II) has been linked to its ability to substitute for Ca(II) under physiological conditions (Aschner and Aschner 1991), and the intestinal transfers of Ca(II) and Mn(II) have been shown to be competitive in vivo (Dupuis et al. 1992). Although the mechanism for Mn(II) transport into brain cells is uncertain, Mn(II) preferentially accumulates in the mitochondria in the areas of the brain that are associated with manganism and neurological symptoms. Manganese is taken up into mitochondria via the calcium uniporter, and once there, Mn(II) inhibits mitochondrial oxidative phosphorylation. Gavin et al. (1992) observed that Mn(II) can inhibit mitochondrial oxidative phosphorylation when incubating isolated mitochondria with Mn(II) at concentrations >1 μM. Recently, it has also been shown that intramitochondiral Mn(II) can inhibit the efflux of Ca(II), which may result in a loss of mitochondrial membrane integrity (Gavin et al. 1999). At the same time, intramitochondrial Mn(II) can also inhibit oxidative phosphorylation and decrease energy production. However, Brouillet et al. (1993) has suggested that the impaired oxidative metabolism induced by manganese is indirectly linked to an excitotoxic process that results in neuronal degeneration. Because manganese accumulates in the mitochondria and is associated with impaired energy production, these authors compared the effects of intrastriatal injection of manganese with effects produced by known mitochodral toxins, aminooxyacetic acid and 1-methyl-4-phenylpyridinium. Lesions produced by these compounds can be blocked through an inhibition of the glutamatergic N-methyl-D-aspartate (NMDA) receptor or by the removal of the cortical glutamatergic input into the striatum by decortication. Thus, these lesions are termed “excitotoxic lesions.” It was shown that decortication or pre-treatment with the NMDA noncompetitive antagonist, MK-801, could reverse or ameliorate neurochemical changes induced by intrastriatal injection of manganese. These authors also showed that intrastriatal manganese treatment also interfered with energy metabolism, ATP concentrations were significantly reduced by 51% and lactate levels were increased by 97%. There is additional evidence that the glutamatergic excitatory system may play a role in manganese toxicity. Recent studies in genetically epilepsy-prone rats have suggested that there are abnormalities in manganese-dependent enzymes. Although the manganese-dependent enzymes are believed to be unrelated to seizure activity in these animals, it is suggested that there is a link between the low manganese concentrations in glial cells and elevated glutamate levels due to low glutamine synthetase activity (Critchfield et al. 1993).
Mn(II) (from manganese chloride) has also been shown to inhibit mitochondrial aconitase activity to a significant level in the frontal cortex of male rats dosed with 6 mg manganese/kg/day for 30 days (Zheng et al. 1998). Aconitase levels in striatum, hippocampus, and substantia nigra were decreased in treated rats, but not to a significant extent. Aconitase, which catalyzes the interconversion of L-citrate to isocitrate, via cis-aconitate, requires iron as a cofactor at its active center (Zheng et al. 1998). When the authors incubated brain mitochondrial fractions with Mn(II), aconitase activity was decreased; the addition of excess iron [Fe(II)] revived the enzyme activity. These data suggest that the similarity of manganese and iron facilitates their proposed interaction at the subcellular level; however, the data do not prove that Mn(II) is the form of manganese that is exerting the inhibitory effect.
Conversely, Suarez et al. (1995) did not observe cytotoxicity in cultured cells exposed to 100 μM Mn(II). The discrepancy noted in this study, and that of Gavin et al. (1992) may have occurred because of a protective effect of the cell membrane; if the cell membrane protects the cytosol, which typically has a low manganese concentration, then the Mn(II) concentration may be too low to affect the mitochondria through uniport uptake (Suarez et al. 1995). Another explanation is that mitochondrial uptake of Mn(II) occurs, but toxic effects require that cells be exposed much longer than isolated mitochondria (Suarez et al. 1995). It has also been established that manganese accumulation in the brain varies between regions, particularly in developing animals; this region-specific accumulation may alter the metabolism and homeostasis of manganese (Chan et al. 1992). In addition, it has been demonstrated that the manganese concentration in the central nervous system, in particular the ventral mesencephalon, can be reduced by cocaine, a dopamine reuptake inhibitor, or by reserpine, a dopamine depleting agent (Ingersoll et al. 1999). This suggests that the dopamine reuptake carrier is linked to a transport mechanism for manganese.
In vitro studies of rat brain mitochondria have demonstrated that there is no apparent mechanism for Mn(II) clearance other than the slow Na+ independent mechanism; it is suggested that Ca(II) and Mn(II) may accumulate in the brain mitochondria during manganese intoxication (Gavin et al. 1990). Other theories regarding the mode of neurotoxicity for manganese (and other metal ions) include toxicity caused by the formation of hydroxyl radicals during the manganese-catalyzed autooxidation of hydrazines (Ito et al. 1992).
It has been suggested that the mechanism of manganese neurotoxicity may in part involve complex interactions with other minerals (Lai et al. 1999). In a developmental rat model of chronic manganese toxicity, administration of manganese in drinking water was associated with increased levels of iron, copper, selenium, zinc, and calcium in various regions of the brain. Moreover, the subcellular distribution of various minerals was differentially altered following manganese treatment. Iron deficiency is associated with increased manganese burden in the central nervous system of rats, while administration of excess iron significantly decreases manganese uptake (Aschner and Aschner 1990). The biochemical mechanisms underlying the interactions between manganese and other minerals are unclear.
Subtle deficits in fine motor and cognitive function in chronically exposed young adult male Cynomologus macaques monkeys have been associated with manganese impairment of in vivo amphetamine-induced dopamine release in the striatum, without detectable changes in markers of striatial dopamine terminal integrity, and with decreased cerebral cortex N-acetylaspartate/creatine ratio (Guilarte et al. 2006a, 2006b; Schneider et al. 2006). In these studies, four monkeys (5–6 years old at the start) were given intravenous injections of manganese sulfate, 10–15 mg/kg or 3.26–4.89 mg manganese/kg, once per week for an average of 34.2 weeks. Three additional monkeys without excess manganese exposure or behavioral evaluations were used as a control group for post-mortem analyses of the brain (Guilarte et al. 2006a). Prior to manganese exposure, the monkeys were trained to perform tests for cognitive and motor function; overall behavior was assessed by ratings and videotaped analysis (Schneider et al. 2006). By the end of the exposure period, monkeys developed deficits in spatial working memory, showed modest decreases in spontaneous activity and manual dexterity, and showed increased frequency of compulsive-type behaviors such as compulsive grooming (Schneider et al. 2006). At study termination, mean manganese concentrations were elevated in exposed monkeys, compared with control monkeys, in the following brain regions: globus pallidus (3.30 versus 0.72 µg/g tissue); caudate (1.18 versus 0.38 µg/g tissue); putamen (1.5 versus 0.48 µg/g tissue); and frontal white matter (0.57 versus 0.17 µg/g tissue) (Guilarte et al. 2006b; Schneider et al. 2006). Positron emission tomography (PET) analysis found changes in amphetamine-induced release of dopamine in the striatum (up to 60% decrease compared with baseline values), but no significant changes in striatal dopamine receptor binding potentials (Guilarte et al. 2006a). Post-mortem chemical and immunohistochemical analysis of caudate and putamen tissue found no evidence for exposure-related changes to levels of D2-dopamine receptor (D2-DAR), dopamine receptor (DAT), tyrosine hydroxylase, or dopamine and its metabolite, homovanillic acid (Guilarte et al. 2006a). Using 1H-magnetic resonance spectroscopy, concentrations of creatine (Cr), N-acetylaspartate (NAA), choline, and myo-inositol were measured. Decreases (relative to baseline) in the NAA/Cr ratio were measured in the parietal cortex and frontal white matter, but not in the striatum (Gulilarte et al. 2006b). Guilarte et al. (2006b) suggested that the changes in the NAA/Cr ratio are indicative of neuronal degeneration or dysfunction in the parietal cortex that may also be associated with the neurobehavioral changes noted in the monkeys. Subsequent gene expression profiling in the frontal cortex of these monkeys found changes consistent with cellular stress responses that the investigators proposed may help to explain the subtle cognitive effects noted (Guilarte et al. 2008). The collective results from these studies suggest that subtle neurobehavioral changes noted in epidemiological studies of chronically exposed workers (see Section 3.2.1.4 and Appendix A) may be similar to those noted in these monkeys and may be related to manganese-induced functional changes and gene expression changes noted in the striatum and the cerebral cortex.
As reviewed by Fitsanakis et al. (2006), most mechanistic research on manganese neurotoxicity has focused on perturbations of the dopaminergic system, but there is evidence to suggest that early consequences of manganese neurotoxicity may involve perturbations of other neurotransmitters including GABA and glutamate in the basal ganglia and other brain regions. For example, there is evidence to suggest that manganese decreases the ability of astrocytes to clear glutamate from extracellular space (Erikson and Aschner 2002, 2003), increases the sensitivity of glutamate receptors to glutamate (see Fitsanakis and Aschner 2005 and Fitsanakis et al. 2006 for review), and perturbs glutamine-glutamate-GABA interconversions in frontal cortex and basal ganglia of rats (Zwingmann et al. 2004, 2007). When rat striatum was perfused with artificial cerebrospinal fluid with 200 nM manganese, GABA levels in the perfusate were decreased by about 60% compared with controls, but no effects on levels of glutamate, aspartate or glycine in the perfusate were observed (Takeda et al. 2003). In the perfused rat hippocampus, 200 nM manganese caused a 50% decrease in the levels of GABA, glutamate, and aspartate in the perfusate (Takeda et al. 2002). The results from the studies of Takeda et al. (2002, 2003) suggest that there are differential regional effects of manganese on the release of different neurotransmitters. Fitsanakis et al. (2006) concluded that additional research is needed to better understand the interdependence of neurotransmitters, including dopamine, glutamate, and GABA and their relationships to manganese neurotoxicity.
3.5.3. Animal-to-Human Extrapolations
As discussed in Section 3.2, the available literature on toxicological analysis of manganese in humans and animals is quite extensive. However, due to the wide dose ranges administered, the variety of responses, and the differences in measured end points, comparisons of effects across species is not straightforward.
Rodent models have primarily been used to study manganese neurotoxicity. These studies have reported mostly neurochemical, rather than neurobehavioral, effects (Brouillet et al. 1993; Chandra 1983; Chandra and Shukla 1978, 1981; Daniels and Abarca 1991; Deskin et al. 1980, 1981; Eriksson et al. 1987a; Gianutsos and Murray 1982; Parenti et al. 1986; Singh et al. 1979; Subhash and Padmashree 1991), as very few studies investigated neurobehavioral effects. It has been suggested that this focus may reflect difficulties in characterizing behavioral changes following basal ganglia damage in the rodent (Newland 1999). Other techniques, such as those used to identify basal ganglia damage as a result of exposure to neuroleptics (Newland 1999), may be refined to further exploit the rodent model as a predictor of neurobehavioral change in the human. The usefulness of the rat model for manganese neurotoxicity is also limited because the distribution of manganese in brain regions is dissimilar to that of the human (Chan et al. 1992; Brenneman et al. 1999; Kontur and Fechter 1988; Pappas et al. 1997). Studies to date have used exposure routes such as inhalation, intravenous, intraperitoneal, or subcutaneous, with few exceptions (Brenneman et al. 1999; Dorman et al. 2000, 2002, 2004a, 2005a, 2006b; Lown et al. 1984; Morganti et al. 1985; Pappas et al. 1997).
The rabbit has also been used as a model for manganese toxicity in a few studies (Chandra 1972; Szakmáry et al. 1995). The only available neurotoxicity study using the rabbit (Chandra 1972) reported that the species, when dosed intratracheally with 253 mg manganese/kg body weight (inferred as a one-time dose), developed hindlimb paralysis (a response not typically observed in humans exposed to excess manganese) after an observation period of 18 months. The animals also exhibited wide-spread neuronal degeneration in the brain. This study suggests that rabbits and humans may be qualitatively similar in the manifestation of neurobehavioral effects. However, further studies are needed to determine if the two species manifest comparable symptoms within the same dose range.
The nonhuman primate has been a useful model for predicting neurotoxicity in the human as the monkey presents neurobehavioral responses to toxicants that are very similar to those observed in humans (Eriksson et al. 1987b; Golub et al. 2005; Gupta et al. 1980; Newland and Weiss 1992; Olanow et al. 1996). Further, the monkey also undergoes neurochemical changes (Bird et al. 1984) as a result of manganese exposure. Studies have shown that monkeys exposed to manganese injected either intravenously or subcutaneously exhibit symptoms very similar to those observed in miners and others exposed to manganese, including ataxia, bradykinesia, unsteady gait, grimacing, and action tremor (Eriksson et al. 1992a, 1992b; Newland and Weiss 1992; Olanow et al. 1996). In addition, monkeys exhibiting these effects show accumulation of manganese in the basal ganglia as observed by MRI (Eriksson et al. 1992b; Newland and Weiss 1992), as do humans who are either exposed to, or are unable to clear, excess manganese (Devenyi et al. 1994; Fell et al. 1996; Hauser et al. 1994; Ono et al. 1995; Pomier-Layrargues et al. 1998; Rose et al. 1999; Spahr et al. 1996). However, primate studies showing these neurobehavioral effects have involved routes of administration that do not mimic environmental exposures, and although the effects in monkeys are qualitatively similar, it is currently unknown whether the effects are seen at the same dose metric as those in humans. Newland (1999) proposes using MRI techniques to relate the administration of certain amounts of manganese with a corresponding MRI signal in the brain and the resultant neurobehavioral effects. This technique might be very useful in developing a true dose-response relationship for manganese neurotoxicity in both the monkey and human.
Mechanisms of manganese toxicity in vivo are likely to be comprised in part by unique characteristics of the exposed individual, as well as by general physiology and exposure route. Multiple route PBPK models have recently been developed for predicting manganese brain concentrations in adult rats, monkeys, and humans and during gestation and lactation (Leavens et al. 2007; Nong et al. 2008; Schroeter et al. 2011; Teeguarden et al. 2007a, 2007b, 2007c; Yoon et al. 2011, 2009a, 2009b). As discussed by Yoon et al. (2011), confidence in predictions from the human models may improve with more information on the normal range and fluctuation of human brain manganese concentrations during early postnatal periods, the relationship between blood manganese concentrations and target tissue dosimetry, and the extent of induction of neonatal biliary excretion. Further extension of the models to other suspected susceptible populations, such as the elderly and individuals with liver dysfunction, also would be useful.
3.6. TOXICITIES MEDIATED THROUGH THE NEUROENDOCRINE AXIS
The potential hazardous effects of certain chemicals on the endocrine system are of current concern because of the ability of these chemicals to mimic or block endogenous hormones. Chemicals with this type of activity are most commonly referred to as endocrine disruptors. However, appropriate terminology to describe such effects remains controversial. The terminology endocrine disruptors, initially used by Thomas and Colborn (1992), was also used in 1996 when Congress mandated the EPA to develop a screening program for “…certain substances [which] may have an effect produced by a naturally occurring estrogen, or other such endocrine effect[s]…”. To meet this mandate, EPA convened a panel called the Endocrine Disruptors Screening and Testing Advisory Committee (EDSTAC), and in 1998, the EDSTAC completed its deliberations and made recommendations to EPA concerning endocrine disruptors. In 1999, the National Academy of Sciences released a report that referred to these same types of chemicals as hormonally active agents. The terminology endocrine modulators has also been used to convey the fact that effects caused by such chemicals may not necessarily be adverse. Many scientists agree that chemicals with the ability to disrupt or modulate the endocrine system are a potential threat to the health of humans, aquatic animals, and wildlife. However, others think that endocrine-active chemicals do not pose a significant health risk, particularly in view of the fact that hormone mimics exist in the natural environment. Examples of natural hormone mimics are the isoflavinoid phytoestrogens (Adlercreutz 1995; Livingston 1978; Mayr et al. 1992). These chemicals are derived from plants and are similar in structure and action to endogenous estrogen. Although the public health significance and descriptive terminology of substances capable of affecting the endocrine system remains controversial, scientists agree that these chemicals may affect the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body responsible for maintaining homeostasis, reproduction, development, and/or behavior (EPA 1997). Stated differently, such compounds may cause toxicities that are mediated through the neuroendocrine axis. As a result, these chemicals may play a role in altering, for example, metabolic, sexual, immune, and neurobehavioral function. Such chemicals are also thought to be involved in inducing breast, testicular, and prostate cancers, as well as endometriosis (Berger 1994; Giwercman et al. 1993; Hoel et al. 1992).
Studies of endocrine effects in humans following manganese exposure are very limited. Alessio et al. (1989) reported the elevation of serum prolactin and cortisol in chronically-exposed workers, while no changes in prolactin, FSH, or LH levels were observed in an occupational study involving shorter exposure periods (Roels et al. 1992). Lucchini et al. (1995) reported elevated serum prolactin levels in ferromanganese workers; 20 of those workers still showed elevated prolactin levels 5 years later after exposure to consistent levels of airborne manganese (Smargiassi and Mutti 1999). In fact, the serum prolactin levels had increased significantly over the previous values. Although these changes are minor, changes in prolactin secretion may have effects on different physiological functions, including loss of libido and impotence in men, and infertility and change in menstrual cycle in women.
No studies of endocrine effects in animals following airborne manganese exposure were located. Short-term animal studies and some of the long-term animal studies were negative for endocrine effects following oral exposure to manganese (NTP 1993). One intermediate study reported a decrease in circulating testosterone and a significant increase in substance P in the hypothalamus and neurotensin in the pituitary in rats dosed intraperitoneally with 6.6 mg manganese/kg/day as manganese chloride (Hong et al. 1984). Two other studies in rats reported that manganese tetroxide in food, given at a dose of 350 mg manganese/kg/day for 224 days (starting on day 1 of gestation and continuing for 224 days) (Laskey et al. 1982) and 214 mg manganese/kg/day given up to 28 days (Laskey et al. 1985), resulted in reduced testosterone levels in male rats. The biological significance of this effect is unknown because the decrease had no result on fertility in the latter study (Laskey et al. 1985), and there were no observed effects on the hypothalamus or pituitary.
A current interest in endocrine effects of manganese revolves around the possibility that developmental manganese exposure may influence the timing of puberty. One study performed on 23-day-old female rats in which manganese was provided by a single, intraventricular administration of 0, 0.01, 0.02, 0.04, or 0.17 mg manganese/kg as manganese chloride found that, at the three highest doses, manganese stimulated a dose-responsive increase in luteinizing hormone (LH) levels (Pine et al. 2005). A dose of 2 mg manganese/kg/day, provided to another group of female pups by daily gavage from PND 12 to 29 significantly advanced the average age of puberty (by approximately 1 day) as well as produced significant increases in serum levels of LH, follicule stimulating hormone (FSH), and estradiol (E2) (Pine et al. 2005). In a follow-up study by Hiney et al. (2011), a dose of 10 mg manganese chloride/kg/day (4.4 mg manganese/kg/day) by gavage from PND 12 to 29 in another group of female pups resulted in elevated gene expression levels of IGF-1, COX-2, and LHRH in the hypothalamus (genes involved in neuroendocrine axis control of puberty onset). Additionally, the release of LHRH and prostaglandin E2 was increased in the median eminence of treated females in vitro. Taken together, the results from these two studies suggest a role for manganese in regulating the timing of puberty in female rats and suggest that excess manganese exposure may accelerate the onset of puberty. Manganese also appears to have pubertal effects in male rats; an oral gavage dose of 11 mg manganese/kg/day provided daily on PNDs 15–48 or 15–55 produced significantly increased LH, FSH, and testosterone at 55 days of age (Lee et al. 2006). Increases in both daily sperm production and efficiency of spermatogenesis were also observed, suggesting that manganese may be a stimulator of prepubertal LHRH/LH secretion and thus facilitate the onset of male puberty. In vitro experiments using medial basal hypothalamic implants from adult male Sprague-Dawley rats showed that manganese at 500 µM increased LHRH release, nitric oxide synthase activity, and the content of cyclic cGMP in the medial basal hypothalamus (Prestifilippo et al. 2007). The inhibition of nitric oxide synthase with a competitive inhibitor prevented the manganese-induced increase in LHRH release. The results of these in vitro studies provide added evidence of the ability of manganese to modulate levels of LHRH, even in adult animals (Prestifilippo et al. 2007).
3.7. CHILDREN’S SUSCEPTIBILITY
This section discusses potential health effects from exposures during the period from conception to maturity at 18 years of age in humans, when all biological systems will have fully developed. Potential effects on offspring resulting from exposures of parental germ cells are considered, as well as any indirect effects on the fetus and neonate resulting from maternal exposure during gestation and lactation. Relevant animal and in vitro models are also discussed.
Children are not small adults. They differ from adults in their exposures and may differ in their susceptibility to hazardous chemicals. Children’s unique physiology and behavior can influence the extent of their exposure. Exposures of children are discussed in Section 6.6, Exposures of Children.
Children sometimes differ from adults in their susceptibility to hazardous chemicals, but whether there is a difference depends on the chemical (Guzelian et al. 1992; NRC 1993). Children may be more or less susceptible than adults to health effects, and the relationship may change with developmental age (Guzelian et al. 1992; NRC 1993). Vulnerability often depends on developmental stage. There are critical periods of structural and functional development during both prenatal and postnatal life, and a particular structure or function will be most sensitive to disruption during its critical period(s). Damage may not be evident until a later stage of development. There are often differences in pharmacokinetics and metabolism between children and adults. For example, absorption may be different in neonates because of the immaturity of their gastrointestinal tract and their larger skin surface area in proportion to body weight (Morselli et al. 1980; NRC 1993); the gastrointestinal absorption of lead is greatest in infants and young children (Ziegler et al. 1978). Distribution of xenobiotics may be different; for example, infants have a larger proportion of their bodies as extracellular water, and their brains and livers are proportionately larger (Altman and Dittmer 1974; Fomon 1966; Fomon et al. 1982; Owen and Brozek 1966; Widdowson and Dickerson 1964). The infant also has an immature blood-brain barrier (Adinolfi 1985; Johanson 1980) and probably an immature blood-testis barrier (Setchell and Waites 1975). Many xenobiotic metabolizing enzymes have distinctive developmental patterns. At various stages of growth and development, levels of particular enzymes may be higher or lower than those of adults, and sometimes unique enzymes may exist at particular developmental stages (Komori et al. 1990; Leeder and Kearns 1997; NRC 1993; Vieira et al. 1996). Whether differences in xenobiotic metabolism make the child more or less susceptible also depends on whether the relevant enzymes are involved in activation of the parent compound to its toxic form or in detoxification. There may also be differences in excretion, particularly in newborns who all have a low glomerular filtration rate and have not developed efficient tubular secretion and resorption capacities (Altman and Dittmer 1974; NRC 1993; West et al. 1948). Children and adults may differ in their capacity to repair damage from chemical insults. Children also have a longer remaining lifetime in which to express damage from chemicals; this potential is particularly relevant to cancer.
Certain characteristics of the developing human may increase exposure or susceptibility, whereas others may decrease susceptibility to the same chemical. For example, although infants breathe more air per kilogram of body weight than adults breathe, this difference might be somewhat counterbalanced by their alveoli being less developed, which results in a disproportionately smaller surface area for alveolar absorption (NRC 1993).
Prenatal and early postnatal developmental effects of manganese have largely been unstudied in humans. Potential developmental effects of manganese were suggested by the results of a study by Hafeman et al. (2007) that reported high mortality among infants <1 year of age in a Bangladesh population where the drinking water supplied by certain local wells contained high levels of manganese. Similarly, Spangler and Spangler (2009) reported increased infant mortality rates in counties in North Carolina with higher groundwater manganese concentrations after accounting for such confounders as low birth weight, economic status, education, and ethnicity. However, it cannot be determined if the observed effects in these studies were solely due to excess manganese alone or could have been influenced by other drinking water or dietary components. An older study by Kilburn (1987) showed that a native population living on an island with rich manganese deposits suffered increased neurological disorders and incidences of birth defects. Manganese exposure was most likely via inhalation and oral routes. However, since this study involved small sample sizes and lacked exposure concentrations and a suitable control group, these effects cannot be ascribed to manganese alone.
Two early studies investigated increased respiratory complaints and symptoms at a junior high school situated 100 m from a manganese alloy plant in Japan (manganese concentrations in total dust at a 200 meter perimeter around the plant were 0.004 mg/m3 [3.7 μg/m3]) (Kagamimori et al. 1973; Nogawa et al. 1973). The initial study showed that the incidences of self-reported respiratory illnesses among children in the exposed school were much higher than those of a control school 7 km away from the plant (Nogawa et al. 1973). Further, evaluations of respiratory fitness showed significant decreases in several parameters. When the installation of dust catchers resulted in a decreased manganese concentration in total dust, complaints of illness decreased, and the test results improved (Kagamimori et al. 1973). These respiratory effects were not unique from those observed in adults exposed to airborne manganese. Further, it was not reported if other compounds were present in the dust generated by the plant, which might have contributed to or caused the reported illnesses. It is possible that these effects might have been triggered by the dust and were not specific to manganese.
The possibility of neurological effects in children environmentally exposed to manganese is a continuing area of epidemiological research.
In early studies, children who have been exposed to elevated levels of inorganic manganese presumably through diet (either a normally ingested diet or through total parenteral nutrition, TPN) have shown signs of motor disorders (e.g., dystonia, dysmetria, propulsion, retropulsion, poor check response bilaterally) similar to those observed in cases of frank manganism (Devenyi et al. 1994; Fell et al. 1996). In a few of the cases, the presence of liver dysfunction indicated a decreased ability to clear excess manganese (Devenyi et al. 1994; Fell et al. 1996), but some of the children with apparently normal livers also exhibited motor disorders (Fell et al. 1996). Several children also exhibited hyperintense signals on MRI resulting from increased exposure to manganese due to cholestatic end-stage liver disease (Devenyi et al. 1994) and from high concentrations of the element in TPN, either in the presence (Fell et al. 1996) or absence (Fell et al. 1996; Ono et al. 1995) of liver disease. The Ono et al. (1995) study involved a child on TPN for more than 2 years; although this child did have increased blood manganese and hyperintense signals in the basal ganglia as shown by MRI, the authors did not report any observable signs of neurotoxicity. A similar lack of observable neurotoxicity was reported in two siblings fed TPN with high manganese concentrations (0.2 mmol manganese/kg/day) for several months (the brother for 63 months total starting at age 4 months; the sister for 23 months total starting at age 1 month) (Kafritsa et al. 1998). Both children had elevated blood manganese levels and showed hyperintense signals in the basal ganglia (especially the globus pallidus and subthalamic nuclei) on MRI. Reduction of manganese concentration in the TPN resulted in a gradual loss of signal on MRI analysis (becoming comparable to normal scans) and a decrease in blood manganese levels as measured in three subsequent annual exams. These equivocal results indicate that there are considerable differences in susceptibility to the neurotoxic effects of excess manganese in children.
Two other earlier studies show that children who drank water containing manganese at average concentrations of at least 0.241 mg/L (Zhang et al. 1995) and ate food with increased manganese content (He et al. 1994) for 3 years performed more poorly in school (as shown by mastery of their native language, mathematics, and overall grade average) and on the WHO neurobehavioral core test battery than those students who drank water with manganese ≤0.04 mg/L. These neurobehavioral tests are among those administered to workers occupationally exposed to manganese to determine the presence of early neurological deficit (Chia et al. 1993a; Iregren 1990; Lucchini et al. 1995; Mergler et al. 1994; Roels et al. 1987a, 1992). These concentrations are much lower than the ones to which adults were exposed in the Kondakis et al. (1989) study. In this study, ingestion of drinking water with excess manganese (1.8–2.3 mg/L) was linked to the onset of unspecified neurological symptoms in an aged population (average age, over 67 years). Though there are limitations, this and other environmental studies in adults (Baldwin et al. 1999; Beuter et al. 1999; Goldsmith et al. 1990; Kawamura et al. 1941; Kondakis et al. 1989; Mergler et al. 1999) and two studies in children (He et al. 1994; Zhang et al. 1995) indicate that both adults and children can manifest similar neurological deficits that are potentially linked to ingesting excess manganese. However, these reports are lacking well-characterized and quantitative exposure data that would indicate whether children and adults experience neurological effects at the same or different exposure levels. Existing studies do not allow estimations of the quantitative susceptibility of children to the preclinical effects of excess manganese exposure. They do indicate, though, that children can develop symptoms of neurotoxicity after oral exposure to manganese that are similar to those effects seen in adults environmentally or occupationally exposed to the metal. Further, these studies indicate that neurological effects may be a concern for children exposed to excess manganese from the environment or from a hazardous waste site.
The investigations by He et al. (1994) and Zhang et al. (1995) showed that children with poorer school performance had higher manganese hair content than children from the control area. Other studies have found that manganese levels in hair are higher in learning disabled children than in normal children (Collipp et al. 1983; Pihl and Parkes 1977). The route of excess exposure is not known, but it is presumed to be mainly oral. These observations are consistent with the possibility that excess manganese ingestion could lead to learning or behavioral impairment in children. However, an association of this sort is not sufficient to establish a cause-effect relationship since a number of other agents, including lead, might also be involved (Pihl and Parkes 1977).
Several recent reports continue to implicate elevated manganese exposure with impaired neurodevelopment. Four epidemiological reports of manganese neurotoxicity in children resulting from manganese exposure in drinking water have been published. In two separate cross-sectional studies, Wasserman et al. (2006, 2011) report statistically significant relationships for decreasing intelligence scores with increasing manganese levels in drinking water in 142–151 children (ages 8–11 years) in Bangladesh. Similarly, in a cross-sectional study conducted by Bouchard et al. (2011), a significant negative association was found between manganese levels in the home tap water and intelligence scores in 362 children from Quebec, Canada. In previous study by Bouchard et al. (2007c), a statistically significant relationship between increased levels of oppositional behaviors and hyperactivity and increased levels of manganese in drinking water in an epidemiological study of 46 children (ages 6– 15 years), also from Quebec, Canada.
The findings from Farias et al. (2010) support of the hyperactivity findings by Bouchard et al. (2007c). This cross-sectional study of 96 students (ages 7–15 years) diagnosed with ADHD and 35 controls reports that students diagnosed with, but not treated for, ADHD had significantly elevated serum manganese levels. However, in students treated for ADHD with stimulants, manganese levels were not different from controls and were significantly lower than untreated ADHD students. The source of manganese exposure in this study was not determined, but is presumed to be primarily oral.
Additionally, three recent case studies suggest that certain children are particularly susceptible to manganese neurotoxicity from high levels in drinking water, including: (1) severe neurotoxic symptoms (inability to walk independently, tendency to fall backward, and development of a “cock-like” walk) and MRI scan findings consistent with a diagnosis of hypermanganism in a previously healthy 5-year-old female that were associated with elevated drinking water concentrations of manganese (1.7–2.4 mg manganese/L), pica, emotional lability, polycythemia, iron deficiency, and elevated levels of plasma manganese (Brna et al. 2011); (2) a similar case of severe manganism-like neurotoxic symptoms in a previously healthy 6-year-old female that were associated with elevated drinking water concentrations of manganese (1.7–2.4 mg manganese/L), pica, a diet high in manganese-rich foods, and elevated levels of plasma manganese (Sahni et al. 2007); and (3) inattentiveness and lack of focus in the classroom and low-percentile performance in tests of memory in a 10-year-old male with no history of learning problems associated with elevated manganese in drinking water (1.21 mg manganese/L) (Woolf et al. 2002).
Increased exposure to elevated airborne manganese near industrial sites has also been associated with altered neurodevelopment. Two studies evaluated 79 children (ages 7–11 years) from the Molango mining district in Mexico exposed to an average manganese air concentration of 0.13 μg/m3 for at least 5 years. Riojas-Rodríguez et al. (2010) reported a significant inverse relationship between manganese exposure and full scale and verbal IQs, while Hernández-Bonilla et al. (2011) reported a subtle negative association of manganese exposure on motor speed and coordination. Similarly, Menezes-Filho et al. (2011) evaluated cognitive performance in 83 children from 55 families living near a ferromanganese alloy plant in Brazil that has been emitting high levels of manganese into the air for 4 decades. Elevated manganese exposure was inversely associated with intellectual function in both children and adults. However, direct correlations between air manganese concentrations and cognitive function were not evaluated in these studies. Likewise, other sources of environmental exposure (i.e., dietary, water) were not considered.
Recent evidence suggests that the critical time-point for adverse neurodevelopmental effects from elevated manganese exposure is as early as 12 months of age. In a prospective study, Claus Henn et al. (2010) reported a U-shaped nonlinear relationship between blood manganese levels and Mental Development Index scores at 12 months in 486 infants from Mexico City. However, by 24 months, manganese levels were not correlated with neurodevelopment using the Bayley Scales of Infant Development-II, Spanish version. These findings are consistent with manganese as both an essential nutrient and a toxicant, and identify 12 months as a potential critical developmental window for manganese exposure.
Taken together, these recent studies provide added weight to the evidence for the neurotoxic potential of excessive manganese in children, but one or more of the following uncertainties preclude the characterization of causal and dose-response relationships between the observed effects and manganese exposure: (1) whether or not the observed effects were solely due to excess manganese alone or could have been influenced by other drinking water or dietary components; (2) the lack of quantitative information about manganese levels from different environmental sources (food, water, and air); and (3) the small sample sizes.
Developmental studies in animals following inhalation exposure to manganese are sparse. One study exists (Lown et al. 1984) in which pregnant mice were exposed to a high concentration of airborne manganese or filtered air for 17 days preconception and then exposed to either the same concentration of manganese or filtered air postconception. Their pups were then fostered to adult females who had experienced the same inhalation exposures as the mothers (no manganese exposure, pre- or post-conception exposure, or both). The pups of exposed mothers had decreased body weight, but exhibited no differences in activity compared to pups from mothers exposed to air, irrespective of exposure history. In neonatal rats orally exposed to 25 or 50 mg manganese/kg/day from PNDs 1 through 21, manganese concentrations in various brain regions were about 2-fold higher than brain manganese concentrations in adult rats exposed to the same oral dose levels for 21 days (Dorman et al. 2000). At the highest dose level, neonatal rats showed an increased acoustic startle response, but exposure-related changes in other neurological end points (clinical signs, motor activity, and passive avoidance) were not found (Dorman et al. 2000). In another study, inhalation exposure of female CD rats to manganese sulfate, starting 28 days prior to breeding through PND 18, caused elevated manganese concentrations in exposed maternal rats (compared with air control rats) in the following tissues: brain and placenta at 0.5 and 1.0 mg manganese/m3 and lung at 0.05, 0.5, and 1.0 mg manganese/m3 (Dorman et al. 2005a). In contrast, statistically significant elevations of manganese concentrations in sampled fetal tissues were observed only in the liver at 0.5 and 1.0 mg manganese/m3, and elevated brain manganese concentrations were only observed in offspring after PND 14. The results from this study suggest that the brain in developing fetuses is partially protected from excess manganese by the placenta, and that the neonatal period is sensitive to increased manganese concentration in brain and other tissues under exposure to elevated airborne manganese concentrations.
Oral studies in animal models, whether involving the dosing of pregnant dams or sucklings, reveal a variety of neurochemical and physiological changes as a result of manganese exposure. The majority of studies have involved manganese chloride. One study in rats reported that pups exposed in utero 11 days during gestation to a relatively low concentration of manganese chloride (22 mg/kg; by gavage in water) did not have any observable decrease in weight gain, nor any gross or skeletal malformations upon necropsy (Grant et al. 1997a). Another study (Szakmáry et al. 1995) that also administered manganese chloride in water by gavage to pregnant rats at the slightly higher concentration of the 33 mg manganese/kg/day throughout the entire gestation period reported a delay of skeletal and organ development as well as an increase in skeletal malformations, such as clubfoot, in unborn pups. These malformations, however, were self-corrected in pups allowed to grow to 100 days of age. In addition, the same dose and route did not result in any observable developmental toxicity in the rabbit (Szakmáry et al. 1995). Rat pups exposed during gestation and after birth to manganese at relatively high concentrations of 120–620 mg/kg in drinking water suffered no observable adverse effects at the low dose and only transient adverse effects (decrease in weight and hyperactivity) at the high dose (Pappas et al. 1997). Similar transient body weight decreases and increases in motor activity were observed in neonatal rats administered 22 mg manganese/kg/day (as manganese chloride), by mouth or gavage, for up to 49 days (Brenneman et al. 1999; Dorman et al. 2000). Jarvinen and Ahlström (1975) fed pregnant rats varying doses of manganese sulfate in food for 8 weeks prior to and during gestation. Fetuses taken at 21 days did not show gross abnormalities, but did have significantly increased body burdens of manganese from mothers fed 187 mg/kg/day.
Rat pups from a generational study in which the male and female parents were exposed to 240–715 mg manganese/kg/day (as manganese chloride in drinking water) in either a diet adequate or deficient in protein (Ali et al. 1983a) suffered a delayed air righting reflex (independent of protein content of diet) and showed significant alterations in the age of eye opening and development of auditory startle when produced by parents fed low-protein diets with 240 mg manganese/kg/day in water. Kontur and Fechter (1988) administered up to 1,240 mg manganese/kg/day as manganese chloride in drinking water to pregnant rats during days 0–20 of gestation. Although the authors found increased manganese levels in the fetus, there were no measurable effects on dopamine or norepinephrine turnover in the pup brain, or in the development of a startle response. However, in a study with both gestational and postnatal exposure (GD 1–PND 24), Molina et al. (2011) reported decreased anxiety behavior on the elevated plus apparatus on PND 24 in rat pups from pregnant dams exposed to 4.79 mg manganese/mL (as manganese chloride in drinking water). Based on body weight and water intake, the study authors calculated daily manganese doses during gestation and lactation as 565 and 1,256 mg/kg/day, respectively. In a postnatal exposure study, an increased amplitude in acoustic startle reflex was observed at PND 21 in neonatal rats administered 22 mg manganese/mg/day (as manganese chloride) by mouth from PND 1 to 21 (Dorman et al. 2000). Significant increases in brain dopamine and DOPAC concentrations in select brain regions in these animals as well as increased brain manganese concentrations were reported. This study demonstrated that neonates treated with manganese showed neurological changes, whereas no effects were observed in the adult animals treated similarly. Lazrishvili et al. (2009) reported marked gliosis in rat pups (PND 40) from dams exposed to 4.4 mg manganese/kg/day (as manganese chloride) before, during, and for 1 month following pregnancy. This is in contrast to the lack of evidence for astrocytic alterations in adult rats exposed to 147 mg manganese/kg/day (as manganese chloride in drinking water) for up to 1 month (Rivera-Mancía et al. 2009).
Neonatal rats given manganese chloride in drinking water for 44 days at a dose of 150 mg manganese/ kg/day developed a transient ataxia on days 15–20 of the treatment and had decreased levels of homovanillic acid in the hypothalamus and striatum on day 15 but not day 60 (Kristensson et al. 1986). Neonatal rats given bolus doses of manganese chloride in water of 1 mg manganese/kg/day for 60 days suffered neuronal degeneration and increased monoamine oxidase on days 15 and 30 of the study, but did not show any clinical or behavioral signs of neurotoxicity (Chandra and Shukla 1978). Similarly, neonatal rats given bolus doses of manganese chloride in 5% sucrose at doses of 0, 1, 10 or 20 mg manganese/kg/day for 24 days after birth showed decreased levels of dopamine, but not norepinephrine, in the hypothalamus (Deskin et al. 1980); doses of 20 mg/kg/day caused a decrease of tyrosine hydroxylase activity and an increase in monoamine oxidase activity in the hypothalamus. In a follow-up study, Deskin et al. (1981) gave 0, 10, 15 and 20 mg manganese/kg/day (as manganese chloride in 5% sucrose by gavage) to neonatal rats from birth to age 24 days. The authors found that the highest dose resulted in increased serotonin in the hypothalamus and decreased acetylcholinesterase in the striatum. However, the authors did not indicate that the acetylcholinesterase decrease was important given other mechanisms involved in the metabolism of this neurochemical. Another neonatal study reported increased locomotor activity when rats were dosed with 10 mg/kg cocaine in adulthood (but no increased locomotor activity without cocaine challenge) following oral exposure to 13.1 mg manganese/kg/day (but not 4.4 mg manganese/kg/day) on PNDs 1–21 (Reichel et al. 2006).
A growing area of research is lasting adverse effects from early exposure to manganese. Tran et al. (2002a) reported an impaired olfactory-mediated homing ability and passive avoidance of footshocks and decreased striatal dopamine levels in male Sprague-Dawley rats exposed to oral doses of 17.2 mg manganese/kg/day (but not 8.6 mg manganese/kg/day) as manganese chloride on PNDs 1–20. Evidence indicates that alterations in passive avoidance behavior and dopamine expression persist into adulthood, long after manganese exposure has ceased (Tran et al. 2002b). Kern and colleagues (Kern and Smith 2011; Kern et al. 2010) reported increased open field activity, impaired spatial learning, increased brain expression of dopamine receptors (D1, D2) and dopamine transporter proteins, and increased glial activation in neonatal (PND 24) Sprague-Dawley rats exposed to oral doses of 50 mg manganese/kg/day (but not 25 mg manganese/kg/day) as manganese chloride on PNDs 1–21. In rats tested as adults (following cessation of exposure at PND 21), open field activity returned to baseline and the only change in the dopaminergic system was increased dopamine D2 receptor in the prefrontal cortex; however, increased glial activation remaine. In another study, Moreno et al. (2009) examined the differential effects of juvenile-only exposure, adult-only exposure, and juvenile followed by adult exposure up to 13.1 mg/kg/day (as manganese chloride via gavage) on both neurochemical and behavioral end points in C57Bl/6 mice. Open-field activity was altered in juvenile-only and juvenile+adult exposure, but not adult-only exposure. All groups had dopaminergic system alterations, with the magnitude of changes being the greatest in juveniles. Only juvenile-exposed mice had alterations in the serotonergic system. Together, these studies suggest that developing mice may be more sensitive to manganese exposure, and that developmental exposure has lasting effects on neurochemical and behavioral end points and later susceptibility to exposure.
Several studies evaluated the effects of manganese in the diet on reproductive development in the pre-weanling rodent. Gray and Laskey (1980) fed mice 1,050 mg manganese/kg/day (as manganese tetroxide) in the diet beginning on PND 15 and continuing for 90 days. The manganese caused decreased growth of the testes, seminal vesicles, and preputial gland. Later studies evaluated the effect of excess manganese via the diet and gavage on development of the rat (Laskey et al. 1982, 1985). These studies reported that 350 mg manganese/kg/day (as manganese tetroxide in food fed to pregnant rats and resulting male offspring for a total of 224 days) (Laskey et al. 1982) or 214 mg manganese/kg/day (as manganese tetroxide by gavage in water given for 28 days) (Laskey et al. 1985) reduced testosterone levels in developing rats.
Studies involving intravenous or subcutaneous exposure routes of pregnant dams indicate that doses of manganese chloride as low as 1.1 mg manganese/kg/day administered on GDs 6–17 in the rat (Grant et al. 1997a; Treinen et al. 1995) and 14 mg/kg/day administered on GDs 9–12 in the mouse (Colomina et al. 1996) can result in decreased fetal body weight and skeletal abnormalities.
The data indicate that animals may suffer adverse developmental effects after inhalation, oral, and intravenous exposures of their pregnant mothers, but results are mixed. Taken together, the evidence from environmental studies in humans and studies in animals suggests that younger children can be affected by exposures to excess manganese. Only one study is available that compared the incidence of adverse neurological effects in neonates and adults exposed to excess manganese (Dorman et al. 2000). Another recent study (Dorman et al. 2005b) showed that fetal brains were protected from excess manganese when their mothers were exposed to air concentrations as high as 1 mg manganese/m3 manganese sulfate for 28 days before mating through PND 18, but increased brain manganese concentrations developed in the offspring by PND 14. Additional information may help to quantitatively characterize the potential differences in susceptibility to manganese-induced effects in young and adult animals.
No studies currently exist on the health effects arising in children as a result of exposure to organic manganese. Therefore, predictions concerning potential effects must be made from extrapolations from existing animal studies.
Weanling mice who ingested 11 mg manganese/kg/day as MMT for 12 months exhibited a significant increase in spontaneous activity at day 80, but no other behavioral differences throughout the exposure period (Komura and Sakamoto 1992b). Concentrations of certain neurotransmitters and dopamine metabolites were modified in different brain regions, but the relationship to manganese levels in the affected regions was weak to none (Komura and Sakamoto 1994).
Developmental studies in rats involving intravenous exposure of pregnant dams to mangafodipir during organogenesis (days 6–17) indicate that the compound targets the skeletal system, resulting in irregularly shaped bones at doses as low as 1 mg manganese/kg/day (Grant et al. 1997a; Treinen et al. 1995). Further, application of specific doses of the compound during segmented time periods in organogenesis causes the same skeletal defects (Treinen et al. 1995). When the compound is administered from 22 days prior to conception until GD 7, at up to 6 mg manganese/kg/day, no developmental effects were observed (Grant et al. 1997a). These data further indicate that animals developing during organogenesis are particularly susceptible to developmental toxicity from mangafodipir exposure. Further, behavioral changes and significant decreases in body weight were observed in rat pups delivered from dams dosed with 1.1 mg manganese/kg/day, while decreased survival was observed in pups from dams given 2.2 mg manganese/kg/day on GDs 6–17.
In contrast to the rat, available studies suggest that the rabbit is far less susceptible to the developmental effects of mangafodipir. One study reported only decreased ossification in fetal sternebrae at 1.1 mg manganese/kg/day when given to dams on GDs 6–17 (Grant et al. 1997a); a similar study in the same species reported no observable developmental toxicity at 2.2 mg manganese/kg/day, but a significant decrease in fetal weight and viable fetuses, with no skeletal abnormalities, at a dose of 3.3 mg manganese/ kg/day also given during organogenesis (Blazak et al. 1996).
In total, these developmental studies indicate that organic manganese can induce adverse developmental effects in the unborn and young, with effects ranging from slight biochemical changes in the brain to structural changes to changes in functional development. However, the majority of studies have involved very high exposure doses.
The developmental toxicity of elemental manganese has been shown in large part by comparison studies between manganese chloride and mangafodipir (Blazak et al. 1996; Grant et al. 1997a; Treinen et al. 1995). While these studies have provided much information as to the targeted teratogenicity of manganese during organogenesis, they have generally involved intravenous exposures, which are not particularly relevant to the general population. Further, it is likely that the majority of women who may be exposed to mangafodipir are beyond child-bearing age, since clinical subjects with suspected liver tumors that merit use of the compound to assist in diagnosis are often over 50 years old (mean values; Bernardino et al. 1992). Should child-bearing women be exposed to the compound in a clinic environment, the doses required to induce developmental toxicity in animals greatly exceed the clinical dose (Blazak et al. 1996; Grant et al. 1997a; Treinen et al. 1995).
The pharmacokinetics of manganese in infants is known to be different than in adults. Balance studies, although limited, show that there is high retention of manganese during the neonatal period (Dorner et al. 1989). Formula-fed infants had an apparent manganese absorption of around 20% (Davidsson et al. 1988; 1989b), compared to absorption in adults, which is shown to be around 3–5% (Mena et al. 1969). The increased absorption may be a compensatory mechanism due to the low concentration of manganese in mother’s milk (Collipp et al. 1983; Dorner et al. 1989; Lönnerdal et al. 1987) and to the increased metabolic needs of infants as compared to adults, since manganese is required for adequate bone mineralization, as well as for connective tissue synthesis (Hurley and Keen 1987). Alternatively, the increased absorption may be due to decreased excretion in the very young (Kostial et al. 1978; Lönnerdal et al. 1987; Miller et al. 1975; Rehnberg et al. 1981), although at least one study indicates that both pre-term and full-term infants actively excrete manganese (Dorner et al. 1989). Some studies have indicated that infants, who acquire all of their manganese in the first 4 months of life from human milk or milk formulas, ingest very different amounts of manganese due to the differing manganese content of these food sources. More specifically, studies showed that due to the low manganese concentration of human milk (4–10 μg/L) and its higher concentration in cow’s milk formulas (30–75 μg/L) and soy formulas (100–300 μg/L) (Dorner et al. 1989; Lönnerdal et al. 1987), more manganese was absorbed from the formula (with absorption rate from all sources being roughly equal). Recent changes in nutritional status of infant formulas have resulted in a more nutritionally balanced absorption of manganese when compared to human milk and cow’s milk formulas (~80–90%), with absorption of manganese from soy milk formulas being slightly lower (~70%; Lönnerdal et al. 1994). However, given the existing differences in inherent manganese concentrations between the different food sources, reports still suggest that infant intake of manganese from milk formulas is 10–50 times that of a breast-fed infant (Lönnerdal 1997). Animal studies show that absorption and/or retention of manganese is similar to that of older animals at approximately post-gestational day 17–18 (Kostial et al. 1978; Lönnerdal et al. 1987; Miller et al. 1975; Rehnberg et al. 1981). However, when this transition takes place in human infants has not been clearly defined.
Animal studies also show increased absorption of manganese in the young. For example, Kostial et al. (1989) found that rat pups retained a greater proportion (67%) of a single oral dose of radiolabeled manganese than adult rats (0.18%). Bell et al. (1989) found that manganese absorption in rat pups (using isolated brush border membrane vesicles from the intestine) is nonsaturable and appears to occur primarily by diffusion. In the older rat, however, a high affinity, low capacity, active-transport mechanism for manganese absorption appears to be present (Garcia-Aranda et al. 1983).
Several elements, including iron (Davis et al. 1992a), phosphorus (Wedekind et al. 1991), and calcium (Wilgus and Patton 1939) are known to decrease manganese absorption in adults and animals. Iron-poor diets result in increased manganese absorption in humans (Mena et al. 1969) and in rats (Pollack et al. 1965). These interactions have not been studied in infants or children, but are expected to occur.
Manganese is known to cross the placenta and has been detected in cord blood in healthy full-term and pre-term infants. It is unknown whether mothers exposed to increased concentrations of manganese will pass on toxic amounts of the metal to their unborn children via the blood. However, as manganese is an essential nutrient and is part of the human body at all times, it is expected to be found in all tissues and fluids of the infant. Manganese is also naturally found in breast milk (typical concentrations in mature milk range from 4 to 10 μg/L) (Collipp et al. 1983). No studies exist concerning breast milk concentrations of mothers exposed to increased concentrations of manganese, but milk manganese concentrations increased with increasing exposure levels in lactating female rats exposed by inhalation to manganese sulfate at 0.05, 0.5, or 1 mg manganese/m3 for 28 days before mating through PND 18 (Dorman et al. 2005a). The mean milk concentration was statistically significantly increased, compared with air control levels, however, only at the highest exposure level. It is unclear if manganese stored in the brain, bone, or in another depot, in excess amounts, could be mobilized to affect a developing fetus. However, one study by Jarvinen and Ahlström (1975) showed that pregnant rats fed 94 mg manganese/ kg/day (as manganese sulfate) for 8 weeks accumulated the metal in their livers in contrast to non-pregnant females. Further, at a daily dose of 187 mg/kg/day, increased manganese concentrations were found in 21-day-old fetuses. These data suggest that homeostatic control of pregnant mothers regulated the distribution of the metal at lower concentrations, but this control was circumvented at high daily concentrations, resulting in liver excesses and distribution in the developing fetus. Although the fetuses in this study showed no physical abnormalities, no neurochemical or neurobehavioral studies were performed to determine potential adverse effects on these relevant end points.
Transferrin is one of the proteins responsible for binding and transporting both iron and manganese throughout the body. One study (Vahlquist et al. 1975) reported no correlation between infant cord blood and maternal blood transferrin levels. The same study reported an increase in plasma transferrin from 1.68±0.60 mg/mL in blood from infants at 6 weeks of age, to a peak of 2.60±0.27 mg/mL at 10 months, with values stabilizing at these adult levels throughout 16 years of age. The authors did not comment as to the statistical difference, if any, of these values.
There are no established biomarkers consistently used as indicators for overexposure to manganese in either adults or children. Elevated blood concentrations and hyperintense signals in the globus pallidus on T1-weighted MRI have been observed in children with increased exposure to manganese (Devenyi et al. 1994; Fell et al. 1996; Kafritsa et al. 1998; Ono et al. 1995). However, the same limitations of these indicators of overexposure in adults (wide range of blood manganese in normal populations, high cost and, hence, low availability of MRI) apply to children. Blood manganese has generally been poorly related to current levels of exposure or cumulative exposure index (Smargiassi and Mutti 1999). Elevated blood manganese alone does not constitute an adequate indicator of manganese overexposure. There are no pediatric-specific biomarkers of exposure or effect. See Section 3.8.1 for further information.
Studies suggest that children may differ from adults in their susceptibility to the toxic effects of manganese due to toxicokinetic differences (i.e., increased absorption and/or retention). Qualitative similarities exist between respiratory and neurological effects seen in adults and children suffering from extreme manganese exposure. While infant and animal studies indicate that the young have an increased uptake of manganese, and distribution of the element in certain tissues may differ with age, studies that reveal quantitative levels of manganese associated with discrete frank effects in both adults and children are lacking. The studies to date (namely absorption, distribution and excretion studies in animals) suggest a pharmacokinetic susceptibility to manganese that is different in children than in adults.
3.8. BIOMARKERS OF EXPOSURE AND EFFECT
Biomarkers are broadly defined as indicators signaling events in biologic systems or samples. They have been classified as markers of exposure, markers of effect, and markers of susceptibility (NAS/NRC 1989).
A biomarker of exposure is a xenobiotic substance or its metabolite(s) or the product of an interaction between a xenobiotic agent and some target molecule(s) or cell(s) that is measured within a compartment of an organism (NAS/NRC 1989). The preferred biomarkers of exposure are generally the substance itself, substance-specific metabolites in readily obtainable body fluid(s), or excreta. However, several factors can confound the use and interpretation of biomarkers of exposure. The body burden of a substance may be the result of exposures from more than one source. The substance being measured may be a metabolite of another xenobiotic substance (e.g., high urinary levels of phenol can result from exposure to several different aromatic compounds). Depending on the properties of the substance (e.g., biologic half-life) and environmental conditions (e.g., duration and route of exposure), the substance and all of its metabolites may have left the body by the time samples can be taken. It may be difficult to identify individuals exposed to hazardous substances that are commonly found in body tissues and fluids (e.g., essential mineral nutrients such as copper, zinc, and selenium). Biomarkers of exposure to manganese are discussed in Section 3.8.1.
Biomarkers of effect are defined as any measurable biochemical, physiologic, or other alteration within an organism that, depending on magnitude, can be recognized as an established or potential health impairment or disease (NAS/NRC 1989). This definition encompasses biochemical or cellular signals of tissue dysfunction (e.g., increased liver enzyme activity or pathologic changes in female genital epithelial cells), as well as physiologic signs of dysfunction such as increased blood pressure or decreased lung capacity. Note that these markers are not often substance specific. They also may not be directly adverse, but can indicate potential health impairment (e.g., DNA adducts). Biomarkers of effects caused by manganese are discussed in Section 3.8.2.
A biomarker of susceptibility is an indicator of an inherent or acquired limitation of an organism's ability to respond to the challenge of exposure to a specific xenobiotic substance. It can be an intrinsic genetic or other characteristic or a preexisting disease that results in an increase in absorbed dose, a decrease in the biologically effective dose, or a target tissue response. If biomarkers of susceptibility exist, they are discussed in Section 3.10, Populations That Are Unusually Susceptible.
3.8.1. Biomarkers Used to Identify or Quantify Exposure to Manganese
Manganese can be measured with good sensitivity in biological fluids and tissues (see Section 7.1), and levels in blood, urine, feces, and hair have been investigated as possible biomarkers of exposure. As a group, workers exposed to a mean concentration of 1 mg manganese/m3 had higher levels of manganese in the blood and the urine than unexposed controls (Roels et al. 1987b). The group average levels in blood appeared to be related to manganese body burden, while average urinary excretion levels were judged to be most indicative of recent exposures. A study by Lucchini et al. (1995) is the only evidence that suggests that blood and urine levels were correlated with manganese exposure on an individual basis. This study differed from others in that it involved exposure to manganese dioxide and measured adverse effects in workers after exposure ceased, whereas other studies involved current exposures, and some, like Roels et al. (1987b) involved exposure to numerous manganese compounds (salts and oxides). The findings of Lucchini et al. (1995) suggest that blood and urine levels of manganese, on an individual basis, are positively correlated with exposure levels in the few weeks following cessation of exposure. In a study of chronically exposed workers who were evaluated while exposure was ongoing, Lucchini et al. (1999) found a positive correlation between manganese levels in total dust and in blood of exposed workers. This correlation did not exist for cumulative exposure index and blood levels of the metal.
Other studies have indicated that on an individual basis, the correlation between the level of workplace exposure and the levels in blood or urine is not a reliable predictor of exposure (Jarvisalo et al. 1992; Roels et al. 1987b, 1992; Smyth et al. 1973). However, two studies (Jarvisalo et al. 1992; Roels et al. 1992) suggest that blood and urinary manganese levels may be used to monitor group exposure, such as exposure in an occupational setting. Also, a study (Siqueira et al. 1991) of ferromanganese workers indicated that exposed workers had elevated levels of plasma and urinary urea and decreased levels of urinary calcium, HDL cholesterol, and plasma inorganic phosphate. The study authors concluded that measurement of these parameters may be useful in the early detection of manganese poisoning. Although manganese may play a role in a metabolic pathway or other biological function involving these products, it is unclear what physiological significance these parameters have as related to manganese toxicity. There was no significant correlation between fecal excretion of manganese and occupational exposure to the metal (Valentin and Schiele 1983). A recent study on environmental exposure to manganese (Baldwin et al. 1999) in southwest Quebec, Canada, indicates that significantly higher levels of blood manganese are correlated with high levels of airborne manganese. In this study, air samples were taken in four geographic areas around a former ferroalloy plant (point source for airborne manganese). The air samples, which were for total dust and PM10 levels, were taken for 3 consecutive days in the summer. Using a geometric algorithm, 297 blood manganese values from nearby residents in seven postal zones were separated into two geographical areas corresponding to the point source. Higher blood manganese values in men and women were located in the geographic area with the higher airborne manganese values. It is notable that the air samples taken were limited in number and were taken only in the summer. However, the authors mentioned that the data were consistent with samples taken in an adjacent urban area and were consistent with potential exposure sources. Further, at the time of sampling, the ferroalloy plant was not in use and exposure data indicated that airborne levels of manganese decreased dramatically at a point 25 km downwind of the plant after the plant closed (Zayed et al. 1994). Thus, manganese exposure of the population in the Baldwin et al. (1999) study is likely to have been greater in the past; current blood manganese levels may be analogous to those observed in occupational workers undergoing a forced layoff (Lucchini et al. 1995). These data, combined with the occupational studies, indicate that there may be a plateau level of homeostatic control of the metal. At low levels, blood manganese concentrations would be related to intake from food, water, and air; large differences in individual blood manganese levels would be observed. At high exposure levels, such as in occupational environments, a higher but still non-toxic level of blood manganese may be maintained by homeostatic control (i.e., a plateau level is reached); alternatively, that level may be exceeded.
These data also indicate that blood manganese levels can be an indicator of exposure to environmental manganese. These data indicate that manganese in blood or urine may be useful in detecting groups with above-average current exposure, but that measurements of manganese in these body fluids in individuals may only be related to exposure dose after the exposure has ceased.
In addition to individual variability, another factor that limits the usefulness of measuring manganese in blood, urine, or feces as a measure of excess manganese exposure is the relatively rapid rate of manganese clearance from the body. As discussed in Section 3.4, excess manganese in blood is rapidly removed by the liver and excreted into the bile, with very little excretion in urine (Klaassen 1974; Malecki et al. 1996b). Thus, levels of manganese in blood or urine are not expected to be the most sensitive indicators of exposure.
Serum prolactin (PRL) has been shown to be a possible biomarker of manganese action of dopamine neurotransmission (Smargiassi and Mutti 1999). Manganese acts on the tuberoinfundibular dopaminergic system, which exerts tonic inhibition of PRL secretion. Serum PRL levels observed in workers occupationally exposed to manganese were shown to be consistent with mechanistic studies as they were distinctly higher than unexposed workers. It is still unclear whether or not serum PRL levels indicate recent or cumulative exposure. The value of PRL as a biomarker is called into question by the Roels et al. (1992) study in which serum PRL levels were not increased in workers chronically exposed to airborne manganese.
Lymphocyte manganese-dependent superoxide dismutase activity increases with increased manganese uptake (Yiin et al. 1996). It has been suggested that this enzyme, in conjunction with serum manganese levels, may be helpful in assessing low and moderate levels of manganese exposure (Davis and Greger 1992; Greger 1999). MnSOD has been shown to be elevated in women ingesting 15 mg of supplemental manganese/day, while levels have been shown to be depressed in the heart and liver of manganese deficient animals. MnSOD is important as a possible biomarker because its levels can be related to oxidative damage. Its sensitivity as a biomarker depends on factors that induce oxidative stress or effect manganese bioavailability including diets high in polyunsaturated fatty acids and strenuous physical exercise (Greger 1999).
Brain MRI scans and a battery of specific neurobehavioral tests (Greger 1998) may be useful in assessing excessive manganese exposure even among industrial workers exposed to airborne manganese (Nelson et al. 1993). These scans also have been successfully used to identify accumulation of manganese in the brains of children exposed to excess manganese (Devenyi et al. 1994; Fell et al. 1996; Ihara et al. 1999; Kafritsa et al. 1998; Ono et al. 1995; Sahni et al. 2007). Levels in feces could be useful in evaluating relatively recent high-level exposures but would not be expected to be helpful in detecting chronic low-level exposures. These methods are potentially useful biomarkers, but require additional evaluation to determine their validity.
While it is well established that exposure to excess manganese can result in increased tissue levels in animals, the correlations among exposure levels, tissue burdens, and health effects have not been thoroughly investigated in humans or animals. Also, since homeostatic mechanisms largely prevent fluctuations of manganese concentration in whole blood and since manganese is mainly excreted by the biliary route, it is not believed possible to identify a biological marker to assess the intensity of exposure or concentration in the target organ (Lauwerys et al. 1992). As noted by Rehnberg et al. (1982), manganese levels in tissues are subject to homeostatic regulation via changes in absorption and/or excretion rates. While exposure to very high levels may overwhelm these mechanisms, continuous exposure to moderate excesses of manganese does not appear to cause a continuous increase in tissue levels (Rehnberg et al. 1982). Moreover, even if tissue levels are increased in response to above-average exposure, levels are likely to decrease toward the normal level after exposure ceases. For example, the level of manganese in the brain of a subject with severe manganism was not different from the normal level (Yamada et al. 1986). For these reasons, measurement of tissue levels of manganese at autopsy or possibly biopsy may be of some value in detecting current exposure levels but is not useful in detecting past exposures. Evaluation of manganese exposure by analysis of tissue levels is also not readily applicable to living persons except through the collection of biopsy samples.
MRI has been used to track manganese distribution in the brains of monkeys (Dorman et al. 2006b; Newland and Weiss 1992; Newland et al. 1989) and humans (Kafritsa et al. 1998; Klos et al. 2005; Nolte et al. 1998; Park et al. 2003; Rose et al. 1999; Uchino et al. 2007; Wolters et al. 1989). In addition, it has been used to assay hyperintense signaling in the globus pallidus and other brain areas of individuals with chronic liver disease (Devenyi et al. 1994; Hauser et al. 1994, 1996; Klos et al. 2005; Nolte et al. 1998; Park et al. 2003; Pomier-Layrargues et al. 1998; Spahr et al. 1996; Uchino et al. 2007), individuals on chronically-administered TPN (Kafritsa et al. 1998; Nagatomo et al. 1999; Ono et al. 1995), and individuals with symptoms characteristic of manganism (Nelson et al. 1993). Although data addressing the sensitivity and specificity of MRI as an indicator for body burden or exposure are limited, the technique is being used to identify individuals who are likely to have increased stores of manganese in brain and potentially in other tissues, as well. For example, the hyperintense signaling in the brain is typically coincident with elevated blood manganese levels (Devenyi et al. 1994; Hauser et al. 1994, 1996; Kafritsa et al. 1998; Klos et al. 2005; Nagatomo et al. 1999; Nolte et al. 1998; Ono et al. 1995; Park et al. 2003; Pomier-Layrargues et al. 1998; Spahr et al. 1996; Uchino et al. 2007). Dorman et al. (2006b) evaluated the use of the pallidal index (PI—ratio of hyperintensities in the globus pallidus and the adjacent subcortical frontal white matter) and the T1 relaxation rate (R1) from MRI to reflect manganese concentrations determined by analytical chemistry in brain regions of monkeys repeatedly exposed by inhalation to aerosols of manganese sulfate at several concentrations ≥0.06 mg. Increases in the PI and R1 were correlated with the pallidal manganese concentration, but increased manganese concentrations in white matter confounded the PI measurements. Dorman et al. (2006b) suggested that R1 can be used to estimate regional brain manganese concentrations and that this technique may be used as a reliable biomarker of occupational manganese exposure.
Neutron activation has been shown to be a possible means of in vivo measurement of manganese in the liver and possibly other tissues and organs, including the brain (Arnold et al. 1999; Rose et al. 1999). Minimum detection levels are low enough to distinguish between normal and elevated concentrations.
Scalp hair has also been investigated as a possible biomarker of manganese exposure. While some studies have found a correlation between exposure level and manganese concentration in hair (Collipp et al. 1983), use of hair is problematic for several reasons. For example, exogenous contamination may yield values that do not reflect absorbed doses, and hair growth and loss limit its usefulness to only a few months after exposure (Stauber et al. 1987). Manganese has also been reported to have a strong affinity for pigmented tissues (Lydén et al. 1984), and Hurley and Keen (1987) and Sturaro et al. (1994) have reported that manganese concentrations in hair vary with hair color. Further, hair may be contaminated by dye, bleaching, or other materials. Thus, it is not surprising that other studies have found no correlation between individual hair levels and the severity of neurological effects in manganese-exposed persons (Stauber et al. 1987). A study that investigated the correlation between potentially toxic metal content in hair and violent behavior found an association between manganese and violent behavior, but it was not conclusively established that manganese was the causative factor (Gottschalk et al. 1991). He et al. (1994) observed that poor performance in school and on neurobehavioral tests was inversely correlated with hair levels of manganese. The manganese exposure in this study was via drinking water and certain foods. Several studies have found that manganese levels in hair are higher in learning disabled children than in nondisabled children (Collipp et al. 1983; Pihl and Parkes 1977). The route of excess exposure is not known but is presumed to be mainly oral. However, an association of this sort is not sufficient to establish a cause-effect relationship since a number of other agents, including lead, might also be involved (Pihl and Parkes 1977). Other studies have found statistically significant associations between hair manganese levels and behavioral deficits (Bouchard et al. 2007c; Wright et al. 2006), subtle motor deficits (Hernández-Bonilla et al. 2011; Standridge et al. 2008) and decreased intellectual function (Bouchard et al. 2011; Menezes-Filho et al. 2011; Riojas-Rodríguez et al. 2010). These studies suggest that hair manganese levels can provide meaningful exposure assessments.
Clara cell protein CC16 is a potential biomarker for exposure to MMT, because the protein decreases in both BALF and serum following MMT exposure (Bernard and Hermans 1997; Halatek et al. 1998), possibly due to decreased synthesis and/or protein secretion due to loss of producing cells (Halatek et al. 1998). The protein can be quantified in serum or urine, but no dose-response studies on the potential biomarker have been performed.
There are no known biomarkers of exposure that are specific for children; any biomarkers applicable for use in adults should be applicable for children. For example, manganese-induced hyperintense signals on MRI have been seen in children (Devenyi et al. 1994; Kafritsa et al. 1998; Ono et al. 1995; Sahni et al. 2007) as well as adults (Hauser et al. 1994, 1996; Nagatomo et al. 1999; Pomier-Layrargues et al. 1998; Spahr et al. 1996).
3.8.2. Biomarkers Used to Characterize Effects Caused by Manganese
The principal adverse health effects associated with exposure to manganese are respiratory effects (lung inflammation, pneumonia, reduced lung function, etc.) and the neurological syndrome of manganism and preclinical neurological effects. Although the respiratory effects are similar in many different exposure studies (Kagamimori et al. 1973; Lloyd Davies 1946; Nogawa et al. 1973), there are no specific biomarkers of effect other than reduced lung function. The fully developed disease can be diagnosed by the characteristic pattern of symptoms and neurological signs (Mena et al. 1967; Rodier 1955), but the early signs and symptoms are not specific for manganese. Careful neurological and psychomotor examination in conjunction with known exposure to manganese may be able to detect an increased incidence of preclinical signs of neurological effects in apparently healthy people (Iregren 1990; Roels et al. 1987a). However, these signs are not sufficiently specific for preclinical effects of manganese to reliably identify whether an individual has been exposed to excess levels for a prolonged period. In addition, no biochemical indicator is currently available for the detection of the early neurotoxic effects of manganese. There are no specific biomarkers that would clearly indicate long-term exposure to excess manganese.
Idiopathic Parkinsonism and manganism can be difficult to distinguish due to some similarity in the symptoms (Kim et al. 1999). Idiopathic Parkinsonism is marked by neurodegeneration in the dopaminergic nigrostriatal pathway, while manganism induced damage occurs postsynaptic to the nigrostriatal system. PET with 18F-dopa afforded a differentiation between manganism and idiopathic Parkinsonism in isolated patients with manganese exposure by indexing the integrity of the dopaminergic nigrostriatal pathway.
Measurement of altered levels of dopamine and other neurotransmitters in the basal ganglia has proven to be a useful means of evaluating central nervous system effects in animals (e.g., Bonilla and Prasad 1984; Eriksson et al. 1987a, 1987b), and these changes are often observed before any behavioral or motor effects are apparent (Bird et al. 1984). No noninvasive methods are currently available to determine whether there are decreased dopamine levels in the brain of exposed humans, but decreased urinary excretion of dopamine and its metabolites has been noted in groups of manganese-exposed workers (Bernheimer et al. 1973; Siqueira and Moraes 1989). However, the relationship between manganese effects on peripheral versus central dopamine levels has not been clearly defined, and given the lack of change in dopamine content in substantia nigra of humans exposed to manganese, the relevance of the animal studies to central nervous system disorder is questionable.
Smargiassi et al. (1995) evaluated platelet monoamine oxidase (MAO) and serum dopamine β-hydroxylase (DBH) activities in 11 men occupationally exposed to manganese via inhalation in a ferroalloy plant. Exposed workers, in general, had lower MAO activities, but similar DBH activities, in comparison to 15 nonexposed control males. However, a positive dose-effect relationship was observed in the exposed group between a Cumulative Exposure Index (CEI) and DBH activity (r2=0.40, p<0.05). The CEI took into account the average annual respirable or total manganese concentrations in dust, the ventilation characteristic of each working area, the number of years that each worker spent in a given area, and all of the areas that a worker had been during his job history. The authors proposed that DBH, which is an expression of catecholamine release, might be increasing dose-dependently in response to reduced turnover of MAO. The authors cautioned however, that while the data appear interesting, they should be investigated in a larger study population, with careful analysis of possible confounding factors (Smargiassi et al. 1995).
Reduced urinary excretion of 17-ketosteroids (perhaps as a consequence of decreased testosterone production) has been noted in many patients with neurological signs of manganism (Rodier 1955), but it has not been determined whether this change is detectable prior to the occurrence of neurological effects. Although the urinary excretion of manganese is generally not related to oral manganese intake, Davis and Greger (1992) have suggested that the concentration of manganese in serum, combined with lymphocyte manganese-dependent superoxide dismutase activity, may be helpful in assessing low and moderate levels of manganese exposure. Manganese superoxide dismutase is activated by manganese, thus it is sensitive to the overall manganese balance. Therefore, increased manganese concentrations will affect an increased manganese superoxide dismutase level. There is no clear link between activity of superoxide dismutase and the harmful effects of manganese. Therefore, the potential usefulness of this technique as a biomarker of effect requires further evaluation.
The Clara cell protein CC16 is a potential biomarker for pulmonary effects from exposure to MMT (Bernard and Hermans 1997; Halatek et al. 1998). Damage of Clara cells by MMT causes a significant reduction in the levels of this protein in the BALF, but does not affect its level in serum. The protein can be quantified in serum or urine as well. However, no dose-response studies on the potential biomarker have been performed. Further, the protein has only been studied following intraperitoneal administration of MMT. It is unknown if CC16 levels will change following other exposure pathways.
For more information on biomarkers for renal and hepatic effects of chemicals see ATSDR/CDC Subcommittee Report on Biological Indicators of Organ Damage (Agency for Toxic Substances and Disease Registry 1990) and for information on biomarkers for neurological effects see OTA (1990).
3.9. INTERACTIONS WITH OTHER CHEMICALS
There is clear evidence from studies in animals that the gastrointestinal absorption (and hence the toxicity) of manganese is inversely related to dietary iron concentrations. That is, high levels of nonheme iron lead to decreased manganese absorption and toxicity, and low levels of iron lead to increased manganese absorption and toxicity (Chandra and Tandon 1973; Davis et al. 1992a, 1992b; Diez-Ewald et al. 1968; Rehnberg et al. 1982). Conversely, high levels of dietary manganese lead to decreased iron absorption (Davis et al. 1992b; Diez-Ewald et al. 1968; Garcia et al. 2006, 2007; Li et al. 2006; Rossander-Hulten et al. 1991; Thomson et al. 1971). Short-term effects of this sort are believed to be the result of kinetic competition between iron and manganese for a limited number of binding sites on intestinal transport enzymes (Thomson et al. 1971), while longer-term effects of iron deficiency or excess are thought to be due to adaptive changes in the level of intestinal transport capacity (Cotzias 1958). The studies reporting competition between iron and manganese in absorption clearly indicate the impact an iron-poor diet will have on manganese uptake in the human (Chandra and Tandon 1973; Davis et al. 1992a, 1992b; Diez-Ewald et al. 1968; Mena et al. 1969; Rehnberg et al. 1982; Thomson et al. 1971). Further, competition between manganese and iron at the blood-brain barrier has been reported (Aschner and Aschner 1990), indicating that excesses of either metal will affect the brain distribution of the other. Johnson and Korynta (1992) found that, in rats, dietary copper can also decrease manganese absorption and increase manganese turnover; dietary ascorbate supplementation had minimal effects on manganese absorption. However, there is insufficient information to determine the significance of these observations for health effects in humans exposed to copper and manganese by the oral route.
Mn(II) pretreatment reduces Cd(II)-induced lethality (Goering and Klaassen 1985). Cadmium has been noted to have an inhibitory effect on manganese uptake (Gruden and Matausic 1989). In addition, manganese appears to be capable of increasing the synthesis of the metal-binding protein metallothionine (Waalkes and Klaassen 1985). Data from a study by Goering and Klaassen (1985) suggest that manganese pretreatment increases the amount of Cd+2 bound to metallothionine, thereby decreasing hepatotoxicity due to unbound Cd+2. The significance of these observations for health effects in humans exposed to cadmium and manganese by the oral or inhalation routes is not clear.
High dietary intakes of phosphorus (Wedekind et al. 1991) and calcium (Wilgus and Patton 1939) were shown to depress manganese utilization in chicks. Low levels of calcium and iron may act synergistically to affect manganese toxicity by increasing absorption, but it is not known whether ensuring iron plus calcium sufficiency will reduce the toxic effects of manganese once it has been absorbed (Cawte et al. 1989). Thus, the importance of these observations to humans exposed to manganese by the oral or inhalation routes is not clear.
Ethanol has been suspected of increasing the susceptibility of humans to manganese toxicity (e.g., Rodier 1955), but evidence to support this is limited. Singh et al. (1979) and Shukla et al. (1976) reported that concomitant exposure of rats to ethanol and manganese (as manganese chloride in drinking water) led to higher levels of manganese in the brain and liver than if manganese were given alone; the higher levels were accompanied by increased effects as judged by various serum or tissue enzyme levels (Shukla et al. 1978). Although the authors referred to these effects as "synergistic," the data suggest that the effects were more likely additive. Based on the report in humans and evidence in animals, the effects of manganese on humans may be enhanced by the consumption of ethanol, but additional investigation is needed.
There is some evidence from a study in animals that chronic administration of drugs such as chlorpromazine (an antipsychotic) results in increased levels of manganese in the brain, including the caudate nucleus (Weiner et al. 1977). Chronic chlorpromazine treatment sometimes results in tardive dyskinesia, and manganese deposition in the brain might contribute to this condition. It has not been determined whether excess manganese exposure increases the risk of chlorpromazine-induced dyskinesia.
Intramuscular injection of animals with metallic nickel or nickel disulfide (Ni3S2) normally leads to a high incidence of injection-site sarcomas, but this increased incidence is reduced when the nickel is injected along with manganese dust (Sunderman et al. 1976). The mechanism of this effect is not clear, but natural killer cell activity normally undergoes a large decrease following nickel injection, and this is prevented by the manganese (Judde et al. 1987). However, the significance that these observations have for human health effects resulting from exposure to nickel and/or manganese by the oral or inhalation routes is not clear.
One study found that allopurinol, when administered orally to rats, antagonized the oxidative effects of manganese in the striatum and brainstem (Desole et al. 1994). The authors suggest that allopurinol, a xanthine oxidase inhibitor, may exert its protective effect by inhibiting both dopamine oxidative metabolism and xanthine oxidase-mediated production of reactive oxygen species.
3.10. POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE
A susceptible population will exhibit a different or enhanced response to manganese than will most persons exposed to the same level of manganese in the environment. Reasons may include genetic makeup, age, health and nutritional status, and exposure to other toxic substances (e.g., cigarette smoke). These parameters result in reduced detoxification or excretion of manganese, or compromised function of organs affected by manganese. Populations who are at greater risk due to their unusually high exposure to manganese are discussed in Section 6.7, Populations with Potentially High Exposures.
A number of researchers have observed that there is a wide range in individual susceptibility to the neurological effects of inhaled manganese dusts (Rodier 1955; Schuler et al. 1957; Smyth et al. 1973; Tanaka and Lieben 1969). For example, Rodier (1955) reported that the majority of manganism cases in miners occurred after 1–2 years of exposure to the metal, with only six cases observed occurring with 1– 3 months exposure. Schuler et al. (1957) showed that in his group of miners, the average time for manifestation of manganism was 8 years, 2 months, with a minimum exposure of 9 months required for symptoms to present. However, the reason for this variable susceptibility is not clear. One likely factor is a difference in work activities and level of exertion. Another is that rates of manganese absorption and/or excretion can vary widely among individuals (Saric et al. 1977a). These toxicokinetic variations may be due to differences in dietary levels of iron and differences in transferrin saturation (Chandra and Tandon 1973; Davis et al. 1992a, 1992b; Mena et al. 1969; Thomson et al. 1971), to differences in dietary levels of other metals (Chowdhury and Chandra 1987; Gruden and Matausic 1989) or of calcium (Cawte et al. 1989), or to different levels of alcohol ingestion (Schafer et al. 1974). Another factor that might be relevant is dietary protein intake: low-level protein intake appears to increase the effect of manganese on brain neurotransmitter levels in exposed animals (Ali et al. 1983a, 1983b, 1985). However, a genetic basis for the wide difference in susceptibility cannot be ruled out.
One group that has received special attention as a potentially susceptible population is the very young. This is mainly because a number of studies indicate that neonates retain a much higher percentage of ingested or injected manganese than adults, both in animals (Keen et al. 1986; Kostial et al. 1978; Rehnberg et al. 1980) and in humans (Zlotkin and Buchanan 1986). The basis for high manganese retention in neonates is not certain, but is presumably a consequence of increased absorption (Mena et al. 1974; Rehnberg et al. 1980) and/or decreased excretion (Kostial et al. 1978; Miller et al. 1975; Rehnberg et al. 1981), possibly because maternal milk is low in manganese (Ballatori et al. 1987). Regardless of the mechanism, the result of the high retention is increased levels of manganese in the tissue of exposed neonatal animals (Miller et al. 1975; Rehnberg et al. 1980, 1981), especially in the brain (Kontur and Fechter 1985, 1988; Kostial et al. 1978; Kristensson et al. 1986; Miller et al. 1975; Rehnberg et al. 1981). This increase has caused several researchers to express concern over possible toxic effects in human infants exposed to manganese in formula (Collipp et al. 1983; Keen et al. 1986; Zlotkin and Buchanan 1986). At least one recent report indicates that an infant’s rate of absorption of manganese from infant formulas, cow’s milk, and breast milk is similar (Lönnerdal et al. 1994), resulting mainly from recent modifications to formulas to optimize the bioavailability of several essential minerals. There is some limited evidence that prenatal or neonatal exposure of animals to elevated levels of manganese can lead to neurological changes in the newborn (Ali et al. 1983a; Chandra and Shukla 1978; Deskin et al. 1980, 1981; Dorman et al. 2000; Kristensson et al. 1986); other studies have either not observed any neurochemical or neurophysiological effects in young animals exposed to excess manganese or the effects have been transient (Kontur and Fechter 1988; Kostial et al. 1978; Pappas et al. 1997). Currently, there is only one report that indicates that neonatal animals showed adverse neurological effects at a dose of manganese that had no effect on adults (Dorman et al. 2000). Brain concentrations of manganese were elevated in the neonates, but not in the adult animals given comparable doses of manganese for similar durations. The concern is that the young may be more susceptible due to increased absorption and/or retention and the potential toxicity from higher circulating levels of the metal. A few studies have reported increased blood and brain levels of the metal, either because of an inability to clear manganese due to chronic liver disease (Devenyi et al. 1994) or to an excess in parenteral nutrition (Kafritsa et al. 1998; Ono et al. 1995). However, observable neurological signs associated with manganese toxicity were only reported in the case of chronic liver disease (Devenyi et al. 1994). Although data suggest that children, particularly infants, are potentially more susceptible to the toxic effects of manganese, available evidence indicates that individual susceptibility varies greatly. Current information is not sufficient to quantitatively assess how susceptibility in children might differ from adults.
Elderly people might also be somewhat more susceptible to manganese neurotoxicity than the general population. Neurological effects were observed in older persons consuming manganese levels similar to levels found in U.S. surface water and groundwater (Deverel and Millard 1988; EPA 1984; Kondakis et al. 1989). The neurological effects observed in a group of families exposed to manganese in their drinking water were reportedly more severe among the older persons, whereas there was little effect in the youngest (Kawamura et al. 1941). Further, occupational studies indicate that older workers represent the largest numbers of manganese poisoning cases (Rodier 1955; Tanaka and Lieben 1969). More recent occupational (Crump and Rousseau 1999; Gibbs et al. 1999) and environmental (Mergler et al. 1999) manganese exposure studies indicate that increasing age was a factor in poorer performance on certain neurobehavioral tests. For example, Beuter et al. (1999) and Mergler et al. (1999) reported that performance on tests that required regular, rapid, and precise pointing movements was significantly decreased in exposed individuals, especially in those 50 years of age and over with high blood manganese levels. These reports suggest that older persons may have a greater susceptibility to adverse effects from inhaled or ingested manganese. One factor that could contribute to this increased susceptibility is a loss of neuronal cells due to aging or to accumulated neurological damage from other environmental neurotoxicants (Silbergeld 1982). Homeostatic mechanisms might become less effective in aged populations, which leads to higher tissue levels of manganese following exposure (Silbergeld 1982).
Mena et al. (1969) noted that the oral absorption of manganese was increased in individuals with iron-deficiency anemia. Altered nutritional status might be another predisposing factor. The inverse relationship of manganese absorption and iron-status has also been reported in animal models (Davis et al. 1992a, 1992b). It has been suggested that anemic persons may be more susceptible to the toxic effects of manganese because of enhanced absorption of iron and manganese through similar uptake mechanisms (Cotzias et al. 1968). Baldwin et al. (1999) reported an inverse relationship between serum iron and blood manganese levels in individuals environmentally exposed to airborne manganese.
Another group of potential concern is people with liver disease. This is because the main route of manganese excretion is via hepatobiliary transport (see Section 3.4.4), so individuals with impaired biliary secretion capacity would be expected to have a diminished ability to handle manganese excesses. In support of this hypothesis, Hambidge et al. (1989) reported that in a group of infants and children receiving parenteral nutrition, children with liver disease had higher average plasma concentrations of manganese than children without liver disease. Devenyi et al. (1994) also observed increased blood manganese concentrations, abnormal MRI scans indicative of increased manganese in the brain, and dystonia similar to that of patients with manganism, in an 8-year-old girl suffering from cholestatic liver disease. Hauser et al. (1994) reported increased blood and brain manganese in two patients with chronic liver disease and one with cirrhosis of the liver and a portacaval shunt. All three exhibited some form of neuropathy, including postural tremor of the upper extremities and a general lack of alertness, along with failure to concentrate and follow simple commands. In a later study, Hauser et al. (1996) did not observe movement disorders, but did observe the increased blood manganese concentrations and abnormal MRI scans in a group of adults with failing livers. Other studies have shown the link between increased deposition of manganese in the blood and/or the brains of humans with cirrhosis of the liver or chronic liver disease (Pomier-Layrargues et al. 1998; Rose et al. 1999; Spahr et al. 1996).
Patients on parenteral nutrition may be at risk for increased exposure to manganese. Forbes and Forbes (1997) observed that 31 of 32 adults treated with total parenteral nutrition (TPN) due to intestinal failure had increased manganese concentrations in their blood. Nagatomo et al. (1999) observed elevated blood manganese levels and hyperintense signals in the basal ganglia upon T1-weighted MRI in two elderly patients receiving TPN. Both patients exhibited severe symptoms associated with manganese exposure (masked facies, marked rigidity, hypokinesia). When manganese supplementation in the TPN was reduced, the blood and brain levels returned to normal.
Children receiving parenteral nutrition have also been shown to have increased blood manganese concentrations with accompanying hyperintense signals in the globus pallidus as observed by MRI (Fell et al. 1996; Kafritsa et al. 1998; Ono et al. 1995). Fell et al. (1996) studied a group of 57 children receiving parenteral nutrition, 11 of whom had a combination of hypermanganesemia and cholestasis.
Four of these 11 patients died; the 7 survivors had whole blood manganese concentrations ranging from 34–101 μg/L. Four months after reduction or removal of manganese from the supplementation, the blood concentration of manganese decreased by a median of 35 μg/L. Two of the seven survivors had movement disorders, one of whom survived to have a MRI scan. The scan revealed bilateral symmetrically increased signal intensity in the globus pallidus and subthalamic nuclei. These signals were also observed in five other children—one from the original group exhibiting cholestasis with hypermanganesemia and five more given parenteral nutrition chronically with no liver disease. These results indicate that the cholestatic condition is not necessary for manganese to accumulate in the brain. A supporting study is provided by Ono et al. (1995) who observed increased blood manganese concentrations and hyperintense signals on MRI in the brain of a 5-year-old child on chronic parenteral nutrition due to a gastrointestinal failure. Five months after the manganese was removed from the parenteral solution, blood manganese levels returned to normal, and the brain MRI scans were almost completely free of abnormal signals. Further, the authors reported no neurological effects from exposure to manganese. Kafritsa et al. (1998) reported results similar to those of Ono et al. (1995). In the latter study, two siblings, one 9 years old and the other 2 years old, had been administered TPN chronically since the ages of 4 and 1 month(s), respectively. While elevated blood and brain manganese levels were reported (via laboratory analyses and MRI), no adverse neurological or developmental effects were observed. Once the manganese supplementation was reduced, the MRI signals abated, and the blood manganese levels returned to a normal range.
Although human interindividual variability is great concerning the ability to tolerate excess amounts of manganese in the body, these data indicate that, in general, children and the elderly may be more susceptible than young and middle-aged adults due to differential toxicokinetics and potential adverse effects superimposed on normal decline in fine motor function with age.
With respect to the respiratory effects of inhaled manganese (e.g., bronchitis, pneumonitis), people with lung disease or people who have exposure to other lung irritants may be especially susceptible. This is supported by the finding that the inhalation of manganese dusts by manganese alloy workers caused an increased incidence of respiratory symptoms (e.g., wheezing, bronchitis) in smokers, but not in nonsmokers (Saric and Lucic-Palaic 1977b).
3.11. METHODS FOR REDUCING TOXIC EFFECTS
This section will describe clinical practice and research concerning methods for reducing toxic effects of exposure to manganese. However, because some of the treatments discussed may be experimental and unproven, this section should not be used as a guide for treatment of exposures to manganese. When specific exposures have occurred, poison control centers and medical toxicologists should be consulted for medical advice. The following texts provide specific information about treatment following exposures to manganese.
Leikin JB, Paloucek JB. 2002. Leikin and Paloucek's poisoning and toxicology handbook. Hudson, OH: Lexi-Comp, Inc., 773-774.
Schonwald S. 2004. Manganese. In: Dart RC, eds. Medical toxicology. 3rd ed. Philadelphia, PA: Lippicott Williams & Wilkins, 1433-1434.
WHO. 1999. Concise international chemical assessment document 12. Manganese and its compounds. Geneva: United Nations Environment Programme. International Labour Organisation. World Health Organization. http://whqlibdoc.who.int/publications/1999/924153012X.pdf. August 04, 2008.
3.11.1. Reducing Peak Absorption Following Exposure
There is substantial evidence to indicate that an interaction between iron and manganese occurs during intestinal absorption (Chandra and Tandon 1973; Diez-Ewald et al. 1968; Keen and Zidenberg-Cher 1990; Mena et al. 1969; Rehnberg et al. 1982). Cawte et al. (1989) cite low levels of iron and calcium as "synergistic factors" that impact on the toxic effects associated with manganese exposures. In a dietary study investigating the effects of copper, iron, and ascorbate on manganese absorption in rats, these substances were all found to influence manganese absorption, depending in part on their relative concentrations (Johnson and Korynta 1992).
Evidence from these reports suggests that it may be possible to reduce the uptake of manganese and thereby circumvent the potential for toxic effects caused by current and future exposure to excess manganese through specific dietary supplementation. For example, sufficient iron or calcium stores, as opposed to a deficiency in these or other minerals, may reduce manganese absorption, and thus reduce potential toxicity. It is not known whether ensuring iron and calcium sufficiency will reduce the toxic effects of manganese once it has been absorbed into the body because information on critical levels of manganese at target sites is not available. No consistent clinical data are available documenting benefit from ipecac or dilution after ingestion of metallic, inorganic, or organic manganese (Schonwald 2004).
3.11.2. Reducing Body Burden
Inhaled manganese is readily absorbed by the lungs, although some may be retained there. Larger particles of dust containing manganese may be transported by mucociliary transport from the throat to the gut (Drown et al. 1986). Manganese in the gut may be directly absorbed either by a simple diffusion process (Bell et al. 1989) or by a high-affinity, low-capacity, active-transport mechanism (Garcia-Aranda et al. 1983). Once in the plasma, manganese is reportedly transported by transferrin; however, information on the mechanism of uptake in extrahepatic tissues is limited (Keen and Zidenberg-Cher 1990).
In severe cases of manganese poisoning, chelation therapy may be recommended in order to reduce the body burden of manganese and to help alleviate symptoms. Chelation therapy with agents such as EDTA may alleviate some of the neurological signs of manganism, but in cases where it has been used, not all patients have shown improvement, and some of the improvements have not always been permanent (Cook et al. 1974; Schonwald 2004). Nagatomo et al. (1999) recently reported the use of Ca-EDTA treatment to reduce the body burden of two elderly patients with increased blood and brain levels of manganese. These patients exhibited masked faces, hypokinesia, and rigidity that are among the clinical signs of manganese poisoning. The potential use of calcium disodium ethylenediaminetetracetate (CaNa2 EDTA) for the management of heavy metal poisoning was investigated in dogs by Ibim et al. (1992). CaNa2 EDTA-treated dogs (without excess manganese exposure) were found to have decreased manganese levels in their hair. It is possible that the decrease was partially associated with mobilization and redistribution of this element from storage as well as from soft tissues. The authors, however, cautioned that the use of CaNa2 EDTA could adversely affect the metabolism of manganese.
In an attempt to treat seven welders with manganism, a solution of 20% CaNa2 EDTA was administered intravenously at the dose of 1.0 g daily for 3 days followed by a pause for 4 days. The therapy continued for 2–4 courses of this treatment, depending upon the improvement of symptoms. The symptoms, as well as blood manganese concentrations and urinary manganese concentrations, were monitored before and after each course of treatment. EDTA treatment resulted in increased manganese excretion in urine and decreased manganese concentrations in the blood; however, the patients did not show significant improvement in their symptoms (Crossgrove and Zheng 2004). A lack of improvement after EDTA chelation has also been observed in an additional case study of an adult worker (Jiang et al. 2006). It is postulated that four carboxyl groups in the EDTA structure, which are essential to its chelating property, render the molecule poorly lipophilic, thus preventing it from effectively crossing the blood-brain barrier.
Thus, EDTA appears to successfully chelate and remove the extracellular manganese ions in the blood, but with limited access to brain parenchyma, it cannot effectively chelate and remove manganese ions from the brain. Because EDTA cannot significantly remove manganese from damaged neurons, it appears to be of very limited therapeutic value for more advanced cases of manganism.
Cyclohexylene-aminotetraacetic acid (CDTA) and dimercaptol-1-propanesulphonic acid sodium salt (DTPA) were shown to decrease tissue manganese content in rats following inhalation exposure, but it is unknown whether the effects of manganese were alleviated (Wieczorek and Oberdörster 1989a, 1989b).
The use of the anti-tuberculosis drug para-aminosalicylic acid (PAS) to treat manganism has been reported (Jiang et al. 2006). The patient in this case study had palpitations, hand tremor, lower limb myalgia, hypermyotonia, and a distinct festinating gait. She received 6 g PAS per day through an intravenous drip infusion for 4 days and rest for 3 days. Fifteen courses of this treatment were administered to the patient. At the end of PAS treatment, the patient’s symptoms were reportedly significantly alleviated, and handwriting recovered to normal. A reexamination at 17 years after PAS therapy found a general normal presentation in clinical, neurologic, brain MRI, and handwriting examinations. Her gait improved, and although it did not improve to an entirely normal status, it could be described as passable. A literature survey of more than 90 cases using PAS (Jiang et al. 2006) indicates a significant therapeutic benefit.
A study in monkeys reported a long half-life of manganese in the brain following inhalation exposure (Newland et al. 1987). Given that neurotoxicity is of concern with manganese exposure, knowledge of the mechanisms behind this longer half-life in the brain may be central to the development of mitigation methods. Newland et al. (1987) reported that this long half-life reflected both redistribution of manganese from other body depots and a slow rate of clearance from the brain. A later study reported that elevated levels in the brain persisted after inhalation exposure (due to redistribution), whereas for subcutaneous exposure, levels declined when administration was stopped (Newland et al. 1989). The authors observed that the accumulation of manganese in the brain was preferential in specific regions, but was unrelated to the route of exposure (Newland et al. 1989). They also reported that there are no known mechanisms or "complexing agents" that have been shown to remove manganese from the brain.
Few data are available regarding the reversibility of the neurological injury produced by prolonged excess manganese exposure. The effects are thought to be largely irreversible, and treatment for manganese intoxication is mainly supportive (Schonwald 2004). However, some evidence indicates that recovery may occur when exposure ceases (Smyth et al. 1973). Anti-Parkinsonian drugs, such as levo-dopa, have been shown to reverse some of the neuromuscular signs of manganism (Ejima et al. 1992; Rosenstock et al. 1971), but these drugs can produce a variety of side effects, and reports have indicated that they are not effective in improving the symptoms of neurotoxicity in manganism patients (Calne et al. 1994; Chu et al. 1995; Cook et al. 1974; Haddad et al. 1998; Huang et al. 1989; Schonwald 2004). Para-aminosalicylic acid was used successfully to treat two patients who exhibited neurological signs of manganese poisoning; one person made an almost complete recovery and the other was significantly improved. The mechanism for this treatment is unknown (Shuqin et al. 1992). Parenti et al. (1988) has proposed the use of antioxidants such as vitamin E, but the effectiveness of this treatment has not been further evaluated.
3.11.3. Interfering with the Mechanism of Action for Toxic Effects
The oxidation state of manganese may influence both its retention in the body (see Section 3.4.3) and its toxicity (see Section 3.5). Therefore, it is possible that interference with the oxidation of manganese could be a method for preventing manganese cellular uptake and toxicity. Regarding retention, one study suggests that clearance is much more rapid for divalent manganese than for trivalent manganese (Gibbons et al. 1976). Regarding neurotoxicity, Mn(III) appears to be more efficient in enhancing the oxidation of catechols than either Mn(II) or Mn(IV) (Archibald and Tyree 1987). Thus, it is plausible that reducing the formation of Mn(III) could possibly both enhance elimination and prevent neurotoxicity, but no studies were located that evaluate this theory.
Ceruloplasmin is involved in the oxidation of iron and has also been involved in the oxidation of divalent manganese ion to the trivalent state (Gibbons et al. 1976). Selective inhibition of this oxidative function may be a method of mitigating the toxic effects of exposure to manganese. However, inhibition of the oxidation of manganese might also result in adverse effects on transport and cellular uptake of other essential metals, especially iron. Furthermore, it is not completely clear how the oxidation state of manganese is related to its normal function in neural cells or how this role is altered in manganese toxicity. Both Mn(II) and Mn(III) have been reported as components of metalloenzymes (Keen and Zidenberg-Cher 1990; Leach and Lilburn 1978; Utter 1976).
Manganese has been shown to catalyze the oxidation of dopamine in vitro; Cawte et al. (1989) reported that the toxicity induced by manganese resulted from the depletion of dopamine and the production of dopamine quinone and hydrogen peroxide through this mechanism. Antioxidants were tested for their ability to inhibit the dopamine oxidation induced by manganese, and it was found that ascorbic acid and thiamine completely inhibited dopamine oxidation both in the presence and absence of manganese. The report did not include data on background oxidation levels nor on the extent of dopamine oxidation in the absence of manganese. Results from treatment with antioxidants were viewed as evidence for their use in mitigating the adverse effects of manganese. However, because dopamine oxidation was inhibited to some degree in the absence of manganese, these data could alternately be interpreted as suggesting a more complex mechanism than the direct action of manganese for inducing dopamine oxidation and subsequent cell toxicity. Further investigation of the inhibition of manganese oxidation as a possible mitigation method should be preceded by additional studies to elucidate the role of manganese in its various oxidation states in normal neuronal cell metabolism and to determine whether oxidative stress is a primary mechanism for neurotoxicity mediated by manganese exposure.
3.12. ADEQUACY OF THE DATABASE
Section 104(I)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the Administrator of EPA and agencies and programs of the Public Health Service) to assess whether adequate information on the health effects of manganese is available. Where adequate information is not available, ATSDR, in conjunction with the National Toxicology Program (NTP), is required to assure the initiation of a program of research designed to determine the health effects (and techniques for developing methods to determine such health effects) of manganese.
The following categories of possible data needs have been identified by a joint team of scientists from ATSDR, NTP, and EPA. They are defined as substance-specific informational needs that if met would reduce the uncertainties of human health assessment. This definition should not be interpreted to mean that all data needs discussed in this section must be filled. In the future, the identified data needs will be evaluated and prioritized, and a substance-specific research agenda will be proposed.
3.12.1. Existing Information on Health Effects of Manganese
The existing data on health effects of inhalation, oral, and dermal exposure of humans and animals to inorganic manganese are summarized in Figure 3-15. The purpose of this figure is to illustrate the existing information concerning the health effects of manganese. Each dot in the figure indicates that one or more studies provide information associated with that particular effect. The dot does not necessarily imply anything about the quality of the study or studies, nor should missing information in this figure be interpreted as a “data need”. A data need, as defined in ATSDR’s Decision Guide for Identifying Substance-Specific Data Needs Related to Toxicological Profiles (Agency for Toxic Substances and Disease Registry 1989), is substance-specific information necessary to conduct comprehensive public health assessments. Generally, ATSDR defines a data gap more broadly as any substance-specific information missing from the scientific literature.
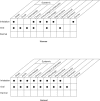
Figure 3-15
Existing Information on Health Effects of Inorganic Manganese. ● Existing Studies
As the upper part of Figure 3-15 reveals, studies in humans exposed to inorganic manganese have focused mainly on intermediate and chronic inhalation exposure and the resulting neurological effects. There are several reports of humans exposed by ingestion and these too have focused on neurological effects. Reproductive effects have been studied in men exposed to manganese by inhalation, but other effects have generally not been formally investigated.
Inorganic manganese toxicity has been investigated in numerous animal studies, both by the oral and the inhalation routes. These studies have included most end points of potential concern. The dermal route for inorganic manganese has not been investigated, but there is no evidence that this exposure pathway is a human health concern. Dermal contact to MMT is expected to occur mainly in occupational settings, and no human dermal contact with mangafodipir is expected to occur. In addition, organic compounds are degraded to some extent in the environment. Thus, dermal effects from organic manganese compounds are not expected to be of great concern for the general population or to persons near hazardous waste sites.
3.12.2. Identification of Data Needs
Presented below is a brief review of available information and a discussion of research needs. Although data are lacking, dermal studies to inorganic manganese are not discussed since there is no evidence that this exposure pathway is a human health concern.
Acute-Duration Exposure. Studies in animals and humans indicate that inorganic manganese compounds have very low acute toxicity by any route of exposure. An exception is potassium permanganate, which is an oxidant that can cause severe corrosion of skin or mucosa at the point of contact (Southwood et al. 1987). Acute inhalation exposure to high concentrations of manganese dusts (manganese dioxide, manganese tetroxide) can cause an inflammatory response in the lung, which can lead to impaired lung function (Maigetter et al. 1976; Shiotsuka 1984). However, this response is characteristic of nearly all inhalable particulate matter (EPA 1985d) and is not dependent on the manganese content of the particle. Large oral doses of highly concentrated solutions of manganese salts given by gavage can cause death in animals (Holbrook et al. 1975; Kostial et al. 1978; Smyth et al. 1969), but oral exposures via food or water have not been found to cause significant acute toxicity (Gianutsos and Murray 1982; NTP 1987a, 1987b). Exposure of mice (Moreno et al. 2009) and rats (Shukakidze et al. 2003) to acute oral doses of about 13 mg manganese/kg as manganese chloride has been associated with behavioral changes, but dietary intakes of manganese in these studies were not measured. Since the acute database is incomplete and studies demonstrating a dose-response are not available, acute MRLs were not derived. In order to derive acute MRL values, further studies may be helpful to define the threshold for adverse effects following acute exposure to manganese. However, any MRL derived for the oral route would have to take into consideration that manganese is an essential nutrient.
Acute-duration exposure studies in animals exposed to MMT via inhalation or via a dermal pathway are lacking. The dermal pathway is very important, because MMT in gasoline that may be spilled on the skin could penetrate and become absorbed. Although the photolability of the compound is an important obstacle for any animal study, carefully planned and executed analyses of the toxicity of this compound to animal models through these exposure pathways are needed.
The likelihood for exposure to mangafodipir is small and clinical trials in humans have shown a great tolerance for a controlled exposure to the compound. Toxicity studies in several different animal species have been performed, including reproductive and developmental studies (and more specifically, teratogenic analysis). Although behavioral data in the young who have been exposed during gestation are relatively limited, human gestational exposure to this compound is not believed to be very likely. Reports of neurological effects have been limited to complaints of headaches in clinical trials. Further evaluation of these effects relative to the distribution of manganese to the brain during clinical use is warranted. Mangafodipir is administered intravenously, which bypasses homeostatic control of the compound. Although animal studies indicate that a single, clinical dose does not cause accumulation of manganese in the brain for longer than 2 weeks (Gallez et al. 1997), human studies have not monitored central nervous system distribution of manganese following mangafodipir injection for longer than half an hour (Lim et al. 1991). In addition, given the neurotoxic effects of excess manganese, evaluation of patients treated with mangafodipir for neurological sequelae are needed.
Intermediate-Duration Exposure. Intermediate-duration inhalation exposure of humans to manganese compounds can lead to central nervous system effects (Rodier 1955). However, reliable estimates of intermediate-duration NOAELs or LOAELs for neurotoxicity in humans are not available. Epidemiological studies in occupationally exposed human populations that help define the intermediate-duration exposure levels (>356 days of exposure) that are associated with neurological effects would be valuable. In the interim, it is expected that the chronic MRL for inhaled inorganic manganese would provide protection for intermediate-duration exposure scenarios. The MRL is based on an analysis of dose-response data for subtle neurological deficits in groups of occupationally exposed workers with average durations of employment from about 5 to 24 years (see Table A-3 in Appendix A); the average duration of employment in workers in the principal study for the MRL was 5.3 years.
Intermediate-duration inhalation studies in animals have yielded NOAEL and LOAEL values for biochemical and neurobehavioral effects (EPA 1977; Morganti et al. 1985; Ulrich et al. 1979a, 1979b), but the range of exposure levels associated with these effects is too wide (an order of magnitude) to define a threshold. Although neurological effects were observed in animals, symptoms characteristic of manganese toxicity (e.g., ataxia, tremor, etc.) are not typically observed in rodent species (with the exception of one study in which ataxia was seen only transiently) (Kristensson et al. 1986). Other rodent studies indicated decreases in motor activity (Gray and Laskey 1980; Komura and Sakamoto 1991), increased activity and aggression (Chandra 1983; Shukakidze et al. 2003), delayed reflexes (Ali et al. 1983a), or deficits in learning (Shukakidze et al. 2003; Vezér et al. 2005, 2007) the effects are not consistent and are observed over a wide dose range. For these reasons, it is concluded that these data are not sufficient to derive an intermediate-duration inhalation MRL. Other animal intermediate-duration studies provide evidence for associations between decreased neuronal cell counts in the globus pallidus and neurobehavioral changes (increased locomotor activity) in rats exposed by inhalation for 13 weeks to a mixture of manganese phosphate/sulfate (at 1.05 mg manganese/m3) or manganese sulfate alone (at concentrations between 0.009 and 0.9 mg manganese/m3), but not to manganese phosphate alone at concentrations up to 1.1 mg manganese/m3 (Normandin et al. 2002; Salehi et al. 2003, 2006; Tapin et al. 2006). Other 13-week rat inhalation exposure studies reported increased brain manganese concentrations and increased locomotor activity after exposure to 3.75 mg manganese/m3 as metallic manganese (St-Pierre et al. 2001) and increased brain manganese concentrations with no increases in olfactory bulb, cerebellar, or striatal concentrations of GFAP after exposure to 0.5 mg manganese/m3 as manganese sulfate or 0.1 mg manganese/m3 as manganese phosphate (Dorman et al. 2004b). Other animal studies have examined the influence of inhalation exposure to manganese sulfate on biochemical end points associated with oxidative stress or inflammation in the brain of rats (Erikson et al. 2005, 2006; HaMai et al. 2006; Taylor et al. 2006) and monkeys (Erikson et al. 2007, 2008). The results from these studies indicate that acute-or intermediate-duration inhalation exposure to manganese sulfate concentrations ranging from about 0.1 to 1.5 mg manganese/m3 can differentially affect brain biochemical markers of neurotoxicity, but understanding of the neurotoxic mechanism of manganese is inadequate to confidently define any one of the observed changes as biologically adverse.
Intermediate-duration oral exposure of humans to manganese has been reported to cause neurotoxicity in two cases (Holzgraefe et al. 1986; Kawamura et al. 1941), but the data for quantitating exposure levels are too limited to define the threshold or to judge whether these effects were due entirely to manganese exposure. An epidemiological investigation of people who have ingested high levels of manganese may provide valuable information on the health risk of intermediate-duration oral exposure and may provide sufficient dose-response data from which to derive an MRL. Additional oral studies in animals including rodents may be valuable in revealing cellular and molecular mechanisms of manganese neurotoxicity; studies on nonhuman primates would probably be the most helpful in estimating a MRL because they appear to be the most suitable animal model for manganese-induced neurological effects comparable to effects observed in humans. However, any MRL derived for the oral route would have to take into consideration that manganese is an essential nutrient and account for manganese intake from daily dietary sources.
Intermediate-duration studies of inhalation and oral exposure to MMT in humans and animals are lacking. Animal studies of this duration evaluating systemic toxicity from exposure to MMT and typical environmental concentrations of its combustion products would be helpful to determine body burdens that might be anticipated for the general population in areas that use this compound. Further, these studies would be helpful in determining mechanisms of toxicity and expected adverse effects in exposed populations.
Due to the nature of mangafodipir administration, which typically occurs only once in a subject, no intermediate-duration studies in humans have been identified for this compound. Although there are a few intermediate-duration studies in animals (Grant et al. 1997a; Larsen and Grant 1997; Treinen et al. 1995), they have focused primarily on reproductive and developmental effects. Studies of the potential neurological effects of exposure to this compound are lacking, although the reason for this may be due to the lack of evidence that the compound distributes in the central nervous system. As discussed previously, the exposure to mangafodipir is expected to be very limited due to the compound’s clinical use. There are no identified data needs for this compound.
Chronic-Duration Exposure and Cancer. As discussed in Sections 2.3 and 3.2.1.4, and Appendix A, a number of epidemiological studies have used sensitive techniques to study the psychological or neurological effects of exposure to low levels of manganese in the workplace (Bast-Pettersen et al. 2004; Beuter et al. 1999; Blond and Netterstrom 2007; Blond et al. 2007; Bouchard et al. 2003, 2005, 2007a, 2007b; Chia et al. 1993a, 1995; Crump and Rousseau 1999; Deschamps et al. 2001; Gibbs et al. 1999, Iregren 1990; Lucchini et al. 1995, 1999; Mergler et al. 1994; Myers et al. 2003a, 2003b; Roels et al. 1987a, 1992, 1999; Summers et al. 2011; Wennberg et al. 1991) or in environmental media close to manganese-emitting industries (Hernández-Bonilla et al. 2011; Lucchini et al. 2007; Kim et al. 2011; Menezes-Filho et al. 2011; Mergler et al. 1999; Riojas-Rodríguez et al. 2010; Rodríguez-Agudelo et al. 2006; Solís-Vivanco et al. 2009; Standridge et al. 2008). Some of the occupational studies have found statistically significant differences between exposed and non-exposed groups or significant associations between exposure indices and neurological effects (Bast-Pettersen et al. 2004; Chia et al. 1993a; Iregren 1990; Lucchini et al. 1995, 1999; Mergler et al. 1994; Roels et al. 1987a, 1992; Wennberg et al. 1991), whereas others have not found significant associations (Deschamps et al. 2001; Gibbs et al. 1999, Myers et al. 2003a, 2003b; Summers et al. 2011; Young et al. 2005). Analyses of dose-response relationships for changes in neurobehavioral tests in several of these studies (Gibbs et al.1999; Iregren et al. 1990; Lucchini et al. 1999; Mergler et al. 1994; Roels et al. 1992; Wennberg et al. 1991) provide the basis of the current chronic inhalation MRL for inorganic manganese (as described in Chapter 2 and Appendix A)
Additional studies involving follow-up evaluation of previously exposed occupational cohorts may be useful to provide information on threshold levels that are correlated with observed preclinical effects. Additional studies of populations living close to manganese-emitting industries that clearly quantify exposure sources (dietary, water consumption, airborne) also may be useful to better describe neurotoxicological potentials of low-level exposure to airborne manganese.
In early animal studies, intermediate or chronic inhalation exposure of monkeys and rats to manganese dusts did not produce neurological signs similar to those seen in humans (Bird et al. 1984; EPA 1983c; Ulrich et al. 1979a, 1979b). For example, Ulrich et al. (1979a) reported that monkeys continually exposed for 9 months to aerosols of manganese dioxide at concentrations as high as 1.1 mg manganese/m3 showed no obvious clinical signs of neurotoxicity, no histopathological changes in brain tissues, and no evidence for limb (leg) tremor or electromyographic effects on flexor and extensor muscles in the arm. However, in a chronic study with Rhesus monkeys, decreased levels of dopamine were found in several regions of the brain (caudate and globus pallidus) (Bird et al. 1984). Behavioral tests detected signs of neurological effects in mice (increased open-field activity and decreased maternal pup retrieval latency), although these are only seen at relatively high exposure levels (60–70 mg manganese/m3) (Lown et al. 1984; Morganti et al. 1985). More recent animal intermediate-duration inhalation studies provide evidence for decreased neuronal cell counts in the globus pallidus and neurobehavioral changes (Normandin et al. 2002; Salehi et al. 2003, 2006; Tapin et al. 2006); increased brain manganese concentrations and increased locomotor activity (St-Pierre et al. 2001); increased brain manganese concentrations without increases in GFAP (Dorman et al. 2004b); and increased biochemical end points associated with oxidative stress or inflammation in the brain (Erikson et al. 2005, 2006; HaMai et al. 2006; Taylor et al. 2006) and monkeys (Erikson et al. 2007, 2008). The results from these studies indicate that acute-or intermediate-duration inhalation exposure to manganese sulfate concentrations ranging from about 0.1 to 1.5 mg manganese/m3 can differentially affect brain biochemical markers of neurotoxicity, but understanding of the neurotoxic mechanism of manganese is inadequate to confidently define any one of the observed changes as biologically adverse.
Chronic inhalation studies in animal models (Bird et al. 1984; EPA 1977; Newland et al. 1989; Olanow et al. 1996) indicate that while non-human primates are very sensitive to the neurological effects of manganese at very low doses (depending on exposure route), rodent models do not exhibit the same neurological symptoms as humans and monkeys despite the administration of high doses through inhalation, oral, and intravenous exposure routes. Although there is an apparent difference in susceptibility, neurological effects have been observed in rodents treated with manganese. Additional studies in animals could be valuable to increase our understanding of the mechanism of manganese-induced disease and the basis for the differences between humans and animals.
Some data on neurological or other health effects in humans from repeated or chronic oral intake of manganese exist (Bouchard et al. 2007c, 2011; Cawte et al. 1987; He et al. 1994; Holzgraefe et al. 1986; Kawamura et al. 1941; Kilburn 1987; Kondakis et al. 1989; Vieregge et al. 1995; Wasserman et al. 2006, 2011; Wright et al. 2006; Zhang et al. 1995). The majority of these studies are limited by uncertainties in the exposure routes, total exposure levels, duration of exposure, or the influence of other confounding factors; none of these studies adequately assessed daily dietary manganese intake. Eight studies (Bouchard et al. 2007c, 2011; Brna et al. 2011; He et al. 1994; Sahni et al. 2007; Wasserman et al. 2006, 2011; Zhang et al. 1995) indicate concentrations of manganese in drinking water that may be associated with preclinical neurological effects in children, but the studies have several limitations.
As discussed in Section 2.3, no oral MRLs were derived for acute-, intermediate-, or chronic-duration oral exposure to manganese, even though the limited human data and extensive animal data clearly identify neurobehavioral changes as the most sensitive effect from intermediate- and chronic-duration oral exposure to excess inorganic manganese. However, inconsistencies in the dose-response relationship information across studies evaluating different neurological end points under different experimental conditions in different species, as well as a lack of information concerning all intakes of manganese (e.g., dietary intakes plus administered doses), make it difficult to derive intermediate- or chronic-duration MRLs using standard MRL derivation methodology from the animal studies. An interim guidance value of 0.16 mg manganese/kg/day is recommended for ATSDR public health assessments. The interim guidance value is based on the Tolerable Upper Intake Level for adults of 11 mg manganese/day established by the FNB/IOM (2001) based on a NOAEL for Western diets. The interim guidance value is necessary because of the prevalence of manganese at hazardous waste sites and the fact that manganese is an essential nutrient.
Additional chronic oral studies, especially epidemiological studies in populations exposed to high levels of either inorganic and organic manganese in the environment, particularly the combustion products of MMT in areas of high traffic density, would be valuable for evaluating the potential for adverse effects from oral exposure to excess manganese from the environment in addition to that ingested through dietary intake.
No studies or anecdotal reports were located that described cancer associated with exposure of humans to inorganic manganese. Chronic oral exposure of rats and mice to high doses of manganese sulfate has provided equivocal evidence of carcinogenic potential (NTP 1993); however, the lack of evidence for the carcinogenic potential of manganese in humans and the equivocal evidence in animals suggest that the potential for cancer may be low. Further animal studies are not needed at this time.
MMT has not been found to induce tumor formation in rodents (Witschi et al. 1981) and additional studies measuring this end point would be useful to corroborate the limited database. Though no studies of carcinogenesis involving mangafodipir exposure were identified, there are no data needs regarding this end point with this compound.
Genotoxicity. One study was located regarding the genotoxic effects of inorganic manganese in humans. An increase in chromosomal aberrations was observed in welders exposed to manganese; however, the welders were also exposed to nickel (known to cause chromosomal aberrations) and iron, so the observed increase could not be attributed solely to manganese (Elias et al. 1989). Some in vivo studies in fruit flies and rats have been negative (Dikshith and Chandra 1978; Rasmuson 1985; Valencia et al. 1985), but manganese has been found to be clastogenic in mice (Joardar and Sharma 1990). In vitro studies in bacteria, yeast, and cultured mammalian cells have yielded mixed, but mainly positive, results (Casto et al. 1979; De Méo et al. 1991; Joardar and Sharma 1990; Kanematsu et al. 1980; Nishioka 1975; NTP 1993; Oberly et al. 1982; Orgel and Orgel 1965; Singh 1984; Ulitzur and Barak 1988; Wong and Goeddel 1988; Zakour and Glickman 1984). Additional studies, especially in cultured mammalian cells, heritable cell types, or in lymphocytes from exposed humans, would be valuable in clarifying the genotoxic potential of manganese. As for organic manganese, no genotoxicity studies were located regarding MMT and studies measuring this end point are needed. Genotoxicity studies for mangafodipir have shown negative effects (Grant et al. 1997a).
Reproductive Toxicity. Men who are exposed to manganese dust in workplace air report decreased libido and impotency (Emara et al. 1971; Mena et al. 1967; Rodier 1955), and may suffer from sexual dysfunction (Jiang et al. 1996b) and decreased sperm and semen quality (Wu et al. 1996). In addition, studies in animals indicate that manganese can cause direct damage to the testes (Chandra et al. 1973; Seth et al. 1973). While the Jiang et al. (1996b) study suggests testicular damage in occupationally exposed men, additional epidemiological studies involving these subjects or other exposed groups to more fully evaluate reproductive function would be valuable. Results from such studies may provide definitive exposure-response data on reproductive function (e.g., impotence, libido, and number of children).
Additional studies in animals are needed to determine whether the testes are damaged directly from exposure to manganese. Information on adverse reproductive effects in women is not available. Data from studies in female animals indicate that manganese can cause post-implantation loss when administered through both oral and subcutaneous exposure routes in female mice and rats (Colomina et al. 1996; Sánchez et al. 1993; Szakmáry et al. 1995; Treinen et al. 1995). To establish more clearly whether or not this is a human health concern, two types of studies would be valuable. First, single-generation reproductive studies of female animals exposed by the inhalation route could be done. Then, if strong evidence for concern is found in animals from these studies, epidemiological studies that included women and men exposed in the workplace would be valuable to assess the effects of manganese on reproductive function.
Developmental Toxicity. There is a growing body of human data on potential developmental effects of excess manganese, although these studies are generally confined to studies of neurodevelopmental effects as observed in children. The incidences of stillbirths and malformations have been studied in an Australian aboriginal population living on an island where environmental levels of manganese are high (Kilburn 1987), but small population size and lack of data from a suitable control group preclude determining whether reported incidence of developmental abnormalities is higher than average. Hafeman et al. (2007) reported high mortality among infants <1 year of age in a Bangladesh population where the drinking water supplied by certain local wells contained high levels of manganese. Similarly, Spangler and Spangler (2009) reported increased infant mortality rates in counties in North Carolina with higher groundwater manganese concentrations after accounting for such confounders as low birth weight, economic status, education, and ethnicity. Two studies investigated neurobehavioral and school performances (He et al. 1994; Zhang et al. 1995) of children exposed to excess levels of manganese in water and food. However, these studies did not report data on either lengths of exposure to the metal or on excess manganese intake compared to control areas. More recent investigations include epidemiological studies that have detected altered behavioral and cognitive performance among children exposed to excess levels of manganese in their local drinking water (Bouchard et al. 2007c, 2011; Wasserman et al. 2006, 2011). These results suggest the neurotoxic potential of excessive manganese exposure to children, but these studies have uncertainties that preclude the establishment of causal relationships between the observed effects and manganese exposure. The studies are limited in their ability to address several important concerns, such as whether manganese alone is responsible for the observed effects and the contribution of dietary manganese levels as well as inhalation exposure levels and small sample sizes. Studies evaluating developmental effects with clear analysis of exposure levels and duration are needed to estimate dose-response relationships of manganese toxicity in children.
Several developmental studies have been performed in animals, but they are mainly limited to rodent species and have measured limited developmental end points. One study in pregnant mice that inhaled manganese resulted in decreased pup weight and a transient increase in activity (Lown et al. 1984). Other studies have indicated that oral exposure to manganese adversely affects reproductive development in male mice (Gray and Laskey 1980) and rats (Laskey et al. 1982, 1985). A single study on rats involving oral exposure indicated that manganese caused a transient decrease in pup weight and increased activity (Pappas et al. 1997). Another study involving gavage dosing reported skeletal abnormalities in unborn pups, but these effects were resolved in pups allowed to grow to 100 days of age (Szakmáry et al. 1995). Neurobehavioral effects have been shown in neonates given excess manganese orally from PND 1 to 21 (Dorman et al. 2000; Reichel et al. 2006; Tran et al. 2002a). Several studies have shown neurochemical changes in offspring of dams exposed to increased manganese concentrations (Lai et al. 1991; Garcia et al. 2006, 2007) or in neonatal animals dosed with excess manganese (Anderson et al. 2007a, 2009; Chandra and Shukla 1978; Deskin et al. 1981; Dorman et al. 2000; Kern and Smith 2011; Kern et al. 2010; Kristensson et al. 1986; Moreno et al. 2009; Reichel et al. 2006; Tran et al. 2002a, 2002b). Also of interest is the possibility that developmental manganese exposure may influence the timing of puberty; such results have been observed in studies of both male and female rats (Lee et al. 2006; Pine et al. 2005).
Studies conducted in infant Rhesus monkeys found that soy-based infant formulas (which contain higher manganese levels than cow’s milk) and a soy-based infant formula supplemented with manganese produced behavioral changes that may be comparable to those implicated in attention deficit-hyperactivity disorders (Golub et al. 2005). Several studies have shown neurobehavioral changes in rodents as well (Dorman et al. 2000; Kern and Smith 2011; Kern et al. 2010; Kristensson et al. 1986; Moreno et al. 2009; Tran et al. 2002a, 2002b).
Other studies indicate that injected manganese is more toxic to a developing fetus than inhaled or ingested manganese. Manganese injected subcutaneously or intravenously during the gestation period causes serious effects on skeletal development and ossification, but studies to date using this exposure pathway have not measured neurological deficits in pups or young rodents. The relevance to humans of results from these injection studies is unclear.
The monkey is increasingly regarded as a more appropriate model for neurological end points; however, monkey studies are extremely expensive and will be limited for this reason. Evaluation of appropriate end points in rodent assays by the oral and inhalation route are needed so that these models can be used to increase the body of knowledge of the developmental toxicity of manganese. Further, the one developmental study involving inhalation exposure (Lown et al. 1984) had many complications; additional studies involving neurobehavioral effects in animals following gestational and postnatal exposure to airborne manganese are necessary. A few developmental studies have involved sectioning fetuses to detect internal malformations (Blazak et al. 1996; Grant et al. 1997a; Szakmáry et al. 1995; Treinen et al. 1995). However, these studies have primarily administered the manganese intravenously, except for Szakmáry et al. (1995). Additional teratogenesis studies that assess bone malformations following inhalation and oral exposures using a wide range of doses are needed given that manganese overexposure affects the developing skeletal system (Blazak et al. 1996; Grant et al. 1997a; Szakmáry et al. 1995; Treinen et al. 1995). In order to improve the accuracy of the development of an oral MRI for manganese, additional developmental neurotoxicology studies using a functional observational battery design and using a wide range of well-established measures in rodents and primates would be useful (Moser 2000).
Immunotoxicity. Studies in animals indicate that injection or consumption of manganese compounds can cause significant changes in the functioning of several cell types of the immune system (NTP 1993; Rogers et al. 1983; Smialowicz et al. 1985, 1987). However, it is not known whether these changes are associated with significant impairment of immune system function. Further studies are needed to etermine whether these effects also occur after inhalation exposure in animals or humans. If so, a battery of immune function tests would be valuable in determining if observed changes result in a significant impairment of immune system function.
Neurotoxicity. Studies in humans exposed to high levels of manganese dust in the workplace provide clear evidence that the chief health effect of concern following manganese exposure is injury to the central nervous system (Emara et al. 1971; Mena et al. 1967; Rodier 1955; Schuler et al. 1957; Smyth et al. 1973). As discussed previously, a number of epidemiological studies have used batteries of neurobehavioral tests of neuromotor, cognition, and mood states to study the neurological effects of exposure to low levels of manganese in the workplace. Analyses of dose-response relationships for changes in neurobehavioral tests in several of these studies (Gibbs et al.1999; Iregren et al. 1990; Lucchini et al. 1999; Mergler et al. 1994; Roels et al. 1992; Wennberg et al. 1991) provide the basis of the current chronic inhalation MRL for inorganic manganese (as described in Chapter 2 and Appendix A). Additional follow-up studies to further evaluate the reversibility of manganese-induced effects and define threshold exposure levels above which manganese-induced neurological effects are irreversible may be useful
Studies in communities surrounding manganese industries have also reported evidence for associations between deficits in neurological end points (such as attention impairments, postural stability, and motor impairments) and increasing biomarkers of manganese exposure in adults and children, but all potential sources of exposure (e.g., air, diet, drinking water) could not be accounted for in these studies and they do not provide useful dose-response data for deriving an MRL for inhaled manganese (Baldwin et al. 1999; Beuter et al. 1999; Bowler et al. 1999; Hernández-Bonilla et al. 2011; Kim et al. 2011; Menezes-Filho et al. 2011; Mergler et al. 1999; Solís-Vivano et al. 2009; Standridge et al. 2008; Riojas-Rodríguez et al. 2010; Rodríguez-Agudelo et al. 2006). More studies that include analyses of both sexes and assess the relationship between environmental sources of excess manganese, altered manganese body burden, and the potential for adverse effects may be useful. Further studies may be useful to determine whether manganese from MMT and/or its unique combustion products contribute to airborne manganese concentrations that can be associated with adverse effects (e.g., respiratory or neurological effects).
The evidence for neurotoxicity in humans following oral exposure to manganese is inconclusive due to several limitations in the majority of these reports (Bouchard et al. 2007c, 2011; Holzgraefe et al. 1986; Kawamura et al. 1941; Kilburn 1987; Kondakis et al. 1989; Wasserman et al. 2006, 2011). One report in Japanese adults (Iwami et al. 1994) showed the link between eating food with concentrations of manganese on the high end of the normal range of a typical Western diet (5.79 mg manganese ingested per day) and low intake concentrations of magnesium associated with an increased incidence of motor neuron disease. Six studies in children (Bouchard et al. 2007c, 2011; He et al. 1994; Wasserman et al. 2006, 2011; Zhang et al. 1995) indicated that those who ingested drinking water and/or who ate food with increased concentrations of manganese (≥0.241 mg/L) for at least 3 years had measurable deficits in performance on certain tests. In addition, the children exposed to manganese performed more poorly in school compared to non-exposed control students (who drank water with manganese concentrations no higher than 0.04 mg/L), as measured in mastery of Chinese, performance in mathematics, and overall grade average (Zhang et al. 1995). These studies show that both adults and children show adverse neurological effects from oral exposure to excess manganese.
Studies in rodents and nonhuman primates indicate that oral intake of high doses of manganese can lead to biochemical and behavioral changes indicative of nervous system effects (Bonilla and Prasad 1984; Chandra 1983; Gupta et al. 1980; Kristensson et al. 1986; Lai et al. 1984; Nachtman et al. 1986), and this is supported by intravenous studies in monkeys (Newland and Weiss 1992). Rodents do not appear to be as susceptible to manganese neurotoxicity as humans; however, a study by Newland and Weiss (1992) indicates that Cebus monkeys would be a reasonable animal model. More recent studies demonstrated LOAEL values of 5.6 mg manganese/kg/day for severely impaired cognitive performance in a maze test following 30-day dietary exposure of adult white rats (Shukakidze et al. 2003); 6.5 mg manganese/kg/day for decreased open-field locomotor activity and acoustic startle response and impaired performance in maze learning (a test of spatial memory) in male adult Wistar rats exposed for 10 weeks by gavage (Vezér et al. 2005, 2007); 11 mg manganese/kg/day for increased pulse-initiated acoustic startle response in Sprague-Dawley rats exposed on PNDs 1–21 (Dorman et al. 2000); and 328 mg manganese/kg/day (but not 107.5 mg manganese/kg/day) for decreased activity during sleep and decreased play activity but no effects on gross motor maturation or performance in cognitive tests in young monkeys (Golub et al. 2005). In contrast, hand steadiness or self-reported scales for assertiveness or anger were not different in adult human female subjects following 8 weeks of exposure to dietary doses of 0.01 or 0.3 mg manganese/kg/day (Finley et al. 2003). Further studies in animals may help determine the basis for the apparent differences in route and species susceptibility.
Additional studies in animals concerning the cellular and biochemical basis of manganese neurotoxicity, including a more detailed analysis of precisely which neuronal cell types are damaged and why, are needed. For example, Lazrishvilli et al. (2009) observed gliosis in the brains of 40-day-old pups of rat dams administered 4.4 or 8.7 mg manganese/kg/day in the diet before, during, and after pregnancy, but Rivera-Mancia et al. (2007) did not find gliosis in the brains of adult rats exposed to 147 mg manganese/kg/day. Further studies may prove helpful in elucidating mechanism(s) of toxic action and could potentially lead to developing methods for mitigating adverse effects induced by manganese.
Epidemiological and Human Dosimetry Studies. As already noted, there are numerous epidemiological studies of workers exposed to manganese dusts in air, and the clinical signs and symptoms of the resulting disease are well established. However, these studies have only involved males and have mostly involved the inhalation route of exposure. Additional epidemiological studies on populations exposed to manganese dust in the workplace or local environments (e.g., such as near foundries, populations exposed to manganese emissions from MMT-burning automobiles, particularly those living in areas of high-traffic density, and populations exposed to above-average oral intakes [either through water and/or food]) may help to strengthen conclusions on dose-response relationships and no-effect exposure levels. This would be helpful in evaluating potential risks to people who may be exposed to above-average manganese levels near hazardous waste sites.
Biomarkers of Exposure and Effect
Exposure. Studies in humans have shown that it is difficult to estimate past exposure to manganese by analysis of manganese levels in blood, urine, feces, or tissues (Roels et al. 1987b; Smyth et al. 1973; Valentin and Schiele 1983; Yamada et al. 1986). This is the result of several factors: (1) manganese is a normal component of the diet and is present in all human tissues and fluids, so above average exposure must be detected as an increase over a variable baseline; (2) manganese is rapidly cleared from the blood and is excreted mainly in the feces, with very little in the urine; and (3) manganese absorption and excretion rates are subject to homeostatic regulation, so above average exposures may result in only small changes in fluid or tissue levels. Probably the most relevant indicator of current exposure is manganese concentrations in tissues, but at present, this can only be measured in autopsy or biopsy samples. Studies on new, noninvasive methods capable of measuring manganese levels in vivo, either in the whole body or in specific organs (e.g., brain), would be very helpful in identifying persons with above average exposure. Dorman et al. (2006b) evaluated the use of the pallidal index (PI—ratio of hyperintensities in the globus pallidus and the adjacent subcortical frontal white matter) and the T1 relaxation rate (R1) from MRI to reflect manganese concentrations determined by analytical chemistry in brain regions and concluded that R1 can be used to estimate regional brain manganese concentrations and be used as a reliable biomarker of occupational manganese exposure.
Effect. The principal biological markers of toxic effects from manganese exposure are changes in the levels of various neurotransmitters and related enzymes and receptors in the basal ganglia (Bird et al. 1984; Bonilla and Prasad 1984; Eriksson et al. 1987a, 1987b). Noninvasive methods to detect preclinical changes in these biomarkers or in the functioning of the basal ganglia need to be developed to help identify individuals in whom neurological effects might result. Research to determine the correlation between urinary excretion levels of neurotransmitters, neurotransmitter metabolites, and/or 17-ketosteroids (Bernheimer et al. 1973; Rodier 1955; Siqueira and Moraes 1989) and the probability or severity of neurological injury in exposed people is also needed. Measurements of MnSOD as a biomarker of effect may also be helpful (Greger 1999), but there is a lack of information concerning the relationship of this enzyme to manganese toxicity.
Research in the use of Clara cell protein CC16 may be useful in identifying populations at risk from exposure to MMT; however, the majority of exposure to this compound is expected to arise from inhalation and ingestion of its combustion products. Therefore, increased use of MMT in gasolines necessitates the development of biomarkers of exposure to inorganic manganese compounds, as discussed previously.
Absorption, Distribution, Metabolism, and Excretion. The toxicokinetics of manganese absorption, distribution, and excretion have been studied in both humans and animals. The oral absorption rate is about 3–5% in humans (Davidsson et al. 1988, 1989a; Mena et al. 1969), but the rate may vary depending on age and dietary iron and manganese intake levels (Chandra and Tandon 1973; Diez-Ewald et al. 1968; Rehnberg et al. 1982; Thomson et al. 1971). Information is needed on the relative proportion of manganese that is absorbed via the gut following mucociliary transport of particles from the lung to the stomach. The oral absorption rate may depend on the chemical form of manganese ingested, but data on this are sparse. Data on the differences in uptake as a function of chemical species (manganese dioxide, manganese tetroxide) and particle size would also be valuable in assessing human health risk from different types of manganese dusts. Absorption of manganese deposited in the lung is expected to be higher for soluble forms of manganese compared with relatively insoluble forms of manganese (Aschner et al. 2005; Roels et al. 1997). Results consistent with nasal uptake of manganese and transport to the brain along neuronal tracts have been obtained in several animal studies (Brenneman et al. 2000; Dorman et al. 2001a, 2002a; Elder et al. 2006; Fechter et al. 2002; Henriksson et al. 1999; Lewis et al. 2005; Normandin et al. 2004; Tjälve and Henriksson 1999; Tjälve et al. 1996; Vitarella et al. 2000). Following nasal instillation of solutions of manganese chloride or sonicated suspensions of ultrafine insoluble manganese oxide particles to rats, similar manganese concentrations were found in the brain olfactory bulb (Elder et al. 2006). These results suggest that ultrafine particles can be distributed from the nasal mucosa to the brain olfactory bulb. Absorption of manganese deposited in the lung or nasal mucosa of rats is expected to be influenced by iron status, with enhanced absorption under iron-deficient conditions and diminished absorption under iron-excess conditions (Thompson et al. 2006, 2007).
Manganese appears to be distributed to all tissues, including the brain (Aschner et al. 2005, 2007; Kristensson et al. 1986; Rehnberg et al. 1980, 1981, 1982). Inhaled manganese appears to be distributed more extensively to the brain than ingested manganese and there are differences in distribution between different forms of manganese (manganese chloride compared with manganese dioxide or manganese phosphate) (Dorman et al. 2001a, 2004b; Roels et al. 1997). Addtional research would be useful in understanding how particle size and solubility of manganese forms influence distribution of manganese to and within the brain. In addition, the metabolism of manganese (specifically, the degree and the rate of oxidation state interconversions) has not been thoroughly investigated. Data on this topic are needed to understand the mechanism of manganese toxicity and would help in evaluating the relative toxicity of different manganese compounds. Excretion of manganese is primarily through the feces (Drown et al. 1986; Klaassen 1974; Mena et al. 1969); because the rate of excretion is an important determinant of manganese levels in the body, further studies would be valuable on the biochemical and physiological mechanisms that regulate manganese excretion.
Additional studies would be useful to more fully elucidate the pharmacokinetic mechanisms responsible for uptake, distribution, and excretion in humans and animals, including studies to determine the following: control rates and processes for uptake of ingested manganese by the intestines and liver, including uptake rates of protein-bound forms by the liver; oxidation rates of manganese in the blood and tissues; relative speciation of Mn(II vs. III) in blood transport mechanisms into the central nervous system, including transfer rates; competition between manganese and iron in terms of transport processes; and distribution following long-term exposures to assess potential storage depots.
Andersen et al. (1999) suggested that an approach to setting acceptable exposure levels for an essential, but neurotoxic, nutrient such as manganese could be based on predicting exposure levels by any route that would increase brain manganese concentrations to a small fraction (e.g., 10–25%) of the variation observed in the general human population. Reliable and validated multiple-route PBPK models for multiple species, including humans, are needed to take this approach to setting acceptable exposure levels. Efforts to develop such models in rats, monkeys, and humans have been described, including the development of models for gestational and postnatal periods (Leavens et al. 2007; Nong et al. 2008; Schroeter et al. 2011; Teeguarden et al. 2007a, 2007b, 2007c; Yoon et al. 2011, 2009a, 2009b). As discussed by Yoon et al. (2011), confidence in predictions from the human models may improve with more information on the normal range and fluctuation of human brain manganese concentrations during early postnatal periods, the relationship between blood manganese concentrations and target tissue dosimetry, and the extent of induction of neonatal biliary excretion.
Data on the pharmacokinetics of mangafodipir are sufficient for environmental assessment purposes. Additional studies concerning absorption, distribution, metabolism, and excretion of MMT, via inhalation, ingestion, and dermal exposures, would be very helpful.
Comparative Toxicokinetics. Several papers have reviewed the fairly extensive literature showing differences in the expression of manganese neurotoxicity in humans, nonhuman primates, and rodents (Aschner et al. 2005; Gwiazda et al. 2007; Newland et al. 1999). Aschner et al. (2005) concluded that manganese-exposed monkeys show overlapping effects to those observed in patients with manganism (e.g., retention of manganese in the basal ganglia and loss of dopamergic neurons), but similar changes in regional brain manganese concentrations, neurochemical concentrations, and neuropathological effects have been observed less consistently in rodents. Likewise, Gwiazda et al. (2007) concluded from their analysis of estimated internal cumulative doses associated with neurobehavioral, histological, and neurochemical changes in manganese-exposed animals that the range of adverse internal cumulative doses extended more than 2 orders of magnitude above the lowest estimated doses associated with subtle neurological deficits in manganese-exposed workers. The reasons for these differences are poorly understood, but may be due to interspecies differences in toxicokinetics or toxicodynamics (i.e., differences in tissue sensitivities). As discussed in the previous section, recent extrapolations of animal PBPK models to humans may be improved by more information on the normal range and fluctuation of human brain manganese concentrations during early postnatal periods, the relationship between blood manganese concentrations and target tissue dosimetry, and the extent of induction of neonatal biliary excretion (Yoon et al. 2011).
Methods for Reducing Toxic Effects. In general, the methods which provide the greatest likelihood of reducing toxic effects are the same as those aimed at reducing body burden (see Section 3.11.2). The recommended methods for the mitigation of manganese toxicity (manganism) are mainly supportive (Schonwald 2004). Administration of anti-Parkinson drugs, such as levo-dopa, is of little use (Calne et al. 1994; Chu et al. 1995; Cook et al. 1974; Schonwald 2004; Huang et al. 1989; Leikin and Paloucek 2002). Chelation therapy with agents such as ethylenediaminetretraacetic acid (EDTA) has reportedly been effective in reducing some of the symptoms (Schonwald 2004; Haddad and Winchester 1990), but was not effective in all cases (Crossgrove and Zheng 2004; Jiang et al. 2006). Studies on the efficacy of newly developed methods to reduce the toxic effects of manganese are needed. The available data indicate that para-aminosalicylate has been successfully used to treat neurological symptoms of manganese poisoning in several patients (Shuqin et al. 1992; Jiang et al. 2006). The use of the antioxidant vitamin E has also been proposed to mitigate manganese-induced effects (Parenti et al. 1988). Additional studies on the efficacy of these treatments are needed. Further evaluation for the mitigation of effects from excess exposure to manganese is also needed.
Methods for reducing toxic effects have not been identified for MMT.
Children’s Susceptibility. Data needs relating to both prenatal and childhood exposures, and developmental effects expressed either prenatally or during childhood, are discussed in detail in the Developmental Toxicity subsection above.
Children have been identified as a potentially susceptible population because of their high absorption and/or retention of manganese as compared to adults. Although some available studies indicate that tissue concentrations of human fetuses are comparable to adults, animal studies indicate that neonates retain higher tissue concentrations than adult animals. Researchers hypothesize that this increased retention of manganese may lead to neurotoxicity. Existing data indicate that the adverse neurological effects of manganese overexposure from intravenous and oral sources are qualitatively similar in children and adults. One study has reported that neonates are more susceptible to the effects of oral exposure to excess manganese than adults (Dorman et al. 2000). Additional quantitative information on the levels of manganese that result in adverse effects in children as compared to adults for inhalation, oral, and intravenous exposures are needed. Further, analysis of existing data from effects observed in the clinical setting might be helpful.
There are inadequate data on the pharmacokinetics of manganese in children. Although two studies provided typical serum manganese levels in differing ages of healthy children (Alarcón et al. 1996; Rükgauer et al. 1997), no studies have provided any data on the distribution of manganese in infants or adolescents. Studies in animals, particularly nonhuman primates, are needed to clearly elucidate the pharmacokinetic handling of manganese in neonates and the young (absorption, metabolism, distribution, elimination). There are no PBPK models for children, embryos, fetuses and pregnant women, infants and lactating women, or adolescents. Such models would be very informative if they could assist in the identification of depots for manganese storage under conditions of excess exposure, as well as the nutritional needs of these age groups for the compound. One study was available that would provide information on the concentrations of manganese that might be found in the developing fetus of a highly-exposed mother (Jarvinen and Ahlström 1975). Further studies of this nature, especially those that measure neurological end points in live offspring following excess exposure, are needed. Similarly, data are needed to determine whether increased amounts of manganese might be present in the breast milk of a mother with significantly elevated blood or tissue manganese concentrations.
There are likely to be multiple mechanisms of manganese toxicity and most of these have probably been elucidated. However, there is a deficiency in our knowledge of how these mechanisms act singly or in combination to explain the different functional deficits observed in children versus adults. There are inadequate data to determine whether metabolism of manganese is different in children than in adults. Manganese is necessary for normal functioning of certain enzymes. However, there are no definitive data to indicate that children might need more manganese than adults for normal body processes. A few studies suggest that children may have a higher need for manganese than adults, based on the increased retention of manganese in the brains of certain neonatal animals, but this hypothesis has not been proven. Additional studies are necessary to determine the nutritional requirements of children for manganese, especially in infants for which FNB/IOM has not provided any guidelines.
Studies indicate that children exposed to increased concentrations of inorganic manganese, either via the diet, due to inability to clear the compound from the body or through parenteral nutrition, develop neurological dysfunction similar to that of adults (Devenyi et al. 1994; Fell et al. 1996; He et al. 1994; Zhang et al. 1995). Other data exist that indicate that children may not be as susceptible as adults to the adverse neurological effects of inorganic manganese (Kawamura et al. 1941), but the limitations in this report make predictions about susceptibility inconclusive. Additional animal studies comparing the potential for inorganic manganese to induce neurological effects in different age groups are needed to help understand the susceptibility of the young compared to adults.
The mechanism of action of inorganic manganese toxicity has not been identified. Studies in humans indicate that children and adults with increased manganese deposition in the globus pallidus and other basal regions suffer neuromuscular deficits. It has been suggested that manganese accelerates the autoxidation of catecholamines and contributes to oxidative stress in these affected regions of the brain. Further research is needed to more completely elucidate the mechanism of inorganic manganese toxicity.
There are no dependable biomarkers of exposure or effect that are consistently used in a clinical setting. However, MRI scans have been used in both adults and children to determine whether manganese is accumulating in certain brain regions. More data are needed to determine the sensitivity and specificity of this method.
Available data do not indicate that there are any interactions of manganese with other compounds that occur only in children. Interactions with compounds in adults are expected to also occur in children. Data concerning the significance of any interactions of manganese with other compounds are needed.
Child health data needs relating to exposure are discussed in Section 6.8.1, Identification of Data Needs: Exposures of Children.
3.12.3. Ongoing Studies
Ongoing studies pertaining to manganese have been identified and are shown in Table 3-20.
Table 3-20
Ongoing Studies on Manganese.
- INTRODUCTION
- DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE
- GENOTOXICITY
- TOXICOKINETICS
- MECHANISMS OF ACTION
- TOXICITIES MEDIATED THROUGH THE NEUROENDOCRINE AXIS
- CHILDREN’S SUSCEPTIBILITY
- BIOMARKERS OF EXPOSURE AND EFFECT
- INTERACTIONS WITH OTHER CHEMICALS
- POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE
- METHODS FOR REDUCING TOXIC EFFECTS
- ADEQUACY OF THE DATABASE
- HEALTH EFFECTS - Toxicological Profile for ManganeseHEALTH EFFECTS - Toxicological Profile for Manganese
Your browsing activity is empty.
Activity recording is turned off.
See more...
