All copyright for chapters belongs to the individual authors who created them. However, for non-commercial, academic purposes, images and content from the chapters portion of Webvision may be used with a non-exclusive rights under a Attribution, Noncommercial 4.0 International (CC BY-NC) Creative Commons license. Cite Webvision, http://webvision.med.utah.edu/ as the source. Commercial applications need to obtain license permission from the administrator of Webvision and are generally declined unless the copyright owner can/wants to donate or license material. Use online should be accompanied by a link back to the original source of the material. All imagery or content associated with blog posts belong to the authors of said posts, except where otherwise noted.
1. Introduction
The mammalian retina has long been a model system for study of development of neural circuits in the CNS because the adult network is well organized into cell-type specific layers, and the anatomy, physiology and function of many of the retinal cell types is well characterized. A major focus of research in the retina is directed toward understanding how functional circuits arise during development.
The development of the retina requires several steps. The first step is to create the right proportion of the 7 cell types that comprise the retina. This process occurs primarily through genesis of the correct number of each cell type. Only ganglion cells have their final number regulated by cell death, which reduces the number of ganglion cells by as much as 50% in some species. The second step is for cells to migrate into the correct location. The third step is for neurons to form synaptic connections with other retinal neurons. Finally, for some of these groups of synaptically coupled cells, synaptic refinement is necessary to generate the circuits that comprise the adult retina.
The process of neuronal migration in the retina has been the focus of developmental biologists since the time of Cajal, who used Golgi staining techniques (Fig. 1). Progenitor cells in the neurepithelium lining the surface of the neural tube later become the ventricular zone of the optic vesicles, optic cup and early retina. Postmitotic cells leave the ventricular zone to migrate to one of three cell layers in the retina remaining attached radially from one side of the retina to the other. The neural cells lie at different levels in the retina and, when in correct position, lose their anchoring radial connections. Then polarity of the differentiating cells occurs and dendrites and axons grow out appropriately. The ganglion cells are the first to emerge as recognizable neurons with axons passing to the optic nerve and central brain structures (Fig. 1a and b). Then amacrine cells (Fig. 1c), Müller cells, bipolar cells (Fig. 1d), and horizontal cells form in the correct layer and finally photoreceptors remain to line the top layers (Fig. 1e and f).
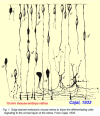
Figure 1.
Golgi stained embryonic mouse retina shows the differentiating cells migrating to the correct layer of the retina. From Cajal, 1894, Translation, 1972 (70).
Before the neural circuits that underlie visual processing emerge, the retina assembles and disassembles a series of intermediate circuits. These transient connections between cells produce the propagating spontaneous activity that is termed retinal waves (1-3). As the retina develops, so do the circuits that underlie retinal waves. The earliest spontaneous retinal waves are propagated via electrical coupling between cells. Then, around birth in mice, waves are produced by a transient network consisting of cholinergic connections between amacrine cells. Finally, just before visual processing begins, they are driven by early glutamatergic signaling.
In this chapter we will discuss how these transient circuits of the inner plexiform layer (IPL) assemble to produce specific patterns of neural activity, and how they transition from one circuit into the next. Finally, we will discuss the role of spontaneous activity in shaping the development of the visual system within both the retina and the brain.
2. Neurotransmitters and Early Retinal Development
Early in development, neurotransmitters can function in the absence of traditional synapses (4). Ultrastructurally identified conventional synapses within the IPL are first formed a few days after birth in mice (5). However, even before that there is evidence of neurotransmitters. Below we discuss the role of early neurotransmitters and their receptors prior to the formation of circuits that mediate vision.
Acetylcholine
Acetylcholine (ACh) signaling plays a key role in the development of the retina. Prior to synapse formation, paracrine action of ACh is essential for regulating early developmental events, such as the regulation of the cell cycle (6) and the growth of neurites (7). In addition, cholinergic synapses are among the earliest to mature and thereby constitute the earliest functional circuits in the retina.
ACh in the retina is produced solely by one cell type, the starburst amacrine cell (SAC), a type of amacrine cell named for its radially symmetric processes (8). In the mature retina, released ACh acts on both muscarinic and nicotinic receptors to modulate the response properties of many different types of ganglion cells (9-13), but it does not affect the SACs themselves (14). However, during development, cholinergic signaling does occur between SACs (14). During the first week after birth in mice, ACh released from SACs activates nicotinic acetylcholine receptors (nAChRs) on neighboring SACs and thus gives rise to a cholinergic network. A key developmental function of this cholinergic network is the generation of retinal waves. This network appears at birth in mice and mediates the initiation and propagation of waves.
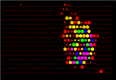
Aside from the effect ACh has on ganglion and amacrine cells via nicotinic acetylcholine receptors, it also acts on the muscarinic acetylcholine receptors (mAChRs) of many cells in the neuroblastic layer (15, 16) (Fig. 2). Figure 2 shows the effect of acetylcholine on progenitor cells in rabbit retina. A one-day old rabbit retina is loaded with Fura-2. Images show responses to bath application of 200μM nicotine (Nic, left), which activates nicotinic acetylcholine receptors, or 100μM carbachol (CCh, right), which activates muscarinic acetylcholine receptors. Red indicates cells that had increases in intracellular calcium whereas blue indicates cells without increases in intracellular calcium.

Figure 2.
Acetylcholine acts on muscarinic receptors in progenitor cells and nicotinic receptors in ganglion and amacrine cells. Adapted from Wong et al, 1995 (16).
The mAChRs are G-protein coupled receptors that lead to an increase in intracellular calcium via release of calcium from internal stores, as opposed to influx through ligand- or voltage-activated channels. Interestingly, the ACh released during retinal waves drives correlated mAChR-dependent calcium transients in undifferentiated cells of the ventricular zone (Fig. 2) (15). Hence, it is possible that ACh released by retinal waves induces signaling that is important for early phases of neurogenesis and for cell migration (17).
GABA
GABA is expressed in more cells during development than during adulthood, thus suggesting that it plays a transient role in circuit formation (18). During the first few postnatal days in rabbit, GABA displays a high transient expression in the ganglion cell layer. Moreover, the IPL of P0 ferret exhibits markers for enzymes involved in the synthesis of GABA (19).
During development, GABA initially serves to depolarize neurons. When activated, ionotropic GABA receptors, GABAA and GABAC, flux chloride (Fig. 3A). Since the chloride concentration within developing retinal neurons is high due to low expression of the potassium-chloride co-transporter, KCC2, receptor activation leads to an efflux of negatively charged chloride ions through the open channels and thus causes depolarization of the cell (Fig. 3A, B left). KCC2 expression gradually increases during the first two weeks after birth in mice (20). Thus there is a ‘switch’ from excitation to inhibition as the reversal potential for chloride drops below the threshold for firing action potentials (Fig. 3 A, B right).
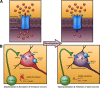
Figure 3.
GABA switches from depolarizing to hyperpolarizing during development. From Ben-Ari et al. 2007 (71).
In turtle retina, the timing of the GABA switch correlates with a decrease in propagating spontaneous activity (21), suggesting that GABA depolarization plays a role in this propagation. In mammalian retina, GABA plays a minor role in correlated spontaneous activity during its excitatory period (1, 22, 23). However, after the GABA switch, GABA’s inhibitory action takes on a prominent role in shaping spontaneous activity. Blocking GABAA receptors greatly increases the frequency of spontaneous retinal waves (22, 24).
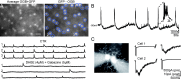
What underlies the switch of GABA’s action from depolarizing to hyperpolarizing? Several environmental factors, including neural activity (25), have been implicated in the timing of this switch in the retina. A recent study used acutely isolated retinas from knockout mice and pharmacological manipulations in retinal explants demonstrates that the timing of the GABA switch in retinal ganglion cells is unaffected by blocking specific neurotransmitter receptors or global activity (26) (Fig. 4). Purified retinal ganglion cells remain depolarized by muscimol for at least two weeks in culture (Fig 4, right), indicating that the GABA switch is not cell-autonomous. Purified ganglion cells co-cultured with other retinal neurons also remain depolarized by muscimol (Fig. 4, middle). However, culturing purified ganglion cells with dissociated cells from the superior colliculus (Fig. 4, right), or treating purified ganglion cells with media conditioned by superior colliculus cultures (Fig. 4C, red), results in a switch to inhibition by muscimol after two weeks in culture, indicating that a diffusible signal independent of local circuit activity regulates the maturation of GABAergic transmission.
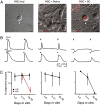
Figure 4.
The GABA switch in retinal ganglion cells is due to a diffusible factor. From Barkis et al., 2010 (26).
Glutamate
Glutamatergic signaling is the last to develop within the IPL. Glutamate is released primarily from bipolar cells and a small subset of amacrine cells (27, 28). In mice, the axons of bipolar cells first express VGLUT1, the enzyme responsible for packaging glutamate into vesicles, around one week after birth (29). Although, ribbon synapses between bipolar cells axons and the dendrites of amacrine and ganglion cells do not form until 11 days after birth (5), glutamatergic currents can be measured before this (24, 29).
State-of-the-art transgenic and imaging techniques have characterized the distribution of glutamatergic synapses onto ganglion cells and between particular subtypes of bipolar and ganglion cells (30, 31). Before the functional maturation of bipolar cell ribbon synapses and long before the structural maturation of the ganglion cell dendritic arbor, adult-like patterns of glutamatergic synapses can be seen. However, between some subtypes of bipolar and ganglion cells, there appears to be an activity dependent remodeling of these synapses (30).
3. Spontaneously active synaptic circuits in the inner plexiform layer
Retinal Waves
Prior to photoreceptor maturation and eye opening, retinal ganglion cells periodically fire bursts of action potentials on the order of once per minute. This spontaneous rhythmic activity was first measured in fetal rat pups and was found to be highly correlated among neighboring ganglion cells (32).
Extracellular recordings using a multielectrode array (2) (Movie 1) and imaging of calcium transients (Movie 2) associated with bursts of action potentials (1, 33) have revealed that these spontaneous bursts propagate from one cell to the next in a wavelike manner. These retinal waves are an extremely robust phenomenon, observed in a large variety of vertebrate species, including chick, turtle, mouse, rabbit, rat, ferret and cat (34).
Movie 2.
Calcium imaging of cholinergic waves. P3 mouse retina loaded with Oregon Green-Bapta-1AM. Playback 10x, size 800x600 µm (unpublished).
Retinal waves persist for an extended period throughout development as shown in Figure 5. However, as the retina matures, the circuitry underlying these retinal waves changes. Thus the wave generating circuits progress through three distinct stages, reflecting the nature of the connections and the cells that are involved (Fig. 5).
Embryonic waves
The earliest waves recorded in mammals are thought to be propagated via gap junctions. Retinal waves before embryonic day 23 in rabbit persist in the presence of antagonists to ionotropic GABA, glycine, ACh, and glutamate receptors, but are completely blocked by 18β-GA, a blocker of gap junction coupling (22). Similarly, mice prior to birth exhibit waves that are insensitive to chemical transmission antagonists (35). How waves are initiated and propagated during this early stage of development is still not clear. During the first week after birth in ferret and mouse, neurobiotin coupling between ganglion cells of the same subtype and amacrine cells has been seen (3, 36). However, ganglion cell coupling is initially weak and becomes stronger with age, while the correlations generated by waves become weaker with age (37). In addition, tracer coupling is found primarily between retinal ganglion cells of the same subtype and many other ganglion cells are not gap junction coupled. Thus, the issue of wave propagation in this early network still remains to be explored.
Neurotransmitters play a modulatory role during early stage waves. At the earliest ages studied in chick retina (E8-E11), wave frequency decreases in the presence of an ACh antagonist and increases with an ACh agonist but waves are not blocked. These waves are unaffected by GABAA and glutamate receptor antagonists (38). Similarly, embryonic waves in mouse are reduced in ACh receptor antagonists (35). In addition, in rabbit, early waves are blocked by the activation of GABAB receptors and are increased in frequency by the inhibition of these receptors (22).
Cholinergic waves
Several experimental results show that chemical synaptic transmission is a prerequisite for cholinergic wave propagation. First, simultaneous whole cell voltage clamp recordings from ganglion cells demonstrate that increases in [Ca2+]i correlated across cells are driven by compound synaptic inputs (1). Second, the compound postsynaptic currents measured from ganglion cells are blocked by bath application of Cd2+, a blocker of calcium channels that are associated with transmitter release (1). Third, the periodic Ca2+ increases, action potentials, and compound postsynaptic currents associated with waves can all be blocked by a variety of nAChRs antagonists (1, 39). Finally, genetic deletion of the beta2 subunit of the nAChR receptor (35) or deletion of the enzyme responsible for synthesis of ACh (40) results in the disruption of normal waves during the first week after birth.
The spatiotemporal properties of cholinergic retinal waves have been well-characterized by fluorescence imaging of calcium indicators, which are reliable markers of cell depolarizations. Figure 6 schematizes these waves (41). Waves initiate in small clusters of coactive neurons and then propagate over spatially restricted areas of the retina. Initiation sites and wave boundaries are distributed randomly across a given retina, indicating that the global patterns of waves are not determined by fixed structures such as pacemaker cells or by repeated activation of the same clusters of neurons. Instead, the propagation boundaries of waves are determined in part by wave-induced refractory regions that last for 40-50 seconds. These observations have led to the hypothesis that every region of the retina is equally likely to initiate or propagate a wave, and therefore the global spatial patterns of waves are determined by the local history of retinal activity (Feller et al., 1997).
Recent studies in rabbit (Zheng et al., 2004; Zheng et al., 2006) and mouse (Ford et al. 2012) retina have revealed the cellular properties of starburst amacrine cells (SACs), the cell type that gives rise to cholinergic waves (Fig. 7) (42). Identifying SACs in mouse retinas was facilitated by the use of a line of mice in which GFP is expressed in SACs (mGluR2-GFP, Fig 7A). Using calcium imaging, spontaneous depolarizations of individual SACs were observed in the absence of synaptic excitation (Fig 7A), indicating that SACs themselves may be the source of initiation for waves. Paired recording between SACs revealed reciprocal cholinergic transmission (Fig 7C), indicating that waves are propagated via these slow, excitatory connections between neighboring SACs. Finally, wave boundaries are thought to arise from a slow after-hyperpolarization in the SACs that recovers over the course of tens of seconds following the depolarization during waves (Fig 7B).
Cholinergic waves may play a role in terminating early stage gap-junction mediated waves. Knock-out animals that lack nAChR receptor subunits still exhibit wave like activity under certain recording conditions, such as elevated temperature [alpha3(-/-) (35), beta2(-/-)(43, 44)]. This activity is likely mediated by gap junctions (44). Moreover, a study using a genetic model that eliminates ChAT in a large portion of the retina found normal cholinergic waves in the spared region, but compensatory waves in the region lacking ChAT (40). These studies suggest a sequential maturation of the retinal circuitry that relies on checkpoints to make transitions from one stage (gap-junction mediated waves) to the next (cholinergic transmission mediated waves). This sort of checkpoint model of neuronal development (45) is further supported by the disassembly of the cholinergic network to make way for glutamatergic signaling (46).
Glutamatergic waves
The synaptic circuitry that drives retinal waves changes postnatally. Though retinal waves early in development require cholinergic neurotransmission, studies in older ferret, rabbit, and mouse indicate that waves become insensitive to cholinergic antagonists and can be blocked by glutamate receptor antagonists (35, 47, 48). This switch in the requisite transmitter occurs at the age that bipolar cells make their initial synaptic connections with ganglion cells and conventional synapses between amacrine and ganglion cells become morphologically mature and numerous. This timing suggests that perhaps waves are mediated by neurotransmitters only when the synapses are first forming.
Glutamatergic waves have distinct spatial and temporal features. Unlike cholinergic waves that drive correlations in all neighboring ganglion cells regardless of subtype, glutamatergic waves occur more frequently in OFF cells than in ON cells (49) (Fig. 8). Waves occur in rapid clusters separated by periods of silence lasting about one minute (24). Within clusters, there is a distinct pattern of firing where ON and OFF cells alternate in firing bursts of action potentials (50) (Fig. 8). These spatial and temporal features are significantly shaped by inhibitory circuits. Blocking ionotropic GABA receptors increases wave frequency (51). Blocking glycine receptors does the same but also eliminates the asynchronous firing between ON and OFF cells.
Similar to the transition from gap-junction to cholinergic waves, the onset of glutamatergic waves may play an active role in the disassembly of the cholinergic circuits (46). Mice lacking the vesicular glutamate transporter VGLUT1 lack glutamate release from bipolar cells. However, these mice still have retinal waves during the time that littermate control animals have glutamatergic waves. Interestingly, these waves in the VGLUT1 knockout mice are unaffected by glutamate receptor antagonists but are blocked by nAChR antagonists (24). Thus, glutamate signaling from bipolar cells seems necessary for dismantling the cholinergic network.
There is some evidence that the transitions between the different circuits that mediate waves are linked. Prior to birth waves are thought to propagate via gap-junctions between ganglion cells (Fig. 9A, left). From postnatal day 1-10 waves are propagated via SAC release of acetylcholine onto other SACs (Fig 9A, Middle, black box). Acetylcholine also depolarizes ganglion cells. During this period of development, the gap-junction signaling between ganglion cells is reduced (Fig 9A, Middle, red box). From P10-P15 bipolar cells release glutamate to propagate waves in a mechanism that is thought to involve spillover of glutamate to excite neighboring bipolar cells (Fig. 9A right, black box). Cholinergic signaling between SACs is reduced (Fig. 9A right, red box).
Genetic disruption of cholinergic or glutamatergic waves result in an extended action of the previous wave generating circuit (Fig. 9B). In wild type mice gap-junction mediated waves (gray) are followed by cholinergic waves (blue) starting at P0, then glutamatergic waves (green) at P10. In mice lacking the Beta2 subunit of the nicotinic acetylcholine receptor, gap-junction mediated waves persist until ~P8. In mice lacking vesicular glutamate transporter VGLUT1, cholinergic waves persist through the second postnatal week.
Gap junctions and waves
Gap junctions are thought to play a role in the generation of embryonic waves, as described above. At later ages in mammals, however, gap junctions also play a minor role in the propagation of retinal waves. Gap junction antagonists reduce or partially block retinal waves after birth in mice (36) and at later stages in rabbit (22) when waves depend critically on chemical transmission. However, these antagonists also have several non-specific effects so these results are inconclusive. A different approach is to study mice in which specific connexins are genetically deleted (Fig. 10). Knockouts of connexins 36 and 45, the two major gap junction forming connexins in the IPL, have normal wave propagation (Fig. 10 A) but the firing between waves at later stages is increased (Fig 10B). Glutamatergic waves and the firing between waves is eliminated by the application of bipolar cell synapse antagonists DNQX and AP5, indicating that bipolar input is important for the generation of waves as in wild type retinas (52).
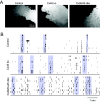
Figure 10.
Spatiotemporal properties of retinal waves are similar in control, Cx45, and Cx36/45 double knockout mice. A, Examples of waves recorded in different genotypes. Each grayscale value represents an active area in one frame, with darker shades corresponding (more...)
In contrast, in the chick retina, gap junctions were found to be involved in wave generation at all ages. Octanol, a significant inhibitor of waves in E8 chick retina, restricts tracer coupling between ganglion cells and other cell types but not between ganglion cells themselves, indicating that the circuitry mediating these waves involves cells other than ganglion cells (38).
4. Role of activity in formation of visual circuitry
Spontaneous activity in the developing retina occurs while functional circuits are forming within the retina and projections from the retina are undergoing refinement at their target regions in the brain. What role do retinal waves play in sculpting the circuits that mediate vision?
ON/OFF circuitry within the retina
Two sub-circuits that have been well characterized in the adult retina are the ON and OFF pathways. The classes of bipolar cells that transmit responses to the onset of light (ON responses) are distinct from those that transmit responses to the cessation of light (OFF responses). These ON and OFF circuits have ganglion cell dendrites, amacrine cell processes and bipolar cell inputs that are physically segregated from each other into what are called the ON and OFF layers of the IPL. The formation of these ON or OFF circuits involves the dendritic maturation of ganglion cell types.
The dendrites of most retinal ganglion cells arborize diffusely within the IPL before restricting their dendrites to distinct lamina (35, 53-57) (Fig. 11). Some studies have suggested that this segregation of ganglion cell dendrites into ON and OFF layers involves bipolar cell activity. First, this segregation is prevented by applying APB to hyperpolarize ON bipolar cells during the period of glutamatergic retinal waves (Fig. 12) (58, 59). Second, mice that lack the MHCI receptor CD3zeta, and thus display altered glutamatergic retinal waves, have ganglion cells with reduced dendritic motility and more diffuse dendrites within the IPL (60) (Fig. 11). However, not all manipulations of spontaneous retinal activity during development alter dendritic stratification. Preventing synaptic release of glutamate from ON bipolar cells by expressing tetanus toxin does not prevent the stratification of ganglion cell dendrites, but it does reduce synapse formation onto the ON bipolar cells (30).
Figure 11.
Retinal waves drive refinement of some retinal ganglion cell dendrites.

Figure 12.
Activity dependent maintenance of ganglion cell dendritic stratification in the developing cat retina. From Bodnarenko and Chalupa, 1993 (58) .
Is there a role for cholinergic waves in this ganglion cell stratification process? Several studies suggest that there is. First, blocking nAChRs during the period of cholinergic waves reduces the motility of filipodia on the dendrites of ganglion cells (61), demonstrating that cholinergic waves can drive structural changes in dendrites. Second, studies in turtle demonstrate that blocking cholinergic waves with nAChR antagonists reduces receptive field sizes (62). Finally, mice lacking the beta2 subunit of the nAChR exhibit a delay in, but not an absence of, the fine stratification of ganglion cell dendrites (35). These findings indicate that cholinergic waves do influence the outgrowth of ganglion cell dendrites but they are not the primary factor that dictates their final organization.
Refinement of retinal projections
Retinal axons undergo a period of refinement before vision begins. At birth in mice, axons from both eyes reside in overlapping regions of the dorsal lateral geniculate nucleus of the thalamus. By about two weeks after birth, the axon terminals from the ganglion cells of each eye separate into non-overlapping regions. Similarly, within the superior colliculus, retinal axons at birth extend over the entire area of the colliculus. However, over the course of about one week, these axons retract to their appropriate retinotopic regions and extensively arborize within a small target zone. These processes of eye-specific segregation and retinotopic map refinement occur during the period of retinal waves. Thus, the hypothesis has emerged that waves might provide cues within their activity pattern to instruct these developmental processes.
The refinement of retinal projections to the brain is thought to be driven by the precise initiation, propagation and termination properties of cholinergic waves (63). The periodic initiation of waves induces depolarizations and calcium transients that may be tuned to drive axon guidance (64, 65) and plasticity mechanisms (66, 67). Propagation speed sets the time scale over which neighboring cells are correlated, and thus may be critical for retinotopic map refinement (68). The spatial extent of wave propagation has been shown to be important for establishing eye-specific segregation of retinal inputs within the thalamus (69).
Retinal waves determine the final size of termination zones of retinal projections to the superior colliculus (SC, Figure 13A). Focal labeling of retinal ganglion cells using DiI labels in retina (Retina) gives small target zones in superior colliculus (WT, Fig. 13A, left). In knockout mice lacking normal cholinergic waves (β2-nAChR KO, Fig. 13A middle), termination zones are less compact. Rescue of the β2-nAChR gene in a subset of retinal cells (Fig. 13 β2 tg) produce small waves, which are sufficient to rescue normal retinotopic map refinement.
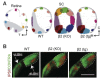
Figure 13.
Retinal waves drive refinement of central projections. A. Schematic showing results of several experiments based on DiI anterograde labeling to determine the retinotopic projection of retinal ganglion cells to the superior colliculus. Results for WT mice, (more...)
Retinal waves also play a role in eye-specific segregation of retinal ganglion cell projections to the lateral geniculate nucleus of the thalamus (Fig. 13b). In wild type mice, there is little overlap between ipsi- and contra-lateral projections. Mice lacking cholinergic waves (β2 KO) and mice with small waves (β2 tg) have significantly overlapping projections from either eye.
Retinal waves also play a role in eye-specific segregation of retinal ganglion cell projections to the lateral geniculate nucleus of the thalamus (Fig. 13b). In wild type mice, there is little overlap between ipsi- and contra-lateral projections. Mice lacking cholinergic waves (β2 KO) and mice with small waves (β2 tg) have significantly overlapping projections from either eye.
5. Conclusion
The neural circuits within the inner plexiform layer are highly organized, making them an ideal system for the study of circuit formation. Understanding how this organized structure and intricate connectivity arise during development is an important endeavor. It is clear that on the path to forming the circuits that mediate vision, the retina creates a series of intermediate circuits that generate spontaneous activity. These transitory circuits are then dismantled as the retina matures. During their brief existence, these transient networks play an important role in shaping the circuits both within the retina and from the retina to the brain.
A wealth of new tools will lead the way to a greater understanding of how these neural circuits develop. Several recent studies have identified lines of mice with GFP or Cre recombinase expression that is restricted to specific classes and even to subsets of retinal neurons. The ability to identify and alter the activity of specific components of a neural circuit will allow future experimentalists to observe how these circuits form and to ask what role spontaneous activity plays in their formation.
About the Authors

Dr. Kevin Ford received his B.S. in Biology from UC San Diego in 2005. He did his graduate work with Marla Feller first at UC San Diego and then UC Berkeley, receiving his Ph.D. in Molecular and Cell Biology from UC Berkeley in 2011. During his graduate career, he researched the cellular mechanisms underlying retinal waves in the developing mouse retina. He is still in Marla’s laboratory at Berkeley.

Dr. Marla Feller received her B.A. and Ph.D. in Physics from University of California at Berkeley in 1985 and 1992 respectively. She was a postdoctoral researcher with at Bell Laboratories with Dr. David Tank (1992-1994) and then at UC Berkeley with Dr. Carla Shatz (1994-1998). She headed a laboratory at the National Institutes of Neurological Disorders (1998-2000), was at UC San Diego (2000-2007) and is now at UC Berkley Neuroscience as an Associate Professor of Neurobiology. She has done research in the elucidating the circuits that mediate retinal waves and also in what role retinal waves play in the establishment of retinal projections to the brain. Currently Marla is investigating the mechanisms underlying the generation of this highly patterned activity and exploring the role it plays in the development and shaping of ganglion cell responses, particularly those generated by a cholinergic amacrine cell network.
References
- 1.
- Feller M.B., et al. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272(5265):1182. [PubMed: 8638165]
- 2.
- Meister M., et al. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252(5008):939–43. [PubMed: 2035024]
- 3.
- Penn A.A., Wong R.O., Shatz C.J. Neuronal coupling in the developing mammalian retina. J Neurosci. 1994;14(6):3805–15. [PMC free article: PMC6576938] [PubMed: 8207489]
- 4.
- Redburn D.A., Rowe-Rendleman C. Developmental neurotransmitters. Signals for shaping neuronal circuitry. Invest Ophthalmol Vis Sci. 1996;37(8):1479–82. [PubMed: 8675389]
- 5.
- Fisher L.J. Development of synaptic arrays in the inner plexiform layer of neonatal mouse retina. J Comp Neurol. 1979;187(2):359–72. [PubMed: 489784]
- 6.
- Pearson R., et al. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(17):7569–7579. [PMC free article: PMC6757998] [PubMed: 12196580]
- 7.
- Lohmann C., Myhr K.L., Wong R.O. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418(6894):177–81. [PubMed: 12110889]
- 8.
- Hayden S.A., Mills J.W., Masland R.M. Acetylcholine synthesis by displaced amacrine cells. Science (New York, N.Y.). 1980;210(4468):435–437. [PubMed: 7433984]
- 9.
- Baldridge W.H. Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1996;16(16):5060–5072. [PMC free article: PMC6579282] [PubMed: 8756436]
- 10.
- Masland R.H., Ames A. 3rd. Responses to acetylcholine of ganglion cells in an isolated mammalian retina. J Neurophysiol. 1976;39(6):1220–35. [PubMed: 993829]
- 11.
- Masland RH, Mills JW, Cassidy C. The functions of acetylcholine in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:121–39. [PubMed: 6151181]
- 12.
- Schmidt M., Humphrey M.F., Wässle H. Action and localization of acetylcholine in the cat retina. Journal of Neurophysiology. 1987;58(5):997–1015. [PubMed: 3694255]
- 13.
- Strang CE, Andison ME, Amthor FR, Keyser KT. Rabbit retinal ganglion cells express functional alpha7 nicotinic acetylcholine receptors. Am J Physiol Cell Physiol. 2005;289:C644–55. [PubMed: 15872006]
- 14.
- Zheng J.J., Lee S., Zhou Z.J. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44(5):851–64. [PubMed: 15572115]
- 15.
- Syed M.M., et al. Spontaneous waves in the ventricular zone of developing mammalian retina. J Neurophysiol. 2004;91(5):1999–2009. [PubMed: 14681336]
- 16.
- Wong R.O., et al. Early functional neural networks in the developing retina. Nature. 1995;374(6524):716–8. [PubMed: 7715725]
- 17.
- Martins R.A.P., Pearson R.A. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Research. 2008;1192:37–60. [PubMed: 17597590]
- 18.
- Sandell J.H. GABA as a developmental signal in the inner retina and optic nerve. Perspect Dev Neurobiol. 1998;5(2-3):269–78. [PubMed: 9777642]
- 19.
- Karne A., et al. Immunocytochemical localization of GABA, GABAA receptors, and synapse-associated proteins in the developing and adult ferret retina. Vis Neurosci. 1997;14(6):1097–108. [PubMed: 9447691]
- 20.
- Zhang L.L., et al. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J Neurophysiol. 2006;95(4):2404–16. [PubMed: 16371454]
- 21.
- Sernagor E., Young C., Eglen S.J. Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(20):7621–7629. [PMC free article: PMC6740765] [PubMed: 12930801]
- 22.
- Syed M.M., et al. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560(Pt 2):533–49. [PMC free article: PMC1665265] [PubMed: 15308679]
- 23.
- Wang C.-T., et al. GABAA Receptor-Mediated Signaling Alters the Structure of Spontaneous Activity in the Developing Retina. J. Neurosci. 2007;27(34):9130. [PMC free article: PMC2933517] [PubMed: 17715349]
- 24.
- Blankenship A.G., et al. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62(2):230–241. [PMC free article: PMC2807181] [PubMed: 19409268]
- 25.
- Leitch E., et al. GABA type-A activity controls its own developmental polarity switch in the maturing retina. J Neurosci. 2005;25(19):4801–5. [PMC free article: PMC6724776] [PubMed: 15888655]
- 26.
- Barkis W.B., Ford K.J., Feller M.B. Non-cell-autonomous factor induces the transition from excitatory to inhibitory GABA signaling in retina independent of activity. Proc Natl Acad Sci U S A. 2010;107(51):22302–7. [PMC free article: PMC3009811] [PubMed: 21135238]
- 27.
- Haverkamp S., Wassle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J Comp Neurol. 2004;468(2):251–63. [PubMed: 14648683]
- 28.
- Johnson J., et al. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. J Comp Neurol. 2004;477(4):386–98. [PMC free article: PMC2586940] [PubMed: 15329888]
- 29.
- Johnson J., et al. Vesicular Neurotransmitter Transporter Expression in Developing Postnatal Rodent Retina: GABA and Glycine Precede Glutamate. The Journal of Neuroscience. 2003;23(2):518–529. [PMC free article: PMC6741860] [PubMed: 12533612]
- 30.
- Kerschensteiner D., et al. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460(7258):1016–20. [PMC free article: PMC2746695] [PubMed: 19693082]
- 31.
- Morgan J.L., Schubert T., Wong R.O. Developmental patterning of glutamatergic synapses onto retinal ganglion cells. Neural Dev. 2008;3:8. [PMC free article: PMC2311295] [PubMed: 18366789]
- 32.
- Galli L., Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988;242(4875):90–1. [PubMed: 3175637]
- 33.
- Wong R.O. Cholinergic regulation of [Ca2+]i during cell division and differentiation in the mammalian retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1995;15(4):2696–2706. [PMC free article: PMC6577749] [PubMed: 7722623]
- 34.
- Wong R.O. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. [PubMed: 10202531]
- 35.
- Bansal A., et al. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20(20):7672. [PMC free article: PMC6772851] [PubMed: 11027228]
- 36.
- Singer J.H., Mirotznik R.R., Feller M.B. Potentiation of L-type calcium channels reveals nonsynaptic mechanisms that correlate spontaneous activity in the developing mammalian retina. J Neurosci. 2001;21(21):8514–22. [PMC free article: PMC6762803] [PubMed: 11606640]
- 37.
- Wong R.O., Meister M., Shatz C.J. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11(5):923–38. [PubMed: 8240814]
- 38.
- Catsicas M., et al. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol. 1998;8(5):283–6. [PubMed: 9501073]
- 39.
- Penn A.A., et al. Competition in retinogeniculate patterning driven by spontaneous activity. Science (New York, N.Y.). 1998;279(5359):2108–2112. [PubMed: 9516112]
- 40.
- Stacy R.C., et al. Disruption and Recovery of Patterned Retinal Activity in the Absence of Acetylcholine. J. Neurosci. 2005;25(41):9347–9357. [PMC free article: PMC6725714] [PubMed: 16221843]
- 41.
- Feller M.B., et al. Dynamic Processes Shape Spatiotemporal Properties of Retinal Waves. Neuron. 1997;19(2):293. [PubMed: 9292720]
- 42.
- Ford K.J., Felix A.L., Feller M.B. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. J Neurosci. 2012;32(3):850–63. [PMC free article: PMC3311224] [PubMed: 22262883]
- 43.
- Stafford B.K., et al. Spatial-Temporal Patterns of Retinal Waves Underlying Activity-Dependent Refinement of Retinofugal Projections. Neuron. 2009;64(2):200–212. [PMC free article: PMC2771121] [PubMed: 19874788]
- 44.
- Sun C., et al. Retinal waves in mice lacking the β2 subunit of the nicotinic acetylcholine receptor. Proceedings of the National Academy of Sciences. 2008;105(36):13638–13643. [PMC free article: PMC2527347] [PubMed: 18757739]
- 45.
- Ben-Ari Y., Spitzer N.C. Phenotypic checkpoints regulate neuronal development. Trends in Neurosciences. 2010;33(11):485–492. [PMC free article: PMC2963711] [PubMed: 20864191]
- 46.
- Blankenship A.G., Feller M.B. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11(1):18–29. [PMC free article: PMC2902252] [PubMed: 19953103]
- 47.
- Wong W.T., et al. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(1):351–360. [PMC free article: PMC6774128] [PubMed: 10627612]
- 48.
- Zhou Z.J., Zhao D. Coordinated transitions in neurotransmitter systems for the initiation and propagation of spontaneous retinal waves. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(17):6570–6577. [PMC free article: PMC6772967] [PubMed: 10964962]
- 49.
- Wong R.O., Oakley D.M. Changing patterns of spontaneous bursting activity of on and off retinal ganglion cells during development. Neuron. 1996;16(6):1087–1095. [PubMed: 8663985]
- 50.
- Kerschensteiner, D. and R.O.L. Wong, A Precisely Timed Asynchronous Pattern of ON and OFF Retinal Ganglion Cell Activity during Propagation of Retinal Waves. 2008. 58(6): p. 851. [PMC free article: PMC2553397] [PubMed: 18579076]
- 51.
- Fischer K.F., Lukasiewicz P.D., Wong R.O. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998;18(10):3767–78. [PMC free article: PMC6793131] [PubMed: 9570807]
- 52.
- Blankenship A.G., et al. The role of neuronal connexins 36 and 45 in shaping spontaneous firing patterns in the developing retina. J Neurosci. 2011;31(27):9998–10008. [PMC free article: PMC3142875] [PubMed: 21734291]
- 53.
- Bodnarenko S.R., et al. The development of retinal ganglion cell dendritic stratification in ferrets. Neuroreport. 1999;10(14):2955–2959. [PubMed: 10549804]
- 54.
- Coombs J.L., Van Der List D., Chalupa L.M. Morphological properties of mouse retinal ganglion cells during postnatal development. The Journal of Comparative Neurology. 2007;503(6):803–814. [PubMed: 17570502]
- 55.
- Kim I.-J., et al. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(4):1452–1462. [PMC free article: PMC2822471] [PubMed: 20107072]
- 56.
- Sernagor E., Eglen S.J., Wong R.O. Development of retinal ganglion cell structure and function. Progress in Retinal and Eye Research. 2001;20(2):139–174. [PubMed: 11173250]
- 57.
- Xu H., Tian N. Pathway-specific maturation, visual deprivation, and development of retinal pathway. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2004;10(4):337–346. [PubMed: 15271261]
- 58.
- Bodnarenko S.R., Chalupa L.M. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364(6433):144–6. [PubMed: 8100613]
- 59.
- Bodnarenko S.R., Jeyarasasingam G., Chalupa L.M. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J Neurosci. 1995;15(11):7037–45. [PMC free article: PMC6578036] [PubMed: 7472459]
- 60.
- Xu H.P., et al. The immune protein CD3zeta is required for normal development of neural circuits in the retina. Neuron. 2010;65(4):503–15. [PMC free article: PMC3037728] [PubMed: 20188655]
- 61.
- Wong W.T., Wong R.O. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nature Neuroscience. 2001;4(4):351–352. [PubMed: 11276221]
- 62.
- Sernagor E., Grzywacz N.M. Influence of spontaneous activity and visual experience on developing retinal receptive fields. Current Biology: CB. 1996;6(11):1503–1508. [PubMed: 8939611]
- 63.
- Huberman A.D., Feller M.B., Chapman B. Mechanisms Underlying Development of Visual Maps and Receptive Fields. Annual Review of Neuroscience. 2008;31(1):479–509. [PMC free article: PMC2655105] [PubMed: 18558864]
- 64.
- Nicol X., et al. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10(3):340–7. [PubMed: 17259982]
- 65.
- Pfeiffenberger C., Yamada J., Feldheim D.A. Ephrin-As and Patterned Retinal Activity Act Together in the Development of Topographic Maps in the Primary Visual System. J. Neurosci. 2006;26(50):12873–12884. [PMC free article: PMC3664553] [PubMed: 17167078]
- 66.
- Butts D.A., Kanold P.O., Shatz C.J., Burst-Based A. “Hebbian” Learning Rule at Retinogeniculate Synapses Links Retinal Waves to Activity-Dependent Refinement. PLoS Biol. 2007;5(3):e61. [PMC free article: PMC1808114] [PubMed: 17341130]
- 67.
- Shah R.D., Crair M.C. Retinocollicular Synapse Maturation and Plasticity Are Regulated by Correlated Retinal Waves. J. Neurosci. 2008;28(1):292–303. [PMC free article: PMC6671137] [PubMed: 18171946]
- 68.
- Chandrasekaran A.R., Shah R.D., Crair M.C. Developmental Homeostasis of Mouse Retinocollicular Synapses. J. Neurosci. 2007;27(7):1746–1755. [PMC free article: PMC6673732] [PubMed: 17301182]
- 69.
- Xu H.P., et al. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron. 2011;70(6):1115–27. [PMC free article: PMC3119851] [PubMed: 21689598]
- 70.
- Ramón y Cajal, S., The structure of the retina. 1894, Translated by S.A. Thorpe and M. Glickstein 1972, Springfield, Ill.,: C. C. Thomas. xxxix, 196 p.
- 71.
- Ben-Ari Y., et al. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87(4):1215–84. [PubMed: 17928584]
- 72.
- Ford K.J., Feller M.B. Assembly and disassembly of a retinal cholinergic network. Vis Neurosci. 2011:1–11. [PMC free article: PMC3982217] [PubMed: 21787461]
- Review Neural remodeling in retinal degeneration.[Prog Retin Eye Res. 2003]Review Neural remodeling in retinal degeneration.Marc RE, Jones BW, Watt CB, Strettoi E. Prog Retin Eye Res. 2003 Sep; 22(5):607-55.
- Strip1 regulates retinal ganglion cell survival by suppressing Jun-mediated apoptosis to promote retinal neural circuit formation.[Elife. 2022]Strip1 regulates retinal ganglion cell survival by suppressing Jun-mediated apoptosis to promote retinal neural circuit formation.Ahmed M, Kojima Y, Masai I. Elife. 2022 Mar 22; 11. Epub 2022 Mar 22.
- Review Synaptology of the inner plexiform layer in the anuran retina.[Microsc Res Tech. 2000]Review Synaptology of the inner plexiform layer in the anuran retina.Gábriel R, Rábl K, Veisenberger E. Microsc Res Tech. 2000 Sep 1; 50(5):394-402.
- An ultrastructural analysis of the development of foetal rat retina transplanted to the occipital cortex, a site lacking appropriate target neurons for optic fibres.[J Neurocytol. 1982]An ultrastructural analysis of the development of foetal rat retina transplanted to the occipital cortex, a site lacking appropriate target neurons for optic fibres.Matthews MA, West LC, Riccio RV. J Neurocytol. 1982 Aug; 11(4):533-57.
- Exploring the retinal connectome.[Mol Vis. 2011]Exploring the retinal connectome.Anderson JR, Jones BW, Watt CB, Shaw MV, Yang JH, Demill D, Lauritzen JS, Lin Y, Rapp KD, Mastronarde D, et al. Mol Vis. 2011 Feb 3; 17:355-79. Epub 2011 Feb 3.
- Formation of Early Retinal Circuits in the Inner-Plexiform Layer - WebvisionFormation of Early Retinal Circuits in the Inner-Plexiform Layer - Webvision
Your browsing activity is empty.
Activity recording is turned off.
See more...

 1.
1.