4.1. Clinical introduction
The risk assessment tools considered in this review are FRAX (with or without BMD), QFracture and BMD (measured by DXA). There are other tools available to assess the risk of fragility fracture, however, during the scoping phase of this short guideline, the stakeholders advised the developers that these three tools are the only validated tools routinely used in the UK. The GDG has therefore agreed to consider the evidence only for FRAX, QFracture and BMD.
FRAX
The FRAX tool was developed in 2008 by the WHO to calculate the risk of fractures in women and men from several clinical risk factors (CRF), with or without the measurement of femoral neck BMD. The clinical risk factors included in the FRAX algorithm are: age, sex, weight, height, previous fracture, parental hip fracture, current smoking, glucocorticoids, rheumatoid arthritis, secondary osteoporosis and alcohol intake (≥ 3units/day). It is applicable to people aged 40–90 years.
The outputs are a 10-year probability of hip fracture and the 10-year probability of a major osteoporotic fracture (clinical spine, forearm, hip or humerus fracture).
FRAX was developed using baseline and follow up data from nine prospective population-based cohorts (including Europe, Australia, Canada and Japan) and validated in 11 prospective population-based cohorts (> 1 million patient years). A UK version of FRAX calibrated to fracture epidemiology in the UK is available. The FRAX tool can be used either with or without BMD results. For clarity, in this guideline we have used the terms ‘FRAX with BMD’ and ‘FRAX without BMD’.
QFracture
QFracture was developed in 2009, and has been internally and externally validated based on large primary care populations in the UK (QResearch and THIN clinical databases). The algorithm is based on variables that are readily available in electronic healthcare records. It estimates an individual’s 10-year risk of developing both hip and major osteoporotic fractures (including hip, spine and wrist), without BMD measurement. It is applicable to people aged 30–85 years.
The clinical risk factors included in the QFracture algorithm in men and women are: age, sex, BMI, smoking, alcohol intake, glucocorticoids, asthma, cardiovascular disease, history of falls, chronic liver disease, rheumatoid arthritis, type 2 diabetes and tricyclic antidepressants. Additional factors used in women only are: hormone replacement therapy, parental history of hip fracture, menopausal symptoms, gastrointestinal malabsorption and other endocrine disorders.
explains which risk factors are included in the two risk assessment algorithms:
Risk factors included in FRAX and QFracture algorithms (as of April 2012).
BMD
BMD is the major criterion used for the diagnosis and monitoring of osteoporosis. BMD gives a quantitative assessment of bone mass per unit area, expressed in g/cm2. Many techniques are available to measure BMD, but the most widely used are based on X-ray absorptiometry in bone, particularly dual-energy X-ray absorptiometry (DXA). DXA may be used to assess bone mineral content at different sites of the skeleton, including those most vulnerable to fracture. The most commonly measured sites are the lumbar spine and the proximal femur. However, the accuracy of lumbar spine measurements may be impaired by scoliosis and vertebral deformity, and so the proximal femur is the preferred site for fragility fracture risk prediction.
The WHO defines osteoporosis as a BMD of 2.5 or more standard deviations below that of a normal young healthy female (a T score of ≤ −2.5 SD) for postmenopausal women and men over 50 years as measured by DXA at the femoral neck. Low BMD is a major risk factor for fragility fracture and other risk factors may act via their effect on BMD. Drugs that target BMD are the most common intervention for people at risk of fragility fracture. The place of BMD measurement in assessment of fragility fracture risk is therefore important.
An estimate of BMD may also be used in the FRAX tool, by either entering the type of DXA scanning equipment used and then the actual femoral neck BMD (in g/cm2) or, in women, entering the T-score based on the NHANES III female reference data and measured at the femoral neck. The FRAX tool can also be used in patients without a BMD test.
4.1.1. Review question
Which risk assessment tools are the most accurate for predicting the risk of fragility fracture in adults, including those without known osteoporosis or previous fragility fracture?
View in own window
| Population | Adult men or women (over 18 years) at risk of fragility fracture, including those without known osteoporosis or previous fragility fracture |
| Index tests (risk assessment tools) |
|
| Reference standard or target conditions | Fractures including:
vertebral hip forearm any fragility fracture.
|
| Outcomes (in terms of discrimination/calibration) |
Area under the curve. Sensitivity, specificity, predictive values. Predicted risk, observed risk. Other outcomes: D statistics, R2 statistic and Brier score.
|
| Study types |
|
4.3. Clinical evidence review on calibration
Calibration refers to how well the predicted risk corresponds to the observed risk in a population. It does not provide information as to whether the correct people are predicted to be at high risk.
Calibration results for FRAX and QFracture tools are reported below, by study.
4.3.1. Leslie 2010A45: calibration of the Canadian FRAX tool
The two tables below ( and ) report the predicted risk for hip and osteoporotic fracture, for the population divided by gender.
Predicted risk for hip fracture.
Predicted risk for major osteoporotic fracture.
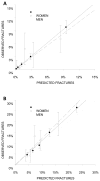
To evaluate calibration of the FRAX tool, patients were stratified by fifth of predicted risk. A graphical representation is given in below, for hip and osteoporotic fracture, divided for men and women.
Predicted 10-year fracture risk from Canadian FRAX tool with BMD (x axis) versus observed Kaplan-Meier 10-year fracture rates (y axis) by fifth of predicted risk for women (solid line) and men (dashed line). The dotted line depicts the line of identity (more...)
The regression slopes for hip fracture are:
Women: 1.03 [1.02–1.04]
Men: 0.92 [0.57–1.27]
For women, there is reasonable agreement between observed and predicted risk of hip fracture, with a slight underestimation of predicted 10-year hip fracture risk. For men, the agreement between observed and predicted risk of hip fracture is reasonable but with some noticeable differences in the last three fifths of predicted risk.
The regression slopes for major osteoporotic fracture are:
Women: 1.13 [1.08–1.19]
Men: 1.24 [1.00–1.48]
For women, there is an under-prediction for the two highest fifths of predicted 10-year fracture risk. For men, there is an underestimation of predicted 10-year fracture risk in the last three fifths of predicted 10-year fracture risks.
4.3.2. Leslie 201248: calibration of the Canadian FRAX in women receiving osteoporosis treatment
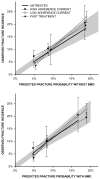
In Leslie 201248, a stepwise gradient in observed 10-year major osteoporotic and hip fracture incidence was plotted as a function of the predicted probability tertile in untreated women (reference subgroup) and each treated subgroup. The authors found that in the untreated group there was good agreement between the predicted and observed 10-year fracture incidence, with the 95% CI containing the line of identity, for both FRAX with and without BMD. Regression slopes were not reported for any of the subgroups, however, none of the 95% CI for the treated subgroups fell below the line of identity ( and ).
Predicted 10-year major osteoporotic fracture probability from FRAX versus observed fracture incidence estimated to 10-years, according to risk tertile. Results are stratified by osteoporosis treatment status with the reference group being untreated women (more...)
Predicted 10-year hip fracture probability from FRAX versus observed fracture incidence estimated to 10-years, according to risk tertile. Results are stratified by osteoporosis treatment status with the reference group being untreated women (heavy solid (more...)
Treatment effects were also assessed in 3047 women with high adherence to at least 5 years of bisphosphonate use (MPR 0.80). The only subgroup where incidence fractures were significantly less than predicted was for hip fractures in the highest risk tertile (observed/predicted ration 0.61, 95% CI 0.40–0.83, p-value<0.001), thought there were good concordance between observed and predicted major osteoporotic fractures (observed/predicted ration 0.92, 95% CI 0.78–1.06, p-value=0.280).
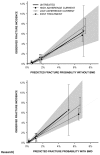
4.3.3. Fraser 201121: calibration of the Canadian FRAX with BMD tool
In Fraser 201121, patients were stratified by fifth of predicted risk and the study compared the mean 10-year fracture probabilities from the Canadian FRAX tool with BMD by quintile subgroups versus the Kaplan-Meier estimates of observed 10-year fracture outcome, divided by men and women. They found that, for major osteoporotic fracture, the predicted probabilities are within the 95% CI for the observed probability in all fifths of predicted risk, except for the middle one for women (in which the observed probability is slightly higher than the predicted) and the second lowest for men (in which the observed probability is slightly lower than the predicted probability). The regression slopes of the 10-year fracture probability predicted by the FRAX tool with BMD (x-axis) vs the Kaplan-Meier estimates of observed 10-year fracture outcome (y-axis) indicate noticeable under-prediction, for both women and men:
For hip fractures, the regression slopes of the 10-year fracture probability predicted by the FRAX tool with BMD (x-axis) vs the Kaplan-Meier estimates of observed 10-year fracture outcome (y-axis) are:
The probability predictions are within the 95% CI for the observed probability in three fifths of predicted risk (probably because of the small number of patients and small numbers of events), while in the highest two fifths of predicted risk for men the model noticeably underestimated the 10-year risk of hip fracture.
4.3.4. Bolland 20114: calibration of the UK FRAX tool in a New Zealand population
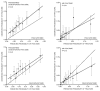
In Bolland 20114, patients were stratified by risk deciles; results are shown in .
Calibration of the calculators. Each panel shows a plot of the observed 10-year probability (expressed as decimals on a scale 0 to 1) of fracture (error bars indicate the 95% CI) versus the mean estimated fracture probability for the cohort divided by (more...)
For osteoporotic fractures, FRAX (with and without BMD) is poorly calibrated (P< 0.01). For hip fracture, the number of events is low (n = 57), therefore, although P = 0.18 for FRAX without BMD, there is a limitation in interpreting the analysis of calibration.
4.3.5. Hippisley-Cox 2009 28: calibration of QFracture in the internal validation study and comparison with FRAX
The population was stratified into tenths of predicted risk (10 categories). For osteoporotic fractures, it showed good calibration overall (ratio predicted vs observed risk ranged from 0.92 to 1.11). For hip fracture, similar results were found, except for over-prediction in the lowest tenth of risk (ratio predicted vs observed risk 1.86 and 2.32 in men and women), and the top tenth risk in men (ratio predicted vs observed risk 1.19).
The study performed a subgroup analysis to directly compare calibration of FRAX and QFracture in the same population. Results in show that FRAX tended to over-predict the risk of hip fracture within each tenth of predicted risk.
Predicted and observed 10-year risk of hip fracture with QFracture and FRAX (Reproduced from BMJ 2009;339:b4229, open access article, with permission of the author).
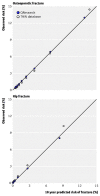
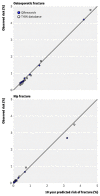
4.3.6. Collins 201110: calibration of QFracture in the external validation study
Observed versus predicted 10-year fracture risks for women. (Reproduced from BMJ 2011;342:d3651, open access article, with permission of the author)
Observed versus predicted 10-year fracture risks for men. (Reproduced from BMJ 2011;342:d3651, open access article, with permission of the author)
Overall, there is good calibration: there is close agreement between predicted and observed risk of osteoporotic and hip fractures across all deciles of risk; there is no over- or under-prediction (Figures 14 and 15). There was also close agreement between predicted and observed fracture risks across all age groups.
No relevant studies were identified for calibration of BMD.
4.4. Clinical evidence review on reclassification
Reclassification is the extent to which a model is superior to another model in terms of correct categorisation of individuals, usually at thresholds that are considered to be important for treatment (for example, using clinical- and cost-effectiveness analysis). The proportion of individuals reclassified largely depends on the threshold selected and the population studied. Reclassification data became available during the development of the guideline showing how people were reclassified between FRAX and QFracture (data prepared by Hippisley-Cox and colleagues, 2011) and with the addition of BMD to FRAX. One other study that examined reclassification when BMD is added to clinical risk factors was available and this is also reported for completeness.
See appendix D for full details of characteristics of included studies and QUADAS II quality assessment.
Table 36. Summary of included studies
Table 37. QUADAS II – Quality assessment of included studies
The study by Johansson et al.33 looked at reclassification using clinical risk factors (CRFs) alone versus CRFs with the addition of BMD. An arbitrary intervention threshold of 35% was selected. Of 2113 women, 17% (n = 354) were classified as high risk and 83% (n = 1759) were classified as low risk based on CRFs alone. After a subsequent recalculation based on CRFs plus BMD, 31% (109/354) of those initially classified as high risk would be reclassified as low risk; 12% (210/1759) of those initially classified as low risk would be reclassified as high risk. In addition, misclassifications were most frequent close to the pre-specified threshold of 35%. Results are summarised in below.
Risk reclassification data for clinical risk factors (CRFs) alone versus CRFs with the addition of BMD – major osteoporotic fracture probability.
The study by Leslie at al.44 conducted an analysis on reclassification, comparing FRAX alone with FRAX plus BMD. An intervention threshold of major osteoporotic fracture of 20% was chosen (from the National Osteoporosis Foundation [NOF] guideline). A total of 22.2% of the population (total N = 39,603) were reclassified. Most reclassification occurred around moderate risk (10–20%) and almost all reclassifications were to the adjacent risk category. Of 39,603 participants, 29% (N = 11,630) were classified as moderate risk based on FRAX alone, respectively. After subsequent recalculation based on FRAX plus BMD, a total of 10.2% (4027/11630) of those that were initially classified as moderate risk (based on FRAX alone) got reclassified as either low (N = 2957) or high (N = 1070) risk. Results are summarised in below.
Risk reclassification data for FRAX alone versus FRAX with the addition of BMD – major osteoporotic fracture probability.
Hippisley-Cox and colleagues have supplied further information, including an additional analysis of reclassification. They defined high risk a 10-year risk of hip fracture in the top tenth for each risk score. The key finding was that patients who were categorised as low risk on FRAX and high on QFracture had higher 10-year observed risks than those who were high on FRAX and low on QFracture and this is consistent in both men and women.
For women, 88.9% are classified as low risk by both scores and 8.8% are classified as high risk by both scores. 1.2% of women would be classified as high risk on FRAX but low risk on QFracture and 1.1% of women would be classified as low risk on FRAX but high risk on QFracture. Their analysis included the 10-year observed risk; however, it is not possible to draw an accurate conclusion.
Similar findings were found in men. Results are summarised in below.
Reclassification statistics – QFracture versus FRAX (data prepared by Hippisley-Cox and colleagues, 2011). Reallocation of subjects based on using the top decile of risk for each score (using Kaplan-Meier plots)
4.5. Health economic evidence review
Five studies 3,26,30,51
32 were found comparing screening strategies; however they were all excluded. Three studies3,26,30 were excluded because they compared risk assessment strategies irrelevant to the current comparison and compared screening strategies for the diagnosis of osteoporosis. One study 30 was excluded because it incorporated a treatment pathway in the economic model where treatment criteria were not applicable to the UK. The final study 32 was excluded because its did not conduct an incremental analysis and as such was not applicable for our purposes. See exclusion list in appendix E for further details of excluded studies.
An original cost analysis of performing risk assessment tools was performed. Comparators include QFracture, FRAX, BMD and FRAX plus BMD. Costs were calculated for a hypothetical cohort of patients presenting to the GP. Details of methods and results are presented in Appendix E.
Our cost analysis showed that risk assessment tools that do not include BMD measurement are less costly than those that include this measurement. Moreover, the cost difference between risk assessment tools that do not include BMD, namely FRAX and QFracture, is negligible.
In a comparison of risk assessment tools that entail assessment with DXA scan, the FRAX plus BMD strategy is less costly than the ‘BMD for all’ strategy if fewer than 68% of people are referred for a DXA scan after a FRAX in the ‘FRAX plus BMD’ strategy. After performing a series of one-way sensitivity analyses on some parameters, results were found to be sensitive to the cost of GP consultation: where the GP consultation cost is smaller than the base case estimate, the FRAX plus BMD strategy is less costly than the BMD-for-all strategy, even at higher patient referral rates for BMD in the FRAX plus BMD strategy.
4.6. Evidence statements
4.6.1. Clinical evidence statements
23 studies (total N ranged from 200 to 2,244,636) reported considerable uncertainty as to whether there was any difference in discrimination amongst FRAX, QFracture and BMD (low to very high risk of bias).
12 studies (total N ranged from 200 to 424,336) reported an AUC between 57% and 85% for FRAX; 2 studies (total N ranged from 1,275,917 to 2,244,636) reported an AUC between 69% and 89% for QFracture; 12 studies (total N ranged from 400 to 16,505) reported an AUC between 63% and 82% for BMD (low to very high risk of bias).
Four studies (total N ranged from 1422 to 39,603) reported (high risk of bias):
sensitivity between 46% and 77% and specificity between 72% and 80% for FRAX with BMD (3% threshold for hip fracture)
sensitivity between 29% and 76% and specificity between 63% and 89% for FRAX with BMD (5% threshold for hip fracture)
sensitivity between 42% and 97% and specificity between 15% and 76% for FRAX with BMD (10% threshold for major osteoporotic fracture)
sensitivity between 50% and 100% and specificity between 0% and 72% for FRAX without BMD (10% threshold for major osteoporotic fracture)
sensitivity between 9% and 28% and specificity between 81% and 96% for FRAX with BMD (20% threshold for major osteoporotic fracture)
sensitivity between 16% and 29% and specificity between 81% and 93% for FRAX without BMD (20% threshold for major osteoporotic fracture)
sensitivity between 0% and 18% and specificity between 94% and 99% for FRAX with BMD (30% threshold for major osteoporotic fracture)
sensitivity between 4% and 10% and specificity between 96% and 99% for FRAX without BMD (30% threshold for major osteoporotic fracture).
Five studies (total N ranged from 1422 to 424,336) reported (high risk of bias):
sensitivity between 59% and 79% and specificity between 39% and 86% for FRAX without BMD (3% threshold for hip fracture)
sensitivity between 39% and 78% and specificity between 50% and 92% for FRAX without BMD (5% threshold for hip fracture).
A subgroup analysis of one study (total N 424,336) reported (high risk of bias):
sensitivity of 55% and specificity of 88% for QFracture (3% threshold for hip fracture)
sensitivity of 39% and specificity of 93% for QFracture (5% threshold for hip fracture)
sensitivity of 22% and specificity of 94% for QFracture (10% threshold for major osteoporotic fracture)
sensitivity of 2% and specificity of 100% for QFracture (20% threshold for major osteoporotic fracture)
sensitivity of 0% and specificity of 100% for QFracture (30% threshold for major osteoporotic fracture).
Three studies for FRAX (total N ranged from 1422 to 39,603) and two for QFracture (total N ranged from 1,275,917 to 2,244,636) suggested that the two tools are overall well calibrated (high risk of bias).
4.6.2. Economic evidence statements
The cost difference between FRAX and QFracture risk stratification tools is negligible.
If fewer than 68% of individuals in the FRAX+BMD strategy are referred for a DXA scan, then this strategy is less costly than performing BMD for all.
4.7. Recommendations and link to evidence
View in own window
| Recommendation |
- 3.
Estimate absolute risk when assessing risk of fracture (for example, the predicted risk of major osteoporotic or hip fracture over 10 years, expressed as a percentage).
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity. |
| Trade-off between clinical benefits and harms | The GDG agreed that the output of the risk assessment should be an absolute risk. The GDG considered that the presence of one risk factor (for example, current use of glucocorticoid) is not sufficient to establish whether a patient is at high risk of fragility fracture; a formal assessment tool should be used to make the transition from RR, based on one risk factor, to absolute risk. The GDG considered that individuals are more likely to be given unnecessary treatment if treatment is based on one known risk factor. Similarly, people who might benefit from interventions might be assumed to be at low risk if an assessment that includes multiple risk factors is not carried out. The GDG considered the distinction between the 10-year fracture probability as output from risk assessment tools and the actual incidence of fractures of an individual over 10 years. Therefore, clinical judgement is needed when interpreting individual risks to patients. |
| Economic considerations | There were no published economic evaluations on the use of risk factor tools. The GDG considered that providing treatment based on RR of one known risk factor may increase costs unnecessarily and may not provide any additional health benefit to the patient. The GDG also considered that even if a patient has a substantial number of risk factors, their absolute risk may still be low and as such the patient would be unlikely to benefit from treatment. |
| Quality of evidence | The recommendation is based on review of risk tools and GDG expert opinion and consensus. |
| Other considerations | The GDG recognised that individual healthcare professionals conducting an assessment might have the expertise to recognise that the risk factors present are enough to classify the patient as high risk (for example, older people with previous fracture). However, given that the outcome of assessment might be long-term pharmacological treatment, the GDG considered that the assessment of absolute risk was preferred and is particularly important for a non-expert in the assessment of fragility fracture risk. Validated assessment tools (FRAX, QFracture) are web-based and freely available for people to use (a person can either carry out self-assessment or a healthcare professional can complete it for them). In cases when internet access and/or a computer are not available (for example, a GP on a home visit in a rural area), a simplified paper version of FRAX is also available, as well as iPhone and iPad applications for both tools.
There is no conclusive evidence that providing treatment on the basis of risk assessment will result in better clinical and cost effective care but the GDG is aware of an ongoing trial in which the efficacy of treatment in individuals identified by FRAX as being at high risk of fracture is being investigated (SCOOP study [see www.scoopstudy.ac.uk]).
The GDG considered it important to differentiate between risk and intervention thresholds. Absolute risk provides a numerical risk estimation in a given time period. The description of this risk (as, for example, low or high) is potentially influenced by a number of factors such as individual patient characteristics and type of risk being predicted.
An intervention threshold will be influenced by the absolute risk, the effect of the intervention on that risk, adverse events and costs these can be summarised as the cost effectiveness of the intervention. Different interventions can therefore have different intervention thresholds.
Different drug therapy intervention thresholds have been proposed, comprising fixed thresholds (for example, 20% 10-year fracture risk of major fracture, 3% 10-year fracture risk of hip fracture as used in the USA), and thresholds that increase with age (as proposed by National Osteoporosis Guideline Group (NOGG) and currently linked to the UK version of the FRAX website).
The GDG had some concerns that they were unable to recommend thresholds for treatment as this guideline did not include clinical and cost effectiveness of treatments. The GDG recognised that until NICE develops further guidance on treatment, the default position for many healthcare practitioners would be to follow guidance set by other organisations or that decisions about which threshold to use will be taken at a local level, in light of characteristics of the high-risk population identified. |
View in own window
| Recommendation |
- 4.
Use either FRAXh (without a bone mineral density [BMD] value, if a dual-energy X-ray absorptiometry [DXA] scan has not previously been undertaken) or QFracturei, within their allowed age ranges, to estimate 10-year predicted absolute fracture risk when assessing risk of fracture. Above the upper age limits defined by the tools, consider people to be at high risk.
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity. The GDG was interested in both calibration and discrimination of risk assessment tools. The available tools have been shown to predict hip, spine, humerus and wrist (FRAX) or hip, wrist and spine (Q-fracture), but not other fractures. |
| Trade-off between clinical benefits and harms | One purpose of risk assessment is to decide on suitability for treatment. Over- prediction will result in unnecessary treatment and anxiety, whereas under- prediction means a person would not be offered potentially preventative treatment. The GDG agreed that as a general rule an assessment tool is better than clinical judgement alone. They acknowledge that practitioners experienced in assessment of fragility risk may find an assessment tool unnecessary but that for the generalist an assessment tool is the preferred way of assessing risk. The evidence indicated that all the tools considered are better than chance at predicting risk and use of an appropriate assessment tool is unlikely to cause harm to a patient. |
| Economic considerations | The original cost analysis developed for this guideline showed that FRAX (without BMD) and QFracture risk assessment tools had similar costs and are less costly than risk assessment tools that include BMD measurement. The clinical review indicated that both FRAX and QFracture are better than chance at predicting risk of fracture. |
| Quality of evidence | All the studies included in the review were classified as being at high or very high risk of bias. The GDG was interested in validation studies of risk assessment tools in the UK. The studies on QFracture were conducted in the UK, while for FRAX only limited evidence for the UK population is available, therefore their applicability is indirect. Two validation studies (one internal and one external validation) were available for QFracture. The authors of QFracture have published a comparison of FRAX with QFracture with their internal validation of QFracture. There are no other validation studies available in the UK for FRAX.
The most common outcome reported was AUC. The evidence available to judge between FRAX and QFracture was limited. Based on the AUC alone, the tools appear to be poor to moderately predictive; however, the GDG recognised that discrimination data based on the AUC alone are not an adequate way of establishing whether one tool performs better than another due to a number of reasons, for example, the AUC is based on the ranks of the predicted probabilities and compares these ranks in people with and without the disease; but the ROC curve does not use the actual predicted probabilities Therefore it is not very sensitive to differences in probabilities between risk scores. In addition, studies included in the review contained individuals of different age ranges which may affect the AUC. Calibration data on FRAX is limited to analysis by Hippisley-Cox and colleagues.
In the direct comparison between FRAX and QFracture using the same population the results are similar. QFracture shows better performance data on all measures reported but this is the database in which QFracture was developed and the difference in risk is small in absolute terms.
The authors of QFracture provided information on reclassification between QFracture and FRAX and although these data favour QFracture the magnitude of the difference is small.
The economic evidence was based on an original cost analysis with potentially serious limitations and partial applicability. |
| Other considerations | The only tools reviewed for the guideline were FRAX and QFracture. These were the tools identified during the scoping phase as appropriate for consideration as they are either already used in the UK and/or they are the tools where evidence of their validity in a UK population might be available. The GDG considered that the information available to assess tools was suboptimal. The method of development and coefficients used in the FRAX equation are not publicly available, how FRAX treats risk factors and interactions between risk factors (for example, causes of secondary osteoporosis and BMD) are not known. The majority of studies provided AUC data only.
The GDG made a research recommendation outlining the evidence they would like to see in the assessment of risk prediction tools.
QFracture is developed and validated in GP databases but this is the setting where most risk assessment will take place. The GDG recognised that FRAX is known to the health community and that the ability to incorporate BMD can be seen as an advantage. However given the lack of evidence for added value of BMD, the GDG did not consider this facility made FRAX the tool of choice. Both tools are available as stand-alone web-based tools that people can use independently of healthcare professionals.
There is no strong evidence to suggest that one tool performs better than the other in people with a specific risk factor; for example, even if QFracture contains data for history of falls, there is no evidence it actually works better than FRAX in predicting risk of fracture in people who fall. |
- h
FRAX, the WHO fracture risk assessment tool, is available from www.shef.ac.uk/FRAX. It can be used for people aged between 40 and 90 years, either with or without BMD values, as specified.
- i
QFracture is available from www.qfracture.org. It can be used for people aged between 30 and 84 years (as of May 2012). BMD values cannot be incorporated into the risk algorithm.
View in own window
| Recommendation |
- 5.
Interpret the estimated absolute risk of fracture in people aged over 80 years with caution, because predicted 10-year fracture risk may underestimate their short-term fracture risk.
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity |
| Trade-off between clinical benefits and harms | Risk assessment tools provide absolute risk over 10 years. In older age groups, death is a competing risk, which means that in the 10-year timeframe, people are more likely to die from any cause than to experience a fragility fracture. People at risk may however benefit from interventions to prevent fracture because their absolute fracture risk over shorter time periods will be high. |
| Economic considerations | Given the lack of clinical evidence for this age group, assessing the cost-effectiveness of different strategies was not feasible. The GDG thought it best to leave decision making to the clinician on a case-by-case basis. |
| Quality of evidence | This recommendation is based on knowledge of risk assessment tools. |
| Other considerations | QFracture includes people up to 85 years and FRAX can include people up to 90 years. The available risk tools generate absolute risk over 10 years but the GDG considered that a shorter time period can be of value in informing decisions for people with short life expectancy at the time of assessment. QFracture can currently be adapted to provide risk estimation for a shorter period of time. The GDG agreed a recommendation using age 80 years as they considered the likelihood of co-morbidities or reduced life expectancy is greatest in this group. Clinical judgement is always required when interpreting risk scores but the GDG considered it particularly important in this group. |
View in own window
| Recommendation |
- 6.
Do not routinely measure BMD to assess fracture risk without prior assessment using FRAX (without a BMD value) or QFracture.
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity. |
| Trade-off between clinical benefits and harms | Measuring BMD requires radiation exposure but the amount of exposure is very low (less than natural daily background radiation). More accurate prediction would increase benefits and reduce harms for individual patients and the population. |
| Economic considerations | The original cost analysis developed for this guideline showed that risk assessment tools without BMD were less costly than risk assessment tools that incorporated BMD assessment. The threshold analysis conducted for this guideline showed that performing FRAX (without BMD) prior to BMD assessment was less costly than performing BMD assessment for all patients when referral for BMD after FRAX was less than 68%. One study32 identified in the literature review reported that the highest referral rate for BMD after FRAX assessment in women aged 50–85 without prior fracture was 14%. The GDG judged that referral rates for BMD assessment in practice would differ according to patient groups. |
| Quality of evidence | All the studies included in the review were classified as being at high or very high risk of bias. The GDG was interested in studies of BMD alone and the comparison between FRAX without BMD and FRAX including BMD in the UK.
13 cohort studies on BMD alone reported AUC as an overall outcome for predictive accuracy. AUC ranges reported by the studies were found to be very similar to those reported in the studies on FRAX and QFracture. Of the 13 studies, three were at low risk of bias, eight were at high risk of bias and one was at very high risk of bias. Reasons for bias assessment include low event rate(< 100 fractures); relatively large percentage loss to follow up and no information on fracture history. Two studies (high risk of bias, indirect applicability) that directly compared FRAX and BMD alone (lumbar spine and femoral neck or hip BMD) found similar AUC ranges. The majority of the included studies were not based on the UK population, which led to indirect applicability.
Only one study investigated the addition of BMD to FRAX and this study was in a Canadian population. An additional study investigated the addition of BMD to clinical risk factors. The overall risk of bias for both studies was high or very high, with indirect applicability. Both studies presented a cross-tabulation table of risk categories (10-year fracture probability) based on the models, which indicates the number of people who move to another risk category or remain in the same category. Reclassification occurred mostly around the set threshold. The studies did not report whether those who were reclassified were reclassified correctly. One study selected arbitrary thresholds.
Results on sensitivity and specificity of the tools at selected thresholds were not sufficient to conclude whether the addition of BMD to FRAX improves the performance of the tool. QFracture showed higher specificity, but also lower sensitivity, for hip fracture compared with FRAX, but there was not enough evidence to decide whether the difference was clinically important.
The economic evidence was based on an original cost analysis with potentially serious limitations and partial applicability. |
| Other considerations | The GDG was aware that risk assessment tools had developed from the recognition that addition of clinical risk factors to BMD improves fracture risk prediction and that measurement of BMD can be costly and difficult to access even in relatively resource-rich countries like the UK. The main outcome from the studies was AUC and the GDG recognised that AUC is typically insensitive in assessing the impact of adding a new predictor (BMD) to a risk score.
The reclassification studies examining addition of BMD to clinical risk factors or FRAX did not report whether the reclassification correctly identified people who did sustain a fracture. Reclassification was rarely from high to low categories or low to high categories and was clustered around the thresholds pre-specified in studies. The rationale for the thresholds chosen was not clear.
The GDG agreed that measurement of BMD alone should not be a routine method of assessment for fracture risk but developed further recommendations for when it would be helpful. |
View in own window
| Recommendation |
- 7.
Following risk assessment with FRAX (without a BMD value) or QFracture, consider measuring BMD with DXA in people whose fracture risk is in the region of an intervention thresholdj for a proposed treatment, and recalculate absolute risk using FRAX with the BMD value.
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity |
| Trade-off between clinical benefits and harms | The aim of assessment is to identify those at high risk and consider appropriate interventions. Measuring BMD requires radiation exposure but the amount of exposure is very low (less than natural daily background radiation). More accurate prediction would increase benefits and reduce harms for individual patients and the population. |
| Economic considerations | The original cost analysis developed for this guideline showed that risk assessment tools without BMD were less costly than risk assessment tools that incorporated BMD assessment. When the benefit of treatment is unclear, the GDG considered risk assessment using BMD is a good use of resources. Risk assessment using BMD can help reduce costs associated with unnecessary treatment and can increase health benefits for those appropriately treated. |
| Quality of evidence | The GDG used the evidence for use of BMD with and without FRAX and consensus to develop this recommendation.
The economic evidence was based on an original cost analysis with potentially serious limitations and partial applicability. |
| Other considerations | The evidence for the reclassification with the addition of BMD indicated that reclassification was most likely around the threshold for treatment. The GDG therefore concluded the BMD measurement could be considered in people whose fracture risk is in the region of an intervention threshold and risk score recalculated. People well above the threshold can be treated without measurement of BMD to assess their risk although the GDG were aware that BMD may be used to monitor treatment. People well below the threshold can be reassured that they do not need treatment, preventing people from receiving investigations and treatment they did not require
No evidence was found specifically relating to risk assessment of people who have taken or are already taking pharmacological treatment for low bone density or osteoporosis. |
- j
An intervention threshold is the level of risk at which an intervention is recommended. People whose risk is in the region from just below to just above the threshold may be reclassified if BMD is added to assessment. It is out of the scope of this guideline to recommend intervention thresholds. Healthcare professionals should follow local protocols or other national guidelines for advice on intervention thresholds.
View in own window
| Recommendation |
- 8.
Consider measuring BMD with DXA before starting treatments that may have a rapid adverse effect on bone density (for example, sex hormone deprivation for treatment for breast or prostate cancer).
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures |
| Trade-off between clinical benefits and harms | The aim of assessment is to identify those at high risk and consider appropriate interventions. Measuring BMD requires radiation exposure but the amount of exposure is very low (less than natural daily background radiation). More accurate prediction would increase benefits and reduce harms for individual patients and the population. |
| Economic considerations | The original cost analysis developed for this guideline identified that fracture risk assessment without BMD is less costly than risk assessment with BMD. However, the GDG considered risk assessment without BMD inappropriate for this patient group. The initial extra cost of BMD assessment is justified because it can help reduce down stream costs associated with fractures resulting from undetected reduction in bone density and can increase health benefit for patients who avoid fractures because of timely and appropriate treatment. |
| Quality of evidence | The GDG used the evidence for use of BMD with and without FRAX and consensus to develop this recommendation. |
| Other considerations | The GDG considered that there are some people who are at risk of dramatic bone loss which will not be captured by FRAX or QFracture. The GDG were particularly concerned about people who are about to start sex hormone deprivation treatments for breast or prostate cancer. Their change in risk as a result of their cancer treatment will not be reflected in clinical risk factors. BMD measurement before and during treatment can be used as a means of quantifying fracture risk. The GDG agreed therefore to make a recommendation that BMD should be considered for this group. The GDG considered that effect on bone density is not sustained once cancer treatment is completed. |
View in own window
| Recommendation |
- 9.
Measure BMD to assess fracture risk in people aged under 40 years who have a major risk factor, such as history of multiple fragility fracture, major osteoporotic fracture, or current or recent use of high-dose oral or systemic glucocorticoids (more than 7.5 mg prednisolone or equivalent per day for 3 months or longer).
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity. |
| Trade-off between clinical benefits and harms | Younger people who have already sustained fragility fractures, particularly at major sites and/or multiple fractures, may be at high risk of future fracture. Exposure to low-dose radiation associated with measurement of BMD is outweighed by the benefit of potentially preventing future fractures in this group. |
| Economic considerations | Both FRAX and QFracture are not applicable to younger patients. However, the GDG believe that even if a person has a substantial number of risk factors, their absolute risk may still be low and as such they would be unlikely to benefit from treatment. While BMD incurs additional cost, this initial cost can reduce additional costs associated with unnecessary treatment. |
| Quality of evidence | This recommendation was informed by the GDG knowledge of risk tools and GDG consensus. |
| Other considerations | FRAX does not include people less than 40 years. Although QFracture includes people between 30 and 40 years, the number of fractures in the dataset for this age group is small. BMD is therefore the only tool available to assess fracture risk in this age group. Multiple fragility fractures or major osteoporotic fractures should be a trigger to consider assessment. People with other factors known to increase fragility fracture incidence (for example, high-dose oral or systemic glucocorticoids, untreated premature menopause) may also be assessed using BMD measurement. The GDG was aware that it is not possible to translate BMD into absolute fracture risk and how to proceed on the basis of results of BMD is unclear. |
View in own window
| Recommendation |
- 10.
Consider recalculating fracture risk in the future:
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity. |
| Trade-off between clinical benefits and harms | Unnecessary repeated assessment will potentially expose people to anxiety about their risk. |
| Economic considerations | The GDG believed that recalculating the risk of fragility fracture any more frequently would increase costs and be unlikely to provide any additional health benefit. Recalculating risk of fracture when there is a change in risk factors will incur additional costs but can reduce long-term costs associated with fractures that have been prevented and can also increase health benefit through appropriate treatment. |
| Quality of evidence | The recommendation is based on GDG knowledge of risk assessment tools and consensus. |
| Other considerations | Absolute risk and BMD usually change slowly. The GDG considered that repeating risk assessment is unnecessary for the majority of people unless there has been a change in risk factors. If an initial assessment indicates people are near a treatment threshold than repeating the assessment after a minimum of 2 years is appropriate. The GDG also considered that if BMD was part of the original assessment, then BMD should be re-measured as part of the repeated risk assessment; however, if BMD was not part of the original assessment, then the decision of measuring BMD will be based on the result of the repeated risk assessment (see ). |
- k
An intervention threshold is the level of risk at which an intervention is recommended. It is out of the scope of this guideline to recommend intervention thresholds. Healthcare professionals should follow local protocols or other national guidelines for advice on intervention thresholds.
View in own window
| Recommendation |
- 11.
Take into account that risk assessment tools may underestimate fracture risk in certain circumstances, for example if a person: has a history of multiple fractures has had previous vertebral fracture(s) has a high alcohol intake is taking high-dose oral or high-dose systemic glucocorticoids (more than 7.5 mg prednisolone or equivalent per day for 3 months or longer) has other causes of secondary osteoporosis l.
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity. |
| Trade-off between clinical benefits and harms | Underestimation of actual risk using risk score could result in people being falsely reassured about their risk and not receiving appropriate interventions. |
| Economic considerations | Underestimation of actual risk would increase long-term costs and reduce health benefits. |
| Quality of evidence | This recommendation was informed by the evidence review of individual risk factors and the review of risk tools. |
| Other considerations | The GDG used their knowledge of how the risk tools work in practice to inform this recommendation. FRAX allows a binary response (yes/no) to history of fracture, smoking, oral or systemic glucocorticoid use, alcohol use and causes of secondary osteoporosis.
The GDG considered that the binary response to previous fracture would potentially result in an underestimation of fracture risk if an individual has had multiple fractures.
The reviews suggested a dose–response relationship with alcohol and smoking. The FRAX equations are not publicly available but the GDG considered the effect seen with heavy alcohol consumption in UK datasets would be unlikely to be captured in the binary response. The IPD analysis included only three cohorts with alcohol data, none of which was from the UK. Adjustment to the risk score may therefore be required. The GDG considered the dose effect with smoking to be of lesser magnitude.
The FRAX website advises that glucocorticoid use is defined as ≥5 mg/day prednisolone or equivalent for at least 3 months. The GDG was aware of evidence of a dose response relationship between glucocorticoids and fracture risk. Glucocorticoids can be used at different doses for differing periods of time and FRAX would underestimate the effect of higher doses on fracture risk.
The GDG considered that risk scores are likely to underestimate risk attributed to causes of secondary osteoporosis. The GDG understands FRAX assumes that all the effect of causes of secondary osteoporosis (other than those which are covered by other questions, for example oral glucocorticoids and rheumatoid arthritis) is mediated through BMD and that by ticking this box an undefined BMD correction is used in the assessment. The GDG considered it likely that at least some causes of secondary osteoporosis affect fracture risk by mechanisms that are partially independent of BMD and fracture risk may therefore be underestimated in such patients. The GDG was also concerned that coding in routine general practices’ databases may not be sufficiently accurate to identify many secondary causes such as hypogonadism and chronic liver disease.
QFracture allows a more detailed quantification of both smoking and alcohol consumption but allows only a yes/no response to oral glucocorticoid use. |
- l
Causes of secondary osteoporosis include endocrine (hypogonadism in either sex including untreated premature menopause and treatment with aromatase inhibitors or androgen deprivation therapy; hyperthyroidism; hyperparathyroidism; hyperprolactinaemia; Cushing’s disease; diabetes), gastrointestinal (coeliac disease; inflammatory bowel disease; chronic liver disease; chronic pancreatitis; other causes of malabsorption), rheumatologival (rheumatoid arthritis; other inflammatory arthropathies), haematological (multiple myeloma; haemoglobinopathies; systemic mastocytosis), respiratory (cystic fibrosis; COPD), metabolic (homocystinuria), chronic renal disease, immobility (due for example to neurological injury or disease).
View in own window
| Recommendation |
- 12.
Take into account that fracture risk can be affected by factors that may not be included in the risk tool, for example living in a care home or taking drugs that may impair bone metabolism (such as anti-convulsants, selective serotonin reuptake inhibitors, thiazolidinediones, proton pump inhibitors and anti-retroviral drugs).
|
|---|
| Relative values of different outcomes | The clinical outcomes that the GDG wished to predict were hip fractures and all osteoporotic fractures. Although hip fractures are particularly associated with significant morbidity and mortality, other osteoporotic fractures, particularly vertebral fractures, are a cause of significant morbidity. |
| Trade-off between clinical benefits and harms | Underestimation of actual risk using risk scores may result in patients being falsely reassured about their risk and/or not receiving appropriate interventions from which they might benefit. |
| Economic considerations | There were no published economic evaluations. Underestimation of risk would increase long-term costs and reduce health benefits. |
| Quality of evidence | This recommendation was informed by the GDG knowledge of risk tools and GDG consensus. |
| Other considerations | Risk estimation tools do not include all factors that can influence fracture risk. Factors that are a significant risk factor for an individual may not be well recorded, not easily measured or not important for much of the population. Some anti-epileptic drugs (for example carbamazepine, primidone, phenobarbital, phenytoin, and sodium valproate ) interfere with vitamin D metabolism and aromatase inhibitors are associated with reduced BMD. Immobility for physical or mental reasons is not included in risk scores but will affect bone density and fracture risk.
Residents of care homes are likely to have many risk factors already included in risk scores (for example, older age, previous fracture, low BMI) but also factors not included such as poor mobility. There is some evidence that their BMD is lower than expected by age, they have a high risk of falls and high fracture rates. The GDG have developed a research recommendation to examine risk prediction for residents of care homes but considered that healthcare professionals should be aware of the combination of risks carried by this population, their high fracture risk and their low median survival. The median survival in a care home is < 600 days) which means 10-year risk is not appropriate. 5,22
1
50 |
4.8. Research recommendations
2. What is the utility of FRAX and QFracture in adults receiving bone protective therapy?
Why this is important
Because of concerns about rare but serious side-effects of long-term anti-resorptive therapy, many physicians prescribe these drugs for a finite period of time, usually 3–5 years. Reassessment of fracture risk at the end of this treatment period is important, since some people remain at high risk of fracture and require continued treatment whereas others may benefit from a ‘drug holiday’ for 1 or more years. Neither FRAX nor QFracture has been examined in treated patients, and it is not known whether the ability of clinical risk factors with or without measurement of BMD to predict fracture risk is similar in untreated and treated patients. There is therefore a need for prospective studies to investigate the predictive power of these tools to assess fracture risk in patients after a period of bone protective therapy.
3. What is the utility of FRAX and QFracture in detecting risk of fragility fracture in adults with causes of secondary osteoporosis?
Why this is important
If secondary osteoporosis is entered as a risk factor in FRAX, the algorithm assumes that the effect is mediated solely through effects on BMD. Input of BMD into the questionnaire in such patients will therefore generate the same fracture risk whether or not secondary osteoporosis is entered. However, it is likely that at least some causes of secondary osteoporosis (for example, inflammatory bowel disease) affect fracture risk by mechanisms that are partially independent of BMD and fracture risk may therefore be underestimated in such patients. There is therefore a need to investigate the accuracy of FRAX in predicting fracture risk in patients with causes of secondary osteoporosis other than rheumatoid arthritis and to establish whether their effect on fracture risk is mediated solely through effects on BMD.
4. What is the added prognostic value of BMD in the assessment of fracture risk with FRAX?
Why this is important
The 10-year fracture risk as estimated by FRAX is calculated using clinical risk factors with or without BMD. The clinical risk factors are routinely available, making calculation of fracture risk possible at the time of consultation. However, refinement of a patient’s 10-year fracture risk using BMD requires assessment using DXA scanning equipment.
Currently, there are no published studies in primary or secondary care evaluating whether the addition of BMD to FRAX improves the accuracy of the predicted fracture risk. There is a need for studies to examine whether adding BMD to FRAX results in the correct reclassification of patients from low risk to high risk (and vice-versa). Furthermore, studies are also needed to evaluate the clinical usefulness (net benefit) of adding BMD to FRAX; that is, how many more patients are correctly classified as high risk (true positives) and low risk (true negatives).
5. What is the utility of FRAX and QFracture in detecting risk of fragility fracture in adults living in residential care?
Why this is important
Care home residents are at high risk of fragility fracture5,22. This is probably related to increased age and frailty with multiple comorbidities, which increase fracture risk. There is also evidence that care home residents have lower BMD, with 70% assessed as having osteoporosis using densitometry criteria alone1. However, tools such as FRAX and QFracture, which only estimate fracture risk up to the ninth decade and use 10-year fracture risk, may under-estimate short-term risk in care home residents, who currently have a mean age of approximately 85 years and a life expectancy of less than 5 years50.
A study is required to assess whether care home residents should have targeted fracture risk assessment and whether residents at higher risk of fracture can be identified, using FRAX or QFracture. This could result in a more effective and efficient strategy for fracture prevention targeting health service resources on those at the very highest fracture risk.
6. What is the accuracy of FRAX, QFracture and BMD in detecting risk of fragility fracture in adults of different ethnic origin in the UK population?
Why this is important
The total population of the UK is around 60 million, with the ethnic minority population making up 7.9 per cent of that total in the 2001 census. The largest category was people of South Asian family origin, who accounted for 2 million people or 3.5% of the population. According to recent research, minority ethnic groups will increase and make up a fifth of Britain’s population by 2051. Fragility fracture risk assessment tools such as FRAX and QFracture were derived from populations that may not reflect this ethnic diversity, and also make assumptions across different racial and ethnic groups that may not be valid. There is concern that these tools will not reliably predict which individuals from minority ethnic groups will or will not sustain a fracture. Further work is therefore needed to determine if these risk assessment tools are accurate and reliable in predicting fracture risk in different ethnic groups in England and Wales.