Table
In vitro Rodents
Background
[PubMed]
5-Hydroxytryptamine (5-HT), commonly known as serotonin, has diverse physiological roles as a neurotransmitter in the central nervous system (1). 5-HT is involved in regulation and modulation of sleep, affective and personality behaviors, and pain. It also is a regulator of smooth muscle function and platelet aggregation. The brain cortical 5-HT system has been implicated in several neuropsychiatric disorders, including major depression, anxiety, schizophrenia, and obsessive-compulsive disorder (2, 3). The effects of 5-HT are mediated by as many as seven classes of receptor populations (5-HT1 to 5-HT7), many of which include several subtypes (4). There are five receptor subtypes within the G-protein–coupled 5-HT1 receptor family: 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F.
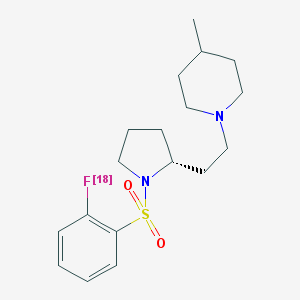
5-HT7 receptors are abundantly present in the hippocampus, thalamus, and hypothalamus; low densities are observed in the cortex and amygdala (5-7). 5-HT7 receptors are involved in the mediation of emotion and the function of the hypothalamus. 5-HT7 receptors are implicated in anxiety, depression, hallucinogenic behavior, circadian rhythms and sleep, memory, epilepsy, and pain. Thus, there is a need for selective ligands to investigate the pharmacological role of 5-HT7 receptors. 1-(2-{(2R)-1-[(2-[18F]Fluorophenyl)sulfonyl]pyrrolidin-2-yl}ethyl)-4-methylpiperidine ([18F]-2FP3) was evaluated as a positron emission tomography (PET) probe for 5-HT7 receptors because the unlabeled 2FP3 was found to be a selective 5-HT7 antagonist with nanomolar affinity for the 5-HT7 receptor.
Synthesis
[PubMed]
The automated radiosynthesis of [18F]-2FP3 involved standard fluoronucleophilic substitution of the corresponding nitro precursor with K[18F]F/Kryptofix2.2.2 in dimethyl sulfoxide for 10 min at 150°C in an automated radiosynthesis unit, followed by solid-phase extraction with C18 cartridge (8, 9). The reported overall radiochemical yield of the radiosynthesis was 48% at the end of bombardment, with a specific activity of 40–130 MBq/nmol (1.1–3.5 mCi/nmol) at the end of synthesis, and a radiochemical purity of >98%. The total synthesis time was 100–120 min. The log P value for [18F]-2FP3 was 1.2–1.4.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
Lemoine et al. (8) performed in vitro autoradiography in rat brains with [18F]-2FP3, which exhibited radioactivity in the cingulate cortex, hippocampus, cerebellum, and thalamus. The radioactivity in the cortex and hippocampus was inhibited by SB269970 (a selective 5-HT7 receptor antagonist), with 43% inhibition at 10 nM and 80% inhibition at 1,000 nM. In vitro binding was performed with CHO cells transfected with human recombinant 5-HT receptors. The binding affinity Kd values of [18F]-2FP3 for 5-HT7 receptors was 1.43 nM with little affinity for 5-HT1A and 5-HT6 receptors.
Animal Studies
Rodents
[PubMed]
Ex vivo stability studies of [18F]-2FP3 in the brains of rats (n = 3/group) were performed at 10, 20, 30, and 40 min after intravenous injection of 55.5 MBq (1.5 mCi) [18F]-2FP3. [18F]-2FP3 remained >95% intact at these time points as determined with HPLC (8).
Rat brains were excised at 20 min after injection of 55.5 MBq (1.5 mCi) [18F]-2FP3. Ex vivo PET imaging studies (n = 3/group) were performed at 45 min after the brains were removed (8). Higher radioactivity levels were observed in the cingulate cortex and hippocampus than in the striatum and cerebellum. Pretreatment with excess SB269970 (30 min, 5 mg/kg) decreased [18F]-2FP3 binding in all brain areas.
Other Non-Primate Mammals
[PubMed]
Lemoine et al. (8) performed in vivo PET imaging studies in the brains of two male cats for 90 min after injection of 74 MBq (2 mCi) [18F]-2FP3. High initial radioactivity levels were observed in the cingulate cortex, thalamus, hippocampus, and cerebellum. Pretreatment with excess SB269970 (30 min, 5 mg/kg) decreased [18F]-2FP3 binding in these brain areas to background levels.
References
- 1.
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44(3):151–62. [PubMed: 9693387]
- 2.
- Fletcher A., Cliffe I.A., Dourish C.T. Silent 5-HT1A receptor antagonists: utility as research tools and therapeutic agents. Trends Pharmacol Sci. 1993;14(12):41–8. [PubMed: 8122313]
- 3.
- Hoyer D., Clarke D.E., Fozard J.R., Hartig P.R., Martin G.R., Mylecharane E.J., Saxena P.R., Humphrey P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994;46(2):157–203. [PubMed: 7938165]
- 4.
- Lanfumey L., Hamon M. 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3(1):1–10. [PubMed: 14965240]
- 5.
- Bard J.A., Zgombick J., Adham N., Vaysse P., Branchek T.A., Weinshank R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem. 1993;268(31):23422–6. [PubMed: 8226867]
- 6.
- Varnas K., Thomas D.R., Tupala E., Tiihonen J., Hall H. Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci Lett. 2004;367(3):313–6. [PubMed: 15337256]
- 7.
- Martin-Cora F.J., Pazos A. Autoradiographic distribution of 5-HT7 receptors in the human brain using [3H]mesulergine: comparison to other mammalian species. Br J Pharmacol. 2004;141(1):92–104. [PMC free article: PMC1574165] [PubMed: 14656806]
- 8.
- Lemoine L., Andries J., Le Bars D., Billard T., Zimmer L. Comparison of 4 radiolabeled antagonists for serotonin 5-HT(7) receptor neuroimaging: toward the first PET radiotracer. J Nucl Med. 2011;52(11):1811–8. [PubMed: 21990574]
- 9.
- Andries J., Lemoine L., Le Bars D., Zimmer L., Billard T. Synthesis and biological evaluation of potential 5-HT(7) receptor PET radiotracers. Eur J Med Chem. 2011;46(8):3455–61. [PubMed: 21620533]
Publication Details
Author Information and Affiliations
Publication History
Created: July 20, 2012; Last Update: November 1, 2012.
Copyright
Publisher
National Center for Biotechnology Information (US), Bethesda (MD)
NLM Citation
Leung K. 1-(2-{(2R)-1-[(2-[18F]Fluorophenyl)sulfonyl]pyrrolidin-2-yl}ethyl)-4-methylpiperidine. 2012 Jul 20 [Updated 2012 Nov 1]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
 In vitro
In vitro