By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsThe paracrine factors are inducer proteins. We now turn to the molecules involved in the response to induction. These molecules include the receptors in the membrane of the responding cell, which binds the paracrine factor, and the cascade of interacting proteins that transmit a signal through a pathway from the bound receptor to the nucleus. These pathways between the cell membrane and the genome are called signal transduction pathways. Several types of signal transduction pathways have been discovered, and we will outline some of the major ones here. As you will see, they appear to be variations on a common and rather elegant theme: Each receptor spans the cell membrane and has an extracellular region, a transmembrane region, and a cytoplasmic region. When a ligand (the paracrine factor) binds its receptor in the extracellular region, the ligand induces a conformational change in the receptor's structure. This shape change is transmitted through the membrane and changes the shape of the cytoplasmic domains. The conformational change in the cytoplasmic domains gives them enzymatic activity—usually a kinase activity that can use ATP to phosphorylate proteins, including the receptor molecule itself. The active receptor can now catalyze reactions that phosphorylate other proteins, and this phosphorylation activates their latent activities in turn. Eventually, the cascade of phosphorylation activates a dormant transcription factor, which activates (or represses) a particular set of genes.
The RTK pathway
The RTK signal transduction pathway was one of the first pathways to unite various areas of developmental biology. Researchers studying Drosophila eyes, nematode vulvae, and human cancers found that they were all studying the same genes. The RTK-Ras pathway begins at the cell surface, where a receptor tyrosine kinase (RTK) binds its specific ligand. Ligands that bind to RTKs include the fibroblast growth factors, epidermal growth factors, platelet-derived growth factors, and stem cell factor. Each RTK can bind only one or a small set of these ligands. (Stem cell factor, for instance, will bind to only one RTK, the Kit protein.) The RTK spans the cell membrane, and when it binds its ligand, it undergoes a conformational change that enables it to dimerize with another RTK. This conformational change activates the latent kinase activity of each RTK, and these receptors phosphorylate each other on particular tyrosine residues. Thus, the binding of the ligand to the receptor causes the autophosphorylation of the cytoplasmic domain of the receptor.
The phosphorylated tyrosine on the receptor is then recognized by an adaptor protein (Figure 6.14). The adaptor protein serves as a bridge that links the phosphorylated RTK to a powerful intracellular signaling system. While binding to the phosphorylated RTK through one of its cytoplasmic domains, the adaptor protein also activates a G protein. Normally, the G protein is in an inactive, GDP-bound state. The activated receptor stimulates the adaptor protein to activate the guanine nucleotide releasing factor. This protein exchanges a phosphate from a GTP to transform the bound GDP into GTP. The GTP-bound G protein is an active form that transmits the signal. After delivering the signal, the GTP on the G protein is hydrolyzed back into GDP. This catalysis is greatly stimulated by the complexing of the Ras protein with the GTPase-activating protein (GAP). In this way, the G protein is returned to its inactive state, where it can await further signaling. One of the major G proteins is called Ras; mutations in the RAS gene account for a large proportion of human tumors (Shih and Weinberg 1982), and the mutations of RAS that make it oncogenic all inhibit the binding of the GAP protein. Without the GAP protein, Ras protein cannot catalyze GTP well, and so remains in its active configuration (Cales et al. 1988; McCormick 1989).
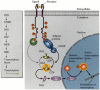
Figure 6.14
The widely used RTK signal transduction pathway. The receptor tyrosine kinase is dimerized by the ligand, which causes the autophosphorylation of the receptor. The adaptor protein recognizes the phosphorylated tyrosines on the RTK and activates an intermediate (more...)
The active G protein associates with a kinase called Raf. The G protein recruits the inactive Raf protein to the cell membrane, where it becomes active (Leevers et al. 1994; Stokoe et al. 1994). The Raf protein is a kinase that activates the MEK protein by phosphorylating it. MEK is itself a kinase, which activates ERK by phosphorylation. And ERK is a kinase that can enter the nucleus and phosphorylate certain transcription factors. This pathway is critical in numerous developmental processes.
In the migrating neural crest cells of humans and mice, the RTK pathway is important in activating the microphthalmia transcription factor (Mitf) to produce the pigment cells. We have been following the Mitf transcription factor for the past two chapters. It is transcribed in the pigment-forming melanoblast cells that migrate from the neural crest into the skin and in the melanin-forming cells of the pigmented retina. But we have not yet discussed what proteins signal this transcription factor to become active. The clue lay in two mouse mutants whose phenotypes resemble those of mice homozygous for microphthalmia mutations. Like those mice, homozygous White mice and homozygous Steel mice are white because their pigment cells have failed to migrate. Perhaps all three genes (Mitf, Steel, and White) are on the same developmental pathway. In 1990, several laboratories demonstrated that the Steel gene encodes a paracrine protein called stem cell factor (see Witte 1990). Stem cell factor binds to and activates the Kit receptor tyrosine kinase encoded by the White gene (Spritz et al. 1992; Wu et al. 2000). The binding of stem cell factor to the Kit RTK dimerizes the Kit protein, causing it to become phosphorylated. The phosphorylated Kit activates the pathway whereby phosphorylated ERK is able to phosphorylate the Mitf transcription factor (Hsu et al. 1997; Hemesath et al. 1998). Only the phosphorylated form of Mitf is able to bind the p300/CBP coactivator protein that enables it to activate transcription of the genes encoding tyrosinase and other proteins of the melanin-formation pathway (Figure 6.15; Price et al. 1998).
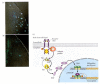
Figure 6.15
Activation of the Mitf transcription factor through the binding of stem cell factor by the Kit RTK protein. The information received at the cell membrane is sent to the nucleus by the RTK signal transduction pathway. (A, B) Demonstration that Kit protein (more...)
The Smad pathway
Members of the TFG-β superfamily of paracrine factors activate members of the Smad family of transcription factors (Figure 6.20; Heldin et al. 1997). The TGF-β ligand binds to a type II TGF-β receptor, which allows that receptor to bind to a type I TGF-β receptor. Once the two receptors are in close contact, the type II receptor phosphorylates a serine or threonine on the type I receptor, thereby activating it. The activated type I receptor can now phosphorylate the Smad proteins. (Researchers named the Smad proteins by eliding the names of the first identified members of this family: the C. elegans Sma protein and the Drosophila Mad protein.) Smads 1 and 5 are activated by the BMP family of TGF-β factors, while the receptors binding activin and the TGF-β family phosphorylate Smads 2 and 3. These phosphorylated Smads bind to Smad 4 and form the transcription factor complex that will enter the nucleus. In vertebrates, the TGF-β superfamily ligand Nodal appears to activate the Smads pathway in those cells responsible for the formation of the mesoderm and for specifying the left-right axis in vertebrates (Graff et al. 1996; Nomura and Li 1998).
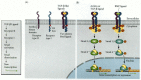
Figure 6.20
The Smad pathway activated by TGF-β superfamily ligands. (A) An activation complex is formed by the binding of the ligand by the type I and type II receptors. This allows the type II receptor to phosphorylate the type I receptor on particular (more...)
The JAK-STAT pathway
Another important pathway transducing information on the cell membrane to the nucleus is the JAK-STAT pathway. Here the set of transcription factors consists of the STAT (signal transducers and activators of transcription) proteins (Ihle 1995Ihle 1996). STATs are phosphorylated by certain receptor tyrosine kinases, including fibroblast growth factor receptors and the JAK family of tyrosine kinases. The JAK-STAT pathway is extremely important in the differentiation of blood cells and in the activation of the casein gene during milk production (Briscoe et al. 1994; Groner and Gouilleux 1995). The role of this pathway in casein production is shown in Figure 6.21. Here, the endocrine factor prolactin binds to the extracellular regions of prolactin receptors, causing them to dimerize. A JAK protein kinase is bound to each of the receptors (in their respective cytoplasmic regions), and these JAK proteins are now brought together, where they can phosphorylate the receptors at several sites. The receptors are now activated and have their own protein kinase activity. Therefore, the JAK proteins convert a receptor into a receptor tyrosine kinase. The activated receptors can now phosphorylate particular inactive STATs and cause them to dimerize. These dimers are the active form of the STAT transcription factors, and they are translocated into the nucleus, where they bind to specific regions of DNA. In this case, they bind to the upstream promoter elements of the casein gene, causing it to be transcribed.

Figure 6.21
A STAT pathway: the casein gene activation pathway activated by prolactin. The casein gene is activated during the last (lactogenic) phase of mammary gland development, and its signal is the secretion of the hormone prolactin from the anterior pituitary (more...)
The STAT pathway is very important in the regulation of human fetal bone growth. Mutations that prematurely activate the STAT pathway have been implicated in some severe forms of dwarfism such as the lethal thanatophoric dysplasia, wherein the growth plates of the rib and limb bones fail to proliferate. The short-limbed newborn dies because its ribs cannot support breathing. The genetic lesion resides in the gene encoding fibroblast growth factor receptor 3 (FGFR3) (Figure 6.22; Rousseau et al. 1994; Shiang et al. 1994). This protein is expressed in the cartilage precursor cells—known as chondrocytes—in the growth plates of the long bones. Normally, the FGFR3 protein is activated by a fibroblast growth factor, and it signals the chondrocytes to stop dividing and begin differentiating into cartilage. This signal is mediated by the STAT1 protein, which is phosphorylated by the activated FGFR3 and then translocated into the nucleus. Inside the nucleus, this transcription factor activates the genes encoding a cell cycle inhibitor, the p21 protein (Su et al. 1997). The mutations causing thanatophoric dwarfism result in a gain-of-function phenotype, wherein the mutant FGFR3 is active constitutively—that is, without the need to be activated by an FGF (Deng et al. 1996; Webster and Donoghue 1996). This causes the chondrocytes to stop proliferating shortly after they are formed, and the bones fail to grow. Mutations that activate FGFR3 to a lesser degree produce achondroplasic (short-limbed) dwarfism, the most prevalent human dominant syndrome.

Figure 6.22
A mutation in the gene for FGFR3 causes the premature constitutive activation of the STAT pathway and the production of phosphorylated Stat1 protein. This transcription factor activates genes that cause the premature termination of chondrocyte cell division. (more...)
WEBSITE
6.6 FGFR mutations. Mutations of the human FGF receptors have been associated with several skeletal malformation syndromes, including syndromes wherein skull cartilage, rib cartilage, or limb cartilage fails to grow or differentiate. http://www.devbio.com/chap06/link0606.shtml
The Wnt pathway
Members of the Wnt family of paracrine factors interact with transmembrane receptors of the Frizzled family. In most instances, the binding of Wnt by the Frizzled protein causes the Frizzled protein to activate the Disheveled protein. Once the Disheveled protein is activated, it inhibits the activity of the glycogen synthase kinase-3 enzyme. GSK-3, if it were active, would prevent the dissociation of the β-catenin protein from the APC protein, which targets β-catenin for degradation. However, when the Wnt signal is given and GSK-3 is inhibited, β-catenin can dissociate from the APC protein and enter the nucleus. Once inside the nucleus, it can form a heterodimer with an LEF or TCF DNA-binding protein, becoming a transcription factor. This complex binds to and activates the Wnt-responsive genes (Figure 6.23A; Behrens et al. 1996; Cadigan and Nusse 1997).
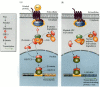
Figure 6.23
The Wnt signal transduction pathway. (A) The Wnt protein binds to its receptor, a member of the Frizzled family of proteins. The Frizzled protein then activates Disheveled, allowing it to become an inhibitor of glycogen synthase kinase 3 (GSK-3). GSK-3, (more...)
This model is undoubtedly an oversimplification, because different cells use this pathway in different ways (see Cox and Peifer 1998). Moreover, its components can have more than one function in the cell. In addition to being part of the Wnt signal transduction cascade, GSK-3 is also a metabolic enzyme regulating glycogen metabolism. The β-catenin protein was first recognized as being part of the cell adhesion complex on the cell surface before it was also found to be a transcription factor. The APC protein also functions as a tumor suppressor in adults. The transformation of normal colon cells into colon cancer is thought to occur when the APC gene is mutated and can no longer keep the β-catenin protein out of the nucleus (Figure 6.23B; Korinek et al. 1997; He et al. 1998). Once in the nucleus, β-catenin can bind with another transcription factor and activate genes for cell division.
One principle that is readily seen in the Wnt pathway (and which is also evident in the Hedgehog pathway) is that activation is often accomplished by inhibiting an inhibitor. Thus, the GSK-3 protein is an inhibitor that is itself repressed by the Wnt signal.
The Hedgehog pathway
Members of the Hedgehog protein family function by binding to a receptor called Patched. The Patched protein, however, is not a signal transducer. Rather, it is bound to a signal transducer, the Smoothened protein. The Patched protein prevents the Smoothened protein from functioning. In the absence of Hedgehog binding to Patched, the Smoothened protein is inactive, and the Cubitus interruptus (Ci) protein is tethered to the microtubules of the responding cell. While on the microtubules, it is cleaved in such a way that a portion of it enters the nucleus and acts as a transcriptional repressor. This portion of the Ci protein binds to the promoters and enhancers of particular genes and acts as an inhibitor of transcription. When Hedgehog binds to the Patched protein, the Patched protein's shape is altered such that it no longer inhibits Smoothened. The Smoothened protein acts (probably by phosphorylation) to release the Ci protein from the microtubules and to prevent its being cleaved. The intact Ci protein can now enter the nucleus, where it acts as a transcriptional activator of the same genes it used to repress (Figure 6.24; Aza-Blanc et al. 1997).

Figure 6.24
The Hedgehog signal transduction pathway. The Patched protein in the cell membrane is an inhibitor of the Smoothened protein. (A) In the absence of Hedgehog binding to Patched, the Ci protein is tethered to the microtubules (by the Cos2 and Fused proteins). (more...)
The Hedgehog pathway is extremely important in limb and neural differentiation in vertebrates. When mice were made homozygous for a mutant allele of Sonic hedgehog, they had major limb abnormalities as well as cyclopia—a single eye in the center of the forehead (Chiang et al. 1996). The vertebrate homologues of the Ci protein in Drosophila are the Gli proteins. Severe truncations of the human GLI3 gene produce a nonfunctional protein that gives rise to Grieg cephalopoly-syndactyly, a condition involving a high forehead and extra digits. A less severe truncation retains the DNA binding domain of the GLI3 protein, but deletes the activion region. Thus, this mutant GLI3 protein can act only as a repressor. This protein is found in patients with Pallister-Hall syndrome, a much more severe syndrome (indeed, lethal soon after birth) involving not only extra digits, but also poor development of the pituitary gland, hypothalamus, anus, and kidneys (see Shin et al. 1999). While mutations that inactivate the Hedgehog pathway can cause malformations, mutations that activate the pathway ectopically can cause cancers. If the Patched protein is mutated in somatic tissues such that it can no longer inhibit Smoothened, it can cause basal cell carcinomas, tumors of the basal cell layer of the epidermis. Heritable mutations of the patched gene cause basal cell nevus syndrome, a rare autosomal dominant condition characterized by both developmental anomalies (fused fingers, rib and facial abnormalities) and multiple malignant tumors such as basal cell carcinoma (Hahn et al. 1996; Johnson et al. 1996).
One remarkable feature of the Hedgehog signal transduction pathway is the importance of cholesterol. First, cholesterol is critical for the catalytic cleavage of Sonic hedgehog protein. Only the amino-terminal portion of the protein is functional and secreted. Second, the Patched protein that binds the Sonic hedgehog protein also needs cholesterol in order to function. It has recently been found (Kelley et al. 1996; Roessler et al. 1996) that some human cyclopia syndromes are caused by mutations in genes that encode either Sonic hedgehog or the enzymes that synthesize cholesterol. Moreover, certain chemicals that induce cyclopia do so by interfering with the cholesterol biosynthetic enzymes (Figure 6.25; Beachy et al. 1997; Cooper et al. 1998). Environmental factors that cause developmental anomalies are called teratogens (from the Greek, meaning “monster-former”), and they will discussed in more detail in Chapter 21. Two teratogens known to cause cyclopia in vertebrates are jervine and cyclopamine. Both substances are found in the plant Veratrum californicum (Keeler and Binns et al. 1968), and both block the synthesis of cholesterol.

Figure 6.25
Head of a cyclopic lamb born of a ewe who had eaten Veratrum californicum early in pregnancy. The cerebral hemispheres fused, forming only one central eye and no pituitary gland. The jervine alkaloid made by this plant inhibits cholesterol synthesis, (more...)
Box
The RTK Pathway and Cell-to-Cell Induction.
Box
The Nature of Human Syndromes.
- Cell Surface Receptors and Their Signal Transduction Pathways - Developmental Bi...Cell Surface Receptors and Their Signal Transduction Pathways - Developmental Biology
Your browsing activity is empty.
Activity recording is turned off.
See more...